1. Introduction
First-trimester metrorrhagia is a very frequent reason for consultation, affecting up to 20-30% of pregnancies. Due to its high prevalence among the population, we have studied visits to the emergency room over a year and we have followed up these pregnancies to assess whether a relation between first trimester vaginal bleeding and the development of further complications throughout the pregnancy could be established. First trimester metrorrhagia refers to bleeding that occurs before 20 weeks of gestation [
1,
2]. Any potential pregnancy-related complication can be extremely distressing for both the patient and her family. For the gynecologist, it is important to be able to identify symptoms related to certain complications that may arise during the first weeks of pregnancy, as well as their potential short and long-term complications, in order to reassure, advise and support the couple at such a difficult time. [
3,
4].
Despite its high prevalence, first trimester bleeding should not be considered physiological, and it is advisable to carry out an adequate differential diagnosis to rule out any possible obstetric, gynecological or systemic underlying pathology.
The aim of the study was to review the cases of bleeding that occurs in the first weeks of gestation and its implications throughout pregnancy. Secondarily, we studied the associated complications and tried to identify possible risk factors that could be used to select those women at higher risk of adverse outcomes that could benefit from early diagnosis and improved follow-up.
2. Materials and Methods
For this study, we selected women who consulted in the Emergency Department of the Hospital QuirónSalud in Málaga throughout the year 2015 for first trimester bleeding. We refer to metrorrhagia in the first trimester as that which occurs during weeks one through 12. Once the pregnant women were identified, we studied risk factors already present before the pregnancy, and others intrinsic to the pregnancy and its natural course.
Among the characteristics of our pregnant women, we collected maternal age in years in the first visit to the emergency room.
Once the gestation reaches weeks 7+6 and 13+6 of amenorrhea, the first trimester analysis is performed, preferably between the 8th and 10th week. Within this analysis, the value of PAPP-A and β-hCG are used as biochemical markers to perform the combined screening of aneuploidies. The values obtained are expressed in multiples of the median (MoM).
Pregnancy-associated plasma protein A (PAPP-A), is secreted by the syncytiotrophoblast and its concentration increases during pregnancy. There are studies that have shown the relationship between low values of PAPP-A and β-hCG detected in the early stages of pregnancy and several complications of pregnancy.
In the second trimester, the O'Sullivan test is performed, consisting of an oral overload with 50g of glucose, and subsequent measurement of blood glucose levels in venous plasma 60 minutes after ingestion.
This determination is performed to every pregnant woman in the second trimester regardless of whether or not they have any risk factors. However, it will only be performed during the first trimester to those women with risk factors, such as maternal age over 35, obesity (BMI>30), a history of gestational diabetes or carbohydrate intolerance, first-degree relatives with diabetes, and history of fetal macrosomia. It is considered pathological when values are ≥140 mg/dl or ≥7.8 mmol/L. In these cases, a further diagnostic confirmation test is carried out using an oral glucose overload with 100gr of glucose.
In our study we have only used the value in mg/dl obtained on the analysis of the second trimester. Even if performed in some cases, we did not take into a count results obtained from the analysis of the first trimester.
Gestational diabetes (GD) is defined as the form of diabetes that is first detected during pregnancy regardless of the need for insulin treatment, the degree of metabolic disorder involved or its persistence after the end of pregnancy. For its diagnosis, the O'Sullivan test is used (pathological when ≥140 mg/dl) and a further confirmation test with an oral glucose overload of 100g. The oral glucose tolerance test (OGTT) reference values are ≥105 - ≥190 - ≥165 - ≥145 (fasting, 1-h, 2-h and 3-h post glucose intake, respectively). For diagnostic confirmation, two of four abnormal values are required. We included in our study patients diagnosed of gestational diabetes during pregnancy using these criteria.
During pregnancy, hypertensive disorder is diagnosed by elevated blood pressure (BP) (systolic ≥ 140 and/or diastolic ≥ 90 mm Hg), in two or more measurements separated by 6 hours. We define proteinuria as the presence of ≥300 mg of protein in a 24-hour urine sample. Preeclampsia is defined as hypertension that appears after 20 weeks of gestation accompanied by proteinuria.
3. Results
3.1. Population
The total number of visits to the emergency room was 9451, 1161 of which were consultations about first-trimester bleeding. Out final sample of study consisted of 696 patients. The average age of the patients studied was 34.1, the median was 34, and it ranged from 18 to 50 years of age.
3.2. Preeclampsia
The calculation of the combined screening of the first trimester is based on several parameters: weeks of gestation according to the first day of the last menstrual period (LMP), crown-rump length (CRL), maternal age, nuchal translucency measured by ultrasound, and biochemical parameters such as PAPP-A and β-hCG expressed in multiples of the median (MoM). In our study we have recorded the value of PAPP-A in MoM.
The mean value of the PAPP-A was 1.0717, with a standard deviation (SD) of ± 0.71. The lowest value was 0.21 and the highest was 5.89.
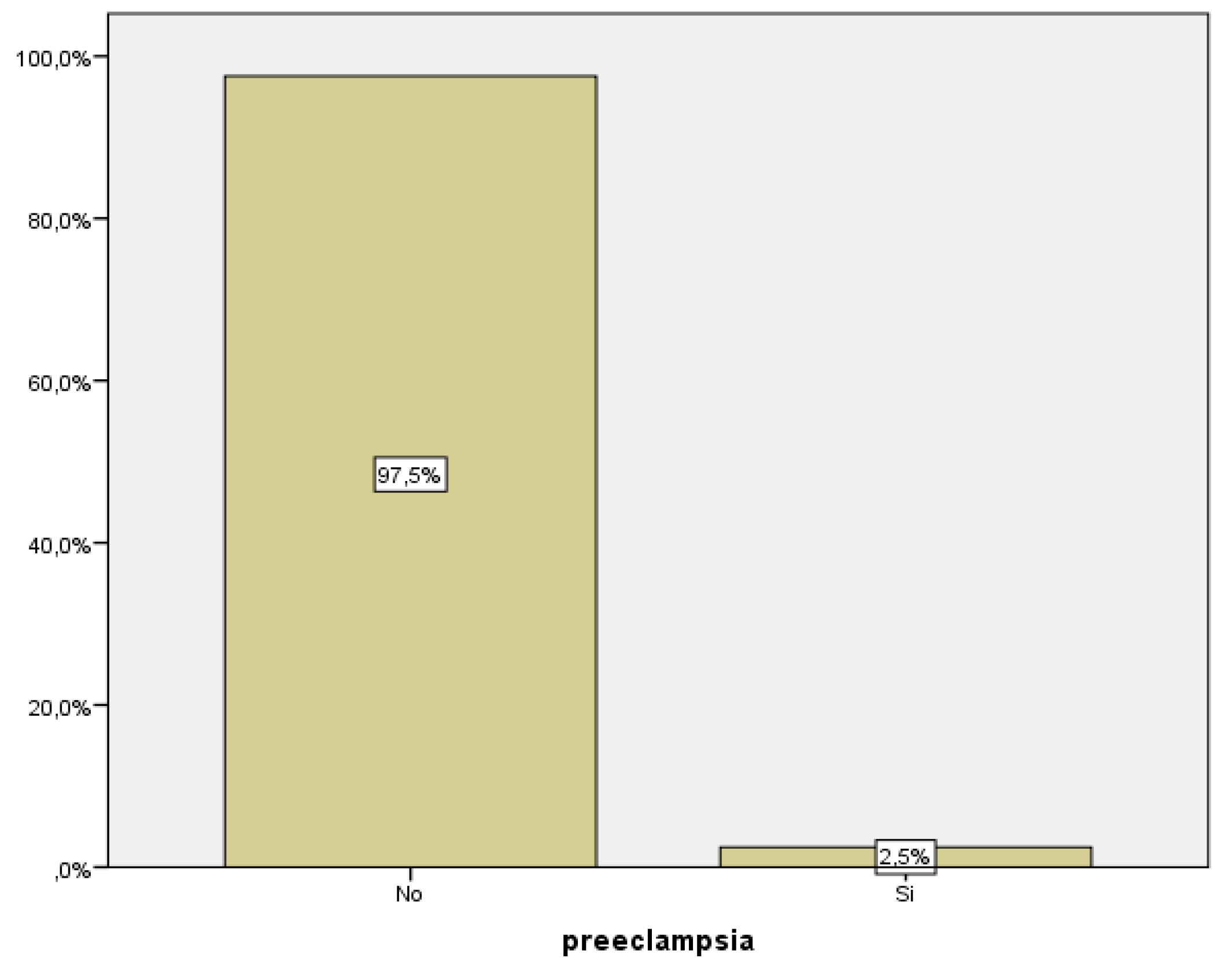
As previously stated, we define preeclampsia as hypertension that appears after 20 weeks of gestation accompanied by proteinuria. In our group of study, we recorded data from 243 pregnant women, 6 of whom were diagnosed with preeclampsia, corresponding to 2.5% of them (
Figure 1).
