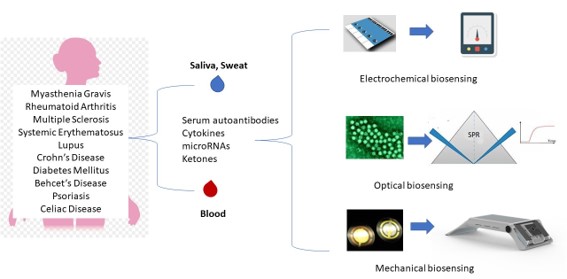
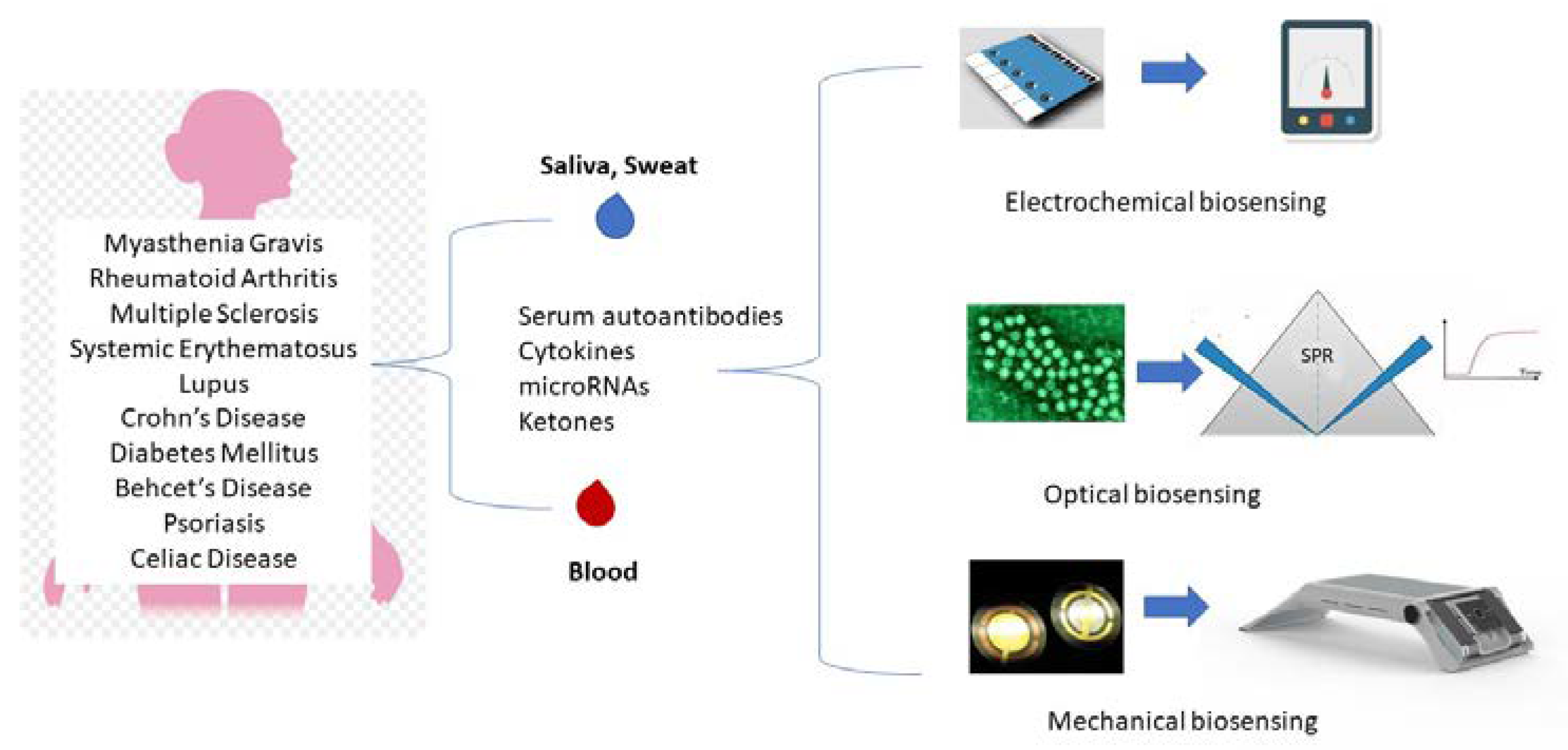
1. Introduction
Autoimmunity refers to long-term disorders that are characterized by the presence of autoantibodies that bind specific proteins, protein complexes or polypeptides resulting in an autoimmune attack that is responsible for the pathogenesis of a disease [
1]. Autoimmune diseases are classified into two categories: organ-specific, like Type I Diabetes Mellitus, Graves’ disease and other thyroid disorders, Myasthenia Gravis etc., and systemic diseases, such as Systemic Lupus Erythematosus, Rheumatoid Arthritis or Systemic Sclerosis.
Systemic disorders are rarer in the global population (overall prevalence of 1.5%), varying from 0.001% (myositis) to 0.9% (rheumatoid arthritis), whereas organ-specific autoimmune disorders are more frequent, varying from 0.004% (chronic autoimmune hepatitis) to 1.7% (psoriasis vulgaris) [
1]. In general, autoimmune disorders affect about 8% of the worldwide population [
2] and are one of the main causes for chronic severe disabilities and mortality, creating significant economic issues for patients and the health care system [
3]. In many cases, serum autoantibodies (AABs) can be detected prior to the clinical onset of a disease and during the course of organ-specific diseases. They are a predictive marker in healthy subjects, because their detection can occur years before clinical manifestations emerge and irreversible damage to the organism and, furthermore, autoantibodies can be a marker for the ongoing course of a disease in patients as a follow-up for monitoring disease activity [
4]. The medical and economic impact of autoimmune diseases is growing and there is a continuous improvement of treatment possibilities through targeted therapies. With this in mind, autoantibody diagnostics have increased over the last decades but there is still a gap between high quality diagnostics and cost-efficiency.
Detection of autoantibodies is mainly conducted by analytical techniques. Currently, Enzyme-linked immunosorbent assay (ELISA), indirect immunofluorescence (IIF) and western blotting are the most common traditional methods which have been developed for AAbs detection in autoimmune disorders [
5]. All these techniques require specialized personnel and equipment, are time-consuming and have a high cost and, in some cases, require for tissue damage to have occurred in order to detect the presence of autoantibodies, making their sensitivity as diagnostic methods less satisfactory [
6]. Considering these limitations, it is important for the development of new and reliable techniques for the detection of autoantibodies to achieve early diagnosis in autoimmune diseases.
Biosensors are integrated receptor-transducer devices that combine a receptor (biochemical recognition system) and a detector (transducer) which transforms the biochemical (biological) response into an output signal that is measurable [
7]. In 1962, Clark and Lyons introduced the first biosensor that meets this definition; an amperometric biosensor designed to monitor glucose that was called “enzyme electrode” [
8]. It was probably the first usable biosensor that led to a widespread use of electrochemical glucose sensors by patients at home and has led to a revolutionary care of patients with Diabetes Mellitus by allowing them to monitor their glucose levels at home instead of a lab [
9]. In this way, patients have more control over their disease and can achieve better management by having the option to measure glucose level at their own convenience, more frequently and at a lower cost [
10]. This is a good example of how modern glucose sensors help achieve the current goals in Point-of-Care (POC) use with a simple measurement protocol that is foolproof and can be used by an untrained patient, with a small portable device, in this case used to draw a small quantity of blood sample, and test strips that are inserted into a meter that offers a read on glucose level and provides the necessary information to the patient in a simple manner. The protocol can be more demanding in some cases, such as with immunosensors used for many types of biomolecules, that often require extra reagents and more steps and would need to be more automated and with a simple packaging suitable for POC use [
11].
