1. Introduction
Sleep-disordered breathing (SDB) defines a heterogeneous group of sleep pathologies encompassing obstructive sleep apnea (OSA), central sleep apnea (CSA) and other rarer sleep-related breathing pathologies.
OSA is a world-wide diffused disorder associated with serious multi-systemic consequences. Conventionally OSA is investigated by means of home-set nocturnal cardio-respiratory recording, and diagnosis is based on the number of respiratory events occurring per hour of sleep, as expressed by the apnea-hypopnea index (AHI). A minimum of 4 recording hours is required to consider the sleep study reliable for diagnosis and, according to the number of respiratory events, the clinical relevance is classified as mild (AHI: 5-14 events/h), moderate (AHI: 15-30 events/h) or severe (AHI > 30 events/h).
As the condition is typically associated with both diurnal and nocturnal consequences, their assessment is also required, according to current diagnostic criteria. In the International Classification of Sleep Disorders (ICSD-3), sleepiness, fatigue, insomnia, snoring, subjective nocturnal respiratory disturbance and observed apneas, associated medical or psychiatric disorders support the diagnosis of OSA.
Notably, the coexistence of other chronic diseases, especially cardiovascular disorders, has been evaluated to assess prognosis, and the novel indicator of C-OSA (comorbid-OSA) has been proposed to ease sleep clinicians in the evaluation of mortality risk in patients with OSA [
1]. In a bidirectional perspective, if the concurrence of other disabling pathologies may worsen prognosis, intermittent hypoxia and sleep-fragmentation induced by OSA can increase the severity of comorbid cardiovascular and metabolic pathologies [
2]. Moreover, C-OSA with cardiovascular diseases may co-exist with central-type respiratory patterns, such as Cheyne-Stokes breathing (CSB). It has been recently proved that the remote monitoring of CPAP devices in OSA patients may reveal early signs of incipient heart failure, such as long cycles of CSB [
3]. The tight association between clinical disorders and sleep apnea, suggest to seek for SDB in all patients.
2. The Limits of AHI
Once sleep apnea has been identified, it is important to measure severity with reliable methods. The predictive value of AHI has been evaluated in a real-world setting, demonstrating a low sensitivity (19%) and a high specificity (84,4%) in the identification of patients affected by mild, moderate or severe OSA [
4]. AHI also fails in the identification of OSA patients at higher risk for diabetes, while the hypoxic burden appears more reliable to predict the metabolic consequences of the disease [
5]. Overall, AHI appears as a poor indicator for disease severity and alternative measures are deemed necessary.
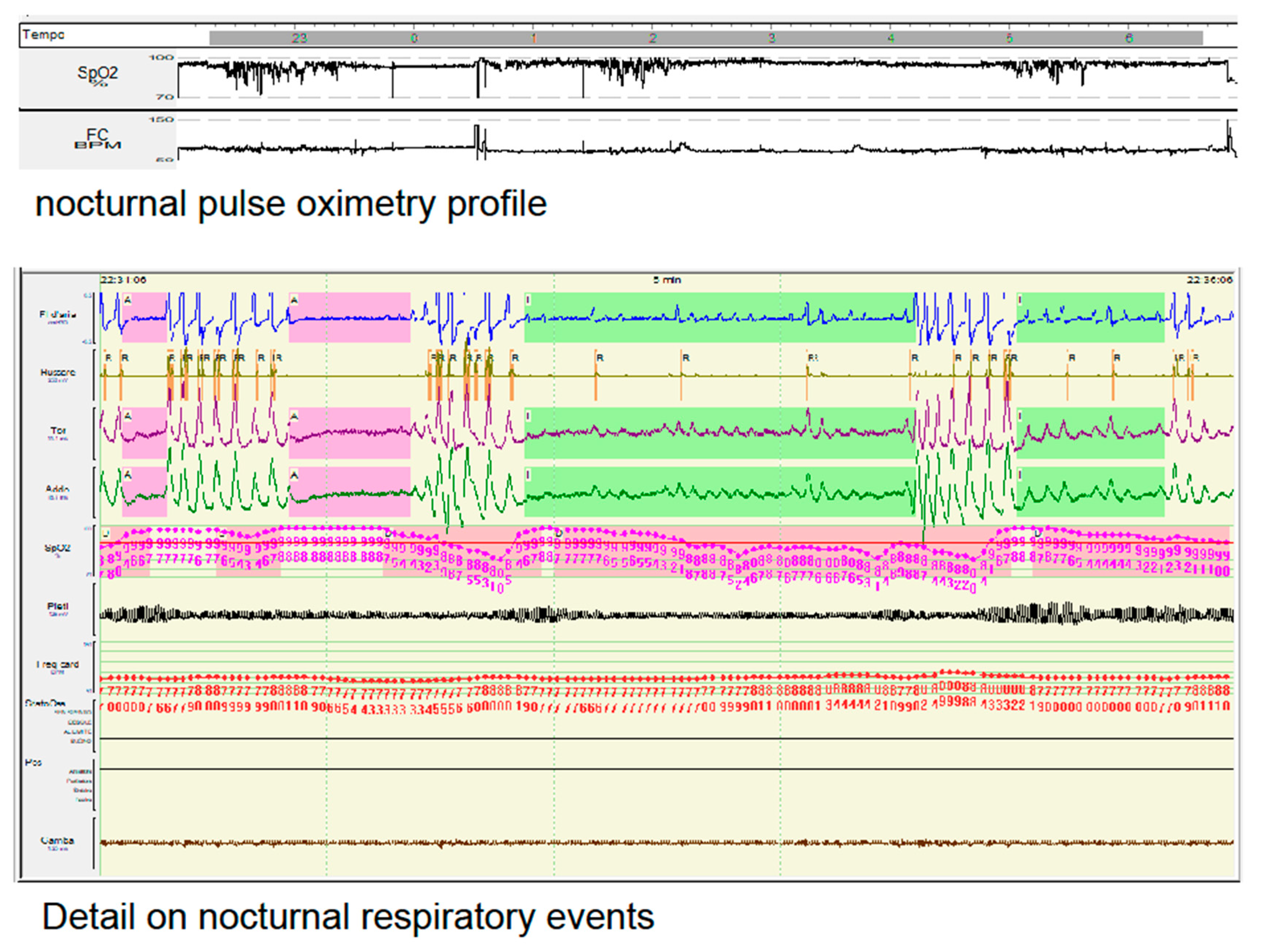
In fig. 1 we summarize the polysomnographic data of a 52 year-old obese Italian woman (body mass index 42), with a personal history of arrhythmia and nocturnal sinus pauses, complaining of snoring and non-refreshing sleep. Her cardio-respiratory recording was consistent with a mild OSA (AHI 12,2 events/h). However looking at the Sat O2 profile and the morphology of her apneas, we could ascertain that the hypoxic load of the patient was compatible with a much more severe clinical condition and thus, regardless of the number of respiratory events measured be the AHI, nocturnal non-invasive ventilation was applied with clinical benefits.
Figure 1.
Example of a nocturnal pulse oximetry profile (upper part of the figure), with a detail on her nocturnal respiratory events (lower part of the figure), in a female patient affected by mild OSA (AHI 12,2 events/h). Despite the lower value of AHI her hypopneas (highlighted in light green) were associated with severe drop of oxygen saturation and respiratory efforts.