We were able to establish a statistically significant relationship between blood levels of PAPP-A and the development of preeclampsia (p<0.005). 75% of women with preeclampsia presented with low PAPP-A levels during the first trimester analysis (
Table 1).
Among all the women with low PAPP-A levels, only 11.1% had developed preeclampsia.
In summary, low PAPP-A levels are not associated with an increased risk of preeclampsia, but preeclampsia associates low blood PAPP-A levels.
3.3. Prematurity
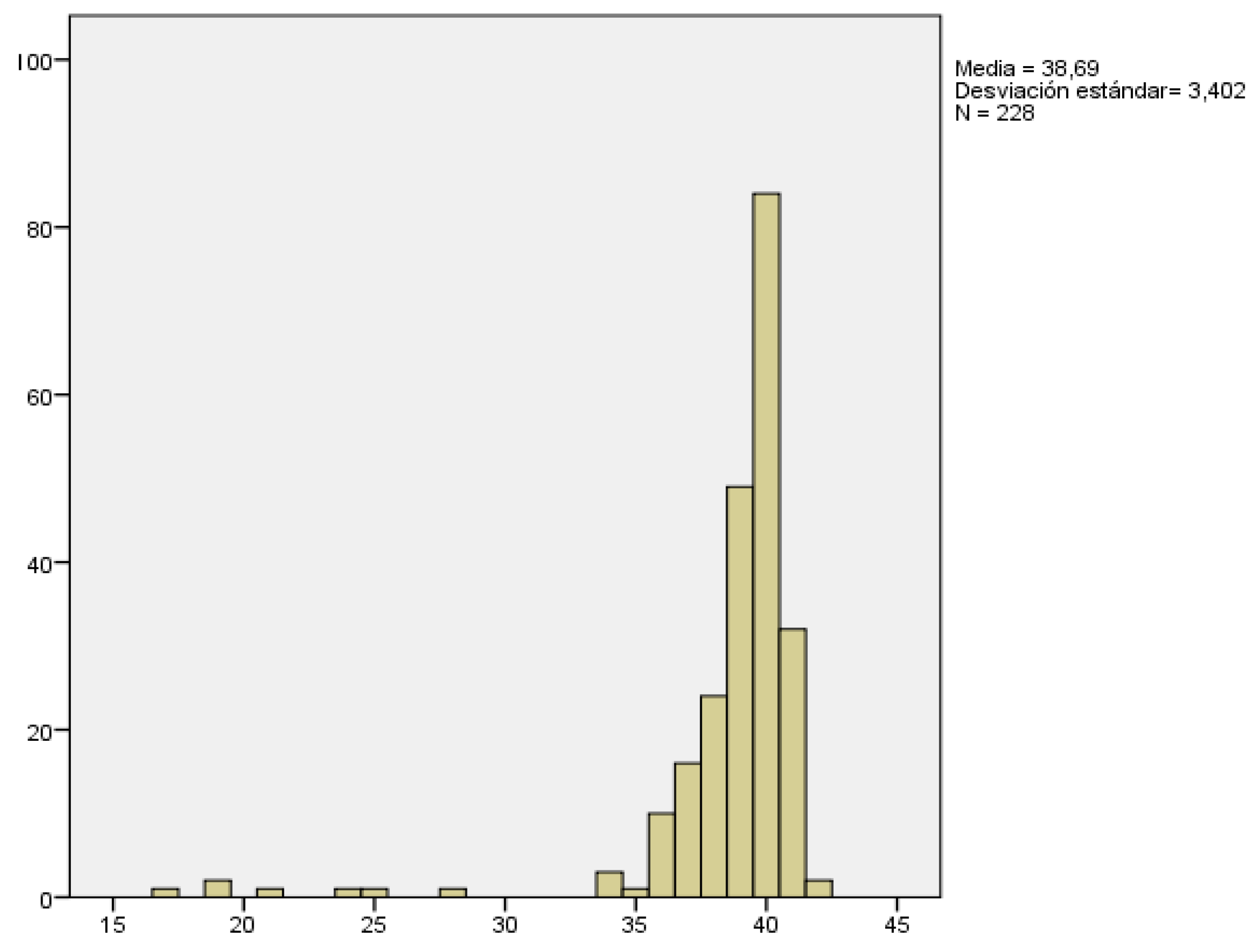
The gestational age of delivery was recorded in all pregnancies. We considered all pregnancies over 12+6 weeks, so this analysis may also include those that resulted in late abortions.
Gestational age at delivery ranged from 17 weeks, which corresponded to a late abortion, to 42 weeks, being 38.69 weeks with a SD of ± 3.4 weeks the mean gestational age at delivery(
Figure 2).
