Introduction
Children with Down Syndrome (DS) and children with Autism Spectrum Disorder (ASD), often experience stress due to impaired social interaction and verbalization, e.g., when exposed to a psychosocial stressor like initiating and maintaining social relationships and difficulties in cognitive switching [
1,
2,
3]. These children seem more fearful than their typically developing peers [
4], use less effective coping strategies in response to stressful situations [
5], and have strong stress reactions to their social environment. Children with ASD or DS show an increased risk of experiencing traumatic events, especially social mistreatment [
1,
6] and anxiety remains one of the most common disorders for children and young people with DS in order to build good mental health [
7].
Some lines of evidence suggest that interactions with animals, specifically dogs, may be stress-reducing for several populations [
8,
9]. First, various studies have revealed positive effects of dog ownership on family functioning as well as decreased child anxiety and stress [
10,
11,
12]. Typically developing children who are supported in a stressful situation by their own dog, experience less stress (as measured by children's self-reported feelings of stress and salivary cortisol) than children who are alone in the same situation, or who are supported by their parents [
13].
Dogs can also offer support in case of developmental or emotional problems [
14,
15]. Beetz and colleagues [
16,
17] measured the cortisol levels of children with insecure attachment styles in the presence of dogs. The support of a dog during the study was associated with significantly lower cortisol levels. This effect was strongly correlated with the time the children spent in physical contact with the dog. There is also some evidence that children admitted to a hospital benefit from interactions with dogs, especially with regard to their experienced anxiety and pain [
18,
19,
20]. However, a critical review concluded that most studies on human-animal interactions in hospital setting suffer from weak methodology and flagged construct validity [
21].
The positive effects of the interactions with dogs on stress symptoms discussed here have mostly been studied in settings in which the children’s families were pet owners. Yet, stress reduction has also been an important hypothesized effect of animal-assisted therapy, and specifically dog-assisted therapy (DAT). DAT consists of structured one-on-one sessions, offered by trained professionals who work with certified therapy dogs. Much like other forms of therapy, the child visits the therapist and dog for a number of sessions, in which he/she works on specific therapeutic goals.
For children with DS or ASD, DAT has been found to improve their social interactions and communication with other people [
22,
23], and to cope with stressful situations [
17,
24,
25]. A recent study on DAT [
26] found a positive effect on behavioral synchrony, and a non-significant trend of decreased problem behavior in children with DS and children with ASD.
A literature review [
24], found two case studies in which stress and arousal were
physiologically measured in children with ASD during animal-assisted therapy with horses [
27] and guinea pigs [
25]. Another study was recently published on the effect of Dog-Assisted interventions in a classroom for children with special educational needs. The conclusion was that the dogs reduced the stress (lower cortisol) levels of the children [
28]. To the best of our knowledge, no studies involving physiological measures on children with DS in the DAT-context were found.
Physiological measurements of stress
A relatively inexpensive and easy way to collect a biomarker of stress is salivary cortisol, which can safely be used in research with children. Research shows a relationship between stress and the hormone cortisol: The more stress a child experiences, the higher its cortisol levels become[
29,
30,
31,
32,
33]. The hypothalamus-pituitary-adrenocortical axis (HPA) is involved in activating the stress response and plays an important role in physiological coping [
33,
34]. As indicated by Hanrahan [
34], a wide range of factors should be considered while using this measure in research with children, including standardizing the time for sample collection, including baseline samples, using consistent collection materials and methods, controlling for certain drinks, foods, medications, and diagnoses, and establishing sound procedures and protocols.
In addition to cortisol, heart rate variability (HRV) is also a viable physiological indicator of stress [
29,
35,
36]. HRV refers to the variation in intervals between successive heartbeats (IBIs: inter beat interval), and is an indicator of the functioning of the autonomic nervous system. The autonomic nervous system (parasympathetic and sympathetic branches) is the part of the nervous system that controls the visceral functions of the body, including the heart, the movements of the gastrointestinal areas and many other vital activities. Mental and emotional feelings directly influence the autonomic nervous system. Research shows that high HRV is related to rest and relaxation, while low HRV is related to effort and stress[
35,
36]. HRV measurements identify the activity of the parasympathetic system by measuring the beat-to-beat alteration in heart rate, e.g., low HRV is indicative of reduced parasympathetic cardiac control [
37,
38].
In summary, children with ASD/DS experience stress in various situations. Dog Assisted Intervention may offer relief. We think that stress reduction happens physiologically, but little research has been done on this, specifically in these populations as no studies involving physiological measures to assess stress reduction on children with DS in the DAT-context were found. The two studies taking physiological measures of children with ASD in the DAT context were case studies involving horses [
27] and guinea pigs [
25]. This research therefore set out to investigate the following two research questions: 1)To what extent is there a difference in the HRV measurements and cortisol levels of children with DS and ASD after following a 6-week DAT program? And 2) To what extent do HRV measurements and cortisol levels change
during DAT sessions (comparing the measurements before and after the session)?
Materials and Methods
Ethical considerations
In our explorative study we will look at how the execution of various tasks in cooperation with a dog influences the experienced stress of children with DS and ASD. Given that this is an explorative study—the first in which physiological measures are used in a DAT context—and because we work with a vulnerable group of children, we have chosen not to use a (non-intervention) control group at this point.
In this study, twenty children with DS or ASD were followed for six weeks while they received dog-assisted therapy (DAT). At the start of the study, written consent was obtained from the parents of the children. The parents were also informed about the aim of the study, as well as about the protocol of the therapy. It was made clear to them that they were free to stop their participation at any moment.
During the research, the dog’s welfare was continually monitored by a handler. All necessary precautions were taken to prevent possible harm to the dogs, such as limiting the time of interacting with the children. The Medical Ethical Review Committee of the University of Amsterdam stated that an official approval of this study was not required concerning the Medical Research Involving Human Subject Act (WMO).
Design
The current study investigates the effect of a 6-week DAT program on the salivary cortisol levels and heart rate variability of 20 children with DS and children with ASD who received a weekly one-hour session of Dog Assisted Therapy. Before and after every session we collected salivary cortisol and before, and after every session we also recorded the heart rate variability.
Participants
Twenty children volunteered in this study, eight children with Down Syndrome (DS) (six males and two females with a mean age of 12, range 10-14 years) and twelve children with autism spectrum disorder (ASD) (eight males and four females with a mean age of 13, range 11-15 years). All children were diagnosed by a pediatrician, and had no psychiatric co-morbidity. All children who participated in the study had an IQ> 40. They all attended education at a school for students with special needs. No allergies and medication use were reported by their parents.
The children were recruited through an invitation letter to the parents in the network of the first author, through the website of the SAM Foundation, (
www.stichtingsam.nl), through an organization for therapy dogs (
www.contacthond.nl) and by referrals from health-care professionals. All parents signed an informed consent and agreed with the procedure. Exclusion criteria were fear of dogs, severe dog allergy, wheel-chair dependency and reported aggression toward animals.
