1. Introduction
The paradigm of mental health treatment is undergoing a significant shift, characterized by a resurgent interest in the therapeutic potential of psychedelic substances [
1]. Concurrently, the role of the gut microbiome in mental health has emerged as a prominent field of study [
2,
3]. The intersection of these two fields has given rise to an exciting frontier of research in the psychedelic renaissance, investigating the symbiotic relationship between the gut microbiome and psychedelics. This review aims to explore the state of knowledge in this burgeoning field, with a focus on understanding how the gut microbiome can modulate the effects of psychedelic substances and how this interaction may shape the future of personalized mental health treatment. [
4,
5,
6]
The intricate relationship between the gut and the brain, often referred to as the gut-brain axis, is the foundation upon which the role of the microbiome in mental health is based. The gut-brain axis is a complex bidirectional communication system that integrates neural, hormonal, and immunological signaling between the gut and the brain. It has been implicated in a variety of psychological and neurological conditions, including anxiety, depression, stress, cognitive impairment, and sleep disorders, and plays a vital role in mental health [
7,
8,
9].
A central player in this communication system is the gut microbiome, a richly diverse ecosystem of microorganisms residing in the human gastrointestinal tract. The microbiome contributes significantly to our overall health, influencing digestion, immunity, and even our mood and cognition. Over the past decade, research has unveiled the profound influence that the gut microbiome can have on the brain and behavior, leading to the emergence of the field of psychobiotics, which explores how modifications of the microbiome can affect mental health [
6,
10].
The gut microbiome's role in modulating the effects of drugs has been well-established in the context of various medications, including antipsychotics and antidepressants [
11]. More recently, research has turned its attention towards the potential role of the gut microbiome in modulating the effects of psychedelic substances. The therapeutic efficacy of psychedelics has gained renewed interest, driven by promising results from clinical trials investigating their potential to treat mental health disorders such as depression, anxiety, and post-traumatic stress disorder (PTSD) [
12,
13].
Evidence suggests that the gut microbiome could be implicated in the metabolism and bioavailability of psychedelic substances, and by extension, their therapeutic effects. For example, recent studies have indicated that specific gut bacteria can modulate the metabolism of
N,
N-dimethyltryptamine (DMT), a psychoactive compound found in ayahuasca, which may influence the bioavailability and pharmacological effects of DMT in the host [
13,
14]. Furthermore, the individual variability in gut microbiome composition may influence the bioavailability and effects of psychedelic substances, emphasizing the potential for a personalized approach in psychedelic therapy.
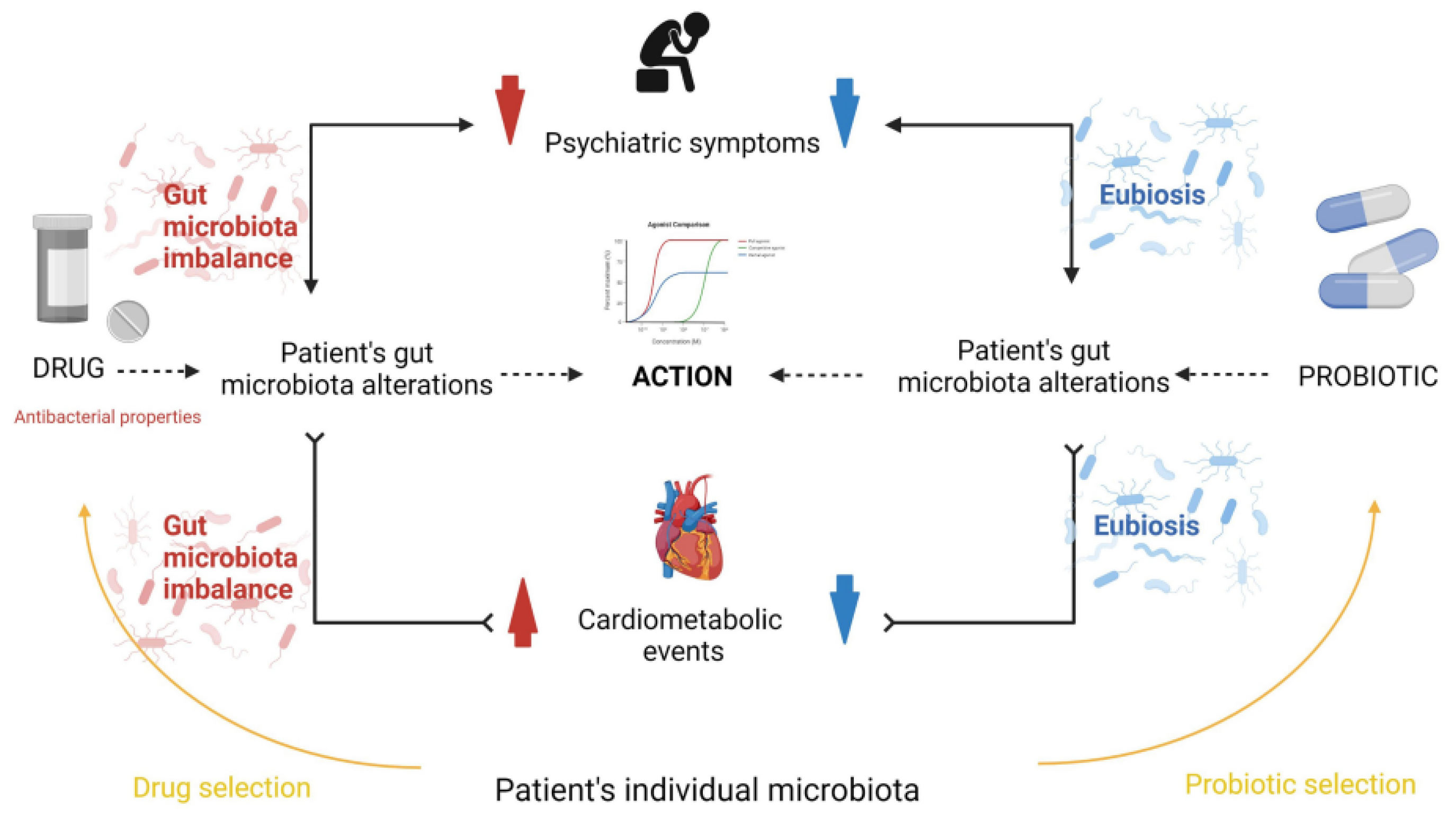
Figure 1, Misera et al. illustrate the gut microbiota influences psychiatric treatment efficacy [
2]. Antipsychotic drugs alter the microbiota composition, which can both mitigate psychiatric symptoms and potentially induce metabolic disorders - a common reason for treatment discontinuation. Probiotic supplementation may alleviate metabolic issues and augment drug effectiveness, highlighting a complex interplay between microbiota, psychopharmacology, and mental health outcomes.
However, our understanding of this intricate interplay between the microbiome and psychedelic substances is in its infancy. There is much we still do not know, including the extent to which the gut microbiome modulates the metabolism and effects of different psychedelic substances, how alterations in the gut microbiome might contribute to individual variability in response to these substances, and the potential risks and benefits of microbiome-mediated effects of psychedelics. This mini-review aims to examine the current evidence, shed light on these questions, and set the direction for future research in this exciting new field of psychedelic science.