Figure 1.
Example of a nocturnal pulse oximetry profile (upper part of the figure), with a detail on her nocturnal respiratory events (lower part of the figure), in a female patient affected by mild OSA (AHI 12,2 events/h). Despite the lower value of AHI her hypopneas (highlighted in light green) were associated with severe drop of oxygen saturation and respiratory efforts.
In the latest years increasing efforts have been dedicated to the identification of non-AHI-dependent measures for OSA severity, the most significant being the Sleep Revolution Project [
6]. Furthermore, current definitions neglect the ‘OSA patients’ pathophysiologic traits’, such as upper airway function, anatomical collapsibility, loop gain, arousal threshold, and sleep structure.
3. OSA and Sleep Architecture
Curiously, SDB occur during sleep, but the recording of brain activity during sleep is not required for diagnosis. This contradiction is due to the high costs and demanding effort to carry out a complete video-polysomnography (PSG) recording, a burden which can be easily by-passed to screen a condition that affects nearly a billion of people world-wide [
7]. However it is well-known that OSA strongly impacts the structural organization of the sleeping brain. Compared to healthy subjects, patients with sleep apnea show reduced amounts of slow-wave sleep (SWS) and increased amounts of lighter sleep stages. Severe OSA also presents a longer latency to stage N3 compared to the mild and moderate subtypes [
8]. Regardless of the OSA severity, the number of respiratory events in stage N3 is significantly lower compared to the other sleep stages [
8]. The lack of respiratory events during SWS probably depends on the higher stability of this sleep stage, which physiologically contains minor amounts of microstructural oscillations [
9]. Human NREM sleep is characterized by periodic oscillatory patterns that reflect its inner adaptability and readiness [
10]. The cyclic alternating pattern (CAP) is the main representative of NREM microstructural dynamics and is deemed as the electroencephalographic (EEG) hallmark of sleep instability [
11].
The restorative power of sleep is influenced by the amount of CAP [
12], expressed by repetitive cycles composed of a phase A (activation) and its subsequent phase B (de-activation), Each phase of the CAP cycle is 2 to 60 s in duration. At least two CAP cycles are required to identify a CAP sequence. The amount of CAP oscillations during NREM sleep is measured by CAP rate.
According to the EEG features, CAP A phases can be further classified as subtypes A1, A2 and A3. In the A1 subtype the EEG synchrony (high-amplitude slow waves) is the predominant activity and, if present, EEG desynchrony (low-amplitude fast waves) occupies less than 20% of the entire phase A duration. Phase A2 subtype is characterized by a mixture of slow and fast rhythms with 20% to 50% of phase A occupied by EEG desynchrony. Finally CAP phases A3 are dominated by rapid low-voltage rhythms with more than 50% of phase A expressed by EEG desynchrony [
13]. The distinction between phase A subtypes is not trivial as they have different roles in sleep organization. Subtypes A1 prevail in the first half of the night and boost the build-up and consolidation of SWS, while subtypes A2 and A3 increase in the second part of the night and prepare the sleeping brain for the appearance of REM sleep or wakefulness. Furthermore, subtypes A3 exert a stronger impact on cardiovascular parameters compared to subtypes A1 and A2 [
14]. Overall, CAP metrics quantify the magnitude of stressful perturbation on the sleeping brain [
15].
