1. Introduction
It seems both important and difficult to develop a purely thermodynamic view of theoretical ecology [
1,
2]. The concept of entropy has received recent attention, in theoretical work such as [
3,
4] as well observational work such as [
5,
6,
7].
Many open questions remain, for example whether to adopt one of the many proposals of minimum and maximum principles of entropy production [
3,
8] and indeed whether entropy is a single unified concept [
9] or a group concepts only superficially related by very similar formalisms [
10].
Perhaps progress can be made if as many of these difficulties as possible are avoided. Population dynamics is well suited to that aim. It can model both steady and transient states, and it spans the gamut from extremely simple to extremely complex models. At the simple end, full analysis is possible while at the complex end the models are capable of expression extreme realism.
It is true that population models do not obviously have thermodynamic meaning. But all are based on birth, growth and death (albeit often merely implicitly), and these involve flows of matter and energy and therefore can and should be understood thermodynamically.
In this paper, I consider a set of models of a simple chemostat with only one population of microbes. This can be viewed as an ecosystem in that it contains many living individuals that are kept alive by a flow of energy and matter. Of course chemostat models can include more and more populations, competing and interacting and constituting several trophic levels. Such models might represent wild ecosystems better and better, but the point here is to have a system that has a manageably small amount of structure.
The only departure from standard chemostat modelling is that growth dynamics is segregated from birth and death dynamics.
2. Models
The models are intentionally as simple as possible.
I first present the base case: a chemostat with a population of n microbes each of mass m, exponential dynamics, constant dilution rate and parameters adjusted to keep biomass constant. Each microbe undergoes binary fission when its initial size has doubled. The only loss of biomass is due to elution, so upon binary fission the two new microbes each has the same biomass as the initial biomass of their parent.
I then present a periodic case: the base case but with a diapause in growth during which binary fission occurs.
Finally, I present the simplest realistic case, with nutrient replenished by the inflow concentration and depleted by the microbe uptake which follows Monod dynamics.
To conclude this section, I briefly discuss age structure, and point out that although the models do not explicitly model age structure, there is a clear sense in which they imply age structure.
Simplicity first: the base case
Microbe mass
m grows at the rate
r, microbe number is reduced only by dilution at the rate
s; initial values are
at
.
Obviously, we have and so that biomass b as a function of time is .
Assume that coincides with binary fission, so that age a is equal to t. Then gives microbe size at age a, and binary fission happens at given by . In this paper, denotes the generation time, that is, the time from one binary fission to the next, and for this model .
The base case + non-zero time for binary fission
Recall that binary fission must happen when
. It is realistic that this process is not instantaneous, and a simple way to model the duration of fission is simply to have growth stop for a while. This leads to the following modification of the base model:
In this case, the generation time is , where is the duration of binary fission and . Since the equations do not include the process of binary fission, for the numerical solution a direct implementation of the duration is necessary.
Minimally realistic: constant resource supply, Monod-like uptake dynamics
Assume nutrient concentration
x with uptake rate
per microbe at and inflow concentration
i. The size growth rate per microbe is proportional to the uptake rate. Then the base model is modified to the following.
This model is minimally realistic for two reasons.
(1) The constant inflow of nutrient provides a realistic (albeit simplistic) model for a driver of the growth of the microbes and thus a source of the energy required to maintain this system in a steady state far from thermodynamic equilibrium. Such a source is necessary for any biological model to qualify as realistic.
(2) The uptake per individual saturates and becomes constant as nutrient concentration increases. This is another requirement for realism, deriving from the fact that nutrient flow into an organism must be constrained by the finite extent of surface area which is able to conduct such a flow.
But the model is not fully realistic (for example, it excludes the non-zero time for binary fission of the previous model). The claim is not that the model meets, in some minimal way, all the requirements of realism but rather that the two requirements it does meet are essential for any model that aims at realism.