Procedure
The sessions with the dog took place at a gymnasium. The children were accompanied by a therapist. They worked together in a 30 min session, once a week for a period of 6 weeks. Activities during the sessions were selected from the CTAC Method [39]. We selected psycho-motor and socialization activities in particular, for example having the dog follow the child’s movements and letting the child exercise his/her balance and be aware of posture and expression. The therapist explained what was expected of the child and what gestures and encouraging words were needed to work with the dog.
During the first phase of each session, the child and the dog, under the care of the therapist and handler, did some exercises to get used to performing the tasks. The second phase of each session was characterized by encouraging the child to build an obstacle course, and to lead the dog through these series of obstacles and ask the dog to perform certain commands, such as sitting and lying on a mat, slalom walk around cones and walking on a bench, or jumping over a low bar [
26].
HRV Measurements
At the beginning of each session, the children were asked to put on a chest belt with heart rate sensor and wear a Polar device V800 wristwatch (Polar Electro Oy, Kempele Finland) to record N-N intervals (Inter beat intervals). The data were read into Polar FlowSync software after the sessions.
The HRV of the children (n = 20) were registered during two periods of 3 minutes, before and at the end of each session. During this registration the children were asked to sit on a chair. Based on visual inspection, we chose the two artifact free periods. The artifacts were due to missing beats because of not right fitted chest belt and movements of the children.
Analysis method HRV - Time Domain Analysis. We first analyzed the global indicators of HRV. The analyzed time domain HRV parameters are the following: Average of the N-N intervals (Mnn) and the Standard deviation of the N-N intervals (SDnn) in seconds. The latter is the most straightforward measure of overall HRV. We also calculated the Root mean square of successive differences (RMSSD), which is the standardized average difference between consecutive beats). RMSSD is the most commonly used time domain measure for short (beat-to-beat) variations.
Analysis method HRV - Frequency domain. A more in-depth analysis of HRV was performed using the frequency bands. To be specific, we analyzed the LF (low frequency) band: 0.04 - 0.15 Hz (period of 6.7 - 25 s). This "blood pressure band" is associated with a relatively large contribution from the sympathetic branch of the autonomic nervous system. The HF (high frequency: 0.15 - 0.4 Hz; period of 2.5 - 6.7 s) "respiratory band", associated with a relatively large contribution from the parasympathetic branch of the autonomic nervous system, was also analyzed. Lastly, we used the LF / HF ratio, which is often used as index for the relative activation of the sympathetic versus the parasympathetic nervous system (van Boxtel et al. 2018).
HRV Data preparation. The HRV data was processed in 3 steps: pre-processing, data analysis and statistical analysis. The first step, pre-processing was intended to reach correct files with N-N intervals (IBIs) for each child, for each session and for the intervals within a session. Some children had less sessions because of insufficient artifact-free data, or had missed sessions due to illness.
Secondly, the actual data analysis was performed. For the raw N-N intervals artifact correction was applied. When a particular IBI was outside the range of + or - 3 standard deviations (SD) around the average, it was removed from the series and interpolated by means of a cubic spline interpolation. In total there were 144 measurements and the intervals averaged 209.1 ms (min. 34.6 – max. 319.5 s, SD = 76.5 ms).
HRV Statistical Analysis. The Root Mean Square of Successive Differences (RMSSD), The mean R-R (Mnn) and Standard Deviation of NN intervals (SDnn) were computed in the time domain, and Low Frequency (LF, 0,04 – 0.15 Hz), High Frequency (HF, 0.15 – 0.4 Hz), and the LF/HF ratio were computed from the Lomb-Scargle spectra. The data of SDnn were not normally distributed. We solved this by applying a logarithmic transformation. The log-transformed data was then analyzed. All variables obtained were analyzed using mixed effect (Non Linear Mixed Effects) models. This was done with the R-statistical package version 3.5.2. NLME models are able to handle missing data, and are able to decrease the residual variance in comparison with a regular repeated ANOVA.
Cortisol Measurements
Samples of salivary cortisol were collected from the children prior to and at 10 minutes after sessions were completed for every session, at a similar time of day (9.45 am, start session 10 am) for each participant during six weeks. The collection was standardized using a Salivette (Sarstedt AG & Co, Numbrecht, Germany), a cotton role packaged in a plastic tube-like container, and the procedure was carried out by the same assistant every time. The child had to chew on the cotton role for 2 minutes. We controlled for foods and drinks for 1 hour before the samples (no caffeinated products, no dairy products and no steroids). In addition, children did not eat or drink for half an hour before each session. The samples were refrigerated within 1 hour after collection. After collecting samples for six weeks, we sent the samples to the laboratory of the Wilhelmina University Children Hospital of Utrecht, the Netherlands for analysis. Cortisol in saliva was measured without extraction using an in-house competitive radio-immunoassay employing a polyclonal anti-cortisol-antibody (K7348). [1,2-3H(N)]-Hydrocortisone (PerkinElmer Inc, Groningen, The Netherlands, NET396250UC) was used as a tracer.
Cortisol Statistical Analysis
The cortisol samples obtained were also analyzed using mixed effect (LME) models with the R-statistical package version 3.5.2.
