Submitted:
29 May 2023
Posted:
31 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Anatomic Evaluation
2.3. MRI technique
3. Results
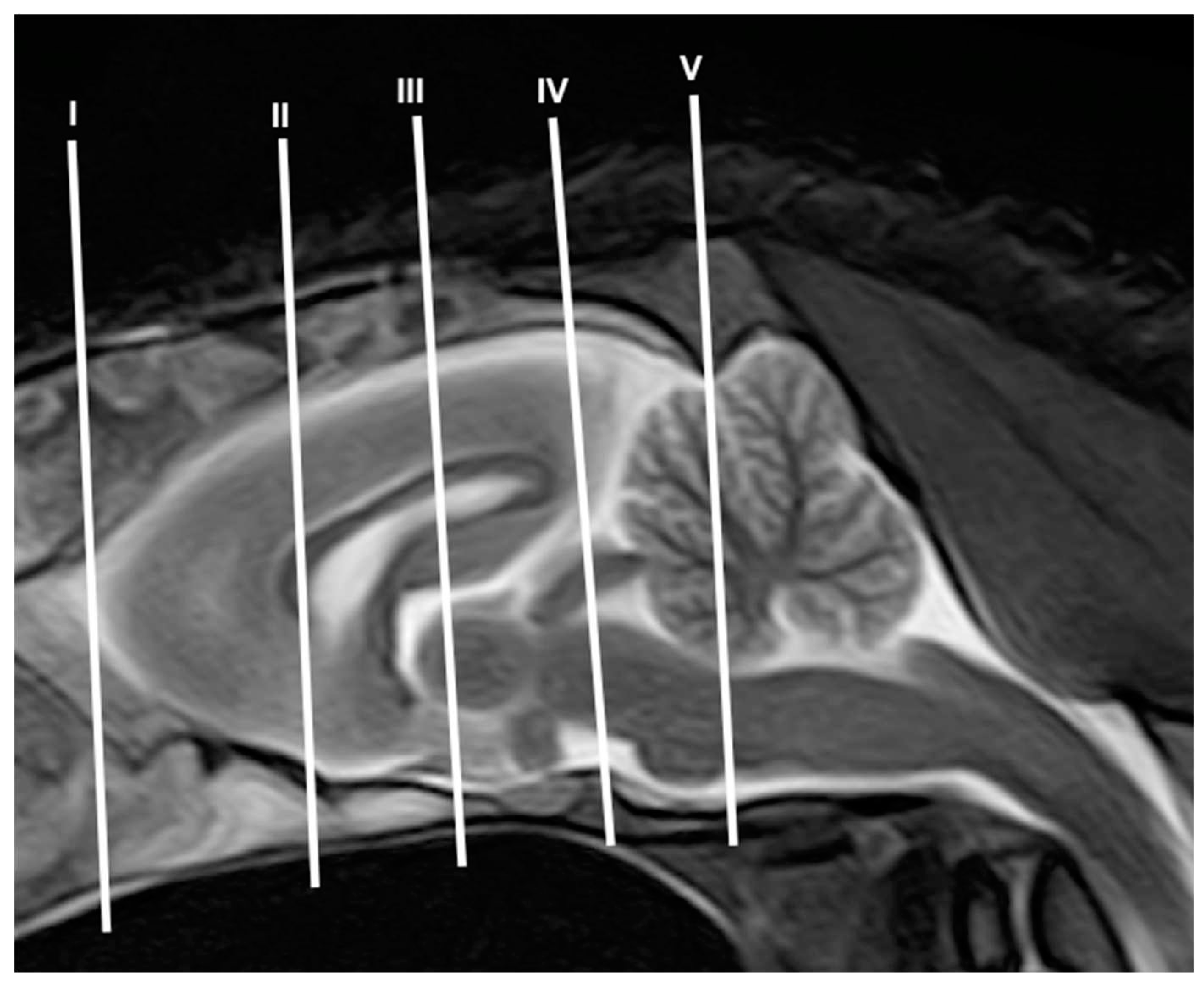
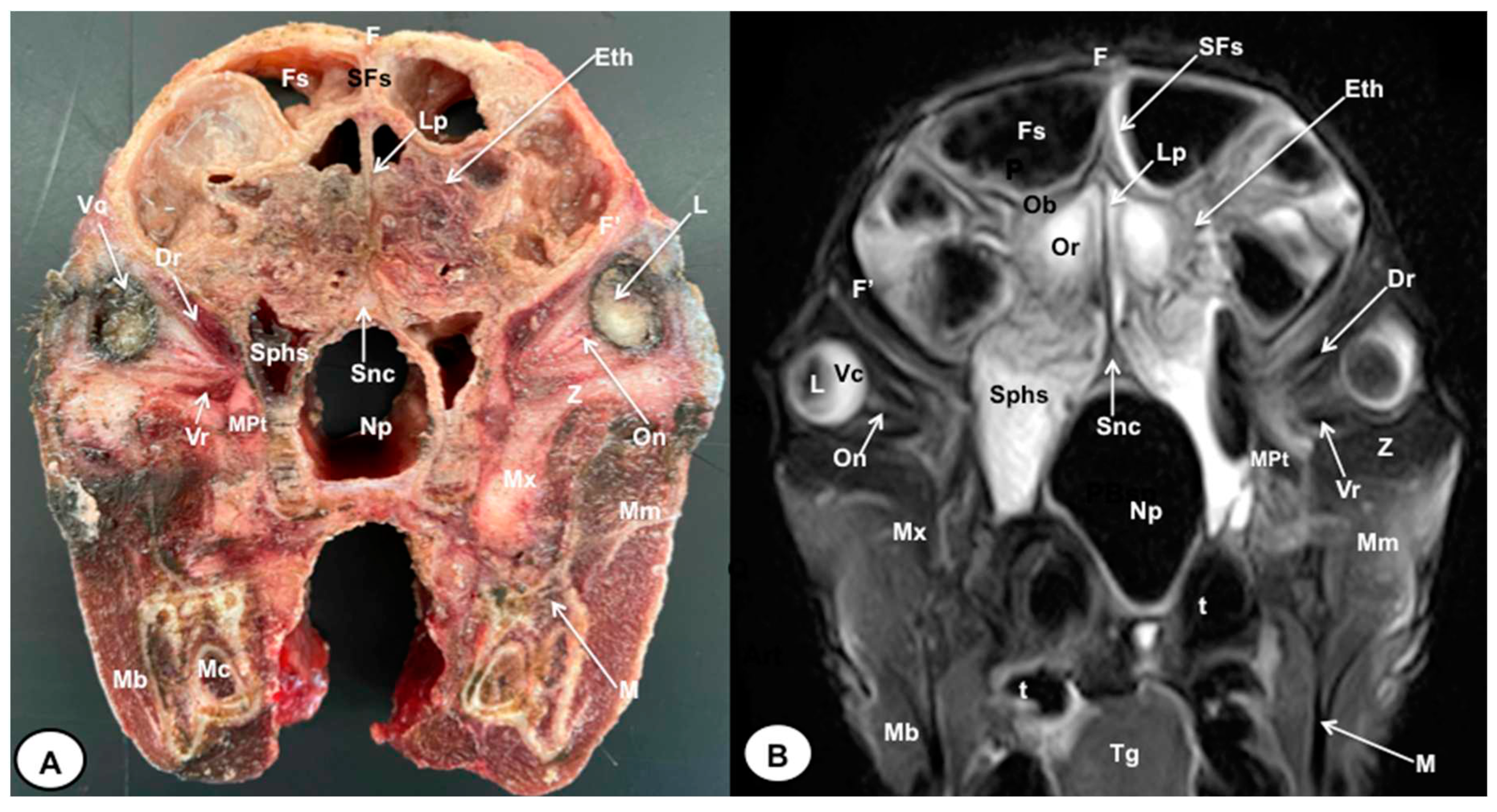
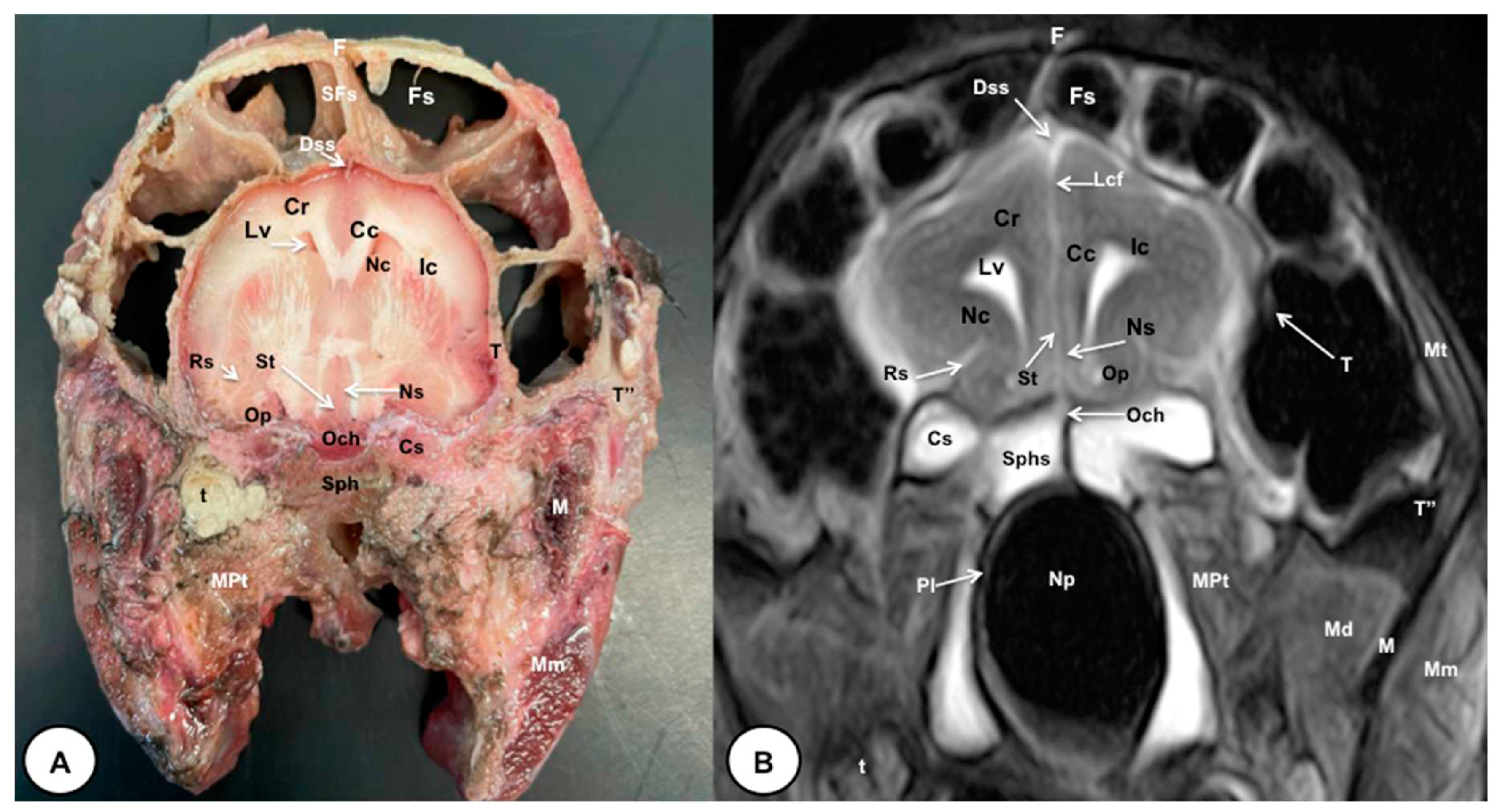
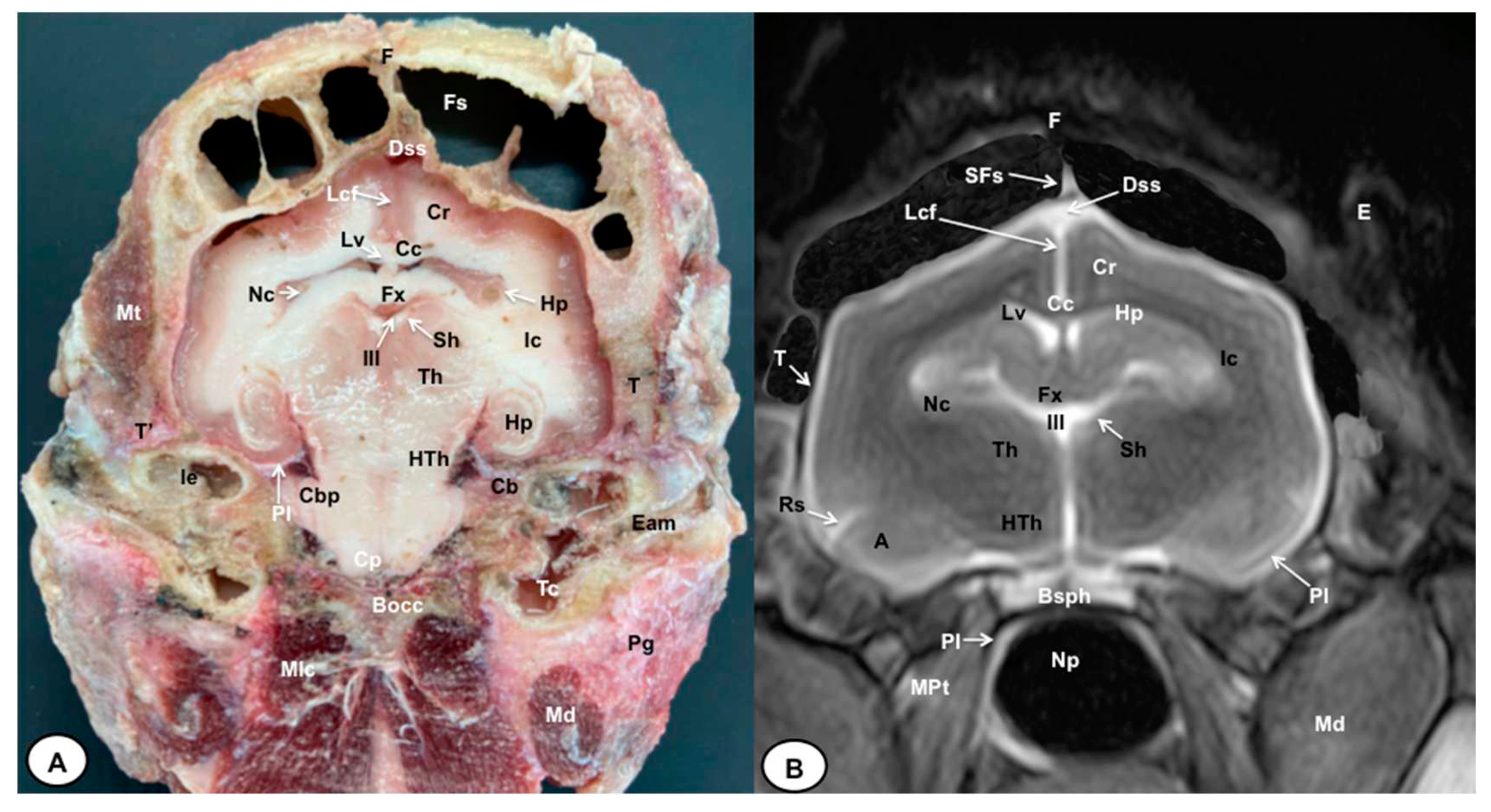
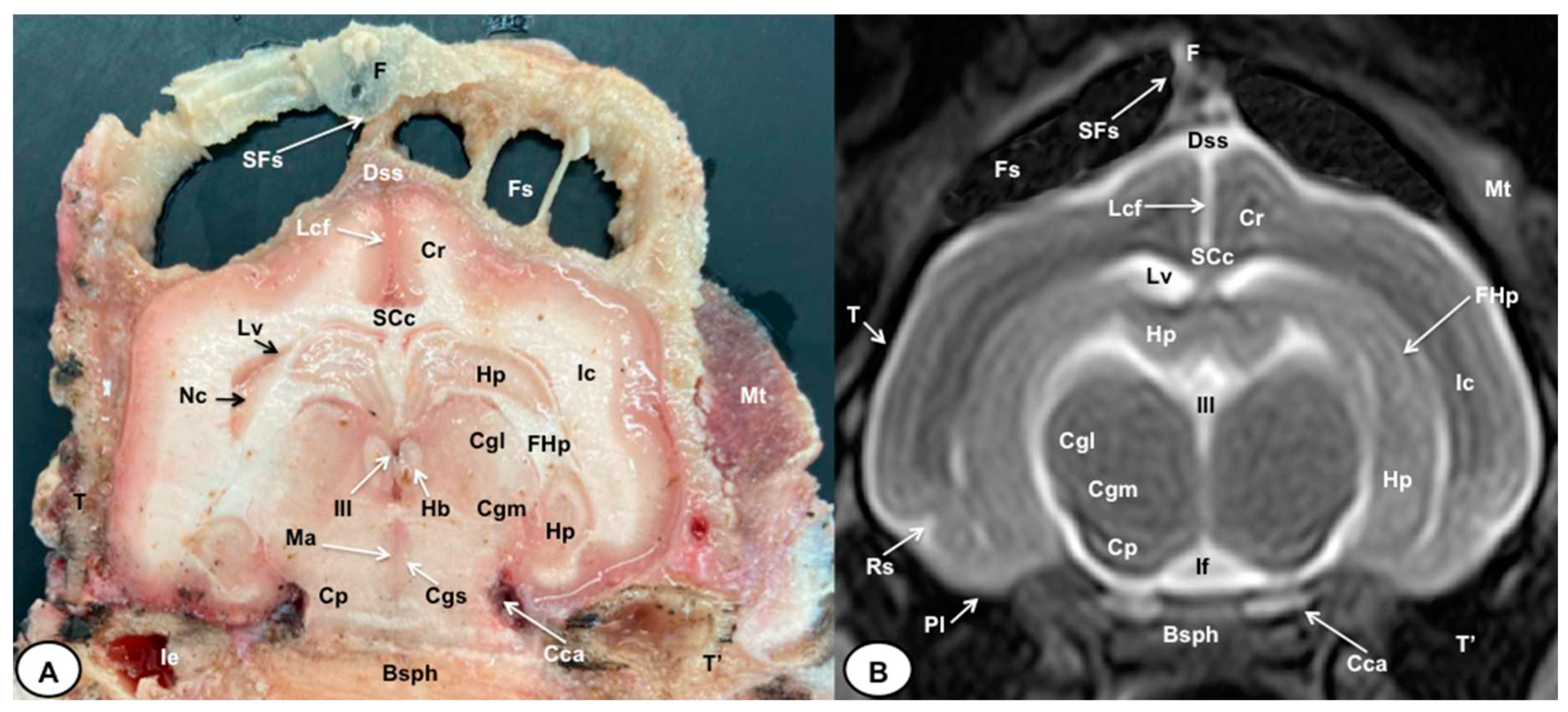
