1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common tumors worldwide. Approximately 75%-85% of liver cancer mortality is the result of from HCC [
1]. Early identification of HCC is difficult; therefore, most cases of HCC are discovered at a late stage [
2]. Early-stage HCC is generally treated with a combination of surgery, radiotherapy, and chemotherapy [
3]. A modern, effective treatment for HCC is liver transplantation; however, organ shortages, perioperative risk, and the strict requirements for appropriate pairing limit the accessibility of liver transplantation. Despite the progress that has been made in therapeutic approaches, HCC recurrence and metastasis rates remain high, thereby leading to unfavorable prognoses [
4,
5]. The development of agents to prevent HCC metastasis is one strategy to increase the survival rate of patients with HCC.
Wild-type p53 protein (WTP53) plays a key role in cell apoptosis and the regulation of the cell cycle after DNA damage [
6]. Cells with a mutated p53 gene may evade apoptosis after DNA damage, and potentially become cancerous. Mutations in the p53 gene are the most common type of gene change in HCC, with an average mutation frequency of 30% [
6]. Cells with a mutated p53 gene lose their tumor suppressing function and promote tumorigenesis and metastasis [
7]. In addition, WTP53 and mutated p53 protein (MTP53) are involved in in the regulation of cell migration and invasion in cancer cell metastasis [
8]. WTP53 and MTP53 have tumor suppressive and oncogenic roles, respectively. MTP53 promotes epithelial-to-mesenchymal transition (EMT), whereas WTP53 prevents EMT [
9]. Therapies that decrease MTP53 expression or target MTP53 may have potential as a means of preventing HCC metastasis.
Benzofuran derivatives are a class of compounds found in higher plants that have attracted the attention of chemists and pharmacologists because of their various biological and pharmacological activities, which include anti-inflammatory, antimicrobial, anti-virus, anti-hyperglycemic, and antitumor activities. In addition to isolating benzofuran derivatives from natural products, medicinal chemists are investigating methods for synthesizing benzofuran rings for application in drugs [
10,
11]. The benzofuran derivative 2-(4-Benzyloxy-3-methoxyphenyl)-5-(carbethoxyethylene)-7-methoxy-benzofuran (BMBF) is an intermediate in the process of total synthesis of ailanthoidol, a neolignan that is isolated from the bark of Zanthoxylum ailanthoidol (Rutaceae) [
12]. Ailanthoidol had an antitumor effect in a multistep skin cancer mouse model [
13]. Ailanthoidol suppressed TGF-β1-promoted migration and invasion in HepG2 cells [
14] and suppressed the proliferation of Huh7 cells [
15]. Though ailanthoidol exhibits antitumor potential, the biological mechanism of BMBF remains unclear. The present study investigated the anti-metastatic and modulatory effects of BMBF in HCC cells with a mutated p53 gene.
2. Materials and Methods
2.1. Materials
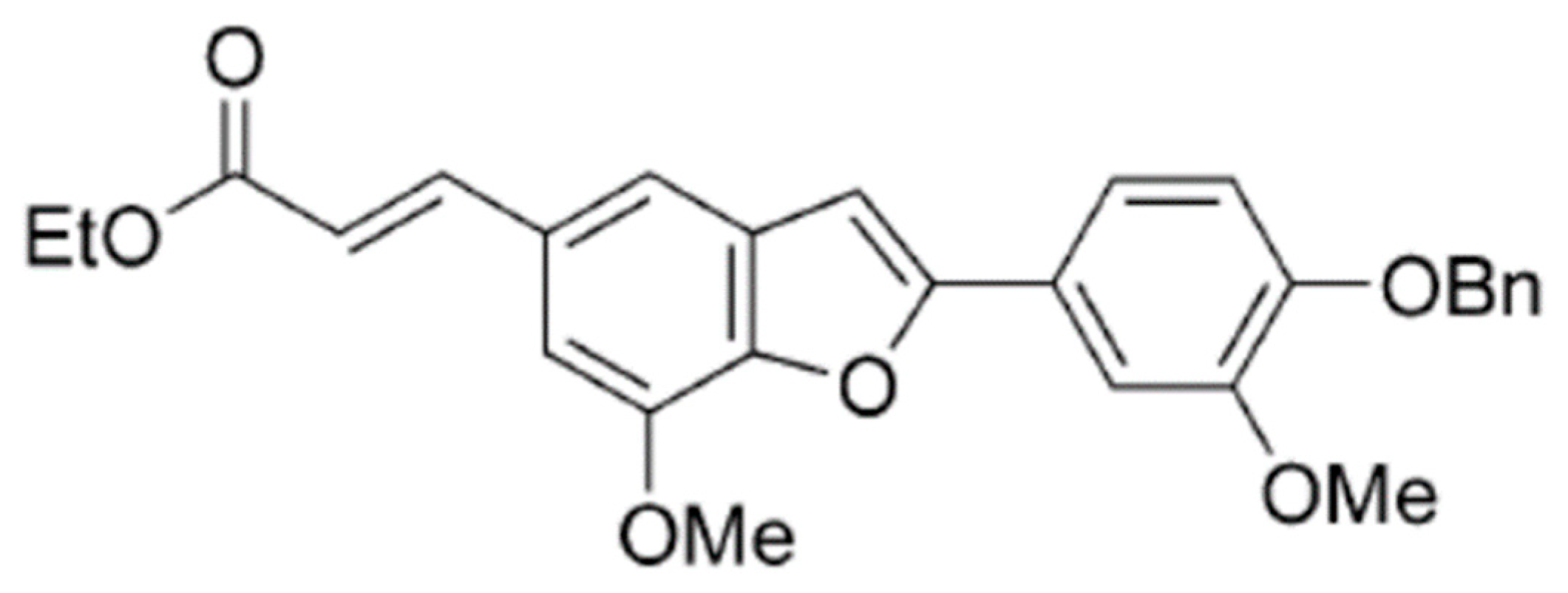
Dulbecco’s modified Eagle’s medium (DMEM), Minimum Essential Media (MEM), phosphate-buffered saline (PBS), fetal bovine serum (FBS), penicillin, streptomycin, and trypsin-EDTA were purchased from Gibco Ltd. (Grand Island, N.Y., USA). Primary antibodies against integrin α7, Slug, E-cadherin, vimentin, MMP9, p53 (DO-1), GADPH, and actin were obtained from Santa Cruz Biotechnology, Inc., CA., USA. Matrigel was obtained from Collaborative Research (Bedford, MA, USA). TRITC-conjugated phalloidin, β-actin antibody, and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). BMBF shown in
Figure 1 was provided by Dr. Yean-Jang Lee and synthesized from 5-bromo-2-hydroxy-3-methoxybenzaldehyde, as previously reported [
13]. Anti-FAK, anti-p-FAK, anti-AKT, and anti-p-AKT were purchased from Cell Signaling Technology (Beverly, MA, USA).
2.2. Cell culture and cell viability assay
The human liver cancer cell line Huh7 (p53 mutant in Y220C) was obtained from the Food Industry Research and Development Institute (Hsinchu, Taiwan), and cultured in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, 1% essential amino acid, and 1mM glutamine. PLC/PRF/5 (p53 mutant in R249S) cells were cultured in MEM supplemented with 10% FBS and 1% penicillin/streptomycin. The cells were maintained at 37˚C in humidified atmosphere of 5% CO2. To evaluate the cytotoxicity of BMBF in HCC cells, Cell Counting Kit-8 (CCK-8, Sigma-Aldrich, St. Louis, MO, USA) was conducted. Briefly, 3×103 of cell was seeded onto 96-well petri dish, then various concentration of BMBF was treated for indicated duration. Sequentially, 10 mL of CCK-8 solution was added to incubate with the medium for 3 hours; the absorbance was read at the wavelength of 450 nm by using ELISA reader (SpectraMax M5, Molecular Devices, Downingtown, PA, USA).