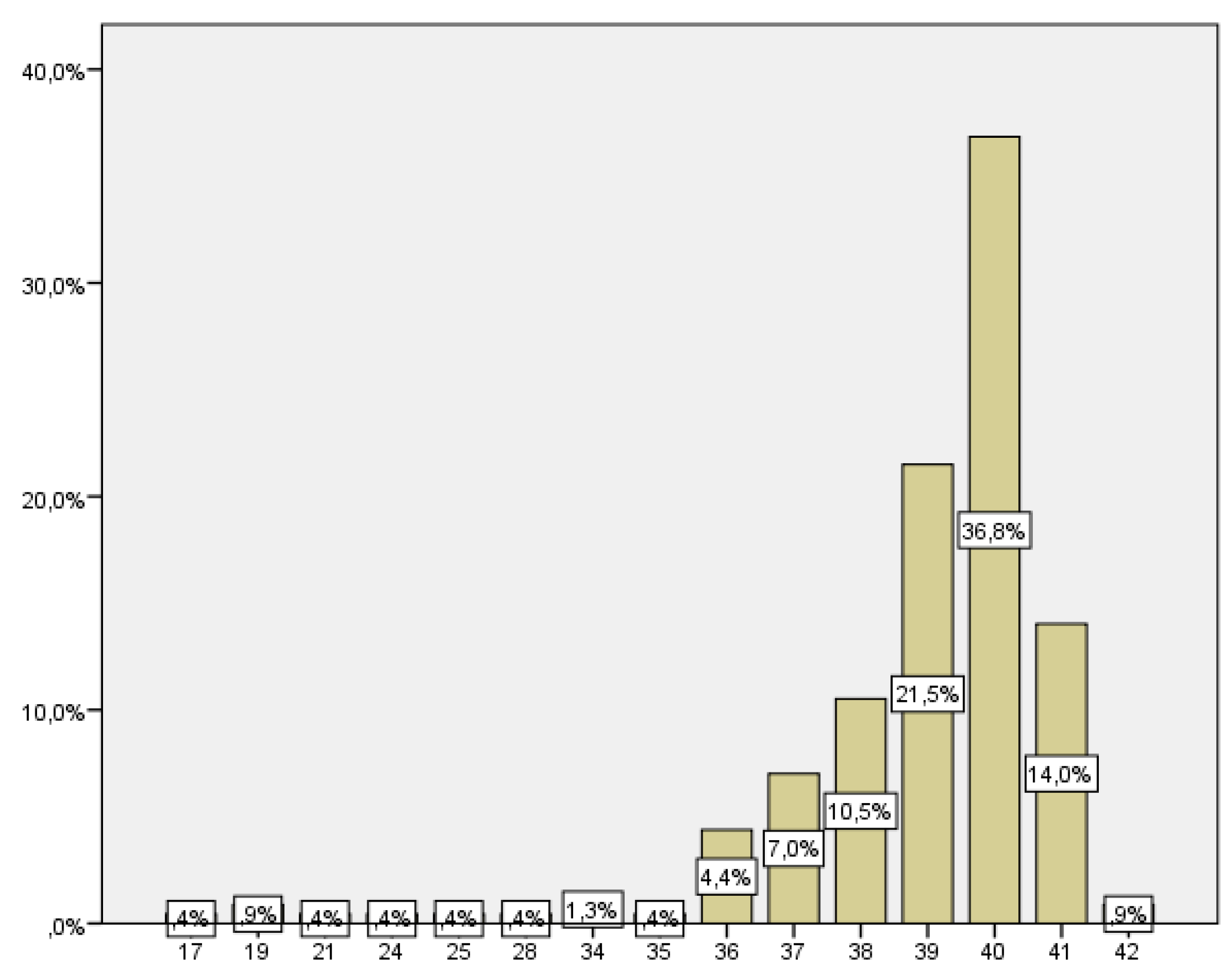
The number of women who delivered before week 37 was 21, which corresponds to 9.2% of the total (
Figure 3).
3.4. Gestational Diabetes
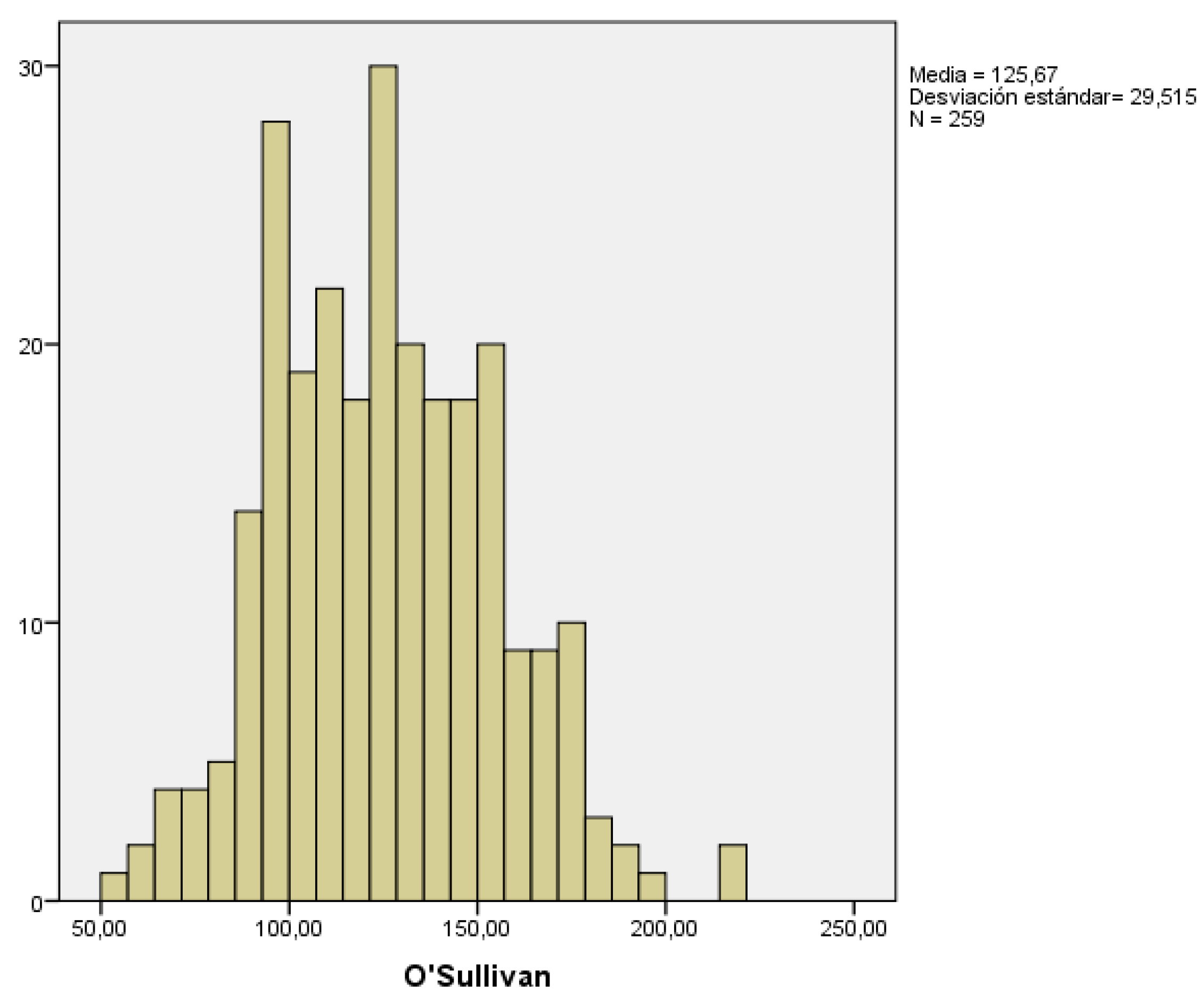
To screen for gestational diabetes, an oral glucose tolerance test using 50g of glucose is performed in the second trimester, known as the O'Sullivan test. In those women with risk factors or history of gestational diabetes in previous pregnancies, this determination is done during the first trimester. In our study, we collected the results obtained in the second trimester, and considered the result pathological when glucose levels were ≥140 mg/dl.
We collected data from 259 pregnant women. The mean glucose value obtained from the O’Sullivan test was 125.67 ± 29.51 mg/dl. The lowest and highest recorded values were 51 and 221 mg/dl, respectively (
Figure 4).
