Submitted:
30 May 2023
Posted:
31 May 2023
You are already at the latest version
Abstract
Keywords:
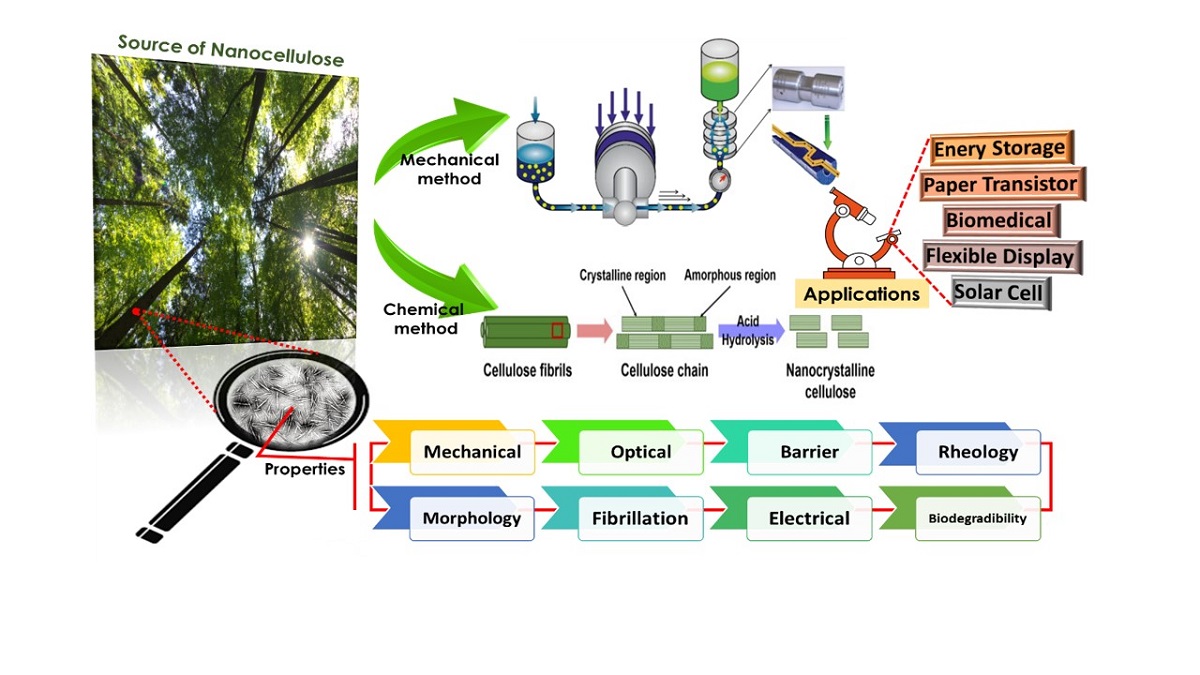
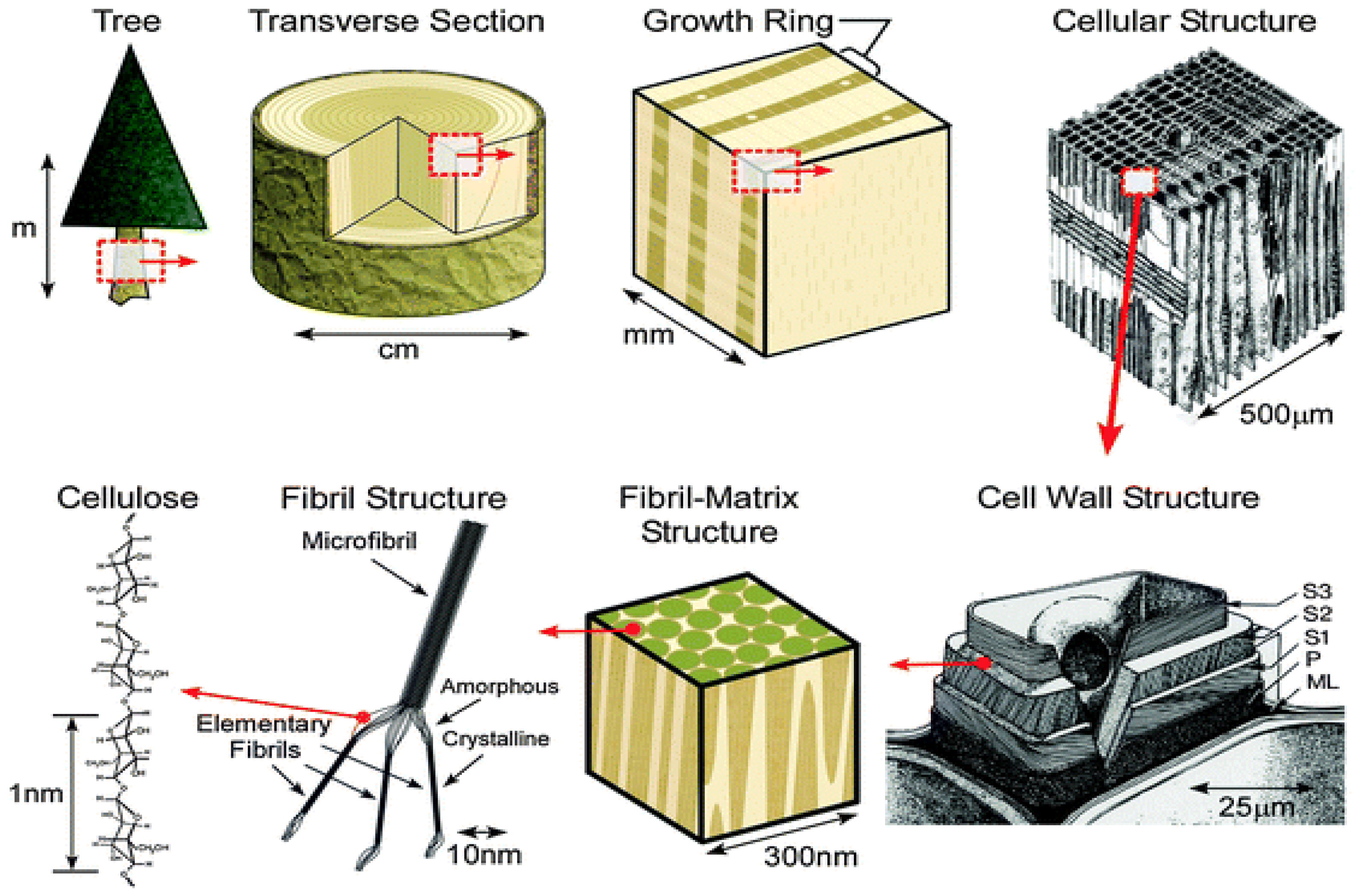
1. Introduction
2. Classification of Nanocellulose
2.1. Cellulose Nanocrystals (CNC) or Nanocrystalline Cellulose (NCC)
2.2. Cellulose Nanofibrils (CNF) or Nano Fibrillated Cellulose (NFC)
2.3. Bacterial Nanocellulose (BNC)
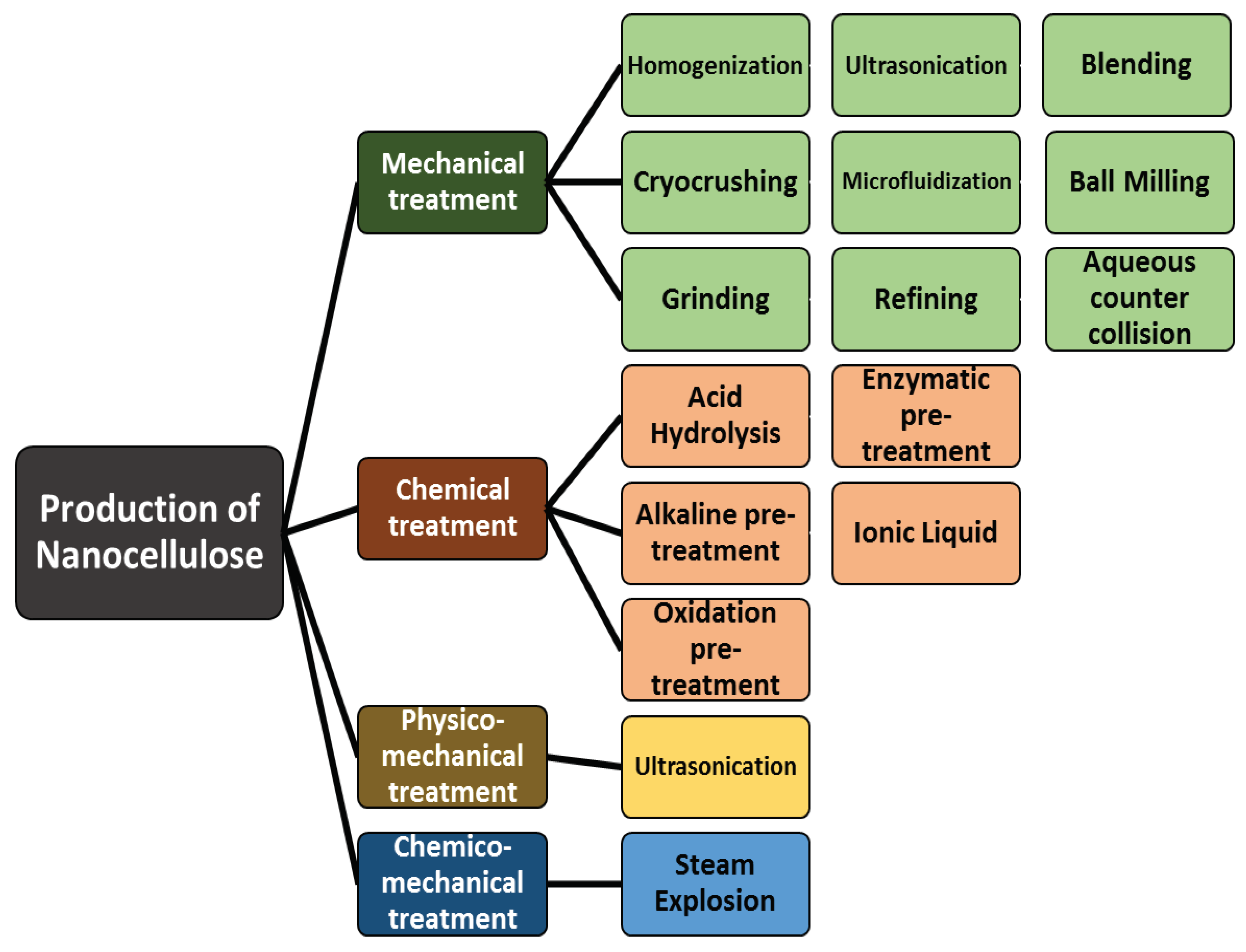
3. Production of Nanocellulose
3.1. Mechanical Method
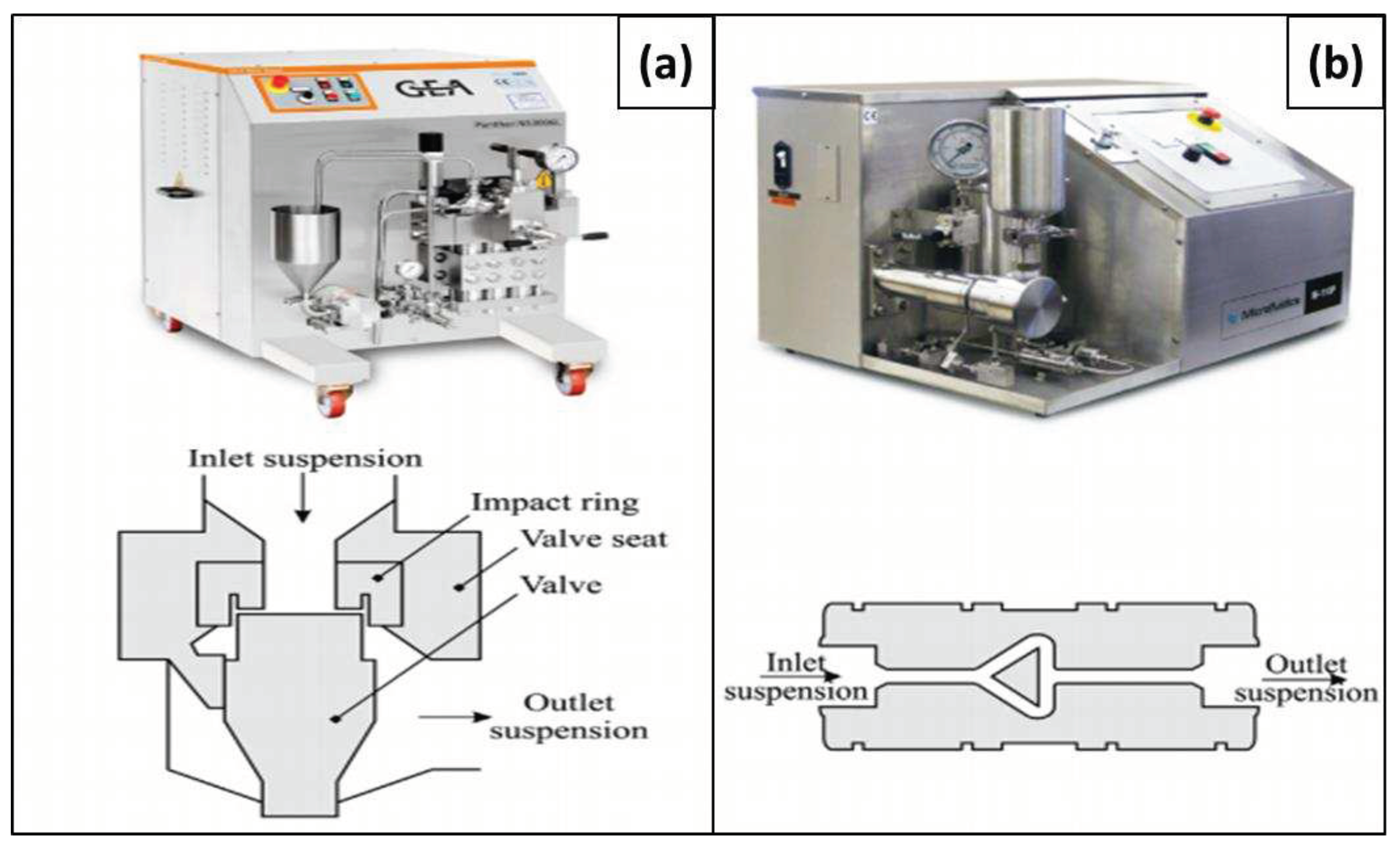
3.1.1. Homogenization
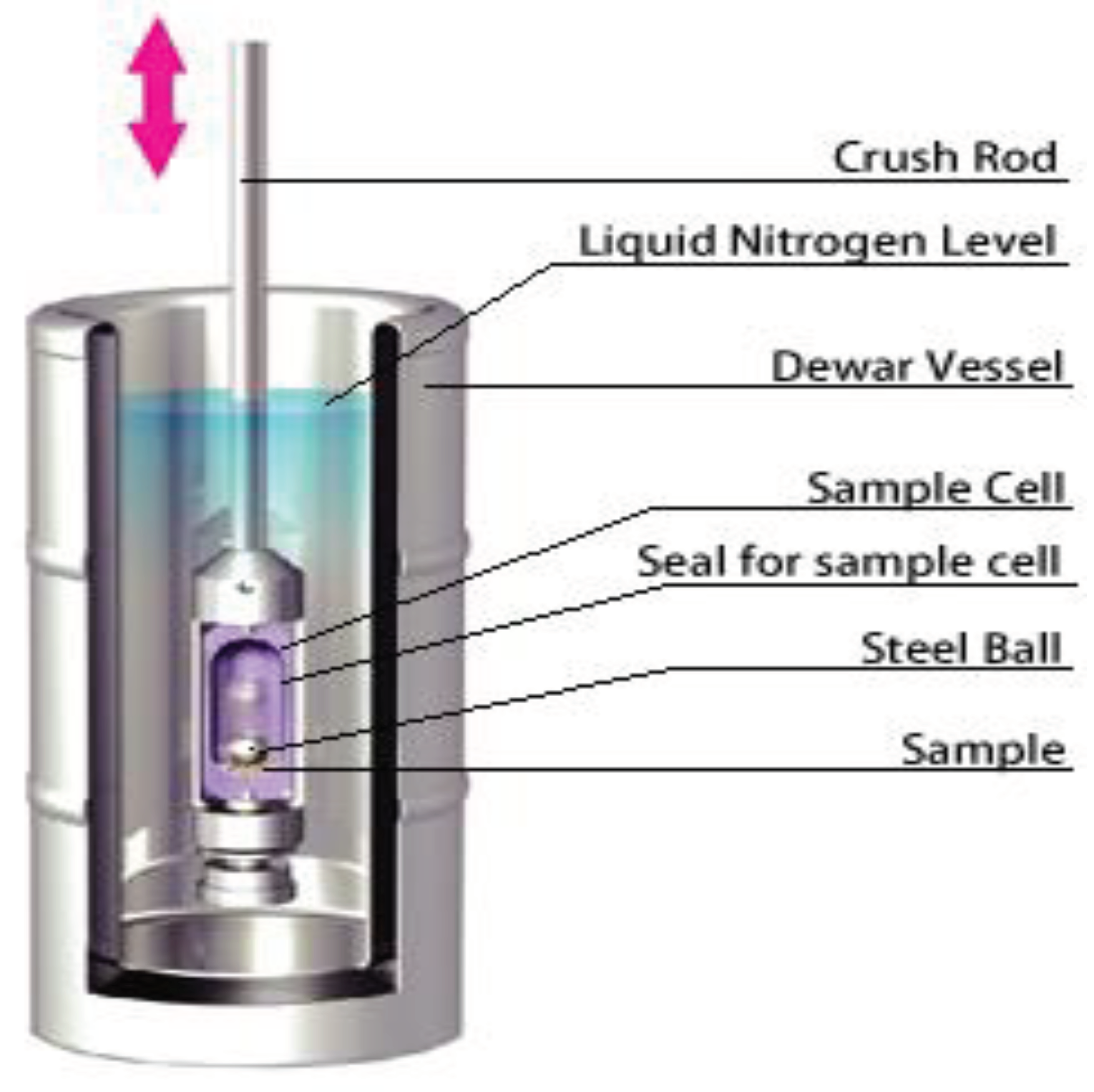
3.1.2. Cryocrushing
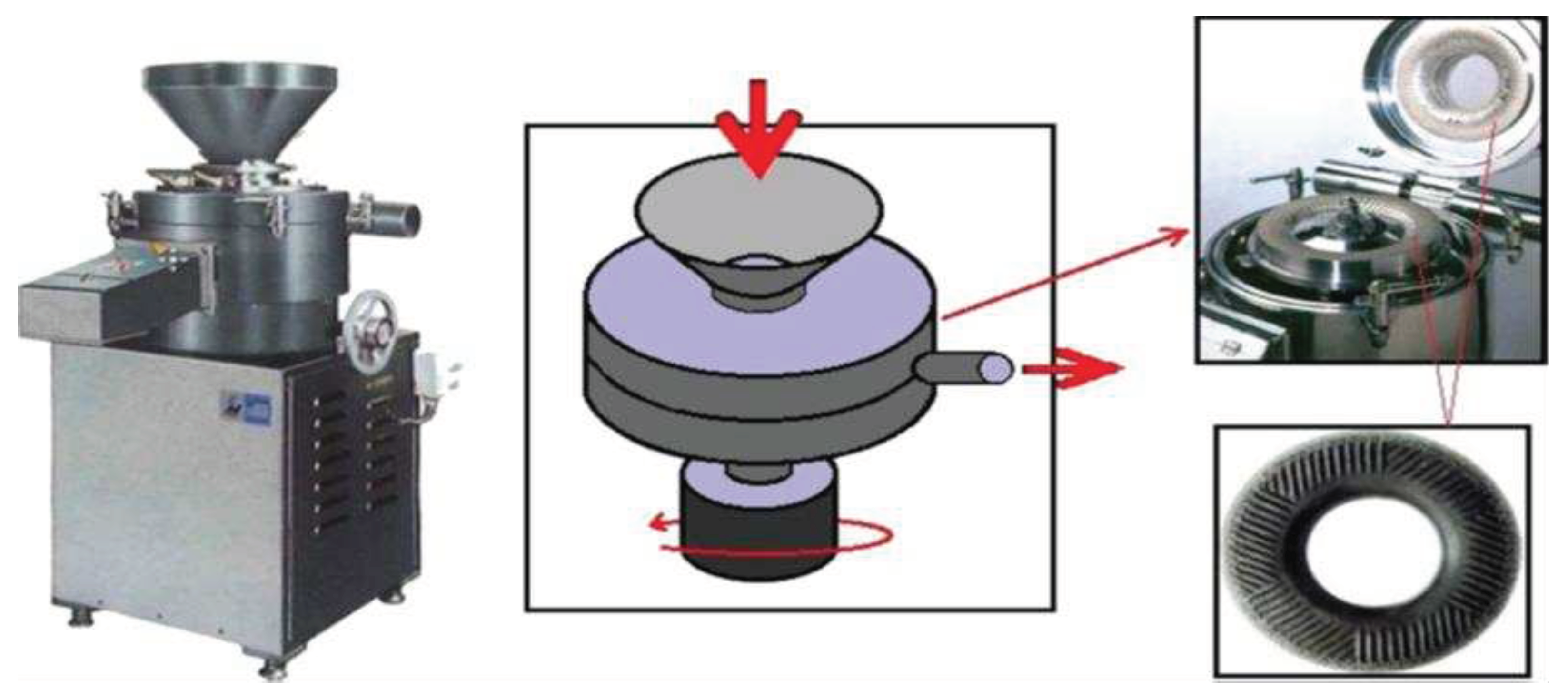
3.1.3. Grinding
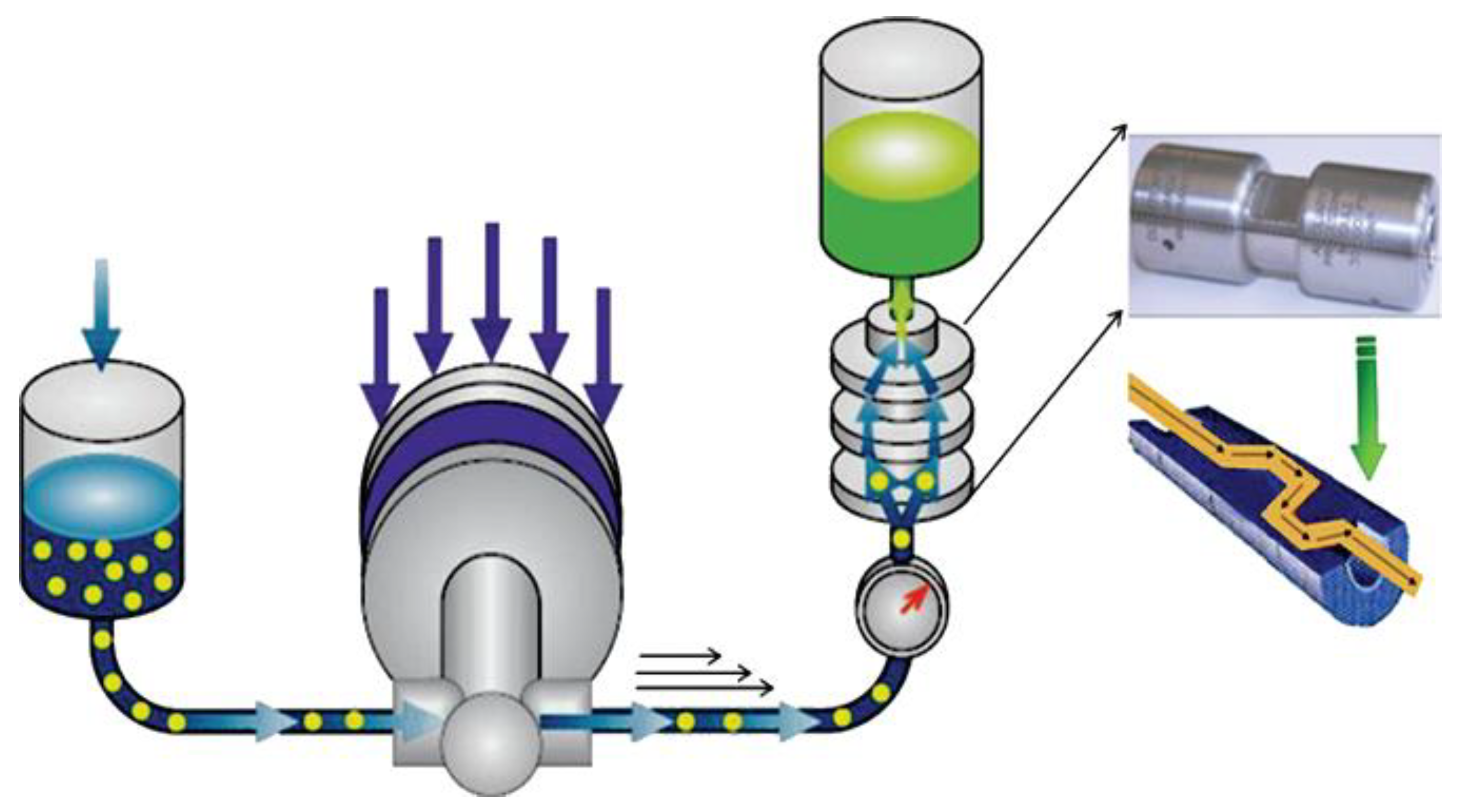
3.1.4. Micro Fluidization
3.1.5. Refining
3.1.6. Blending
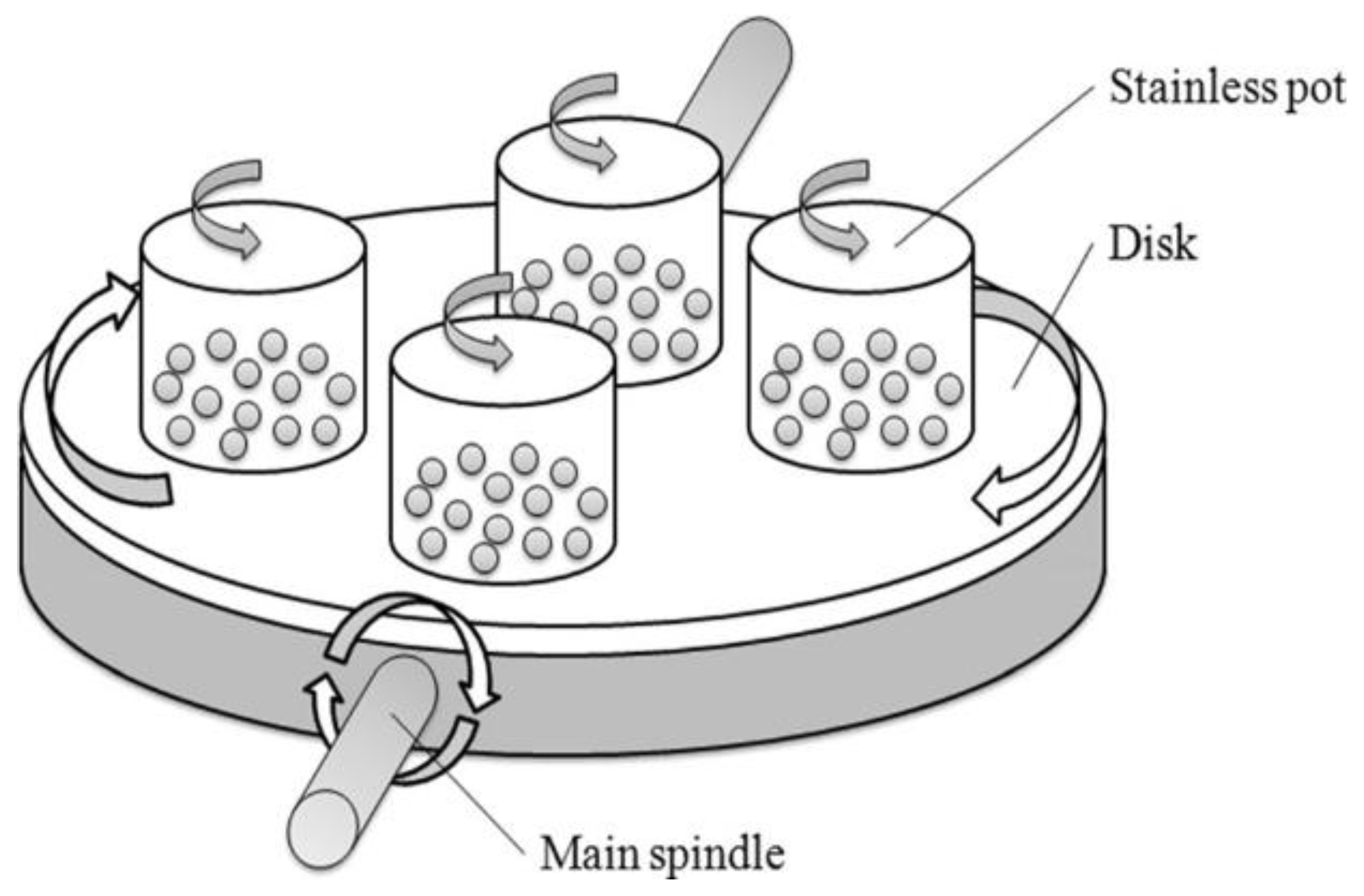
3.1.7. Ball Milling
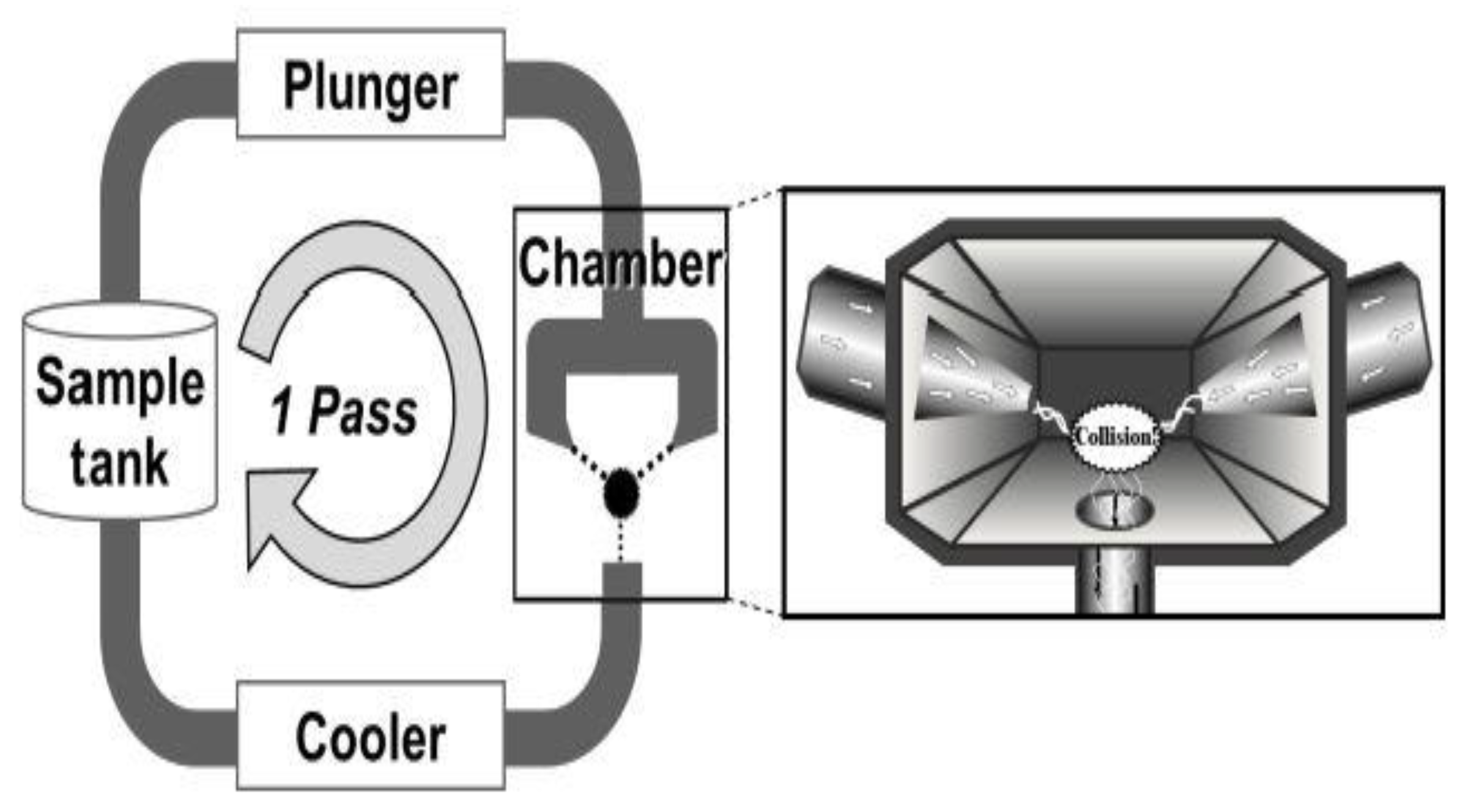
3.1.8. Aqueous Counter Collision (ACC)
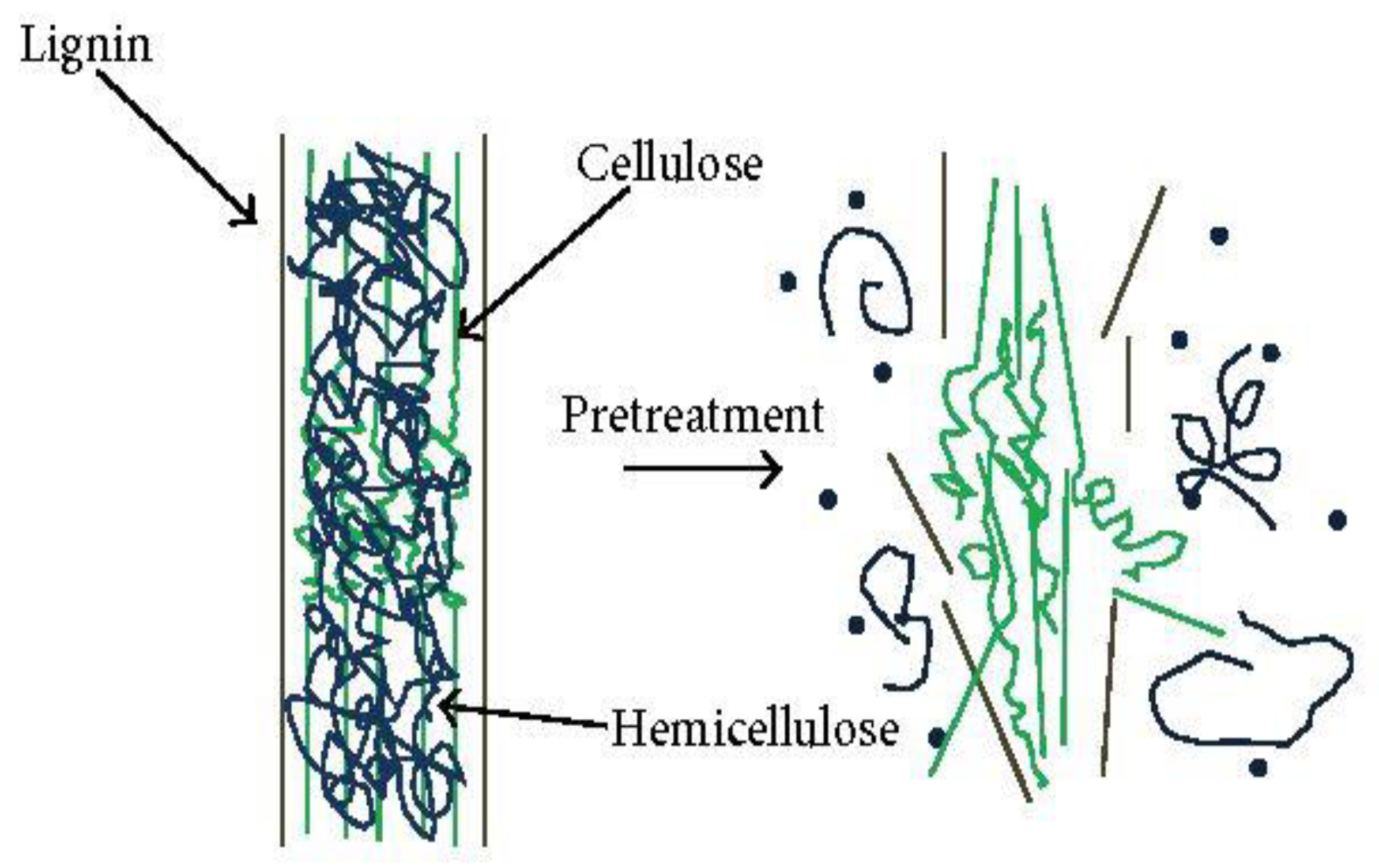
3.2. Chemical Method
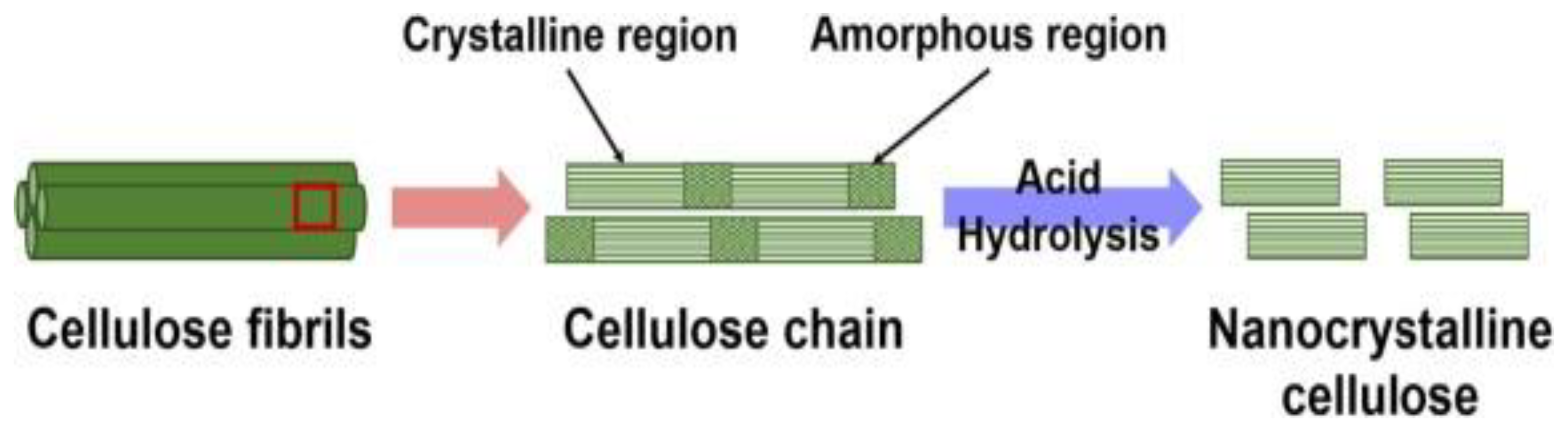
3.2.1. Acid Hydrolysis
3.2.2. Alkaline Pre-treatment
3.2.3. Oxidation Pre-treatment
3.2.4. Enzymatic Pre-treatment
3.2.5. Ionic Liquid
3.3. Physico-mechanical Treatment