2. Biosensors
Biosensors, as self-contained analytical devices, combine both a biological element and a signal transducer as mentioned. The biological element is on a solid-state surface and enables a bio specific interaction with an analyte. The output signal caused by the analyte binding can be obtained by using labeled compounds or not, thus classifying the biosensors respectively as labeled and label-free types. One of the commonly used methods for labeling involves the use of enzymes, radionuclides, nanoparticles, and fluorescent or electrochemiluminescent probes. There are various test formats available, including sandwich assays, competitive assays, and indirect assays [
12]. The primary benefit of labeled test formats is their ability to detect lower concentrations with greater accuracy, although higher operational costs are usually implied with longer assay times in comparison to most label-free test formats, that are performed without any labeled compound and are distinguished between direct and indirect assays [
13].
Direct assays are a simpler and inexpensive approach, as the signal response changes when analyte molecules bind to the transducer surface with no need for further steps or reagents in the procedure. In some cases, this simplicity could be a disadvantage when dealing with complex samples as there could be binding of other non-analyte components onto the sensor surface and a false-positive result may arise. This kind of unwanted effects can be resolved by ensuring that a significant change in the biosensor signal response is achieved only by the analyte binding to the corresponding element that is immobilized on the biosensor surface or using labeled test formats where the labeled compound usually determines the final biosensor signal response.
When considering the use of a biosensor for diagnostic applications the core question of single-use or multiuse arises. It is more than the biosensor itself that needs to be considered during the development stage in order to create a user-friendly and reliable device. The degree of specificity is determined by the biorecognition element used in the biosensor, the majority of which bind with high affinity to the analyte molecules. The sensitivity is primarily influenced by the detector element that turns the biological response into an output signal that can be quantified [
14]. In the multiuse option, the sensing capability of the biosensor needs to be regained and often a regeneration step that leads to dissociating the binding between the biorecognition element and the analyte is necessary. This regeneration procedure may require additional expenses that exceed the savings that the use of multiuse biosensors provides. When considering the development of a POC use sensor, single-use devices that are disposable are usually preferred [
15] and similar considerations are applied to all system elements that come into contact with the sample provided.
One of the major challenges of creating a biosensor is to detect the disease biomarker selectively among a vast number of proteins and other elements in the sample that could potentially interfere with the analysis and the result. A patient’s prognosis can be benefited by the quantification of the appropriate biomarkers for that specific disease that can be detected in tissue or body fluids [
16]. Selectively detecting these biomarkers requires meticulous consideration of the efficiency of the bioreceptor itself, its immobilization on the sensors surface and the way the signal is transduced, so that the output signal can be maximized and the response to components bound nonspecifically is minimized.
2.1. Categories
2.1.1. Electrochemical
Due to their sensitivity, selectivity, quick response and cost effectiveness, electrochemical devices are the most common biosensors used for the early detection of disease-related biomarkers. Additionally, these biosensors have drawn a lot of interest as suitable POC tests for diagnostic purposes. Numerous nanobiosensors and electrochemical biosensors have been reported in the literature for the detection of different biomarkers in autoimmune diseases. The most frequently used techniques include amperometric, impedimetric and voltametric techniques [
17]. Functionalized nanomaterials, such as metallic nanoparticles, conducting polymers and others, in association with electrochemical systems improved electron transfer, consequently improving the electrical signal transduction.
Amperometric biosensors use a fixed voltage to detect the amount of an analyte by measuring the produced current. Regarding autoimmune diseases and Rheumatoid Arthritis in particular, an amperometric nanobiosensor (NGP-NTiP-Thi) based on gold nanoparticles, titanium dioxide nanoparticles, and thionine was designed by Li et al in 2008 to detect macrophage migration inhibitory factor (MIF) in the serum of RA patients [
18]. Villa et al in 2011 [
19] conducted a study creating an amperometric immunosensor for the measurement of anti-citrullinated peptide antibodies (ACPAs), a possible biomarker for the diagnosis of Rheumatoid Arthritis, in which a composite made of multiwalled carbon nanotubes and polystyrene (MWCNT-PS) was used for the platform’s sensitivity. Concerning Systemic Lupus Erythematosus (SLE), in 2018 Fagúndez et al. [
20] developed a sandwich-format electrochemical immunosensor for the anti-double stranded DNA (anti-dsDNA) autoantibodies assay in serum samples from patients.
A potent and non-destructive approach that has been lately become more widely utilized as diagnostic tool is the electrical impedance spectroscopy (EIS) biosensor. In Multiple Sclerosis (MS) two label-free EIS biosensors have been fabricated. The first is a cytokine immunosensor by Bhavsar et al (2009) designed for MS diagnosis by detecting Interleukin-12 (IL-12) [
21]. In a study conducted by Derkus et al. in 2013 a label-free electrochemical impedance immunosensor to determine anti-myelin basic protein (anti-MBP) autoantibodies in patients with MS was produced. In this biosensor, the use of titaniumdioxide (TiO2) nanoparticles resulted in a satisfactory detection range [
22] .
Regarding voltametric biosensors, they utilize a variety of techniques, including square wave voltammetry (SWV), linear sweep voltammetry (LSV), differential pulse voltammetry (DPV), and cyclic voltammetry (CV). Their high sensitivity, selectivity, cost-effectiveness, and their capacity for simultaneous quantification of their targets, classify them among the most extensively used and available methods for the detection of specific biomarkers in autoimmune disorders. In 2017, a new nanoimmunosensor based on graphene oxide (GO)/[poly(propyleneglycol)] (pPG) nanocomposite was published by Derkus et al. for detecting MBP and Tau proteins simultaneously in MS patients using serum and cerebrospinal fluid (CSF) as samples [
23]. Another autoimmune disease for which voltametric biosensors have been studied is celiac disease (CD), an inflammatory disorder mediated by T-cells in the upper small intestine caused by consuming gluten [
24]. Two examples for the early detection of anti-tTG antibodies in patients, include the nanobiosensor developed by Gupta et al [
25], a GQD/PAMAM nanohybrid modified AuNP embedded in MWCNT (Multiwalled carbon nanotube) and another nanoimmunosensor based on cyclic voltammetry developed by Neves et al [
26]. With the use of cyclic voltammetry and square wave voltammetry, the team of Bellagha-Chenchah et al., developed an electrochemical biosensor in order to detect autoantibodies against CSG114(Glc), a synthetic glycopeptide with the potential to detect antibodies in patients with MS [
27]. The synthetic glycopeptide was modified in order to be suitable for bioelectrochemical detection and a lower sample volume could be used due to a series of platinum microband electrodes employed on microfluidic channels, which offer other advantages as well towards the development of this type of system as a POC test device [
28].
Another detection method that also has high sensitivity, fast response and low background signals is the photoelectrochemical (PEC) method [
29]. This method has been studied for use in the early diagnosis of Rheumatoid Arthritis by the team of Pang et al, who developed a PEC biosensor based on ZnO nanorods (NRs)/CH3NH3PbI3/nitrogen-doped carbon quantum dots (NCQDs) nanocomposites for rapid determination of fibroblast-like synoviocyte (FLS) cells [
30]. Overall, biosensors based on electrochemical methods are frequently used as a tool for early detection of autoimmune diseases due to their several advantages, including their rapid detection capability, the fact that they can be reusable and that they require a low sample volume. The quantitative analysis is feasible but false positive results can occur originating from matrix electrolytes and not adequate control on the working electrode at higher currents [
31].