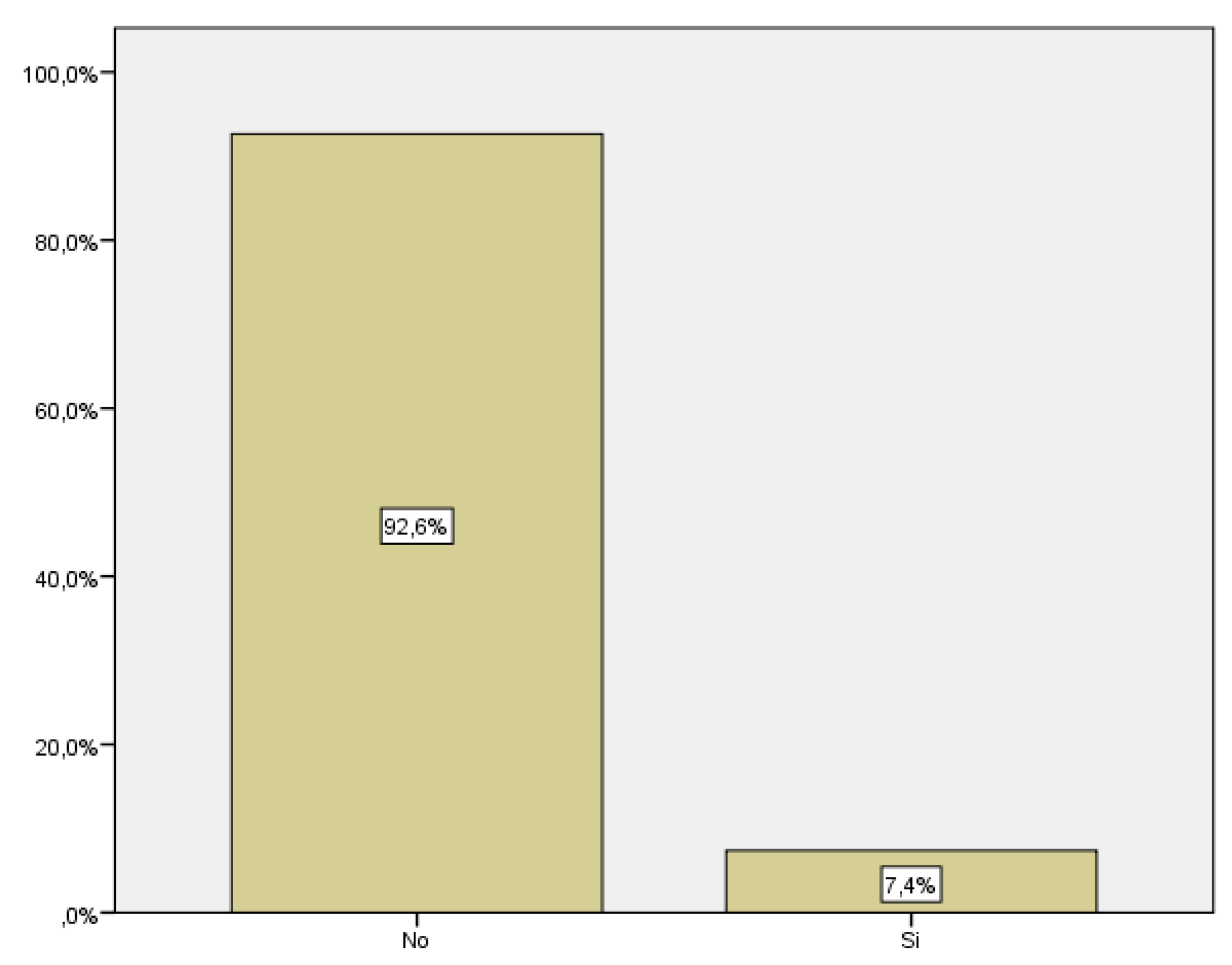
We only took into account cases of gestational diabetes diagnosed during pregnancy; cases of diabetes diagnosed before were not included in the study. Among the women included in the study, 18 developed gestational diabetes, which corresponds to 7.4% of the total (
Figure 5).
We have expanded the study by selecting the patients based on maternal age to assess whether maternal ages relates to certain obstetric complications.
3.5. Threatened preterm labor
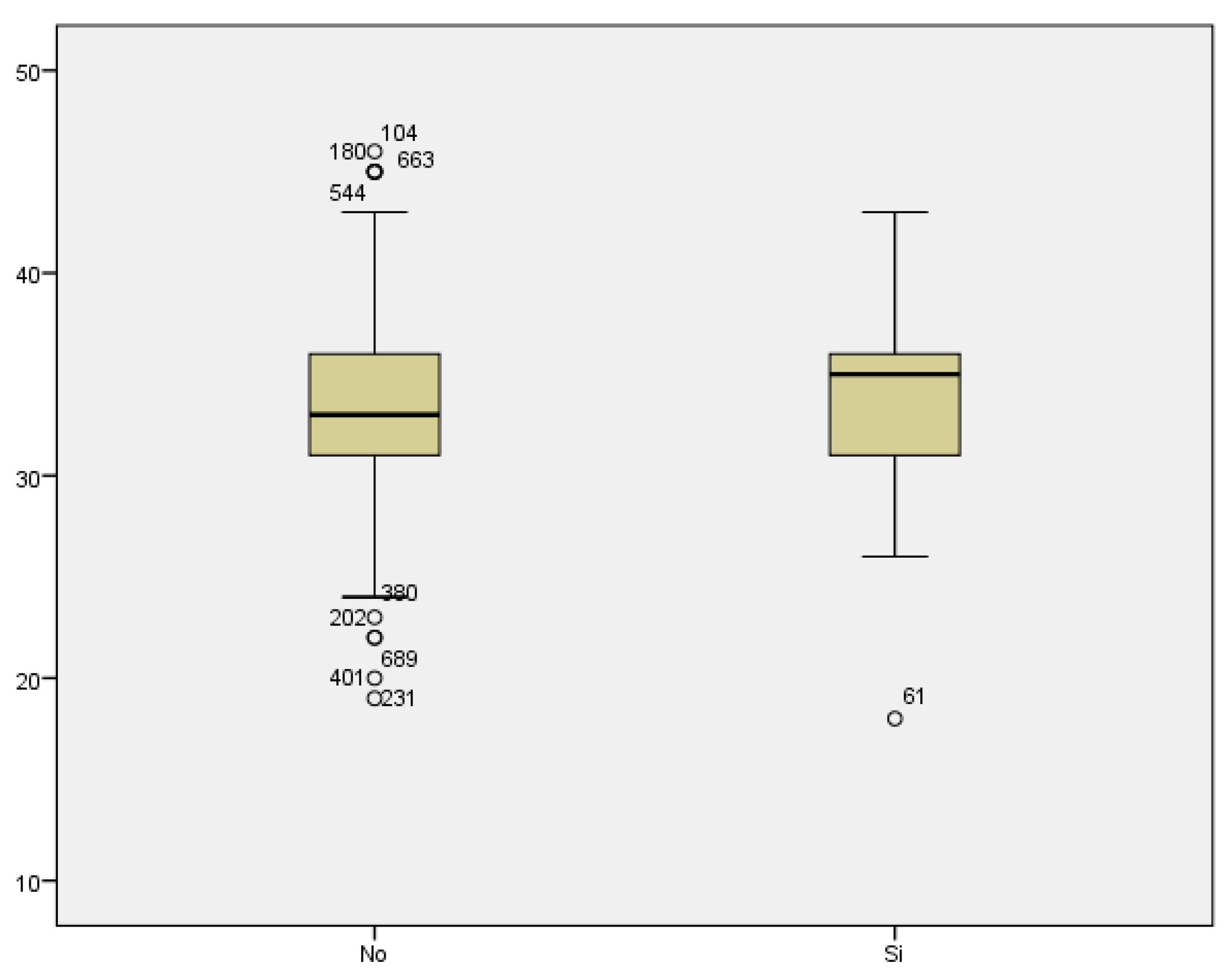
We reviewed the cases of threatened preterm labor. We did not find a statistically significant relationship between maternal age threatened preterm labor (33.40 ± 4.462 vs 33.41 ± 5.335 years) (
Figure 6).