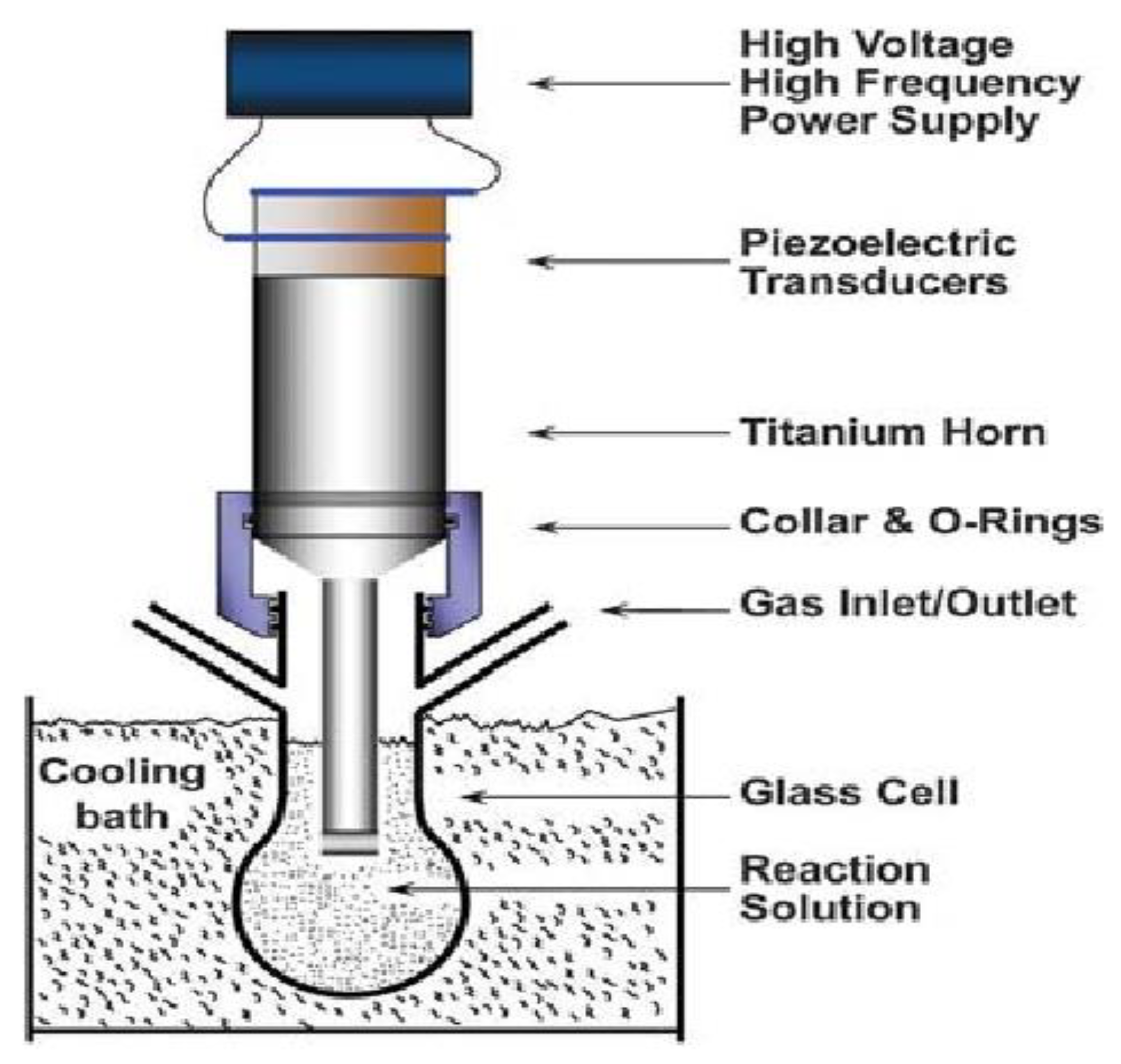
3.3.1. Ultrasonication
3.4. Chemico-mechanical Treatment
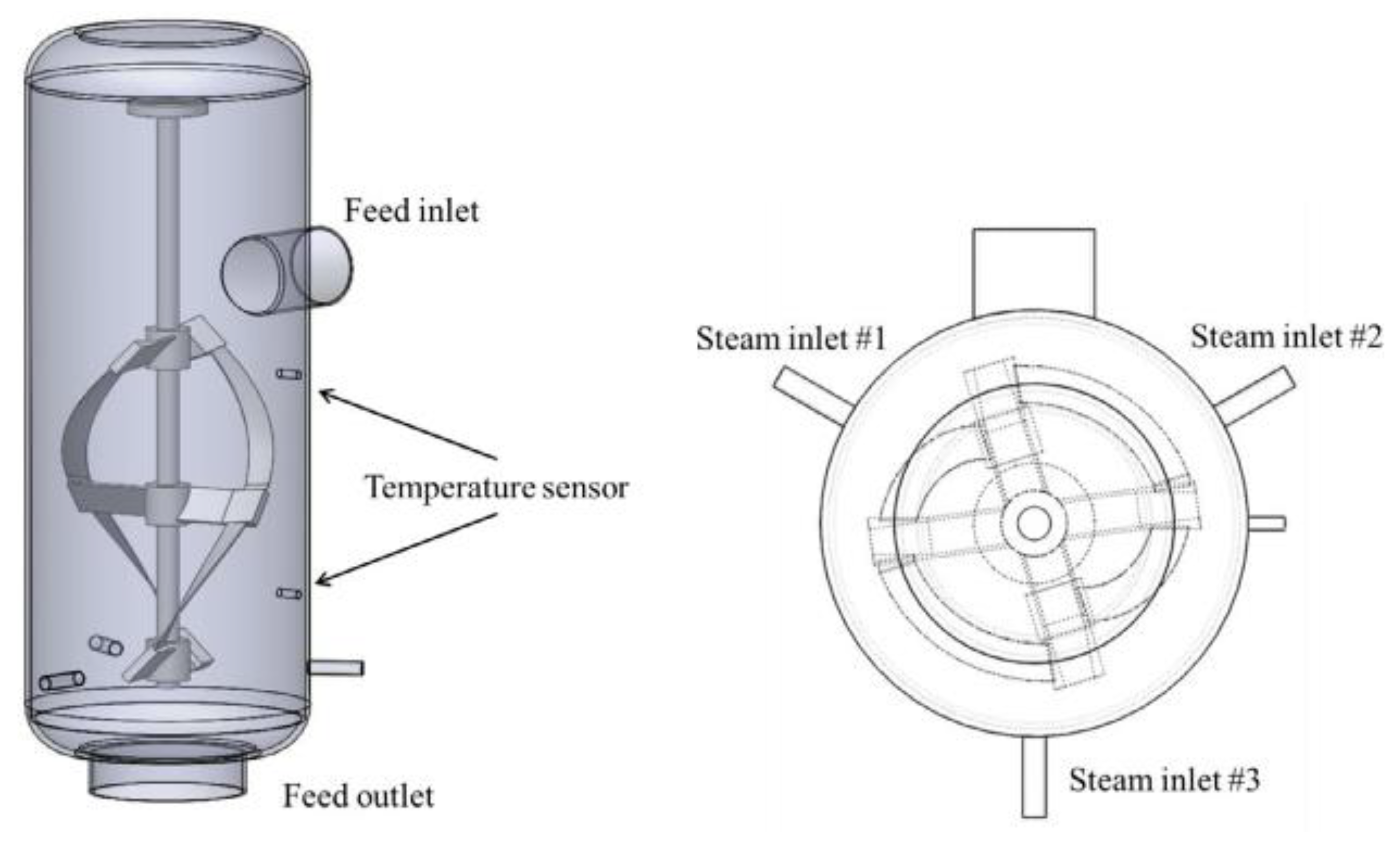
3.4.1. Steam Explosion
3.5. Summary of Other Preparation Methods
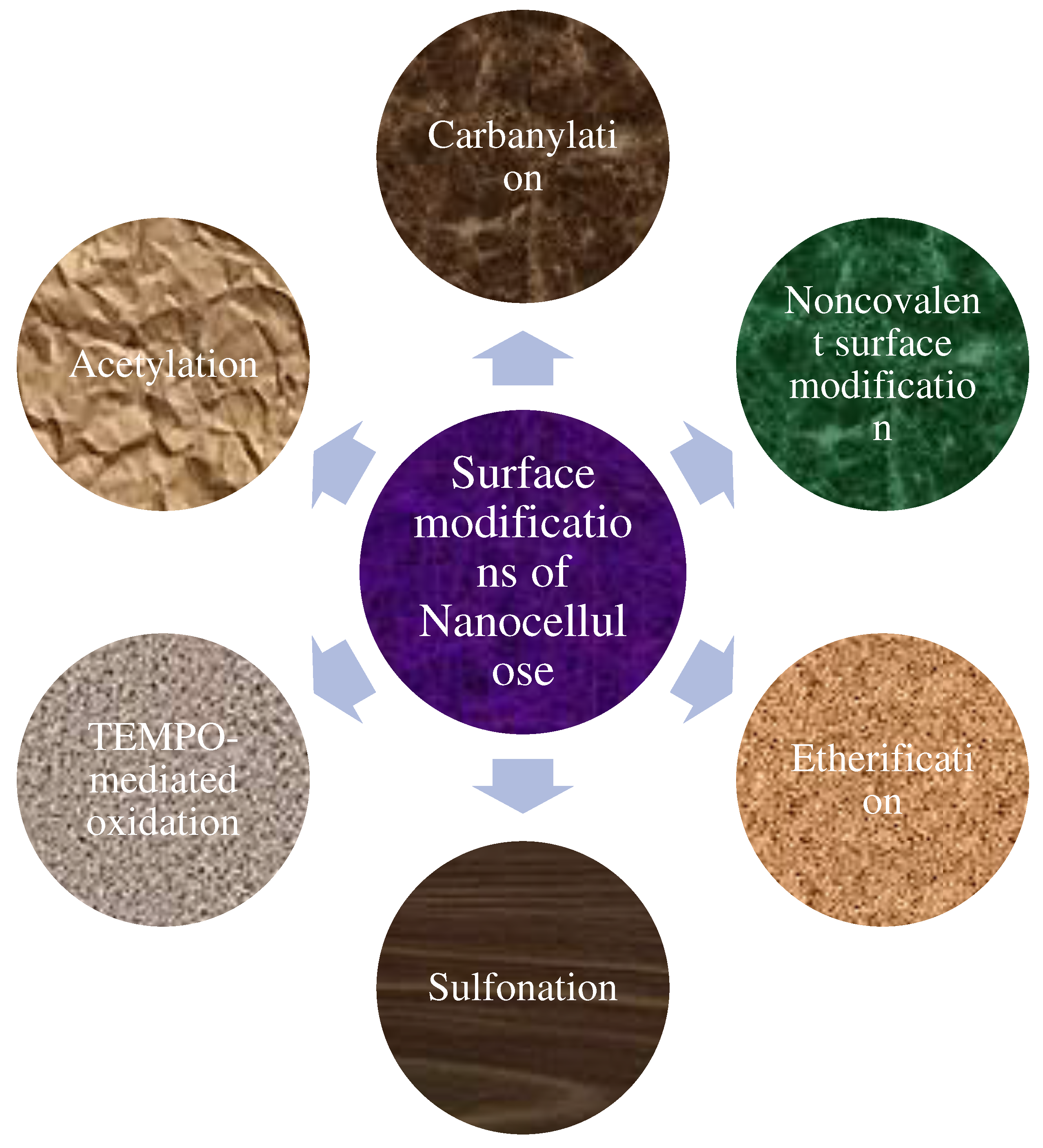
4. Surface Modifications of Nanocellulose
4.1. Noncovalent Surface Modification
4.2. Carbonylation
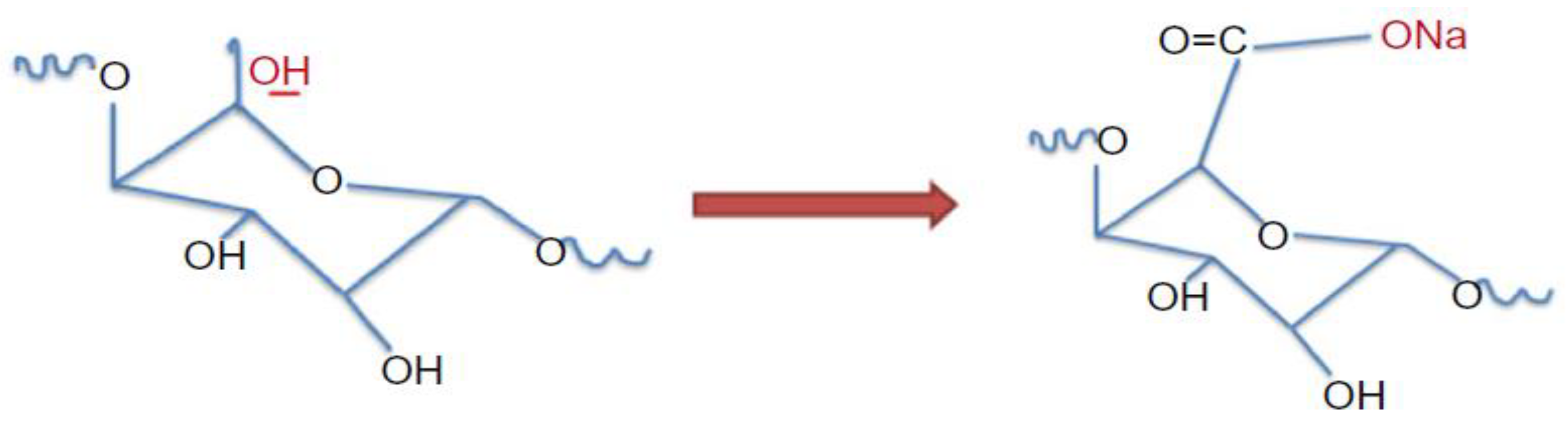
4.3. TEMPO-mediated Oxidation
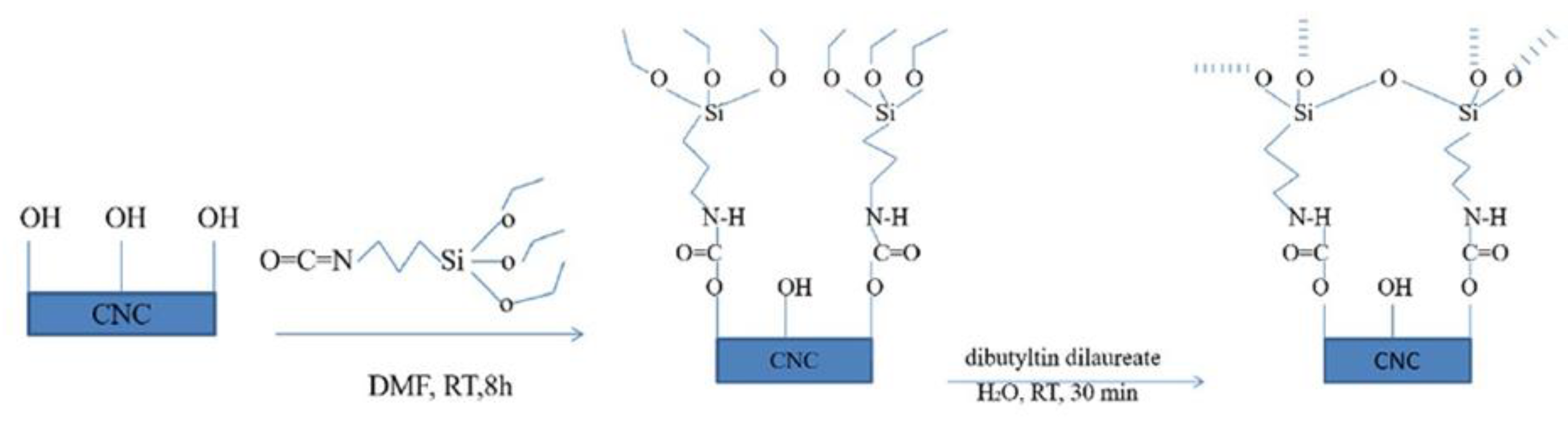
4.4. Esterification
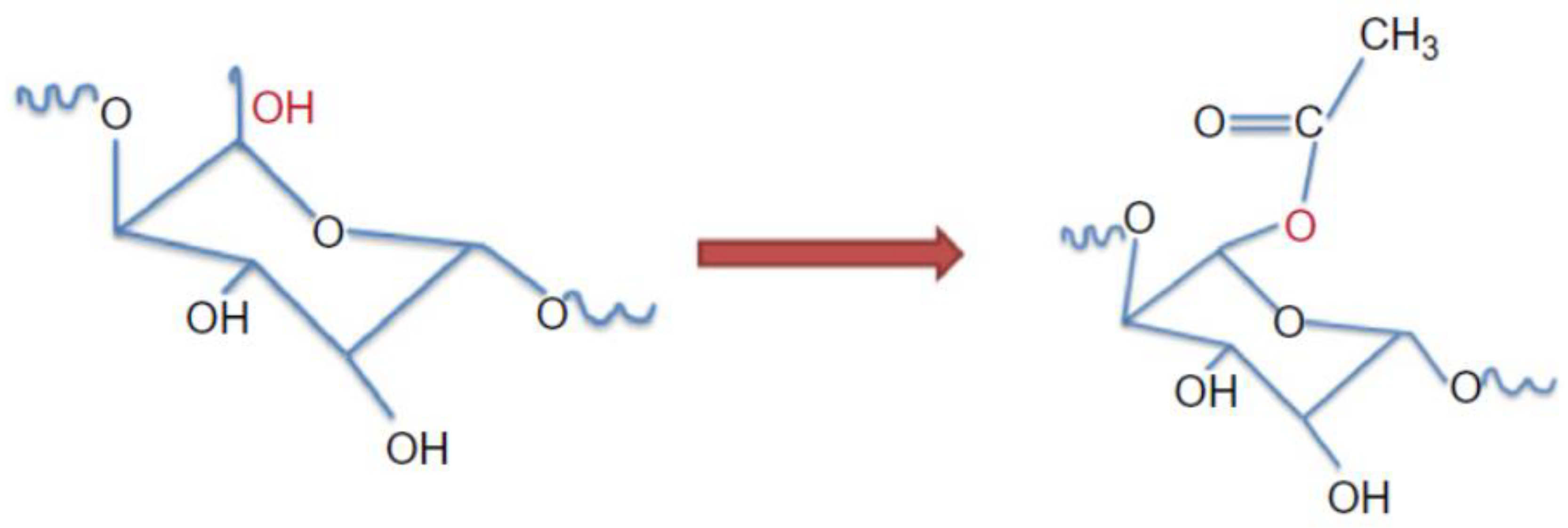
4.5. Acetylation
4.6. Sulfonation
4.7. Summary of Nanocellulose Surface Modification
5. Processing-property Correlation of Nanocellulose
5.1. Mechanical Properties
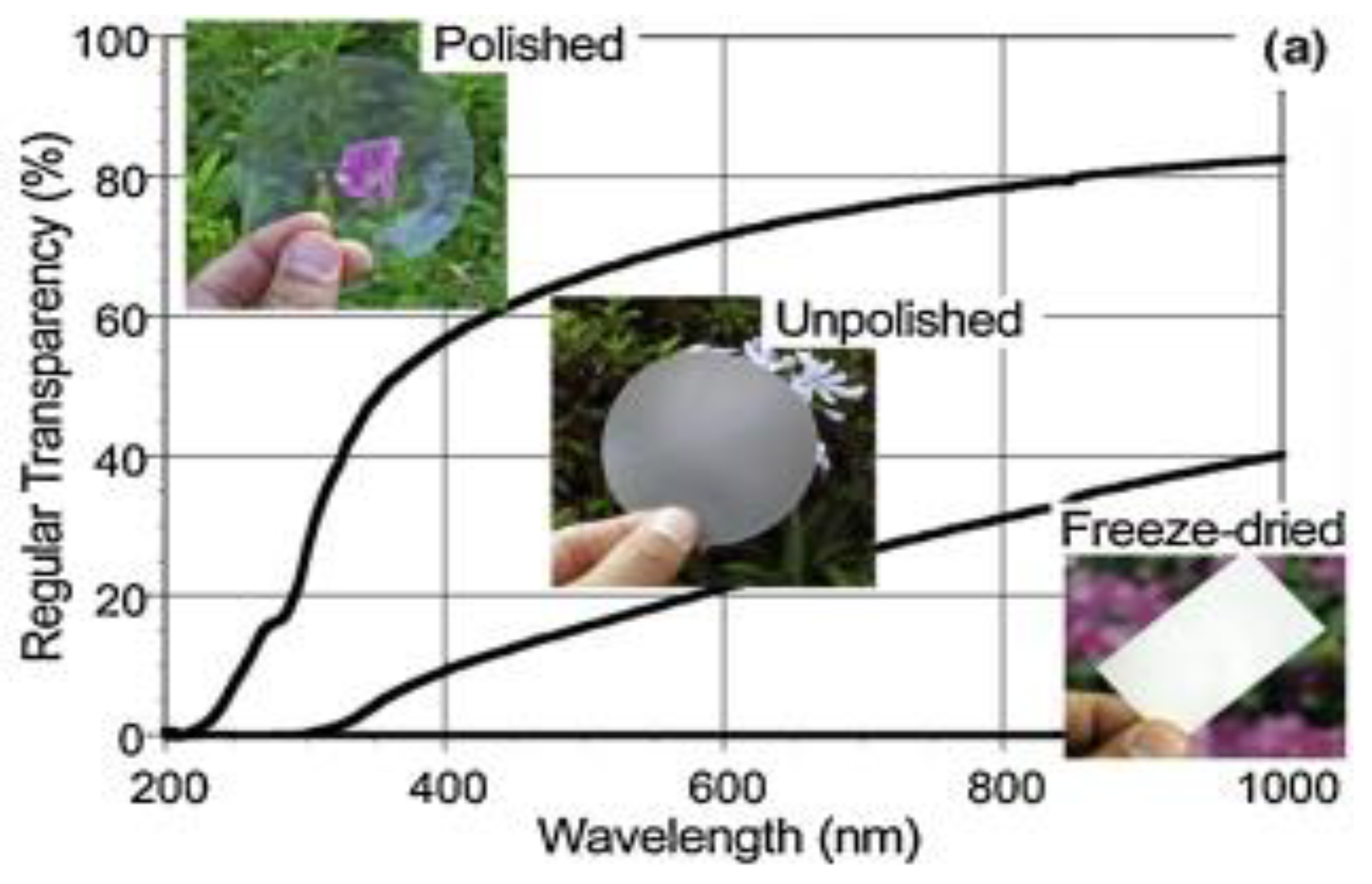
5.2. Optical Properties
5.3. Barrier Properties
5.4. Rheology of Nanocellulose
5.5. Morphology
5.6. Degree of Fibrillation
5.7. Electrical Properties
5.8. Biodegradability
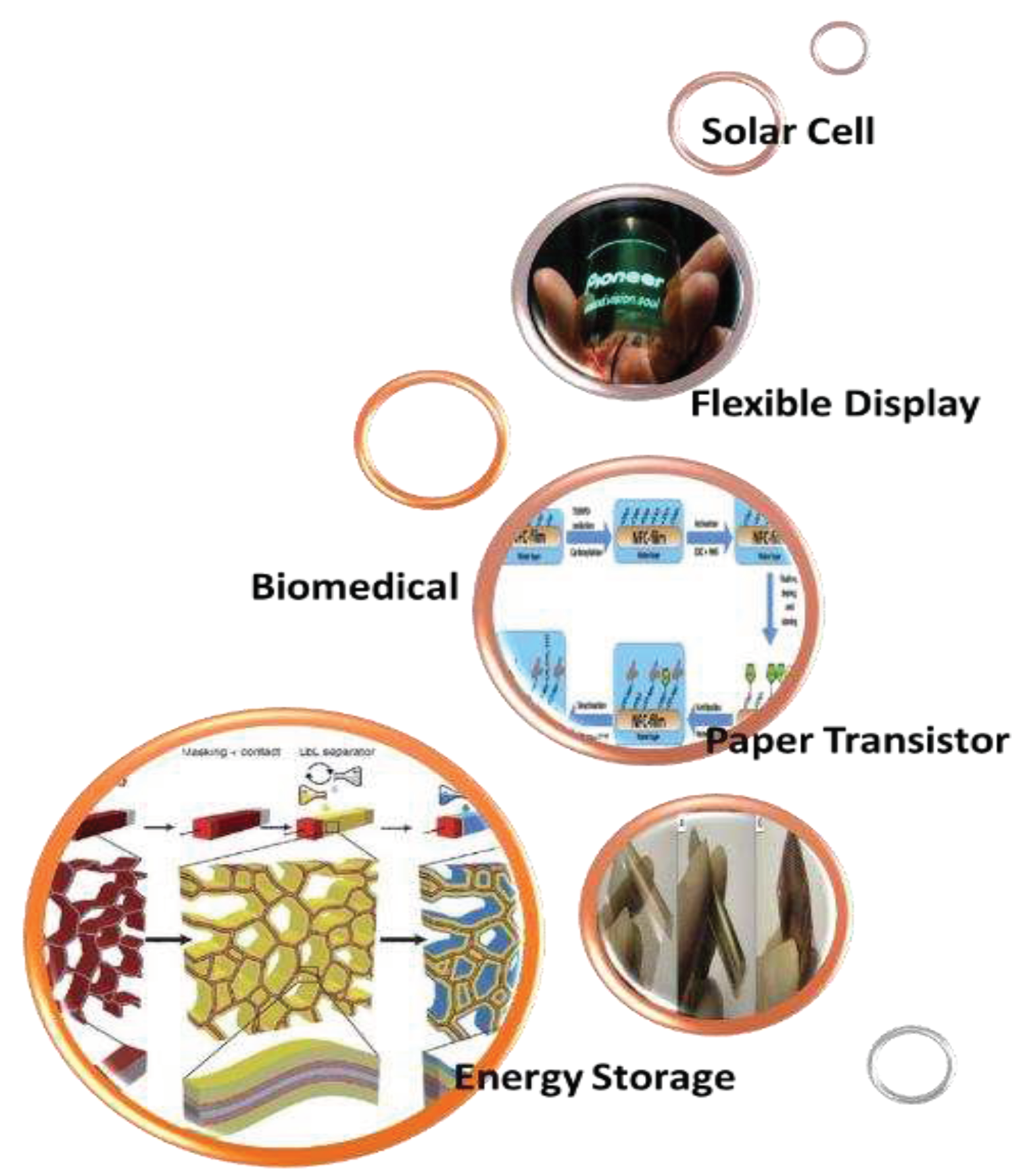
6. Applications of Nanocellulose
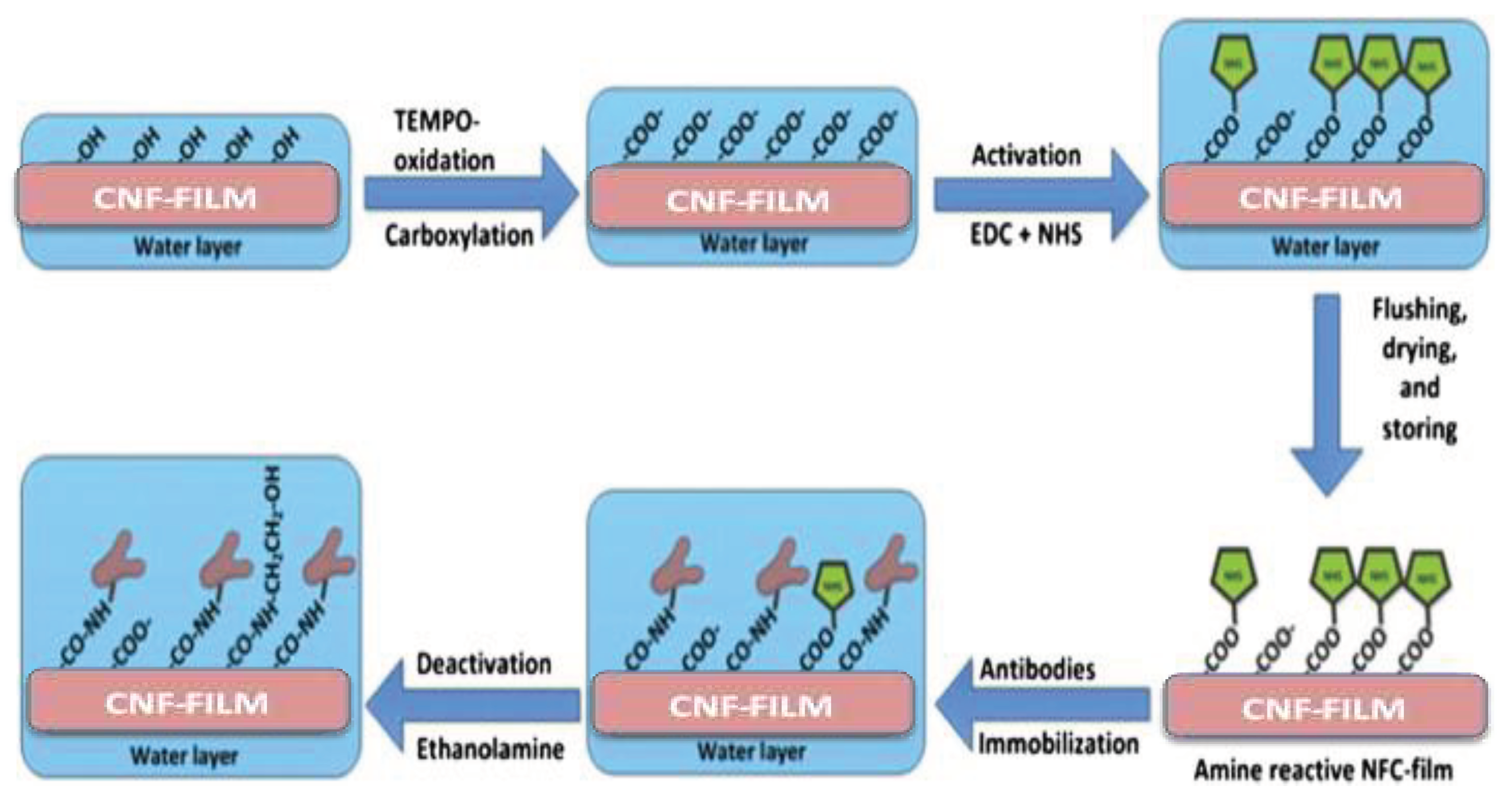
6.1. Biomedical
6.2. Flexible Display
6.3. Energy Storage
6.4. Paper Transistor
6.5. Solar Cell
6.6. Overview of Nanocellulose Application
7. Future Perspectives and Challenges
8. Conclusions