2.1.2. Optical biosensors
A compact analytical devise that contains a biorecognition sensing element that is integrated with an optical transducer system is characterized as an optical biosensor and its main objective is to produce a proportionate signal to the concentration of the analyte (the measured substance). The exploitation of the interaction of the optical field with a biorecognition element provides optical detection. As a category they can be divided in label-based and label-free. The first category, label-based biosensors, involve using a specific label and a method (fluorescent, luminescent or colorimetric) to generate the optical signal. On the other hand, the label-free biosensors generate the signal directly by the interaction of the transducer with the sample [
32]. The advantages of label-free detection can be offered by optical biosensors as well and in the field of autoimmune diseases the screening of biomarkers for diagnostic purposes has recently evolved. A number of studies for the detection of specific biomarkers have been conducted regarding optical biosensors.
The use of electrochemiluminescence (ECL) has advantages including high sensitivity, a wide detection range, simple controllability, and a rapid response and, thus, ECL biosensors have a wide use to detect biomarkers [
33]. Rheumatoid Arthritis has been one of the autoimmune diseases targeted with an attempt to have early diagnosis by sensitive quantification of the anti-CCP (anti-cyclic citrullinated peptide) antibody by the team of Zhao et al [
34]. Their team developed a label-free ECL sensor that is based on asymmetric heterogeneous polyaniline-gold (PANI-Au) nanomaterial, which was incorporated with graphite-like carbon nitride (g-C3N4) in order to improve stability. Another common and widely used optical method is fluorescence. In the common autoimmune disease MS, the team of Mansourian et al. [
35] developed a biosensor for determining microRNA-145 as a biomarker in patients’ plasma, based on fluorescent DNA hosted silver nanoclusters (AgNCs) and hybridization chain reaction (HCR) amplification. The use of silver nanoclusters is considered to have many advantages, including simple synthesis, low toxicity, high stability and biocompatibility [
36].
Surface plasmon resonance (SPR) and surface plasmon resonance imaging (SPRi) are also techniques used in optical biosensors for detecting specific biomarkers in patients with autoimmune diseases. The SPR phenomenon occurs when the surface of metal is illuminated by polarized light at a specific angle (resonant angle) and electrons get excited (plasmon) when they absorb light energy. The intensity of the reflected laser beam decreases with the resonance angle and the number of absorbed molecules on the metal surface strongly influence resonance, thus, allowing for a calibration curve for a specific analyte by a resonance angle change [
37,
38].
These biosensors have a wide range of applications in the area of diagnosis and for measuring molecular interactions. In RA patients, the real-time detection of chemokine CXCL12 in urine samples was achieved by the development of an SPR biosensor by Vega et al [
39], using a SAM-coated gold chip covalently attached to lentiviral particles that contained CXCL4 (chemokine ligand 4) through amine coupling. In MS there was a recent enzyme-free strategy developed with single nanostructured enhancer of SPRi for the simultaneous multiple microRNAs assay using as a sample patients’ blood. Four microRNAs were detected in this study by Sguassesero et al. through neutravidin-coated gold nanospheres (nGNSs) that were functionalized with biotinylated antibody against DNA/RNA hybrids [
40]. This type of biosensors have been used in research for POC diagnostics regarding interferon-γ, a cytokine secreted by immune cells that can be considered an early indicator for different autoimmune disorders [
41], such as Crohn’s Disease [
42], Rheumatoid Arthritis [
43], Multiple sclerosis [
44] and Systemic Erythematosus Lupus [
43]. By immobilizing IFN-γ on the electrodes, Sipova et al used an engineered protein that adsorbed to the IFN-γ bound on the surface but mentioned that the presence and amount of IFN-γ in the solution indirectly altered the SRP signal [
45,
46]. The SPR technique is very sensitive to the binding of molecules from a solution, and it is important for the nonspecific molecules binding to be eliminated. Stigter et al. in a research paper, also on intereferon-gamma isolation and quantification, presented that metal surfaces that were modified with dextran-mercaptoundecanoic acid (MUA) showed minimum nonspecific adsorption from patients’ plasma [
47]. SPR biosensors are used for detecting autoimmune diseases and are a rather common optical methods option due to their sensitivity, specificity, their small size and their cost-effectiveness [
32]. Compared to conventional techniques analyses with SPR is less time-consuming, can be easily adapted to automated systems and repetitive measurements can occur on the chip surfaces. Usually, optical sensors based on SPR need laser sources and optical components that are well-aligned. Nevertheless, the possibility of small SPR sensors that are cost-effective exists as the team of Chuang et al. presented by using cyclic olefin copolymer miniature prisms and paper in order to establish a multistep quantitative analysis [
48].
In general, optical biosensors by enabling the direct, real-time and label-free detection of many different biomarkers provide advantages over analytical techniques used towards detecting autoimmunity. Currently, they are a widespread technology and scientists focus on improving the sensitivity and resolution of a conventional SPR, with modifications including surface plasmon resonance imaging (SPRi), long range surface plasmon (LRSP) and localized surface plasmon resonance (LSPR) in the area of biosensing with a direction to make the technology compatible with miniaturization for portable devices [
49]. A new analytical tool may emerge through their application for autoantibody detection in several autoimmune diseases, common or rarer, such as Myasthenia Gravis in which patients present autoantibodies against AChR and other proteins of the neuromuscular junction [
50]. They may be of great diagnostic interest as there can be an analysis of the serum level of the autoantibody in question and, furthermore, of its binding characteristics, making them applicable to a wide spectrum of autoimmune disorders and their serological evaluation [
6].
2.1.3. Mechanical Biosensors
In interactions such as biorecognition, mechanical transducers may detect changes in mechanical parameters like mass, surface stress and viscoelasticity [
51]. Their complexity makes them less common and popular than optical or electrochemical biosensors. This type of biosensors can be categorized into four broad categories depending on the chemical interactions of the sensor and the analyte: 1) affinity-based assays that achieve highly selective target identification by applying high specificity between the device surface and the target, 2) separation-based assays where spatiotemporal separation of analytes is permitted due to chemical affinities between immobilized molecules and flowing analytes, 3) fingerprint assays where the target is identified through specific binding affinities to sensors and 4) spectrometric assays, where the target’s identification is enabled through deducing it’s optical properties or its mass [
52].
Quartz crystal microbalance (QCM) sensors are the most established ones, which are based on quartz crystal resonators [
53]. They are centimeter-scale mechanical resonators that measure the mass of analytes on their surface in fluid, gas or vacuum. When the crystal is deformed with the use of piezoelectric technique, a mass change occurs when the analyte binds to the biorecognition element that is immobilized on the crystal surface. There have been attempts to develop RA-specific peptide-coated single walled carbon nanotube (SWCNT)-based QCM biosensors [
54]. By binding an RA-specific peptide (containing citric citrulline) to a functionalized SWCNT and depositing it on a QCM sensing crystal, it was possible to identify the related autoantibodies in patients’ serum. The finding demonstrated that the QCM sensor detected 34.4% more RA patients with anti-citrullinated peptide antibodies that those detected by the classic analytical method of ELISA and 37.5% more patients than microarray analysis [
54].
