Submitted:
25 May 2023
Posted:
02 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Design and Period
2.3. Study Population
2.4. Sample size determination
2.5. Sampling Method
2.6. Data Collection
Specimen Collection
2.7. Laboratory Procedures
2.7.1. Culture and Identification of Group B Streptococci
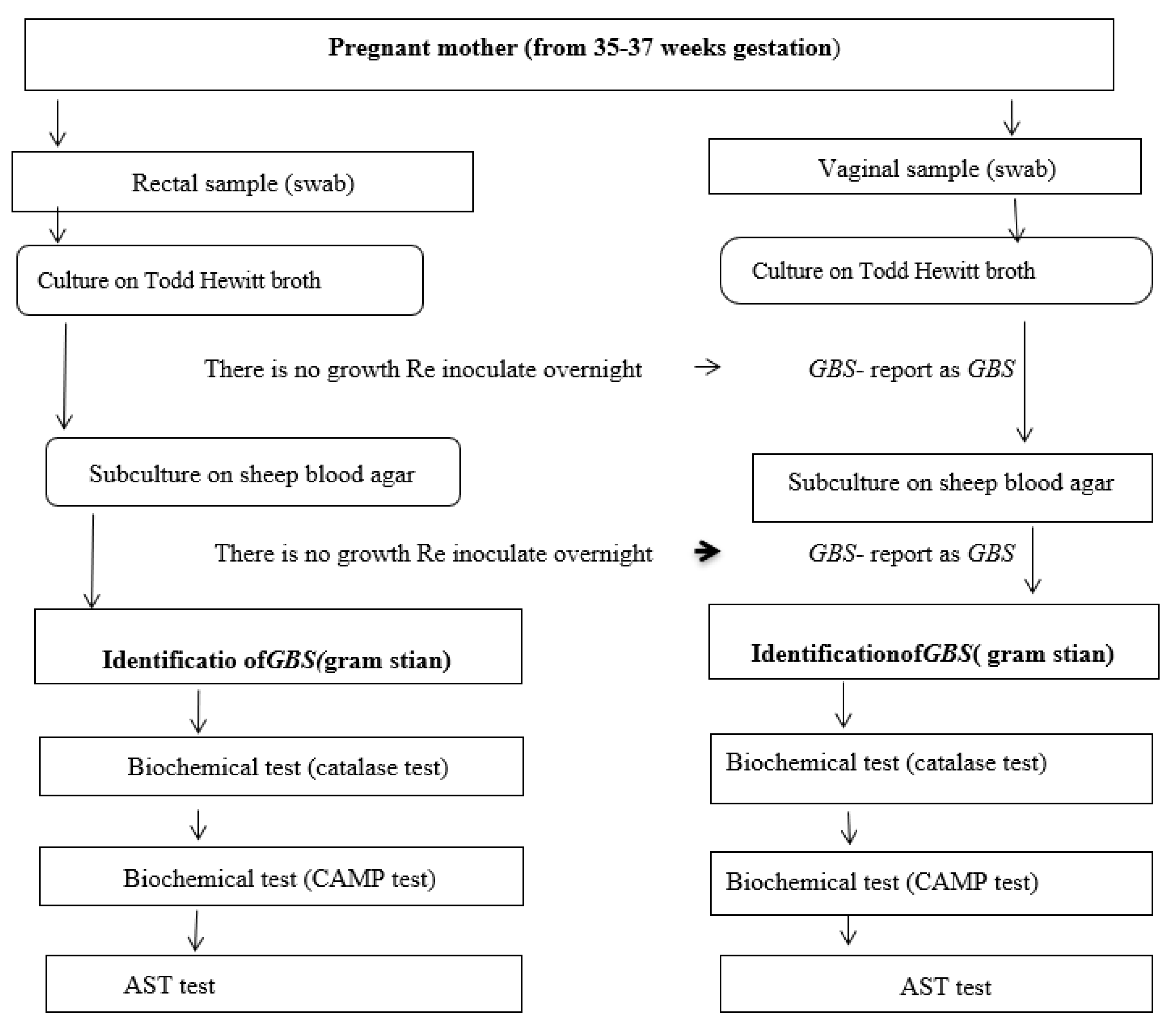
2.7.2. Antimicrobial Susceptibility Testing (AST)
2.8. Data Quality Control
2.9. Methods of Data Analysis
3. Results
3.1. Socio-Demographic Characteristics
3.2. Obstetric and Clinical Characteristics
3.3. Group B Streptococcus Colonization
3.4. Factors Associated with Maternal Group B Streptococci Colonization
3.5. Antimicrobial Susceptibility pattern of GBS Isolates
4. Discussion
4.1. The Strengths of This Study
4.2. Limitations of the Study
- No serotyping was done and using only disc diffusion for antibiotic susceptibility test was conducted.