Supplementary Materials
Acknowledgments
Nomenclature
| Abbreviations | |||
| CNC | Cellulose nanocrystals | MCC | Microcrystalline cellulose |
| CNF | Cellulose nanofibrils | ACC | Aqueous counter collision |
| BNC | Bacterial nanocellulose | ILs | Ionic liquids |
| SEM | Scanning electron microscope | HIUS | High intensity ultrasonication |
| TEM | Transmission electron microscope | WAXS | Wide-angle X-ray scattering |
| CAGR | Compound annual growth rate | PLA | Polylactic acid |
| TEMPO | (2,2,6,6-Tetramethylpiperidin-1yl)oxyl | AFM | Atomic force microscopy |
| NaOCl | Sodium hypochlorite | IPTS | Isocyanatepropltriethoxysilane |
| CAGA | Compound annual growth rate | USA | United States of America |
| PVA | Polyvinyl alcohol | OLED | Organic light-emitting diode |
| LIB | Li-ion battery | Symbols | |
| MWCNT | Multi-walled carbon nanotube | $ | United States dollar |
| IgG | Immunoglobulin | µ | micro |
| PANI | Polyaniline | Å | Angstrom |
| CTE | Coefficient of thermal expansion | nm | Nanometer |
| APS | Ammonium persulfate | ppm | Parts per million |
References
- Wang, J.; Han, X.; Zhang, C.; Liu, K.; Duan, G. Source of nanocellulose and its application in nanocomposite packaging material: A review. Nanomaterials 2022, 12, 3158. [Google Scholar] [CrossRef]
- Tafete, G.A.; Abera, M.K.; Thothadri, G. Review on nanocellulose-based materials for supercapacitors applications. Journal of Energy Storage 2022, 48, 103938. [Google Scholar] [CrossRef]
- Shi, Z.; Phillips, G.O.; Yang, G. Nanocellulose electroconductive composites. Nanoscale 2013, 5, 3194–3201. [Google Scholar] [CrossRef]
- Sun, X.; Xu, W.; Zhang, X.; Lei, T.; Lee, S.-Y.; Wu, Q. ZIF-67@ Cellulose nanofiber hybrid membrane with controlled porosity for use as Li-ion battery separator. Journal of Energy Chemistry 2021, 52, 170–180. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, L.; Lu, Y.; Zhang, X.; Yang, D. Research progress of nanocellulose for electrochemical energy storage: A review. Journal of Energy Chemistry 2020, 51, 342–361. [Google Scholar] [CrossRef]
- Hentze, H.-P. From nanocellulose science towards applications. Developments in advanced biocomposites 2010, 71. [Google Scholar]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. Journal of Applied Polymer Science 2015, 132. [Google Scholar] [CrossRef]
- Greenstreet, E.; Ramsey, H. Properties of Nanocellulose.