CAP also reflects the specific impact of diseases on sleep organization providing additional information compared to the American Academy of Sleep Medicine (AASM) arousal rules in OSA patients, the physiological behavior of sleep instability (involving both respiratory events and EEG activation) overcomes the limited quantification of conventional ‘fast arousals’ [
16].
Accordingly sleep instability, which represent one of the main responsible for excessive daytime sleepiness in OSA patients, cannot be measured only with the count of AASM conventional arousal, but should also include the (commonly neglected) ’subcortical arousals’, which are frequently accompanied by various degree of autonomic stimulation [
17]. In this perspective sleep microstructure may be highly informative. In the clinical domain, CAP subtypes A1 prevail in the milder OSA phenotype, whilst subtypes A2 and A3 abound in patients with moderate-to-severe OSA [
18]. If the CAP system still promotes sleep continuity in milder cases, the situation becomes unbalanced in moderate-severe OSA, where the CAP system turns into an intrusive phenomenon, perturbing sleep dynamics [
18]. The existence of two distinct arousal-related mechanisms in human sleep is similar to what has been described in rodents [
19]. According to this model, depending on the stimulated regions of the parabrachial nucleus (PBN), arousal may yield to either fast frequency bursts, strongly similar to A2/A3 CAP subytpes (when the more lateral regions of the PBN are stimulated) or high-amplitude slow-waves, in response to the medial PBN stimulation [
20]. In this perspective, CAP can phenotype the ‘arousal-gate’ in patients with sleep apnea.
Sleep microstructure can also informe on the neurobehavioral adaptability to sleep fragmentation in OSA patients. In fact it has been suggested that the AHI is inconsistently associated with behavioral performances in patients affected by sleep apnea: while some patients may be highly vulnerable to sleep loss, some other can be impressively resistant to the negative effects of sleep deprivation [
21]. This is not trivial as, for instance, individual resilience to sleep loss might influence driving performance and subsequent risk for car crashes [
21]. While conventional polysomnographic metrics nor AASM arousal index were predictive of neurobehavioral impairment, it has been documented that higher EEG slowing ratio during REM and lower spindle density at the frontal EEG were significantly associated with slower reaction time on psychomotor vigilance task and driving simulator test [
22](Parekh et al., 2019). In parallel it has been demonstrated that, in ventilated OSA patients, the utilization of suboptimal continuous positive airway pressure (CPAP) may favor the recurrence of sustained inspiratory airflow limitation, which in turns is associated with abnormal K-complexes (coupled with bursts of alpha as arousal surrogates). Hence suboptimal CPAP therapy can lead to subtle/‘nonvisible’ sleep fragmentation [
23].
Another interesting measure adopted to indicate sleep continuity is represented by the Odds ratio products (ORP) [
24]. The ORP represent an attempt to capture the transitions within sleep/wakefulness states during clinical polysomnography, in order to overcome the rigid subdivisions imposed by the conventional Rechtschaffen and Kales rules for PSG scoring. ORP correlate with sleep depth and quality, this metric is calculated as a ratio of the absolute power of different frequency bands over 3-s segments. ORP ratio ranges from 0 to 2.5, with 0 indicative of very deep sleep and 2.5 related to wide awake state [
24]. The utilization of ORP might serve as novel biomarkers for PSG in various sleep conditions. In OSA for instance it has been proved that, compare to healthy sleepers, ORP are usually lower during wake (suggesting increased sleep propensity), but higher NREM (consistent with lighter NREM sleep) [
25]. Among the advantages of using ORP there is the possibility to verify the arousal effects’ towards sleep depth, which can be inferred by the speed with which sleep deepens following an arousal, to so-called postarousal sleep dynamics [
26]. When ORP is high, arousal stimuli can easily trigger arousal, whereas when ORP is low strong stimuli are required to provoke an arousal. In other words, the metric of ORP inform us to what extent patients can recover sleep after arousal intrusions [
26].
Although the disorder is typically evaluated using cardio-respiratory recording, some informative features can be inferred on the ongoing brain activity: for instance the pulse wave amplitude has been associated to the amount of EEG response (including CAP features) to respiratory events, suggesting a strong coupling between cortical and autonomic activation [
27].
4. Breathing Oscillations, Daytime Sleepiness and Treatment Outcomes
The temporal association between CAP oscillations and respiratory events is predictable: most apneas/hyponeas in fact co-occur with CAP phase B and thus the disease is frequently defined as a prototypical example of a CAP phase-B disorder [
28]. As a translation of the lesser arousal branch of arousal swings, phase B represents a highly vulnerable background for upper airway collapse and for attenuation of biochemical and neural mechanisms in the control of the ventilatory drive, while the A phase is mandatory to restore breathing. It has been demonstrated that the vast majority of respiratory events are coupled with CAP (96% in NREM and 80% in REM sleep). In details, the apneas and hypopneas are commonly temporally associated with a CAP phase B, while breathing restoration is typically recovered during phase A [
29]. Recently, a large study analyzing over 1.6 million cortical arousals, 350,000 apneas and over 1.9 million hypopneas collected from 11,400 manually scored PSG recordings available online, demonstrated that the duration of 95-99% of all respiratory events falls beneath 60 seconds [
30]. This finding is supported by a solid physiological background as the length of CAP phase B (which triggers and supports breathing interruption) spans within the 1 min interval [
12].