- Failure to assess the outcome on neonates whose mother detected to be colonized by GBS on the study.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Disclosure
References
- Liesse Iyamba J-M, Mongane PM, Lukukula CM, Ngbandani BK, Tshimpangila JD, Vihembo GM; et al. Vaginal Colonization and Antibiotic Susceptibility Pattern of Group B Streptococcus Isolated from Pregnant Women in Maternit Des Soeurs de Pauvres de Bergame de Kimbanseke, Kinshasa, Democratic Republic of Congo. Advances in Microbiology 2021, 11, 335–341.
- Mengist A, Kannan H, Abdissa A. Prevalence and antimicrobial susceptibility pattern of anorectal and vaginal group B Streptococci isolates among pregnant women in Jimma, Ethiopia. BMC research notes 2016, 9, 351.
- Mengist HM, Zewdie O, Belew A, Dabsu R. Prevalence and drug susceptibility pattern of group B Streptococci (GBS) among pregnant women attending antenatal care (ANC) in Nekemte Referral Hospital (NRH), Nekemte, Ethiopia. BMC research notes 2017, 10, 388.
- LAMMLER SS, I.W. T. WIBAWAN, E. OTT, V. BOPP and A. MARTINEZ-TAGLE. Comparison of streptococci of serological group B isolated from healthy carriers and active disease in Chile. J Med Microbiol 1995, 42.
- Leykun Y., Genet C, Mulu W. Group B Streptococci Vaginal-Recto Colonization, Vertical Transmission to Newborns, Antimicrobial Susceptibility Profile and Associated Factors in Selected Health Facilities of Bahir Dar City. Infection and Drug Resistance 2021, 14, 5457–5472. [CrossRef]
- Centers for Disease Control and Prevention (CDC). Perinatal group B streptococcal disease after universal screening recommendations--United States, 2003-2005. 2007 Jul 20; 56(28):701-5. MMWR Morbidity & Mortal Weekly Report 2007, 56, 701–708.
- Nishihara Y, Dangor Z, French N, Madhi S, Heyderman R. Challenges in reducing group B Streptococcus disease in African settings. Arch Dis Child 2017, 102, 72–77. [CrossRef] [PubMed]
- Kwatra G, Cunnington MC, Merrall E, Adrian PV, Ip M, Klugman KP; et al. Prevalence of maternal colonisation with group B streptococcus: A systematic review and meta-analysis. The Lancet Infectious Diseases 2016, 16, 1076–1084. [CrossRef]
- Perinatal group B streptococcal disease after universal screening recommendations--United States, 2003-2005. MMWR Morbidity and mortality weekly report 2007, 56, 701–705.
- Plainvert C, Anselem O, Joubrel C, Marcou V, Falloukh A, Frigo A; et al. Persistence of group B Streptococcus vaginal colonization and prevalence of hypervirulent CC-17 clone correlate with the country of birth: A prospective 3-month follow-up cohort study. European journal of clinical microbiology & infectious diseases : Official publication of the European Society of Clinical Microbiology 2021, 40, 133–140.