2.3. Microscopic examination
After treatment the indicated conditions, Huh7 cells were fixed with 4% paraformaldehyde for 10 min and permeabilized with 0.1% triton X-100 in PBS for 5 min. The cell morphology was assessed by using phase-contrast microscopy. In addition, cytoskeletal changes (F-actin) were analyzed through fluorescence microscopy by staining with TRITC-conjugated phalloidin (500 ng/mL) for 1 h. Images were acquired using a fluorescence microscope (Nikon Microscope SE, Nippon Kogaku KK, Tokyo, Japan) at 400ⅹor 200ⅹ magnification.
2.4. Scratch motility assay
Huh7 cells (2.5 × 105 cells/mL) were seeded onto a 6-well plate, and grown overnight to confluence. The monolayer was scratched with a yellow pipette tip, washed with PBS to remove floating cells, and photographed (0 h), then treated with BMBF (0–5 μM). After photographed (24 h), the cells that motile into the scratched area was counted in 5 randomly selected fields (100× magnification) by the digital planimetry using the ImageJ software. The area of cell migration was expressed as a percentage of the initial area (0 h). Data represented as mean ± SD of three independent experiments.
2.5. Cell migration and invasion assay
Cell migration and invasion assays were performed using a Boyden chemotaxis chamber. The upper culture chamber consisted of a polycarbonate filter (pore size, 8 μm) coated with (for invasion) or without (for migration) a uniform layer of 40 μg/cm2 of Matrigel basement membrane matrix was placed in the upper compartment of the chemotaxis chamber. Huh7 cells were pretreated with BMBF (0-5 μM) for 24 h. The cells were harvested and 6 × 104 cells/well were suspended in serum-free media, then placed into the upper chamber. The complete growth medium with 10% FBS was placed in the lower chamber. After incubation for 24 h, the cells on the upper surface of the filter were wiped with a cotton swab. The cells on the lower surface of the filter were fixed for 10 min with methanol and stained with Giemsa for 1 h, and the cells that had migrated or invaded into the lower surface of the filter were sequentially counted by light microscopy (200×). The experiment was performed in triplicate; in each filter, the cells from 5 randomly selected fields were counted to represent the data as mean ± SD.
2.6. Preparation of total cell extracts and immunoblot analysis
Cells were plated onto 10-cm2 dishes at a density of 1 × 106 cells/mL and treated with BMBF for 24 h. To prepare the whole-cell extract, the cells were harvested and suspended in a lysis buffer (50 mM Tris, 5 mM EDTA, 150 mM NaCl, 1% NP40, 0.5% deoxycholic acid, 1 mM sodium orthovanadate, 81 μg/mL aprotinin, 170 mg/mL leupeptin, 100 mg/mL PMSF; pH 7.5). After reacted for 30 min at 4°C, the mixtures were centrifuged at 10,000 g for 10 min, and the supernatants were collected as the whole-cell extracts. The protein content was determined by Bradford protein assay (Kenlor Industries, Costa Mesa, CA, USA). Equal amount of protein sample was subjected into 8%–12% SDS-polyacrylamide gel electrophoresis to separate, and then electrotransferred to nitrocellulose membrane (Sartorius Co., Goettingen Germany). They subsequently reacted with the primary antibodies (i.e., anti-E-cadherin, anti-vimentin, anti-Slug, anti-MMP-9, and anti-integrin α7). Anti-GADPH or anti-β-actin was used as the internal control. The secondary antibody was a peroxidase-conjugated goat anti-mouse or -rabbit antibody. After completing the procedures, the bands were exposed by enhanced chemiluminescence using a commercial Enhanced chemiluminescence (ECL) kit (ImmobilonTM Western, Millipore Co., Billerica, MA, USA).
2.7. Transfection of p53siRNA
3×103 Huh7 cells were seeded on 96-well dishes or 4×105 on 10 cm-dish. Following incubation for overnight, p53 siRNA (40 nM and 80 nM) or control siRNA (40 nM) (Santa Cruz Biotecnology, Santa Cruz, CA, USA) were transfected using T-Pro NTR II transfection reagent according to the manufacturer’s instructions (T-Pro Biotechnology Co., New Taipei City, Taiwan). p53 siRNAs (sense: 5’-AGA-CCU-AUG-GAA-ACU-ACU-Utt-3’) were purchased from GeneDireX Inc. (Taoyuan City, Taiwan). Following incubation for 48 h, the cells were treated with or without BMBF for 24 h, then the viable cells were added to the upper chamber of Boyden chamber for invasion assay or the total cell lysate was prepared for immunoblotting analysis.
2.8. Statistical analysis
Statistical significance was determined by one-way analysis of variance with the post hoc Dunnett’s test. P values lower than 0.05 were considered statistically significant.
3. Results
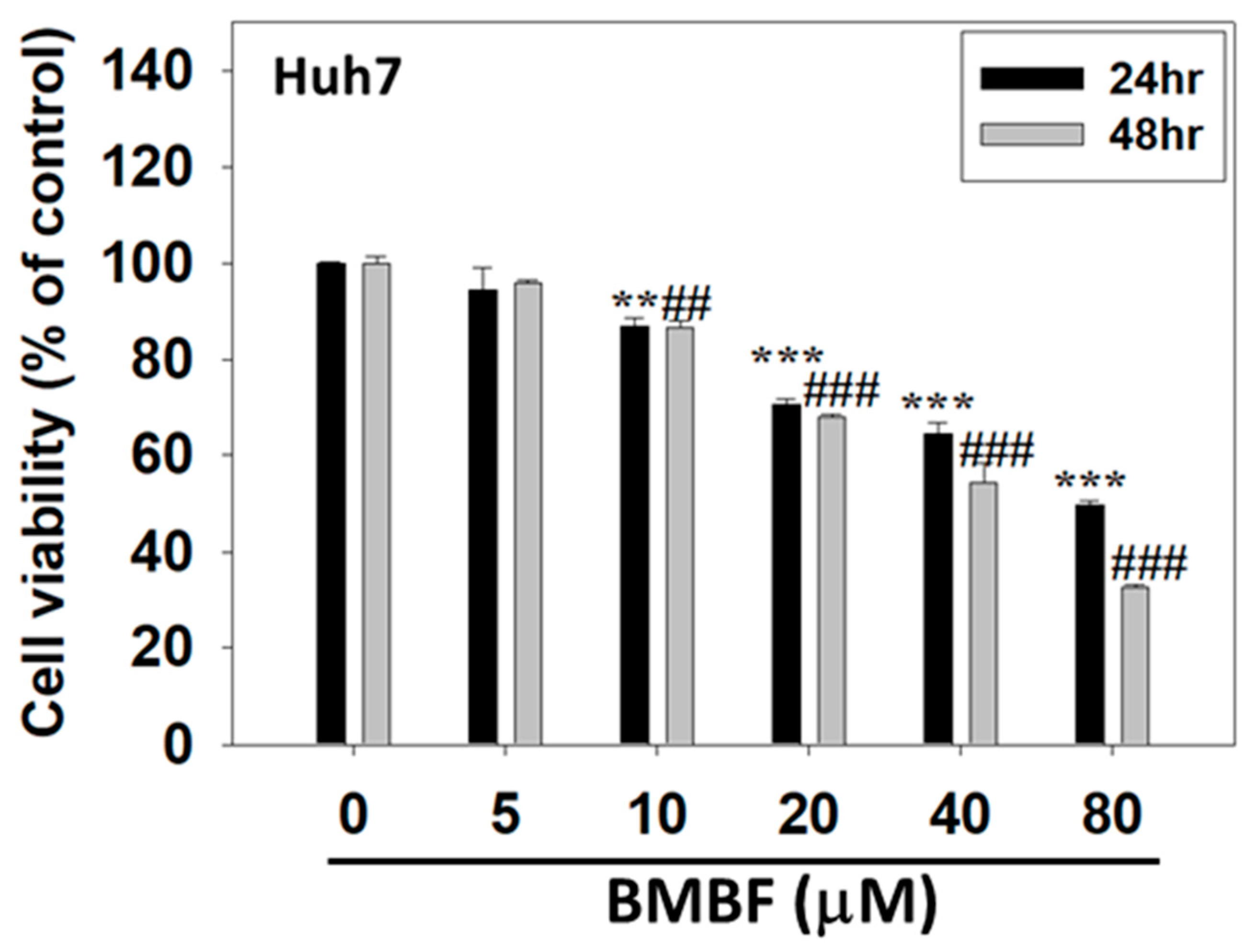
3.1. BMBF suppressed the viability of Huh7 cells
The cytotoxicity of BMBF in Huh7 cells was assessed using CCK-8 assay. Huh7 cells were treated with various concentrations of BMBF (0, 5, 10, 20, 40, and 80 μM) for 24 and 48 h. Treatment with concentrations of BMBF greater than 5 μM for 24 and 48 h significantly suppressed the viability of the Huh7 cells (
Figure 2). In the Huh7 cells, the IC50 value of BMBF at 24 h was 48.22 μM, and that at 48 h was 38.15 μM.