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Materials Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Randhawa, A.; Dutta, S.D.; Ganguly, K.; Patil, T.V.; Patel, D.K.; Lim, K.-T. A review of properties of nanocellulose, its synthesis, and potential in biomedical applications. Applied Sciences 2022, 12, 7090. [Google Scholar] [CrossRef]
- Balat, M.; Ayar, G. Biomass energy in the world, use of biomass and potential trends. Energy sources 2005, 27, 931–940. [Google Scholar] [CrossRef]
- Norizan, M.N.; Shazleen, S.S.; Alias, A.H.; Sabaruddin, F.A.; Asyraf, M.R.M.; Zainudin, E.S.; Abdullah, N.; Samsudin, M.S.; Kamarudin, S.H.; Norrrahim, M.N.F. Nanocellulose-Based Nanocomposites for Sustainable Applications: A Review. Nanomaterials 2022, 12, 3483. [Google Scholar] [CrossRef]
- Peng, B.L.; Dhar, N.; Liu, H.; Tam, K. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. The Canadian Journal of Chemical Engineering 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chemical Society Reviews 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angewandte Chemie International Edition 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Habibi, Y.; Goffin, A.-L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites based on poly (ε-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J Mater Chem 2008, 18, 5002–5010. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Mohammadkazemi, F.; Doosthoseini, K.; Ganjian, E.; Azin, M. Manufacturing of bacterial nano-cellulose reinforced fiber− cement composites. Construction and Building Materials 2015, 101, 958–964. [Google Scholar] [CrossRef]
- Wang, H.D.; Roeder, R.D.; Whitney, R.A.; Champagne, P.; Cunningham, M.F. Graft modification of crystalline nanocellulose by Cu (0)-mediated SET living radical polymerization. Journal of Polymer Science Part A: Polymer Chemistry 2015, 53, 2800–2808. [Google Scholar] [CrossRef]
- Milanez, D.H.; Amaral, R.M.d.; Faria, L.I.L.d.; Gregolin, J.A.R. Assessing nanocellulose developments using science and technology indicators. Materials Research 2013, 16, 635–641. [Google Scholar] [CrossRef]
- Batmaz, R.; Mohammed, N.; Zaman, M.; Minhas, G.; Berry, R.M.; Tam, K.C. Cellulose nanocrystals as promising adsorbents for the removal of cationic dyes. Cellulose 2014, 21, 1655–1665. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Iannoni, A.; Saino, E.; Visai, L.; Berglund, L.A.; Kenny, J. Multifunctional bionanocomposite films of poly (lactic acid), cellulose nanocrystals and silver nanoparticles. Carbohyd Polym 2012, 87, 1596–1605. [Google Scholar] [CrossRef]
- Peresin, M.S.; Habibi, Y.; Zoppe, J.O.; Pawlak, J.J.; Rojas, O.J. Nanofiber composites of polyvinyl alcohol and cellulose nanocrystals: Manufacture and characterization. Biomacromolecules 2010, 11, 674–681. [Google Scholar] [CrossRef]
- Kelly, J.A.; Shukaliak, A.M.; Cheung, C.C.; Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Responsive photonic hydrogels based on nanocrystalline cellulose. Angewandte Chemie International Edition 2013, 52, 8912–8916. [Google Scholar] [CrossRef]
- Shopsowitz, K.E.; Qi, H.; Hamad, W.Y.; MacLachlan, M.J. Free-standing mesoporous silica films with tunable chiral nematic structures. Nature 2010, 468, 422. [Google Scholar] [CrossRef]
- Tang, J.; Lee, M.F.X.; Zhang, W.; Zhao, B.; Berry, R.M.; Tam, K.C. Dual responsive pickering emulsion stabilized by poly [2-(dimethylamino) ethyl methacrylate] grafted cellulose nanocrystals. Biomacromolecules 2014, 15, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Marway, H.S.; Kasem, H.; Pelton, R.; Cranston, E.D. Dried and redispersible cellulose nanocrystal Pickering emulsions. ACS Macro Letters 2016, 5, 185–189. [Google Scholar] [CrossRef]
- Sunasee, R.; Hemraz, U.D.; Ckless, K. Cellulose nanocrystals: A versatile nanoplatform for emerging biomedical applications. Expert opinion on drug delivery 2016, 13, 1243–1256. [Google Scholar] [CrossRef]
- Li, F.; Biagioni, P.; Finazzi, M.; Tavazzi, S.; Piergiovanni, L. Tunable green oxygen barrier through layer-by-layer self-assembly of chitosan and cellulose nanocrystals. Carbohyd Polym 2013, 92, 2128–2134. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Sui, L.; Elkasabi, Y.; Burgardt, P.; Lee, J.; Miryala, A.; Kusumaatmaja, W.; Carman, M.R.; Shtein, M.; Kieffer, J. Layer-by-layer assembled films of cellulose nanowires with antireflective properties. Langmuir 2007, 23, 7901–7906. [Google Scholar] [CrossRef] [PubMed]
- Cerclier, C.; Guyomard-Lack, A.; Moreau, C.; Cousin, F.; Beury, N.; Bonnin, E.; Jean, B.; Cathala, B. Coloured Semi-reflective Thin Films for Biomass-hydrolyzing Enzyme Detection. Adv Mater 2011, 23, 3791–3795. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lai, C.; Marchewka, R.; Berry, R.; Tam, K. Use of CdS quantum dot-functionalized cellulose nanocrystal films for anti-counterfeiting applications. Nanoscale 2016, 8, 13288–13296. [Google Scholar] [CrossRef]
- Habibi, Y.; Chanzy, H.; Vignon, M.R. TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 2006, 13, 679–687. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Lu, C.; Deng, Y. Aerogels from crosslinked cellulose nano/micro-fibrils and their fast shape recovery property in water. J Mater Chem 2012, 22, 11642–11650. [Google Scholar] [CrossRef]
- Roco, M.C.; Bainbridge, W.S. Societal implications of nanoscience and nanotechnology: Maximizing human benefit. J Nanopart Res 2005, 7, 1–13. [Google Scholar] [CrossRef]
- Buch, N.; Rehman, O.; Hiller, J. Impact of processed cellulose fibers on portland cement concrete properties. Transportation Research Record: Journal of the Transportation Research Board 1999, 72–80. [Google Scholar] [CrossRef]
- Lokhande, P.; Singh, P.P.; Vo, D.-V.N.; Kumar, D.; Balasubramanian, K.; Mubayi, A.; Srivastava, A.; Sharma, A. Bacterial nanocellulose: Green polymer materials for high performance energy storage applications. Journal of Environmental Chemical Engineering 2022, 108176. [Google Scholar] [CrossRef]
- Iguchi, M.; Yamanaka, S.; Budhiono, A. Bacterial cellulose—A masterpiece of nature’s arts. J Mater Sci 2000, 35, 261–270. [Google Scholar] [CrossRef]
- Okiyama, A.; Motoki, M.; Yamanaka, S. Bacterial cellulose II. Processing of the gelatinous cellulose for food materials. Food Hydrocolloids 1992, 6, 479–487. [Google Scholar] [CrossRef]
- Nishi, Y.; Uryu, M.; Yamanaka, S.; Watanabe, K.; Kitamura, N.; Iguchi, M.; Mitsuhashi, S. The structure and mechanical properties of sheets prepared from bacterial cellulose. J Mater Sci 1990, 25, 2997–3001. [Google Scholar] [CrossRef]
- Watanabe, K.; Eto, Y.; Takano, S.; Nakamori, S.; Shibai, H.; Yamanaka, S. A new bacterial cellulose substrate for mammalian cell culture. Cytotechnology 1993, 13, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Helenius, G.; Bäckdahl, H.; Bodin, A.; Nannmark, U.; Gatenholm, P.; Risberg, B. In vivo biocompatibility of bacterial cellulose. J Biomed Mater Res A 2006, 76, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Progress in Polymer Science 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M. Microbial cellulose—The natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Chandana, A.; Mallick, S.P.; Dikshit, P.K.; Singh, B.N.; Sahi, A.K. Recent developments in bacterial nanocellulose production and its biomedical applications. Journal of Polymers and the Environment 2022, 1–28. [Google Scholar] [CrossRef]
- Yasuda, K.; Gong, J.P.; Katsuyama, Y.; Nakayama, A.; Tanabe, Y.; Kondo, E.; Ueno, M.; Osada, Y. Biomechanical properties of high-toughness double network hydrogels. Biomaterials 2005, 26, 4468–4475. [Google Scholar] [CrossRef]
- Nakayama, A.; Kakugo, A.; Gong, J.P.; Osada, Y.; Takai, M.; Erata, T.; Kawano, S. High mechanical strength double-network hydrogel with bacterial cellulose. Adv Funct Mater 2004, 14, 1124–1128. [Google Scholar] [CrossRef]
- Hong, L.; Wang, Y.; Jia, S.; Huang, Y.; Gao, C.; Wan, Y. Hydroxyapatite/bacterial cellulose composites synthesized via a biomimetic route. Mater Lett 2006, 60, 1710–1713. [Google Scholar] [CrossRef]
- Chen, S.; Zou, Y.; Yan, Z.; Shen, W.; Shi, S.; Zhang, X.; Wang, H. Carboxymethylated-bacterial cellulose for copper and lead ion removal. J Hazard Mater 2009, 161, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.; Othman, N.E.A.; Wahab, N.A.; Aziz, A.A. The Effect of Chemical and High Pressure Homogenization Treatment Conditions on the Morphology of Nanocellulose. Journal of Advanced Research in Applied Mechanics 2022, 93, 1–7. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind Crop Prod 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Li, J.; Wei, X.; Wang, Q.; Chen, J.; Chang, G.; Kong, L.; Su, J.; Liu, Y. Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohyd Polym 2012, 90, 1609–1613. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Sain, M.; Kortschot, M. Cellulose microfibrils: A novel method of preparation using high shear refining and cryocrushing. Holzforschung 2005, 59, 102–107. [Google Scholar] [CrossRef]
- Sihag, S.; Pal, J.; Yadav, M. Extraction and Characterization of Nanocellulose from Wheat Straw: Facile Approach. Journal of Water and Environmental Nanotechnology 2022, 7, 317–331. [Google Scholar]
- Bhatnagar, A.; Sain, M. Processing of cellulose nanofiber-reinforced composites. Journal of Reinforced Plastics and Composites 2005, 24, 1259–1268. [Google Scholar] [CrossRef]
- Wang, B.; Sain, M. Dispersion of soybean stock-based nanofiber in a plastic matrix. Polymer International 2007, 56, 538–546. [Google Scholar] [CrossRef]
- Wang, B.; Sain, M. Isolation of nanofibers from soybean source and their reinforcing capability on synthetic polymers. Composites Science and Technology 2007, 67, 2521–2527. [Google Scholar] [CrossRef]
- Gulipalli, P.; Borle, S.; Chivukula, K.; Adusumalli, R.B. Processing of nanocellulose sheet for capturing fine particulate matter. Materials Today: Proceedings 2022. [Google Scholar] [CrossRef]
- Taniguchi, T.; Okamura, K. New films produced from microfibrillated natural fibres. Polymer International 1998, 47, 291–294. [Google Scholar] [CrossRef]
- Iwamoto, S.; Nakagaito, A.; Yano, H. Nano-fibrillation of pulp fibers for the processing of transparent nanocomposites. Applied Physics A: Materials Science & Processing 2007, 89, 461–466. [Google Scholar]
- Wang, Q.; Zhu, J.; Gleisner, R.; Kuster, T.; Baxa, U.; McNeil, S. Morphological development of cellulose fibrils of a bleached eucalyptus pulp by mechanical fibrillation. Cellulose 2012, 19, 1631–1643. [Google Scholar] [CrossRef]
- Foo, M.L.; Ooi, C.W.; Tan, K.W.; Chew, I.M. Preparation of black cumin seed oil Pickering nanoemulsion with enhanced stability and antioxidant potential using nanocrystalline cellulose from oil palm empty fruit bunch. Chemosphere 2022, 287, 132108. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, A.; Salas, C.; Rojas, O.J. Physical, thermal, chemical and rheological characterization of cellulosic microfibrils and microparticles produced from soybean hulls. Ind Crop Prod 2016, 84, 337–343. [Google Scholar] [CrossRef]
- Bharimalla, A.K.; Deshmukh, S.; Patil, S.; Nadanathangam, V.; Saxena, S. Development of energy efficient nanocellulose production process by enzymatic pretreatment and controlled temperature refining of cotton linters. Cellulose 2022, 1–15. [Google Scholar] [CrossRef]
- Hamada, H.; Tahara, K.; Uchida, A. The effects of nano-fibrillated cellulose as a coating agent for screen printing. Proceedings of 12th TAPPI Advanced Coating Fundamentals Symposium.
- Nakagaito, A.; Yano, H. The effect of morphological changes from pulp fiber towards nano-scale fibrillated cellulose on the mechanical properties of high-strength plant fiber based composites. Applied Physics A 2004, 78, 547–552. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.A. Structure and properties of cellulose nanocomposite films containing melamine formaldehyde. Journal of Applied Polymer Science 2007, 106, 2817–2824. [Google Scholar] [CrossRef]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses, and commercial potential. J. Appl. Polym. Sci.: Appl. Polym. Symp. 1983, 37, 815–827. [Google Scholar]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose. Google Patents: 1983.
- Joseleau, J.-P.; Chevalier-Billosta, V.; Ruel, K. Interaction between microfibrillar cellulose fines and fibers: Influence on pulp qualities and paper sheet properties. Cellulose 2012, 19, 769–777. [Google Scholar] [CrossRef]
- Spence, K.L.; Venditti, R.A.; Rojas, O.J.; Habibi, Y.; Pawlak, J.J. The effect of chemical composition on microfibrillar cellulose films from wood pulps: Water interactions and physical properties for packaging applications. Cellulose 2010, 17, 835–848. [Google Scholar] [CrossRef]
- Hassan, M.L.; Hassan, E.A.; Oksman, K.N. Effect of pretreatment of bagasse fibers on the properties of chitosan/microfibrillated cellulose nanocomposites. J Mater Sci 2011, 46, 1732–1740. [Google Scholar] [CrossRef]
- Stelte, W.; Sanadi, A.R. Preparation and characterization of cellulose nanofibers from two commercial hardwood and softwood pulps. Ind Eng Chem Res 2009, 48, 11211–11219. [Google Scholar] [CrossRef]
- Karande, V.; Bharimalla, A.; Hadge, G.; Mhaske, S.; Vigneshwaran, N. Nanofibrillation of cotton fibers by disc refiner and its characterization. Fibers and Polymers 2011, 12, 399–404. [Google Scholar] [CrossRef]
- Bharimalla, A.; Patil, P.; Deshmukh, S.; Vigneshwaran, N. ENERGY EFFICIENT PRODUCTION OF NANO-FIBRILLATED CELLULOSE (NFC) FROM COTTON LINTERS BY TRI-DISC REFINING AND ITS CHARACTERIZATION. CELLULOSE CHEMISTRY AND TECHNOLOGY 2017, 51, 395–401. [Google Scholar]
- Uetani, K.; Yano, H. Nanofibrillation of wood pulp using a high-speed blender. Biomacromolecules 2010, 12, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Hsieh, Y.-L. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohyd Polym 2013, 95, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Chaker, A.; Alila, S.; Mutjé, P.; Vilar, M.R.; Boufi, S. Key role of the hemicellulose content and the cell morphology on the nanofibrillation effectiveness of cellulose pulps. Cellulose 2013, 20, 2863–2875. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Ikenaga, K.; Takagi, H. Cellulose nanofiber extraction from grass by a modified kitchen blender. Mod Phys Lett B 2015, 29, 1540039. [Google Scholar] [CrossRef]
- Verma, Y.K.; Singh, A.K.; Paswan, M. Preparation of Bamboo-Based Nano-Cellulose by Ball Milling. In Advances in Material Science and Metallurgy; Springer, 2003; pp. 89–98. [Google Scholar]
- Zhang, L.; Tsuzuki, T.; Wang, X. Preparation of cellulose nanofiber from softwood pulp by ball milling. Cellulose 2015, 22, 1729–1741. [Google Scholar] [CrossRef]
- Kekäläinen, K.; Liimatainen, H.; Biale, F.; Niinimäki, J. Nanofibrillation of TEMPO-oxidized bleached hardwood kraft cellulose at high solids content. Holzforschung 2015, 69, 1077–1088. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, S.; Mao, Q.; Buekens, A.; Wang, Y.; Yan, J. Energy transfer and kinetics in mechanochemistry. Environmental Science and Pollution Research 2017, 24, 24562–24571. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, D.; Kanda, A.; Kondo, T. Characterization of mercerized cellulose nanofibrils prepared by aqueous counter collision process. Journal of Wood Science 2022, 68, 1–9. [Google Scholar] [CrossRef]
- Kose, R.; Mitani, I.; Kasai, W.; Kondo, T. “Nanocellulose” as a single nanofiber prepared from pellicle secreted by gluconacetobacter xylinus using aqueous counter collision. Biomacromolecules 2011, 12, 716–720. [Google Scholar] [CrossRef]
- Kondo, T.; Kose, R.; Naito, H.; Kasai, W. Aqueous counter collision using paired water jets as a novel means of preparing bio-nanofibers. Carbohyd Polym 2014, 112, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, K.; Yokota, S.; Kondo, T. Difference between bamboo-and wood-derived cellulose nanofibers prepared by the aqueous counter collision method. Nordic Pulp & Paper Research Journal 2014, 29, 69–76. [Google Scholar]
- Almashhadani, A.Q.; Leh, C.P.; Chan, S.-Y.; Lee, C.Y.; Goh, C.F. Nanocrystalline cellulose isolation via acid hydrolysis from non-woody biomass: Importance of hydrolysis parameters. Carbohydrate Polymers 2022, 119285. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Natural fibers, biopolymers, and biocomposites; CRC press, 2005. [Google Scholar]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resources Conversion 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Wang, B.; Sain, M. The effect of chemically coated nanofiber reinforcement on biopolymer based nanocomposites. Bioresources 2007, 2, 371–388. [Google Scholar] [CrossRef]
- Wang, B.; Sain, M.; Oksman, K. Study of structural morphology of hemp fiber from the micro to the nanoscale. Applied Composite Materials 2007, 14, 89. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues–Wheat straw and soy hulls. Bioresource technology 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Lee, H.; Hamid, S.B.A.; Zain, S. Conversion of lignocellulosic biomass to nanocellulose: Structure and chemical process. The Scientific World Journal 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nishiyama, Y.; Putaux, J.-L.; Vignon, M.; Isogai, A. Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Vignon, M.R. Optimization of cellouronic acid synthesis by TEMPO-mediated oxidation of cellulose III from sugar beet pulp. Cellulose 2008, 15, 177–185. [Google Scholar] [CrossRef]
- Janardhnan, S.; Sain, M.M. Targeted disruption of hydroxyl chemistry and crystallinity in natural fibers for the isolation of cellulose nano-fibers via enzymatic treatment. BioResources 2011, 6, 1242–1250. [Google Scholar]