- Tumuhamye J, Steinsland H, Bwanga F, Tumwine JK, Ndeezi G, Mukunya D; et al. Vaginal colonization with antimicrobial-resistant bacteria among women in labor in central Uganda: Prevalence and associated factors. Antimicrobial resistance and infection control 2021, 10, 37. [CrossRef] [PubMed]
- Dilrukshi GN, Kottahachchi J, Dissanayake D, Pathiraja RP, Karunasingha J, Sampath MKA; et al. Group B Streptococcus colonisation and their antimicrobial susceptibility among pregnant women attending antenatal clinics in tertiary care hospitals in the Western Province of Sri Lanka. Journal of obstetrics and gynaecology : The journal of the Institute of Obstetrics and Gynaecology 2021, 41, 1–6.
- Jisuvei SC, Osoti A, Njeri MA. Prevalence, antimicrobial susceptibility patterns, serotypes and risk factors for group B streptococcus rectovaginal isolates among pregnant women at Kenyatta National Hospital, Kenya; a cross-sectional study. BMC infectious diseases 2020, 20, 302.
- Nishihara Y, Dangor Z, French N, Madhi S, Heyderman R. Challenges in reducing group B Streptococcus disease in African settings. Archives of disease in childhood 2017, 102, 72–77. [CrossRef] [PubMed]
- Assefa S, Desta K, Lema T. Group B streptococci vaginal colonization and drug susceptibility pattern among pregnant women attending in selected public antenatal care centers in Addis Ababa, Ethiopia. BMC pregnancy and childbirth 2018, 18, 135.
- Gizachew M, Tiruneh M, Moges F, Adefris M, Tigabu Z, Tessema B. Newborn colonization and antibiotic susceptibility patterns of Streptococcus agalactiae at the University of Gondar Referral Hospital, Northwest Ethiopia. BMC pediatrics 2018, 18, 378.
- Arain FR, Al-Bezrah NA, Al-Aali KY. Prevalence of maternal genital tract colonization by group B streptococcus from Western Province, Taif, Saudi Arabia. Journal of Clinical Gynecology and Obstetrics 2015, 4, 258–264. [CrossRef]
- WSUCSH, Co. Wolaita Sodo University Comprehensive Specialized Hospital Healthcare Quality Progress. 2022. [Google Scholar]
- Musa Mohammed1 DA, Yimtubezinash Woldeamanuel2, Demissie A. Prevalence of group B Streptococcus colonization among pregnant women attending antenatal clinic of Hawassa Health Center. thiopian Journal of Health Development 2012, 26, 36–42.
- Shiferawu S, Mekonen M, Baza D, Lera T. Prevalence of Group b Streptococcus, Its Associated Factors and Antimicrobial Susceptibility Pattern Among Pregnant Women Attending Antenatal Care at Arbaminch Hospital, South Ethiopia. American Journal of Health Research 2019, 7, 104. [CrossRef]
- M100 Performance Standards for Antimicrobial Susceptibility Testing. Juanita Smit. 1/13/2021.;31st Edition.
- Mengist A, Kannan H, Abdissa A. Prevalence and antimicrobial susceptibility pattern of anorectal and vaginal group B Streptococci isolates among pregnant women in Jimma, Ethiopia. BMC research notes 2016, 9, 1–5.
- Melo SCCSd, Costa AB, Silva FTRd, Silva NMMG, Tashima CM, Cardoso RF; et al. Prevalence of Streptococcus agalactiae colonization in pregnant women from the 18 th Health Region of Paraná State. Revista do Instituto de Medicina Tropical de São Paulo 2018, 60.
- Bolukaoto JY, Monyama CM, Chukwu MO, Lekala SM, Nchabeleng M, Maloba MR; et al. Antibiotic resistance of Streptococcus agalactiae isolated from pregnant women in Garankuwa, South Africa. BMC research notes 2015, 8, 1–7.
- Poyart C, Jardy L, Quesne G, Berche P, Trieu-Cuot P. Genetic basis of antibiotic resistance in Streptococcus agalactiae strains isolated in a French hospital. Antimicrobial agents and chemotherapy 2003, 47, 794–797. [CrossRef]
- Cockerill FR, Wikler MA, Alder J, Dudley M, Eliopoulos G, Ferraro M; et al. Performance standards for antimicrobial susceptibility testing: Twenty-second informational supplement. Clinical and Laboratory Standards Institute 2012, 32, M100–S22.