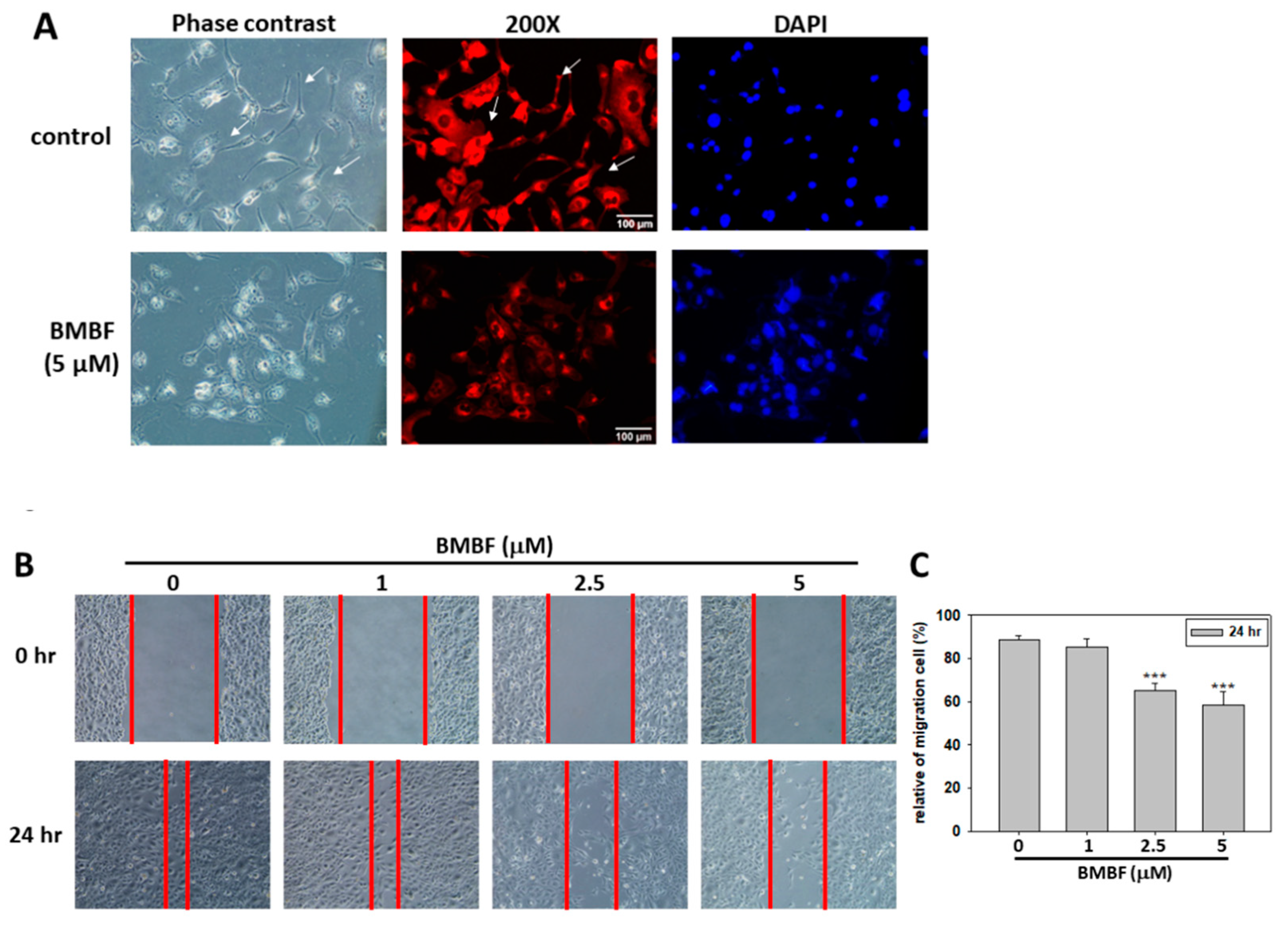
3.2. BMBF reduced the Cytoskeletons change and inhibited the motility, migration, and invasion in Huh7 cells
Metastasis occurs in the vast majority of cancer deaths and is a complex process consisting of tumor cell motility away from the primary site, migration into vasculature, invasion into surrounding parenchyma, and growth at the metastatic sites. A crucial element of this process is the remodeling of the cytoskeleton [
16]. To evaluate the antimetastatic potential of BMBF, this study used noncytotoxic concentrations of BMFB. First, we investigated the effect of BMBF on the actin cytoskeleton of Huh7 cells by using TRITC-conjugated phalloidin to stain the F-actin. In our preliminary observation, Huh7 cells, cells from an aggressive HCC cell line, exhibited lamellipodium protrusion and a more intensely stained F-actin cytoskeleton. Nonetheless, the F-actin cytoskeleton was reduced when treated with BMBF (
Figure 3A). Cytoskeleton alterations are associated with cell motility; therefore, we investigated the effect of BMBF on the motility of Huh7 cells by using a scratch motility assay. BMBF dose-dependently inhibited wound closure (
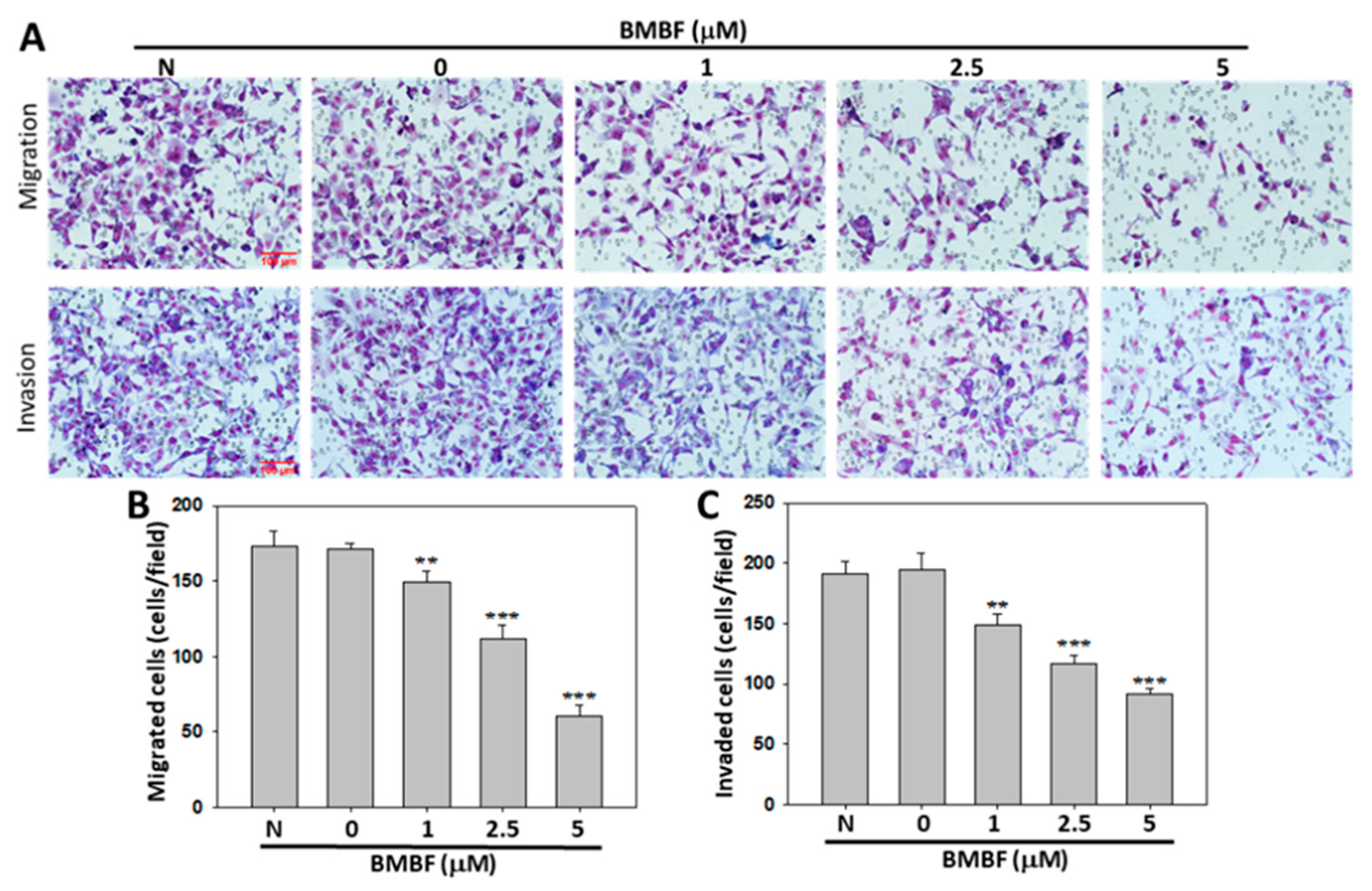
Figure 3B and 3C). Furthermore, Boyden chamber assay revealed that BMBF at a concentration of 1, 2.5, or 5 μM significantly suppressed Huh7 cell migration and invasion (
Figure 4A and 4B). These findings indicate that BMBF has in vitro antimetastatic potential in HCC cells.
