Submitted:
30 May 2023
Posted:
30 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Spodoptera frugiperda
2.2. Diet Source
| Host Plant | Family | Common Name | Diet form |
|---|---|---|---|
| Zea mays | Poaceae | Corn | Baby corn |
| Cucumis sativus | Cucurbitaceae | Cucumber | Leaf |
| Brassica oleracea var. italica | Brassicaceae | Broccoli | Bud |
| Brassica oleracea var. botrytis | Brassicaceae | Cauli flower | Bud |
| Brassica oleracea var. capitata | Brassicaceae | Cabbage | Bud |
| Brassica rapa subsp. chinensis var. parachinensis | Brassicaceae | Choy sum | Leaf |
| Brassica rapa subsp. chinensis | Brassicaceae | Bok choy | Leaf |
| Phaseolus vulgaris | Fabaceae | Bean | Pod |
| Vigna unguiculata ssp. sesquipedalis | Fabaceae | Long bean | Pod |
| Amaranthus viridis | Amaranthaceae | Spinach/green amaranth | Leaf |
| Ipomoea aquatica | Convolvulaceae | Water spinach | Leaf |
| Musa sp. | Musaceae | Banana | Leaf |
| Carica papaya | Caricaeae | Papaya | Leaf |
| Cyperus rotundus | Cyperaceae | Coco grass | Leaf |
2.3. Effect of different diet on the survival, development, and fecundity of S. frugiperda
2.4. Data Analysis