Biology is fundamentally based on mechanical interactions. On a cellular level, mechanical forces of chemical origin control adhesion and motility and on a molecular level they control transport and affinity. Unique opportunities to detect forces, displacements and changes in mass from cellular and subcellular activities are provided by biological sensing in the mechanical domain [
52]. Mechanical biosensors often benefit from properties that scale advantageously as physical size is decreased. Exquisite mass resolution is provided by nanoscale mechanical sensors. Nanoelectromechanical systems (NEMS) have achieved nanogram resolution in a fluid environment and zeptogramscale mass resolution when operating in a vacuum. The ability of a device’s mechanical compliance to be displaced or disformed is significantly increased by uniform reduction of its dimensions [
52]. One of the biggest challenges for all NEMS devices has been the development of transduction and actuation methods that are efficient, but significant advances have been made over those past years [
55].
Mechanical biosensors, like QCM, have the benefit of being very sensitive, having a large dynamic range of detection ranging from nanomolar to femtomolar ranges. Another benefit is that they are flexible enough to utilize almost any surface coating for tests [
52]. The main disadvantage of mechanical biosensors is the fact that handling samples is often cumbersome and susceptible to measurement artifacts [
3]. There are still many challenges, from integrating arrays of advanced nanosensors with conventional techniques to developing better capture agents but developing tools capable of high-throughput studies at the level of single cell for understanding biological systems remain the ultimate goal for developing tools that will bring advances in the field.
A schematical overview of the main biosensing approaches for the diagnosis and monitoring of autoimmune diseases is presented in
Figure 1.
3. Nanomaterials in biosensors
Nanomaterials are often used in biosensors to improve the electrical, mechanical and optical characteristics, regardless of the kind of sensor, in order to increase the detection limits [
56]. The modification of the nanoparticles’ physical and chemical characteristics, alterations in their size, shape or composition, is beneficial. The use of nanomaterials along with multianalyte detection and platform integration to create POC devices present a promising future for therapy monitoring and early detection of chronic autoimmune diseases. In the last years there has been important progress in the use of nanomaterials regarding the methods of immobilization. The optimal goal with the integration of nanomaterials into immunosensors is to obtain surfaces that are nanostructured for the immobilization of antibodies or antigens, thus enhancing the biosensors’ performance. High conductivity materials, such as metallic particles, nanotubes and graphene, have electrocatalytic effects and give higher signals by improving the electron transfer [
57].
Graphene is considered a “wonder material” due to its great physicochemical characteristics, including high conductivity, high surface-volume ratio and low charge carrier resistance [
58], all of which categorize graphene as an ideal candidate in order to design a transducing material. The graphene sheets have increased conductivity and their dimensionality makes it possible for every atom to be easily exposed to environmental fluctuations, an important characteristic for a biosensor [
59]. Another material that offers excellent platform in biosensing are Gold Nanoparticles (GNPs) that have unique colorimetric properties, depending on their size, their shape, and their state of aggregation [
60] and they are utilized in developing electrochemical, optical and piezoelectric biosensors.
CNT, the allotropic form of carbon is also known for its excellent electrical, mechanical, thermal and electrocatalytic properties [
61] and their geometry has attracted many potential biosensor applications. When they are doped with polyaniline, known for its improved redox activity, CNTs present better biosensing properties. CNTs have been utilized in dermal biosensors paving the way for cost-effective health monitoring in a manner that is not invasive. Another material used in nanobiosensors are covalent organic frameworks (COFs) that have been developed from organic molecules with covalently linked nano/microporous structures. They have a fully conjugate structure, a large surface area and one atomic thickness dimension and, with these advantages, they have potential use in sensors and electrochemical devices [
62]. Metal–organic frameworks (MOFs) have an interesting architecture and possess a large surface area with high level of porosity, they are customizable, and they have tunable characteristics, all excellent properties for their application in biosensors [
63].
4. Bioelement immobilization
In chronic autoimmune diseases, bioelements specific for biomarkers could be immobilized through different techniques and the method used for the immobilization is of paramount importance for the performance of the sensor. The bioelement used can be either linked via self-assembled monolayers (SAMs) or directly absorbed onto the electrode. Even though the adsorption of the bioelement is a simple procedure, a controlled orientation of the recognition element is not allowed for the proper binding of the antibody or the antigen and, therefore, it is preferred to use methods that allow an oriented and controlled immobilization. An example of this are SAMs that, through AU-S linking, can be readily formed onto gold electrodes. A strategy could be to utilize polymer composites for linking covalently in an oriented manner the antibody [
64] or by linking the antibody directly onto the electrode by electroaddressing [
65]. Polymers that are employed for oriented immobilization include poly (sodium-4-styrensulfonic acid) [
66] and polydopamine [
18].
As support for the immobilization of the antibody, protein A or G and avidin could be used as they have the advantages of high loading capacity and easy separation along with easy washing steps [
67]. In electrochemical biosensors improvement of the signal-to-noise ratio can be achieved by nanoparticles of gold (Au) and Zinc oxide (ZnO) [
68], nanorods, graphene [
69] and graphene oxide (GO) [
70] that can act as electron mediators by lowering the applied potential. Immunosensors with integrated nanomaterials enhance the performance of the biosensors by achieving the goal of having nanostructured surfaces for the immobilization of the antigen or the antibody. The biocompatibility of the nanomaterials is also important to mention [
71].
Multiple antibodies or aptamers can be immobilized on nanoparticles, therefore increasing sensitivity. Silica nanoparticles were employed by Taghdisi et al to immobilize aptamers, while copper nanoparticles were utilized to immobilize aptamers, as they interact with the poly-T strand of the aptamer increasing the fluorescent signal [
72]. According to Wen et al. [
73], the introduction of magnetic nanoparticles additionally makes it easier for separation and concentration multiple biomarker immobilization. By altering the distribution of electric fields on the particle surface, nanoparticles can enhance the localized surface plasmons, a technique demonstrated by Jeong et al who fabricated a fiber optic LSPR sensor based on spherical gold nanoparticles (Au NPs) for a real-time label-free immunoassays of IFN-gamma and a specific antigen [
74]. This technique could be examined towards its application on autoimmune diseases. Towards IFN-gamma detection, in order to enhance chemiluminescent signal amplification, the team of Jiang used luminol-tagged gold nanoparticles (AuNPs) and by this strategy the aptasensor showed high sensitivity pointing to significant possible applications in clinical analysis [
75], while Zhu et al employed CdS quantum dots for their photoluminescent properties and their lesser toxicity in comparison to other nanoparticles that are Cd-based [
76]. This latter sensitive electrochemiluminescent (ECL) immunosensor possesses a 3-dimensional structure nanocomposite that enhances the intensity of ECL as it is beneficial for immobilizing primary antibodies. Secondary labeled with CdS quantum dots antibodies are used to generate ECL on a sandwich-type immunoreaction. In theory, it may be used to monitor the biomarkers in samples in response to drug stimulation [
76].
5. Chronic diseases and biosensors
One of the leading causes of death and of severe disability worldwide are chronic diseases. Apart from autoimmunity that has been analyzed earlier, research has been made on biosensors regarding other chronic conditions as well. Due to the large variety of chronic diseases, examples of some of the most common ones will be provided in this article. Diabetes Mellitus (DM) is a metabolic disorder that results in hyperglycemia or nerve and kidney dysfunctions that are triggered by high glucose levels and the impaired biological action or defective secretion of insulin in the patients [
77]. The detection of DM currently is performed by testing the glucose serum levels, including fasting plasma glucose (FPG) test and oral glucose tolerance test (OGTT) [
78]. Over the past few years, a new method for detection of specific blood components is being researched. Terahertz (THz) microstructured fiber (MSF) biosensing has a high accuracy, is a fast technique and there can be compact device size [
79,
80]. THz imaging and spectroscopy have become increasingly popular for in vivo applications due to the non-ionizing and non-invasive nature of THz radiation, in combination with its high sensitivity to water.