3.3. Inhibitory effect of BMBF on EMT-related proteins and integrin α7 in Huh7 cells
EMT is a biological process in which polarized epithelial cells undergo multiple internal biological changes and transition into a mesenchymal phenotype; the process is highly mobile and invasive [
17]. EMT plays an essential role in the progression and metastasis of HCC [
18]. EMT involves the loss of E-cadherin and the production of vimentin, which enables cells to migrate and invade surrounding tissue. Matrix metalloproteinases (MMPs) are also involved in this process [
19]. We investigated the effect of BMBF on the levels of E-cadherin, vimentin, MMP-9, and Slug, which is the transcription factor involved in EMT-related protein expression in Huh7 cells. BMBF upregulated the expression of E-cadherin and suppressed vimentin, Slug, and MMP-9 (
Figure 5A). Integrins are membrane protein receptors that trigger distinct signaling and play a key role in cancer propagation and progression [
20]. Integrin α7 expression was reported to be higher in metastatic HCC cells than in nonmetastatic cells [
21], and integrin α7 was reported to be overexpressed in Huh7 cells [
22]. We analyzed the effect of BMBF on the expression of integrin α7 and the phosphorylation of its downstream signal mediators, such as FAK and AKT [
20]. BMBF suppressed the expression of integrin α7 and decreased the phosphorylation of FAK and AKT (
Figure 5B).
3.4. BMBF suppressed the invasion in Huh7 cells with p53 knock-down
EMT in HCC cells involves p53 [
23]. We investigated the effect of BMBF on p53 expression in Huh7 cells. BMBF decreased p53 expression in the Huh7 cells (
Figure 6A). The invasion ability of Huh7 cells transfected with p53 siRNA was assessed by using Boyden chamber assay; p53 siRNA significantly inhibited invasion and suppressed the expression of integrin α7, Slug, and MMP9 in Huh7 cells. BMBF-induced downregulation of p53 has antimetastatic potential.
3.5. Anti-invasion of BMBF in PLC/PRF/5 cells
We evaluated the anti-invasion potential of BMBF in PLC/PRF/5 cells, cells from another aggressive HCC cell line, with the p53 mutant R249S by using Boyden chamber assay. BMBF significantly inhibited the invasion effect (
Figure 7A and 7B). Consistent with the effect of BMBF in the Huh7 cells, BMBF suppressed the expression of p53, integrin α7, and MMP9. In addition, BMBF upregulated E-cadherin and downregulated vimentin and EMT-related transcription factor Slug (
Figure 7C).
4. Discussion
In the present study, the benzofuran derivative BMBF suppressed migration and invasion in HCC cells with a mutated p53 gene. The underlying mechanisms involve the upregulation of E-cadherin and the downregulation of vimentin, Slug, and MMP-9. In addition, BMBF decreased integrin α7 expression, deactivated FAK/AKT, and inhibited expression of p53, slowing metastasis (
Figure 8). Our findings indicate that BMBF is a potential antimetastatic agent in HCC cells with a p53 mutation.
Metastasis is the main reason for the failure of cancer therapy. EMT is an essential process in cancer metastasis. EMT allows normal hepatic epithelial cells to undergo multiple biological changes that enable them to assume a mesenchymal phenotype, which enhances the cells’ migration and invasion capacity and increases their resistance to apoptosis [
18]. Aberrant activation of EMT is crucial in cancer metastasis and involves multiple molecular mechanisms and signal transduction pathways, the hallmark of which is the downregulation of E-cadherin and upregulation of vimentin. Transcription factors Slug and twist induce EMT [
24]. MTP53 promotes the expression of several EMT-related transcription factors [
23]. In the present study, BMBF reduced the expression of p53 in Huh7 and PLC/PRF/5 cells with mutated p53 genes. Furthermore, BMBF upregulated the expression of E-cadherin and downregulated the expression of vimentin and the EMT-associated transcription factor Slug. MTP53 protein levels may be affected by miRNA or enzyme-controlled stability [
25]. Our results indicate that BMBF has antimetastatic properties in HCC cells; however, its underlying mechanisms in reducing MTP53 warrant further investigation.
Integrins are transmembrane receptors built up by the αβ-heterodimer. Several integrins are downregulated in tumor tissues [
20]. Integrin α7 is a key regulator in tumor propagation and has cancer stem cell properties [
26,
27]. Integrin α7 expression is high in various cancer cells, including mesothelioma and Huh7 cells [
22,
28]. Wu et. al., reported that integrin α7 knockdown suppressed HCC progression and inhibited EMT in HCCs [
22]. Hass et al. observed that integrin α7 regulates several signal pathways, including the FAK/AKT pathway, promoting cell proliferation and metastasis [
29]. Moreover, integrin α7 is associated with negative clinical outcomes in patients with HCC and regulates cancer stem cell markers [
30]. In the present study, BMBF reduced the integrin α7 levels and deactivated the downstream FAK/AKT signaling pathway. This demonstrates that BMBF-induced downregulation of integrin α7 prevents HCC metastasis. Whether BMBF regulates cancer stem cell markers in HCC warrants further investigation.
Deletion or mutation of p53 occurs in approximately 50% of patients with cancer and results in the loss of its tumor suppression function. Mutation of p53 leads to oncogenic gain-of-function properties and results in cancer metastasis [
8]. Ailanthoidol, a natural benzofuran, has exhibited antitumor potential [
13,
14,
15]. Ailanthoidol, through downregulation of MTP53 and deactivation of the STAT3 pathway, has an antiproliferation effect in Huh7 cells. Benzofuran derivatives, through HIF-1 inhibition, have an antiproliferation effect, especially against p53-independent (p53 deleted) malignant tumors [
30]. The role of benzofuran derivatives in preventing tumor metastasis remains unclear. Benzofuran is considered a critical class of heterocyclic compounds or fragments, which are present in many drugs [
10]. Due to benzofuran’s biological and medicinal importance, benzofuran derivatives have attracted the attention of scientists [
10,
31]. In the present study, noncytotoxic concentrations of BMBF exhibited antimigration and anti-invasion effects and downregulated MTP53 levels in HCC cells. Mutations of R249S in p53, which represent a gain of function, are phosphorylated by CDK4/cyclin D1 and then translocated into the nucleus. In the nucleus, R249S binds to and augments c-Myc activity, resulting in an increase in ribosome biogenesis and proliferation [
32]. Y220C mutations in p53 can cause the dedifferentiation of hepatocytes in response to oncogenic stimuli, which may result in the growth of malignant reprogrammed progenitor cells [
33].