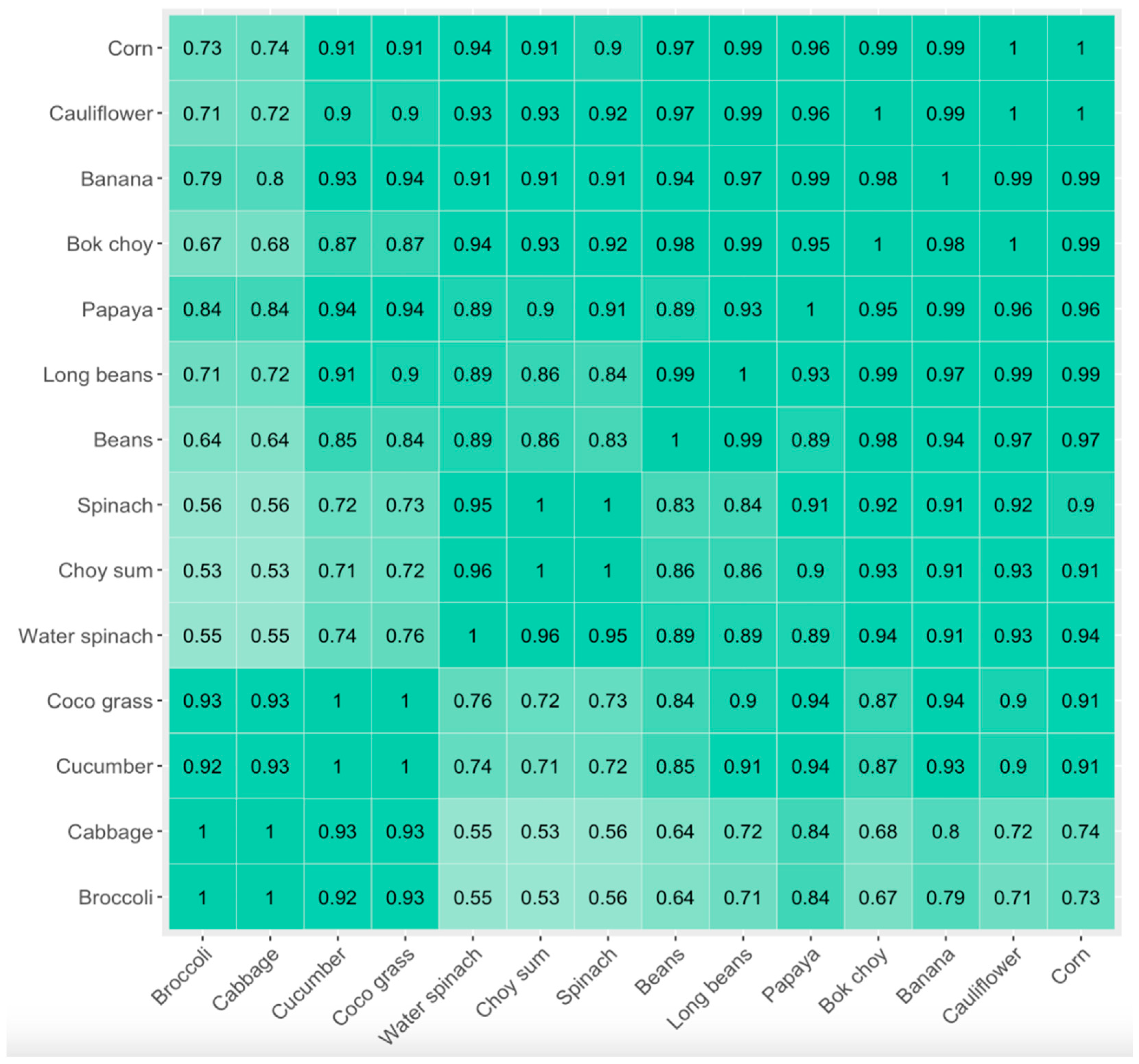
3. Results
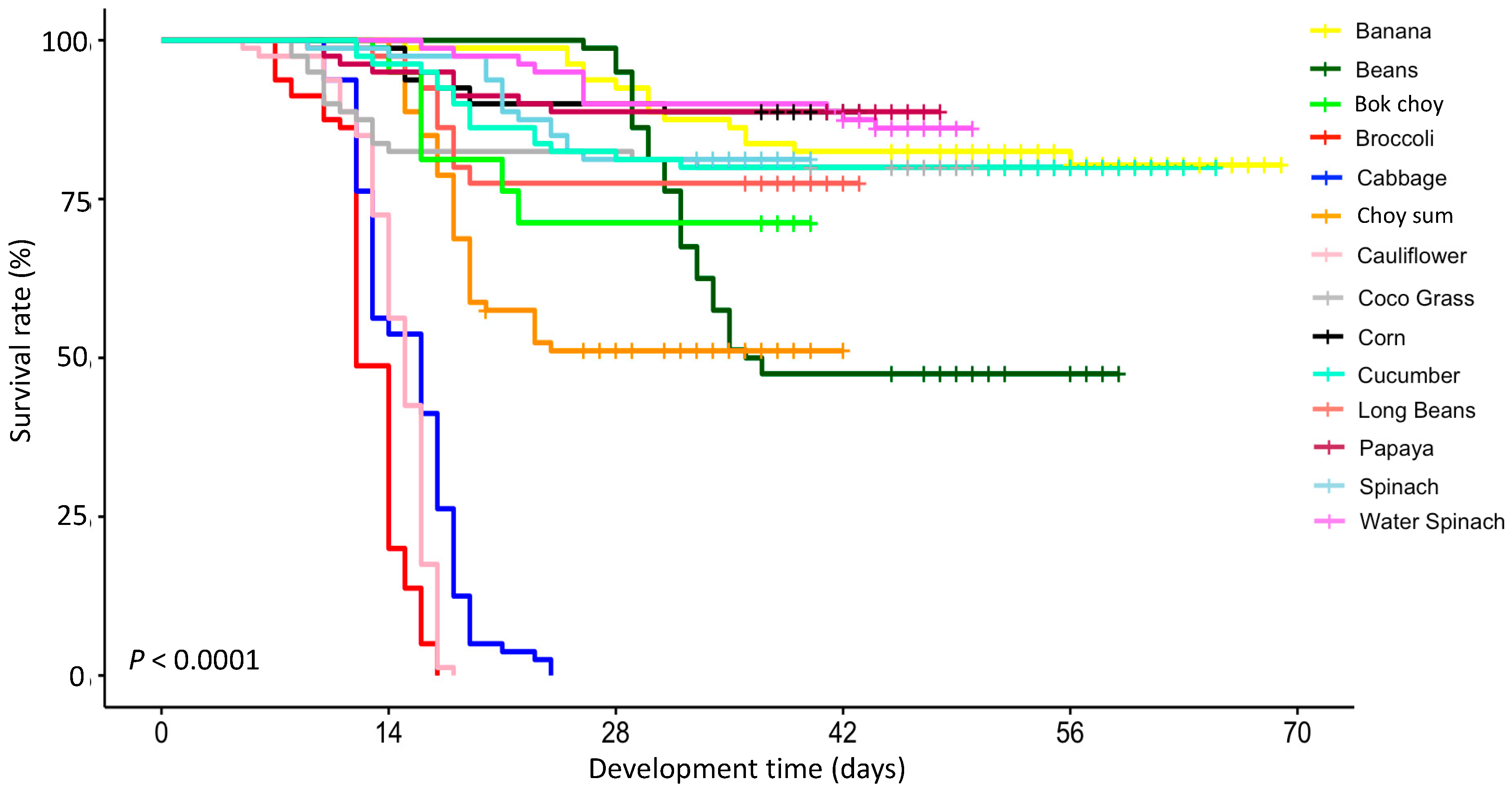
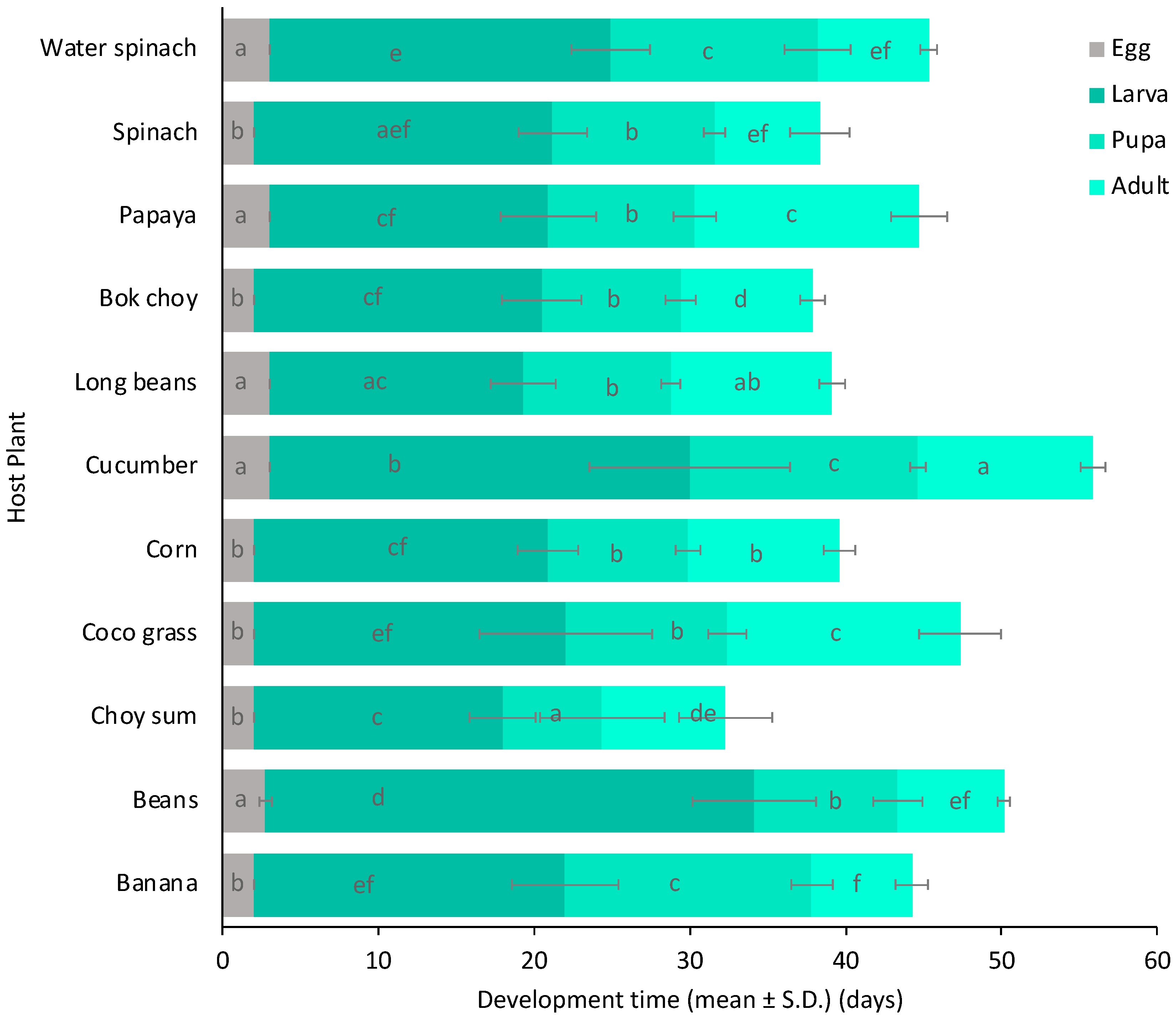
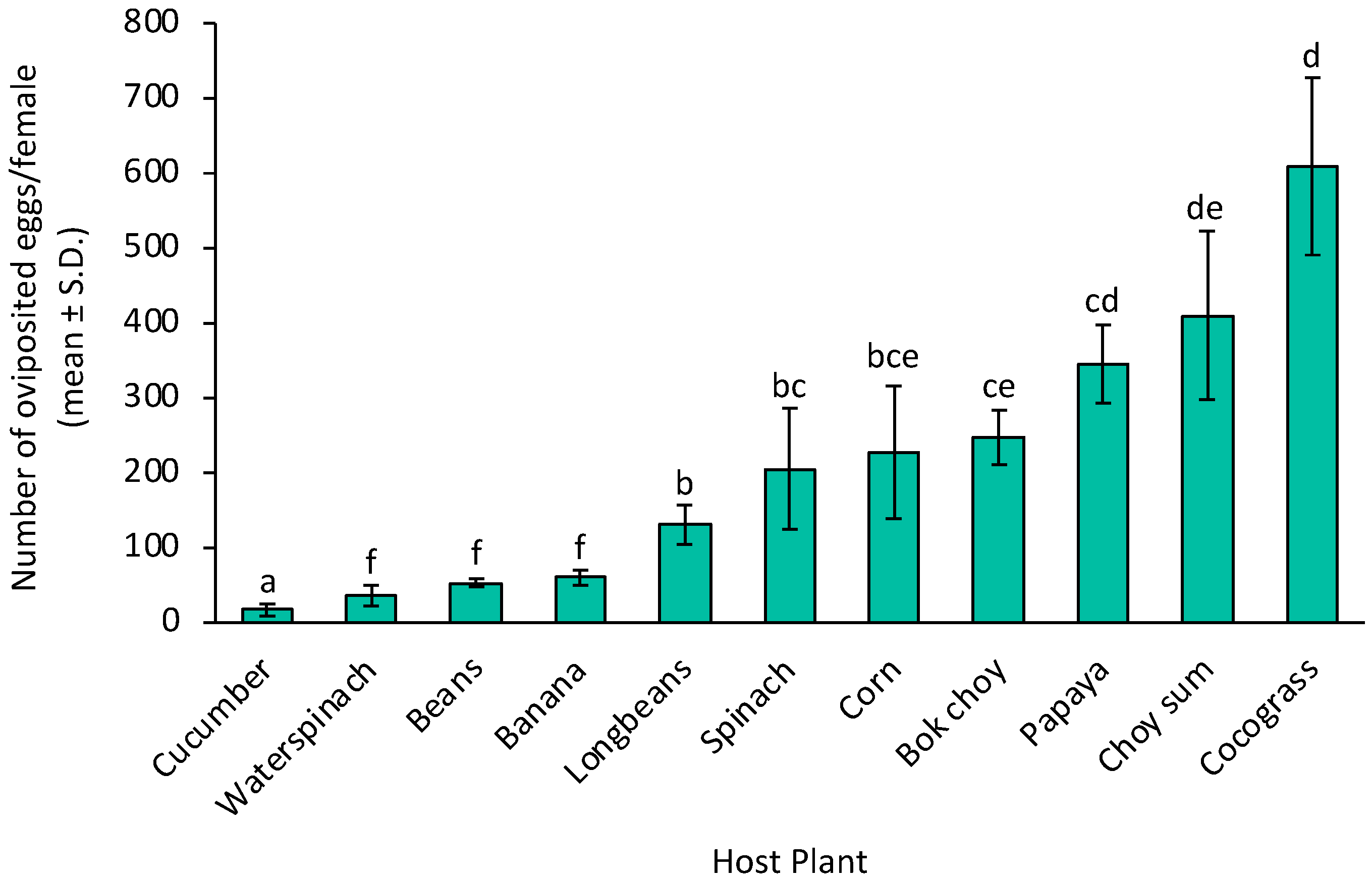
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sagar, G.C.; Aastha, B.; Laxman, K. An introduction of fall armyworm (Spodoptera frugiperda) with management strategies: a review paper. Nippon J. Environ. Sci. 2020, 1(4), 1010. [CrossRef]
- Maruthadurai, R.; Ramesh, R. Occurrence, damage pattern and biology of fall armyworm, Spodoptera frugiperda (J.E. smith) (Lepidoptera: Noctuidae) on fodder crops and green amaranth in