3.6. Maternal age
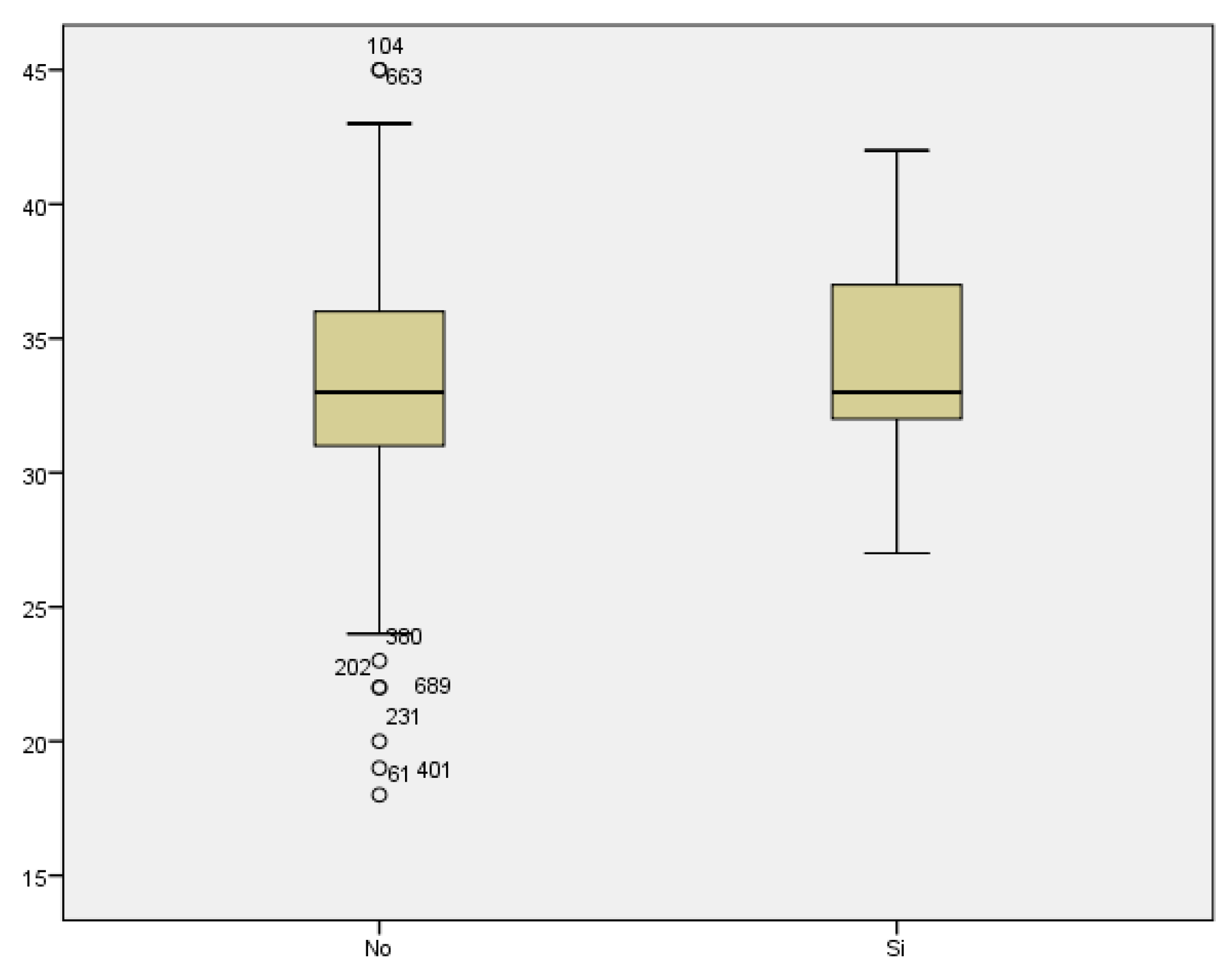
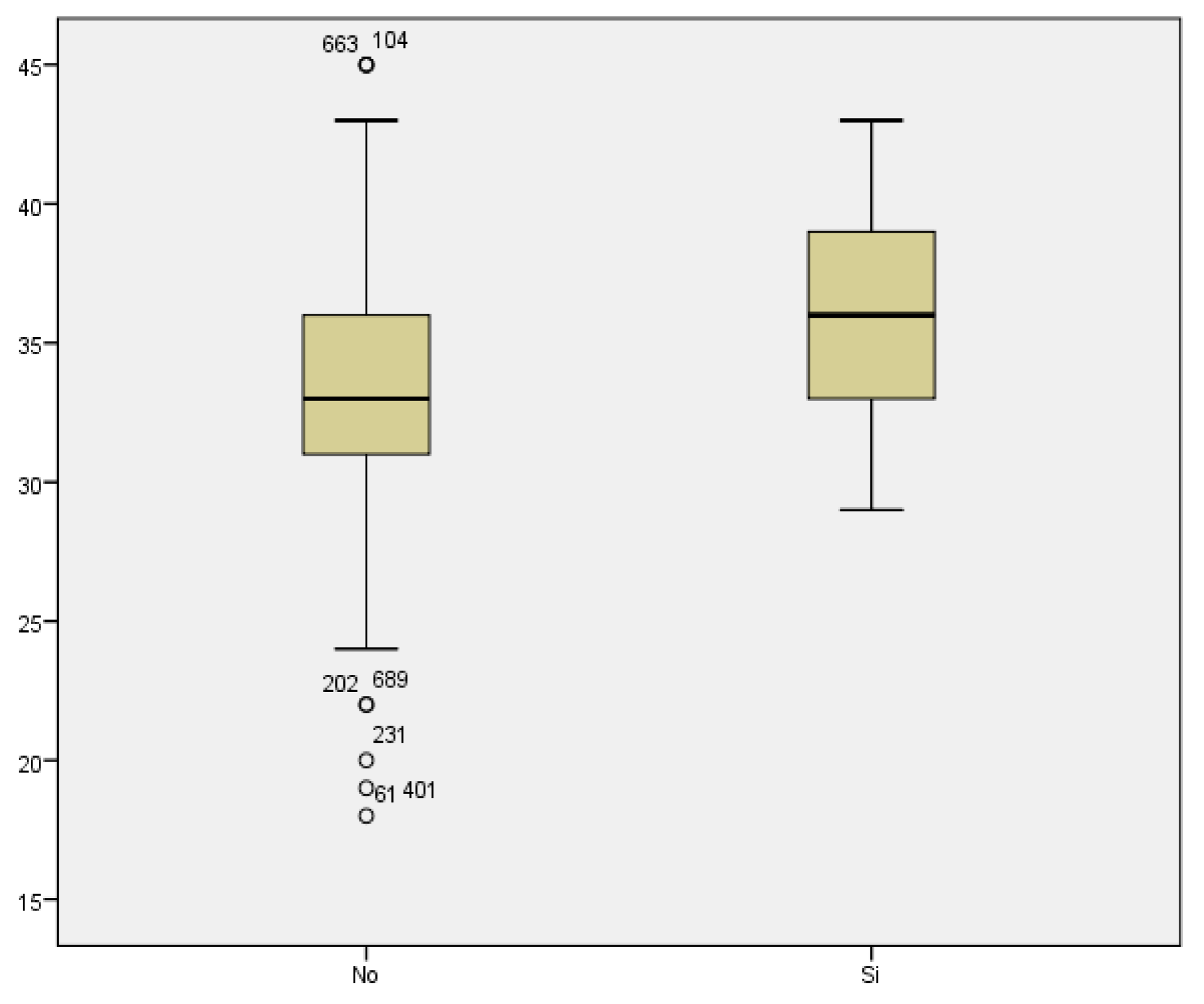
Regarding prematurity, we assessed maternal age in relation to having a preterm delivery. The mean maternal age of women who delivered before week 37 was 34.48 years, while it was 33.11 years for those who delivered at term. We found no statistically significant difference between both groups (p<0.187) (
Figure 7).