5. Conclusions
Our findings indicate that mutations in p53 involve tumor growth and the regulation of metastasis. In vivo studies that investigate physiological responses should be conducted to verify the effects and mechanisms of BMBF. Whether BMBF inhibits the metastasis of p53-independent (p53 deleted) malignant tumors requires further clarification.
Author Contributions
Conceptualization, T.H.T. and H.J.L.; Data curation, T.H.T., Y.C.S. and H.J.L.; Funding acquisition, T.H.T.; Methodology, T.H.T., Y.C.S. and H.J.L.; Supervision, Writing – original draft, T.H.T. and H.J.L.; Writing – review & editing, T.H.T. and H.J.L.; Resource, Y.J.L.; Formal analysis, T.H.T., Y.C.S. and H.J.L.; Supervision, H.J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Technology Grant (MOST 108-2320-B-040-016-MY3), Taiwan, and partially by the Ministry of Science and Technology Grant (MOST 111-2320-B-040-015).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Digital Image Analyzer was performed in the Instrument Center of Chung Shan Medical University, which was supported by the Ministry of Science and Technology, Ministry of Education and Chung Shan Medical University, Taiwan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Pastushenko, I.; Blanpain, C. EMT transition states during tumor progression and metastasis. Trends Cell Biol 2019, 29, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Shu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Bertuccio, P.; Turati, F.; Carioli, G.; Rodriguez, T.T.; Vecchia, C.L.; Malvezzi, M.; Negri, E. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol 2017, 67, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Bucci, L.; Garuti, F.; Brunacci, M. Patients with advanced hepatocellular carcinoma need a personalized management: a lesson from clinical practice. Hepatology 2018, 67, 1784–1796. [Google Scholar] [CrossRef]
- Long, J.; Wang, A.; Bai, Y.; Lin, J.; Yang, Xu.; Wang, D.; Yang, X.; Jiang, Y.; Zhao, H. Development and validation of a TP53-associated immune prognostic model for hepatocellular carcinoma. EBioMedicine 2019, 42, 363–374. [Google Scholar] [CrossRef]
- Brosh, R.; Rotter, V. When mutants gain new powers: news from the mutant P53 field. Nat Rev Cancer 2009, 9, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Roszkowska, K.A.; Piecuch, A.; Sady, M.; Gajewski, Z.; Flis, S. Gain-of-function mutations in p53 in cancer invasiveness and metastasis. Int J Mol Sci 2020, 21, 1334. [Google Scholar] [CrossRef]
- Semenov, O.; Daks, A.; Fedorova, O.; Shuvalov, O.; Barlev, N.A. Opposing roles of wild-type and mutant p53 in the process of epithelial to mesenchymal transition. Front Mol Biosci 2022, 9, 928399. [Google Scholar] [CrossRef]
- Nevagi, R.J.; Dighe, S.N.; Dighe, S.N. Biological and medicinal significance of benzofuran. Eur J Med Chem 2015, 97, 561–581. [Google Scholar] [CrossRef]
- Khanam, H.; Shamsuzzaman. Bioactive benzofuran derivatives: a review. Eur J Med Chem 2015, 97, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Chen, C.L.; Lee, Y.J. Total synthesis of ailanthoidol and precursor XH14 by Stille coupling. J Org Chem 2003, 68, 2968–2971. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kao, E.S.; Chu, C.Y.; Lin, W.L.; Chiou, Y.H.; Tseng, T.H. Inhibitory effect of ailanthoidol on 12-O-tetradecanoyl-phorbol-13-acetate-induced tumor promotion in mouse skin. Oncol Rep 2006, 16, 921–927. [Google Scholar] [CrossRef]
- Tseng, T.H.; Lee, H.J.; Lee, Y.J.; Lee, K.C.; Shen, C.H.; Kuo, H.C. Ailanthoidol, a neolignan, suppresses TGF-β1-induced HepG2 heptoblastoma cell progression. Biomedicines 2021, 9, 1110. [Google Scholar] [CrossRef]
- Tseng, T.H.; Wang, C.J.; Lee, Y.J.; Shao, Y.C.; Shen, C.H.; Lee, K.C.; Tung, S.Y.; Kuo, H.C. Suppression of the proliferation of Huh7 hepatoma cells involving the downregulation of mutant p53 protein and inactivation of the STAT 3 pathway with ailanthoidol. Int J Mol Sci 2022, 23, 5102. [Google Scholar] [CrossRef]
- Fife, C.M.; McCarroll, J.A.; Kavallaris, M. Movers and shakers: cell cytoskeleton in cancer metastasis. Br J Pharmacol 2014, 171, 5507–5523. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J Clin Invest 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- van Zijl, F.; Zulehner, G.; Petz, M.; Schneller, D.; Kornauth, C.; Hau, M.; et al. Epithelial-mesenchymal transition in hepatocellular carcinoma. Future Oncol 2009, 5, 1169–1179. [Google Scholar] [CrossRef]
- Scheau, C.; Badarau, I.A.; Costache, Raluca.; Caruntu, C. The role of matrix metalloproteinases in the epithelial-mesenchymal transition of hepatocellular carcinoma. Anal Cell Pathol 2019, 9423907. [CrossRef]
- Hamidi, H.; Ivaska, J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Ge, J.; Wang, Y.; Chen, Z.; Chen, D. Integrin alpha 7 correlates with poor clinical outcomes, and it regulates cell proliferation, apoptosis and stemness via PTK2-PI3K-Akt signaling pathway in hepatocellular carcinoma. Cell Signal 2020, 66, 109465. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Kong, X.; Wang, Z. Integrin α7 knockdown suppresses cell proliferation, migration, invasion and EMT in hepatocellular carcinoma. Exp Ther Med 2021, 21, 309. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, Y.; Guan, D.; Li, J.; Yin, H.; Pan, Y.; Xie, D.; Chen, Yan. Critical roles of p53 in epithelial-mesenchymal transition and metastasis of hepatocellular carcinoma cells. PLoS One 2013, 8, e72846. [CrossRef]
- Guttilla, R.I. Mechanism and regulation of epithelial–mesenchymal transition in cancer. Cell Health Cytoskelet 2015, 7, 155–166. [Google Scholar] [CrossRef]
- Vijayakumaran, R.; Tan, K.H.; Miranda, P.J.; Haupt, S.; Haupt, Y. Regulation of mutant p53 protein expression. Front Oncol 2015, 5, 284. [Google Scholar] [CrossRef]
- Ming, X.; Fu, Li.; Zhang, L.; Qin, Y.; Cao, T.; Chan, K.; et al. Integrin α7 is a functional cancer stem cell surface marker in oesophageal squamous cell carcinoma. Nat Commun 2016, 7, 13568. [Google Scholar] [CrossRef]
- Nunes, A.M.; Barraza-Flores, P.; Smith, C.R.; Burkin, D.J. Integrin α7: A major driver and therapeutic target for glioblastoma malignancy. Stem Cell Investig 2017, 4, 97. [Google Scholar] [CrossRef]
- Burkin, D.J.; Fontelonga, T.M. Mesothelioma cells breaking bad: Loss of integrin α7 promotes cell motility and poor clinical outcomes in patients. J Pathol 2015, 237, 282–284. [Google Scholar] [CrossRef]
- Haas, T.L.; Sciuto, M.R.; Brunetto, L.; Valvo, C.; Signore, M.; et al. Integrin α7 Is a Functional Marker and Potential Therapeutic Target in Glioblastoma. Cell Stem Cell 2017, 21, 35–50. [Google Scholar] [CrossRef]
- Yang, Y.R.; Wei, J.; Mo, X.; Yuan, Z.; Wang, J.; Zhang, C.; Xie, Y.; You, Q.; Sun, H. Discovery and optimization of new benzofuran derivatives against p53-independent malignant cancer cells through inhibition of HIF-1 pathway. Bioorg Med Chem Lett 2016, 26, 2713–2718. [Google Scholar] [CrossRef]
- Miao, Y.; Hu, Y.; Yang, J.; Liu, T.; Sun, J.; Wang, X. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv 2019, 9, 27510–27540. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hao, Q.; Lu, H. Mutant p53 in cancer therapy—the barrier or the path. J Mol Cell Biol 2019, 11, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Tschaharganeh, D.F.; Xue, W.; Calvisi, D.F.; Evert, M.; Michurina, T.V.; et al. p53-Dependent nestin regulation links tumor suppression to cellular plasticity in liver cancer. Cell 2014, 158, 579–592. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Chemical structure of BMBF.