Goa, India. Phytoparasitica 2019, 48(1), 15-23. https://doi.org/10.1007/s12600-019-00771-w. [CrossRef]
- Nonci, N.; Kalgutny, S.H.; Hary, S.; Mirsam, H.; Muis, A.; Azrai, M.; Aqil, M. Pengenalan fall armyworm (Spodoptera frugiperda J.E. Smith) hama baru pada tanaman jagung di Indonesia; Balai Penelitian Tanaman Serealia: Maros, Indonesia, 2019. [in Indonesian].
- Khatri, S.; Pakuwal, P.; Khanal, S. Archives of agriculture and environmental science. Arch. Agric. Environ. Sci. 2020, 5, 583–591.
- FAO. Global action for fall armyworm control. Available online: https://www.fao.org/fall-armyworm/monitoring-tools/faw- map/en/ (accessed on 03 March 2023).
- Everington, K. Fall Armyworms from China Invade Taiwan. Available online: https://www.taiwannews.com.tw/en/news/3721661 (accessed on 20 May 2023).
- Sarwono. Waspadai Ulat Grayak Spodoptera frugiperda. Available online: http://cybex.pertanian.go.id/mobile/artikel/80172/WASPADAI-ULAT-GRAYAK-SPODOPTERA-FRUGIPERDA/ (accessed on 03 May 2023).
- Prasanna, B.M.; Huesing, J.E.; Eddy, R.; Peschke, V.M. Fall armyworm in Africa: A Guide for Integrated Pest Management, 1st ed.; CDMX: CIMMYT, Mexico, 2018; pp: 1-120.
