Submitted:
28 May 2023
Posted:
31 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
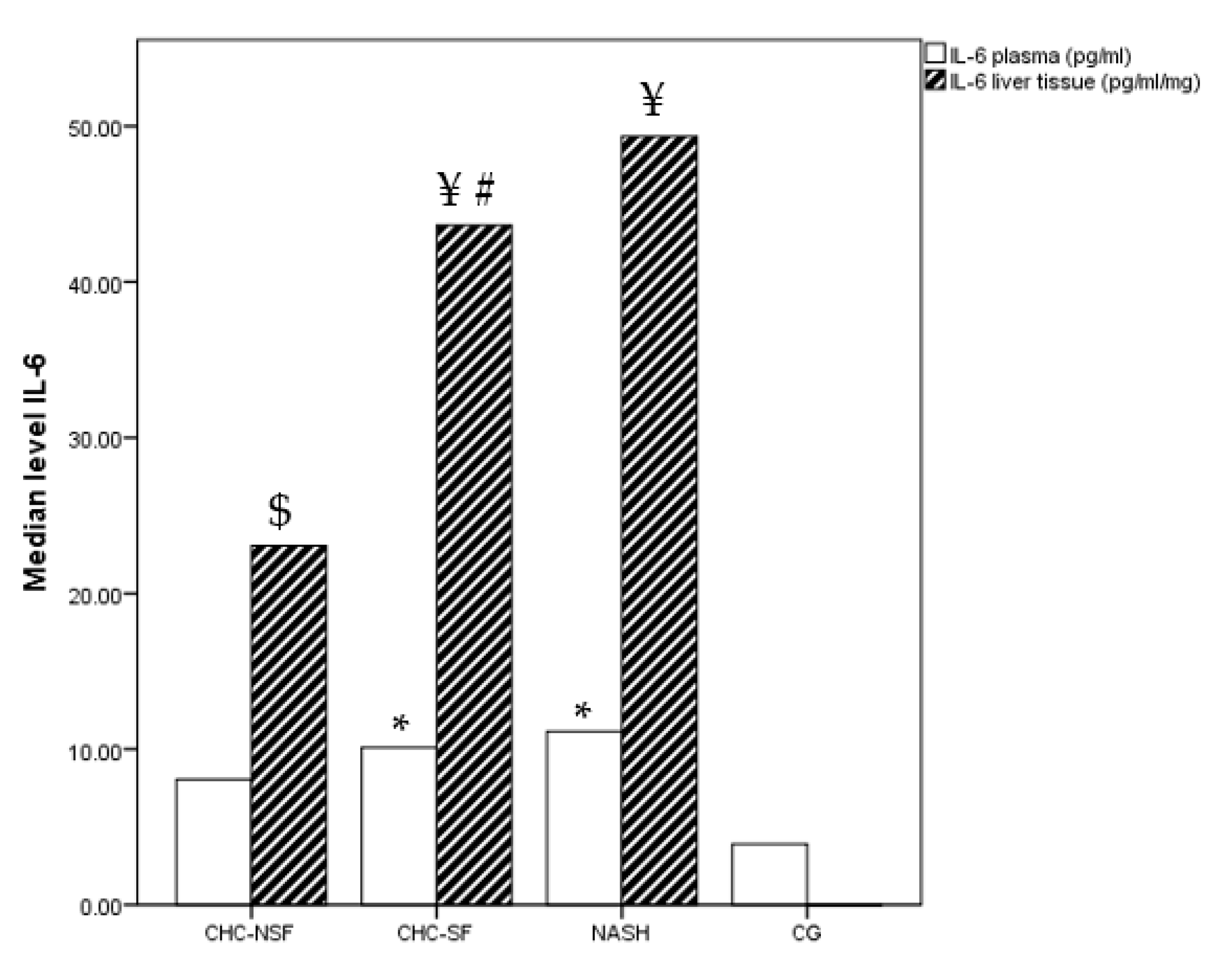
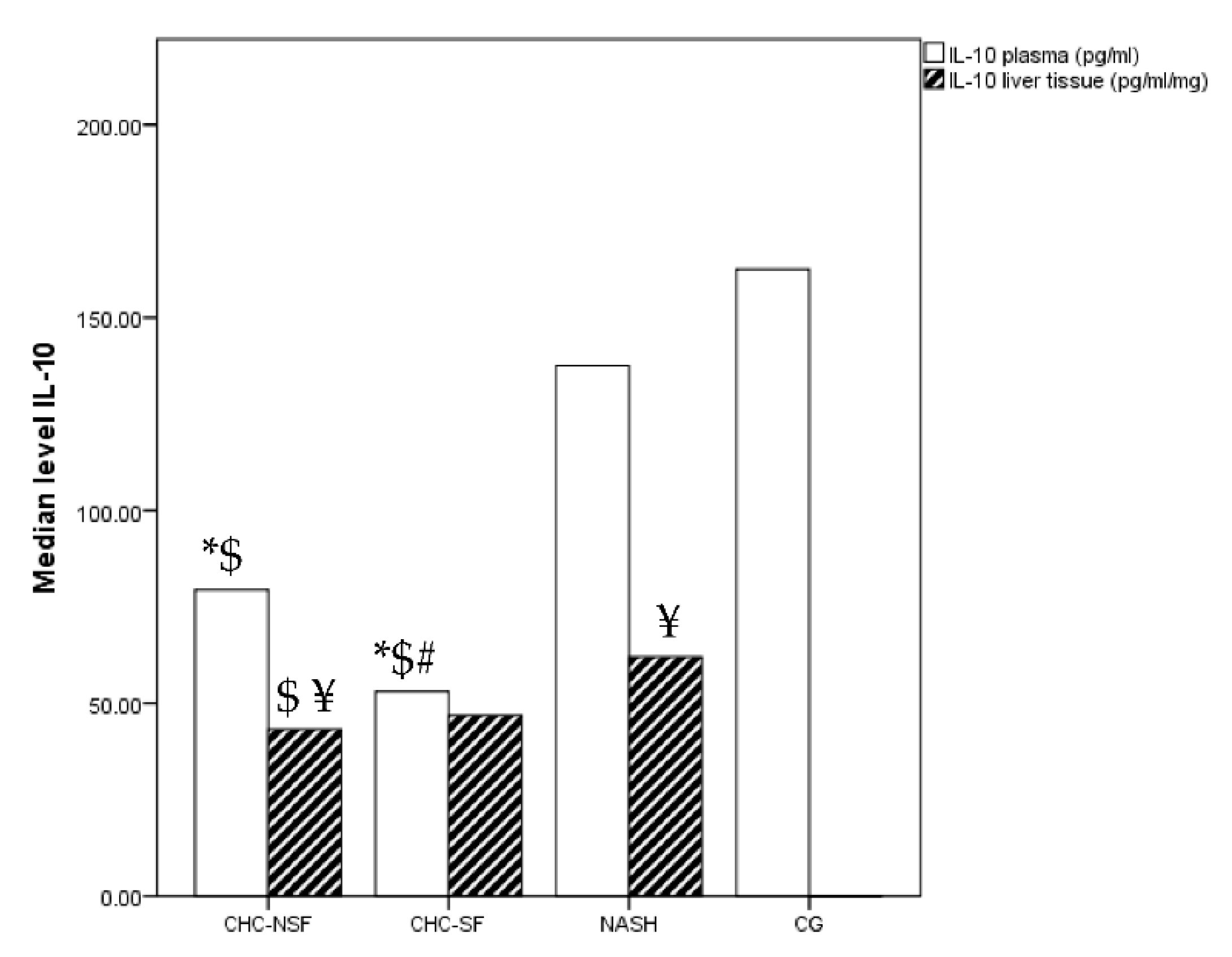
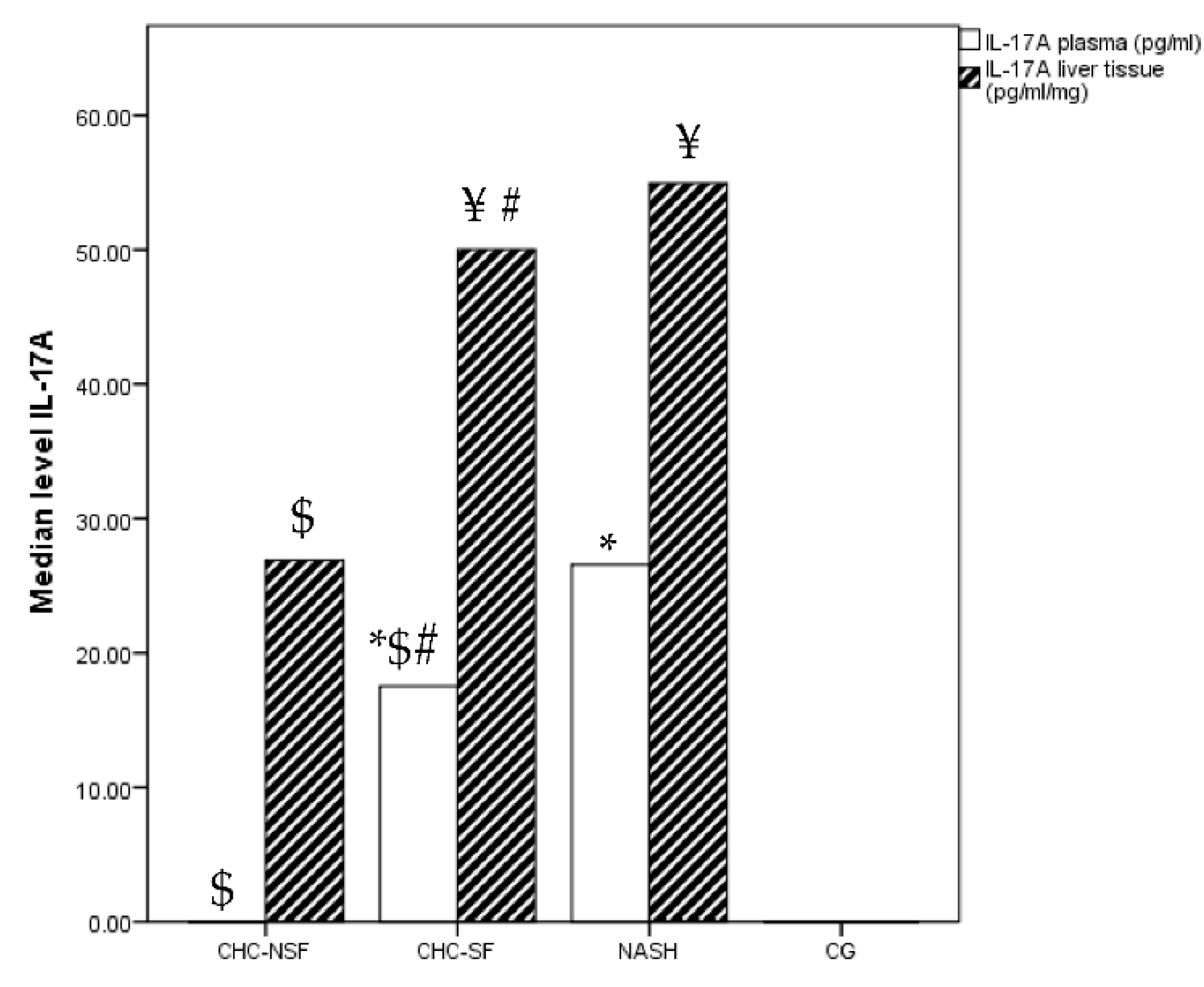
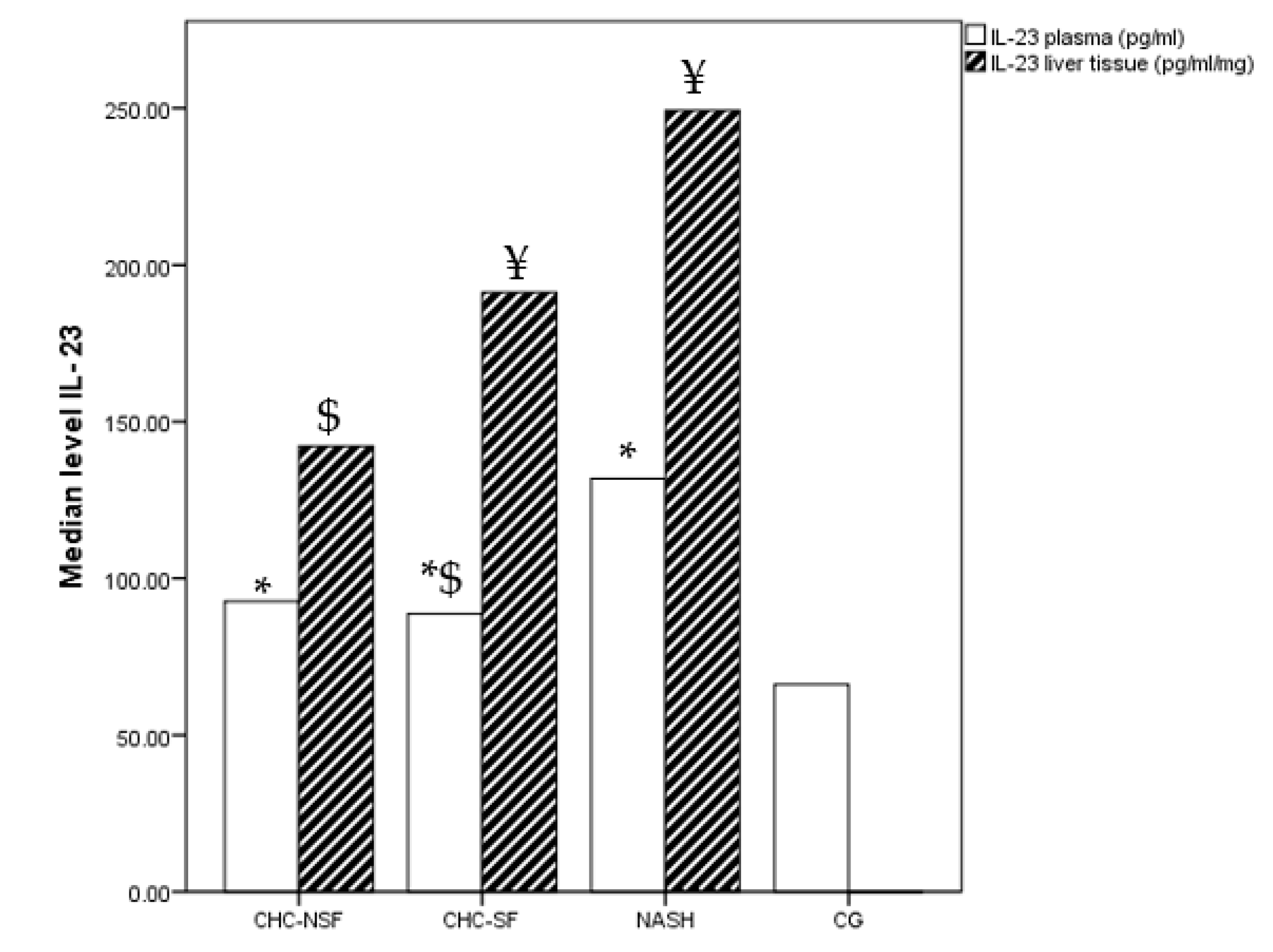
2. Results
3. Discussion
4. Materials and Methods
4.1. Subjects
- pregnancy
- presence of decompensated cirrhosis;
- co-infection with human immunodeficiency virus (HIV) and co-infection with hepatitis A, B, or D virus for CHC group of patients; infection with HIV, hepatitis A, B, C or D for NASH and control group of patients;
- other chronic or acute liver disease (autoimmune/toxic);
- presence of any of an immunocompromised state
- patients with HCC.
4.2. Pathohistological analysis of liver tissue
- Steatosis was graded as follows: <5% of liver parenchyma- 0; 5–33%- 1; >33–66%- 2; >66%- 3;
- Fibrosis was staged: none- 0; perisinusoidal or periportal- 1; perisinusoidal and portal/periportal- 2; bridging fibrosis- 3; cirrhosis- 4;
- Inflammation: lobular, portal (0-3);
- Hepatocellular ballooning degenaration (0-2)
4.3. Detection of HCV Antibody, Viral Load and Genotypes of HCV
4.4. Biochemical Assays
4.5. Measurement of Plasma and Tissue Cytokines (IL-6, IL-10, IL-17A and IL-23)
4.6. Anthropometric measurements
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Informed Consent Statement
Conflicts of Interest
References
- Cheemerla, S.; Balakrishnan, M. Global Epidemiology of Chronic Liver Disease. Clin. Liver Dis. 2021, 17, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.M.; Singal, A.G.; Tapper, E.B. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin. Gastroenterol. Hepatol. 2019, 18, 2650–2666. [Google Scholar] [CrossRef] [PubMed]
- CDC National Vital Statistics Reports, July, 2021. https://www.cdc.gov/nchs/nvss/leading-causes-of-death.htm.
- Sherlock, S. The immunology of liver disease. Am. J. Med. 1970, 49, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Vierling, J.; Gershwin, M.E.; Milich, D.; Chisari, F.V.; Hoofnagle, J.H. Immunology and the liver. Hepatology 1991, 13, 977–994. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.; Zhao, S.; Niu, X.; Fu, N.; Su, S.; Wang, R.; Zhang, Y.; Qiao, L.; Nan, Y. Involvement of the Interleukin-23/Interleukin-17 Axis in Chronic Hepatitis C Virus Infection and Its Treatment Responses. Int. J. Mol. Sci. 2016, 17, 1070. [Google Scholar] [CrossRef]