Population structure in the three models above
Although the models do not have any parameters that depend on age nor any mechanisms that vary by age, every individual microbe has an age in the sense of the time it has lived since it came into being by binary fission. So every individual has an age, and the population as a whole has an age structure. Since size is a function of age, population structure also includes age structure.
One can describe the population structure in terms of cohorts (here, a cohort is the set of all individuals of a given age). The models above are formulated as if there is only one cohort, but can be extended to multiple cohorts, as follows.
Assume that at there are k cohorts with ages, individual mass and number denoted by , and , where . To study the evolution of this cohort structure, it will be convenient to use the cohort biomasses and the total biomass . To avoid confusion, the initial biomass is always written as .
In the first two models, the cohorts do not interact at all and each have exactly the same dynamics. In the last model, the cohorts interact indirectly by sharing the nutrient supply. But we shall see that there is a critical level of biomass at the cohort dynamics effectively decouple.
3. Results
In what follows, the term “steadily cyclic state” denotes a system state where m and n are periodic and is either periodic or constant.
The diagrams illustrating the results require numerical values to be chosen for the parameters and initial values. For convenience, these are chosen to so that mass varies approximately from 1 to 2 and the period is not too far from 1. Clearly, this implies a unit of mass of a few nano- or micrograms, a similarly arbitrary unit of for the number of perhaps a few millions or billions and careful adjustment of parameters.