Results
Heart Rate Variability, time domain:
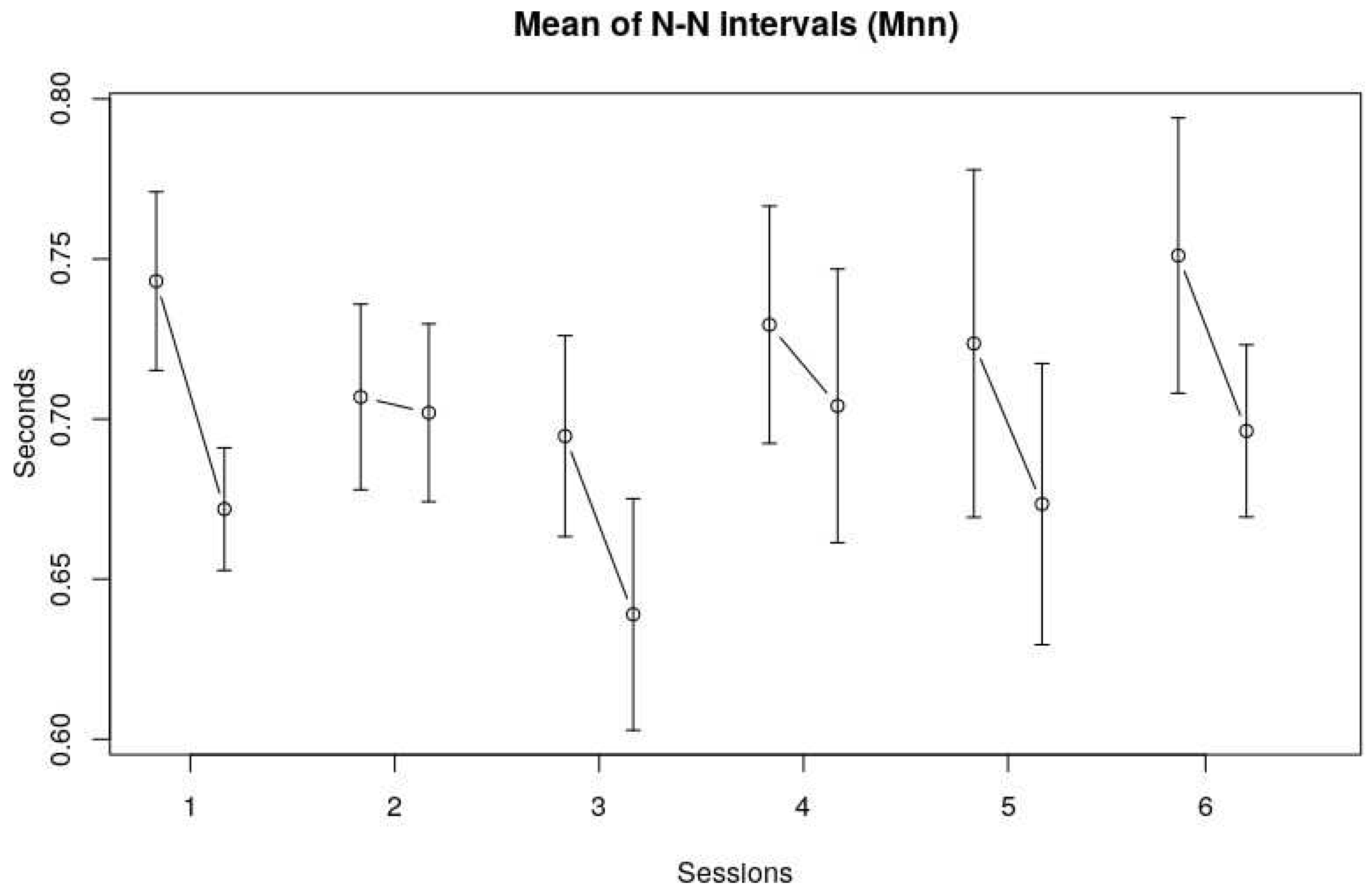
The overall average length of the N-N intervals (Mnn) was 0.69 s (SE 0.11 s). This corresponds to an average heart rate of 86.30 bpm. The averages were fairly normally distributed. Mnn does seem to decrease slightly in the third session, but a visual inspection of
Figure 1 shows no clear trend in Mnn over the course of six sessions. Although there seemed no difference over all sessions, the Mnn before and after a session did vary.
Figure 1 shows that the Mnn are somewhat higher at the beginning of each session, meaning that the heart rate is slightly lower at the beginning.
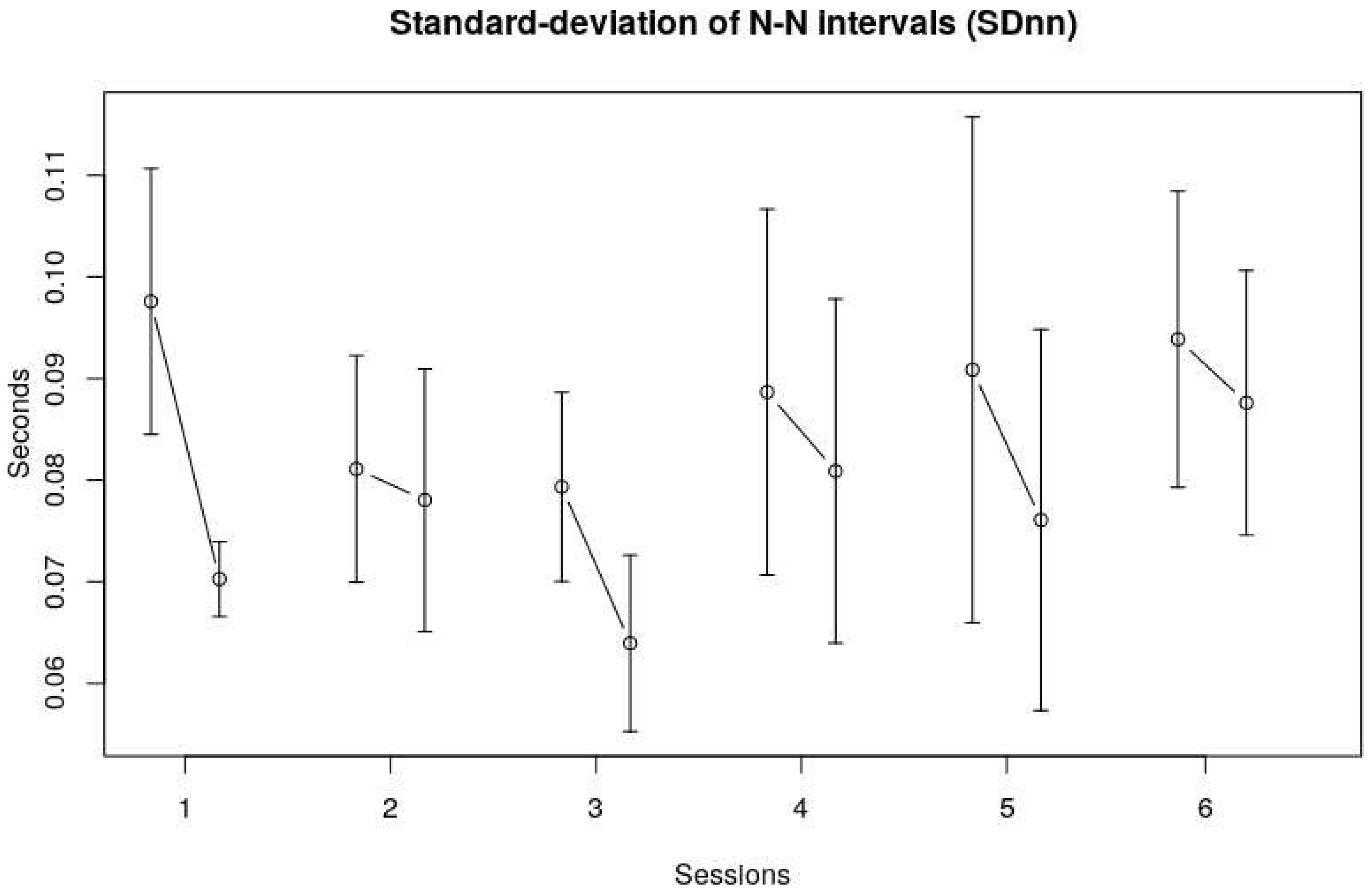
The standard deviations of the R-R intervals (SDnn) provide a global measure for HRV in the time domain. The average SDnn was 0.078 (SE 0.01) Again, no clear trend could be observed over the six sessions, although a visual inspection of
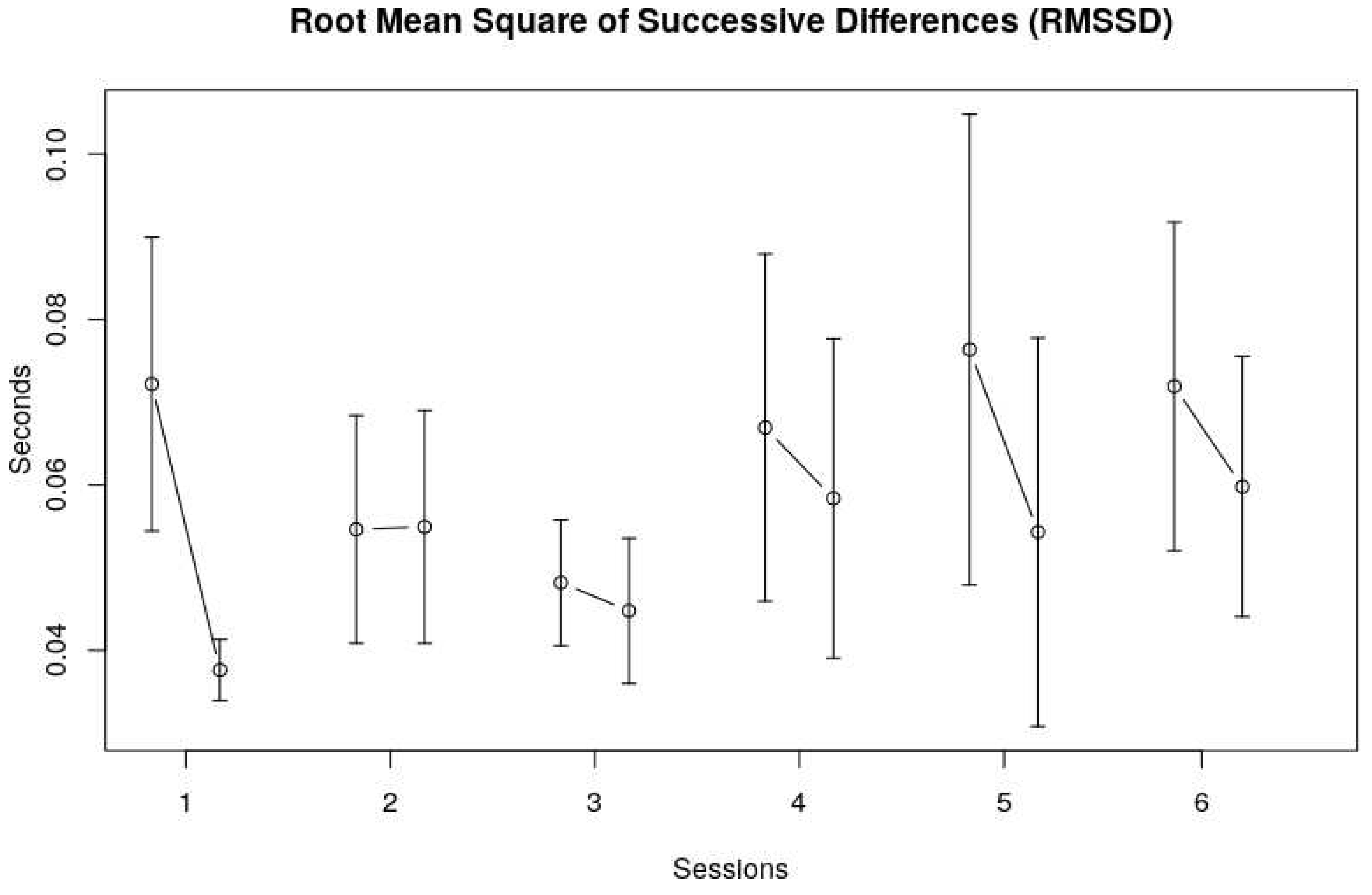