- Li, H.; Tsokos, G.C. IL-23/IL-17 Axis in Inflammatory Rheumatic Diseases. Clin. Rev. Allergy Immunol. 2020, 60, 31–45. [Google Scholar] [CrossRef]
- Cătană, C.-S.; Neagoe, I.B.; Cozma, V.; Magdaş, C.; Tăbăran, F.; Dumitraşcu, D.L. Contribution of the IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2015, 21, 5823–5830. [Google Scholar] [CrossRef]
- Menter, A.; Krueger, G.G.; Paek, S.Y.; Kivelevitch, D.; Adamopoulos, I.E.; Langley, R.G. Interleukin-17 and Interleukin-23: A Narrative Review of Mechanisms of Action in Psoriasis and Associated Comorbidities. Dermatol. Ther. 2021, 11, 385–400. [Google Scholar] [CrossRef]
- Bankir, M.; Acik, D.Y. IL-17 and IL-23 levels in patients with early-stage chronic lymphocytic leukemia. North. Clin. Istanb. 2020, 8, 24–30. [Google Scholar] [CrossRef]
- Kolls, J.K.; Lindén, A. Interleukin-17 Family Members and Inflammation. Immunity 2004, 21, 467–476. [Google Scholar] [CrossRef]
- Iwakura, Y.; Ishigame, H. The IL-23/IL-17 axis in inflammation. J. Clin. Investig. 2006, 116, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.; Zhao, S.; Niu, X.; Fu, N.; Su, S.; Wang, R.; Zhang, Y.; Qiao, L.; Nan, Y. Involvement of the Interleukin-23/Interleukin-17 Axis in Chronic Hepatitis C Virus Infection and Its Treatment Responses. Int. J. Mol. Sci. 2016, 17, 1070. [Google Scholar] [CrossRef] [PubMed]
- Heredia, J.E.; Sorenson, C.; Flanagan, S.; Nunez, V.; Jones, C.; Martzall, A.; Leong, L.; Martinez, A.P.; Scherl, A.; Brightbill, H.D.; et al. IL-23 signaling is not an important driver of liver inflammation and fibrosis in murine non-alcoholic steatohepatitis models. PLOS ONE 2022, 17, e0274582. [Google Scholar] [CrossRef]