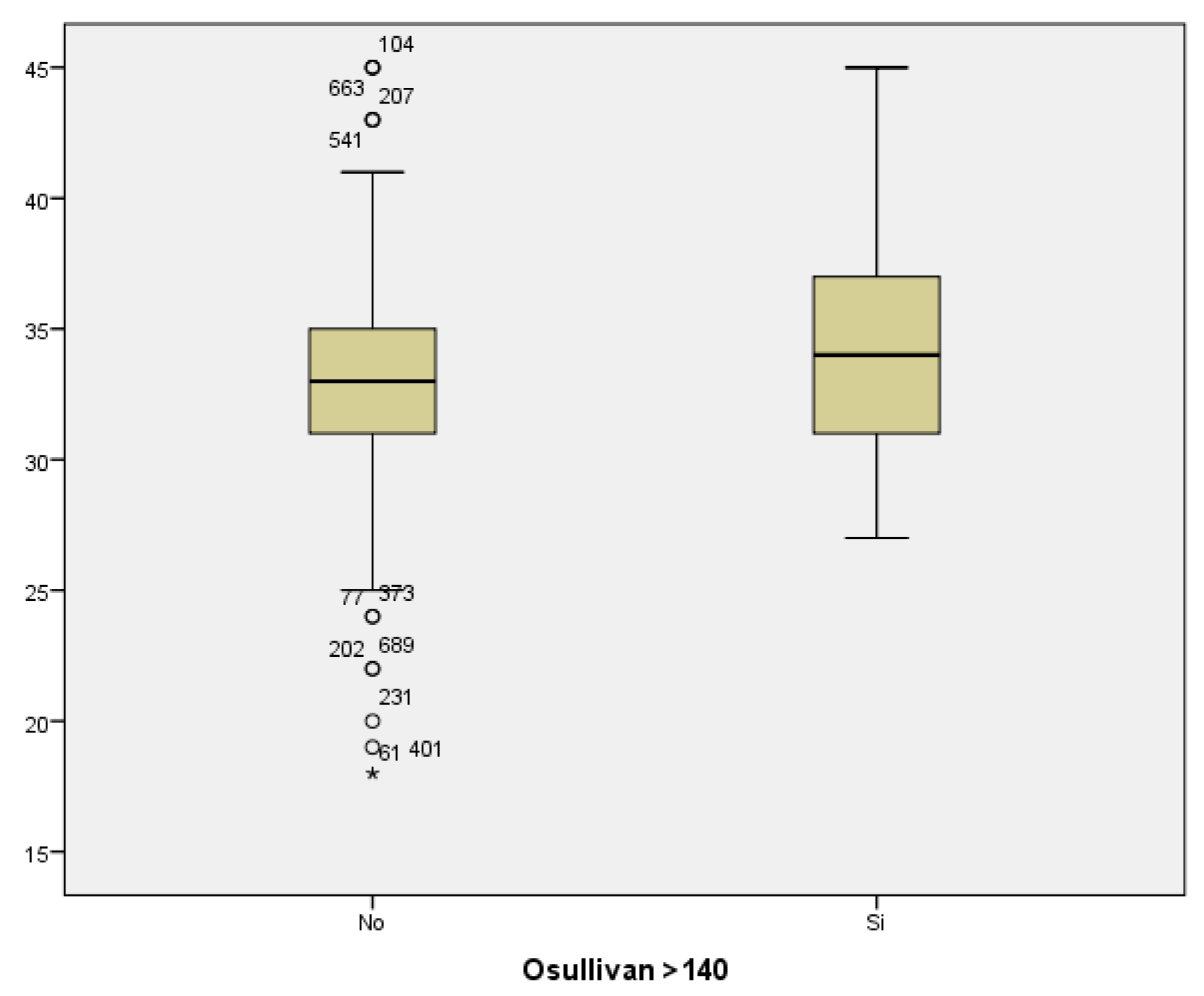
If we analyze the results of the O’Sullivan test (using >140 mg/dl as the cut-off value) in relation to maternal age, we conclude that the mean maternal age for those women with test results within the normal range was 33.04; whilst the mean maternal age among women with pathological test results was 34.12 years. Therefore, the result seems to be influenced by maternal age, with higher prevalence of pathological test results with increased maternal age. However, statistical significance was not found (p<0.069)
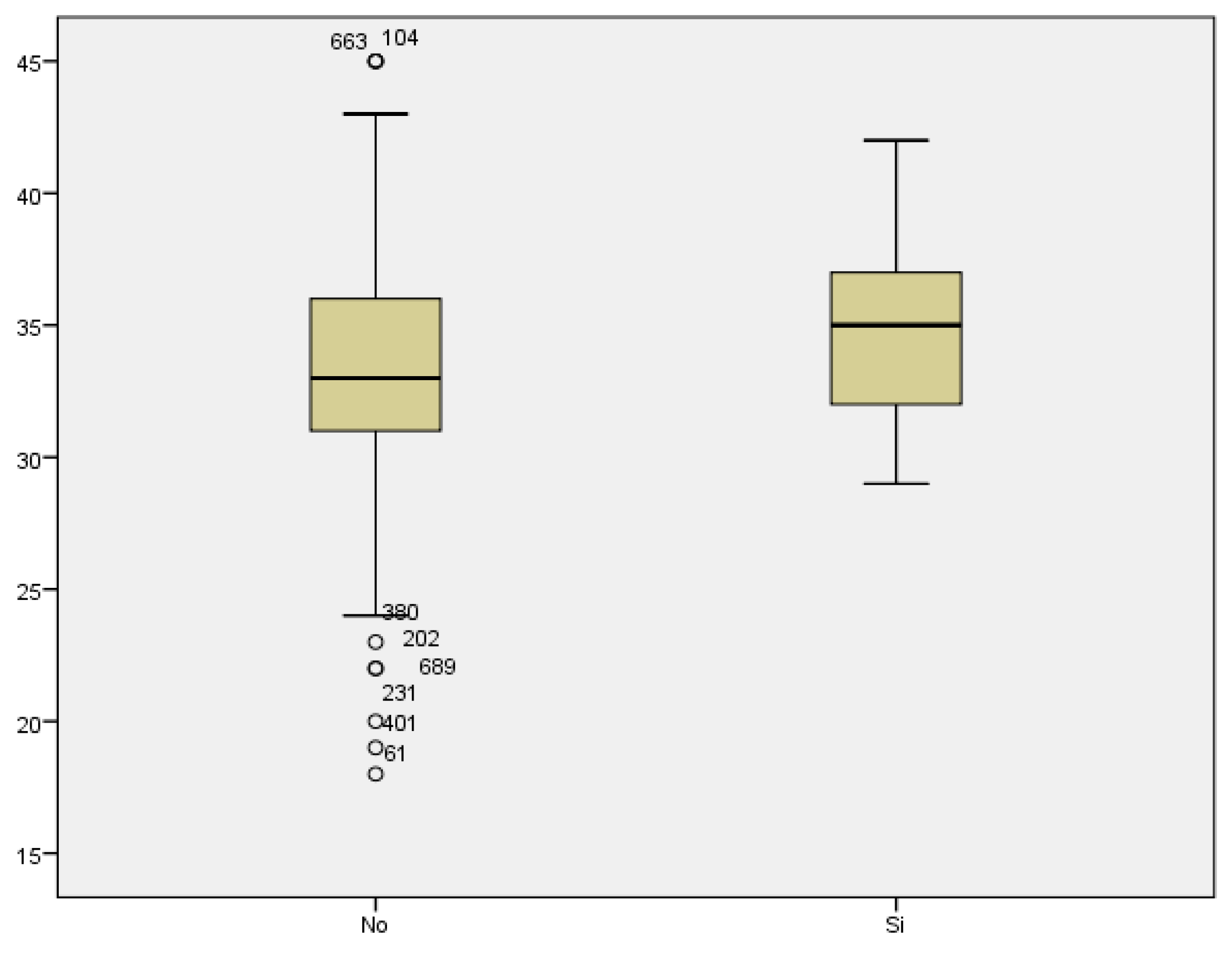
Comparing the mean maternal age in women who developed gestational diabetes with respect to those who did not, we found a statistically significant difference, with p<0.007. The mean maternal age of those who presented with GD was 36.06 years, compared to a mean age of 33.11 years of those who did not suffer from GD. Therefore, the prevalence of GD increased with maternal age (
Figure 9).
We did not find a statistically significant difference between the mean maternal age of women who developed preeclampsia, and women who did not (35 and 33.25 years of age, respectively) with a value of p<0.350 (
Figure 10).
4. Discussion
4.1. Preeclampsia
The rate of preeclampsia in our patients was 2.5%. A lower value than that estimated globally, approximately between 5 and 10%. According to the WHO, this rate is seven times higher in developing countries and in areas of greater prevalence of cardiovascular disease [
5,
6,
7].
In our study we did not find an increase in cases of preeclampsia. However, we observed that 75% of patients with preeclampsia presented low blood levels of PAPP-A in the laboratory tests performed on the first trimester.
We have found studies showing similar results, in which they also observed a greater risk of preeclampsia, preterm labor and low birth weight, in pregnant women with low levels of PAPP-A [
8,
9,
10].
As previously stated, low levels of PAPP-A do not relate to an increased risk of preeclampsia, but preeclampsia is related to having low blood PAPP-A levels.
In our sample, we have used a value of 0.6 MoM as a cuff-off for PAPPA-A, with values lower than 0.6 being considered low. Other authors have used values of 0.4 and 0.2 MoM as the cuff-off, observing more striking statistically significant differences in terms of adverse outcomes in these two groups. The most severe cases, associated with intrauterine fetal death or placental abruption, presented for the most part values of PAPPA-A below 0.2 MoM [
11].
For the above-mentioned reasons, the measurement of serum PAPP-A during the first trimester analysis may be useful to predict future adverse outcomes in pregnancy [
12].
4.2. Prematurity
Regarding pre-term delivery, understood as that which takes place before the end of week 37 of gestation, we found a rate of 9.2% preterm births among our patients.
According to data from the National Institute of Statistics, the prematurity rate represents 6.5-9% of all births, and may increase to and reach 12.5% in reference centers. We compared the prematurity rate obtained in our study with that published by other work groups and found similar results, with a rate of pre-term birth around 10.24% [
13,
14].
We have studied maternal age as a risk factor for prematurity; finding no statistically significant differences in the rate of prematurity with increasing maternal age.