Monitoring blood glucose levels plays a significant role in managing the disease as mentioned, but there is also an interesting correlation between sweat glucose and blood glucose levels both in patients and in healthy subjects [
81]. It has been mentioned that glucose concentrations are approximately 100 times lower in a person’s sweat than in the blood [
82,
83] and a precise measure of the glucose concentration in the sweat could provide a non-invasive estimation of the blood glucose levels. Another condition related to DM is Diabetic Ketoacidosis, caused in patients with hyperglycemia by the accumulation of ketone bodies. A dominant physiological ketone, β-hydroxybutyrate, can be detected in sweat and can be used as a biomarker for this condition [
84].
Another example of a chronic autoimmune disease is Behcet’s disease (BD), a rare autoimmune disorder that causes inflammation of blood vessels and tissues. Patients often experience a different odor that could be a result of the abnormal composition of their sweat and a metabolomics analysis revealed that some metabolites could be candidate biomarkers [
85]. Several of the cytokines found in sweat were thought to be connected to a number of chronic conditions. For example, interleukin-31 (IL-31) has a role in autoimmune skin conditions, such as psoriasis and alopecia [
86] and is considered the most accurate predictor of all-cause mortality in older people compared to other cytokines [
87]. The most significant biomarker in sweat is Cl- , which is considered a gold standard biomarker for the diagnosis of cystic fibrosis[
88].
For all these diseases that have as a common characteristic the possible biomarkers in sweat, epidermal wearable biosensors is a current technological challenge. This category of portable detection devices can be worn directly onto the epidermis for in situ detection of the determined biomarkers sweat and other body fluids. Real-time analysis of the biomarkers can be facilitated, and patients can have continuous monitoring of their health and, furthermore, this monitoring can provide sufficient information for biomedical research, clinical diagnosis and treatment [
89]. An in-depth understanding of the biochemical process of various biomarkers may be achieved though this type of monitoring and provide valuable information that will strengthen the management of chronic diseases. A variety of substrates, such as smart bandages, wristbands and textile, have been used for the manufacture of wearable biosensors [
89,
90,
91], making them comfortable and convenient to wear.
Despite the difficulty of integrating liquid handling and immunobiosensing devices in a wearable platform, many types of biosensors have been used for biomarker detection in sweat with electrochemical biosensors being the most commonly used method [
92,
93,
94], including enzyme-based biosensors [
95], aptamer-based biosensors [
96] and immune-based biosensors [
92]. Another option is colorimetric biosensors that, with the use of electronic equipment, capture the sensor’s color changes in response to different analytes and quantify them by performing an image analysis [
94,
97,
98]. Of course, the ultimate clinical application would be met by the continuous monitoring combined with real-time sampling, as the number of biomarkers that can be monitored continuously with accuracy is still very limited and it is necessary to further develop these methods.
6. Biosensors for autoimmune diseases based on antibodies, antigens and peptides
The vast range of autoimmune diseases and the limited knowledge on their pathogenesis leads to symptomatic medical treatments instead of curing the diseases. As mentioned earlier, early diagnosis and detection of the diseases is important to reduce the severity of the symptoms and to prevent irreversible damage to tissues and organs. In most cases, serological tests conducted in laboratories are needed for disease confirmation in order to measure objectively and evaluate specific markers for each disease as an indicator of the disease’s progress or the pharmacologic response. Interesting concepts towards POC diagnostics on several autoimmune diseases based on Abs and Ags have been reported.
In Celiac Disease (CD) biosensors examples for the early detection of anti-tTG antibodies in patients, include the nanobiosensor developed by Gupta et al [
25] and by Neves et al [
26], as mentioned in the electrochemical biosensors section. Another team, Cosa-Garcia et al, developed a biosensor that can detect two different biomarkers, with two isotypes each (IgA AGA, IgB AGA, IgA anti-tTG and IgB anti-tTG). Nanostructured carbon-metal hybrid was used to modify screen-printed electrodes and the antigens (AGA and tTG) were immobilized onto the electrodes in order to capture specific Abs [
99]. Several examples of indirect assays regarding IgA and IgB anti-tTG have been published, such as the team of Zhang [
100] that used cyclic voltammetry, electrochemical impedance spectroscopy and differential pulse voltammetry as detection methods to develop a double signal electrochemical immunosensor for the fast and sensitive detection of human IgG antibodies. This sensor was based on AgNPs/carbon nanocomposite (Ag/C NC) as the signal probe and a catalytic substrate was developed. This double-signal strategy was successfully implemented in analysis of human IgG in patients’ serum and could provide an effective and relatively simple method for developing other immunosensors [
100]. New diagnostic tools like biosensors are needed especially in cases of common autoimmune disorders, like Multiple Sclerosis, a disease in which patients develop a series of autoantibodies and their presence in biological fluids can be used as diagnosis and prognosis biomarkers [
101], and Rheumatoid Arthritis, where the early stages diagnosis is crucial for a positive outcome for the disease [
102].
Another versatile tool towards the creation of flexible frameworks concerning autoimmune diseases are peptides, due to their capacity to assemble themselves in highly ordered structures in all three dimensions. Novel biosensors can be developed with the use of peptides, as they can modify the amino-acid sequence and therefore their secondary structure, optimizing in this way the interactions between adjacent peptides [
57]. Short peptides offer short response time in electrochemical detection, better chemical stability and have a good biocompatibility and they can be easily obtained by synthesis [
103]. The development of biosensors that are based on peptides instead of immunosensors or aptasensors is an increasing tactic in biosensors for autoimmune diseases.
In
Table 1, examples are given of the most known biosensor techniques and related biomarkers for chronic disease.
7. Discussion and General Considerations
Using biomedical equipment and biosensors in specific is an innovative method to provide personalized monitoring, disease management and treatment according to the biochemical characteristics and needs of each person and it is expected to revolutionize medical practice. Taking into account the importance of early diagnosis, especially in autoimmune diseases that appear to have significantly growing incidence, biosensors could represent a significant alternative and a viable option to the diagnostic methods that are currently being used. Autoimmunity is currently an important health issue as the number of cases reported in significantly increasing and, in most cases, there is no cure. Both practitioners and patients would benefit from early diagnosis, as mentioned, and it is essential for proper tools to be developed. These tools need to be simple, to be affordable and suitable for decentralized analysis by any type of user and not only specialized personnel.
Great advancements in immunology and molecular biology have been made leading to a set of biomarkers for almost every chronic disease, either proteins, antibodies or peptides. Implementing POC devices able to detect these biomarkers remains the biggest challenge, especially before the manifestation of symptoms. In literature, there have been many reports of such attempts, as mentioned in this review, most of them dedicated to electrochemical biosensors that appear to be viable alternatives for developing POC tests.
The incorporation of nanomaterials and other types of chemicals and attractive bioassay formats into a biosensor’s design is also important in electrochemical biosensing. Using magnetic particles for implementing bioassay configurations, combined with Screen-printed electrodes (SPE) has shown that multiomic determination can be performed with simple test protocols. When it comes to integrated forms, the incorporation of nanomaterials and the modification of electrode surfaces with the appropriate chemicals are particularly significant. Whether nanomaterials are used singly or combined in hybrid nanostructures, their variety has been utilized as electrode modifiers to improve bioreceptor immobilization and charge transfer. In many cases mentioned, high sensitivity has been achieved and many strategies have turned into microfluidic devices.