- Yan, S.; Wang, L.; Liu, N.; Wang, Y.; Chu, Y. Critical role of interleukin-17/interleukin-17 receptor axis in mediating Con A-induced hepatitis. Immunol. Cell Biol. 2011, 90, 421–428. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hwang, S.; Ahmed, Y.A.; Feng, D.; Li, N.; Ribeiro, M.; Lafdil, F.; Kisseleva, T.; Szabo, G.; Gao, B. Immunopathobiology and therapeutic targets related to cytokines in liver diseases. Cell. Mol. Immunol. 2020, 18, 18–37. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; on behalf of theInternational Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014. [Google Scholar] [CrossRef]
- Dixon, J.B.; Bhathal, P.S.; O’Brien, P.E. Nonalcoholic Fatty Liver Disease: Predictors of Nonalcoholic Steatohepatitis and Liver Fibrosis in the Severely Obese. Gastroenterology 2001, 121, 91–100. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- Kountouras, J. Innate immunity and nonalcoholic fatty liver disease. Ann. Gastroenterol. 2023, 36, 244–256. [Google Scholar] [CrossRef]
- Yuan, L.; Hanlon, C.L.; Terrault, N.; Alqahtani, S.; Tamim, H.; Lai, M.; Saberi, B. Portrait of Regional Trends in Liver Transplantation for Nonalcoholic Steatohepatitis in the United States. Am. J. Gastroenterol. 2022, 117, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Gkouvatsos, K.; Pantopoulos, K. Chronic hepatitis C and liver fibrosis. World J. Gastroenterol. 2014, 20, 11033–53. [Google Scholar] [CrossRef] [PubMed]
- Edmison, J.; McCullough, A.J. Pathogenesis of Non-alcoholic Steatohepatitis: Human Data. Clin. Liver Dis. 2007, 11, 75–104. [Google Scholar] [CrossRef] [PubMed]
- Afify, M.; Hamza, A.H.; Alomari, R.A. Correlation Between Serum Cytokines, Interferons, and Liver Functions in Hepatitis C Virus Patients. J. Interf. Cytokine Res. 2017, 37, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Klinker, H.; Boeker, K.H.W.; Merle, U.; Link, R.; Buggisch, P.; Hüppe, D.; Cornberg, M.; Sarrazin, C.; Wedemeyer, H.; et al. Elevated liver enzymes predict morbidity and mortality despite antiviral cure in patients with chronic hepatitis C: Data from the German Hepatitis C-Registry. Hepatol. Commun. 2022, 6, 2488–2495. [Google Scholar] [CrossRef]
