Submitted:
26 May 2023
Posted:
30 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. Participants
2.4. Data Sources
2.5. Variables
2.6. Statistical Analysis
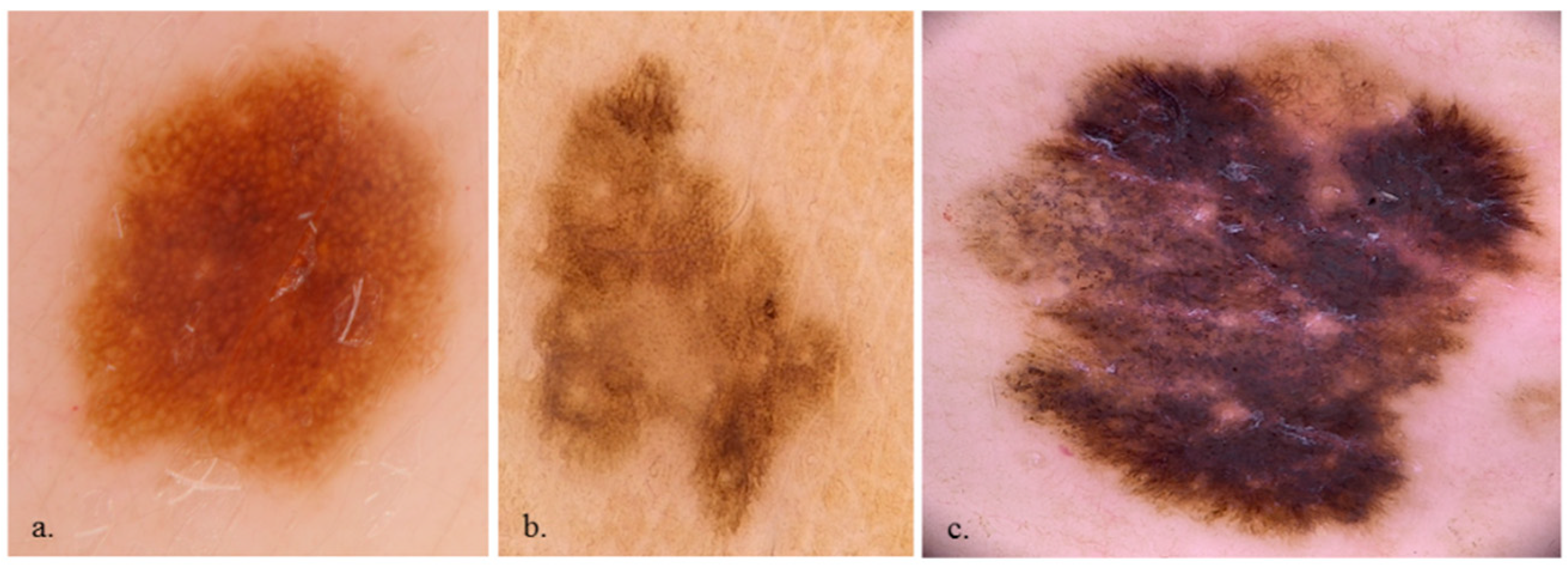
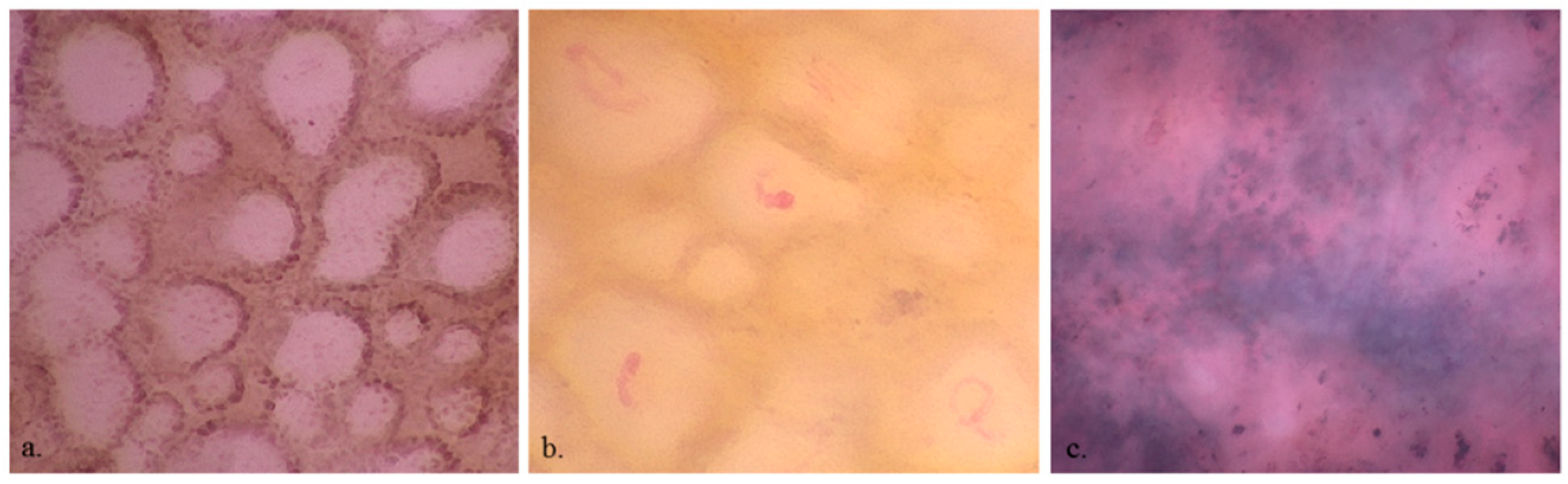
3. Results
3.1. Participants and Lesion Data
3.4. Multivariate Analysis
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Garbe, C.; Peris, K.; Hauschild, A.; Saiag, P.; Middleton, M.; Spatz, A.; Grob, J.J.; Malvehy, J.; Newton-Bishop, J.; Stratigos, A.; et al. Diagnosis and treatment of melanoma: European consensus-based interdisciplinary guideline. Eur J Cancer 2010, 46, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Sacchetto, L.; Zanetti, R.; Comber, H.; Bouchardy, C.; Brewster, D.H.; Broganelli, P.; Chirlaque, M.D.; Coza, D.; Galceran, J.; Gavin, A.; et al. Trends in incidence of thick, thin and in situ melanoma in Europe. Eur J Cancer 2018, 92, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015, 136, E359–386. [Google Scholar] [CrossRef] [PubMed]
- Iannacone, M.R.; Youlden, D.R.; Baade, P.D.; Aitken, J.F.; Green, A.C. Melanoma incidence trends and survival in adolescents and young adults in Queensland, Australia. Int J Cancer 2015, 136, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet (London, England) 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Jerant, A.F.; Johnson, J.T.; Sheridan, C.D.; Caffrey, T.J. Early detection and treatment of skin cancer. American family physician 2000, 62, 357–368. [Google Scholar] [PubMed]
- Kittler, H.; Pehamberger, H.; Wolff, K.; Binder, M. Follow-up of melanocytic skin lesions with digital epiluminescence microscopy: patterns of modifications observed in early melanoma, atypical nevi, and common nevi. Journal of the American Academy of Dermatology 2000, 43, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Keung, E.Z.; Gershenwald, J.E. The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: implications for melanoma treatment and care. Expert review of anticancer therapy 2018, 18, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Deeks, J.J.; Chuchu, N.; Ferrante di Ruffano, L.; Matin, R.N.; Thomson, D.R.; Wong, K.Y.; Aldridge, R.B.; Abbott, R.; Fawzy, M.; et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. The Cochrane database of systematic reviews 2018, 12, Cd011902. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.E.; Macaskill, P.; Holt, P.E.; Menzies, S.W. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol 2008, 159, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Cortonesi, G.; Rubegni, P. High magnification and fluorescence advanced videodermoscopy for hypomelanotic melanoma. Skin research and technology : official journal of International Society for Bioengineering and the Skin (ISBS) [and] International Society for Digital Imaging of Skin (ISDIS) [and] International Society for Skin Imaging (ISSI) 2020, 26, 766–768. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Labeille, B.; Debarbieux, S.; Carrera, C.; Lacarrubba, F.; Witkowski, A.M.; Moscarella, E.; Arzberger, E.; Kittler, H.; Bahadoran, P.; et al. Dermoscopy vs. reflectance confocal microscopy for the diagnosis of lentigo maligna. J Eur Acad Dermatol 2018, 32, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Rossi, R.; Ferrara, G.; Tognetti, L.; Rubegni, P.; Perrot, J.L. Image Gallery: Super-high magnification dermoscopy can identify pigmented cells: correlation with reflectance confocal microscopy. Br J Dermatol 2019, 181, e1. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Tognetti, L.; Campoli, M.; Liso, F.; Cicigoi, A.; Cartocci, A.; Rossi, R.; Rubegni, P.; Perrot, J.L. Super-high magnification dermoscopy can aid the differential diagnosis between melanoma and atypical naevi. Clinical and experimental dermatology 2021, 46, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Perrot, J.L.; Labeille, B.; Cambazard, F. [Diagnosis of scabies by high-magnification dermoscopy: the “delta-wing jet” appearance of Sarcoptes scabiei]. Annales de dermatologie et de venereologie 2013, 140, 722–723. [Google Scholar] [CrossRef] [PubMed]
- Puppin, D., Jr.; Salomon, D.; Saurat, J.H. Amplified surface microscopy. Preliminary evaluation of a 400-fold magnification in the surface microscopy of cutaneous melanocytic lesions. Journal of the American Academy of Dermatology 1993, 28, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Dusi, D.; Rossi, R.; Simonacci, M.; Ferrara, G. Image Gallery: the new age of dermoscopy: optical super-high magnification. British Journal of Dermatology 2018, 178, e330–e330. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Rossi, R.; Ferrara, G.; Tognetti, L.; Rubegni, P.; Perrot, J.L. Image Gallery: Super-high magnification dermoscopy can identify pigmented cells: correlation with reflectance confocal microscopy. 2019, 181, e1-e1. [CrossRef]
- Cinotti, E.; Ekinde, S.; Labeille, B.; Raberin, H.; Tognetti, L.; Rubegni, P.; Perrot, J.L. Image Gallery: Pigmented hyphae can be identified in vivo by high and super-high magnification dermoscopy. Br J Dermatol 2019, 181, e4. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Bertello, M.; Donelli, C.; Rossi, R.; Tognetti, L.; Perrot, J.L.; Rubegni, P. Super-high magnification dermoscopy can detect Demodex folliculorum. Journal of the European Academy of Dermatology and Venereology : JEADV 2023, 37, e96–e97. [Google Scholar] [CrossRef] [PubMed]