- Blumberg HM, Stephens DS, Modansky M, Erwin M, Elliot J, Facklam RR; et al. Invasive group B streptococcal disease: The emergence of serotype V. Journal of Infectious Diseases 1996, 173, 365–373. [CrossRef] [PubMed]
- Flaherty RA, Borges EC, Sutton JA, Aronoff DM, Gaddy JA, Petroff MG; et al. Genetically distinct Group B Streptococcus strains induce varying macrophage cytokine responses. PLoS ONE 2019, 14, e0222910.
- Khan MA, Faiz A, Ashshi AM. Maternal colonization of group B streptococcus: Prevalence, associated factors and antimicrobial resistance. Annals of Saudi medicine 2015, 35, 423–427. [CrossRef] [PubMed]
- Valkenburg-Van Den Berg AW, Sprij AJ, Oostvogel PM, Mutsaers JA, Renes WB, Rosendaal FR; et al. Prevalence of colonisation with group B Streptococci in pregnant women of a multi-ethnic population in The Netherlands. European Journal of Obstetrics & Gynecology and Reproductive Biology 2006, 124, 178–183.
- El Aila NA, Tency I, Claeys G, Saerens B, Cools P, Verstraelen H; et al. Comparison of different sampling techniques and of different culture methods for detection of group B streptococcus carriage in pregnant women. BMC infectious diseases 2010, 10, 1–8.
- Alemseged G, Niguse S, Hailekiros H, Abdulkadir M, Saravanan M, Asmelash T. Isolation and anti-microbial susceptibility pattern of group B Streptococcus among pregnant women attending antenatal clinics in Ayder Referral Hospital and Mekelle Health Center, Mekelle, Northern Ethiopia. BMC Research Notes 2015, 8, 1–8.
- Ayata A, Güvenç H, Felek S, Aygün AD, Kocabay K, Bektas S. Maternal carriage and neonatal colonisation of group B streptococci in labour are uncommon in Turkey. Paediatric and perinatal epidemiology 1994, 8, 188–192. [CrossRef] [PubMed]
- El-Kersh TA, Al-Nuaim LA, Kharfy TA, Al-Shammary FJ, Al-Saleh SS, Al-Zamel FA, Detection of genital colonization of group B streptococci during late pregnancy. Saudi medical journal 2002, 23, 56–61.
- Strus M, Pawlik D, Brzychczy-Włoch M, Gosiewski T, Rytlewski K, Lauterbach R; et al, Group B streptococcus colonization of pregnant women and their children observed on obstetric and neonatal wards of the University Hospital in Krakow, Poland. Journal of medical microbiology 2009, 58, 228–233. [CrossRef]
- Rausch A-V, Gross A, Droz S, Bodmer T, Surbek DV. Group B Streptococcus colonization in pregnancy: Prevalence and prevention strategies of neonatal sepsis. 2009.
- Schmidt J, Halle E, Halle H, Mohammed T, Gunther E, Colonization of pregnant women and their newborn infants with group B streptococci in the Gondar College of Medical Sciences. Ethiopian medical journal 1989, 27, 115–119.
- Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease: Revised guidelines from CDC, 2010. Department of Health and Human Services, Centers for Disease Control and …; 2010.
- Savoia D, Gottimer C, Crocilla C, Zucca M. Streptococcus agalactiae in pregnant women: Phenotypic and genotypic characters. Journal of Infection 2008, 56, 120–125. [CrossRef] [PubMed]
- Fatemi F, Chamani L, Pakzad P, Zeraati H, Rabbani H, Asgari S. Colonization rate of group B Streptococcus (GBS) in pregnant women using GBS agar medium. Acta Medica Iranica 2009, 25–30.
- Assefa S, Desta K, Lema T. Group B streptococci vaginal colonization and drug susceptibility pattern among pregnant women attending in selected public antenatal care centers in Addis Ababa, Ethiopia. BMC pregnancy and childbirth 2018, 18, 1–9.