- Vonghia, L.; Magrone, T.; Verrijken, A.; Michielsen, P.; Van Gaal, L.; Jirillo, E.; Francque, S. Peripheral and Hepatic Vein Cytokine Levels in Correlation with Non-Alcoholic Fatty Liver Disease (NAFLD)-Related Metabolic, Histological, and Haemodynamic Features. PLOS ONE 2015, 10, e0143380. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Stewart, A.G.; Woodman, O.L.; Ritchie, R.H.; Qin, C.X. Non-Alcoholic Steatohepatitis: A Review of Its Mechanism, Models and Medical Treatments. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Castro-Narro, G.; Moctezuma-Velázquez, C.; Male-Velázquez, R.; Trejo-Estrada, R.; Bosques, F.J.; Moreno-Alcántar, R.; Rodríguez-Hernández, H.; Bautista-Santos, A.; Córtez-Hernández, C.; Cerda-Reyes, E.; et al. Position statement on the use of albumin in liver cirrhosis. Ann. Hepatol. 2022, 27, 100708. [Google Scholar] [CrossRef]
- Rawi, S.; Wu, G.Y. Pathogenesis of Thrombocytopenia in Chronic HCV Infection: A Review. J. Clin. Transl. Hepatol. 2020, 8, 184–191. [Google Scholar] [CrossRef]
- Manuc, D.; Preda, C.M.; Sandra, I.; Baicus, C.; Cerban, R.; Constantinescu, I.; Olteanu, A.O.; Ciora, C.A.; Manuc, T.; Chiriac, D.E.; et al. Signification of Serum Alpha-Fetoprotein Levels in Cases of Compensated Cirrhosis and Hepatitis C Virus without Hepatocellular Carcinoma. J. Med. Life 2020, 13, 68–74. [Google Scholar] [CrossRef]
- Chu, C.-W.; Hwang, S.-J.; Luo, J.-C.; Lai, C.-R.; Tsay, S.-H.; Li, C.-P.; Wu, J.-C.; Chang, F.-Y.; Lee, S.-D. Clinical, Virologic, and Pathologic Significance of Elevated Serum Alpha-fetoprotein Levels in Patients with Chronic Hepatitis C. J. Clin. Gastroenterol. 2001, 32, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Falleti, E.; Fabris, C.; Toniutto, P.; Fontanini, E.; Cussigh, A.; Bitetto, D.; Fumolo, E.; Fornasiere, E.; Bragagnini, W.; Pinato, D.J.; et al. Interleukin-6 Polymorphisms and Gender: Relationship with the Occurrence of Hepatocellular Carcinoma in Patients with End-Stage Liver Disease. Oncology 2009, 77, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.; Collins, M.; Kenny, C.; Ryan, E.; Keane, C.; Crowe, J. Polymorphisms in tumour necrosis factor-α, transforming growth factor-β, interleukin-10, interleukin-6, interferon-γ, and outcome of hepatitis C virus infection. J. Med Virol. 