- Scarfì, F.; Gori, A.; Topa, A.; Trane, L.; Dika, E.; Broganelli, P.; Massi, D.; De Giorgi, V. Image Gallery: In vivo fluorescence-advanced videodermatoscopy for the characterization of skin melanocytic pigmented lesions. Br J Dermatol 2019, 180, e104. [Google Scholar] [CrossRef] [PubMed]

| Benign lesions n = 117 |
Malignant lesions n = 73 |
p-value | |
|---|---|---|---|
| Age (years; SD) | 48.79 (19.19%) | 64.98 (16.70%) | <0.001 |
| Male | 68 (61.3%) | 31 (56.4%) | 0.846 |
| Female | 49 (38.7%) | 42 (43.6%) |
| Benign lesions | Malignant lesions | p-value | |
|---|---|---|---|
| 7-point checklist | |||
| Atypical pigment network | 57 (48.7%) | 59 (80.8%) | <0.001 |
| Blue-whitish veil | 18 (15.4%) | 49 (67.1%) | <0.001 |
| Atypical vascular pattern | 1 (0.9%) | 12 (16.4%) | <0.001 |
| Irregular streaks | 11 (9.4%) | 22 (30.1%) | 0.001 |
| Regression structures | 33 (28.2%) | 49 (67.1%) | <0.001 |
| Irregular pigmentations | 40 (34.2%) | 23 (31.5%) | 0.823 |
| Irregular dots/globules | 36 (30.8%) | 18 (24.7%) | 0.457 |
| General pattern | |||
| homogeneous | 32 (27.4%) | 43 (58.9%) | <0.001 |
| globular | 41 (35.0%) | 7 (9.6%) | <0.001 |
| network | 67 (57.3%) | 41 (56.2%) | 1.000 |
| Benign lesions | Malignant lesions | p-value | |
|---|---|---|---|
| Cell presence | 113 (96.6%) | 72 (98.6%) | 0.695 |
| Keratinocytes | 109 (93.2%) | 64 (87.7%) | 0.304 |
| Roundish melanocytes | 35 (29.9%) | 36 (49.3%) | 0.011 |
| Dendritic melanocytes | 19 (16.2%) | 22 (30.1%) | 0.037 |
| Melanophages | 19 (16.2%) | 17 (23.3%) | 0.310 |
| Cell colour | |||
| Black | 13 (11.1%) | 7 (9.6%) | 0.929 |
| Brown | 107 (91.5%) | 66 (90.4%) | 1.000 |
| violet/blue | 45 (38.5%) | 29 (39.7%) | 0.983 |
| Cell irregularity in shape and size | 21 (17.9%) | 38 (52.1%) | <0.001 |
| Cell distribution: irregular arrangement | 19 (16.2%) | 30 (41.1%) | <0.001 |
| Roundish nests | 42 (35.9%) | 22 (30.1%) | 0.510 |
| Out-of-focus structureless areas | |||
| bluish | 36 (30.8%) | 24 (32.9%) | 0.886 |
| grey/brown | 23 (19.7%) | 11 (15.1%) | 0.543 |
| Vessels | 26 (22.2%) | 23 (31.5%) | 0.210 |
| Network | |||
| with edged papillae | 30 (25.6%) | 3 (4.1%) | <0.001 |
| without edged papillae | 18 (15.4%) | 19 (26.0%) | 0.107 |
| Angled nest | 9 (7.7%) | 16 (22.2%) | 0.008 |
| OR | p-value | |
|---|---|---|
| Age | 1.05 (1.03-1.09) | <0.001 |
| Irregular melanocyte distribution | 4.69 (1.58-15.45) | 0.007 |
| Network with edged papillae | 0.16 (0.03-0.57) | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