- De Steenwinkel FD, Tak HV, Muller AE, Nouwen JL, Oostvogel PM, Mocumbi SM. Low carriage rate of group B streptococcus in pregnant women in Maputo, Mozambique. Tropical Medicine & International Health 2008, 13, 427–429.
- Tsolia M, Psoma M, Gavrili S, Petrochilou V, Michalas S, Legakis N. et al. Group B streptococcus colonization of Greek pregnant women and neonates: Prevalence, risk factors and serotypes. Clinical microbiology and infection 2003, 9, 832–838. [CrossRef] [PubMed]
- YektaKooshali MH, Hamidi M, Tousi SMTR, Nikokar I. Prevalence of group B streptococcus colonization in Iranian pregnant women: A systematic review and meta-analysis. International Journal of Reproductive BioMedicine, 2018; 16.
- Garland SM, Kelly N, Ugoni AM. Is antenatal group B streptococcal carriage a predictor of adverse obstetric outcome? Infectious diseases in obstetrics and gynecology 2000, 8, 138–42. [CrossRef]
- Tsui M, Ip M, Ng P, Sahota DS, Leung T, Lau T. Change in prevalence of group B Streptococcus maternal colonisation in Hong Kong. Hong Kong Med J 2009, 15, 414–419.
| Socio-demographic characteristics | Categories | Frequency | Percentage (%) |
|---|---|---|---|
| Health Institutions | |||
| WSU Comprehensive Specialized Hospital | -- | 169 | 60.6% |
| Sodo Health Center | -- | 110 | 39.4% |
| Age groups | 15-19 | 23 | 8.2% |
| 20-24 | 53 | 19.0% | |
| 25-29 | 135 | 48.8% | |
| 30-34 | 48 | 17.2% | |
| ≥ 35 | 20 | 7.1% | |
| Residence | Urban | 260 | 93.2% |
| Rural | 19 | 6.8% | |
| Educational status | Primary | 166 | 59.5% |
| Secondary | 55 | 19.7% | |
| College& above | 58 | 20.8% | |
| Marital status | Married | 249 | 89.2% |
| Divorced | 6 | 2.2 | |
| Single | 21 | 7.5% | |
| Widowed | 3 | 1.1 | |
| Occupational status | Housewife | 120 | 43.01 |
| Civil servant | 64 | 22.9 | |
| Student | 50 | 17.9 | |
| Merchant | 45 | 16.0 | |
| Monthly income | 1300-3000 | 221 | 79.2 |
| 3100-5000 | 35 | 12.5 | |
| 5100-1000 | 20 | 20 | |
| ≥ 10000 | 3 | 1.1 |
| Variables | Categories | Frequency | Percent (%) |
|---|---|---|---|
| Number of gravidity | primigravida | 173 | 62.0 |
| Multigravida | 106 | 38 | |
| Gestational age (in weeks) | 35 | 58 | 20.8 |
| 36 | 146 | 52.3 | |
| 37 | 75 | 26.9 | |
| History of Contraceptive usage | Yes | 223 | 79.9 |
| No | 56 | 20.1 | |
| History of abortion | Yes | 51 | 18.3 |
| No | 228 | 81.7 | |
| History of preterm labor | Yes | 13 | 4.7 |
| No | 266 | 95.3 | |
| History of Pretermprolonged rupture of membranes | Yes | ----- | ----- |
| No | 279 | 100 | |
| Diagnosis of UTI during pregnancy | Yes | 33 | 11.8 |
| No | 246 | 88.2 | |
| Diagnosis of STI during pregnancy | Yes | 3 | 1.1 |
| No | 276 | 98.9 | |