2003, 71, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Dolganiuc, A.; Norkina, O.; Kodys, K.; Catalano, D.; Bakis, G.; Marshall, C.; Mandrekar, P.; Szabo, G. Viral and Host Factors Induce Macrophage Activation and Loss of Toll-Like Receptor Tolerance in Chronic HCV Infection. Gastroenterology 2007, 133, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Wang, K.; Aoyama, T.; Grivennikov, S.I.; Paik, Y.; Scholten, D.; Cong, M.; Iwaisako, K.; Liu, X.; Zhang, M.; et al. Interleukin-17 Signaling in Inflammatory, Kupffer Cells, and Hepatic Stellate Cells Exacerbates Liver Fibrosis in Mice. Gastroenterology 2012, 143, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Robak, E.; Gerlicz-Kowalczuk, Z.; Dziankowska-Bartkowiak, B.; Wozniacka, A.; Bogaczewicz, J. Serum concentrations of IL-17A, IL-17B, IL-17E and IL-17F in patients with systemic sclerosis. Arch. Med Sci. 2019, 15, 706–712. [Google Scholar] [CrossRef]
- Meng, P.; Zhao, S.; Niu, X.; Fu, N.; Su, S.; Wang, R.; Zhang, Y.; Qiao, L.; Nan, Y. Involvement of the Interleukin-23/Interleukin-17 Axis in Chronic Hepatitis C Virus Infection and Its Treatment Responses. Int. J. Mol. Sci. 2016, 17, 1070. [Google Scholar] [CrossRef]
- Belinchón-Romero, I.; Bellot, P.; Romero-Pérez, D.; Herraiz-Romero, I.; Marco, F.; Frances, R.; Ramos-Rincón, J.-M. Non-alcoholic fatty liver disease is associated with bacterial translocation and a higher inflammation response in psoriatic patients. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Tang, Y.; Bian, Z.; Zhao, L.; Liu, Y.; Liang, S.; Wang, Q.; Han, X.; Peng, Y.; Chen, X.; Shen, L.; et al. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin. Exp. Immunol. 2011, 166, 281–290. [Google Scholar] [CrossRef]
- de Souza-Cruz, S.; Victória, M.B.; Tarragô, A.M.; da Costa, A.G.; Pimentel, J.P.D.; Pires, E.F.; Araújo, L.d.P.; Coelho-Dos-Reis, J.G.; Gomes, M.d.S.; Amaral, L.R.; et al. Liver and blood cytokine microenvironment in HCV patients is associated to liver fibrosis score: a proinflammatory cytokine ensemble orchestrated by TNF and tuned by IL-10. BMC Microbiol. 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Rau, M.; Schilling, A.-K.; Meertens, J.; Hering, I.; Weiss, J.; Jurowich, C.; Kudlich, T.; Hermanns, H.M.; Bantel, H.; Beyersdorf, N.; et al. Progression from Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis Is Marked by a Higher Frequency of Th17 Cells in the Liver and an Increased Th17/Resting Regulatory T Cell Ratio in Peripheral Blood and in the Liver. J. Immunol. 