2. Understanding the Gut-Brain Axis
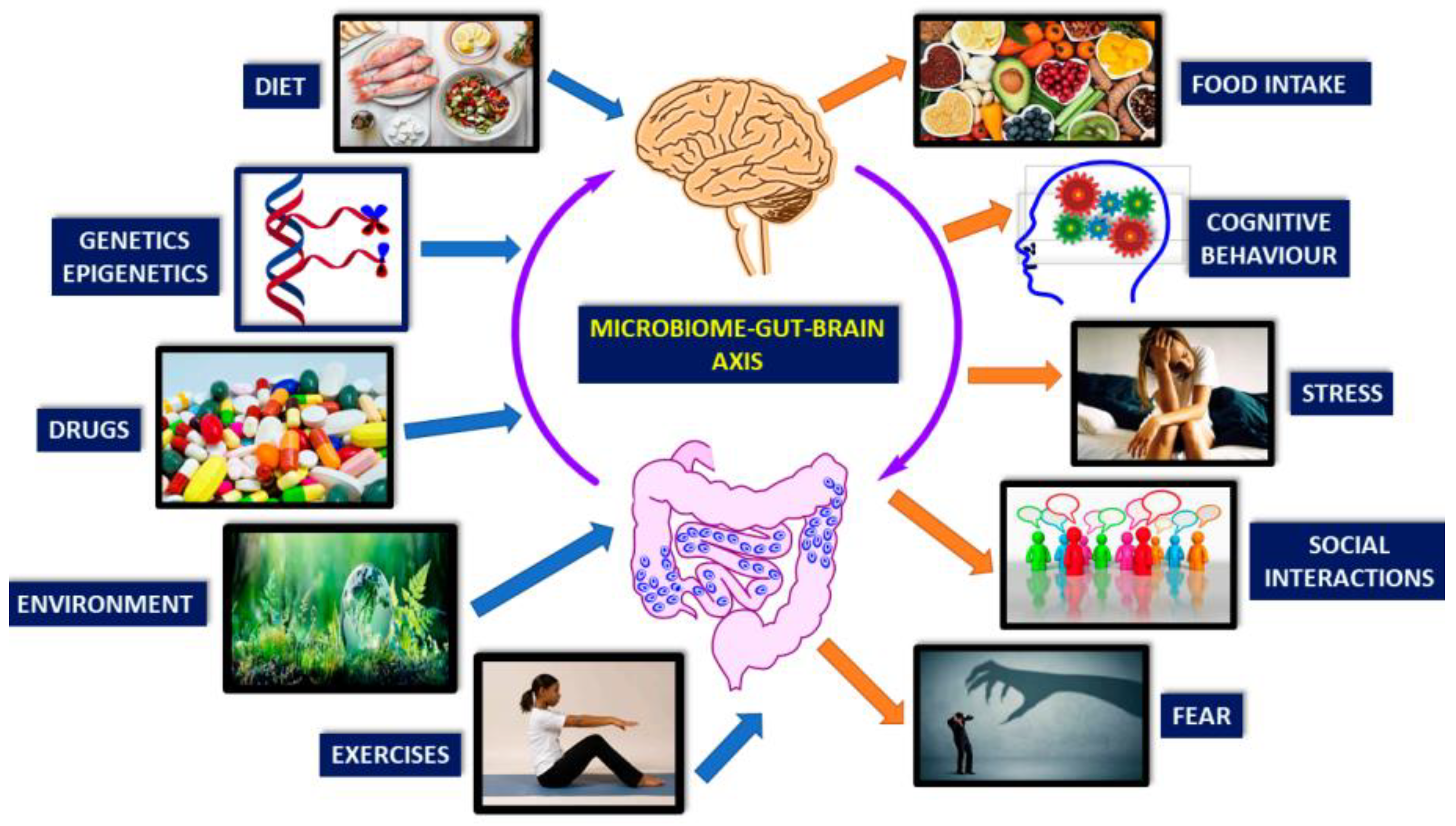
The gut-brain axis represents a complex bidirectional communication system that establishes a connection between the central and enteric nervous systems, playing an indispensable role in maintaining homeostasis [
15,
16]. The gut-brain axis encompasses multiple communication channels, including the immune system, the vagus nerve, and the production of various hormones and neurotransmitters [
17]. Importantly, recent studies have highlighted the crucial role that the gut microbiota plays in modulating this axis [
18].
The gut microbiota, a complex community of microorganisms residing in the human gastrointestinal tract, profoundly influences various aspects of human physiology, including metabolism, immunity, and neurobiology. The brain can influence the composition of the gut microbiota through the autonomic nervous system (ANS), affecting gut motility and secretion, as well as immune cell function [
19]. Conversely, the gut microbiota can affect the brain's activity through the production of metabolites, modulation of immune responses, and direct interactions with enteroendocrine cells and the enteric nervous system (ENS) [
20].
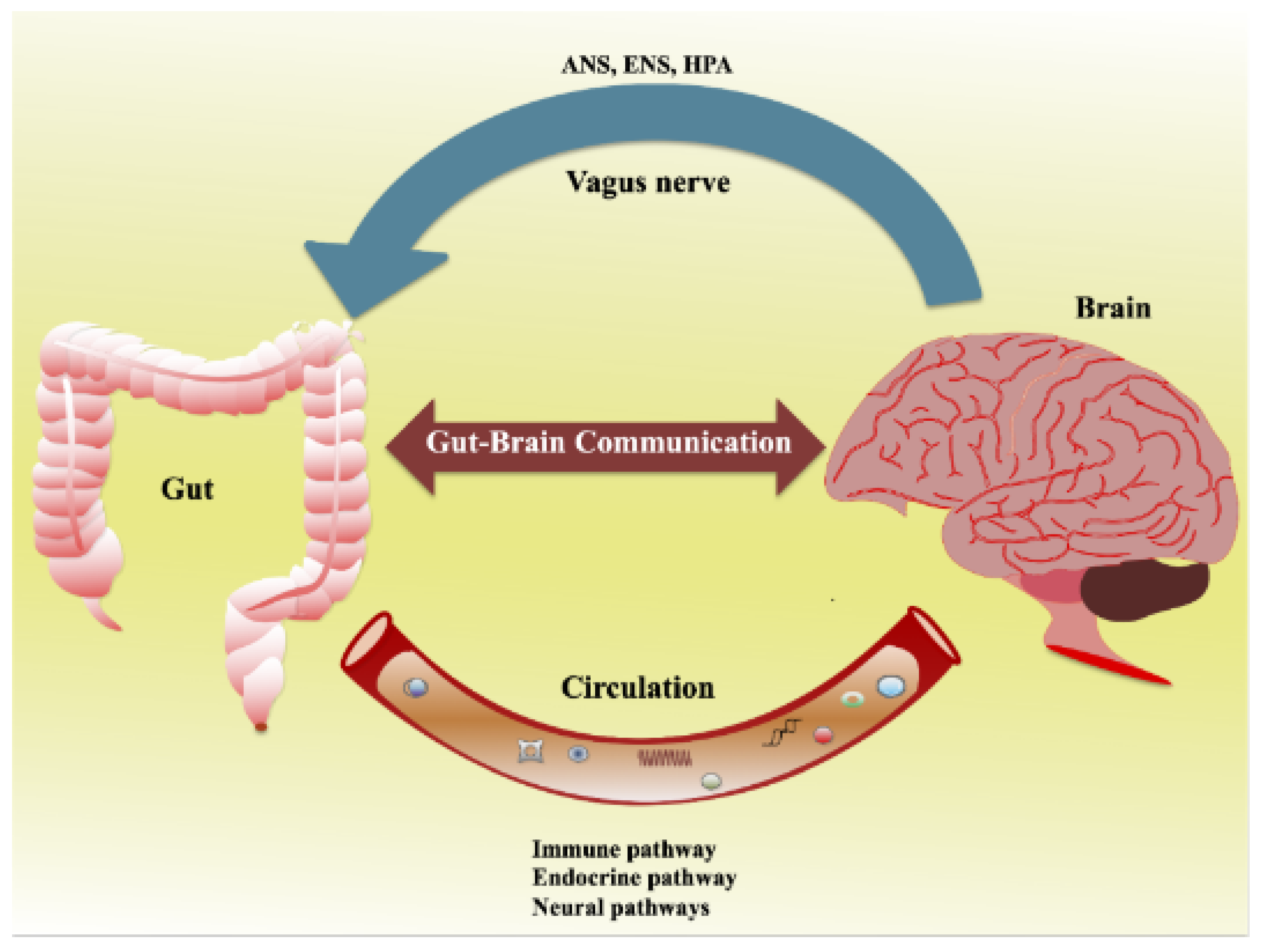
Figure 2 shows the bidirectional communication between the gut and the brain [
21]. Afferent and efferent brain neurons connect and signal through multiple pathways, including the ANS, ENS, and hypothalamic–pituitary–adrenal (HPA) axis.
The ENS is a complex network that controls intrinsic gut functions like motility, secretion, and absorption. Communication between the gut and brain is influenced by multiple pathways such as the ANS, ENS, HPA axis, immune, endocrine, and neural pathways. The ENS communicates with the CNS via intestinofugal neurons and sensory information travels via primary afferent neurons. The ANS, consisting of sympathetic and parasympathetic nerves, in conjunction with neuronal and neuroendocrine signaling, controls vital functions and CNS-mediated gut changes. The ANS impacts gut physiology directly via the CNS, while gut microbiota communicates through their metabolites and interacts with the ANS gut synapses. Furthermore, the ANS can modulate gut immune responses directly or indirectly via microbial interactions.