- Trisyono, Y.A.; Suputa, Aryuwandari, V.E.F.; Hartaman, M.; Jumari. Occurrence of heavy infestation by the fall armyworm Spodoptera frugiperda, a new alien invasive pest, in corn in Lampung Indonesia. J. Perlind. Tanam. Indones. 2019, 23(1), 156–160. https://doi.org/10.22146/jpti.46455. [CrossRef]
- Bagariang, W.; Tauruslina, E.; Kulsum, U.; Murniningtyas, T.; Suyanto, H.; Surono; Cahyana, N.A.; Mahmuda, D. Effectivity of Chlorantraniliprole insecticide against the larva of Spodoptera frugiperda (JE Smith). JPT: Jurnal Proteksi Tanaman 2020, 4(1), 29–37. https://doi.org/10.25077/jpt.4.01.29-37.2020. [in Indonesian]. [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.; Roque-Specht, V.F.; Paula-Moraes, S.V.de; Peterson, J.A.; Hunt, T.E. Developmental parameters of Spodoptera frugiperda (Lepidoptera: Noctuidae) immature stages under controlled and standardized conditions. J. Agric. Sci. 2019, 11(8), 76. https://doi.org/10.5539/jas.v11n8p76. [CrossRef]
- Sudrajat, S.; Kurniawan, A.M. Diversifikasi pemanfaatan lahan sawah di Desa Tambakrejo Kecamatan Tempel Kabupaten Sleman Daerah Istimewa Yogyakarta. Jurnal Bumi Indonesia 2017, 6(4), 228849. [in Indonesian].
- Syahidah, T.; Rizali, A.; Prasetyo, L. B.; Pudjianto, P.; & Buchori, D. Relationship between landscape structure and Hymenoptera diversity: An interaction model on long bean fields. J. Entomol. Indones. 2021, 18(1), 456087. https://doi.org/10.5994/jei.18.1.43 [in Indonesian]. [CrossRef]
- Rwomushana. Spodoptera frugiperda (fall armyworm). Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.29810 (acessed on 25 May 2023).
- Putra, I.L.I.; Wulanda, A. Life cycle of Spodoptera frugiperda J.E. Smith with green spinach and green thorn spinach leaves in the laboratory. Bioma: Jurnal Ilmiah Biologi 2021, 10(2), 201-216. https://doi.org/10.26877/bioma.v10i2.7928 [in Indonesian]. [CrossRef]
- Hutagalung, R.P.S.; Sitepu, S.F. Biology of Fall Armyworm (Spodoptera frugiperda J. E. Smith) (Lepidoptera: Noctuidae) in laboratory. Jurnal Pertanian Tropik 2021, 8(1), 1-10. https://doi.org/10.32734/jpt.v8i1.5584 [in Indonesian]. [CrossRef]
- Arifin, S.H.A.; Ainun S.H. Morfologi dan siklus hidup Spodoptera frugiperda JE Smith (Lepidoptera: Noctuidae) dengan pakan daun kedelai (Glycine max L) di Laboratorium. Bacelor’s thesis, Universitas Hasanuddin, Makassar, Indonesia, 2021.
- Megasari, D.; Putra, I.L.I.; Martina, N.D.; Wulanda, A.; Khotimah, K. Biology of Spodoptera frugiperda JE Smith on some types of feed in the laboratory. Agrovigor: Jurnal Agroekoteknologi 2022, 15(1), 63-67. https://doi.org/10.21107/agrovigor.v15i1.11978 [in Indonesian]. [CrossRef]
- Putra, I.L.I., & Martina, N.D. Life cycle of Spodoptera frugiperda with feeding kale and leeks in laboratory. J. Ilmu Pertanian Indones. 2021, 26(3), 386-391. https://doi.org/10.18343/jipi.26.3.386 [in Indonesian]. [CrossRef]
- Chen, W.H.; Itza, B.; Kafle, L.; Chang, T.Y. Life table study of fall armyworm (Spodoptera frugiperda) (Lepidoptera: Noctuidae) on three host plants under laboratory conditions. Insects 2023, 14, 329. https://doi.org/10.3390/insects14040329. [CrossRef]
- Ashok, K.; Kennedy, J. S.; Geethalakshmi, V.; Jeyakumar, P.; Sathiah, N.; Balasubramani, V. Lifetable study of fall army worm Spodoptera frugiperda (JE Smith) on maize. Indian J. Entomol. 2020, 82(3), 574-579.