2016, 196, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Cachem, F.C.O.F.; Dias, A.S.; Monteiro, C.; Castro, J.R.; Fernandes, G.; Delphim, L.; Almeida, A.J.; Tavares, F.; Maciel, A.M.A.; Amendola-Pires, M.M.; et al. The proportion of different interleukin-17-producing T-cell subsets is associated with liver fibrosis in chronic hepatitis C. Immunology 2017, 151, 167–176. [Google Scholar] [CrossRef]
- Cachem, F.C.; Dias, A.S.; Monteiro, C.; Fernandes, G.; Delphim, L.; Tavares, F.; Maciel, A.M.; Amendola-Pires, M.M.; Brandão-Mello, C.E.; Andrade, R.M.; et al. Different core-specific T cell subsets are expanded in chronic hepatitis C with advanced liver disease. Cytokine 2018, 124, 154456. [Google Scholar] [CrossRef] [PubMed]
- Askoura, M.; Abbas, H.A.; Al Sadoun, H.; Abdulaal, W.H.; Abu Lila, A.S.; Almansour, K.; Alshammari, F.; Khafagy, E.-S.; Ibrahim, T.S.; Hegazy, W.A.H. Elevated Levels of IL-33, IL-17 and IL-25 Indicate the Progression from Chronicity to Hepatocellular Carcinoma in Hepatitis C Virus Patients. Pathogens 2022, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- El-Emshaty, H.M.; Nasif, W.A.; Mohamed, I.E. Serum Cytokine of IL-10 and IL-12 in Chronic Liver Disease: The Immune and Inflammatory Response. Dis. Markers 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Owusu, D.O.; Phillips, R.; Owusu, M.; Sarfo, F.S.; Frempong, M. Increased levels of circulating IL-10 in persons recovered from hepatitis C virus (HCV) infection compared with persons with active HCV infection. BMC Res. Notes 2020, 13, 1–7. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Kawaratani, H.; Kitazawa, T.; Yoshiji, H.; Fujimoto, M.; Uemura, M.; Fukui, H. Immunotherapy for nonalcoholic steatohepatitis using themultiple cytokine production modulator Y-40138. World J. Gastroenterol. 2009, 15, 5533–40. [Google Scholar] [CrossRef]
- Aboushousha, T.; Emad, M.; Rizk, G.; Ragab, K.; Hammam, O.; Fouad, R.; Helal, N.S. IL-4, IL-17 and CD163 Immunoexpression and IL-6 Gene Polymorphism in Chronic Hepatitis C Patients and Associated Hepatocellular Carcinoma. Asian Pac. J. Cancer Prev. 2021, 22, 1105–1113. [Google Scholar] [CrossRef]