Nevertheless, application on real samples is still quite limited and at a research level. Both existing biomarkers and candidate ones that have been or will be identified, need to be validated exhaustively along with developed devices. It is essential for a combined effort to be made in order for the strategies mentioned to be implemented in the near future in clinical and daily life. The advancement of the development of other strategies as well is essential simultaneously with validating biomarkers and respectively developing biosensors. An option in the future could be biodevices with no reagent that could have the capacity for real-time determination at a lower cost.
Biosensors can provide a new hope for the next generation technology of clinical diagnosis, especially if they can achieve the detection of low or even ultralow concentrations especially in the early stages of chronic diseases, and particularly autoimmune ones, such as SLE or other disorders with antinuclear antibodies, like Myasthenia Gravis. Their ability to detect various biomolecules makes them versatile and that is a great advantage towards identifying novel biomarkers and prognosing chronic diseases or monitoring a patient’s progress and pharmaceutical response. Hopefully, in the future the increased need to cure and to prevent permanent organ and tissue damage from chronic autoimmune diseases will be met by new highly sensitive analytical devices, that have unique advantages of rapid, low-cost, real-time detection compared to traditional assays.
Author Contributions
Conceptualization, R.G. and S.K.; methodology, R.G.; investigation, R.G.; writing—original draft preparation, R.G.; writing—review and editing, S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tozzoli, R., The diagnostic role of autoantibodies in the prediction of organ-specific autoimmune diseases. Clin Chem Lab Med 2008, 46(5), 577-87. [CrossRef]
- Zharkova, O., et al., Pathways leading to an immunological disease: systemic lupus erythematosus. Rheumatology (Oxford) 2017, 56(suppl_1), i55-i66. [CrossRef]
- Zhang, X., et al., Immunosensors for Biomarker Detection in Autoimmune Diseases. Arch Immunol Ther Exp (Warsz), 2017, 65(2), 111-121. [CrossRef]
- Conrad, K., et al., Autoantibody diagnostics in clinical practice. Autoimmun Rev, 2012, 11(3), 207-11. [CrossRef]
- Campuzano Susana, P.M., González-Cortés Araceli, Yáñez-Sedeño Paloma, Pingarrón M.José, Electrochemical biosensors for autoantibodies in autoimmune and cancer diseases. Analytical Methods 2019, 11(7), 871-887.
- Thaler, M., et al., Biosensor analyses of serum autoantibodies: application to antiphospholipid syndrome and systemic lupus erythematosus. Anal Bioanal Chem, 2009, 393(5), 1417-29. [CrossRef]
- Thevenot, D.R., et al., Electrochemical biosensors: recommended definitions and classification. Biosens Bioelectron, 2001, 16(1-2), 121-31. [CrossRef]
- Clark, L.C., Jr. and C. Lyons, Electrode systems for continuous monitoring in cardiovascular surgery. Ann N Y Acad Sci, 1962, 102, 29–45. [CrossRef]
- Forster, J. Robert, C.R.L., ed. Optimizing glucose sensing for diabetes monitoring. Electronic and Optical Materials, Bioelectronics and Medical Devices, ed. H.-B.K. Kunal Pal, Anwesha Khasnobish, Sandip Bag, Indranil Banerjee, Usha Kuruganti. 2019, Woodhead Publishing. 765-778.
- Jones, A., et al., Multiplexed Immunosensors and Immunoarrays. Anal Chem, 2020, 92(1), 345-362. [CrossRef]
- Biomarkers Definitions Working, G., Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther, 2001, 69(3), 89-95.
- Jiang, X., et al., Immunosensors for detection of pesticide residues. Biosens Bioelectron, 2008, 23(11), 1577-87. [CrossRef]
- Gauglitz, G., Direct optical sensors: principles and selected applications. Anal Bioanal Chem 2005, 381(1), 141-55. [CrossRef]
- Leca-Bouvier, B. and L.J. Blum, Biosensors for Protein Detection: A Review. Analytical Letters 2005, 38(10), 1491-1517. [CrossRef]
- Mascini, M. and S. Tombelli, Biosensors for biomarkers in medical diagnostics. Biomarkers, 2008, 13(7), 637-57. [CrossRef]
- Spain, E., T.E. Keyes, and R.J. Forster, DNA sensor based on vapour polymerised pedot films functionalised with gold nanoparticles. Biosens Bioelectron, 2013, 41, 65–70. [CrossRef]
- Huang, Y., et al., Disease-Related Detection with Electrochemical Biosensors: A Review. Sensors (Basel), 2017, 17(10). [CrossRef]
- Li, S., et al., Development of a novel method to measure macrophage migration inhibitory factor (MIF) in sera of patients with rheumatoid arthritis by combined electrochemical immunosensor. Int Immunopharmacol 2008, 8(6), 859-65. [CrossRef]
- Villa Mde, G., et al., Carbon nanotube composite peptide-based biosensors as putative diagnostic tools for rheumatoid arthritis. Biosens Bioelectron, 2011, 27(1), 113-8.
- Fagundez, P., et al., An electrochemical biosensor for rapid detection of anti-dsDNA antibodies in absolute scale. Analyst 2018, 143(16), 3874-3882.
- Bhavsar, K., et al., A cytokine immunosensor for Multiple Sclerosis detection based upon label-free electrochemical impedance spectroscopy using electroplated printed circuit board electrodes. Biosens Bioelectron 2009, 25(2), 506-9. [CrossRef]
- Derkus, B., et al., Myelin basic protein immunosensor for multiple sclerosis detection based upon label-free electrochemical impedance spectroscopy. Biosens Bioelectron 2013, 46, 53-60. [CrossRef]
- Derkus, B., et al., Simultaneous quantification of Myelin Basic Protein and Tau proteins in cerebrospinal fluid and serum of Multiple Sclerosis patients using nanoimmunosensor. Biosens Bioelectron 2017, 89(Pt 2), 781-788. [CrossRef]
- Pasinszki, T. and M. Krebsz, Advances in celiac disease testing. Adv Clin Chem, 2019, 91, 1–29.
- Gupta, S., et al., Ultrasensitive transglutaminase based nanosensor for early detection of celiac disease in human. Int J Biol Macromol 2017, 105(Pt 1), 905-911. [CrossRef]
- Neves, M.M., et al., Celiac disease detection using a transglutaminase electrochemical immunosensor fabricated on nanohybrid screen-printed carbon electrodes. Biosens Bioelectron 2012, 31(1), 95-100. [CrossRef]
- Lolli, F., et al., The glycopeptide CSF114(Glc) detects serum antibodies in multiple sclerosis. J Neuroimmunol, 2005, 167(1-2), 131-7. [CrossRef]
- Bellagha-Chenchah, W., et al., Interactions between Human Antibodies and Synthetic Conformational Peptide Epitopes: Innovative Approach for Electrochemical Detection of Biomarkers of Multiple Sclerosis at Platinum Electrodes. Electrochimica Acta, 2015, 176, 1239–1247. [CrossRef]
- Abolhasan, R., et al., Application of hairpin DNA-based biosensors with various signal amplification strategies in clinical diagnosis. Biosens Bioelectron, 2019, 129, 164–174. [CrossRef]
- Pang, X., et al., A photoelectrochemical biosensor for fibroblast-like synoviocyte cell using visible light-activated NCQDs sensitized-ZnO/CH3NH3PbI3 heterojunction. Biosens Bioelectron, 2016, 77, 330–8. [CrossRef]
- Ghorbani, F., et al., Application of various optical and electrochemical aptasensors for detection of human prostate specific antigen: A review. Biosens Bioelectron, 2019, 142, 111484. [CrossRef]
- Damborsky, P., J. Svitel, and J. Katrlik, Optical biosensors. Essays Biochem 2016, 61(1), 91-100.