- Wu, L.H.; Zhou, C; Long, G.Y.; Yang, X.B.; Wei, Z.Y.; Liao, Y.J.; Yang, H.; Hu, C.X. Fitness of fall armyworm, Spodoptera frugiperda to three solanaceous vegetables. J. Integr. Agric. 2021, 20(3), 755-763. [CrossRef]
- Niassy, S.; Agbodzavu, M. K.; Kimathi, E.; Mutune, B.; Abdel-Rahman, E.F.M.; Salifu, D.; Hailu, G.; Belayneh, Y.T.; Felege, E.; Tonnang, H.E.Z.; Ekesi, S.; Subramanian, S. Bioecology of fall armyworm Spodoptera frugiperda (JE Smith), its management and potential patterns of seasonal spread in Africa. PloS one 2021, 16(6), e0249042. [CrossRef]
- He, L.M.; Wang, T.L.; Chen, Y. C.; Ge, S.S.; Wyckhuys, K.A.; Wu, K.M. Larval diet affects development and reproduction of East Asian strain of the fall armyworm, Spodoptera frugiperda. J. Integr. Agric. 2021, 20(3), 736-744. [CrossRef]
- Unbehend, M.; Hänniger, S.; Vásquez, G.M.; Juárez, M.L.; Reisig, D.; McNeil, J. N.; Meagher, R.L; Jenkins, D.A.; Heckel, D.G.; Groot, A.T. Geographic variation in sexual attraction of Spodoptera frugiperda corn-and rice-strain males to pheromone lures. PloS one 2014, 9(2), e89255. [CrossRef]
- Golikhajeh, N.; Naseri, B.; Razmjou, J. Geographic origin and host cultivar influence on digestive physiology of Spodoptera exigua (Lepidoptera: Noctuidae) larvae. J. Insect Sci. 2017, 17(1), 12. [CrossRef]
- Dudley, W.N.; Wickham, R.; Coombs, N. An introduction to survival statistics: Kaplan-Meier analysis. J. Adv. Pract. Oncol. 2016, 7(1), 91. [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. 2008. Simultaneous inference in general parametric models. Biom. J. 2008, 50(3), 346–363. https://doi.org/10.1002/BIMJ.200810425. [CrossRef]
- R Core Team. R language definition. R foundation for statistical computing: Vienna, Austria, 2013.
- Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer; 2009.
- Chimweta, M.; Nyakudya, I.W.; Jimu, L.; Bray Mashingaidze, A. Fall armyworm [Spodoptera frugiperda (JE Smith)] damage in maize: management options for flood-recession cropping smallholder farmers. Int. J. Pest Manag. 2020, 66(2), 142-154. [CrossRef]
- Harrison, R; Banda, J.; Chipabika, G.; Chisonga, C.; Katema, C.; Ndalamei, D.M.; Nyirenda, S.; Tembo, H. Low impact of fall armyworm (Spodoptera frugiperda) (Lepidoptera: Noctuidae) across smallholder fields in Malawi and Zambia. J. Econ. Entomol. 2022, 115(6), 1783-1789. [CrossRef]
- Hill, D.S. Pests of crops in warmer climates and their control. Springer: Dordrecht, Netherland, 2008.