Figure 1.
Chemical structure of BMBF.
Figure 2.
Anti-proliferation effect of BMBF in the Huh7 cells. After treatment with various concentrations of BMBF for 24 h and 48 h, the viable cells were determined by CCK-8 kit. After treatment with the kit reagents, the optical density was measured at 450 nm using ELISA multi-well plate reader. Data represented as means ± SD (n=3). The asterisks indicated statistic changes (** p<0.01, ***p<0.001, compared to the 24 h control; ## p<0.01, ###p<0.001, compared to the 48 h control).
Figure 2.
Anti-proliferation effect of BMBF in the Huh7 cells. After treatment with various concentrations of BMBF for 24 h and 48 h, the viable cells were determined by CCK-8 kit. After treatment with the kit reagents, the optical density was measured at 450 nm using ELISA multi-well plate reader. Data represented as means ± SD (n=3). The asterisks indicated statistic changes (** p<0.01, ***p<0.001, compared to the 24 h control; ## p<0.01, ###p<0.001, compared to the 48 h control).
Figure 3.
Effect of BMBF on the cytoskeleton and motility of Huh7 cells. (A) After treatment with or without BMBF (5 μM) for 48 h, the cytoskeleton of Huh7 cells was stained with TRITC-conjugated phalloidin and nuclear was stained with DAPI. The microscopy image was taken (400ⅹ). (B) The cell was scratched with a yellow pipet tip and photographed by a phase contrast microscope under the field of 100ⅹ magnification (0 h). In the following, the Huh7 cells were treated with BMBF for 24 h, then observed and photographed (24 h). (C) The area of cells migrated into scratched area was determined at 5 randomly selected field by the digital planimetry using ImageJ software. The area of cell migration was expressed as a percentage of the initial area (0 h). Data represented as means ± SD of three independent experiments (** p<0.01, ***p<0.001).
Figure 3.
Effect of BMBF on the cytoskeleton and motility of Huh7 cells. (A) After treatment with or without BMBF (5 μM) for 48 h, the cytoskeleton of Huh7 cells was stained with TRITC-conjugated phalloidin and nuclear was stained with DAPI. The microscopy image was taken (400ⅹ). (B) The cell was scratched with a yellow pipet tip and photographed by a phase contrast microscope under the field of 100ⅹ magnification (0 h). In the following, the Huh7 cells were treated with BMBF for 24 h, then observed and photographed (24 h). (C) The area of cells migrated into scratched area was determined at 5 randomly selected field by the digital planimetry using ImageJ software. The area of cell migration was expressed as a percentage of the initial area (0 h). Data represented as means ± SD of three independent experiments (** p<0.01, ***p<0.001).
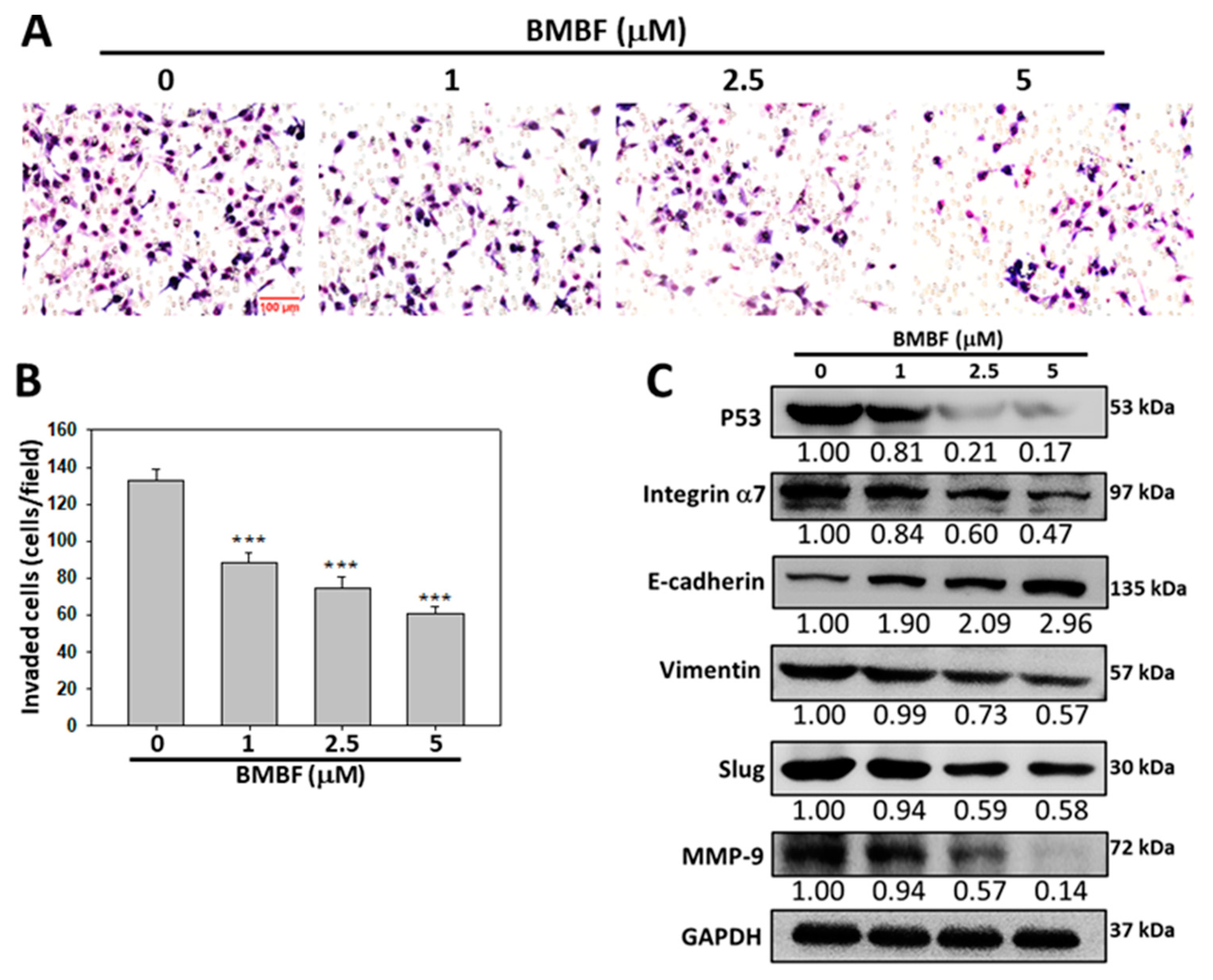
Figure 4.
Inhibitory effect of BMBF on the migration and invasion of Huh7 cells. Huh7 cells (6 x 104) were seeded onto upper chamber consisting of 8 μm pore-size filter coated without (upper panel) and with Matrigel matrix and the complete growth medium was placed in the lower chamber. After incubation for 24 h with or without BMBF, the filters were fixed for 10 min with methanol and stained with Giemsa for 1 h. The cell migrated or invaded into the lower surface of the filter were observed (200ⅹ) under microscopy and photographed (A) and counted in 5 randomly selected fields (B). Data represented as means ± SD of three independent experiments (**p<0.01; ***p<0.001). Scale bar=100 μm.
Figure 4.
Inhibitory effect of BMBF on the migration and invasion of Huh7 cells. Huh7 cells (6 x 104) were seeded onto upper chamber consisting of 8 μm pore-size filter coated without (upper panel) and with Matrigel matrix and the complete growth medium was placed in the lower chamber. After incubation for 24 h with or without BMBF, the filters were fixed for 10 min with methanol and stained with Giemsa for 1 h. The cell migrated or invaded into the lower surface of the filter were observed (200ⅹ) under microscopy and photographed (A) and counted in 5 randomly selected fields (B). Data represented as means ± SD of three independent experiments (**p<0.01; ***p<0.001). Scale bar=100 μm.