We analyzed the data published by other authors on this matter and found disparate results. There are publications with similar results to ours, in which they did not find an increase in prematurity [
15]. However, other authors have documented an increase in prematurity among pregnant women of advanced aged. This increase could be influenced by the greater degree of associated maternal pathology, which in turn could result in a premature delivery [
16].
A recent study shows the same conclusions; it assessed the association between maternal age and prematurity and found no increase in maternal age among pregnancies that terminated spontaneously, while they demonstrated a rise in prematurity with increased maternal age among pregnancies that required a planned delivery due to associated medical complications. Therefore, there was a correlation between increased maternal age and prematurity but as a result of maternal medical conditions that required planned delivery.
4.3. Threatened preterm labor
Regarding threatened preterm labor, as we have previously stated, the data we obtained was in line with that previously published in other studies. Sorting the patients based on maternal age, we did not observe a statistically significant increase in the cases of threatened preterm labor with increasing maternal age. However, other studies have found greater risk of threatened preterm labor, which translated into an increase in hospitalizations rates and the need for specific tocolytic treatment [
15].
4.4. Gestational Diabetes
The rate of gestational diabetes in our sample (7.4%) is similar to that previously reported by other studies. The latest studies show rates ranging from 7 to 14%, with a significant increase in recent years [
17]. However, other studies suggest rates somewhat higher than ours, for instance, it is estimated that 15% of pregnancies are affected by DG in Australia [
18].
In line with the rest of our study, we classified women according to age, in order to assess whether there is an association between increases in maternal age and the development of GD. In this case, we found that there is also a correlation between maternal age and GD, with increasing maternal age being a risk factor for GD.
Likewise, many authors have previously studied the relationship between increased maternal age and poor perinatal outcomes. Montori et al [
19], found in their analysis a statistically significant increase in the prevalence of GD, hypertensive disorders, induction of labor and cesarean sections in women older than 35 years [
20].
As it has been well-established, advanced maternal age increases the risk of several gestation-related complications. Consequently, we should make emphasis on the need to perform GD screening tests and keep strict control of BP levels in all pregnant women, especially among the older ones.
5. Strengths and limitations
Our study had several strengths. First, the sample of our study was constituted by all the patients that visited the emergency department of our hospital throughout a year, and their reasons for consultation were analyzed. Week 12+6 was established as the time limit for vaginal bleeding to be considered as first-trimester metrorrhagia, which allows us to establish clear inclusion and exclusion criteria.