- Liu, S., et al., Detection of Abrin by Electrochemiluminescence Biosensor Based on Screen Printed Electrode. Sensors (Basel) 2018, 18(2), 357. [CrossRef]
- Zhao, Y., et al., Label-free ECL immunosensor for the early diagnosis of rheumatoid arthritis based on asymmetric heterogeneous polyaniline-gold nanomaterial. Sensors and Actuators B: Chemical, 2018, 257, 354–361. [CrossRef]
- Mansourian, N., M. Rahaie, and M. Hosseini, A Nanobiosensor Based on Fluorescent DNA-Hosted Silver Nanocluster and HCR Amplification for Detection of MicroRNA Involved in Progression of Multiple Sclerosis. J Fluoresc, 2017, 27(5), 1679-1685. [CrossRef]
- Dong, H., et al., Label-free and ultrasensitive microRNA detection based on novel molecular beacon binding readout and target recycling amplification. Biosens Bioelectron, 2014, 53, 377–83. [CrossRef]
- Nguyen, H.H., et al., Surface plasmon resonance: a versatile technique for biosensor applications. Sensors (Basel), 2015, 15(5), 10481-510. [CrossRef]
- Shrivastav, A.M., U. Cvelbar, and I. Abdulhalim, A comprehensive review on plasmonic-based biosensors used in viral diagnostics. Commun Biol, 2021, 4(1), 70. [CrossRef]
- Vega, B., et al., Real-time detection of the chemokine CXCL12 in urine samples by surface plasmon resonance. Talanta, 2013, 109, 209–15. [CrossRef]
- Sguassero, A., et al., A simple and universal enzyme-free approach for the detection of multiple microRNAs using a single nanostructured enhancer of surface plasmon resonance imaging. Anal Bioanal Chem, 2019, 411(9), 1873-1885. [CrossRef]
- Yerrapragada, R.M. and D. Mampallil, Interferon-gamma detection in point of care diagnostics: Short review. Talanta 2022, 245, 123428. [CrossRef]
- ,Breese, E. et al., Interleukin-2- and interferon-gamma-secreting T cells in normal and diseased human intestinal mucosa. Immunology, 1993, 78(1), 127-31.
- Sarangi, S., et al., Interferon-gamma (IFN-gamma) intronic variant (rs2430561) is a risk factor for systemic lupus erythematosus: Observation from a meta-analysis. Lupus, 2023, 32(2), 284-294. [CrossRef]
- Manivasagam, S., et al., Targeting IFN-lambda Signaling Promotes Recovery from Central Nervous System Autoimmunity. J Immunol 2022, 208(6), 1341-1351.
- Šípová, H., et al., Surface plasmon resonance biosensor based on engineered proteins for direct detection of interferon-gamma in diluted blood plasma. Sensors and Actuators B: Chemical, 2012, 174, 306–311. [CrossRef]
- Šípová, H., et al., Sensitive Detection of Interferon-Gamma with Engineered Proteins and Surface Plasmon Resonance Biosensor. Procedia Engineering, 2011, 25, 940–943. [CrossRef]
- Stigter, E.C., G.J. de Jong, and W.P. van Bennekom, An improved coating for the isolation and quantitation of interferon-gamma in spiked plasma using surface plasmon resonance (SPR). Biosens Bioelectron, 2005, 21(3), 474-82.
- Chuang, T.L., et al., Disposable surface plasmon resonance aptasensor with membrane-based sample handling design for quantitative interferon-gamma detection. Lab Chip 2014, 14(16), 2968-77. [CrossRef]
- Chen, C. and J. Wang, Optical biosensors: an exhaustive and comprehensive review. Analyst, 2020, 145(5), 1605-1628. [CrossRef]
- Golfinopoulou, R., et al., Clinical Genomic, phenotype and epigenetic insights into the pathology, autoimmunity and weight management of patients with Myasthenia Gravis (Review). Mol Med Rep 2021, 24(1), 512. [CrossRef]
- Tamayo, J., et al., Biosensors based on nanomechanical systems. Chem Soc Rev 2013, 42(3), 1287-311. [CrossRef]
- Arlett, J.L., E.B. Myers, and M.L. Roukes, Comparative advantages of mechanical biosensors. Nat Nanotechnol, 2011, 6(4), 203-15. [CrossRef]
- Lange, K., B.E. Rapp, and M. Rapp, Surface acoustic wave biosensors: a review. Anal Bioanal Chem 2008, 391(5), 1509-19. [CrossRef]
- Drouvalakis, K.A., et al., Peptide-coated nanotube-based biosensor for the detection of disease-specific autoantibodies in human serum. Biosens Bioelectron 2008, 23(10), 1413-21. [CrossRef]
- Ekinci, K.L., Electromechanical transducers at the nanoscale: actuation and sensing of motion in nanoelectromechanical systems (NEMS). Small, 2005, 1(8-9), 786-97. [CrossRef]
- Holzinger, M., A. Le Goff, and S. Cosnier, Nanomaterials for biosensing applications: a review. Front Chem, 2014, 2, 63. [CrossRef]
- Florea, A., et al., Electrochemical Biosensors as Potential Diagnostic Devices for Autoimmune Diseases. Biosensors (Basel), 2019, 9(1), 38. [CrossRef]
- Schedin, F., et al., Detection of individual gas molecules adsorbed on graphene. Nat Mater, 2007, 6(9), 652-5. [CrossRef]
- Afsahi, S., et al., Novel graphene-based biosensor for early detection of Zika virus infection. Biosens Bioelectron, 2018, 100, 85–88. [CrossRef]
- Aldewachi, H., et al., Gold nanoparticle-based colorimetric biosensors. Nanoscale 2018, 10(1), 18-33. [CrossRef]
- Iijima, S., Helical microtubules of graphitic carbon. Nature, 1991, 354(6348), 56-58. [CrossRef]
- Wang, L., et al., Covalent organic frameworks (COFs)-based biosensors for the assay of disease biomarkers with clinical applications. Biosensors and Bioelectronics, 2022, 217, 114668. [CrossRef]
- Rasheed, T. and K. Rizwan, Metal-organic frameworks-based hybrid nanocomposites as state-of–the-art analytical tools for electrochemical sensing applications. Biosensors and Bioelectronics, 2022, 199, 113867.