Figure 2 shows that the SDnn seemed to decrease from the first to third session, and then increased again to a somewhat stable level over the last three sessions.The SDnn at the end of each session seemed lower than at the beginning of each session, indicating less variability over the course of a single session, see also the RMSSD data in
Figure 3.
Figure 2 Standard-deviation of N-N intervals (SDnn). The vertical axis displays the Standard deviation of N-N intervals of the children in seconds, the horizontal axis represents the session number.
The Root mean square of successive differences (RMSSD) shows roughly the same picture, namely no clear trend over sessions, and a decrease of the heart rate variability within a session.
Table 1 shows the NLME model results for the Time domain measures (Mnn, LogSDnn and RMSSD). No significant changes in the Time domain measures were found over the six sessions. However, with regard to measures taken within a session, we found significant changes in Mnn (
F(1,113) = 12.86,
p < .01), SDnn (
F(1,113) = 5.45,
p = .02) and RMSSD (
F(1,113) = 12.96,
p < .01). No interaction effects were found.
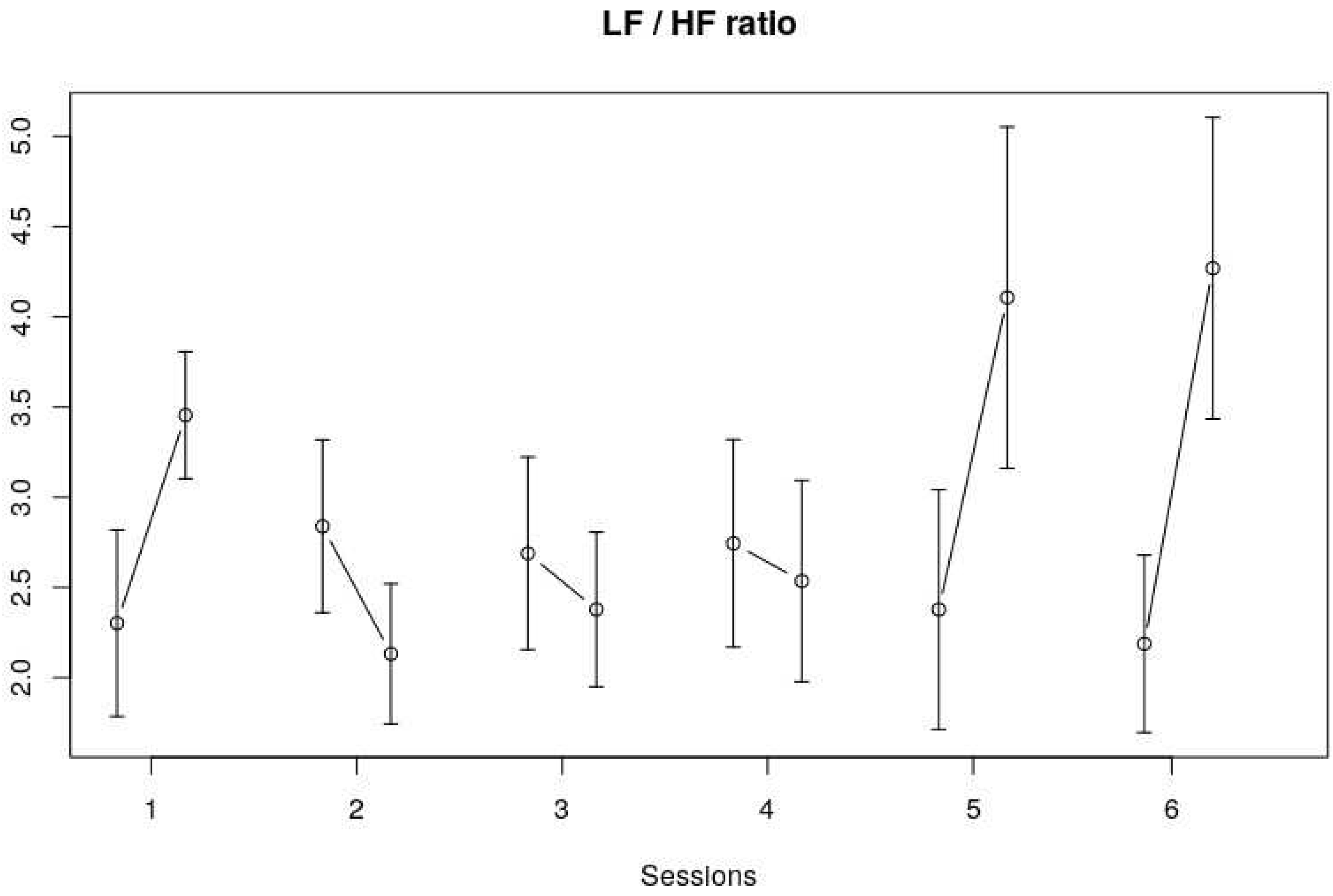
Frequency Domain
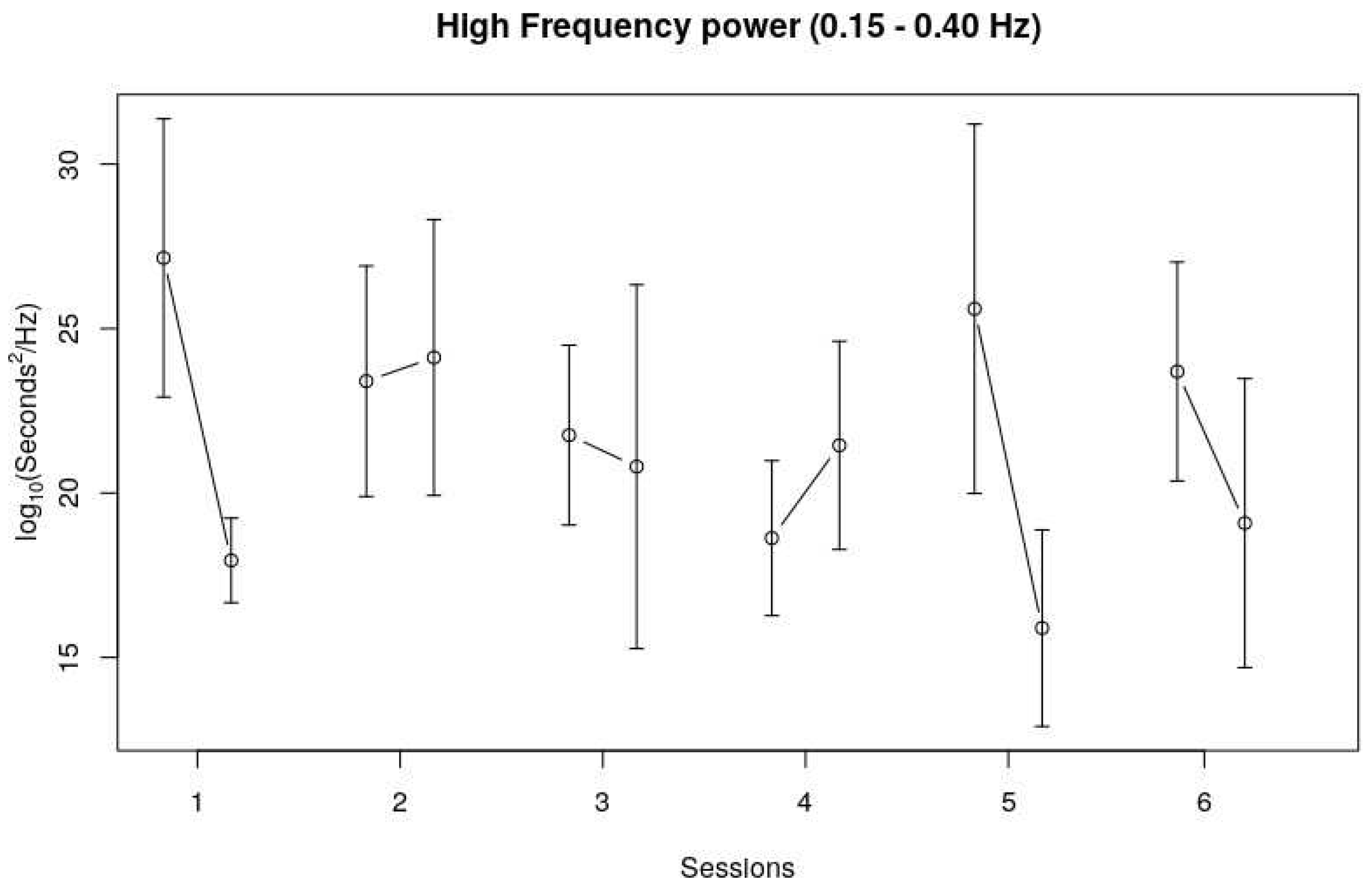
With regard to the Low Frequency band (LF), no trend could be observed over the course of six sessions, as well as no clear trend within sessions. The High Frequency band (HF) showed a similar pattern as the Time Domain measures, and especially the RMSSD pattern. No clear trend over the six sessions could be observed, but HF seemed to decrease during four out of six sessions (see
Figure 4).
Table 2 shows the NLME model results for the Frequency domain measures (LF band, HF band and LF/HF). No significant findings for the LF band were observed. The within-session trend that could be observed for the HF band fell short of significance (
F(1,113) = 3.38,
p = .07). The within-session LF/HF did show a significant change (
F(1,113) = 4.96,
p = 03). The session*within session LF/HF also showed a significant change (
F(5,113) = 3.07,
p = 0.01) which indicates that the within-session effects of the LF/HF ratio differed across the sessions. This is also visible in
Figure 5, that is, the changes in LF/HF ratio within the first, fifth and sixth session were bigger and in a different direction compared to the LF/HF ratio changes within the other sessions.