One of the most striking examples of the gut microbiota's influence on brain function comes from studies of germ-free mice. These animals exhibit various alterations in brain chemistry and behavior compared to conventionally raised mice, suggesting a fundamental role for the gut microbiota in brain development and function [
22].
Certain members of the gut microbiota can produce neurotransmitters and neuromodulators, including gamma-aminobutyric acid (GABA), serotonin, dopamine, and short-chain fatty acids (SCFAs), which can influence brain function both directly and indirectly [
23]. For instance, SCFAs can modulate the function of microglia, the primary immune cells in the brain, thereby influencing neuroinflammation and neurodegeneration [
24]. Furthermore, some gut bacteria can metabolize dietary tryptophan into indole derivatives, which can activate the aryl hydrocarbon receptor (AHR), a ligand-activated transcription factor that regulates immune responses and maintains the integrity of the gut barrier [
25].
The gut-brain axis also plays a crucial role in stress responses. Exposure to stress can alter the composition of the gut microbiota, a phenomenon termed dysbiosis, leading to increased intestinal permeability, also known as "leaky gut" [
26]. This can lead to the translocation of bacterial components into the bloodstream, triggering systemic inflammation, which, in turn, can affect brain function and behavior [
27].
The gut-brain axis is a multifaceted communication system linking the brain with the gut microbiota, playing a fundamental role in health and disease. An improved understanding of the gut-brain axis could pave the way for novel therapeutic strategies in the treatment of various psychiatric and neurological disorders, including those potentially modulated by psychedelic substances.
3. Microbiome and Psychedelic Interaction
The human gut microbiome has a profound influence on our health and well-being, not only in terms of physical health, but also as a modulator of brain function and behavior, including mood, cognition, and stress responses [
17]. With the ongoing psychedelic renaissance exploring the therapeutic potentials of psychedelic substances like psilocybin, lysergic acid diethylamide (LSD), and ayahuasca, it is essential to consider the role of the gut microbiome in this narrative.
Psychedelic substances can induce potent changes in consciousness, leading to significant alterations in perception, mood, and cognitive processes [
28]. The profound effects of these substances have sparked renewed interest in their therapeutic potential, particularly for mental health disorders such as depression, anxiety, and PTSD [
29].
Figure 3, Kelly et al. illustrate how the microbiota-gut-brain (MGB) axis may modulate responses to psychedelic therapy, acting as a biofeedback system [
30]. Initial MGB activity could help identify individuals more likely to benefit from such therapy. Prior to treatment, adjustments to the MGB axis could enhance responsiveness. During treatment, the MGB axis might affect psychedelic drug metabolism variability, while post-treatment, reinforcing MGB signaling could promote and sustain beneficial behavioral changes towards homeostasis.
Furthermore, recent evidence suggests the gut microbiome might play a role in the metabolism and bioavailability of these psychedelic substances, which in turn, could impact their pharmacological effects. For instance, Nichols et al. found that the gut bacterium Bifidobacterium modulates the metabolism of
N,
N-dimethyltryptamine (DMT), a psychoactive compound found in ayahuasca [
31]. This finding hints at the possibility that the therapeutic effects of psychedelics may, in part, be contingent upon the composition of an individual's gut microbiota.
This metabolic process of psychedelic substances by the gut microbiota can be expanded further when considering other substances such as psilocybin, LSD, and mescaline. Research by Mezquita et al. indicated that certain bacterial strains possess the necessary enzymes to convert psilocybin, found in "magic mushrooms," into its active metabolite, psilocin [
32]. Furthermore, Nichols et al. found that the gut bacterium Enterococcus faecalis possesses the enzyme lysergic acid diethylamide
N-demethylase, which can degrade LSD [
33].
Another line of investigation focuses on mescaline, a psychoactive compound found in the peyote cactus. Preliminary research by Saito et al. suggested that specific bacterial strains could metabolize mescaline into different metabolites
in vitro [
34]. While these studies provide preliminary evidence of microbiome involvement in the metabolism of various psychedelic compounds, further research is necessary to understand the extent and implications of these interactions for the bioavailability, effects, and individual variability in response to these substances.
In addition to metabolism, the gut microbiome can influence the effects of psychedelic substances on mood and behavior through its interactions with the gut-brain axis. A bidirectional communication system between the enteric and central nervous systems, the gut-brain axis is modulated by the gut microbiota and can affect various brain functions [
20]. For example, alterations in the gut microbiota have been linked to mood disorders like depression and anxiety, suggesting a role for the microbiome in modulating emotional responses [
35].
Given the known effects of psychedelics on emotional and cognitive processes, it is plausible to speculate that they might exert some of their effects through alterations in the gut microbiota. A recent study by Flanagan & Nichols suggested that the gut microbiota might interact with psychedelics to modulate their effects on mood and cognition [
14]. However, much more research is needed to test these hypotheses and to understand the precise mechanisms involved.
The potential interaction between the gut microbiome and psychedelic substances represents a promising area of research within the psychedelic renaissance. By investigating these interactions, we might gain novel insights into how psychedelics work, the factors that influence their effects, and new ways to optimize their therapeutic potential.
4. Interpersonal Variability in Psychedelic Response
Psychedelic substances, despite their demonstrated therapeutic potential, have long been recognized to exhibit a high degree of interpersonal variability in response [
36]. Individuals who use the same dosage of the same substance can experience vastly different subjective effects. This variability can be manifested in several ways, such as intensity and duration of effects, subjective experiences, and potential for therapeutic outcomes. Recognizing and understanding these differences is crucial, especially when considering the therapeutic applications of psychedelic substances in personalized medicine.
This variability has been traditionally attributed to a variety of factors. One of the most recognized is "set and setting" [
37]. "Set" refers to the mindset, expectation, and psychological state of the individual at the time of taking the psychedelic, while "setting" pertains to the physical and social environment in which the substance is used. This concept suggests that both the individual's internal mental state and the external environment significantly influence the individual's experience with the psychedelic. Psychedelic drugs offer therapeutic potential but can also induce adverse effects, making it crucial to predict individual reactions.
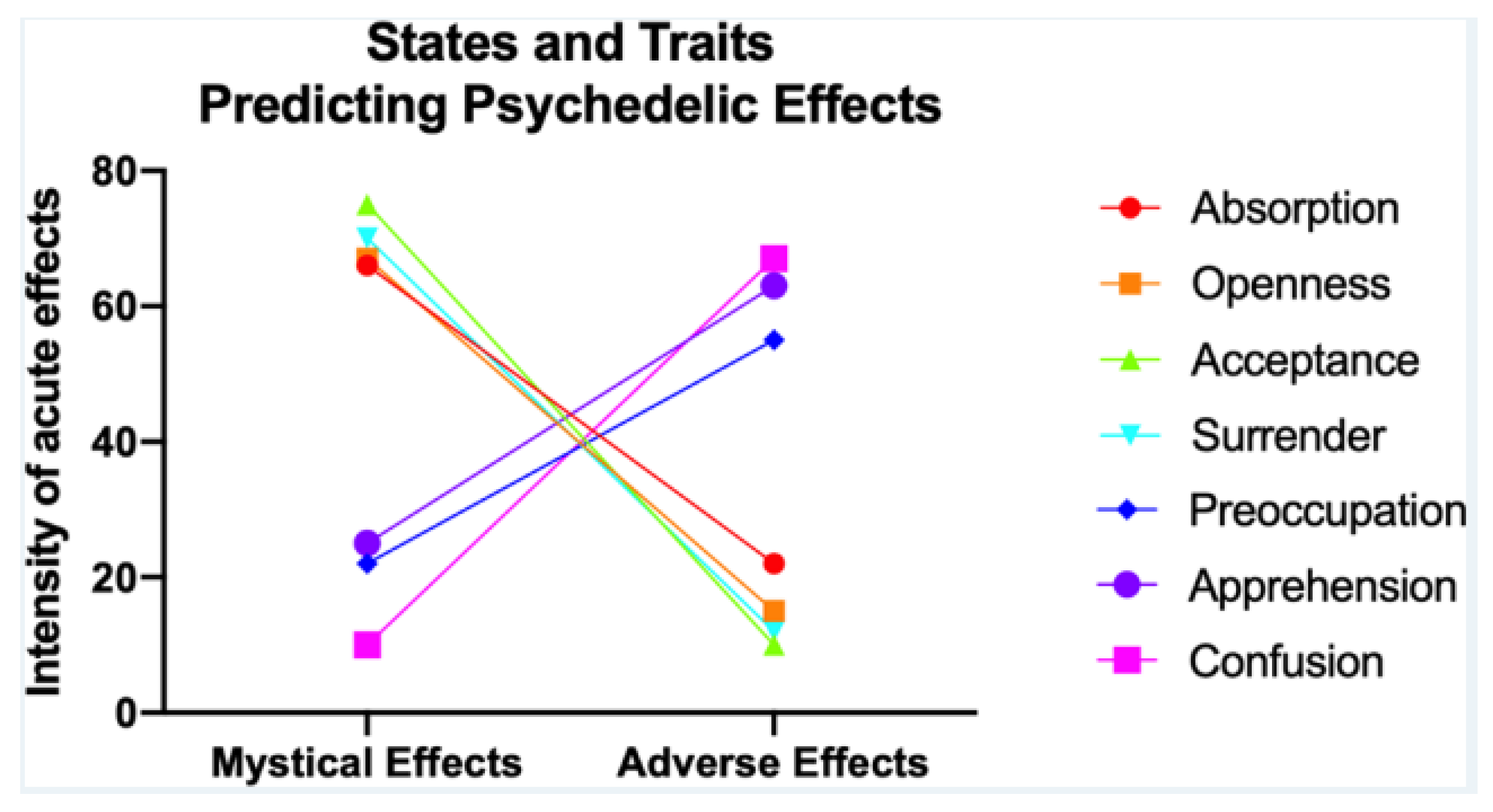
Figure 4 illustrate a systematic review of 14 studies, which found that traits like absorption, openness, acceptance, and a state of surrender correlate with positive experiences, while those low in openness and surrender or in certain negative psychological states are more likely to have adverse reactions [
38]. Age, experience with psychedelics, 5-HT2AR binding potential, executive network node diversity, and rACC volume might influence reactions.