CAP metrics correlate with subjectively perceived daytime sleepiness in patients with OSA [
31]. In male subjects with moderate to severe OSA, CAP rate, CAP duration, the number of CAP cycles, and the duration and percentage of the subtypes A2 are significantly higher in patients with excessive sleepiness (ES) compared to those non-ES. In contrast, the two groups (ES vs non-ES) showed no differences in conventional sleep parameters. In other words, higher levels of arousal activation induced by respiratory events determine a more aggressive sleep fragmentation which leads to a more somnolent daytime setting.
PSG can also be used to assess treatment benefits on patients with severe OSA. It has been proved that continuous positive airway pressure (CPAP), the first-line treatment for moderate-to-severe OSA, increases the duration of N3 and decreases the lightest NREM sleep stages. With respect to microstructural PSG parameters, the reduction of respiratory events promotes an attenuation of CAP fluctuations, in particular, during stage N3, and a parallel increase of the CAP oscillations that occur independent from apneas/hypopneas [
32]. In this perspective we can summarize that CPAP promotes the restoration of a more physiological CAP oscillatory pattern, with oscillations that are disengaged from breathing constraints. Interestingly, the timing of CPAP-dependent PSG variations is not the same for all sleep features: for instance CPAP treatment induces an immediate restoration of sleep continuity, a more consolidated REM sleep, a curtailment of sleep latency and an enhancement of SWS, while the amount of CAP cycles and A1 subtypes remain below normal values even one month after the introduction of ventilatory support [
33](Fig.2). An impressive increase in the SWS occurs already during the first night of CPAP, together with a parallel normalization in the overall duration of CAP phases A and B, both significantly longer in patients with severe OSA [
34]. Furthermore, in patients with severe OSA treated with CPAP, variations of CAP rate significantly correlate with daytime vigilance [
34].
Figure 2.
Polysomnographic example of a patient affected by severe OSA before (panel A) and after (panel B) the introduction of CPAP. Panel A: note the tight association between apneas and cyclic alternating pattern (CAP) oscillations, with strong prevalence of subtypes A2 and A3, with high autonomic impact; panel B: observe the restoration of physiologic CAP oscillations, with predominance of subtypes A1.
Figure 2.
Polysomnographic example of a patient affected by severe OSA before (panel A) and after (panel B) the introduction of CPAP. Panel A: note the tight association between apneas and cyclic alternating pattern (CAP) oscillations, with strong prevalence of subtypes A2 and A3, with high autonomic impact; panel B: observe the restoration of physiologic CAP oscillations, with predominance of subtypes A1.
Sleep recordings may also provide information on the impairment of cognitive processes, a detrimental consequence in patients affected by untreated OSA [
35,
36]. The extent of cognitive impairment, assessed with Montreal Cognitive Assessment (MoCA), has been found to be related to the severity of sleep fragmentation as expressed by the CAP A3 rate in patients with sleep apnea [
37]. A similar association between CAP metrics and cognitive deterioration has been described by Karimzadeh et al. [
38], who demonstrated a direct correlation between the quantity of CAP phase A1 fluctuations and cognitive performances, especially in the verbal fluency, memory and visuo-spatial skills, in patients affected by OSA. Patients suffering from sleep apnea presented higher density of CAP phase A oscillations within the fronto-parietal regions, a conditions that may interfere with NREM sleep-dependent cognitive processes. Recently, impaired social cognition has also been identified in middle-age patients affected by OSA with no other relevant neurological or psychiatric conditions, potentially associated to the pathology-dependent impairment of REM sleep stage [
35].
The application of CAP scoring can support the phenotyping of various diseases as demonstrated for Down syndrome, Fragile-X syndrome and attention deficit hyperactivity disorder, where the evaluation of sleep microstructure can help in the identification of patients suffering between ‘primary disease forms’ (lower amounts of CAP oscillations) from those with coexistent sleep-related breathing disorders (higher CAP rate values) [
39,
40]. In the latest years, the European Sleep Apnea Database (ESADA) has tried to detail OSA heterogeneity using clustering procedures. The OSA clusters identified from the ESADA consortium are based on gender, age, symptoms, comorbidities and respiratory features, but so far no conventional PSG measures have been included. The possibility to identify OSA phenotypes might improve patients’ prognostication and ease the identification of targeted therapies. From an operational point of view ‘phenotypes’ can be described as:
“A category of patients with OSA distinguished from others by a single or combination of disease features, in relation to clinically meaningful attributes (symptoms, response to therapy, health outcomes, quality of life’’. The possibility to reveal through phenotypes individual differences is fundamental to tailor treatment prescription and to increase long-term compliance [
41]. For instance, it has been demonstrated that patients with REM-dominant OSA present reduced total sleep time, decreased sleep efficiency and lack of REM sleep representation [
42]. REM-dominant OSA patients also present reduced response to mandibular advancement splints [
43] and, due to the association between REM-related AHI indices and incident hypertension, they likely deserve non-invasive ventilation, which should be guaranteed in the second half of the night, when REM sleep is largely prevalent [
44,
45]. Conversely, patients with NREM-dominant OSA present higher ventilator control instability during NREM sleep (which paradoxically improves during REM sleep), a condition that may co-exist with low arousal threshold and which might benefit from pharmacological –sleep-stabilizing—approaches [
46,
47,
48].
In this perspective, the utilization of polysomnographic features (with combined macro and microstructure analysis) may reinforce the reliability of any attempt of OSA phenotypization, leading to strongly predictive approaches, with potential clinical implications.