- López-Rubio, A.; Lagaron, J.; Ankerfors, M.; Lindström, T.; Nordqvist, D.; Mattozzi, A.; Hedenqvist, M.S. Enhanced film forming and film properties of amylopectin using micro-fibrillated cellulose. Carbohyd Polym 2007, 68, 718–727. [Google Scholar] [CrossRef]
- Svagan, A.J.; Azizi Samir, M.A.; Berglund, L.A. Biomimetic polysaccharide nanocomposites of high cellulose content and high toughness. Biomacromolecules 2007, 8, 2556–2563. [Google Scholar] [CrossRef]
- Tanpichai, S.; Quero, F.; Nogi, M.; Yano, H.; Young, R.J.; Lindstrom, T.; Sampson, W.W.; Eichhorn, S.J. Effective Young’s modulus of bacterial and microfibrillated cellulose fibrils in fibrous networks. Biomacromolecules 2012, 13, 1340–1349. [Google Scholar] [CrossRef]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of cellulose with ionic liquids and its application: A mini-review. Green Chemistry 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Kuzmina Olga, G.; Sashina Elena, S.; Svetlana, T.; Dariusz, W. Dissolved state of cellulose in ionic liquids-the impact of water. Issues 2017. [Google Scholar]
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem Rev 2009, 109, 6712–6728. [Google Scholar] [CrossRef]
- Fukaya, Y.; Hayashi, K.; Wada, M.; Ohno, H. Cellulose dissolution with polar ionic liquids under mild conditions: Required factors for anions. Green Chemistry 2008, 10, 44–46. [Google Scholar] [CrossRef]
- Vitz, J.; Erdmenger, T.; Haensch, C.; Schubert, U.S. Extended dissolution studies of cellulose in imidazolium based ionic liquids. Green chemistry 2009, 11, 417–424. [Google Scholar] [CrossRef]
- Mohd Ishak, N.A.; Abdullah, F.Z.; Muhd Julkapli, N. Production and characteristics of nanocellulose obtained with using of ionic liquid and ultrasonication. Journal of Nanoparticle Research 2022, 24, 1–22. [Google Scholar] [CrossRef]
- Abramov, O. High Intensity Ultrasonics. Theory and Industrial Applications, Gordon and Breach Science Publishers. America 1998. [Google Scholar]
- Wang, S.; Cheng, Q. A novel process to isolate fibrils from cellulose fibers by high-intensity ultrasonication, Part 1: Process optimization. Journal of applied polymer science 2009, 113, 1270–1275. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of ultrasound to the synthesis of nanostructured materials. Adv Mater 2010, 22, 1039–1059. [Google Scholar] [CrossRef]
- Gemmer, R.E.; Borsoi, C.; Hansen, B.; Dahlem Júnior, M.A.; Francisquetti, E.L.; Rossa Beltrami, L.V.; Zattera, A.J.; Catto, A.L. Extraction of Nanocellulose from Yerba Mate Residues Using Steam Explosion, TEMPO-mediated Oxidation and Ultra-fine Friction Grinding. Journal of Natural Fibers 2022, 19, 10539–10549. [Google Scholar] [CrossRef]
- Liao, Y.-C.; Yen, F.-Y.; Hung, F.; Su, C.-H.; Chen, W.-H. Intermittent pressurized operation of steam explosion pretreatment system. Journal of the Taiwan Institute of Chemical Engineers 2016, 67, 285–291. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Vignon, M.R. Microfibrillated cellulose from the peel of prickly pear fruits. Food Chemistry 2009, 115, 423–429. [Google Scholar] [CrossRef]
- Savadekar, N.; Mhaske, S. Synthesis of nano cellulose fibers and effect on thermoplastics starch based films. Carbohyd Polym 2012, 89, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohyd Polym 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Hamid, S.B.A.; Zain, S.K.; Das, R.; Centi, G. Synergic effect of tungstophosphoric acid and sonication for rapid synthesis of crystalline nanocellulose. Carbohyd Polym 2016, 138, 349–355. [Google Scholar] [CrossRef]
- Theivasanthi, T.; Christma, F.A.; Toyin, A.J.; Gopinath, S.C.; Ravichandran, R. Synthesis and characterization of cotton fiber-based nanocellulose. International journal of biological macromolecules 2018, 109, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Zarei, S.; Niad, M.; Raanaei, H. The removal of mercury ion pollution by using Fe3O4-nanocellulose: Synthesis, characterizations and DFT studies. J Hazard Mater 2018, 344, 258–273. [Google Scholar] [CrossRef]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Synthesis and characterization of chitosan-PVP-nanocellulose composites for in-vitro wound dressing application. International Journal of Biological Macromolecules 2017, 105, 111–120. [Google Scholar] [CrossRef]
- Benini, K.C.C.d.C.; Voorwald, H.J.C.; Cioffi, M.O.H.; Rezende, M.C.; Arantes, V. Preparation of nanocellulose from Imperata brasiliensis grass using Taguchi method. Carbohyd Polym 2018, 192, 337–346. [Google Scholar] [CrossRef]
- Wang, H.; Kong, L.; Ziegler, G.R. Fabrication of starch - Nanocellulose composite fibers by electrospinning. Food Hydrocolloids 2019, 90, 90–98. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Pieczywek, P.M.; Zdunek, A. Tailored nanocellulose structure depending on the origin. Example of apple parenchyma and carrot root celluloses. Carbohyd Polym 2019, 210, 186–195. [Google Scholar] [CrossRef]
- Franco, T.S.; Potulski, D.C.; Viana, L.C.; Forville, E.; de Andrade, A.S.; de Muniz, G.I.B. Nanocellulose obtained from residues of peach palm extraction (Bactris gasipaes). Carbohyd Polym 2019, 218, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Hse, C.-Y.; Cornelis, F.; Hu, T.; Qi, J.; Shupe, T.F. Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohyd Polym 2016, 151, 725–734. [Google Scholar] [CrossRef]
- Chandra, J.; George, N.; Narayanankutty, S.K. Isolation and characterization of cellulose nanofibrils from arecanut husk fibre. Carbohyd Polym 2016, 142, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.B.d.; Bras, J.; Pimenta, M.T.B.; Curvelo, A.A.d.S.; Belgacem, M.N. Production of cellulose nanocrystals from sugarcane bagasse fibers and pith. Ind Crop Prod 2016, 93, 48–57. [Google Scholar] [CrossRef]
- Lamaming, J.; Hashim, R.; Sulaiman, O.; Leh, C.P.; Sugimoto, T.; Nordin, N.A. Cellulose nanocrystals isolated from oil palm trunk. Carbohyd Polym 2015, 127, 202–208. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Menegalli, F.C. Cellulose nanofibers produced from banana peel by chemical and enzymatic treatment. LWT-Food Science and Technology 2014, 59, 1311–1318. [Google Scholar] [CrossRef]
- Thomas, M.G.; Abraham, E.; Jyotishkumar, P.; Maria, H.J.; Pothen, L.A.; Thomas, S. Nanocelluloses from jute fibers and their nanocomposites with natural rubber: Preparation and characterization. International Journal of Biological Macromolecules 2015, 81, 768–777. [Google Scholar] [CrossRef]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chemical Society Reviews 2014, 43, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ma, X.; Yang, G.; Alain, D. Introduction to nanocellulose. Nanocellulose: From Fundamentals to Advanced Materials 2019, 1–20. [Google Scholar]
- Lu, J.; Sun, C.; Yang, K.; Wang, K.; Jiang, Y.; Tusiime, R.; Yang, Y.; Fan, F.; Sun, Z.; Liu, Y. Properties of polylactic acid reinforced by hydroxyapatite modified nanocellulose. Polymers 2019, 11, 1009. [Google Scholar] [CrossRef]
- Li, B.; Wu, C.; Zhang, Y.; Cao, X.; Luo, Z. Microstructure and thermal and tensile properties of poly (vinyl alcohol) nanocomposite films reinforced by polyacrylamide grafted cellulose nanocrystals. Journal of Macromolecular Science, Part B 2020, 59, 223–234. [Google Scholar] [CrossRef]
- Tang, C.; Chen, Y.; Luo, J.; Low, M.Y.; Shi, Z.; Tang, J.; Zhang, Z.; Peng, B.; Tam, K.C. Pickering emulsions stabilized by hydrophobically modified nanocellulose containing various structural characteristics. Cellulose 2019, 26, 7753–7767. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose processing properties and potential applications. Current Forestry Reports 2019, 5, 76–89. [Google Scholar] [CrossRef]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. Journal of Polymer Science Part B: Polymer Physics 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Camarero-Espinosa, S.; Endes, C.; Mueller, S.; Petri-Fink, A.; Rothen-Rutishauser, B.; Weder, C.; Clift, M.J.D.; Foster, E.J. Elucidating the potential biological impact of cellulose nanocrystals. Fibers 2016, 4, 21. [Google Scholar] [CrossRef]
- Roman, M. Toxicity of cellulose nanocrystals: A review. Industrial Biotechnology 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. New process of chemical grafting of cellulose nanoparticles with a long chain isocyanate. Langmuir 2010, 26, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Bras, J.; Viet, D.; Bruzzese, C.; Dufresne, A. Correlation between stiffness of sheets prepared from cellulose whiskers and nanoparticles dimensions. Carbohyd Polym 2011, 84, 211–215. [Google Scholar] [CrossRef]
- Sattar, M.A.; Patnaik, A. Role of interface structure and chain dynamics on the diverging glass transition behavior of SSBR-SiO2-PIL elastomers. ACS omega 2020, 5, 21191–21202. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Thomas, S.; Dufresne, A. Handbook of nanocellulose and cellulose nanocomposites; John Wiley & Sons, 2017. [Google Scholar]
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. Journal of Saudi Chemical Society 2018, 22, 949–978. [Google Scholar] [CrossRef]
- Heux, L.; Chauve, G.; Bonini, C. Nonflocculating and chiral-nematic self-ordering of cellulose microcrystals suspensions in nonpolar solvents. Langmuir 2000, 16, 8210–8212. [Google Scholar] [CrossRef]
- Zhou, Q.; Brumer, H.; Teeri, T.T. Self-Organization of Cellulose Nanocrystals Adsorbed with Xyloglucan Oligosaccharide—Poly (ethylene glycol)—Polystyrene Triblock Copolymer. Macromolecules (Print) 2009, 42, 5430–5432. [Google Scholar] [CrossRef]
- Follain, N.; Belbekhouche, S.; Bras, J.; Siqueira, G.; Marais, S.; Dufresne, A. Water transport properties of bio-nanocomposites reinforced by Luffa cylindrica cellulose nanocrystals. Journal of membrane science 2013, 427, 218–229. [Google Scholar] [CrossRef]
- De Nooy, A.; Besemer, A.; Van Bekkum, H. Highly selective TEMPO mediated oxidation of primary alcohol groups in polysaccharides. Recueil des Travaux Chimiques des Pays-Bas 1994, 113, 165–166. [Google Scholar] [CrossRef]
- Xhanari, K.; Syverud, K.; Chinga-Carrasco, G.; Paso, K.; Stenius, P. Reduction of water wettability of nanofibrillated cellulose by adsorption of cationic surfactants. Cellulose 2011, 18, 257–270. [Google Scholar] [CrossRef]
- Qing, Y.; Sabo, R.; Zhu, J.; Agarwal, U.; Cai, Z.; Wu, Y. A comparative study of cellulose nanofibrils disintegrated via multiple processing approaches. Carbohyd Polym 2013, 97, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Osong, S.H.; Norgren, S.; Engstrand, P. Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: A review. Cellulose 2016, 23, 93–123. [Google Scholar] [CrossRef]
- Chin, K.M.; Sung Ting, S.; Ong, H.L.; Omar, M. Surface functionalized nanocellulose as a veritable inclusionary material in contemporary bioinspired applications: A review. Journal of Applied Polymer Science 2018, 135, 46065. [Google Scholar] [CrossRef]
- Eyholzer, C.; Tingaut, P.; Zimmermann, T.; Oksman, K. Dispersion and reinforcing potential of carboxymethylated nanofibrillated cellulose powders modified with 1-hexanol in extruded poly (lactic acid)(PLA) composites. Journal of Polymers and the Environment 2012, 20, 1052–1062. [Google Scholar] [CrossRef]
- Hasani, M.; Cranston, E.D.; Westman, G.; Gray, D.G. Cationic surface functionalization of cellulose nanocrystals. Soft Matter 2008, 4, 2238–2244. [Google Scholar] [CrossRef]
- Eyholzer, C.; Lopez-Suevos, F.; Tingaut, P.; Zimmermann, T.; Oksman, K. Reinforcing effect of carboxymethylated nanofibrillated cellulose powder on hydroxypropyl cellulose. Cellulose 2010, 17, 793–802. [Google Scholar] [CrossRef]
- Daud, J.B.; Lee, K.Y. Surface modification of nanocellulose. Handbook of nanocellulose and cellulose nanocomposites 2017, 1, 101–122. [Google Scholar]
- Nechita, P.; Panaitescu, D. Improving the dispersibility of cellulose microfibrillated structures in polymer matrix by controlling drying conditions and chemical surface modifications. Cell Chem Technol 2013, 47, 711–719. [Google Scholar]
- Ashori, A.; Babaee, M.; Jonoobi, M.; Hamzeh, Y. Solvent-free acetylation of cellulose nanofibers for improving compatibility and dispersion. Carbohyd Polym 2014, 102, 369–375. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, J.; Gong, J.; Li, J.; Mo, L. Preparation, characterization and acetylation of cellulose nanocrystal allomorphs. Cellulose 2018, 25, 4905–4918. [Google Scholar] [CrossRef]
- Fathi, M.; Karim, M.; Ahmadi, N. Nanostructures of cellulose for encapsulation of food ingredients. In Biopolymer Nanostructures for Food Encapsulation Purposes; Elsevier, 2019; pp. 493–519. [Google Scholar]
- Sirviö, J.A.; Visanko, M.; Liimatainen, H. Deep eutectic solvent system based on choline chloride-urea as a pre-treatment for nanofibrillation of wood cellulose. Green Chemistry 2015, 17, 3401–3406. [Google Scholar] [CrossRef]
- Zhu, G.; Lin, N. Surface chemistry of nanocellulose. Nanocellulose: From fundamentals to advanced materials 2019, 115–153. [Google Scholar]
- Thakur, V.; Guleria, A.; Kumar, S.; Sharma, S.; Singh, K. Recent advances in nanocellulose processing, functionalization and applications: A review. Materials Advances 2021, 2, 1872–1895. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem Rev 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Liimatainen, H.; Visanko, M.; Sirvio, J.A.; Hormi, O.E.; Niinimaki, J. Enhancement of the nanofibrillation of wood cellulose through sequential periodate–chlorite oxidation. Biomacromolecules 2012, 13, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Semenikhin, N.; Chang, H.; Moon, R.J.; Kumar, S. Post-sulfonation of cellulose nanofibrils with a one-step reaction to improve dispersibility. Carbohyd Polym 2018, 181, 247–255. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Jin, L.; Wang, Y.; Qin, M. Adsorption of Cu (II), Pb (II) and Cr (VI) from aqueous solutions using black wattle tannin-immobilized nanocellulose. J Hazard Mater 2017, 339, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.; Mariano, M.; Rabelo, S.; Gouveia, R.; Lona, L. Isolation and surface modification of cellulose nanocrystals from sugarcane bagasse waste: From a micro-to a nano-scale view. Appl Surf Sci 2018, 436, 1113–1122. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef]
- Syverud, K.; Chinga-Carrasco, G.; Toledo, J.; Toledo, P.G. A comparative study of Eucalyptus and Pinus radiata pulp fibres as raw materials for production of cellulose nanofibrils. Carbohyd Polym 2011, 84, 1033–1038. [Google Scholar] [CrossRef]
- Sassi, J.-F.; Chanzy, H. Ultrastructural aspects of the acetylation of cellulose. Cellulose 1995, 2, 111–127. [Google Scholar] [CrossRef]
- Qu, J.; Yuan, Z.; Wang, C.; Wang, A.; Liu, X.; Wei, B.; Wen, Y. Enhancing the redispersibility of TEMPO-mediated oxidized cellulose nanofibrils in N, N-dimethylformamide by modification with cetyltrimethylammonium bromide. Cellulose 2019, 26, 7769–7780. [Google Scholar] [CrossRef]
- Henriksson, M.; Henriksson, G.; Berglund, L.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. European Polymer Journal 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Zimmermann, T.; Pöhler, E.; Geiger, T. Cellulose fibrils for polymer reinforcement. Adv Eng Mater 2004, 6, 754–761. [Google Scholar] [CrossRef]
- Leitner, J.; Hinterstoisser, B.; Wastyn, M.; Keckes, J.; Gindl, W. Sugar beet cellulose nanofibril-reinforced composites. Cellulose 2007, 14, 419–425. [Google Scholar] [CrossRef]
- Bruce, D.; Hobson, R.; Farrent, J.; Hepworth, D. High-performance composites from low-cost plant primary cell walls. Composites Part A: Applied Science and Manufacturing 2005, 36, 1486–1493. [Google Scholar] [CrossRef]
- Dufresne, A.; Cavaille, J.-Y.; Vignon, M.R. Mechanical behavior of sheets prepared from sugar beet cellulose microfibrils. Journal of applied polymer science 1997, 64, 1185–1194. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.A.; Isaksson, P.; Lindstrom, T.; Nishino, T. Cellulose nanopaper structures of high toughness. Biomacromolecules 2008, 9, 1579–1585. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 2008, 10, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Hirota, M.; Tamura, N.; Kimura, S.; Fukuzumi, H.; Heux, L.; Isogai, A. Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under neutral conditions. Biomacromolecules 2009, 10, 1992–1996. [Google Scholar] [CrossRef]
- Syverud, K.; Stenius, P. Strength and barrier properties of MFC films. Cellulose 2009, 16, 75. [Google Scholar] [CrossRef]
- Junior, C.P.; De Carvalho, L.; Fonseca, V.; Monteiro, S.; d’Almeida, J. Analysis of the tensile strength of polyester/hybrid ramie–cotton fabric composites. Polymer Testing 2004, 23, 131–135. [Google Scholar] [CrossRef]
- Stevens, C. Industrial applications of natural fibres: Structure, properties and technical applications; John Wiley & Sons, 2010; Volume 10. [Google Scholar]
- Morais, J.P.S.; de Freitas Rosa, M.; Nascimento, L.D.; do Nascimento, D.M.; Cassales, A.R. Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohydrate Polymers 2013, 91, 229–235. [Google Scholar] [CrossRef]
- Nishino, T.; Hirao, K.; Kotera, M.; Nakamae, K.; Inagaki, H. Kenaf reinforced biodegradable composite. Composites science and technology 2003, 63, 1281–1286. [Google Scholar] [CrossRef]
- Rao, D.V.; Srinivas, K.; Naidu, A.L. A review on Jute stem Fiber and its Composites. International Journal of Engineering trends and Technology (IJETT) 2017, 6, 1–9. [Google Scholar]
- Rao, P.D.; Rao, D.V.; Naidu, A.L.; Bahubalendruni, M.R. Mechanical Properties of Banana fiber Reinforced Composites and Manufacturing Techniques: A Review.