The steadily cyclic states of the three models
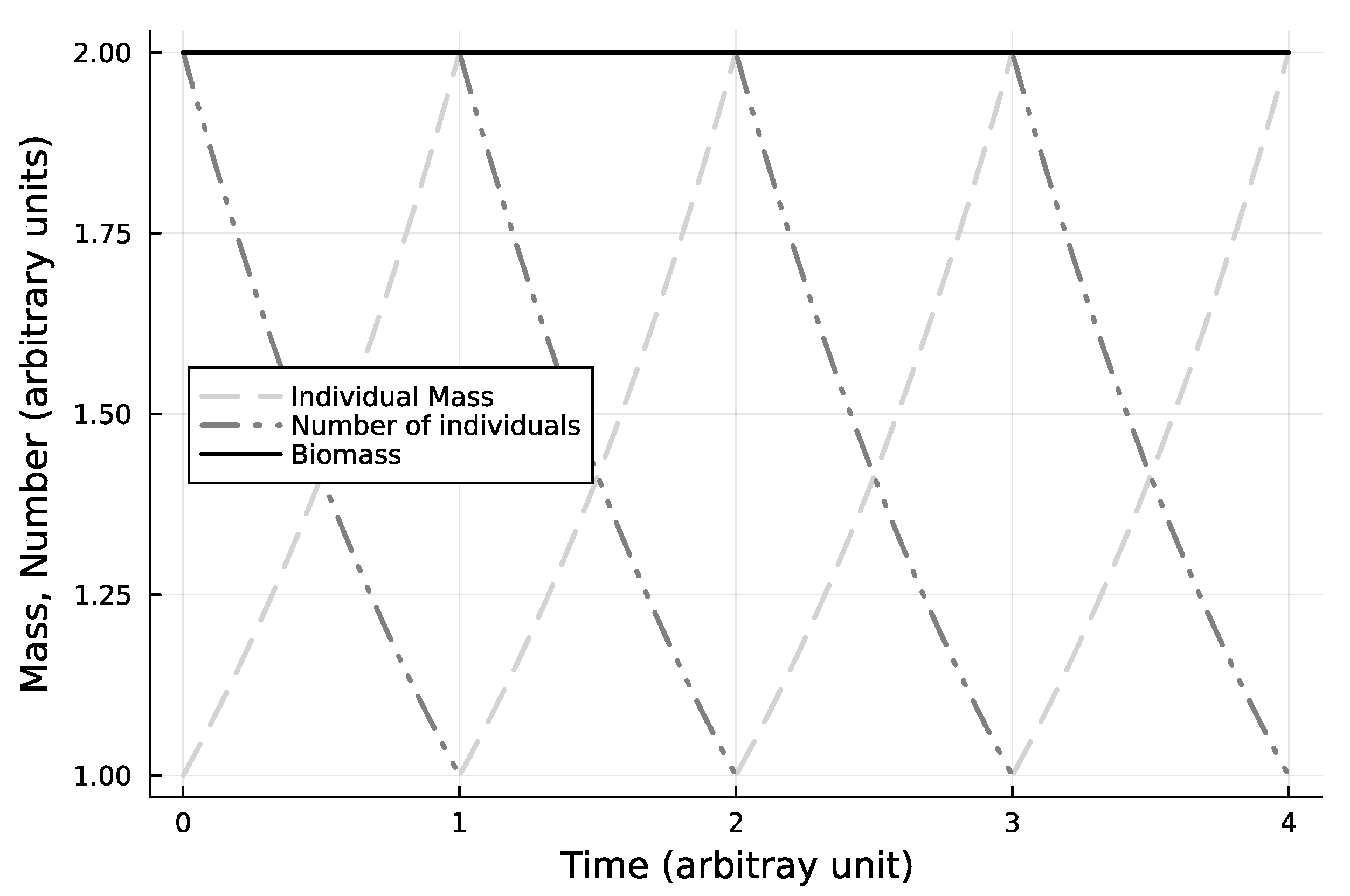
For the base case,
. So if and only
do we have the steady state
, and this applies to every cohort. Denoting the initial state of the
i-th cohort by
, we have
. On the other hand,
are periodic with period
as defined above, see
Figure 1.
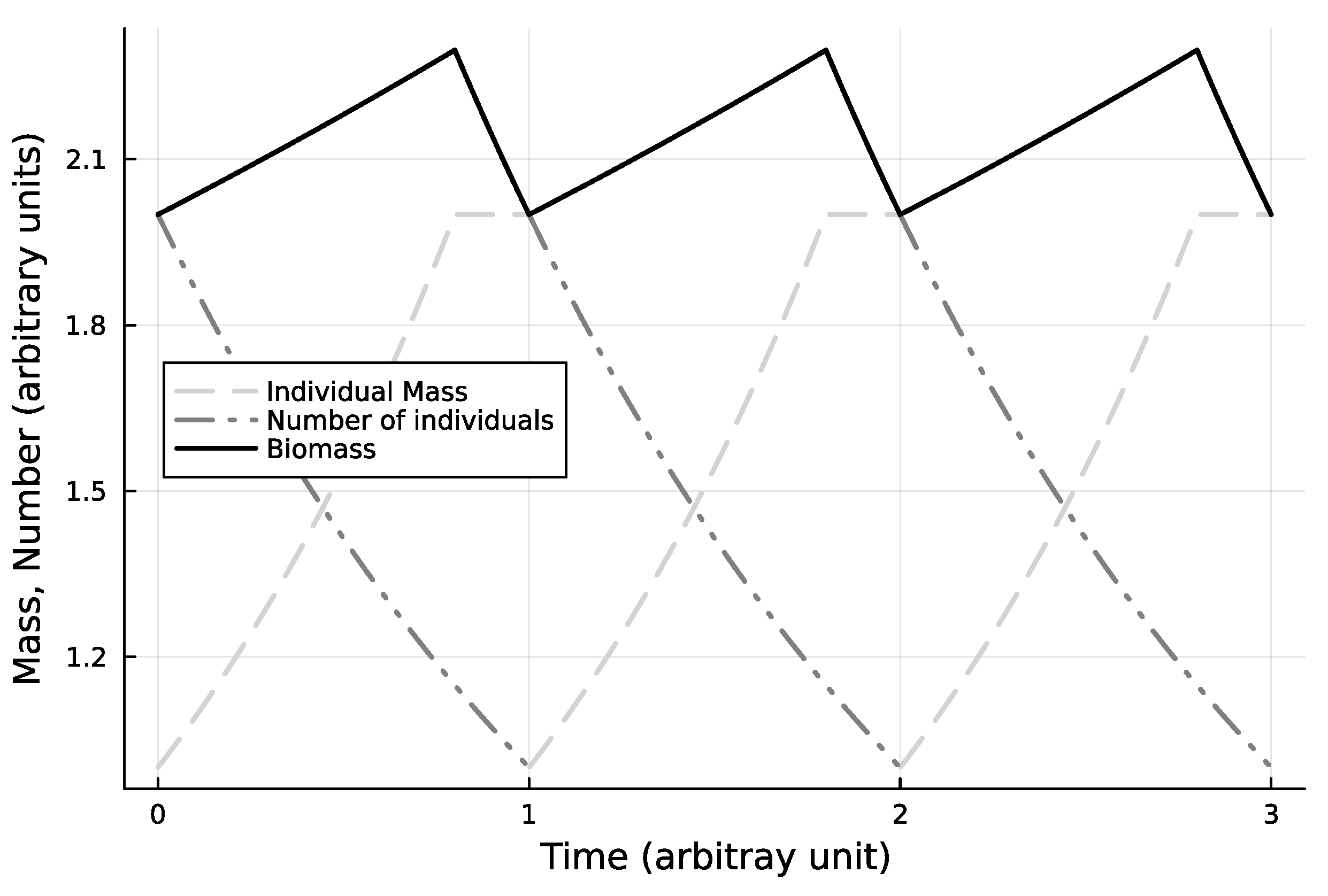
In model two, the base case + pause for fission, size doubles in time
and then growth stops for time
while binary fission takes place. Generation time
and for a steady state this must be the time it takes for the population number to be halved by dilution. Hence
in the steady state. During the pause in size growth, biomass declines as there is no pause in dilution. But since
during the pause, after binary fission we have
so the individual size is
at the start of each new period of size growth. As a result, although biomass
is not constant, it is periodic, see
Figure 2.
Population structure is more complicated than in the base case, as each cohort biomass has periods of increase and decrease. However, the changes in structure are themselves periodic and in that sense population structure is persistent.
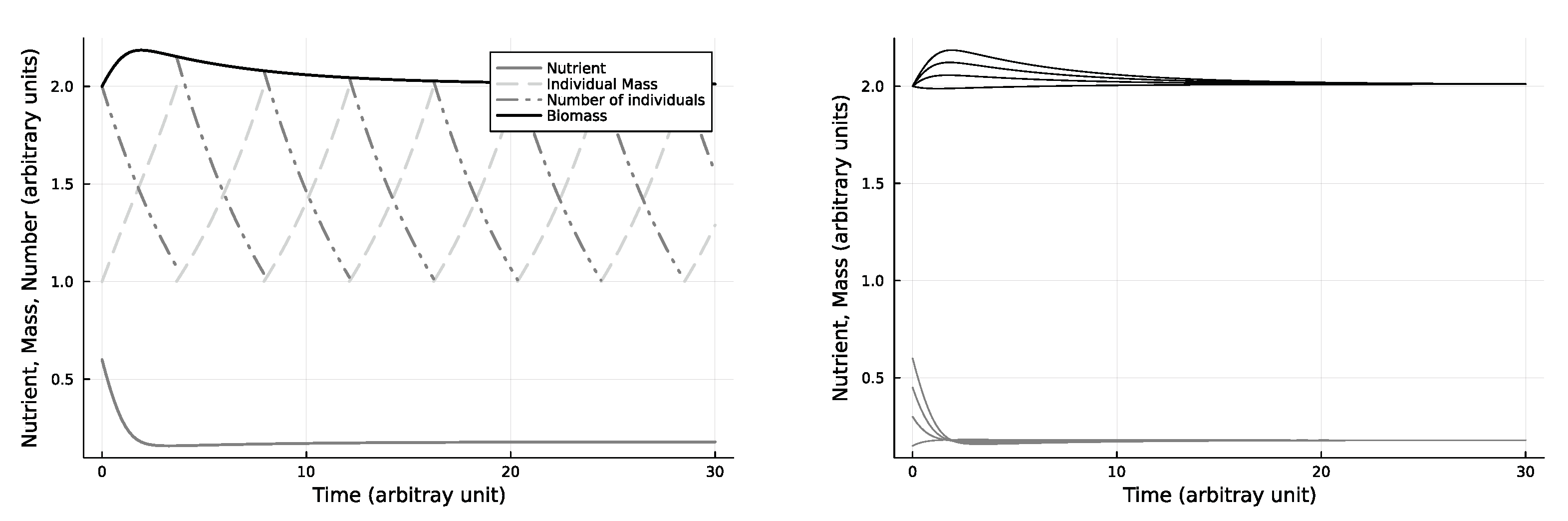
For model three, the minimally realistic case, the biomass
and nutrient concentration
of the steadily cycling state can be found by solving
simultaneously. Noting that
we deduce that for
,
if and only if
. Applying this to
we obtain
. Then size growth is at the rate
, and solving
we obtain
, so that
and the doubling time for size growth equals the halving time for in population number due to dilution. Thus
are periodic with generation time
. This is illustrated in
Figure 3, which also shows that the steadily cycling state is a limit cycle.