5. The Contribution of Machine Learning
In order to curtail a timely OSA diagnosis, a number of portable/wearable hardware devices, based on machine learning approaches, have been proposed to screen for this condition. Their lower costs and the ease of use compared to conventional polysomnography stand out among the major advantages. However the absence of a medical supervision may expose patients to the risk of less accurate diagnosis. Most of these devices work on photoplethysmographic (PPG) sensors to detect oxygen desaurations. In this case the information regarding breathing events is indirectly collected from blood flow patterns in the microvascular tissue bed [
52]. Although still insufficient to ascertain diagnosis, the utilization of wearable devices has gained increasing importance in the screening of subjects with a high-risk for OSA [
53].Artificial intelligence can be adopted to evaluate polysomnographic findings in order to screen for sleep apnea. The automated classification of polysomnographic features (including macro and microstructure data) has already been tested for various sleep pathologies including narcolepsy, rapid eye movement behaviour disorder, periodic leg movement disorder, sleep-related epilepsy and insomnia [
54,
55]. Considering the strong impact of OSA on the sleep organization, the availability of automated methods to score sleep recordings might ease the identification of clusters of electrophysiological data, potentially related to distinct clinical phenotypes. In this perspective machine learning could represent a relevant step forward in the intricate scenario of sleep apnea diagnosis and phenotypization. So far the most important applications of artificial intelligence in obstructive sleep apnea have been: 1) predicting treatment outcomes of various treatment options, 2) improving treatment options, and 3) personalizing treatment thanks to the enhanced understanding of underlying mechanisms of the disease [
56]. This new ‘personalized’ approach to sleep apnea may increase patients’ compliance and increase the efficacy of proposed approaches. The utility of machine learning has been been also suggested to select patients addressed to CPAP therapy, surgical treatment and/or oral appliances. The possibility to predict therapeutic success with non-invasive techniques represents an ambitious goal for sleep specialists.Due to unavoidable links between autonomic system and electroencephalographic fin-dings, few authors explored the bidirectional association between cardiopulmonary functioning and EEG signal. Simplifying all the NREM sleep stages into two opposite dimensions, i.e., stable versus unstable NREM sleep, it has been proved that stable breathing periods result in high frequency coupling of respiration and HRV, with simultaneous presence of higher EEG delta power. In parallel, the so-called unstable NREM sleep may be recognized by a sleep fragmentation phenotype [
57]. Sleep apneas are strongly associated to cardiovascular consequences and autonomic imbalance. The periodic alterations in sympathetic nervous activity and parasympathetic nerve activity during each respiratory event define a peculiar heart rate pattern, which seems like a signature of the condition. Typically we can notice a drop in heart rate, which is a physiological reaction to the apnea (known as the “diving reflex”), immediately followed by a phasic acceleration in heart rate (the relative tachycardia), restoring blood gas exchange in the lung. This alternation can also be schematized in terms of periodic shifts between parasympathetic and sympathetic tone dominance, Given the periodic reproducibility of these cardiological variations, mathematical approaches and machine learning techniques have been adopted to asses their dynamics in sleep apnea. However, due to inter and intra-individual variability of cardiovascular activity related to physical training conditions, age, weight, and concomitant diseases, the utilization of the sole cardiac signal is still largely insufficient to represent OSA severity [
57,
58].
6. Conclusion
Although international guidelines promote the ‘one-size-fits-all’ strategy for OSA management, it is important to remember that the disease may present important inter-individual differences, reasonably warranting a more tailored approach. In this framework, video-PSG and sleep microstructure could provide additional information in the clinical management of patients with SDB. In addition, CAP-related arousal instability seems to be one of the key mechanisms explaining cognitive decline and vigilance impairment in sleep apnea [
17]. The cost and complexity of CAP scoring can be overcome by the availability of reliable automatic scoring systems [
59], which will help both researchers and clinicians to better define the impact of SDB on the sleep texture as well as neurological and extra-neurological consequences. The vertical integration of combined approaches might ease our understanding of this complex sleep pathology.
Author Contributions
LP and CM; Writing: CM and LP—Original Draft Preparation, CM, IZ, LP; Writing—Review & Editing, all the authors. Conceptualization: LP. and CM; methodology, CM; data curation, LP, CM.; writing—original draft preparation, CM, LP; writing—review and editing,CM, LP, FR, IP, MS; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the absence of newly collected clinical data (review article).
Informed Consent Statement
Not applicable (review article).