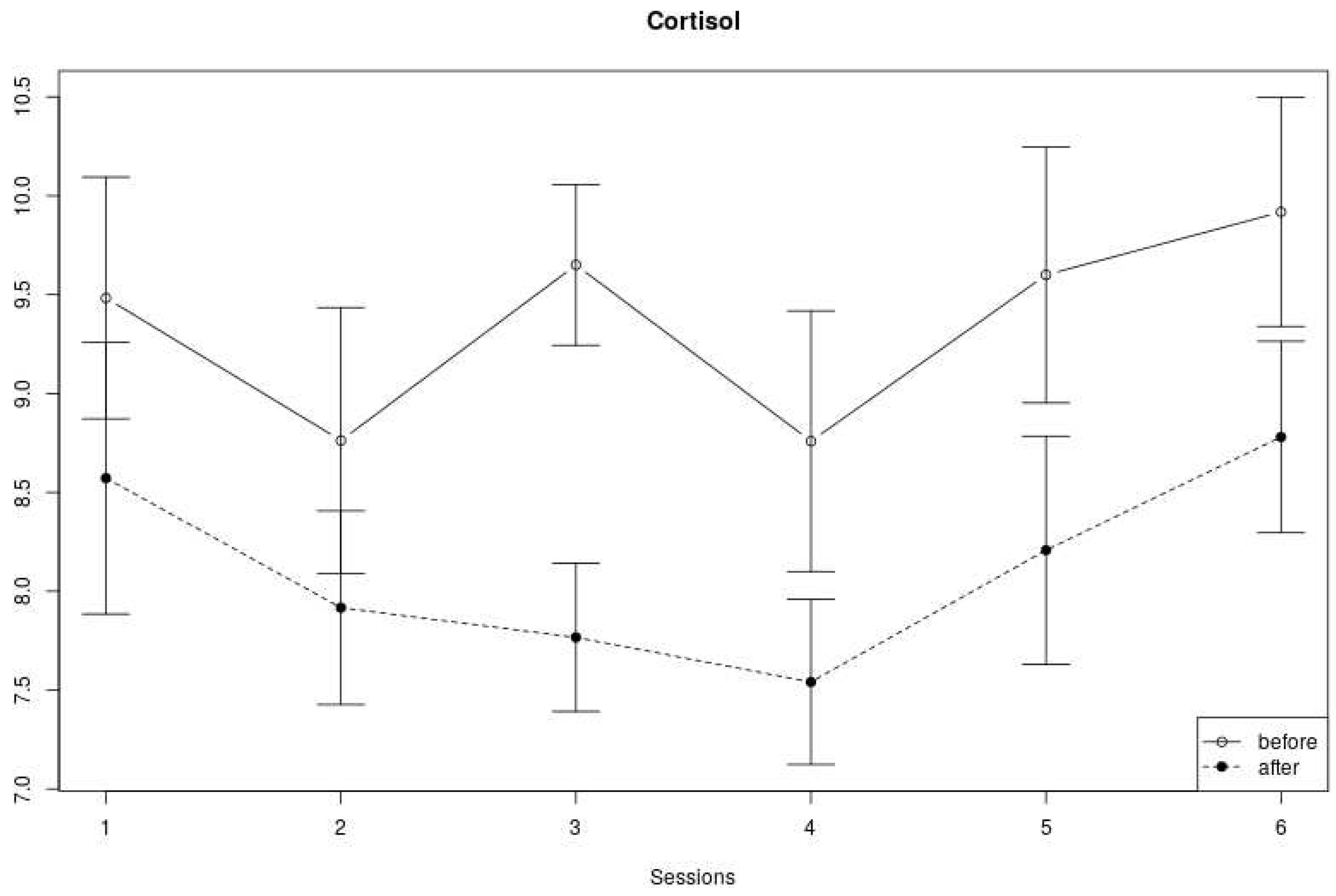
Cortisol measures We found no clear trend with regard to the changes in cortisol levels over the six sessions (see
Figure 6). Cortisol at the beginning of a session alternately decreased and increased over the course of six weeks, and cortisol levels at the end of a session decreased from the first to fourth session, and then increased again in the last two sessions. Yet, the cortisol levels measured within a session were always lower at the end of each session compared to levels at the start of the session.
In line with the visual inspection of
Figure 6,
Table 3 shows no significant effect of cortisol across sessions, but a significant within-session effect (F(1,159) = 25.36, p < .01). No significant interaction effect was found.
Discussion
In this study, we analyzed physiological changes in 8 children with Down syndrome (SD) and 12 children with Autism Spectrum Disorder (ASD) who took part in a 6-week Dog-Assisted Therapy (DAT) program. The children worked with the same dog during the 6-week program. To be specific, we measured Heart Rate Variability (HRV) and salivary cortisol at the beginning and end of 6 weekly sessions of DAT. To our knowledge, only recently one study is published measuring cortisol during dog assisted intervention in a classroom [
28] while this is the first study in which these physiological measures (HRV and cortisol) are collected during a DAT program for children with ASD and DS, although note that in previous (case) studies on animal-assisted therapy with horses [
27] and guinea pigs [
25], physiological data was also used.
Our results show that both HRV (Time and Frequency Domain) and cortisol measures decreased significantly within sessions. Over the course of the full program (6 sessions), however, no significant changes in HRV and salivary cortisol could be found. In addition, the data mostly showed no significant interaction effects, apart from the LF/HF ratio, which showed a different within-session effect across sessions. That is, the changes in LF/HF ratio within the fifth and sixth session were bigger compared to the changes within the first four sessions.
Interestingly, there seems to be a discrepancy between the two types of physiological data. To be specific, the Time-Domain HRV measurements decrease within sessions, which generally indicates a higher level of arousal. At the same time, the cortisol levels decrease within sessions, which corresponds to lower levels of arousal. A reason for this discrepancy might be that during the course of the session, the movement activity of the children increased, which makes the heart rate increase as well. Although the children were seated on a chair when taking the HRV measurements, it takes time for an increased heart rate to return to a baseline level. It is well-known that HRV decreases when the heart rate is increased [40].
This means that the higher activity levels within DAT sessions confounded the HRV results. From the HF Frequency results it also appears that the children were breathing faster over the course of a session. Yet, since breathing is not directly measured, and since this interpretation of the HF variance is not undisputed [
37], we cannot state this with confidence. Cortisol levels do not depend on movement, provided that the movement is not caused by a flight reaction [41]. Cortisol levels are therefore possibly a more reliable measure of stress reduction during an active form of therapy such as DAT.
To our knowledge, HRV has not previously been measured in similar AAI contexts. Although cortisol was also not measured during DAT, it has been measured in pet owners [43], as well as in a study in which children with insecure attachment styles responded to a stressful situation in presence of a dog [
16]. The results of those studies are in line with this study, i.e., less arousal in the presence of a dog. Yet, a study on salivary cortisol levels taken from students during their final exams in presence of a dog showed no detectable differences, even though students’ own perceptions of their stress levels decreased [42]. This discrepancy between studies shows that we should be cautious when interpreting cortisol levels in the context of Animal-Assisted Interventions. Based on the current study and previous studies [
16,42,43], we can cautiously conclude that cortisol levels decrease on the short term (during a session) when interacting with animals.
Contrary to our expectations, there were no significant HRV and cortisol changes over the full course of a 6-week DAT program. This means that this DAT program did not succeed to bring children to lower stress levels on the longer term (6 weeks). Note however, that a DAT program of a longer duration, and/or of a higher intensity (several times a week) could possibly lead to different results. This would correspond to the finding that dog owners (who have frequent interactions with their dogs) appear to have lower cortisol levels [43].