- Awmack, C.S.; Leather S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817-844. https://doi.org/10.1146/annurev.ento.47.091201.145300. [CrossRef]
- Silva, D.M.D.; Bueno, A.D.F.; Andrade, K.; Stecca, C.D.S.; Neves, P.M.O.J.; Oliveira, M.C.N.D.; 2017. Biology and nutrition of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on different food sources. Sci. Agric. 2017, 74, 18-31. https://doi.org/10.1590/1678-992X-2015-0160. [CrossRef]
- Subiono, T. Preferences of Spodoptera frugiperda (Lepidoptera: Noctuidae) in several feed sources. Jurnal Agroekoteknologi Tropika Lembab 2020, 2(2), 130-134. https://doi.org/10.35941/JATL. [in Indonesian]. [CrossRef]
- Russianzi, W.; Anwar, R.; Triwidodo, H. 2021. Biostatistics of fall armyworm Spodoptera frugiperda in maize plants in Bogor, West Java, Indonesia. Biodiversitas 2021, 22(6), 3463-3469. https://doi.org/10.13057/biodiv/d220655. [CrossRef]
- Gopalakrishnan, R.; Kalia, V.K. Biology and biometric characteristics of Spodoptera frugiperda (Lepidoptera: Noctuidae) reared on different host plants with regard to diet. Pest Manag. Sci. 2022, 78(5), 2043-2051. https://doi.org/10.1002/ps.6830. [CrossRef]
- Zhou, S.; Qin, Y.; Wang, X.; Zheng, X.; Lu, W. Fitness of the fall armyworm Spodoptera frugiperda to a new host plant, banana (Musa nana Lour.). Chem. Biol. Technol. Agric. 2022, 9 (1), 78. https://doi.org/10.1186/s40538-022-00341-z. [CrossRef]
- Franco Archundia, S.L.; Valdés-Estrada, M.E.; Jiménez-Pérez, A.; Figueroa Brito, R. Evaluation of the toxic effect of seeds of four varieties of Carica papaya against Spodoptera frugiperda. Proceedings of the Interamerican Society for Tropical Horticulture 2005, 49, 202-204.
- Pérez-Gutiérrez, S.; Zavala-Sánchez, M.A.; González-Chávez, M.M.; Cárdenas-Ortega, N.C.; Ramos-López, M.A. Bioactivity of Carica papaya (Caricaceae) against Spodoptera frugiperda (Lepidoptera: Noctuidae). Molecules 2011, 16(9), 7502-7509. https://doi.org/10.3390/molecules16097502. [CrossRef]
- Juleha, S.; Afifah, L.; Surjana, T.; Yustiano, A. Potency of papaya leaves (Carica papaya L.) as contact poison and repellent against Spodoptera frugiperda. Jurnal Agrotech 2022, 12(2), 66-72. https://doi.org/10.31970/agrotech.v12i2.95. [in Indonesian]. [CrossRef]
- Wijerathna, D.M.I.J.; Ranaweera, P.H.; Perera, R.N.N.; Dissanayake, M.L.M.C.; Kumara, J.B.D.A.P. Biology and feeding preferences of Spodoptera frugiperda (Lepidoptera: Noctuidae) on maize and selected vegetable crops. J. Agric. Sci. - Sri Lanka 2021, 16(1), 126-134. http://doi.org/10.4038/jas.v16i1.9190. [CrossRef]
- Wang, W.; He, P.; Zhang, Y.; Liu, T.; Jing, X.; Zhang, S. The population growth of Spodoptera frugiperda on six cash crop species and implication for its occurrence and damage potential in China. Insects 2020, 11(9), 639: http://doi.org/10.3390/insects11090639. [CrossRef]
- Putra, I.L.I.; Khotimah, K. Life cycle Spodoptera frugiperda JE Smith with lettuce (Lactuca sativa L.) and pakcoy (Brassica rapa L.) in the laboratory. J. Trop. Crop Prot. 2021, 2(1), 8-13. https://doi.org/10.19184/jptt.v2i1.21459. [in Indonesian]. [CrossRef]
- Kaur, S.; Kaur, R.; Chauhan, B.S. Understanding crop-weed-fertilizer-water interactions and their implications for weed management in agricultural systems. Crop Prot. 2018, 103, 65-72. [CrossRef]
- Barros, E.M.; Torres, J.B.; Ruberson, J.R.; Oliveira, M.D. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomol. Exp. Appl. 2010, 137(3), 237-245. https://doi.org/10.1111/j.1570-7458.2010.01058.x. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