- Cernat, A., et al., Nanostructured Platform Based on Graphene-Polypyrrole Composite for Immunosensor Fabrication. International Journal of Electrochemical Science, 2015, 10, 4718–4731. [CrossRef]
- Baraket, A., et al., A fully integrated electrochemical biosensor platform fabrication process for cytokines detection. Biosens Bioelectron, 2017, 93, 170–175. [CrossRef]
- Balkenhohl, T. and F. Lisdat, Screen-printed electrodes as impedimetric immunosensors for the detection of anti-transglutaminase antibodies in human sera. Anal Chim Acta, 2007, 597(1), p. 50-7. [CrossRef]
- Taleat, Z., et al., CA 125 Immunosensor Based on Poly-Anthranilic Acid Modified Screen-Printed Electrodes. Electroanalysis, 2013, 25, 269–277. [CrossRef]
- Wang, X., Fabrication of Electrochemical Immunosensor for Interferon-γ Determination and Its Application of Tuberculosis Diagnosis. International Journal of Electrochemical Science, 2017, 12, 7262–7271. [CrossRef]
- Yan, G., et al., A highly sensitive label-free electrochemical aptasensor for interferon-gamma detection based on graphene controlled assembly and nuclease cleavage-assisted target recycling amplification. Biosens Bioelectron, 2013, 44, 57–63. [CrossRef]
- Yang, Z., et al., Graphene oxide based ultrasensitive flow-through chemiluminescent immunoassay for sub-picogram level detection of chicken interferon-gamma. Biosens Bioelectron, 2014, 51, 356–61. [CrossRef]
- Felix, F.S. and L. Angnes, Electrochemical immunosensors - A powerful tool for analytical applications. Biosens Bioelectron, 2018, 102, 470–478. [CrossRef] [PubMed]
- Taghdisi, S.M., et al., An amplified fluorescent aptasensor based on single-stranded DNA binding protein, copper and silica nanoparticles for sensitive detection of interferon-gamma. Anal Chim Acta, 2017, 984, 162–167. [CrossRef]
- Wen, D., et al., DNA based click polymerization for ultrasensitive IFN-γ fluorescent detection. Sensors and Actuators B: Chemical, 2018, 276, 279–287. [CrossRef]
- Jeong, H.H., et al., Real-time label-free immunoassay of interferon-gamma and prostate-specific antigen using a Fiber-Optic Localized Surface Plasmon Resonance sensor. Biosens Bioelectron, 2013, 39(1), 346-51. [CrossRef]
- Jiang, J., et al., A homogeneous hemin/G-quadruplex DNAzyme based turn-on chemiluminescence aptasensor for interferon-gamma detection via in-situ assembly of luminol functionalized gold nanoparticles, deoxyribonucleic acid, interferon-gamma and hemin. Anal Chim Acta, 2013, 791, 60–4. [CrossRef] [PubMed]
- Zhu, M., et al., Dynamic evaluation of cell-secreted interferon gamma in response to drug stimulation via a sensitive electro-chemiluminescence immunosensor based on a glassy carbon electrode modified with graphene oxide, polyaniline nanofibers, magnetic beads, and gold nanoparticles. Microchimica Acta, 2016, 183, 1739–1748. [CrossRef]
- Xue, J., et al., Ultra-High Sensitivity Terahertz Microstructured Fiber Biosensor for Diabetes Mellitus and Coronary Heart Disease Marker Detection. Sensors (Basel), 2023, 23(4). [CrossRef]
- Khan, R.M.M., et al., From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina (Kaunas), 2019, 55(9), 546. [CrossRef] [PubMed]
- Wang, J., et al., THz Sensing of Human Skin: A Review of Skin Modeling Approaches. Sensors, 2021, 21, 3624. [CrossRef]
- Habib, M., et al., Hollow core photonic crystal fiber for chemical identification in terahertz regime. Optical Fiber Technology, 2019, 52, 101933. [CrossRef]
- Karpova, E.V., E.E. Karyakina, and A.A. Karyakin, Wearable non-invasive monitors of diabetes and hypoxia through continuous analysis of sweat. Talanta, 2020, 215, 120922. [CrossRef]
- Moyer, J., et al., Correlation Between Sweat Glucose and Blood Glucose in Subjects with Diabetes. Diabetes Technology & Therapeutics, 2012,, 14(5), 398-402. [CrossRef]
- Karpova, E.V., et al., Noninvasive Diabetes Monitoring through Continuous Analysis of Sweat Using Flow-Through Glucose Biosensor. Anal Chem, 2019, 91(6), 3778-3783. [CrossRef]
- Zhang, X., et al., Integrated Wearable Sensors for Sensing Physiological Pressure Signals and β-Hydroxybutyrate in Physiological Fluids. Analytical Chemistry, 2022,, 94(2), 993-1002. [CrossRef]
- Cui, X., et al., Specific sweat metabolite profile in ocular Behcet’s disease. International Immunopharmacology, 2021, 97, 107812. [CrossRef] [PubMed]
- Hladek, M., et al., High Social Coping Self-Efficacy Associated With Lower Sweat Interleukin-6 in Older Adults With Chronic Illness. Journal of Applied Gerontology, 2022, 41(2), 581-589. [CrossRef]
- Varadhan, R., et al., Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci, 2014, 69(2), 165-73. [CrossRef]
- Farrell, P.M., et al., Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J Pediatr, 2017, 181S, S4-S15 e1.
- Kim, J., et al., Wearable biosensors for healthcare monitoring. Nature Biotechnology, 2019, 37(4), 389-406. [CrossRef]
- Kim, J., et al., Wearable temporary tattoo sensor for real-time trace metal monitoring in human sweat. Electrochemistry Communications, 2015, 51, 41–45. [CrossRef]
- Sato, K., R. Leidal, and F. Sato, Morphology and development of an apoeccrine sweat gland in human axillae. Am J Physiol 1987, 252(1 Pt 2), R166-80. [CrossRef]
- Huynh, V.L., et al., Hollow Microfibers of Elastomeric Nanocomposites for Fully Stretchable and Highly Sensitive Microfluidic Immunobiosensor Patch. Advanced Functional Materials, 2020, 30(46), 2004684. [CrossRef]
- Sempionatto, J.R., et al., Epidermal Enzymatic Biosensors for Sweat Vitamin C: Toward Personalized Nutrition. ACS Sensors 2020, 5(6), 1804-1813. [CrossRef]
- Jia, W., et al., Electrochemical Tattoo Biosensors for Real-Time Noninvasive Lactate Monitoring in Human Perspiration. Analytical Chemistry, 2013, 85(14), 6553-6560. [CrossRef]
- Bollella, P. and L. Gorton, Enzyme based amperometric biosensors. Current Opinion in Electrochemistry, 2018, 10, 157–173. [CrossRef]
- An, J.E., et al., Wearable Cortisol Aptasensor for Simple and Rapid Real-Time Monitoring. ACS Sens 2022, 7(1), 99-108. [CrossRef]
- Bae, C.W., et al., Fully Stretchable Capillary Microfluidics-Integrated Nanoporous Gold Electrochemical Sensor for Wearable Continuous Glucose Monitoring. ACS Applied Materials & Interfaces, 2019, 11(16), 14567-14575. [CrossRef]
- Santiago-Malagon, S., et al., A self-powered skin-patch electrochromic biosensor. Biosens Bioelectron, 2021, 175, 112879. [CrossRef]
- Neves, M.M.P.S., et al., Multiplexed electrochemical immunosensor for detection of celiac disease serological markers. Sensors and Actuators B: Chemical, 2013, 187, 33–39. [CrossRef]
- Zhang, S., et al., A double signal electrochemical human immunoglobulin G immunosensor based on gold nanoparticles-polydopamine functionalized reduced graphene oxide as a sensor platform and AgNPs/carbon nanocomposite as signal probe and catalytic substrate. Biosens Bioelectron, 2016, 77, 1078–85. [CrossRef]
- Real-Fernandez, F., et al., Glycopeptide-based antibody detection in multiple sclerosis by surface plasmon resonance. Sensors (Basel), 2012, 12(5), 5596-607. [CrossRef]
- Takeuchi, T., Biomarkers as a treatment guide in rheumatoid arthritis. Clin Immunol, 2018, 186, 59–62. [CrossRef] [PubMed]
- Hu, H., et al., A photoelectrochemical immunoassay for tumor necrosis factor-α using a GO-PTCNH2 nanohybrid as a probe. Journal of Electroanalytical Chemistry, 2018, 824, 195–200. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).