- Tan, Z.; Qian, X.; Jiang, R.; Liu, Q.; Wang, Y.; Chen, C.; Wang, X.; Ryffel, B.; Sun, B. IL-17A Plays a Critical Role in the Pathogenesis of Liver Fibrosis through Hepatic Stellate Cell Activation. J. Immunol. 2013, 191, 1835–1844. [Google Scholar] [CrossRef]
- Gomes, A.L.; Teijeiro, A.; Burén, S.; Tummala, K.S.; Yilmaz, M.; Waisman, A.; Theurillat, J.-P.; Perna, C.; Djouder, N. Metabolic Inflammation-Associated IL-17A Causes Non-alcoholic Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell 2016, 30, 161–175. [Google Scholar] [CrossRef]
- Wieckowska, A.; Papouchado, B.G.; Li, Z.; Lopez, R.; Zein, N.N.; Feldstein, A.E. Increased Hepatic and Circulating Interleukin-6 Levels in Human Nonalcoholic Steatohepatitis. Am. J. Gastroenterol. 2008, 103, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wu, L.; Xie, W.; Shao, Y.; Jiang, J.; Zhao, Z.; Yan, M.; Chen, Z.; Cui, D. The imbalance of Th17/Treg cells is involved in the progression of nonalcoholic fatty liver disease in mice. BMC Immunol. 2017, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Wang, Y.; Zhao, Q.; Wang, C.; Hu, Y.; Wu, B. Th17 cells are increased with severity of liver inflammation in patients with chronic hepatitis C. J. Gastroenterol. Hepatol. 2011, 27, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Macek Jilkova, Z.; Afzal, S.; Marche, H.; Decaens, T.; Sturm, N.; Jouvin-Marche, E.; Huard, B.; Marche, P.N. Progression of fibrosis in patients with chronic viral hepatitis is associated with IL-17(+) neutrophils. Liver Int. Off. J. Int. Assoc. Study Liver 2016, 36, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Negro, F. ; European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. J. Hepatol. 2014, 60, 392–420. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Bedossa, P.; Poynard, T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
| Variable | CHC-NSF (n=20) |
CHC-SF (n=16) |
NASH (n=19) |
CG (n=20) |
p value |
|---|---|---|---|---|---|
| Age (years) | 46.3 ± 13.6 | 54.2 ± 16.3 | 43.9 ± 9.2 | 42.5 ± 13.3 | ns |
| GenderFemale/ Male (%) | 49/51 | 52/48 | 34/66 | 47/53 | ns |