Now for the question of age structure. Unlike the previous two, in the minimally realistic case the different cohorts interact (albeit indirectly) because they share the nutrient supply. However, when steadily cycling, the nutrient level is constant at and the parameter values are the same for all cohorts. Therefore in this case, as in the previous two, age structure is persistent.
Entropy production in the steadily cycling states
Every metabolically active organism produces entropy (see [
11] for a discussion of entropy production in a model of the life cycle of an aerobic organism). Thus the size growth in each of the three models in this paper implies entropy production. As noted above, entropy production in non-linear thermodynamics is a very active area of research and many issues remain to be resolved. However, if we restrict our attention to the steadily cycling case of each model, then the rate of entropy production is periodic and we can for the moment ignore the causal role of entropy in how that state was reached. All that matters is that entropy production is guaranteed by individual growth.
However, entropy production is not necessarily constant throughout the life cycle of a microbe. In these models, under steady cycling, this difficulty is avoided quite simply: each microbe grows from to before fission occurs later (where for the base model and the minimally realistic model) and at a constant rate. Therefore all microbes produce the same amount of entropy during their lifetimes; in fact, entropy production for a single microbe over a period equal to the generation time is a constant, no matter at what size it starts (that is, this includes the growth pattern to followed by to ). Summing over all cohorts the entropy produced over a single generation time is therefore therefore a constant, whatever time the interval starts.
Moreover, this applies to any age distribution, as follows. Consider the interval and a cohort of size initially. This cohort will grow to size , then double in number and halve in size and grow back to size at the end of the interval. All this time the undergo dilution such that the enumerated size of the cohort at is equal to its size at . Therefore cohort i exports the entropy that corresponds to an overall mass gain of for all i, irrespective of initial indivdidual size.
In other words, in these models the entropy produced by the metabolism of the microbes is decoupled from the age structure—entropy production over an interval of length is the same for all age structures.
4. Discussion
These results illuminate the following question: do changes to ecosystem structure result in changes to entropy production? For the models presented above, the answer is no: no matter what the age structure, the population is steadily cycling and the entropy production per cycle is invariant. It seems possible that some aspects of ecosystem structure may escape from entropic constraint.
How far might these results generalise?
Many models could be written analogously to the ones above. All that is needed is for biomass b to be decomposed into , with models that imply births and deaths such that solutions for b are the same for all age structures.
Moreover, introducing a death rate would not change the results. Simply replace the dilution rate s with the loss rate . The individuals in a given cohort will still have the same age (by definition), so that deaths actually do not influence the age structure. Neither to they influence the production of entropy, which (in these models, anyway) is due solely to the metabolism of living individuals.