Another aspect contributing to the interpersonal variability is genetic predisposition [
33]. It is well established that genetic factors can affect the metabolism of various substances, including psychedelics, which in turn, may influence their pharmacokinetics and pharmacodynamics. For instance, polymorphisms in enzymes involved in the metabolism of psychedelic substances, such as monoamine oxidases and cytochrome P450 enzymes, can lead to differences in the metabolism rate among individuals, thereby affecting the substances' bioavailability and effects [
39].
The emerging role of the gut microbiome adds another layer of complexity to this equation. As discussed earlier, the gut microbiome can affect the metabolism of psychedelic substances, suggesting it may play a role in mediating the interpersonal variability in psychedelic response. Differences in gut microbiome composition between individuals could lead to variability in the bioavailability and effects of psychedelic substances. For example, if one individual's gut microbiota metabolizes a psychedelic substance more efficiently than another's, this could lead to differences in the effects experienced by the two individuals, even if they consume the same dose [
32].
Additionally, the gut microbiome's role in modulating the gut-brain axis implies that it could potentially influence an individual's psychological response to psychedelics. It's plausible that an individual's gut microbiota could influence their mood and cognition, which could, in turn, influence their response to psychedelic substances [
35]. Moreover, recent research suggests that the gut microbiota can influence the brain's serotonergic system, which is a primary target of many psychedelic substances [
20].
While we are just beginning to understand how the gut microbiome might influence the interpersonal variability in psychedelic response, this area of research has profound implications. By elucidating the factors that contribute to this variability, we may be able to optimize psychedelic therapy to better suit individual needs. This could involve tailoring the dosage or type of psychedelic used based on an individual's genetic makeup, gut microbiome composition, or psychological state.
The variability in the psychedelic response among individuals is a complex phenomenon influenced by numerous factors, including mindset, environment, genetics, and potentially, the gut microbiome. More research is needed to understand these factors and their interactions, but the potential payoff is substantial: a more personalized and effective approach to psychedelic therapy.
5. Implications for Mental Health Treatment
Psychedelics, once stigmatized and marginalized in the medical community, are now in the spotlight as a potentially transformative treatment for various mental health disorders [
40]. The recent rediscovery of the therapeutic potential of these substances, sometimes referred to as the 'psychedelic renaissance', has offered new hope for patients suffering from treatment-resistant mental health conditions such as depression, anxiety, PTSD, and addiction [
41,
42].
The understanding that the gut microbiome may influence the response to psychedelic substances brings about a new frontier in the application of these substances for mental health treatment. As mentioned, variability in psychedelic experiences is significant, and this individuality of response could be partially modulated by the unique microbial composition of each person's gut [
43]. If further research confirms this hypothesis, we could soon be at the cusp of tailoring psychedelic therapy to the individual's microbiome, thus making treatment more effective and personalized.
For example, alterations in the gut microbiome are increasingly recognized as contributing factors in the development and manifestation of various mental health conditions, such as depression and anxiety [
18]. This is mostly attributed to the role of the gut microbiome in modulating the gut-brain axis, affecting mood, cognition, and behavior [
20]. If psychedelics' effects can be modulated by the gut microbiome, there is the possibility that tailoring these substances to the individual's microbial composition could enhance their therapeutic efficacy.
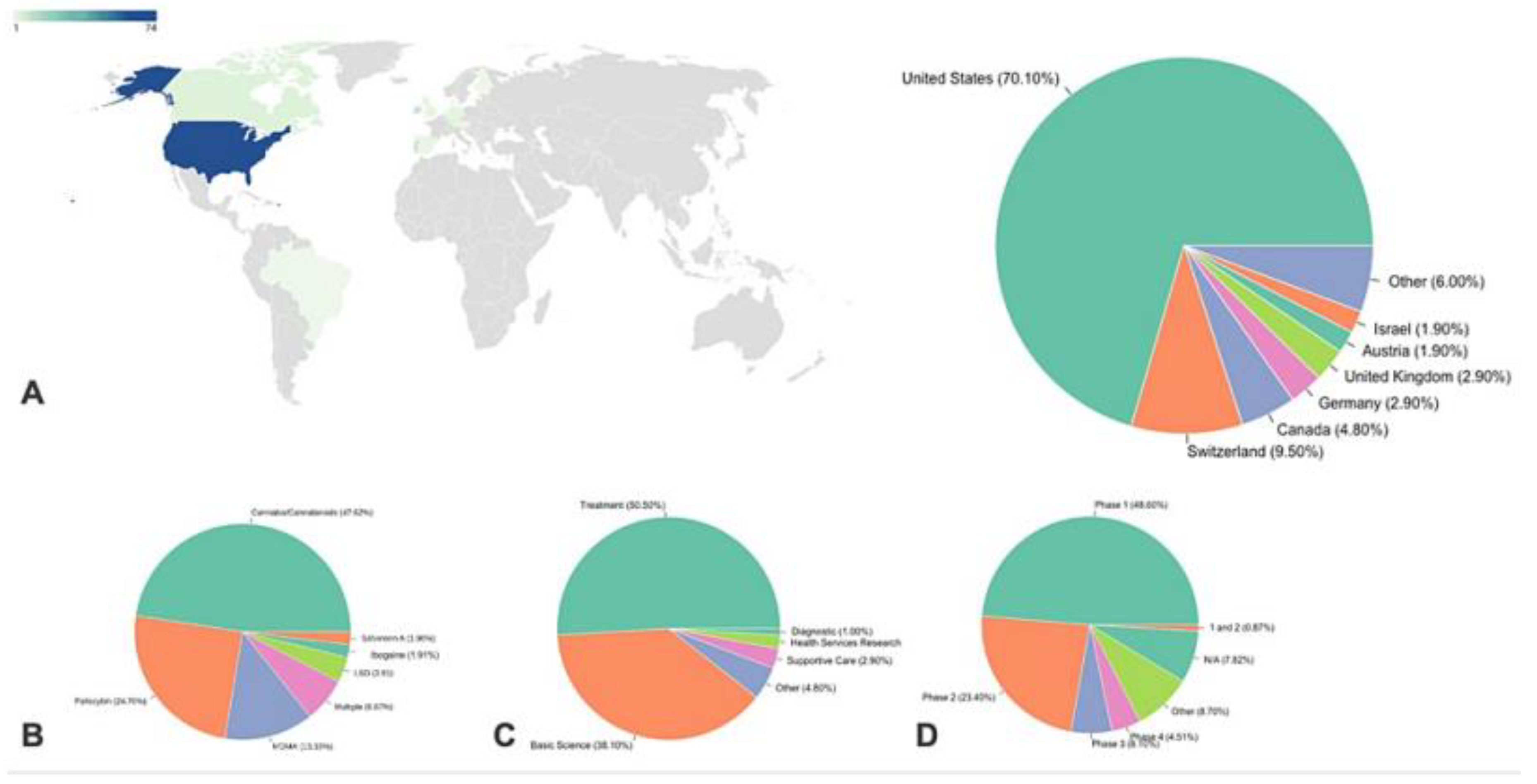
The US has the most ongoing clinical trials on psychedelics despite strict regulations (
Figure 5), hinting at possible future easing of these laws. Switzerland, more accepting of psychedelics, leads in per-capita studies, reflecting the influence of cultural acceptance on research [
44]. The predominance of US trials in this study may be due to a bias from using the Clinicaltrials.gov database, while other databases also exist. Reviewing clinical trials over time reveals an evolution in hypotheses, with initial trials before 2010 primarily focused on using psychedelics to improve mood in patients undergoing other treatments. More recently, psychedelics are being used as the primary treatment for various diseases, particularly psychiatric ones. The methodological robustness of these studies has also improved over time, with more trials using quadruple masking.