There are some limitations of this study. First, we decided to include no control group for ethical reasons (i.e., we worked with a vulnerable group of children and there was no precedent). However, this limits the generalizability of our results. Second, because we worked with a specific group of children, we also needed profesionally trained therapists to guide these children. This resulted in a relatively small sample size. We therefore recommend to conduct this study in a larger context, possibly combined with a DAT program of a longer duration (see above), as there are some indications from previous studies that prolonged exposure to dogs could influence cortisol levels [43]. Lastly, even though we used a HRV chest strap with a special size for children, the artifacts in our recordings could indicate that the fit was not optimal. As mentioned in the method section, we tried to solve this by measuring HRV while children sat in a chair before and at the end of each session. Future studies could overcome this by using wrist devices that register HRV, or other equipment that registers respiratory frequencies and blood pressure in addition to HRV. Despite these limitations, we believe that this study sets an important precedent for the use of physiological measures in research on Animal Assisted Interventions.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- Allen, Karen, Barbara E. Shykoff, and Joseph L. Izzo. 2001. “Pet Ownership, but Not ACE Inhibitor Therapy, Blunts Home Blood Pressure Responses to Mental Stress.” Hypertension 38 (4).
- Almela, Mercedes, Vanesa Hidalgo, Carolina Villada, Leander van der Meij, Laura Espín, Jesús Gómez-Amor, and Alicia Salvador. 2011. “Salivary Alpha-Amylase Response to Acute Psychosocial Stress: The Impact of Age.” Biological Psychology 87 (3): 421–29. https://doi.org/10.1016/J.BIOPSYCHO.2011.05.008. [CrossRef]
- Barker, Sandra B., Randolph T. Barker, Nancy L. McCain, and Christine M. Schubert. 2016. “A Randomized Cross-over Exploratory Study of the Effect of Visiting Therapy Dogs on College Student Stress before Final Exams.” Anthrozoos 29 (1). https://doi.org/10.1080/08927936.2015.1069988. [CrossRef]
- Barker, Sandra B., Janet S. Knisely, Christine M. Schubert, Jeffrey D. Green, and Suzanne Ameringer. 2015. “The Effect of an Animal-Assisted Intervention on Anxiety and Pain in Hospitalized Children.” Anthrozoös 28 (1): 101–12. https://doi.org/10.2752/089279315X14129350722091. [CrossRef]
- Beetz, A. 2017. “Theories and Possible Processes of Action in Animal Assisted Interventions.” Applied Developmental Science. https://doi.org/10.1080/10888691.2016.1262263. [CrossRef]
- Beetz, Andrea, Henri Julius, Dennis Turner, and Kurt Kotrschal. 2012. “Effects of Social Support by a Dog on Stress Modulation in Male Children with Insecure Attachment.” Frontiers in Psychology 3: 352. https://doi.org/10.3389/fpsyg.2012.00352. [CrossRef]
- Berry, Alessandra, Marta Borgi, Nadia Francia, Enrico Alleva, and Francesca Cirulli. 2013. “Use of Assistance and Therapy Dogs for Children with Autism Spectrum Disorders: A Critical Review of the Current Evidence.” The Journal of Alternative and Complementary Medicine 19 (2): 73–80. https://doi.org/10.1089/acm.2011.0835. [CrossRef]
- Boxtel, Geert J. M. van, Pierre J. M. Cluitmans, Roy J. E. M. Raymann, Martin Ouwerkerk, Ad J. M. Denissen, Marian K. J. Dekker, and Margriet M. Sitskoorn. 2018. “Heart Rate Variability, Sleep, and the Early Detection of Post-Traumatic Stress Disorder.” In Sleep and Combat-Related Post Traumatic Stress Disorder, 253–63. New York, NY: Springer New York. https://doi.org/10.1007/978-1-4939-7148-0_22. [CrossRef]
- Chamchad, D., J.C. Horrow, L. Nakhamchik, and V.A. Arkoosh. 2007. “Heart Rate Variability Changes during Pregnancy: An Observational Study.” International Journal of Obstetric Anesthesia 16 (2): 106–9. https://doi.org/10.1016/J.IJOA.2006.08.008. [CrossRef]
- Chur-Hansen, Anna, Michelle McArthur, Helen Winefield, Emma Hanieh, and Susan Hazel. 2014. “Animal-Assisted Interventions in Children’s Hospitals: A Critical Review of the Literature.” Anthrozoös 27 (1): 5–18. https://doi.org/10.2752/175303714X13837396326251. [CrossRef]
- Evans, Subhadra, Laura C Seidman, Jennie Ci Tsao, Kirsten C Lung, Lonnie K Zeltzer, and Bruce D Naliboff. 2013. “Heart Rate Variability as a Biomarker for Autonomic Nervous System Response Differences between Children with Chronic Pain and Healthy Control Children.” Journal of Pain Research 6: 449–57. https://doi.org/10.2147/JPR.S43849. [CrossRef]
- Finck, K.S. 1993. “Finck - 1993 - Children, Their Pet Dogs, and Affect Attument.Pdf.”.