- Dang, L.N.; Seppälä, J. Electrically conductive nanocellulose/graphene composites exhibiting improved mechanical properties in high-moisture condition. Cellulose 2015, 22, 1799–1812. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Yang, Z.; Liu, L.; Wang, H. Flexible electrically conductive nanocomposite membrane based on bacterial cellulose and polyaniline. The Journal of physical chemistry B 2011, 115, 8453–8457. [Google Scholar] [CrossRef] [PubMed]
- Gea, S.; Reynolds, C.T.; Roohpour, N.; Wirjosentono, B.; Soykeabkaew, N.; Bilotti, E.; Peijs, T. Investigation into the structural, morphological, mechanical and thermal behaviour of bacterial cellulose after a two-step purification process. Bioresource technology 2011, 102, 9105–9110. [Google Scholar] [CrossRef]
- Taokaew, S.; Seetabhawang, S.; Siripong, P.; Phisalaphong, M. Biosynthesis and characterization of nanocellulose-gelatin films. Materials 2013, 6, 782–794. [Google Scholar] [CrossRef]
- Reddy, J.P.; Rhim, J.-W. Characterization of bionanocomposite films prepared with agar and paper-mulberry pulp nanocellulose. Carbohyd Polym 2014, 110, 480–488. [Google Scholar] [CrossRef]
- Jabbar, A.; Militký, J.; Wiener, J.; Kale, B.M.; Ali, U.; Rwawiire, S. Nanocellulose coated woven jute/green epoxy composites: Characterization of mechanical and dynamic mechanical behavior. Composite Structures 2017, 161, 340–349. [Google Scholar] [CrossRef]
- Le Bras, D.; Strømme, M.; Mihranyan, A. Characterization of Dielectric Properties of Nanocellulose from Wood and Algae for Electrical Insulator Applications. The Journal of Physical Chemistry B 2015, 119, 5911–5917. [Google Scholar] [CrossRef]
- Savadekar, N.; Karande, V.; Vigneshwaran, N.; Bharimalla, A.; Mhaske, S. Preparation of nano cellulose fibers and its application in kappa-carrageenan based film. International journal of biological macromolecules 2012, 51, 1008–1013. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Characterization of microfibrillated cellulose (MFC) films made of different types of raw material. Nordic Polymer Days 2008, 11–13. [Google Scholar]
- Nogi, M.; Iwamoto, S.; Nakagaito, A.N.; Yano, H. Optically transparent nanofiber paper. Adv Mater 2009, 21, 1595–1598. [Google Scholar] [CrossRef]
- Nogi, M.; Yano, H. Optically transparent nanofiber sheets by deposition of transparent materials: A concept for a roll-to-roll processing. Appl Phys Lett 2009, 94, 233117. [Google Scholar] [CrossRef]
- Lu, J.; Wang, T.; Drzal, L.T. Preparation and properties of microfibrillated cellulose polyvinyl alcohol composite materials. Composites Part A: Applied Science and Manufacturing 2008, 39, 738–746. [Google Scholar] [CrossRef]
- Aulin, C.; Ahola, S.; Josefsson, P.; Nishino, T.; Hirose, Y.; Osterberg, M.; Wagberg, L. Nanoscale Cellulose Films with Different Crystallinities and Mesostructures Their Surface Properties and Interaction with Water. Langmuir 2009, 25, 7675–7685. [Google Scholar] [CrossRef]
- Dufresne, A.; Dupeyre, D.; Vignon, M.R. Cellulose microfibrils from potato tuber cells: Processing and characterization of starch–cellulose microfibril composites. Journal of Applied Polymer Science 2000, 76, 2080–2092. [Google Scholar] [CrossRef]
- Svagan, A.J.; Hedenqvist, M.S.; Berglund, L. Reduced water vapour sorption in cellulose nanocomposites with starch matrix. Composites Science and Technology 2009, 69, 500–506. [Google Scholar] [CrossRef]
- Lasseuguette, E.; Roux, D.; Nishiyama, Y. Rheological properties of microfibrillar suspension of TEMPO-oxidized pulp. Cellulose 2008, 15, 425–433. [Google Scholar] [CrossRef]
- Rusli, R.; Eichhorn, S.J. Determination of the stiffness of cellulose nanowhiskers and the fiber-matrix interface in a nanocomposite using Raman spectroscopy. Appl Phys Lett 2008, 93, 033111. [Google Scholar] [CrossRef]
- George, J.; Janardhan, R.; Anand, J.; Bhagawan, S.; Thomas, S. Melt rheological behaviour of short pineapple fibre reinforced low density polyethylene composites. Polymer 1996, 37, 5421–5431. [Google Scholar] [CrossRef]
- Colson, J.; Bauer, W.; Mayr, M.; Fischer, W.; Gindl-Altmutter, W. Morphology and rheology of cellulose nanofibrils derived from mixtures of pulp fibres and papermaking fines. Cellulose 2016, 23, 2439–2448. [Google Scholar] [CrossRef]
- Moberg, T.; Sahlin, K.; Yao, K.; Geng, S.; Westman, G.; Zhou, Q.; Oksman, K.; Rigdahl, M. Rheological properties of nanocellulose suspensions: Effects of fibril/particle dimensions and surface characteristics. Cellulose 2017, 24, 2499–2510. [Google Scholar] [CrossRef]
- Serizawa, S.; Inoue, K.; Iji, M. Kenaf-fiber-reinforced poly (lactic acid) used for electronic products. Journal of Applied Polymer Science 2006, 100, 618–624. [Google Scholar] [CrossRef]
- Mohanty, S.; Verma, S.K.; Nayak, S.K. Rheological characterization of PP/jute composite melts. Journal of applied polymer science 2006, 99, 1476–1484. [Google Scholar] [CrossRef]
- Twite-Kabamba, E.; Mechraoui, A.; Rodrigue, D. Rheological properties of polypropylene/hemp fiber composites. Polymer Composites 2009, 30, 1401–1407. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, H.; Sun, H.-J.; Zhao, Y.; Yang, X.; Cheng, S.Z.; Yang, G. Thermoresponsive bacterial cellulose whisker/poly (NIPAM-co-BMA) nanogel complexes: Synthesis, characterization, and biological evaluation. Biomacromolecules 2013, 14, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Liimatainen, H.; Ezekiel, N.; Sliz, R.; Ohenoja, K.; Sirviö, J.A.; Berglund, L.; Hormi, O.; Niinimäki, J. High-strength nanocellulose–talc hybrid barrier films. Acs Appl Mater Inter 2013, 5, 13412–13418. [Google Scholar] [CrossRef]
- Olszewska, A.; Eronen, P.; Johansson, L.-S.; Malho, J.-M.; Ankerfors, M.; Lindström, T.; Ruokolainen, J.; Laine, J.; Österberg, M. The behaviour of cationic nanofibrillar cellulose in aqueous media. Cellulose 2011, 18, 1213. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Pignon, F.; Belgacem, M.N. Morphological properties of nanofibrillated cellulose produced using wet grinding as an ultimate fibrillation process. J Mater Sci 2015, 50, 531–541. [Google Scholar] [CrossRef]
- Besbes, I.; Vilar, M.R.; Boufi, S. Nanofibrillated cellulose from alfa, eucalyptus and pine fibres: Preparation, characteristics and reinforcing potential. Carbohyd Polym 2011, 86, 1198–1206. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D.; Hedenqvist, M.; Ankerfors, M.; Lindström, T. Highly transparent films from carboxymethylated microfibrillated cellulose: The effect of multiple homogenization steps on key properties. Journal of Applied Polymer Science 2011, 119, 2652–2660. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G. Optical methods for the quantification of the fibrillation degree of bleached MFC materials. Micron 2013, 48, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Hoeng, F.; Denneulin, A.; Bras, J. Use of nanocellulose in printed electronics: A review. Nanoscale 2016, 8, 13131–13154. [Google Scholar] [CrossRef] [PubMed]
- El Baradai, O.; Beneventi, D.; Alloin, F.; Bongiovanni, R.; Bruas-Reverdy, N.; Bultel, Y.; Chaussy, D. Microfibrillated cellulose based ink for eco-sustainable screen printed flexible electrodes in lithium ion batteries. Journal of Materials Science & Technology 2016, 32, 566–572. [Google Scholar]
- Leijonmarck, S.; Cornell, A.; Lindbergh, G.; Wågberg, L. Single-paper flexible Li-ion battery cells through a paper-making process based on nano-fibrillated cellulose. J Mater Chem A 2013, 1, 4671–4677. [Google Scholar] [CrossRef]
- Flandin, L.; Cavaillé, J.; Bidan, G.; Brechet, Y. New nanocomposite materials made of an insulating matrix and conducting fillers: Processing and properties. Polymer Composites 2000, 21, 165–174. [Google Scholar] [CrossRef]
- Schroers, M.; Kokil, A.; Weder, C. Solid polymer electrolytes based on nanocomposites of ethylene oxide–epichlorohydrin copolymers and cellulose whiskers. Journal of Applied Polymer Science 2004, 93, 2883–2888. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, X.; Wu, X.; Wang, S.; Lu, C. Cellulose nanocrystals mediated assembly of graphene in rubber composites for chemical sensing applications. Carbohyd Polym 2016, 140, 88–95. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Wu, X.; Lu, C. Tailoring percolating conductive networks of natural rubber composites for flexible strain sensors via a cellulose nanocrystal templated assembly. Soft Matter 2016, 12, 845–852. [Google Scholar] [CrossRef]
- Wu, X.; Tang, J.; Duan, Y.; Yu, A.; Berry, R.M.; Tam, K.C. Conductive cellulose nanocrystals with high cycling stability for supercapacitor applications. Journal of Materials Chemistry A 2014, 2, 19268–19274. [Google Scholar] [CrossRef]
- Rubino, S.; Razaq, A.; Nyholm, L.; Strømme, M.; Leifer, K.; Mihranyan, A. Spatial mapping of elemental distributions in polypyrrole-cellulose nanofibers using energy-filtered transmission electron microscopy. The Journal of Physical Chemistry B 2010, 114, 13644–13649. [Google Scholar] [CrossRef]
- Silva, M.J.; Sanches, A.O.; Malmonge, L.F.; Medeiros, E.S.; Rosa, M.F.; McMahan, C.M.; Malmonge, J.A. Conductive Nanocomposites Based on Cellulose Nanofibrils Coated with Polyaniline-DBSA Via In Situ Polymerization. Proceedings of Macromolecular Symposia; 2012; pp. 196–202. [Google Scholar]
- Luong, N.D.; Korhonen, J.T.; Soininen, A.J.; Ruokolainen, J.; Johansson, L.-S.; Seppälä, J. Processable polyaniline suspensions through in situ polymerization onto nanocellulose. European Polymer Journal 2013, 49, 335–344. [Google Scholar] [CrossRef]
- Wu, M.; Kuga, S.; Huang, Y. Quasi-one-dimensional arrangement of silver nanoparticles templated by cellulose microfibrils. Langmuir 2008, 24, 10494–10497. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, O.; Schroeter, M.; Capadona, J.R.; Weder, C. Nanocomposites based on cellulose whiskers and (semi) conducting conjugated polymers. Journal of Materials Chemistry 2007, 17, 2746–2753. [Google Scholar] [CrossRef]
- Wu, X.; Chabot, V.L.; Kim, B.K.; Yu, A.; Berry, R.M.; Tam, K.C. Cost-effective and scalable chemical synthesis of conductive cellulose nanocrystals for high-performance supercapacitors. Electrochimica Acta 2014, 138, 139–147. [Google Scholar] [CrossRef]
- Liu, D.Y.; Sui, G.; Bhattacharyya, D. Synthesis and characterisation of nanocellulose-based polyaniline conducting films. Composites Science and Technology 2014, 99, 31–36. [Google Scholar] [CrossRef]
- Choi, J.; Park, S.; Cheng, J.; Park, M.; Hyun, J. Amphiphilic comb-like polymer for harvest of conductive nano-cellulose. Colloids and Surfaces B: Biointerfaces 2012, 89, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Elbi, P.; Deepa, B.; Jyotishkumar, P.; Pothen, L.; Narine, S.; Thomas, S. X-ray diffraction and biodegradation analysis of green composites of natural rubber/nanocellulose. Polymer degradation and stability 2012, 97, 2378–2387. [Google Scholar] [CrossRef]
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.; Luong, J.H. Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends in biotechnology 2012, 30, 283–290. [Google Scholar] [CrossRef]
- Dong, S.; Cho, H.J.; Lee, Y.W.; Roman, M. Synthesis and cellular uptake of folic acid-conjugated cellulose nanocrystals for cancer targeting. Biomacromolecules 2014, 15, 1560–1567. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Ruottinen, V.; Ruotsalainen, J.; Rönkkö, S.; Johansson, L.-S.; Hinkkanen, A.; Järvinen, K.; Seppälä, J. Synthesis of cellulose nanocrystals carrying tyrosine sulfate mimetic ligands and inhibition of alphavirus infection. Biomacromolecules 2014, 15, 1534–1542. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N.; French, A.; Concha, M.; DeLucca, A.; Wu, Q. Nanocellulose-based biosensors: Design, preparation, and activity of peptide-linked cotton cellulose nanocrystals having fluorimetric and colorimetric elastase detection sensitivity. Engineering 2013, 5, 20. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N.; Sethumadhavan, K.; Ullah, A.; Condon, B. Peptide conjugated cellulose nanocrystals with sensitive human neutrophil elastase sensor activity. Cellulose 2013, 20, 1223–1235. [Google Scholar] [CrossRef]
- Ullah, M.W.; Adhikari, M.; Atta, O.M.; Farooq, U.; Ul-Islam, M.; Shahzad, A.; Manan, S.; Yang, G. Biomedical Applications of Nanocellulose. In Emerging Nanotechnologies in Nanocellulose; Springer, 2023; pp. 367–406. [Google Scholar]
- Orelma, H.; Filpponen, I.; Johansson, L.-S.; Österberg, M.; Rojas, O.J.; Laine, J. Surface functionalized nanofibrillar cellulose (NFC) film as a platform for immunoassays and diagnostics. Biointerphases 2012, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Carbonell, R.G.; Rojas, O.J. Bioactive cellulose nanofibrils for specific human IgG binding. Biomacromolecules 2013, 14, 4161–4168. [Google Scholar] [CrossRef]
- Zhang, Y.; Nypelö, T.; Salas, C.; Arboleda, J.; Hoeger, I.C.; Rojas, O.J. Cellulose nanofibrils. Journal of Renewable Materials 2013, 1, 195–211. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, J.; Zhong, J. Nanocellulose Paper for Flexible Electronic Substrate. In Emerging Nanotechnologies in Nanocellulose; Springer, 2023; pp. 211–235. [Google Scholar]
- Okahisa, Y.; Yoshida, A.; Miyaguchi, S.; Yano, H. Optically transparent wood–cellulose nanocomposite as a base substrate for flexible organic light-emitting diode displays. Composites Science and Technology 2009, 69, 1958–1961. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Juntaro, J.; Sain, M.; Manuspiya, H. Development of transparent bacterial cellulose nanocomposite film as substrate for flexible organic light emitting diode (OLED) display. Ind Crop Prod 2012, 35, 92–97. [Google Scholar] [CrossRef]
- Sabo, R.; Yermakov, A.; Law, C.T.; Elhajjar, R. Nanocellulose-enabled electronics, energy harvesting devices, smart materials and sensors: A review. , 4, 5 2016, 4, 297–312. [Google Scholar] [CrossRef]
- Wang, Z.; Nyholm, L. Energy Storage Applications. In Emerging Nanotechnologies in Nanocellulose; Springer, 2023; pp. 237–265. [Google Scholar]
- Sun, Z.; Eyley, S.; Guo, Y.; Salminen, R.; Thielemans, W. Synergistic effects of chloride anions and carboxylated cellulose nanocrystals on the assembly of thick three-dimensional high-performance polypyrrole-based electrodes. Journal of Energy Chemistry 2022, 70, 492–501. [Google Scholar] [CrossRef]
- Zheng, G.; Cui, Y.; Karabulut, E.; Wågberg, L.; Zhu, H.; Hu, L. Nanostructured paper for flexible energy and electronic devices. Mrs Bull 2013, 38, 320–325. [Google Scholar] [CrossRef]