Moreover, if the gut microbiome can metabolize psychedelic substances and consequently affect their bioavailability, it may be possible to modulate the gut microbiome to enhance the bioavailability of these substances [
32]. This could result in more efficient and potent treatments, minimizing the required dosage of the substance and potentially reducing side effects.
Lastly, considering the emerging concept of 'psychedelic diet' or pre- and probiotic supplementation prior to psychedelic therapy, it might be possible to prepare the gut microbiome to maximize the therapeutic effects of psychedelics [
45]. However, further research is needed to validate the efficacy of such approaches.
The interactions between the gut microbiome and psychedelic substances could potentially revolutionize the way we approach mental health treatment. While more research is needed to fully understand these interactions, the possibility of a more personalized and effective mental health treatment paradigm is certainly on the horizon.
6. Microbiome-Targeted Interventions in Psychedelic Therapy
The symbiotic relationship between humans and the trillions of microbial organisms residing in the gut, collectively known as the gut microbiome, has received increased scientific interest over the past decade. The gut microbiome plays a crucial role in overall human health, influencing many physiological processes from nutrient absorption and immune system function to neurotransmitter production and neurodevelopment [
6]. However, the gut microbiome's potential role in psychedelic therapy, a growing field in mental health treatment, remains a largely unexplored frontier.
Psychedelic substances, such as psilocybin, LSD), and 3,4-Methylenedioxymethamphetamine (MDMA), have shown promising results in the treatment of mental health disorders, including depression, anxiety, and PTSD [
41,
42]. Yet, the therapeutic effects of these substances vary considerably among individuals, which may, in part, be due to differences in the gut microbiome composition [
43].
Psychedelic substances are exogenous compounds or xenobiotics, which can be metabolized by the gut microbiota, potentially affecting their bioavailability and therapeutic effects. Therefore, microbiome-targeted interventions, aimed at manipulating the gut microbiome, could theoretically modulate the therapeutic efficacy of psychedelics.
There are several potential microbiome-targeted interventions, including prebiotics, probiotics, and fecal microbiota transplantation (FMT). Prebiotics are dietary substances that promote the growth of beneficial gut bacteria, enhancing overall gut health. Probiotics are live bacteria that, when consumed in adequate amounts, confer a health benefit to the host. Both prebiotics and probiotics could potentially influence the metabolism and effects of psychedelic substances by altering the gut microbiome composition [
46].
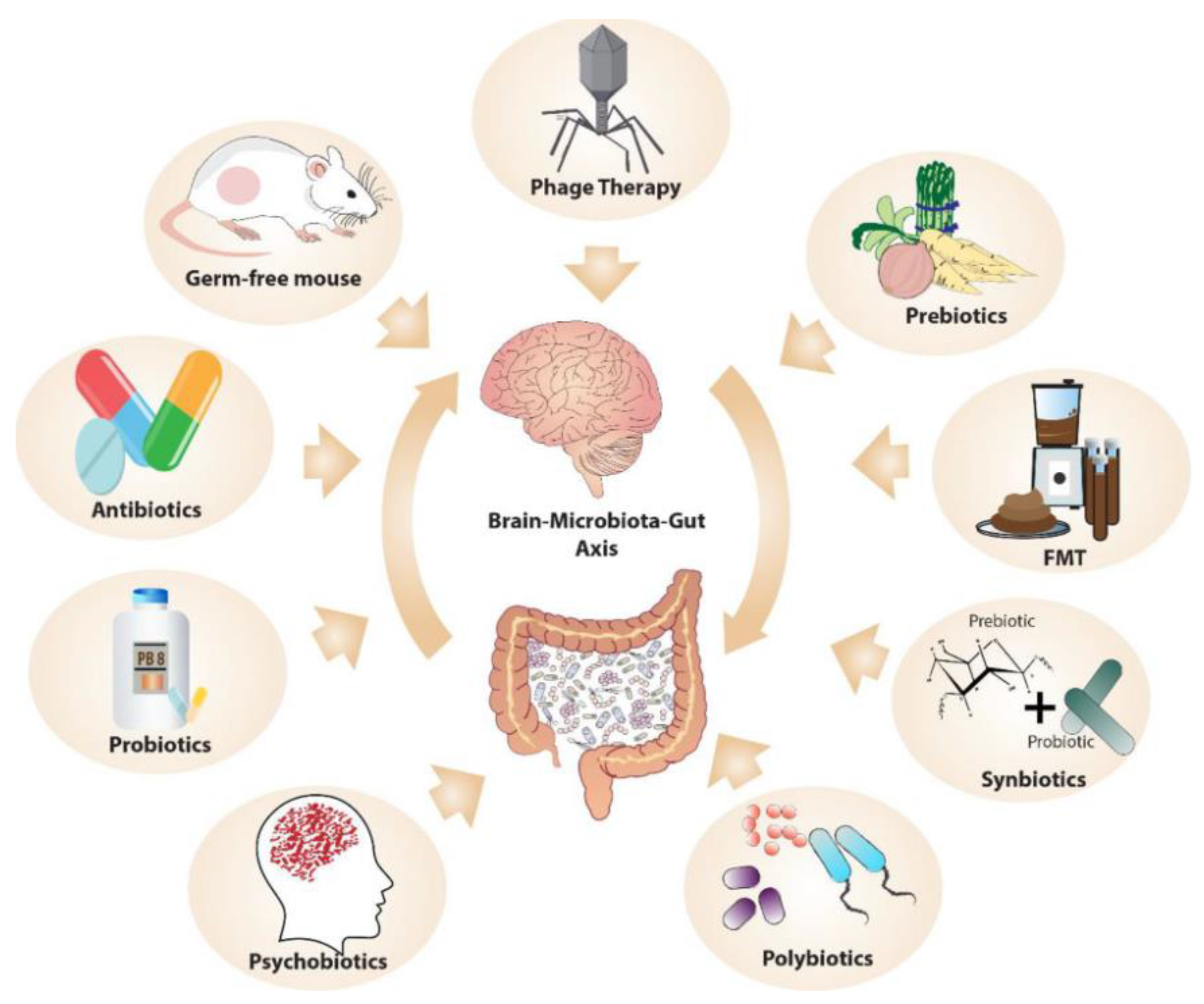
MGB communication is being studied by manipulating the microbiota, using methods such as germ-free (GF) animal models, microbiome depletion with antibiotics, and FMT (
Figure 6) [
47]. GF models reveal the microbiome's role in stress response, anxiety, social behavior, and cognition. FMT allows transfer of microbiota, potentially transmitting disorders like depression, but also shows therapeutic promise, as seen in gastrointestinal and psychiatric treatments. Phage therapy, involving viruses that infect specific bacteria, offers potential in modulating microbiome composition, although its use is currently limited to research. 'Postbiotics', nonviable bacterial products or metabolites, notably short-chain fatty acids, also play a significant role in the host, with their production encouraged by high-fiber diets. These methods could be employed to target the MGB axis in psychedelic therapy.