Data Availability Statement
not applicable (review article, no new data had been collected).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chiang CL, Chen YT, Wang KL, Su VY, Wu LA, Perng DW, Chang SC, Chen YM, Chen TJ, Chou KT. Comorbidities and risk of mortality in patients with sleep apnea. Ann Med. 2017 Aug;49(5):377-383. [CrossRef] [PubMed]
- Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest 2015, 147, 266–274. [CrossRef]
- Saito K, Takamatsu Y. Cheyne-Stokes Breathing as a Predictive Indicator of Heart Failure in Patients With Obstructive Sleep Apnea; A Retrospective Case Control Study Using Continuous Positive Airway Pressure Remote Monitoring Data. Front Cardiovasc Med. 2022 Feb 7;9:790331. [CrossRef]
- Silva GE, Rojo-Wissar DM, Quan SF, Haynes PL. Predictive ability of the International Classification of Sleep Disorders-3 in identifying risk of obstructive sleep apnea among recently unemployed adults. Sleep Breath. 2021 Sep;25(3):1325-1334. [CrossRef]
- Wojeck BS, Inzucchi SE, Qin L, Yaggi HK. Polysomnographic predictors of incident diabetes and pre-diabetes: an analysis of the DREAM study. J Clin Sleep Med. 2023 Apr 1;19(4):703-710. [CrossRef]
- Arnardottir ES, Islind AS, Óskarsdóttir M, Ólafsdóttir KA, August E, Jónasdóttir L, Hrubos-Strøm H, Saavedra JM, Grote L, Hedner J, Höskuldsson S, Ágústsson JS, Jóhannsdóttir KR, McNicholas WT, Pevernagie D, Sund R, Töyräs J, Leppänen T; Sleep Revolution. The Sleep Revolution project: the concept and objectives. J Sleep Res. 2022 Aug;31(4):e13630. [CrossRef]
- Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pépin JL, Peppard PE, Sinha S, Tufik S, Valentine K, Malhotra A. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019 Aug;7(8):687-698. [CrossRef]
- Wu B, Cai J, Yao Y, Pan Y, Pan L, Zhang L, Sun Y. [Relationship between sleep architecture and severity of obstructive sleep apnea]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020 Aug 25;49(4):455-461. Chinese. [CrossRef]
- Terzano MG, Parrino L, Spaggiari MC. The cyclic alternating pattern sequences in the dynamic organization of sleep. Electroencephalogr Clin Neurophysiol. 1988 May;69(5):437-47. [CrossRef] [PubMed]
- Halász P, Bódizs R, Parrino L, Terzano M. Two features of sleep slow waves: homeostatic and reactive aspects--from long term to instant sleep homeostasis. Sleep Med. 2014 Oct;15(10):1184-95. [CrossRef] [PubMed]
- Parrino L, Ferri R, Bruni O, Terzano MG. Cyclic alternating pattern (CAP): the marker of sleep instability. Sleep Med Rev. 2012 Feb;16(1):27-45. [CrossRef] [PubMed]
- Parrino L, Grassi A, Milioli G. Cyclic alternating pattern in polysomnography: what is it and what does it mean? Curr Opin Pulm Med. 2014 Nov;20(6):533-41. [CrossRef] [PubMed]
- Terzano MG, Parrino L, Sherieri A, Chervin R, Chokroverty S, Guilleminault C, Hirshkowitz M, Mahowald M, Moldofsky H, Rosa A, Thomas R, Walters A. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med. 2001 Nov;2(6):537-53; Erratum in: Sleep Med. 2002 Mar;3(2):185. [CrossRef] [PubMed]
- Hartmann S, Ferri R, Bruni O, Baumert M. Causality of cortical and cardiovascular activity during cyclic alternating pattern in non-rapid eye movement sleep. Philos Trans A Math Phys Eng Sci. 2021 Dec 13;379(2212):20200248. [CrossRef] [PubMed]
- Mutti C, Azzi N, Halasz P, Szucs A, Parrino L. Intra period CAP kinetics to stressful perturbation: a message from obstructive sleep apnea. Sleep Med. 2021 Apr;80:226-227. [CrossRef] [PubMed]
- Milioli G, Bosi M, Grassi A, Riccardi S, Terzano MG, Cortelli P, Poletti V, Parrino L. Can sleep microstructure improve diagnosis of OSAS? Integrative information from CAP parameters. Arch Ital Biol. 2015 Jun-Sep;153(2-3):194-203. [CrossRef] [PubMed]
- Punjabi NM, Lim D. Reply to Dr. Gold's commentary con: Sleep fragmentation causes hypersomnolence in OSA. Sleep Med Rev. 2021 Feb;55:101398. [CrossRef] [PubMed]
- Gnoni V, Drakatos P, Higgins S, Duncan I, Wasserman D, Kabiljo R, Mutti C, Halasz P, Goadsby PJ, Leschziner GD, Rosenzweig I. Cyclic alternating pattern in obstructive sleep apnea: A preliminary study. J Sleep Res. 2021 Dec;30(6):e13350. [CrossRef]
- Anaclet, C., Ferrari, L., Arrigoni, E., Bass, C. E., Saper, C. B., Lu, J., & Fuller, P. M. (2014). The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nature Neuroscience, /: https, 1224.