Second, we have an acceptable sample size with a total of 696 pregnant women. Third, we carried out of a comprehensive assessment of each patient, reviewing previous medical and obstetric-gynecological history (abortions, uterine surgeries), current gestational data (first trimester screening and analytical values, cervical length, history of obstetric complications) and data regarding the delivery and the newborn (weeks of gestation, mode of delivery, newborn weight, Apgar test value, birth weight, etc.).
However, this study also has some limitations. As a first limitation, this is a retrospective study, therefore the data we used was limited to the information included in the digitized clinical history. Secondly, our study is solely based on visits to the emergency department of Hospital QuironSalud Málaga, and therefore the follow-up of the gestation and the subsequent analysis of obstetric history was performed on this same center. In consequence, data regarding patients with prenatal care at different centers was missing. We have been able to retrieve some of the data lost by external monitoring from the collection of medical histories from the Andalusian Health System.
Another limitation was the absence of a control group with which to compare the results and assess the rate of complications among women who did not present with vaginal bleeding during the first trimester. Due to the high prevalence of first-trimester bleeding, and its potential implications for the pregnancy, it would be interesting to further continue the study and perform it prospectively, allowing for the establishment and control of potential risk factors that may lead to poor gestational outcomes.
6. Conclusions
In views of the results obtained in our study, which are in line with those previously observed by others, we can establish a positive correlation between advanced maternal age and the increased prevalence of various comorbidities and associated complications among pregnancies in which first-trimester bleeding was reported.
Author Contributions
Conceptualization, L.B.C, D.A.D. and E.G.M.; methodology, L.B.C, D.A.D and E.G.M.; software, L.B.C.; validation, D.A.D and E.G.M.; formal analysis, L.B.C.; investigation, L.B.C and D.A.D; data curation, L.B.C.; writing—original draft preparation, L.B.C; writing—review and editing, L.B.C, D.A.D, E.G.M and L.C.F; visualization, L.B.C, D.A.D. and E.G.M.; supervision, D.A.D and E.G.M.; translation, L.C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank all of the authors included in this original paper. This research contributes to the doctoral work of L.B.C. We would like to thank all the participants in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martonffy, A. I., Rindfleisch, K., Lozeau, A. M., & Potter, B. (2012). First trimester complications. Primary Care: Clinics in Office Practice, 39(1), 71-82. [CrossRef]
- Matamoros, C. M., & Rodríguez, O. M. (2011). Una actualización en aborto recurrente. Revista médica de Costa Rica y Centroamérica, 68(599), 423-433.
- Lamb, E. H. (2002). The impact of previous perinatal loss on subsequent pregnancy and parenting. The Journal of perinatal education, 11(2), 33-40. DOI: 10.1891/1058-1243.11.2.33.
- Griebel CP, Halvorsen J, Golemon TB, Day AA. (2005). Management of spontaneous abortion. American Family Physician, 72:1243–1250.
- Curiel, E., Prieto, M. A., & Mora, J. (2008). Factores relacionados con el desarrollo de preeclampsia. Revisión de la bibliografía. Clín. investig. ginecol. obstet. (Ed. impr.), 87-97.
- Maynard, S. E., & Karumanchi, S. A. (2008). Preeclampsia. In Molecular and Genetic Basis of Renal Disease (pp. 441-451). WB Saunders.
- Vargas, V. M., Acosta, G., & Moreno, M. A. (2012). La preeclampsia un problema de salud pública mundial. Revista chilena de obstetricia y ginecología, 77(6), 471-476. http://dx.doi.org/10.4067/S0717-75262012000600013.
- Boutin, A., Gasse, C., Demers, S., Blanchet, G., Giguère, Y., & Bujold, E. (2018). Does low PAPP-A predict adverse placenta-mediated outcomes in a low-risk nulliparous population? The great obstetrical syndromes (GOS) study. Journal of Obstetrics and Gynaecology Canada, 40(6), 663-668. [CrossRef]
- Turner, J. M., & Kumar, S. (2020). Low first trimester pregnancy-associated plasma protein-A levels are not associated with an increased risk of intrapartum fetal compromise or adverse neonatal outcomes: a retrospective cohort study. Journal of Clinical Medicine, 9(4), 1108. [CrossRef]
- Pakniat, H., Bahman, A., & Ansari, I. (2019). The relationship of pregnancy-associated plasma protein A and human chorionic gonadotropin with adverse pregnancy outcomes: A prospective study. The Journal of Obstetrics and Gynecology of India, 69, 412-419. [CrossRef]
- Livrinova, V., Petrov, I., Samardziski, I., Jovanovska, V., Simeonova-Krstevska, S., Todorovska, I., ... & Gjeorgjievska, M. (2018). Obstetric outcome in pregnant patients with low level of pregnancy-associated plasma protein A in first trimester. Open Access Macedonian Journal of Medical Sciences, 6(6), 1028. DOI: 10.3889/oamjms.2018.238.
- Jafari, R. M., Masihi, S., Barati, M., Maraghi, E., Sheibani, S., & Sheikhvatan, M. (2019). Value of pregnancy-associated plasma protein-A for predicting adverse pregnancy outcome. Archives of Iranian Medicine, 22(10), 584-587.
- Nogueira da Gama, S. G., Martinelli, K. G., Soares Dias, B. A., Pereira-Esteves, A. P., do Carmo Leal, M., & dos Santos-Neto, E. T. (2022). A population-based study of the relationship between advanced maternal age and premature/early-term birth in Brazil. International Journal of Gynecology & Obstetrics, 159(1), 173-181. [CrossRef]
- Sociedad Española de Ginecología y Obstetricia. Parto Pretérmino. (2020). Protocolos SEGO: http://www.prosego.com.
- Pérez, B. H., Tejedor, J. G., Cepeda, P. M., & Gómez, A. A. (2011). La edad materna como factor de riesgo obstétrico. Resultados perinatales en gestantes de edad avanzada. Progresos de Obstetricia y ginecología, 54(11), 575-580. [CrossRef]
- Luque Fernández, M. Á. (2008). Evolución del riesgo de mortalidad fetal tardía, prematuridad y bajo peso al nacer, asociado a la edad materna avanzada, en España (1996-2005). Gaceta Sanitaria, 22, 396-403. [CrossRef]
- Chen, P., Wang, S., Ji, J., Ge, A., Chen, C., Zhu, Y., ... & Wang, Y. (2015). Risk factors and management of gestational diabetes. Cell biochemistry and biophysics, 71, 689-694. [CrossRef]
- Ford, H. L., Champion, I., Wan, A., Reddy, M., Mol, B. W., & Rolnik, D. L. (2022). Predictors for insulin use in gestational diabetes mellitus. European Journal of Obstetrics & Gynecology and Reproductive Biology, 272, 177-181. [CrossRef]
- Montori, M. G., Martínez, A. Á., Álvarez, C. L., Cuchí, N. A., Alcalá, P. M., & Ruiz-Martínez, S. (2021). Advanced maternal age and adverse pregnancy outcomes: A cohort study. Taiwanese Journal of Obstetrics and Gynecology, 60(1), 119-124. [CrossRef]
- Attali, E., & Yogev, Y. (2021). The impact of advanced maternal age on pregnancy outcome. Best Practice & Research Clinical Obstetrics & Gynaecology, 70, 2-9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).