FMT, the process of transferring fecal bacteria from a healthy donor to a recipient, represents another potential method to alter the gut microbiome and, possibly, the response to psychedelic substances. It has been successfully used in the treatment of Clostridium difficile infections and is currently being explored as a treatment for other disorders associated with gut microbiota imbalances, such as irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) [
48].
There is a growing interest in the gut microbiome's role in modulating the effects of psychedelic therapy. Future research should focus on understanding the complex interactions between the gut microbiome and psychedelic substances and explore the potential of microbiome-targeted interventions to enhance the therapeutic efficacy of psychedelics.
7. Personalized Medicine and Psychedelics
The current era of medicine is witnessing a shift from the conventional one-size-fits-all treatment approaches to more personalized, patient-specific strategies. This transition is being fueled by advancements in genomics, proteomics, and other molecular diagnostic tools that enable the identification of individual-specific disease risk factors, prognostic indicators, and therapeutic targets. Psychedelic therapies, despite their historic, socio-cultural roots, are no exception to this trend.
Personalized psychedelic therapy refers to the tailoring of psychedelic treatment to meet the unique physiological, psychological, and experiential needs of an individual patient. The consideration for such customization ranges from genetic and metabolic differences that influence the pharmacokinetics of psychedelic substances to distinct psychosocial contexts that can shape an individual's subjective psychedelic experience and subsequent therapeutic outcomes.
Genetic variations play a critical role in the metabolism and effects of psychedelics. Cytochrome P450 enzymes, primarily CYP2D6, metabolize many psychedelics, including psilocybin and DMT. Genetic polymorphisms in the CYP2D6 gene are associated with variations in enzyme activity, leading to significant interindividual variability in drug metabolism. For instance, poor metabolizers can experience intensified and prolonged psychedelic effects due to slow drug metabolism, while ultra-rapid metabolizers may require higher doses for therapeutic efficacy [
49].
Further, there is considerable variability in the 5-HT2A receptor – the primary target of psychedelics – due to genetic polymorphisms. Differences in receptor density, signaling efficacy, and downstream effects may lead to differential responses to psychedelics [
50]. Research also suggests that the HTR2A gene polymorphisms might be linked with susceptibility to psychological disorders and the therapeutic response to psychedelic treatment [
51].
Psychedelic experiences are deeply personal and highly influenced by the individual's mindset and environment – a concept known as 'set and setting.' Individual personality traits, mental state, expectations, cultural background, and the physical and social environment can significantly modulate the subjective experience and therapeutic outcomes of psychedelic therapy. Recognizing these factors and tailoring the set and setting to the individual's needs can enhance the safety and efficacy of psychedelic therapy [
52].
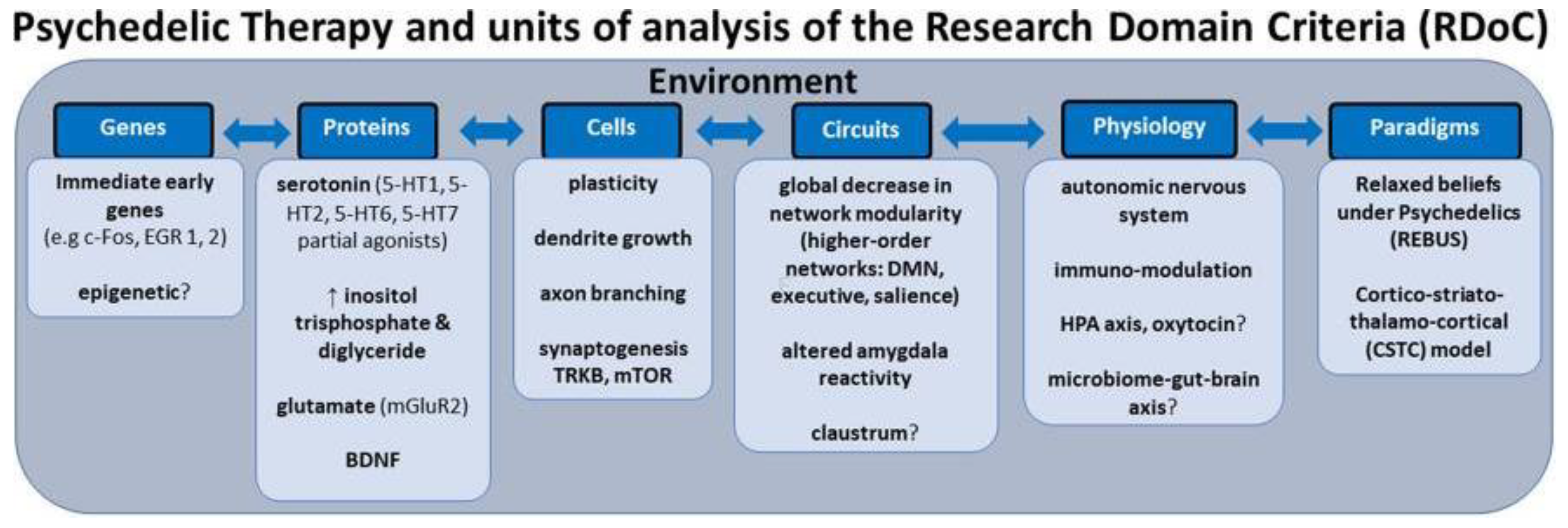
Personalized psychiatry faces challenges due to the reliance on categorical diagnostic systems and small-scale studies, which fail to capture mental health complexity. Categorical labels, though useful in clinical settings, often overlap and do not provide specific biological markers of mental health. Small effect sizes in mental health studies necessitate an integration of multiple variables for accurate prediction models. A shift toward a transdiagnostic, dimensional approach, deconstructing diagnoses into dimensional constructs, may enhance treatment precision. The RDoC (Research Domain Criteria) neuroscientific framework integrates developmental processes and environmental inputs, aiming to identify specific biosignatures for better mental health outcomes (
Figure 7) [
53]. This could revolutionize the precision of psychedelic therapy.
The microbiome-gut-brain axis, as discussed earlier, adds another level of complexity to personalized psychedelic therapy. The gut microbiome's composition and function, influenced by diet, lifestyle, antibiotics use, and other factors, can affect the metabolism and neuropharmacology of psychedelics. Personalizing psychedelic therapy might involve modulating the gut microbiome to optimize psychedelic effects [
54].
Integration, the process of making sense and deriving insights from the psychedelic experience, is a crucial part of psychedelic therapy. The process is highly individualized, depending on the person's cognitive styles, emotional processing, cultural context, and personal narratives. Ensuring the integration process aligns with the individual's unique needs can improve therapeutic outcomes [
55].
Despite its potential, personalized psychedelic therapy poses several challenges. It requires an interdisciplinary approach integrating psychopharmacology, genetics, microbiology, psychology, and other fields. In-depth knowledge of the individual patient is required, which necessitates thorough pre-treatment assessments. Also, while some personalization aspects, such as set and setting, can be addressed relatively easily, others, such as genetic testing and microbiome modulation, require more resources and advanced technologies. Further research is needed to understand the best practices for personalizing psychedelic therapy and to ensure that these personalized approaches are accessible and equitable [
56].
Personalized psychedelic therapy represents a promising frontier in the psychedelic renaissance. It capitalizes on the unique characteristics of psychedelic therapy - its physiological effects, subjective experiences, and potential for profound personal insights. By considering the unique needs and contexts of each patient, personalized psychedelic therapy has the potential to maximize therapeutic benefits, minimize risks, and contribute to the evolving understanding of psychedelic medicines.
8. Limitations and Future Research Directions
While the renaissance of psychedelic research is undeniably exciting, yielding promising results in treating various mental health disorders, it is important to remain cognizant of the limitations and challenges that currently exist in this field. Recognizing these challenges can help inform future research directions, ensuring the responsible and effective development and application of psychedelic therapies.
One primary limitation is the heterogeneity of the psychedelic experience, which can lead to inconsistent outcomes in clinical trials. The psychedelic experience is influenced by numerous factors including the individual's mindset, the environment ('set and setting'), and the therapeutic relationship [
52]. Thus, standardizing these aspects across diverse patient populations and treatment settings poses a significant challenge. Future research should aim to identify reliable and feasible methods for controlling these factors in clinical trials to achieve more consistent results.
Furthermore, the pharmacokinetics of psychedelics varies greatly among individuals, partly due to genetic polymorphisms affecting drug metabolism and target receptor function [
49]. This variability can affect the safety and efficacy of psychedelic therapies, necessitating dose adjustments or alternative treatment options for certain individuals. Future research could explore pharmacogenomic approaches to personalize psychedelic therapy, while also investigating the safety and efficacy of different dosing regimens.