- Kaur, S.; Saper, C.B. Neural circuitry underlying waking up to hypercapnia. Front Neurosci 2019, 13, 401. [Google Scholar] [CrossRef] [PubMed]
- Vakulin A, Catcheside PG, Baulk SD, Antic NA, Banks S, Dorrian J, McEvoy RD. Individual variability and predictors of driving simulator impairment in patients with obstructive sleep apnea. J Clin Sleep Med. 2014 Jun 15;10(6):647-55. [CrossRef]
- Parekh A, Mullins AE, Kam K, Varga AW, Rapoport DM, Ayappa I. Slow-wave activity surrounding stage N2 K-complexes and daytime function measured by psychomotor vigilance test in obstructive sleep apnea. Sleep. 2019 Mar 1;42(3):zsy256. [CrossRef]
- Parekh A, Kam K, Mullins AE, Castillo B, Berkalieva A, Mazumdar M, Varga AW, Eckert DJ, Rapoport DM, Ayappa I. Altered K-complex morphology during sustained inspiratory airflow limitation is associated with next-day lapses in vigilance in obstructive sleep apnea. Sleep. 2021 Jul 9;44(7):zsab010. [CrossRef]
- Younes M, Ostrowski M, Soiferman M, Younes H, Younes M, Raneri J, Hanly P. Odds ratio product of sleep EEG as a continuous measure of sleep state. Sleep. 2015 Apr 1;38(4):641-54. [CrossRef]
- Younes M, Azarbarzin A, Reid M, Mazzotti DR, Redline S. Characteristics and reproducibility of novel sleep EEG biomarkers and their variation with sleep apnea and insomnia in a large community-based cohort. Sleep. 2021 Oct 11;44(10):zsab145. [CrossRef]
- Younes M, Hanly PJ. Immediate postarousal sleep dynamics: an important determinant of sleep stability in obstructive sleep apnea. J Appl Physiol (1985). 2016 Apr 1;120(7):801-8. [CrossRef] [PubMed]
- Bosi M, Milioli G, Riccardi S, Melpignano A, Vaudano AE, Cortelli P, Poletti V, Parrino L. Arousal responses to respiratory events during sleep: the role of pulse wave amplitude. J Sleep Res. 2018 Apr;27(2):259-267. [CrossRef] [PubMed]
- Parrino L, Rausa F, Azzi N, Pollara I, Mutti C. Cyclic alternating patterns and arousals: what is relevant in obstructive sleep apnea? In Memoriam Mario Giovanni Terzano. Curr Opin Pulm Med. 2021 Nov 1;27(6):496-504. [CrossRef] [PubMed]
- Terzano MG, Parrino L, Boselli M, Spaggiari MC, Di Giovanni G. Polysomnographic analysis of arousal responses in obstructive sleep apnea syndrome by means of the cyclic alternating pattern. J Clin Neurophysiol. 1996 Mar;13(2):145-55. [CrossRef] [PubMed]
- Zitting KM, Lockyer BJ, Azarbarzin A, Sands SA, Wang W, Wellman A, Quan SF. Association of cortical arousals with sleep-disordered breathing events. J Clin Sleep Med. 2023 ;19(5):899-912. 1 May. [CrossRef]
- Korkmaz S, Bilecenoglu NT, Aksu M, Yoldas TK. Cyclic Alternating Pattern in Obstructive Sleep Apnea Patients with versus without Excessive Sleepiness. Sleep Disord. 2018 ;2018:8713409. 16 May. [CrossRef]
- Chen S, Li Q, Zou X, Zhong Z, Ouyang Q, Wang M, Luo Y, Yao D. Effects of CPAP Treatment on Electroencephalographic Activity in Patients with Obstructive Sleep Apnea Syndrome During Deep Sleep with Consideration of Cyclic Alternating Pattern. Nat Sci Sleep. 2022 Nov 21;14:2075-2089. [CrossRef]
- Parrino L, Thomas RJ, Smerieri A, Spaggiari MC, Del Felice A, Terzano MG. Reorganization of sleep patterns in severe OSAS under prolonged CPAP treatment. Clin Neurophysiol. 2005 Sep;116(9):2228-39. [CrossRef] [PubMed]
- Parrino L, Smerieri A, Boselli M, Spaggiari MC, Terzano MG. Sleep reactivity during acute nasal CPAP in obstructive sleep apnea syndrome. Neurology. 2000 Apr 25;54(8):1633-40. [CrossRef] [PubMed]
- 35. Gnoni V, Mesquita M, O’Regan D, Delogu A, Chakalov I, Antal A, Young AH, Bucks RS, Jackson ML and Rosenzweig I (2023) Distinct cognitive changes in male patients with obstructive sleep apnoea without co-morbidities. Front. Sleep. [CrossRef]
- Gu Y, Gagnon JF, Kaminska M. Sleep electroencephalography biomarkers of cognition in obstructive sleep apnea. J Sleep Res. 2023 Mar 20:e13831. [CrossRef] [PubMed]
- Li N, Wang J, Wang D, Wang Q, Han F, Jyothi K, Chen R. Correlation of sleep microstructure with daytime sleepiness and cognitive function in young and middle-aged adults with obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2019 Dec;276(12):3525-3532. [CrossRef] [PubMed]
- Karimzadeh F, Nami M, Boostani R. Sleep microstructure dynamics and neurocognitive performance in obstructive sleep apnea syndrome patients. J Integr Neurosci. 2017;16(2):127-142. [CrossRef] [PubMed]
- Miano S, Castelnovo A, Bruni O, Manconi M. Sleep microstructure in attention deficit hyperactivity disorder according to the underlying sleep phenotypes. J Sleep Res. 2022 Feb;31(1):e13426. Erratum in: J Sleep Res. 2023 Apr;32(2):e13780. [CrossRef]