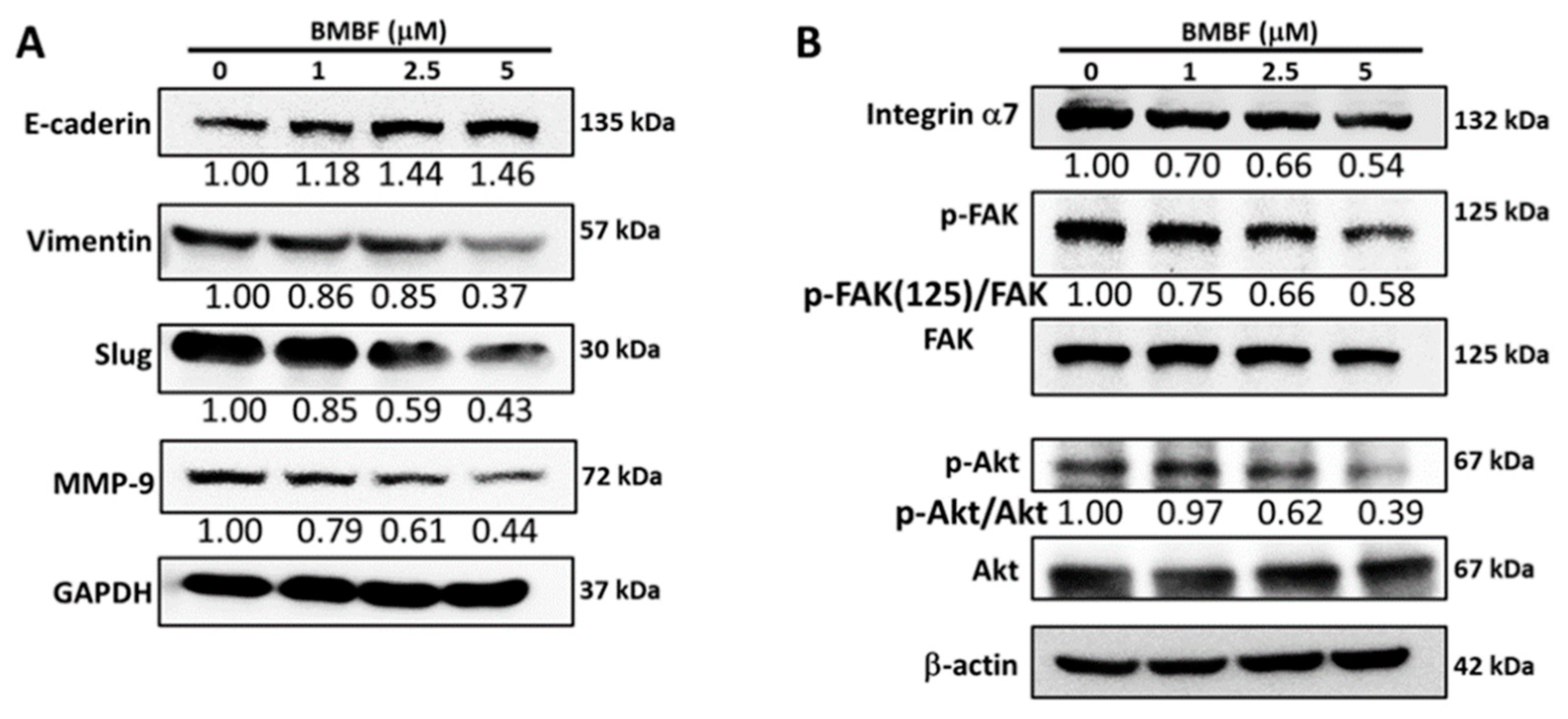
Figure 5.
Effect of BMBF on the expression of EMT- related proteins, MMP9, integrin α7, and its down-stream signal proteins. After treatment with BMBF for 24 h, the total cell lysates were prepared and subjected to the western blot analysis against specific antibody as indicated in the figure. The GADPH or β-actin was used as the loading control. The relative image density of image was quantified by the densitometer.
Figure 5.
Effect of BMBF on the expression of EMT- related proteins, MMP9, integrin α7, and its down-stream signal proteins. After treatment with BMBF for 24 h, the total cell lysates were prepared and subjected to the western blot analysis against specific antibody as indicated in the figure. The GADPH or β-actin was used as the loading control. The relative image density of image was quantified by the densitometer.
Figure 6.
Anti-invasion effect of BMBF associated with down-regulation of p53 mediated suppressing the expression of integrin α7, Slug, and MMP-9. (A) Effect of BMBF on the protein expression of p53 in Huh7 cells evaluated by immunoblotting analysis. (B) Transfection of p53 siRNA affecting the invasion of Huh7 cells by Boyden chamber assay. After transfection with p53 siRNA for 48 h, the cells were seeded onto upper chamber consisting of 8 μm pore-size filter coated with Matrigel matrix, then the complete growth medium was placed in the lower chamber and incubation for 24 h. The cell invaded into the lower surface of the filter were observed (200ⅹ) under microscopy and photographed and counted in 5 randomly selected fields. Scale bar=100 μm. (C) Data represented as means ± SD of three independent experiments (**p<0.01, ***p<0.001). (D) After transfection with p53 siRNA for 48 h, the total cell lysate was prepared then the expression of integrin α7, Slug, and MMP-9 was evaluated by immunoblotting analysis. The β-actin was used as the loading control. The relative density of image was quantified by the densitometer.
Figure 6.
Anti-invasion effect of BMBF associated with down-regulation of p53 mediated suppressing the expression of integrin α7, Slug, and MMP-9. (A) Effect of BMBF on the protein expression of p53 in Huh7 cells evaluated by immunoblotting analysis. (B) Transfection of p53 siRNA affecting the invasion of Huh7 cells by Boyden chamber assay. After transfection with p53 siRNA for 48 h, the cells were seeded onto upper chamber consisting of 8 μm pore-size filter coated with Matrigel matrix, then the complete growth medium was placed in the lower chamber and incubation for 24 h. The cell invaded into the lower surface of the filter were observed (200ⅹ) under microscopy and photographed and counted in 5 randomly selected fields. Scale bar=100 μm. (C) Data represented as means ± SD of three independent experiments (**p<0.01, ***p<0.001). (D) After transfection with p53 siRNA for 48 h, the total cell lysate was prepared then the expression of integrin α7, Slug, and MMP-9 was evaluated by immunoblotting analysis. The β-actin was used as the loading control. The relative density of image was quantified by the densitometer.