Figure 8 illustrates the brain-gut-microbiota axis, which allows for bidirectional communication between the gut and CNS via complex, not fully understood mechanisms like neural, endocrine, immune, and metabolic pathways. Gut microbes can produce most brain neurotransmitters, influencing the CNS via multiple mechanisms. For example, probiotic Bifidobacteria can increase the amount of serotonin precursor, tryptophan. Lactobacilli species can modify GABA metabolism, changing brain GABA receptor expression and behavior. Gut-brain interaction also stimulates the hypothalamic-pituitary-adrenal (HPA) axis, inducing cortisol secretion, the body's main stressor system. Environmental factors, psychological or physical stress can affect this system, subsequently impacting gut microbiota/barrier function (
Figure 8) [
57]. Further research is required to fully understand and exploit these mechanisms in clinical settings.
Another critical limitation is the limited understanding of the long-term effects of psychedelic therapy. While short-term therapeutic effects are well-documented, more research is needed to assess the duration of these effects and potential long-term risks. Long-term follow-up studies are essential to determine the sustainability of therapeutic outcomes and identify any late-onset adverse effects [
58].
Additionally, the integration of psychedelic experiences into one's daily life is a vital but often overlooked aspect of psychedelic therapy. Integration involves the interpretation of the psychedelic experience and the application of any insights gained to promote positive changes in one's life. The lack of standardized guidelines and professional training for integration therapy is a significant challenge that future research needs to address [
59].
There are also legal and regulatory challenges to psychedelic research. Despite recent changes in some regions, the use of psychedelics remains heavily restricted in many countries, hindering the conduct of research and the accessibility of psychedelic therapy^5^. Continued advocacy for policy reform, backed by rigorous scientific evidence, is needed to reduce these barriers.
Looking at the microbiome's influence on psychedelic therapy, a novel but rapidly evolving area of research, there is still much to learn. Existing evidence points to a potential role of the gut microbiota in modulating the effects of psychedelics, suggesting the potential of microbiome-targeted interventions to enhance psychedelic therapy. However, our understanding of the intricate relationships between the gut microbiota, the brain, and psychedelic substances remains limited. Future research should focus on elucidating these mechanisms and conducting controlled trials of microbiome-targeted interventions in conjunction with psychedelic therapy [
33].
Furthermore, there is a need for research into methods for mitigating potential adverse effects of psychedelic therapy, such as the development of persistent psychosis or hallucinogen persisting perception disorder (HPPD) in susceptible individuals [
60].
Finally, an important future direction for psychedelic research is to continue exploring the therapeutic potential of these substances for a broader range of disorders. While most research has focused on mental health disorders, emerging evidence suggests potential applications in neurology, immunology, and other medical fields [
14].
9. Summary and Conclusion
Psychedelics have gained substantial attention in recent years for their potential therapeutic applications in treating mental health disorders, particularly depression, anxiety, and PTSD. This renewed interest, often referred to as the psychedelic renaissance, is rooted in the acknowledgment of the unique capacity of psychedelics to catalyze profound changes in perception, emotion, and cognition that can contribute to transformative therapeutic experiences.
There is a substantial body of evidence suggesting the therapeutic potential of psychedelics, with a variety of studies indicating their efficacy in treating mental health disorders. Interpersonal variability, however, is a key factor that can modulate these outcomes, with elements such as set and setting, gut microbiota, and genetic factors playing a role in influencing the therapeutic efficacy of psychedelics.
Set and setting, referring to an individual's mindset and the environmental context, are recognized as critical aspects that shape the nature and quality of the psychedelic experience. This variability emphasizes the importance of a carefully prepared and supportive environment in clinical and therapeutic settings to maximize positive outcomes and minimize adverse reactions.
In terms of the gut-brain axis, emerging research has demonstrated that the gut microbiota can influence brain function and behavior, potentially modulating the response to psychedelics. This insight opens up new opportunities for microbiome-targeted interventions to optimize the therapeutic benefits of psychedelics, highlighting the role of dietary interventions, probiotics, prebiotics, and fecal microbiota transplantation.
Moreover, understanding the genetics of psychedelic response is important for the development of personalized medicine approaches in psychedelic therapy. Pharmacogenomic strategies, involving genetic testing to predict an individual's drug response, could potentially guide the selection of appropriate psychedelic substances and doses for each individual, thereby maximizing therapeutic effects and minimizing adverse effects.
Yet, while the therapeutic potential of psychedelics is exciting, it's crucial to be aware of the limitations and future directions of this field. Limitations include the heterogeneity of the psychedelic experience, the lack of long-term studies, the current legal and regulatory hurdles, and the limited understanding of the precise role of the gut microbiota. Future research should aim to overcome these limitations, particularly in developing personalized and safe psychedelic therapies, standardizing integration therapy, understanding the long-term effects, and exploring the potential of these substances for a broader range of disorders.
In conclusion, the psychedelic renaissance holds significant promise for the future of mental health treatment. However, for this promise to be realized, it's important to conduct comprehensive and rigorous research to fully understand the complex dynamics involved in the psychedelic experience. This involves not only exploring the profound changes in consciousness induced by these substances but also understanding the impact of the interpersonal variability in the psychedelic response, the role of the gut-brain axis, and the potential of personalized medicine. If navigated thoughtfully and responsibly, psychedelics could revolutionize mental health treatment, providing powerful new tools to promote healing and growth.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Roth, B.L.; Gumpper, R.H. Psychedelics as Transformative Therapeutics. Am. J. Psychiatry. https://doi.org/10.1176/appi.ajp.20230172. PMID: 37122272. Psychedelic history available online: https://blog.retreat.guru/the-history-of-psychedelics & https://www.portasophia.org/timelines/addiction-timeline (accessed 27th May, 2023). 2023, 180, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Misera, A.; Łoniewski, I.; Palma, J.; Kulaszyńska, M.; Czarnecka, W.; Kaczmarczyk, M.; Liśkiewicz, P.; Samochowiec, J. Clinical significance of microbiota changes under the influence of psychotropic drugs. An updated narrative review. Front. Microbiol. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Kargbo, R.B. Microbiome-Gut-Brain Axis Modulation: New Approaches in Treatment of Neuropsychological and Gastrointestinal Functional Diorders. ACS Med. Chem. Lett 2023. [Google Scholar] [CrossRef] [PubMed]
- Angoa-Pérez, M.; Kuhn, D.M. Evidence for Modulation of Substance Use Disorders by the Gut Microbiome: Hidden in Plain Sight. Pharmacol. Rev. 2021, 73, 571–596. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Bolstridge, M.; Day, C.M.J.; et al. Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacol. 2018, 235, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O'Riordan, K.J.; Cowan, C. S, M.; Sandhu, K.V. et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Guo, K.; Zeng, L.; Zeng, B.; Huo, R.; Luo, Y.; et al. Metabolite identification in fecal microbiota transplantation mouse livers and combined proteomics with chronic unpredictive mild stress mouse livers. Transl. Psychiatry 2018, 8, 34. [Google Scholar] [CrossRef]
- Sen, T.; Cawthon, C.R.; Ihde, B.T.; Hajnal, A.; DiLorenzo, P.M.; de La Serre, C.B.; Czaja, K. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol. Behav. 2017, 173, 305–317. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stilling, R.M.; Stanton, C.; Cryan, J.F. Collective unconscious: How gut microbes shape human behavior. J. Psychiatr. Res. 2015, 63, 1–9. [Google Scholar] [CrossRef]