- Germone, Monique M, Robin L Gabriels, Noémie A Guérin, Zhaoxing Pan, Tiffany Banks, and Marguerite E O’Haire. 2019. “Animal-Assisted Activity Improves Social Behaviors in Psychiatrically Hospitalized Youth with Autism.” Autism 23 (7): 1740–51. https://doi.org/10.1177/1362361319827411. [CrossRef]
- Hall, Sophie S., Hannah F. Wright, Annette Hames, and Daniel S. Mills. 2016. “The Long-Term Benefits of Dog Ownership in Families with Children with Autism.” Journal of Veterinary Behavior 13. https://doi.org/10.1016/j.jveb.2016.04.003. [CrossRef]
- Handlin, Linda, Eva Hydbring-Sandberg, Anne Nilsson, Mikael Ejdebäck, Anna Jansson, and Kerstin Uvnäs-Moberg. 2011. “Short-Term Interaction between Dogs and Their Owners: Effects on Oxytocin, Cortisol, Insulin and Heart Rate—An Exploratory Study.” Anthrozoos: A Multidisciplinary Journal of The Interactions of People & Animals 24 (3): 301–15. https://doi.org/10.2752/175303711X13045914865385. [CrossRef]
- Hanrahan, Kirsten, Ann Marie McCarthy, Charmaine Kleiber, Susan Lutgendorf, and Eva Tsalikian. 2006. “Strategies for Salivary Cortisol Collection and Analysis in Research with Children.” Applied Nursing Research 19 (2): 95–101. https://doi.org/10.1016/j.apnr.2006.02.001. [CrossRef]
- Hart, Lynette A. 2006. “Psychosocial Benefits of Animal Companionship.” In Handbook on Animal-Assisted Therapy, 59–78. Elsevier. https://doi.org/10.1016/B978-012369484-3/50006-2. [CrossRef]
- Jansen, Lucres M.C., Christine C. Gispen-De Wied, Victor M Wiegant, Herman G.M. Westenberg, Bertine E Lahuis, and Herman Van Engeland. 2006. “Autonomic and Neuroendocrine Responses to a Psychosocial Stressor in Adults with Autistic Spectrum Disorder.” Journal of Autism and Developmental Disorders 36 (7): 891–99. https://doi.org/10.1007/s10803-006-0124-z. [CrossRef]
- Jansen, Lucres M C, Christine C Gispen-de Wied, Rutger-Jan van der Gaag, and Herman van Engeland. 2003. “Differentiation between Autism and Multiple Complex Developmental Disorder in Response to Psychosocial Stress.” Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology 28 (3): 582–90. https://doi.org/10.1038/sj.npp.1300046. [CrossRef]
- Janssen, C. G. C., C. Schuengel, and J. Stolk. 2002. “Understanding Challenging Behaviour in People with Severe and Profound Intellectual Disability: A Stress-Attachment Model.” Journal of Intellectual Disability Research 46 (6): 445–53. https://doi.org/10.1046/j.1365-2788.2002.00430.x. [CrossRef]
- Kertes, Darlene A, Jingwen Liu, Nathan J Hall, Natalie A Hadad, Clive D L Wynne, and Samarth S Bhatt. n.d. “Effect of Pet Dogs on Children’s Perceived Stress and Cortisol Stress Response.” Accessed June 11, 2018. https://doi.org/10.1111/sode.12203. [CrossRef]
- Marques, Andrea H., Marni N. Silverman, and Esther M. Sternberg. 2010. “Evaluation of Stress Systems by Applying Noninvasive Methodologies: Measurements of Neuroimmune Biomarkers in the Sweat, Heart Rate Variability and Salivary Cortisol.” Neuroimmunomodulation 17 (3): 205–8. https://doi.org/10.1159/000258725. [CrossRef]
- Martin, François, and Jennifer Farnum. 2002. “Animal-Assisted Therapy for Children with Pervasive Developmental Disorders.” Western Journal of Nursing Research 24 (6): 657–70. https://doi.org/10.1177/019394502320555403. [CrossRef]
- May, Dana K, Nicholas P Seivert, Annmarie Cano, Rita J Casey, Amy Johnson, and Dana May. 2016. “Animal-Assisted Therapy for Youth: A Systematic Methodological Critiqu.” Human-Animal Interaction Bulletin 4 (1): 1–18. https://wwwp.oakland.edu/Assets/Oakland/nursing/files-and-documents/AAT/HAIB Article - MAY-SIEVERT-JOHNSON.pdf.
- MOTOMURA, Naoyasu, Takayoshi YAGI, and Hitomi OHYAMA. 2004. “Animal Assisted Therapy for People with Dementia.” Psychogeriatrics 4 (2): 40–42. https://doi.org/10.1111/j.1479-8301.2004.00062.x. [CrossRef]
- O’Haire, Marguerite E. 2017. “Research on Animal-Assisted Intervention and Autism Spectrum Disorder, 2012–2015.” Applied Developmental Science 21 (3): 200–216. https://doi.org/10.1080/10888691.2016.1243988. [CrossRef]
- O’Haire, Marguerite E, Samantha J McKenzie, Alan M Beck, and Virginia Slaughter. 2015. “Animals May Act as Social Buffers: Skin Conductance Arousal in Children with Autism Spectrum Disorder in a Social Context.” Developmental Psychobiology 57 (5): 584–95. https://doi.org/10.1002/dev.21310. [CrossRef]
- Pinheiro, J., and D. Bates. 2019. “Linear and Nonlinear Mixed Effects Models.” https://bugs.r-project.org.
- Rai, Balwant, Jasdeep Kaur, and Bernard H. Foing. 2012. “Salivary Amylase and Stress during Stressful Environment: Three Mars Analog Mission Crews Study.” Neuroscience Letters 518 (1): 23–26. https://doi.org/10.1016/J.NEULET.2012.04.034. [CrossRef]
- Ramirez, Sylvia Z., and Thomas R. Kratochwill. 1997. “Self-Reported Fears in Children With and Without Mental Retardation.” Mental Retardation 35 (2): 83–92. https://doi.org/10.1352/0047-6765(1997)035<0083:SFICWA>2.0.CO;2. [CrossRef]
- Redefer, Laurel A., and Joan F. Goodman. 1989. “Brief Report: Pet-Facilitated Therapy with Autistic Children.” Journal of Autism and Developmental Disorders 19 (3): 461–67. https://doi.org/10.1007/BF02212943. [CrossRef]
- Rieger, Annika, Regina Stoll, Steffi Kreuzfeld, Kristin Behrens, and Matthias Weippert. n.d. “Heart Rate and Heart Rate Variability as Indirect Markers of Surgeons’ Intraoperative Stress.” Accessed June 11, 2018. https://doi.org/10.1007/s00420-013-0847-z. [CrossRef]
- Silva, Karine, Rita Correia, Mariely Lima, Ana Magalhães, and Liliana de Sousa. 2011. “Can Dogs Prime Autistic Children for Therapy? Evidence from a Single Case Study.” The Journal of Alternative and Complementary Medicine 17 (7): 655–59. https://doi.org/10.1089/acm.2010.0436. [CrossRef]
- Tabares, C., F. Vicente, S. Sánchez, A. Aparicio, S. Alejo, and J. Cubero. 2012. “Quantification of Hormonal Changes by Effects of Hippotherapy in the Autistic Population.” Neurochemical Journal 6 (4): 311–16. https://doi.org/10.1134/S1819712412040125. [CrossRef]
- Takai, Noriyasu, Masaki Yamaguchi, Toshiaki Aragaki, Kenji Eto, Kenji Uchihashi, and Yasuo Nishikawa. 2004. “Effect of Psychological Stress on the Salivary Cortisol and Amylase Levels in Healthy Young Adults.” Archives of Oral Biology 49 (12): 963–68. https://doi.org/10.1016/j.archoralbio.2004.06.007. [CrossRef]
- Thayer, Julian F., Fredrik Åhs, Mats Fredrikson, John J. Sollers, and Tor D. Wager. 2012. “A Meta-Analysis of Heart Rate Variability and Neuroimaging Studies: Implications for Heart Rate Variability as a Marker of Stress and Health.” Neuroscience & Biobehavioral Reviews 36 (2): 747–56. https://doi.org/10.1016/J.NEUBIOREV.2011.11.009. [CrossRef]
- Viau, Robert, Geneviève Arsenault-Lapierre, Stéphanie Fecteau, Noël Champagne, Claire-Dominique Walker, and Sonia Lupien. 2010. “Effect of Service Dogs on Salivary Cortisol Secretion in Autistic Children.” Psychoneuroendocrinology 35 (8): 1187–93. https://doi.org/10.1016/j.psyneuen.2010.02.004. [CrossRef]
- Wright, Hannah, Sophie Hall, Annette Hames, Jessica Hardiman, Richard Mills, and Daniel Mills. 2015. “Pet Dogs Improve Family Functioning and Reduce Anxiety in Children with Autism Spectrum Disorder.” Anthrozoos 28 (4). https://doi.org/10.1080/08927936.2015.1070003. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).