Might non-deterministic dynamics be used? Certainly. Let’s interpret the dynamics as expected values of stochastic processes. Then the expected biomass is still a steadily cycling variable, and hence the expected rate of entropy export is constant, irrespective of age distribution. However, the strict synchrony of individuals in a cohort would be lost, and some sort of drift in age distribution would result.
It seems plausible that other structures might also allow for escape from entropic constraint. For a spatial example, consider cattle in a pasture. Suppose that over longish time intervals, they spatial distribution of grazing pressure is constant. This can obviously be achieved in many different spatial arrangements. For example, a tight herd could move steadily around the paddock, or individuals could move randomly, independently and ergodically.
However, it seems less easy to construct examples for intra- and interspecific interactions, because as mass m changes with age, these interactions could not plausibly have unchanged effects.
What about genetics? Could it be that some changes in genetic structure have no implications for entropy production? Again, yes. For example, this follows from the genetic code itself, whereby a give amino acid is coded for by several different codons, so that there are genetic changes which do not result in any changes to the resulting protein.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Jørgensen, S.E.; Fath, B.D. Application of thermodynamic principles in ecology. Ecological Complexity 2004, 1, 267–280. [Google Scholar] [CrossRef]
- Nielsen, S.N.; Müller, F.; Marques, J.C.; Bastianoni, s.; Jørgensen, S.E. Thermodynamics in Ecology — An Introductory Review. Entropy 2020, 22, 820. [Google Scholar] [PubMed]
- Endres, R.G. Thermodynamics of switching in multistable non-equilibrium systems. Nature Scientific Reports 2017, 7, 14437. [Google Scholar]
- Roach, T.N.F. Use and abuse of entropy in biology: a case for caliber. Entropy 2020, 22, 1335. [Google Scholar] [PubMed]
- Meysman, F.J.R.; Bruers, S. Ecosystem functioning and maximum entropy production: a quantitative test of hypotheses. Phil. Trans. R. Soc. B 2010, 365, 1405–1416. [Google Scholar] [CrossRef]
- Ludovisi, A. Effectiveness of entropy-based functions in the analysis of ecosystem state and development. Ecological Indicators 2014, 36, 617–623. [Google Scholar]
- Worrall, F. ; Morrison, R; Evans, C. ; Kaduk, J.; Page, S.; Cumming, A.; Rayment, M.; Kettridge, N. Are peatlands in different states with respect to their thermodynamic behaviour? A simple test of peatland energy and entropy budgets. Hydrological Processes 2021, 35(12), e14431. [Google Scholar]
- Di Vita, A. The Garden of Forking Paths. In Non-equilibrium Thermodynamics. Lecture Notes in Physics; Springer: Switzerland, 2022; Volume 1007, pp. 201–212. [Google Scholar]
- Jaynes, E.T. Information theory and statistical mechanics. Physical Review 2002, 106, 620–630. [Google Scholar]
- Popović, M. E. Research in Entropy Wonderland: A Review of the Entropy Concept. Thermal Science 2018, 22, 1163–1178. [Google Scholar] [CrossRef]
- Jusup, M.; Sousa, T.; Domingos, T.; Labinac, V.; Marn, N.; Wang, Z.; Klanjšček, T. Physics of metabolic organization. Physics of Life Reviews 2017, 20, 1–39. [Google Scholar] [CrossRef]
- Michaelian, K.; Cano Mateo, R.E. A Photon Force and Flow for Dissipative Structuring: Application to Pigments, Plants and Ecosystems. Entropy 2022, 24, 76. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, E. What is life? Cambridge University Press: London, 1944. [Google Scholar]
- Demetrius, L.; Gundlach, V. Directionality Theory and the Entropic Principle of Natural Selection, Entropy 2014 16, 5428–5522.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).