| History of ANC visit | Yes | 279 | 100 |
| No | 0 | 0 | |
| History of any antibiotic use | Yes | 0 | 0 |
| No | 279 | 100 | |
| History of any chronic medical illness | Yes | 16 | 5.7 |
| No | 263 | 94.3 |
| Characteristics | GBS | COR 95% CI | AOR 95% CI | P-value | ||
|---|---|---|---|---|---|---|
| Variables | Categories | Positive (%) | Negative (%) | |||
| Age group | 15-19 | 6(30.4) | 16(69.6) | 0.403(0.089,1.835 | 0.380(0.078,1.861) | 0.240 |
| 20-24 | 13(24.5) | 40(75.5) | 0.543(0.137,2.153) | 0.385 | ||
| 25-29 | 32(15.6) | 114(84.4) | 0.9589(0.258,3.560 | 0.949 | ||
| 30-34 | 12(47.9) | 25(52.1) | 0.192(0.050,0.741) | 0.300(0.072,1.254) | 0.099 | |
| >35 | 4 (15.0) | 17(85.0) | 1 | 1 | ||
| Educational Status | Primary | 49(30.7) | 117(69.3) | 1 | 1 | |
| Secondary | 3(3.6) | 52(96.4) | 0.812(0.413,1.596) | 0.545 | ||
| College & above | 15(25.9) | 43(74.1) | 6.047(1.642,22.370 | 6.610(1.724, 25.349) | 0.01 | |
| Occupational Status | Housewife | 32(26.7) | 88(73.3) | 0.770(0.376,1.578) | 0.475 | |
| Civil servant | 14(21.9) | 50(78.1) | 1.276(0.501,3.245) | 0.609 | ||
| Student | 10(20.0) | 40(80.0) | 0.770(0.317,1.870 | 0.564 | ||
| Merchant | 11(24.4) | 34(75.6 | 1 | 1 | ||
| Gravidity | Primigravida | 34(19.7) | 139(80.3) | 1 | 1 | |
| Multigravida | 33(31.1) | 73(68.9) | 0.499(0.286,0.871) | 1.761(0.941,3.296) | 0.077 | |
| Gestational age | 35 | 14(24.1) | 44(75.9) | 1 | 1 | |
| 36 | 28(19.2) | 118(80.8) | 1.433(0.671,3.061) | 0.352 | ||
| 37 | 25(33.3) | 50(66.7) | 2.204(1.166,4.164) | 1.509 (0.700,3.254) | 0.15 | |
| Contraception | Yes | 53(23.8) | 170(76.5) | 0.946(0.474,1.890) | 0.875 | |
| No | 14(25.0) | 42(75.0) | 1 | 1 | ||
| Abortion | Yes | 12(23.5) | 39(76.5) | 1.184(0.569,2.462) | 0.651 | |
| No | 55(24.1) | 173(75.9) | 1 | |||
| Preterm-labor | Yes | 5(38.5) | 8(61.5) | 0.698(0.208,2.345) | 0.561 | |
| No | 62(23.3) | 204(76.7) | 1 | 1 | ||
| UTI | Yes | 6(18.2) | 26(81.8) | |||
| No | 61(24.8) | 186(75.2) | 0.835(0.345,2.020) | 0.689 | ||
| Antibiotics with disc potency | Susceptible (%) | Intermediate (%) | Resistant (%) |
|---|---|---|---|
| Penicillin G (10 IU) | 62(92.5%) | ----- | 5 (7.46%) |
| Ampicillin (10μg) | 60 (89.6%) | ----- | 7 (10.4%) |
| Erythromycin (15μg) | 52/ (77%) | 5/67 (7.46%) | 10/ (14.92%) |
| Clindamycin (2μg) | 51/ (76.11%) | ---- | 16/ (23.88%) |
| Vancomycin (30μg) | 50/ (74.62%) | 6 (8.9%) | 11 (16.41%) |
| Ceftriazone (30μg) | 60/ (89.6%) | ----- | 7/ (10.4%) |
| Ciprofloxacin (5μg) | 30/ (44.77%) | ----- | 37 (55.22%) |
| Chloramphenicol (30μg) | 62/ (92.5%) | 5/ (7.5%) | ------ |
| Tetracycline (2μg) | 6/ (8.9%) | 2/ (2.98%) | 59/ (88%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