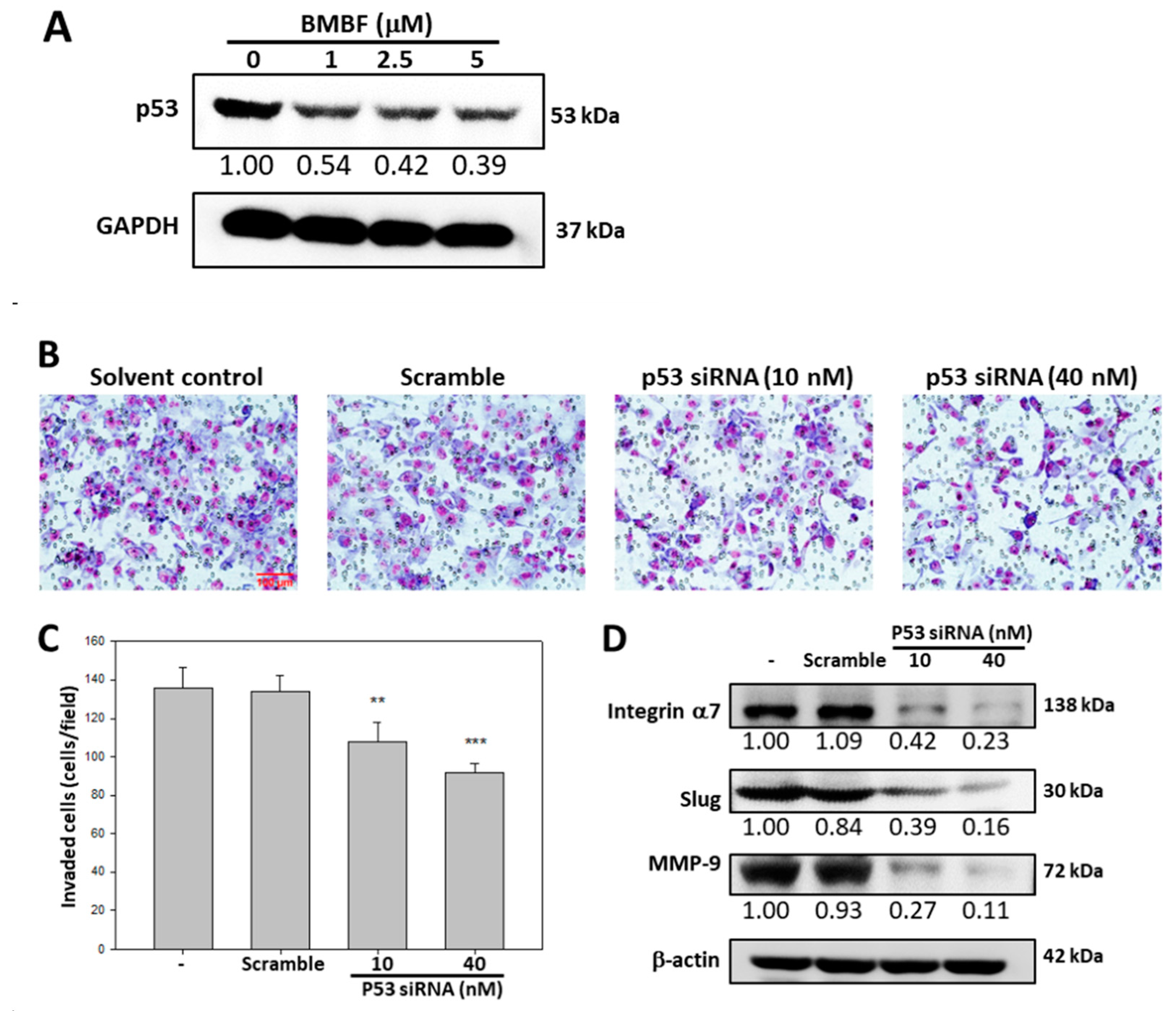
Figure 7.
Inhibitory effect of BMBF on cell invasion of PLC/PRF/5 cells. (A) PLC/PRF/5 cells (2 x 105) were seeded onto upper chamber consisting of 8 μm pore-size filter coated with Matrigel matrix and the complete growth medium was placed in the lower chamber. After incubation with BMBF for 24 h, the filters were fixed for 10 min with methanol and stained with Giemsa for 1 h. The cell invaded into the lower surface of the filter were observed (200ⅹ) under microscopy and photographed and counted in 5 randomly selected fields. (B) Data represented as means ± SD of three independent experiments (***p<0.001). Scale bar=100 μm. (C) After treatment with BMBF for 24 h, the total cell lysates were prepared and subjected to the immunoblotting analysis against specific antibody as indicated in the figure. The GADPH was used as the loading control. The relative image density was quantified by the densitometer.
Figure 7.
Inhibitory effect of BMBF on cell invasion of PLC/PRF/5 cells. (A) PLC/PRF/5 cells (2 x 105) were seeded onto upper chamber consisting of 8 μm pore-size filter coated with Matrigel matrix and the complete growth medium was placed in the lower chamber. After incubation with BMBF for 24 h, the filters were fixed for 10 min with methanol and stained with Giemsa for 1 h. The cell invaded into the lower surface of the filter were observed (200ⅹ) under microscopy and photographed and counted in 5 randomly selected fields. (B) Data represented as means ± SD of three independent experiments (***p<0.001). Scale bar=100 μm. (C) After treatment with BMBF for 24 h, the total cell lysates were prepared and subjected to the immunoblotting analysis against specific antibody as indicated in the figure. The GADPH was used as the loading control. The relative image density was quantified by the densitometer.
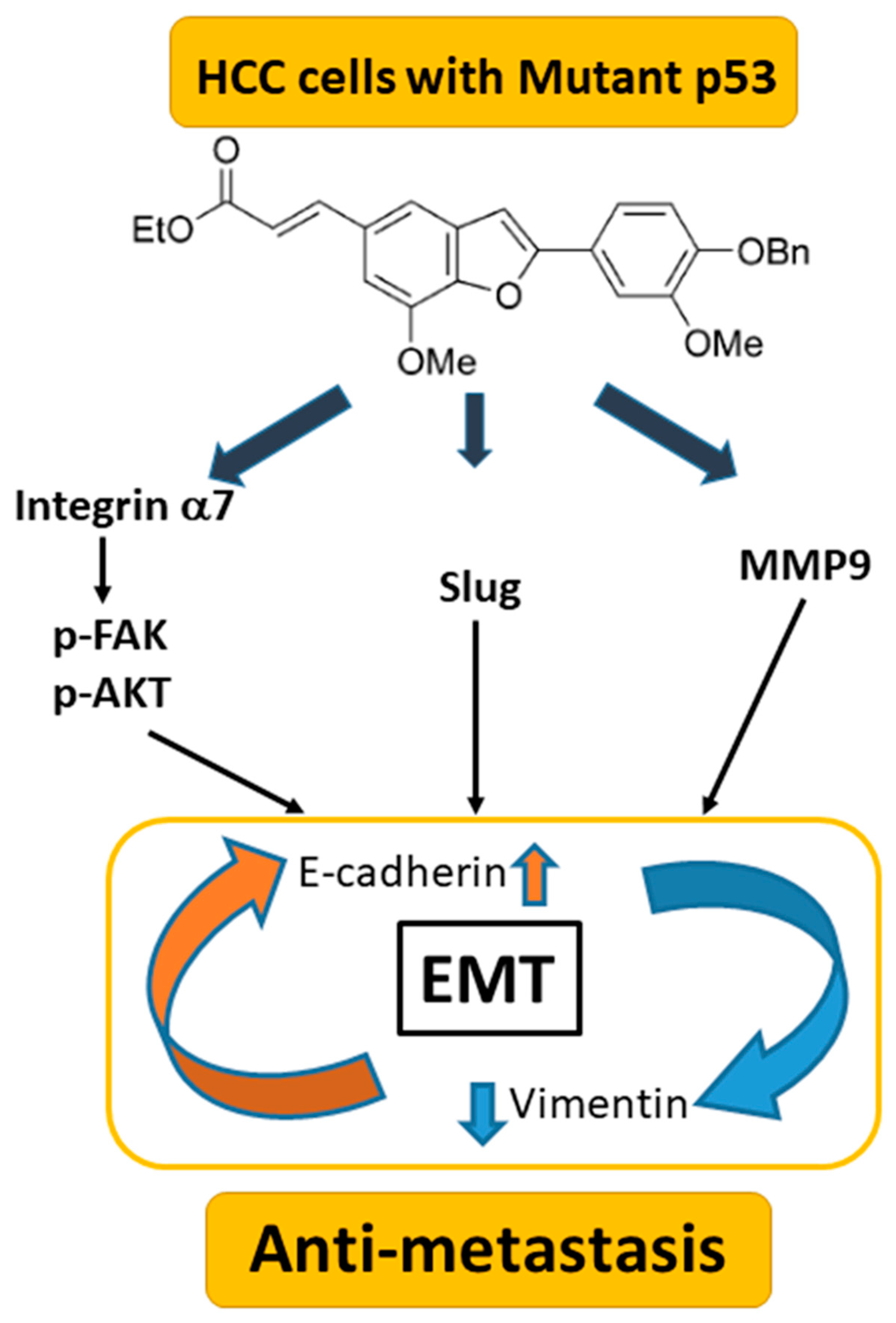
Figure 8.
Summary of BMBF in HCCs. BMBF possesses anti-metastasis potential involving down-regulated mutant p53 mediating alternations of integrin α7, EMT and MMP9 in HCCs.
Figure 8.
Summary of BMBF in HCCs. BMBF possesses anti-metastasis potential involving down-regulated mutant p53 mediating alternations of integrin α7, EMT and MMP9 in HCCs.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).