| Body mass (kg) | 70.0±9.6 | 70.1±9.5 | 96.7±19.4 *#$ | 67.8±11.1 | 0.000 |
| Body hight (cm) | 177.8±10.9 | 176.4±11.6 | 179.0±7.9 | 176.6±11.6 | ns |
| BMI (kg/cm2) | 22.0±0.9 | 22.4±0.8 | 30.1±5.5*#$ | 21.5±1.2 | 0.000 |
| Erythrocytes (cells/ml) | 4.9 ± 0.5 | 4.8 ± 0.6 | 5.0 ± 0.5 | 4.4 ±0.5 | ns |
| Hemoglobin (g/L) | 147.7 ± 13.7 | 142.1 ± 19.3 | 144.3 ± 14.2 | 137.8 ± 7.6 | ns |
| Leucocytes (cells/ml) | 7.8 ± 2.5 | 6.5 ± 2.2 | 6.6 ± 1.6 | 5.6 ± 1.4 | ns |
| PLT (cells/ml) | 230.6 ± 63.2 | 182.2 ± 57.4*# | 220.1 ± 41.0 | 263.7 ±66.7 | 0.000 |
| AST (U/l) | 41.3 ± 39.9* | 104.5 ± 38.8*# | 50.1 ±15.7*& | 22.5 ± 5.6 | 0.000 |
| ALT (U/l) | 61.1 ± 28.7* | 157.1 ± 29.9*# | 88.8 ± 44.2*& | 33.9 ± 11.1 | 0.000 |
| γGTP (U/l) | 67.8 ± 94.0 | 81.0 ± 82.8 | 61.1 ± 45.9 | 24.3 ± 5.1 | ns |
| AF (u/L) | 66.6 ± 24.9 | 80.0 ± 53.9 | 67.1 ± 17.5 | 71.4 ± 14.3 | ns |
| AFP (ng/ml) | 4.4 ± 2.4 | 11.8 ± 8.2*# | 2.9 ± 1.1& | 1.9 ± 0.6 | 0.000 |
| TSH (microU/ml) | 1.5 ± 0.8 | 1.6 ± 0.6 | 1.7 ± 0.9 | 2.5 ± 0.5 | ns |
| Glucose (mmol/l) | 5.7 ± 1.5 | 5.8 ± 1.1 | 5.4 ± 0.7 | 4.7 ± 0.5 | ns |
| Uric acide (µmol/l) | 5.3 ± 2.1 | 6.2 ± 1.6 | 5.6 ± 1.5 | 4.7 ± 1.2 | ns |
| Creatinine (µmol/l) | 79.8 ± 19.6 | 76.6 ± 17.2 | 81.5 ± 14.6 | 67.4 ± 10.8 | ns |
| Total cholesterol (mmol/l) | 4.7 ± 1.1 | 4.2 ± 1.0 | 7.1 ± 0.8*#& | 3.6 ± 0.7 | 0.000 |
| LDL (mmol/l) | 2.6 ± 1.0 | 2.4 ± 0.8 | 4.7 ± 0.8*#& | 2.5 ± 0.5 | 0.000 |
| HDL (mmol/l) | 1.5 ± 0.5* | 1.4 ± 0.3* | 1.3 ± 0.7* | 2.0 ± 0.5 | 0.000 |
| Triglycerides (mmol/l) | 1.25±0.8 | 1.20±0.8 | 4.13±1.13*#& | 1.14±0.2 | 0.000 |
| Total proteins (g/l) | 76.6 ± 6.5 | 75.1 ± 7.6 | 76.3 ± 7.7 | 75.9 ± 6.1 | ns |
| Albumin (g/l) | 38.9 ± 3.1* | 31.1 ± 2.4*# | 43.3 ± 5.5& | 42.6 ± 3.9 | 0.000 |
| Total bilirubin (mg/dl) | 10.6 ± 4.5 | 10.9 ± 2.7 | 13.7 ± 9.3 | 10.5 ± 3.3 | ns |
| Direct bilirubin (mg/dl) | 3.9 ± 1.8 | 4.6 ± 1.4 | 3.9 ± 1.5 | 2.1 ± 1.5 | ns |
| INR | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.1 | 1.0 ± 0.1 | ns |
| Fibrinogen (mg/dl) | 2.9 ± 0.5 | 2.8 ± 0.5 | 3.1 ± 0.4 | 2.6 ± 0.4 | ns |
| CRP (mg/dl) | 2.5 ± 2.0 | 3.7 ± 2.0 | 3.8 ± 2.3 | 1.3 ± 0.6 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