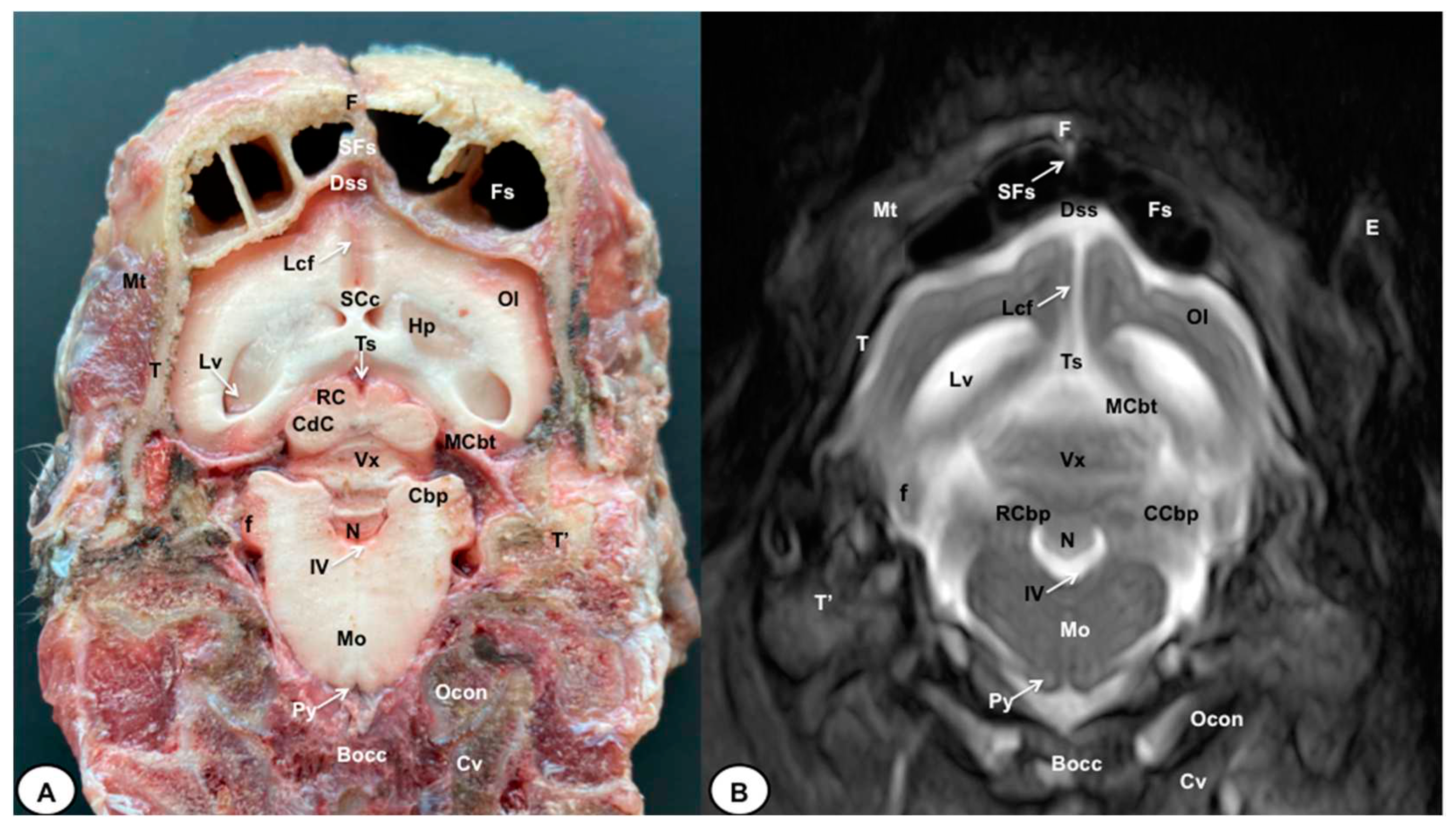
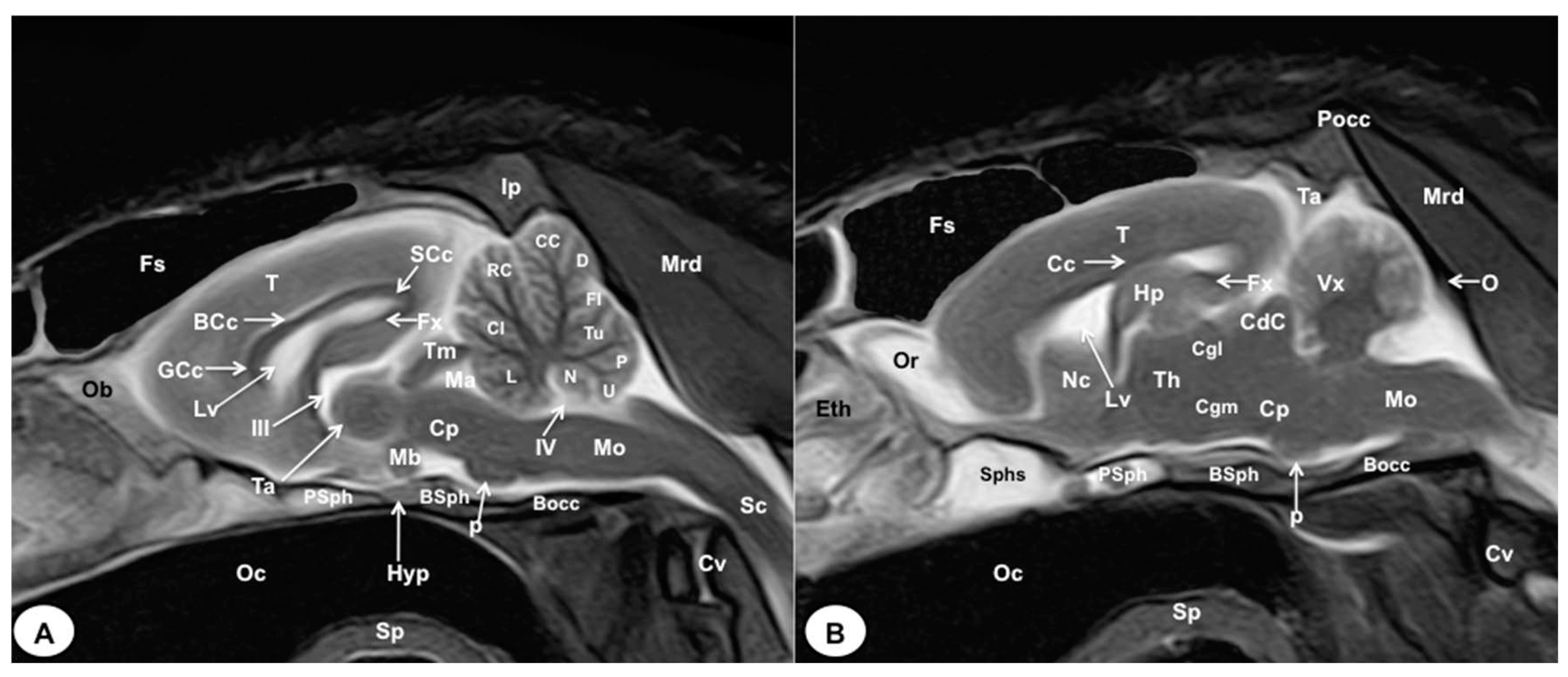
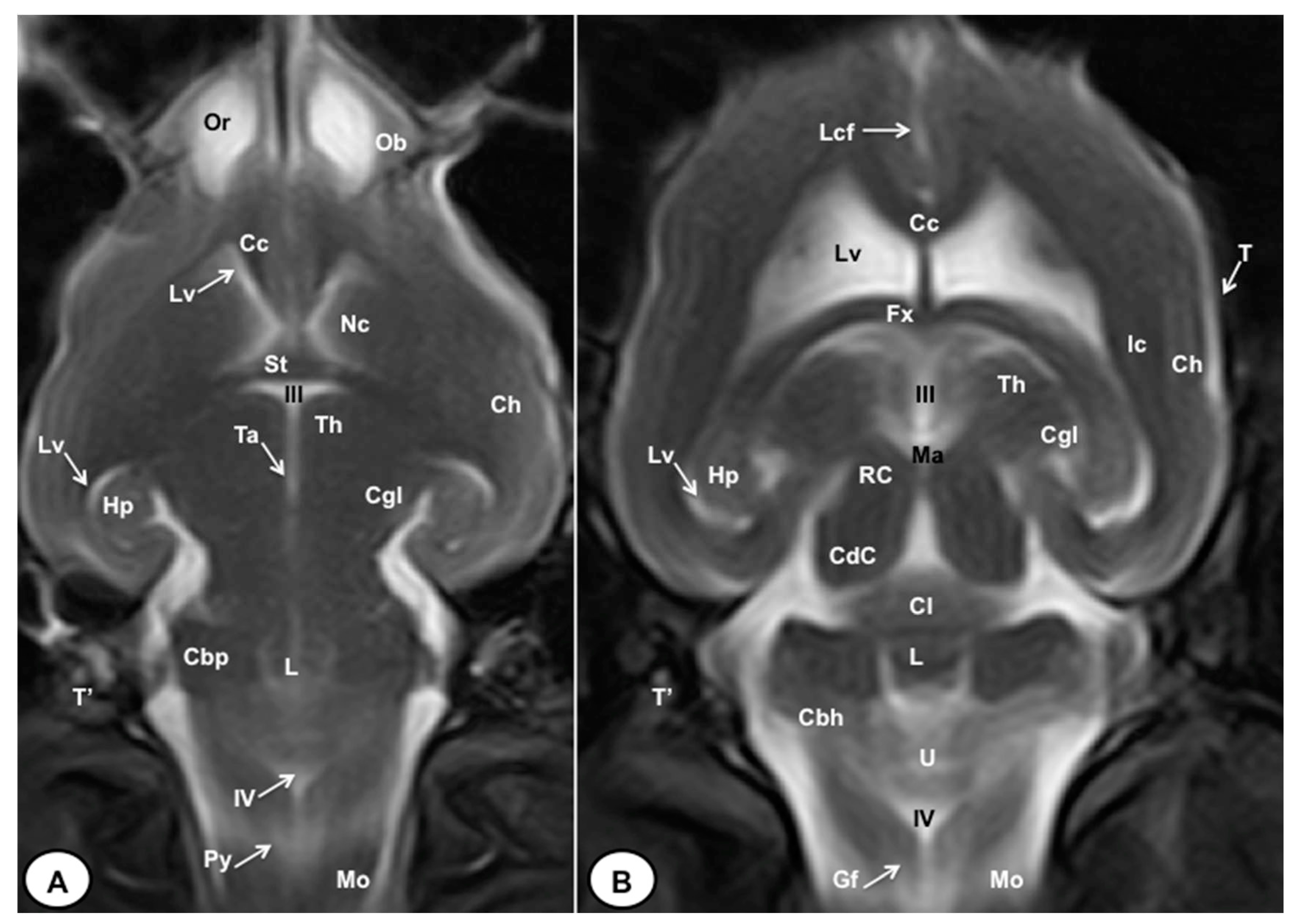
3.1. Anatomical gross-sections
3.2. Magnetic Resonance Imaging (MRI)
4. Discussion
5. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Banzato T, Russo E, Di Toma A, Palmisano G, Zotti A. Anatomic imaging of the Boa constrictor head: A comparison between radiography, computed tomography and cadaver anatomy. Am J Vet Res 2011, 72, 1592–1599. [Google Scholar]
- Van Caelenberg AI, De Rycke LM, Hermans K, et al. Comparison of radiography and CT to identify changes in the skulls of four rabbits with dental disease. J Vet Dent 2011, 28, 172–181. [CrossRef] [PubMed]
- González Rodríguez E, Encinoso Quintana M, Morales Bordon D, Garcés JG, Artiles Nuez H, Jaber JR. Anatomical Description of Rhinoceros Iguana (Cyclura cornuta cornuta) Head by Computed Tomography, Magnetic Resonance Imaging and Gross-Sections. Animals 2023, 13, 955.
- Arencibia A, Corbera JA, Ramírez G, Díaz-Bertrana ML, Pitti L, Morales M, Jaber JR. Anatomical Assessment of the Thorax in the Neonatal Foal Using Computed Tomography Angiography, Sectional Anatomy, and Gross Dissections. Animals 2020, 10, 1045. [Google Scholar] [CrossRef] [PubMed]
- Zafra R, Carrascosa C, Rivero M, Peña S, Fernández T, Suarez-Bonnet A, Jaber JR. Analysis of equine cervical spine using three-dimensional computed tomographic reconstruction. J Appl Anim Res 2012, 40, 108–111. [Google Scholar] [CrossRef]
- Amori G, De Smet K. "Hystrix cristata". IUCN Red List of Threatened Species. 2016, e.T10746A22232484.
- Wilson DE, Reeder DM. eds. "Species Hystrix (Hystrix) cristata". Mammal Species of the World: A Taxonomic and Geographic Reference (3rd ed.) 2005. Johns Hopkins University Press. p. 1543.
- Angelici FM, Colangelo P, Gippoliti S. Out of Europe: Investigating Hystrix cristata (Rodentia: Hystricidae) skull morphometric geographic variability in Africa. Biogeographia 2021, 36, a001. [Google Scholar]
- Storch, G. Porcupines. Pages 300-307 in B. Grzimek, editor. Grzimek1s Encyclopedia of Mammals 1990. McGraw-Hill, New York.