- Miano S, Bruni O, Elia M, Scifo L, Smerieri A, Trovato A, Verrillo E, Terzano MG, Ferri R. Sleep phenotypes of intellectual disability: a polysomnographic evaluation in subjects with Down syndrome and Fragile-X syndrome. Clin Neurophysiol. 2008 Jun;119(6):1242-7. [CrossRef] [PubMed]
- Bailly S, Grote L, Hedner J, Schiza S, McNicholas WT, Basoglu OK, Lombardi C, Dogas Z, Roisman G, Pataka A, Bonsignore MR, Pepin JL; ESADA Study Group. Clusters of sleep apnoea phenotypes: A large pan-European study from the European Sleep Apnoea Database (ESADA). Respirology. 2021 Apr;26(4):378-387. [CrossRef] [PubMed]
- Zinchuk, A. V. , Gentry, M. J., Concato, J., & Yaggi, H. K. (2017). Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches. Sleep medicine reviews. [CrossRef]
- Sutherland K, Takaya H, Qian J, Petocz P, Ng AT, Cistulli PA. Oral Appliance Treatment Response and Polysomnographic Phenotypes of Obstructive Sleep Apnea. J Clin Sleep Med. 2015 Aug 15;11(8):861-8. [CrossRef]
- Appleton, S. L. , Vakulin, A., Martin, S. A., Lang, C. J., Wittert, G. A., Taylor, A. W., McEvoy, R. D., Antic, N. A., Catcheside, P. G., & Adams, R. J. (2016). Hypertension Is Associated With Undiagnosed OSA During Rapid Eye Movement Sleep. Chest, 150(3), 495–505. [CrossRef]
- Aurora RN, Crainiceanu C, Gottlieb DJ, Kim JS, Punjabi NM. Obstructive Sleep Apnea during REM Sleep and Cardiovascular Disease. Am J Respir Crit Care Med. 2018 Mar 1;197(5):653-660. [CrossRef]
- Joosten, S. A. , Landry, S. A., Wong, A. M., Mann, D. L., Terrill, P. I., Sands, S. A., Turton, A., Beatty, C., Thomson, L., Hamilton, G. S., & Edwards, B. A. (2021). Assessing the Physiologic Endotypes Responsible for REM- and NREM-Based OSA. Chest, 2007. [Google Scholar] [CrossRef]
- Thomas RJ, Terzano MG, Parrino L, Weiss JW. Obstructive sleep-disordered breathing with a dominant cyclic alternating pattern--a recognizable polysomnographic variant with practical clinical implications. Sleep. 2004 Mar 15;27(2):229-34. [CrossRef]
- Smales, E. T. , Edwards, B. A., Deyoung, P. N., McSharry, D. G., Wellman, A., Velasquez, A., Owens, R., Orr, J. E., & Malhotra, A. (2015). Trazodone Effects on Obstructive Sleep Apnea and Non-REM Arousal Threshold. Annals of the American Thoracic Society, 12(5), 758–764. [CrossRef]
- Bozkurt S, Bostanci A, Turhan M. Can Statistical Machine Learning Algorithms Help for Classification of Obstructive Sleep Apnea Severity to Optimal Utilization of Polysomnography Resources? Methods Inf Med. 2017 Aug 11;56(4):308-318. [CrossRef] [PubMed]
- Tsai CY, Liu WT, Hsu WH, Majumdar A, Stettler M, Lee KY, Cheng WH, Wu D, Lee HC, Kuan YC, Wu CJ, Lin YC, Ho SC. Screening the risk of obstructive sleep apnea by utilizing supervised learning techniques based on anthropometric features and snoring events. Digit Health. 2023 Mar 6;9:20552076231152751. [CrossRef]
- Ferreira-Santos D, Amorim P, Silva Martins T, Monteiro-Soares M, Pereira Rodrigues P. Enabling Early Obstructive Sleep Apnea Diagnosis With Machine Learning: Systematic Review. J Med Internet Res. 2022 Sep 30;24(9):e39452. [CrossRef]
- Puranik, S. , Morales A.W. Heart rate estimation of PPG signals with simultaneous accelerometry using adaptive neural network filtering. IEEE Trans. Consum. Electron. [CrossRef]
- Khor YH, Khung SW, Ruehland WR, Jiao Y, Lew J, Munsif M, Ng Y, Ridgers A, Schulte M, Seow D, Soon W, Churchward T, Howard ME. Portable evaluation of obstructive sleep apnea in adults: A systematic review. Sleep Med Rev. 2023 Apr;68:101743. [CrossRef] [PubMed]
- Murarka S, Wadichar A, Bhurane A, Sharma M, Acharya UR. Automated classification of cyclic alternating pattern sleep phases in healthy and sleep-disordered subjects using convolutional neural network. Comput Biol Med. 2022 Jul;146:105594. [CrossRef] [PubMed]
- Sharma M, Patel V, Tiwari J, Acharya UR. Automated Characterization of Cyclic Alternating Pattern Using Wavelet-Based Features and Ensemble Learning Techniques with EEG Signals. Diagnostics (Basel). 2021 Jul 30;11(8):1380. [CrossRef]
- Brennan HL, Kirby SD. The role of artificial intelligence in the treatment of obstructive sleep apnea. J Otolaryngol Head Neck Surg. 2023 Feb 7;52(1):7. [CrossRef]
- Parrino L, Halasz P, Szucs A, Thomas RJ, Azzi N, Rausa F, Pizzarotti S, Zilioli A, Misirocchi F, Mutti C. Sleep medicine: Practice, challenges and new frontiers. Front Neurol. 2022 Oct 14;13:966659. [CrossRef]
- Penzel T, Kantelhardt JW, Bartsch RP, Riedl M, Kraemer JF, Wessel N, Garcia C, Glos M, Fietze I, Schöbel C. Modulations of Heart Rate, ECG, and Cardio-Respiratory Coupling Observed in Polysomnography. Front Physiol. 2016 Oct 25;7:460. [CrossRef]
- Parrino, L. Now that automatic processing makes CAP scoring fast and reliable is the sleep field ready for a paradigm shift? Sleep. 2023 Jan 11;46(1):zsac255. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).