- Bahr, S.M.; Tyler, B.C.; Wooldridge, N.; Butcher, B.D.; Burns, T.L.; Teesch, L.M.; Oltman, C.L.; Azcarate-Peril, M.A.; Kirby, J.R.; Calarge, C.A. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl. Psychiatry. 2015, 5, 652. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Muthukumaraswamy, S.; Roseman, L.; Kaelen, M.; Droog, W.; Murphy, K.; Tagliazucchi, E.; Schenberg, E.E.; Nest, T.; Orban, C.; Leech, R.; et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc. Natl. Acad. Sci. U S A. 2016, 113, 4853–4858. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E.; Johnson, M.W.; Nichols, C.D. Psychedelics as Medicines: An Emerging New Paradigm. Clin. Pharmacol. Ther. 2017, 101, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, T.W.; Nichols, C.D. Psychedelics as anti-inflammatory agents. Int. Rev. Psychiatry. 2018, 30, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut–Brain Axis. Annu. Rev. Med 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress. 2017, 7, 124–136. [Google Scholar] [CrossRef]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Invest. 2015, 125, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol Clin. North Am. 2017, 46, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Suganya, K.; Koo, B. Gut–Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef]
- Luczynski, P.; Whelan, S.O.; O'Sullivan, C.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Eur. J, Neurosci. 2016, 44, 2654–2666. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.P.; Michel, M.L.; Da Costa, G.; et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Söderholm, J.D.; Yates, D.A.; Gareau, M.G.; Yang, P.C.; MacQueen, G.; Perdue, M.H. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, 1257–1263. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.C. The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro. Endocrinol. Lett. 2008, 29, 117–124. [Google Scholar]
- Carhart-Harris, R.L.; Nutt, D.J. Serotonin and brain function: A tale of two receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef]
- Bogenschutz, M.P.; Forcehimes, A.A.; Pommy, J.A.; Wilcox, C.E.; Barbosa, P.C.R.; Strassman, R.J. Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J. Psychopharmacol. 2015, 29, 289–299. [Google Scholar] [CrossRef]
- Kelly, J.R.; Clarke, G.; Harkin, A.; Corr, S.C.; Galvin, S.; Pradeep, V.; Cryan, J.F.; Dinan, T.G. Seeking the Psilocybiome: Psychedelics meet the microbiota-gut-brain axis. IJCHP 2023, 23, 100349. [Google Scholar] [CrossRef]
- Nichols, D.E.; Barker, S.A.; McKenna, D.J.; Romanelli, F.; Canfield, D.V.; Clark, M.R. The endogenous hallucinogen and trace amine N,N-dimethyltryptamine (DMT) displays potent protective effects against hypoxia via sigma-1 receptor activation in human primary iPSC-derived cortical neurons and microglia-like immune cells. Front. Neurosci. 2020, 14, 1–11. [Google Scholar] [CrossRef]
- Mezquita, G.A.; Correia, B.L.; Bastos, I.M.; Bentes, A.S.; de Carvalho, J.G. The missing link: Does the gut microbiota influence the efficacy of psychedelics? J. Psychopharmacol. 2021, 35, 495–507. [Google Scholar] [CrossRef]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef]
- Saito, Y.; Hashimoto, K.; Cyranoski, D.; Yokoyama, A.; Suzuki, K.; Kawase, Y.; Takeda, T.; Hongo, S. The gut microbiota and psychiatric disorders: Implications for clinical practice. J. Clin. Psychiatry. 2019, 80, 1–11. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Hartogsohn, I. Set and setting, psychedelics and the placebo response: An extra-pharmacological perspective on psychopharmacology. J. Psychopharmacol. 2016, 30, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Winkelman, M.J. The Evolved Psychology of Psychedelic Set and Setting: Inferences Regarding the Roles of Shamanism and Entheogenic Ecopsychology. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Aday, J.S.; Davis, A.K.; Mitzkovitz, C.M.; Bloesch, E.K.; Davoli, C.C. Predicting Reactions to Psychedelic Drugs: A Systematic Review of States and Traits Related to Acute Drug Effects. ACS Pharmacol. Transl. Sci. 2021, 4, 424–435. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.P.; Childress, A.R.; Ehrman, R.; Robbins, S.J. Conditioning factors in drug abuse: Can they explain compulsion? J. Psychopharmacol. 1998, 12, 15–22. [Google Scholar] [CrossRef]
- Johnson, M.W.; Griffiths, R.R. Potential therapeutic effects of psilocybin. Neurotherapeutics. 2017, 14, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Goodwin, G.M. The therapeutic potential of psychedelic drugs: Past, present, and future. Neuropsychopharmacology. 2017, 42, 2105–2113. [Google Scholar] [CrossRef]
- Mithoefer, M.C.; Mithoefer, A.T.; Feduccia, A.A.; et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: A randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018, 5, 486–497. [Google Scholar] [CrossRef]
- Skosnik, P.D.; Cortes-Briones, J.A. Targeting the ecology within: The role of the gut-brain axis and human microbiota in drug addiction. Med. Hypotheses. 2016, 93, 77–80. [Google Scholar] [CrossRef]
- Kurtz, J.S.; Patel, N.A.; Gendreau, J.L.; Yang, C.; Brown, N.; Bui, N.; Picton, B.; Harris, M.; Hatter, M.; Beyer, R.; Sahyouni, R.; Diaz-Aguilar, L.D.; Castellanos, J.; Schuster, N.; Abraham, M.E. The Use of Psychedelics in the Treatment of Medical Conditions: An Analysis of Currently Registered Psychedelics Studies in the American Drug Trial Registry. Cureus 2022, 14, 29167. [Google Scholar] [CrossRef]
- Luna, R.A.; Foster, J.A. Gut brain axis: Diet microbiota interactions and implications for modulation of anxiety and depression. Curr. Opin. Biotechnol. 2015, 32, 35–41. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; Calder, P.C.; Sanders, M.E. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.I.; Mörkl, S.; Sandhu, K.V.; Cryan, J.F.; Dinan, T.G. The Gut Microbiome and Mental Health: What Should We Tell Our Patients? Can. J. Psychiatry 2019, 64, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Kirchherr, H.; Kühn-Velten, W.N. Quantitative determination of forty-eight antidepressants and antipsychotics in human serum by HPLC tandem mass spectrometry: A multi-level, single-sample approach. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2006, 843, 100–113. [Google Scholar] [CrossRef]
- Nichols, C.D.; Sanders-Bush, E. A phenylisopropylamine derivative of metabotropic glutamate receptor antagonist produces a hallucinogen-specific-like discriminative stimulus in mice. Pharmacol. Biochem. Behav. 2004, 79, 739–746. [Google Scholar] [CrossRef]
- Gonzalez-Maeso, J.; et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007, 53, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Hartogsohn, I. Constructing drug effects: A history of set and setting. Drug Sci. Policy Law. 2017, 3, 2050324516683325. [Google Scholar] [CrossRef]
- Kelly, J.R.; Gillan, C.M.; Prenderville, J.; Kelly, C.; Harkin, A.; Clarke, G. Psychedelic Therapy's Transdiagnostic Effects: A Research Domain Criteria (RDoC) Perspective. Front. Psychiatry 2020, 12, 800072. [Google Scholar] [CrossRef] [PubMed]
- Skosnik, P.D.; Cortes-Briones, J.A. Targeting the ecology within: The role of the gut-brain axis and human microbiota in drug addiction. Med. Hypotheses. 2016, 93, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.; Day, C.; Krzanowski, J.; Nutt, D.; Carhart-Harris, R. Patients' accounts of increased "connectedness" and "acceptance" after psilocybin for treatment-resistant depression. J. Humanist Psychol. 2017, 57, 520–564. [Google Scholar] [CrossRef]
- Koenig, J.; Parzer, P.; Reichl, C.; Andritzky, B.; Brunner, R.; Resch, F.; Kaess, M. Cortisol and self-harm in adolescents: A community study. J. Psychiatr. Res. 2016, 83, 78–85. [Google Scholar] [CrossRef]
- Karakan, T.; Ozkul, C.; Küpeli Akkol, E.; Bilici, S.; Capasso, R. Gut-Brain-Microbiota Axis: Antibiotics and Functional Gastrointestinal Disorders. Nutrients 2021, 13, 389. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.W.; Garcia-Romeu, A.; Griffiths, R.R. Long-term follow-up of psilocybin-facilitated smoking cessation. Am. J. Drug Alcohol Abuse. 2017, 43, 55–60. [Google Scholar] [CrossRef]
- Watts, R.; Day, C.; Krzanowski, J.; Nutt, D.; Carhart-Harris, R. Patients' accounts of increased "connectedness" and "acceptance" after psilocybin for treatment-resistant depression. J. Humanist Psychol. 2017, 57, 520–564. [Google Scholar] [CrossRef]
- Halpern, J.H.; Lerner, A. G.; Passie, T. A review of hallucinogen persisting perception disorder (HPPD) and an exploratory study of subjects claiming symptoms of HPPD. Curr. Top Behav. Neurosci. 2018, 36, 333–360. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).