- Capello V, Cauduro A. Comparison of diagnostic consistency and diagnostic accuracy between survey radiography and computed tomography of the skull in 30 rabbits with dental disease. J Exot Pet Med 2016, 25, 115–127. [Google Scholar] [CrossRef]
- Capello, V. Diagnostic Imaging of Dental Disease in Pet Rabbits and Rodents. Vet Clin North Am Exot Anim Pract 2016, 19, 757–782. [Google Scholar]
- Glodek J, Adamiak Z, Przeworski A. Magnetic Resonance Imaging of Reptiles, Rodents, and Lagomorphs for Clinical Diagnosis and Animal Research. Comp Med 2016, 66, 216–219. [Google Scholar]
- Fornazari F, Guimaraes FF, Teixeira CR, Langoni H. Isolation of staphylococcus epidermidis from inflamed upper respiratory tract of an orange-spined hairy dwarf porcupine (Sphiggurus villosus). J Venom Anim Toxins Incl Trop Dis 2012, 18, 455–458. [Google Scholar]
- Van Caelenberg AI, De Rycke LM, Hermans K, Verhaert L, van Bree HJ, Gielen IM. Low-field magnetic resonance imaging and cross-sectional anatomy of the rabbit head. Vet J 2011, 188, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Müllhaupt D, Augsburger H, Schwarz A, Fischer G, Kircher P, Hatt JM, Ohlerth S. Magnetic resonance imaging anatomy of the rabbit brain at 3 T. Acta Vet Scand 2015, 28, 47. [Google Scholar]
- Capello V, Lennox A. Advanced diagnostic imaging and surgical treatment of an odontogenic retromasseteric abscess in a guinea pig. J Small Anim Pract 2015, 56, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Moreno E, Arbat-Plana A, Batalle D, Soria G, Illa M, Prats-Galino A, Eixarch E, Gratacos E. A magnetic resonance image based atlas of the rabbit brain for automatic parcellation. PLoS One 2013, 8, e67418. [Google Scholar]
- Shek JW, Wen GY, Wisniewski HM. Atlas of the rabbit brain and spinal cord. Basel: Karger; 1986.
- Popesko P, Rajtova V, Horak J. Anatomy of small laboratory animals. London: Wolfe Publishing Ltd; 1990; pp. 14–53.
- Gray-Edwards HL, Salibi N, Josephson EM, Hudson JA, Cox NR, Randle AN, McCurdy VJ, Bradbury AM, Wilson DU, Beyers RJ, Denney TS, Martin DR. High resolution MRI anatomy of the cat brain at 3 Tesla. J Neurosci Methods 2014, 227, 10–17. [Google Scholar]
- International Committee on Veterinary Gross Anatomical Nomenclature. Nomina anatomica veterinaria. 5. New York: Ithaca; 2012.
- Arencibia A, Encinoso M, Jaber JR, Morales D, Blanco D, Artiles A, Vázquez JM. Magnetic resonance imaging study in a normal Bengal tiger (Panthera tigris) stifle joint. BMC Vet Res 2015, 11, 192. [Google Scholar]
- Jaber JR, Encinoso M, Morales D, Artiles A, Santana M, Blanco D, Arencibia A. Anatomic study of the normal Bengal tiger (Panthera tigris tigris) brain and associated structures using low field magnetic resonance imaging. Eur J Anat 2016, 20, 195–203. [Google Scholar]
- Arencibia A, Hidalgo M, Vázquez JM, Contreras S, Ramírez G, Oros J. Sectional anatomic and magnetic resonante imaging features of the head of juvenile loggerhead sea turtles (Caretta careta). Am J Vet Res 2012, 73, 1119–1127. [Google Scholar] [CrossRef]
- Valente AL, Cuenca R, Zamora M, Parga M, Lavin S, Alegre F, Marco I. Sectional anatomic and magnetic resonance imaging of coelomic structures of loggerhead sea turtles. Am J Vet Res 2006, 67, 1347–1353. [Google Scholar] [CrossRef]
- Arencibia A, Matos J, Encinoso M, Gil F, Artiles A, Martínez-Gomariz F, Vázquez JM. Computed tomography and magnetic resonance imaging study of a normal tarsal joint in a Bengal tiger (Panthera tigris). BMC Vet Res 2019, 15, 126. [Google Scholar]
- Snow TM, Litster AL, Gregory RJ. Big cat scan: Magnetic resonance imaging of the tiger. Australas Radiol 2004, 48, 93–95. [Google Scholar]
- Encinoso M, Oros J, Ramírez G, Jaber JR, Artiles A, Arencibia A. Anatomic Study of the Elbow Joint in a Bengal Tiger (Panthera tigris tigris) Using Magnetic Resonance Imaging andGross Dissections. Animals 2019, 9, 1058. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Moreno E, Arbat-Plana A, Batalle D, Soria G, Illa M, Prats-Galino A, Eixarch E, Gratacos E. A Magnetic Resonance Image Based Atlas of the Rabbit Brain for Automatic Parcellation.
- Chuang N, Mori S, Yamamoto A, Jiang H, Ye X, et al. An MRI-based atlas and database of the developing mouse brain. NeuroImage 2011, 54, 80–89. [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