- Pushparaj, V.L.; Shaijumon, M.M.; Kumar, A.; Murugesan, S.; Ci, L.; Vajtai, R.; Linhardt, R.J.; Nalamasu, O.; Ajayan, P.M. Flexible energy storage devices based on nanocomposite paper. Proceedings of the National Academy of Sciences 2007, 104, 13574–13577. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, L.; Bongiovanni, R.; Chaussy, D.; Gerbaldi, C.; Beneventi, D. Cellulose-based Li-ion batteries: A review. Cellulose 2013, 20, 1523–1545. [Google Scholar] [CrossRef]
- Chiappone, A.; Nair, J.R.; Gerbaldi, C.; Jabbour, L.; Bongiovanni, R.; Zeno, E.; Beneventi, D.; Penazzi, N. Microfibrillated cellulose as reinforcement for Li-ion battery polymer electrolytes with excellent mechanical stability. J Power Sources 2011, 196, 10280–10288. [Google Scholar] [CrossRef]
- Willgert, M.; Leijonmarck, S.; Lindbergh, G.; Malmström, E.; Johansson, M. Cellulose nanofibril reinforced composite electrolytes for lithium ion battery applications. J Mater Chem A 2014, 2, 13556–13564. [Google Scholar] [CrossRef]
- Sun, Z.; Thielemans, W. Interconnected and high cycling stability polypyrrole supercapacitors using cellulose nanocrystals and commonly used inorganic salts as dopants. Journal of Energy Chemistry 2023, 76, 165–174. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Z.; Chen, Y.; Weadock, N.; Wan, J.; Vaaland, O.; Han, X.; Li, T.; Hu, L. Tin anode for sodium-ion batteries using natural wood fiber as a mechanical buffer and electrolyte reservoir. Nano Lett 2013, 13, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, G.; Kandasubramanian, B. Fabrication of transparent paper devices from nanocellulose fiber. Materials Chemistry and Physics 2022, 281, 125707. [Google Scholar] [CrossRef]
- Fortunato, E.; Correia, N.; Barquinha, P.; Pereira, L.; Goncalves, G.; Martins, R. High-performance flexible hybrid field-effect transistors based on cellulose fiber paper. Ieee Electr Device L 2008, 29, 988–990. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yun, S.; Ko, H.-U.; Kim, J. A flexible paper transistor made with aligned single-walled carbon nanotube bonded cellulose composite. Current Applied Physics 2013, 13, 897–901. [Google Scholar] [CrossRef]
- Fujisaki, Y.; Koga, H.; Nakajima, Y.; Nakata, M.; Tsuji, H.; Yamamoto, T.; Kurita, T.; Nogi, M.; Shimidzu, N. Transparent nanopaper-based flexible organic thin-film transistor array. Adv Funct Mater 2014, 24, 1657–1663. [Google Scholar] [CrossRef]
- Hassinen, T.; Alastalo, A.; Eiroma, K.; Tenhunen, T.-M.; Kunnari, V.; Kaljunen, T.; Forsström, U.; Tammelin, T. All-Printed Transistors on Nano Cellulose Substrate. MRS Advances 2015, 1, 645–650. [Google Scholar] [CrossRef]
- Li, C.; Wang, F.; Jimmy, C.Y. Semiconductor/biomolecular composites for solar energy applications. Energy & Environmental Science 2011, 4, 100–113. [Google Scholar]
- Hu, L.; Zheng, G.; Yao, J.; Liu, N.; Weil, B.; Eskilsson, M.; Karabulut, E.; Ruan, Z.; Fan, S.; Bloking, J.T. Transparent and conductive paper from nanocellulose fibers. Energy & Environmental Science 2013, 6, 513–518. [Google Scholar]
- Zhou, Y.; Fuentes-Hernandez, C.; Khan, T.M.; Liu, J.-C.; Hsu, J.; Shim, J.W.; Dindar, A.; Youngblood, J.P.; Moon, R.J.; Kippelen, B. Recyclable organic solar cells on cellulose nanocrystal substrates. Sci Rep-Uk 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Yuwawech, K.; Wootthikanokkhan, J.; Tanpichai, S. Enhancement of thermal, mechanical and barrier properties of EVA solar cell encapsulating films by reinforcing with esterified cellulose nanofibres. Polymer Testing 2015, 48, 12–22. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Zhang, Z.; Sèbe, G.; Rentsch, D.; Zimmermann, T.; Tingaut, P. Ultralightweight and flexible silylated nanocellulose sponges for the selective removal of oil from water. Chem Mater 2014, 26, 2659–2668. [Google Scholar] [CrossRef]
- Jiang, Q.; Kacica, C.; Soundappan, T.; Liu, K.-k.; Tadepalli, S.; Biswas, P.; Singamaneni, S. An in situ grown bacterial nanocellulose/graphene oxide composite for flexible supercapacitors. J Mater Chem A 2017, 5, 13976–13982. [Google Scholar] [CrossRef]
- Zhu, H.; Xiao, Z.; Liu, D.; Li, Y.; Weadock, N.J.; Fang, Z.; Huang, J.; Hu, L. Biodegradable transparent substrates for flexible organic-light-emitting diodes. Energy & Environmental Science 2013, 6, 2105–2111. [Google Scholar]
- Huang, L.; Chen, X.; Nguyen, T.X.; Tang, H.; Zhang, L.; Yang, G. Nano-cellulose 3D-networks as controlled-release drug carriers. Journal of Materials Chemistry B 2013, 1, 2976–2984. [Google Scholar] [CrossRef]
- Yan, C.; Wang, J.; Kang, W.; Cui, M.; Wang, X.; Foo, C.Y.; Chee, K.J.; Lee, P.S. Highly stretchable piezoresistive graphene–nanocellulose nanopaper for strain sensors. Adv Mater 2014, 26, 2022–2027. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, J.T.; Kettunen, M.; Ras, R.H.; Ikkala, O. Hydrophobic nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents. Acs Appl Mater Inter 2011, 3, 1813–1816. [Google Scholar] [CrossRef] [PubMed]
- Nimeskern, L.; Ávila, H.M.; Sundberg, J.; Gatenholm, P.; Müller, R.; Stok, K.S. Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. Journal of the mechanical behavior of biomedical materials 2013, 22, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohyd Polym 2018, 184, 453–464. [Google Scholar] [CrossRef]




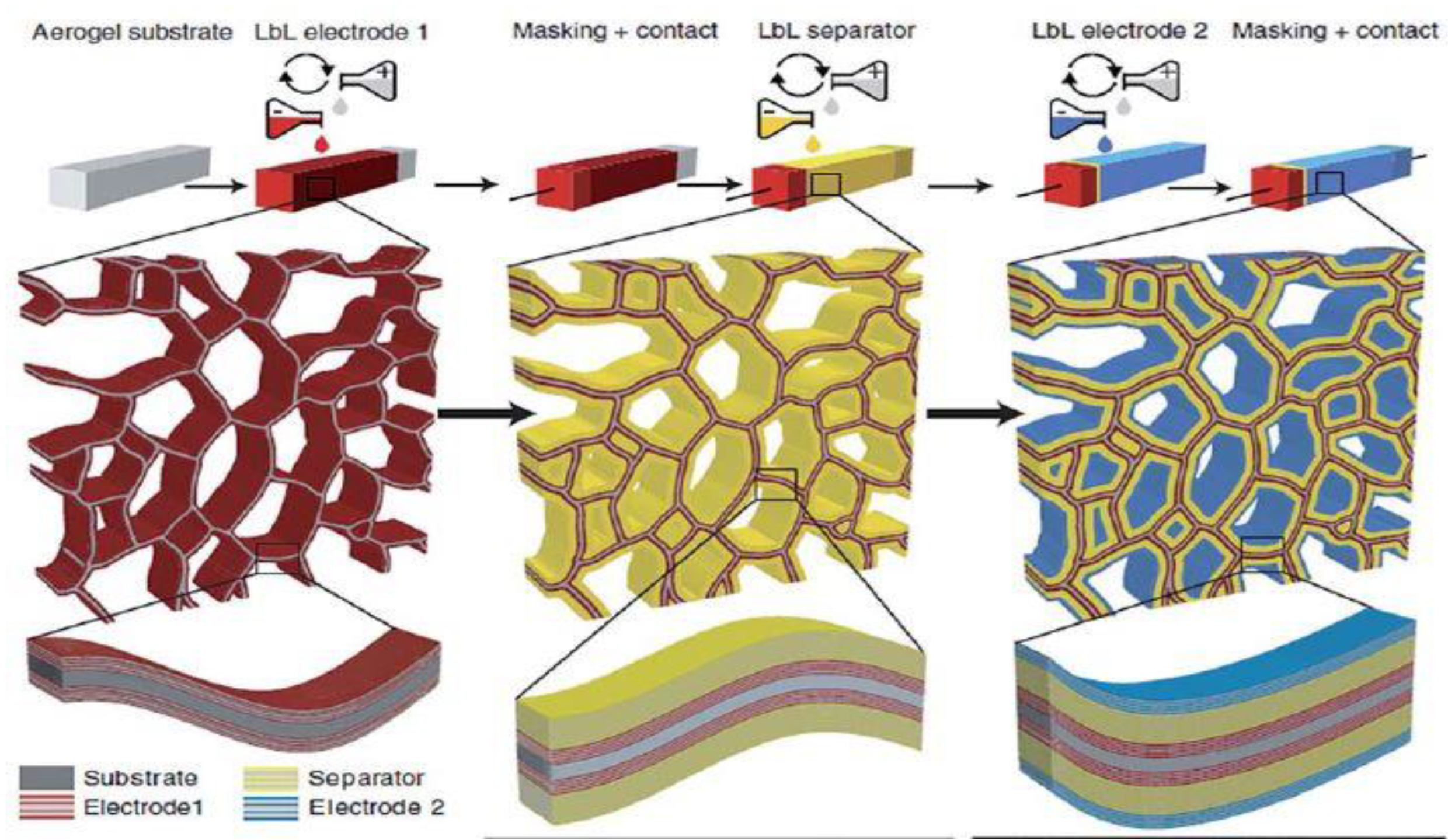
| Type of Nanocellulose | Synonyms | Typical Sources | Formation and average size |
|---|---|---|---|
| Cellulose nanocrystals (CNC) | Cellulose nanocrystals, crystallites, whiskers, rod-like cellulose microcrystals | Ramie tunicin, wood, wheat straw, mulberry bark | Method used: Acid Hydrolysis. Ø=5-70 nm L=100-250 nm |
| Cellulose nanofibrils (CNF) | Micro fibrillated cellulose, nanofibrils, and microfibrils | Sugar beet, hemp, wood, flax | Mechanical treatment and chemical treatment Ø=5-60 nm L=several micrometers |
| Bacterial Nanocellulose (BNC) | Bacterial cellulose, microbial cellulose, bio-cellulose | Low molecular weight sugar and alcohols | Bacterial based approach Ø=20-100 nm |
| Ref | Raw Materials | Preparation Method | Dimension |
|---|---|---|---|
| [116] | Cladodes of Opuntia Ficus Indica | Homogenization | ~ 5nm in width |
| [99] | Sugar beet pulp | TEMPO mediated oxidation | Not reported |
| [96] | Wheat straw | Cryocrushing and Homogenization | 20-120 nm in width |
| [63] | Kraft pulp | Refining and Homogenization | 50-100 nm in width |
| [117] | Cotton fibers | Refining | 242 ± 158 nm in diameter |
| [118] | Sugarcane bagasse | Acid Hydrolysis | ~32.84 nm |
| [119] | Cotton linter | Ultrasonication | 15-35 nm in diameter |
| [120] | Raw Cotton | Acid Hydrolysis and Alkaline pre-treatment | Not reported |
| [121] | Cystoseria myricaas algae | Acid Hydrolysis | 10-30 nm |
| [122] | Hibiscus cannabinus | Alkaline pre-treatment and Acid Hydrolysis | mean diameter of 6.1 ± 5 nm |
| [123] | Imperata brasiliensis grass | Acid Hydrolysis | diameters were 10–60 nm and length 150–250 nm. |
| [124] | Amylose maize starch | Electrospinning | 1-4 μm in diameter |
| [125] | Apple and carrot pomaces | Ultrasonication | 3.31-3.54 nm |
| [126] | Peach palm extraction (Bactris gasipaes) | Delignification treatments | Not reported |
| [127] | Moso bamboo culms | Microwave liquefaction and Ultrasonication | 567 ± 149 μm in diameter |
| [128] | Areca nut husk | Acid Hydrolysis and homogenization | 1-10 nm in diameter |
| [129] | Sugarcane bagasse | Acid hydrolysis | 69-117nm in length, 6-7nm in diameter |
| [130] | Oil palm trunk | Acid hydrolysis | 7.67-7.97 nm in diameter, 397- 367 nm in length |
| [131] | Banana peel | Alkaline pre-treatment and Acid hydrolysis | 7.6-10.9nm in diameter, 454.9-2889.7 nm in length |
| [132] | Raw jute fibers | Alkaline pre-treatment and steam explosion | ~50 nm in diameter |
| References | Effect of surface modification on various properties | Before surface modification | After surface modification | Reason |
|---|---|---|---|---|
| [138,139] | Crystallinity of nanocellulose | Lower crystalline value | Enhance the crystalline value | A greater hydrolysis time disintegration or remove the amorphous phase and improve the crystalline value |
| [140,141] | Toxicity of nanocellulose | Toxicity | As per the ecotoxicological evaluation, the nanocellulose has lower toxic and lower environmental damage | Proinflammatory and Cytotoxicity reactions are minimizing toxicity. |
| [142] | Specific surface area | Lower specific surface area 950-200 m2/g) | Excellent specific surface area (250-350 m2/g) | H2SO4 treatment |
| [143] | Aspect ratio | Low or medium aspect ratio | Higher aspect ratio | TEMPO oxidation method |
| [144,145] | Mechanical property | Poor mechanical property | Enhanced rigidity, strength, toughness, barrier features, and even flame retardancy | Collagen-based composite films reinforced with CNCs. |
| [146] | Thermal property | Lower thermal expansion coefficient due to its higher crystallinity and strength of nanocellulose network | Excellent thermal property | H2SO4 hydrolyzed method |
| [139] | Rheological property | Tendency to shear-thinning and pseudo-plasticity depends on the pH of the environment. | Enhancement in shear rate with lower viscosity of nanocellulose. | TEMPO-oxidation method |
| [147] | Stability dispersion and agglomeration | Agglomeration and clustering of nanocellulose problem | Minimize the agglomeration problem | Freeze drying or supercritical dying of CO2. |
| References | Nanocellulose | Method | Key findings | Applications |
|---|---|---|---|---|
| [170] | CNC | H2SO4 Hydrolysis | High metal absorbing capability and good regeneration capacity | Better nanocomposite to remove the contaminant from industrial waste |
| [171] | CNC | H2SO4 hydrolysis | Improved dispersion and thermodynamic wetting | Reinforcements for hydrophobic materials |
| [148] | Nanocellulose | Noncovalent surface modification | Dispersion ability improved | Thermal energy storage |
| [172] | Nanocellulose | sulfonation | Improving formation of stable colloidal suspension | Determine aviation energies for the dehydration process |
| [158] | CNC | Esterification | Cationic charges over the surface of nanocellulose | - |
| [151,173] | CNF | TEMPO-medicated oxidation | Formation of stable colloidal suspensions | Thermal energy storage |
| [150] | Nanocellulose | Carbonylation | Improve the cellulose hydrophobicity | Packing applications |
| [174] | Nanocellulose | Acetylation | Improve the cellulose hydrophobicity | Packing applications |
| [175] | CNF | TEMPO-mediated oxidation | Improved hydrophobicity and thermal stability | Thermal storage |
| References | Raw material | Preparation method | Max. Stress (MPa) | Modulus of elasticity (GPa) |
|---|---|---|---|---|
| [181] | Softwood dissolving pulp | Vacuum filtering | 104 | 14.0 |
| [182] | Softwood and hardwood bleached kraft pulp | Vacuum filtering | 222-233 | 6.2-6.9 |
| [183] | Hardwood bleached kraft pulp | Vacuum filtering | 222-312 | 6.2-6.5 |
| [184] | Bleached spruce sulfite pulp | Vacuum filtering | 104-154 | 15.7-17.5 |
| [178] | Sugar beet pulp chips | Casting | 104 | 9.3 |
| [185,186] | Ramie | Retting | 393-870 | 7.3 |
| [185,187] | Cotton | Acidic hydrolysis | 128-597 | 5.5-12.6 |
| [188] | Kenaf | Retting | 930 | 53 |
| [189] | Jute | Retting | 393-800 | 10-30 |
| [190] | Banana | Chemical treatment | 600 | 17.85 |
| [191] | Bleached birch pulp | Mechanical disintegration | 172 | 5.3 |
| [192] | Bacterial Nanocellulose | Not reported | 357.3 | 20.8 |
| [193] | A. xylinum | Two-step purification | 88.9 | 7.6 |
| [194] | Gelatin (A. xylinum) | Static cultivation | 63 | Not reported |
| [195] | Murlberry pulp | Acid Hydrolysis | 33.3-41.3 | 0.77-1.11 |
| [196] | Tossa jute fiber | Acid Hydrolysis | 32.94 – 48.66 | 4.81-5.76 |
| [197] | Softwood pulp | Ultrasonication | 141.6 | 12.27 |
| [197] | Algae | Ultrasonication | 77.97 | 8.12 |
| [198] | Cotton | Disc refiner | 23-26 | Not reported |
| References | Raw material | Shear rate (s-1) | Viscosity | Run Temp (oC) |
|---|---|---|---|---|
| [208] | Pineapple | 22.2 | 3.5 x 104 Pa.s | 125 |
| [209] | Softwood sulphite pulp | 20 | 260 mPa.s | 20 |
| [210] | Cellulose nanofibrils | 0.1-1.0 | 10 – 100 mPa.s | 25 |
| [211] | Kenaf/PLA | 103-104 | 50-300 Pa.s | 200 |
| [212] | Jute/PP | 10-2-104 | 10 – 104Pa.s | 180 |
| [213] | Hemp/PP | 10-1-103 | 102-105 Pa.s | 180 |
| [214] | Gluconacetobacter xylinus | 0-400 | 170-400 Pa.s | 25 |
| References | Nanocellulose type | Conductive structure | Conductivity (S cm-1) |
|---|---|---|---|
| [228] | CNC | PPy | Up to 36 |
| [229] | CNF | PPy | 1.5 |
| [230] | CNC | PANI | Up to 10-1 |
| [231] | CNF | PANI | 2.6 x 10-5 |
| [232] | CNF | silver | 5 |
| [233] | CNC | PANI + PFE | 0.01 – 0.5 |
| [234] | CNC | PPy | Up to 4 |
| [235] | CNC | PANI | 2.6 x 10-5 |
| [236] | BC | CNT | 0.13 x 10-3 |
| [191] | CNF | GO | 7.3 x10-2 -15.4 |
| [192] | BC | PANI | 2.0x10-4-9.5 10-3 |
| Ref | Class of Nanocellulose | Raw materials | Special Properties | Field of application |
|---|---|---|---|---|
| [181] | CNF | Softwood Pulp | High Toughness | Nanopaper |
| [269] | CNF | Not reported | Cell-friendly | 3D Bioprinting Human Chondrocytes |
| [270] | CNF | Oat Straw | High Porosity | Selective Removal of Oil from Water |
| [271] | BNC | Not reported | Natural Abundance | Energy Storage Device |
| [272] | CNF | Bleached softwood pulp | Not reported | Organic Light Emitting Diodes |
| [228] | CNC | Not reported | Not reported | Supercapacitor |
| [249] | BNC | Nata de coco (A. xylinum) | Flexible | Organic Light Emitting Diodes |
| [273] | BNC | Gluconacetobacter xylinum | Not reported | Drug delivery system |
| [274] | CNF | Not reported | Highly Stretchable | Strain Sensor |
| [266] | CNF | Softwood Cellulose fibers | Superior Optical properties | Conductive paper |
| [275] | CNF | Not reported | High Porosity | Oil absorbent |
| [276] | BNC | Bacteria suspension | Good tensile mechanical properties | Ear cartilage replacement |
| [239] | CNC | Bleached softwood sulfite pulp | Oblong geometry, lack of cytotoxicity, numerous surface hydroxyl groups | Chemotherapeutic agents to cancer cells |
| [277] | CNC | Not reported | Eco-friendliness and biodegradability | Anti-bacterial food packaging |
| [198] | CNF | Cotton | Not reported | Food-packaging |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





