1. Introduction
Ulcerative colitis (UC) is rising worldwide, reaching a prevalence of over 5 per 1000 people and 2 per 1000 people in Europe and the US, respectively1. This rise, along with the concomitant increase in life expectancy, means more patients will experience disease burden for a longer time. Therefore, long-term therapy is needed with both effective and safe drugs. The introduction of biologics in the past two decades has had a profound effect on the treatment landscape of UC. Nevertheless, many patients do not respond to therapy induction and lose response over time2,3. Although switching to the next biologic drug is possible, loss-of-response rates are even higher for biologic-experienced patients4. Hence, it is important to evaluate DS, both as a clinical target and as a surrogate marker for drug efficacy, especially when choosing the first biologic. This is particularly important as head-to-head studies of biologic drugs in IBD are scarce. Unfortunately, data regarding DS are limited, often derived from clinical-phase drug studies that substantially differ from real-world clinical settings5. Therefore, we aimed to assess the DS of two biologic drugs used to treat UC: IFX and VDZ.
2. Materials and Methods
This retrospective cohort study was conducted at the Rabin Medical Center, a large tertiary referral center in Israel.
1.1. Patient Selection and Follow-Up Period
We included patients 18 years old and above who received IFX or VDZ at the RMC infusion center between Dec 1st, 2017, and May 1st, 2021. Patients with acute severe colitis, Crohn's disease, those with IBD-U, or those that underwent colectomy were excluded. Patients were identified using the combination of ICD-9 codes for UC diagnosis and the internal health maintenance operator (HMO)s' codes for IV treatment of the specific biologic drug. Patients were followed from the first documentation of therapy until the last follow-up date.
1.2. Outcomes
We assessed the DS of IFX and VDZ. DS was defined as long as corticosteroid, surgical, and hospitalization-free treatment was documented. We assessed DS in treatment-naïve, treatment-experienced, and in patients treated with one of the investigated drugs after failing the other.
1.3. Data Collection
An extensive medical chart review was performed. Patients' data were collected from the first treatment documentation to the end of follow-up. Data collected included sex, age at diagnosis, age at treatment start, length of disease until treatment was started, disease extent (proctitis, left-sided or extensive), history of previous treatments with biologics, and comprehensive clinical laboratory data. We also documented if treatment was in combination with an immunomodulatory drug. We separately compared those previously naïve to biologic drugs and those who were experienced (2nd line treatment) separately.
1.4. Statistical Analysis
We used the Fisher-exact test for categorical variables and students' T-test or Wilcoxon test for continuous variables when comparing the two groups and then constructed a Kaplan-Meier survival curve to depict comparative DS (depicted by length of drug treatment in weeks). We performed a univariate analysis for factors associated with treatment cessation (sustainability) to calculate the factor specific hazard ratio (HR). We then adjusted for confounding factors (such as factors associated with disease severity as described below in the results section) by performing a multivariate COX-regression model and calculated the HR for DS. All results are reported in a 95% confidence limit. All statistical analysis was done using the SAS software version 9.4.
3. Results
2.1. Baseline Population Characteristics
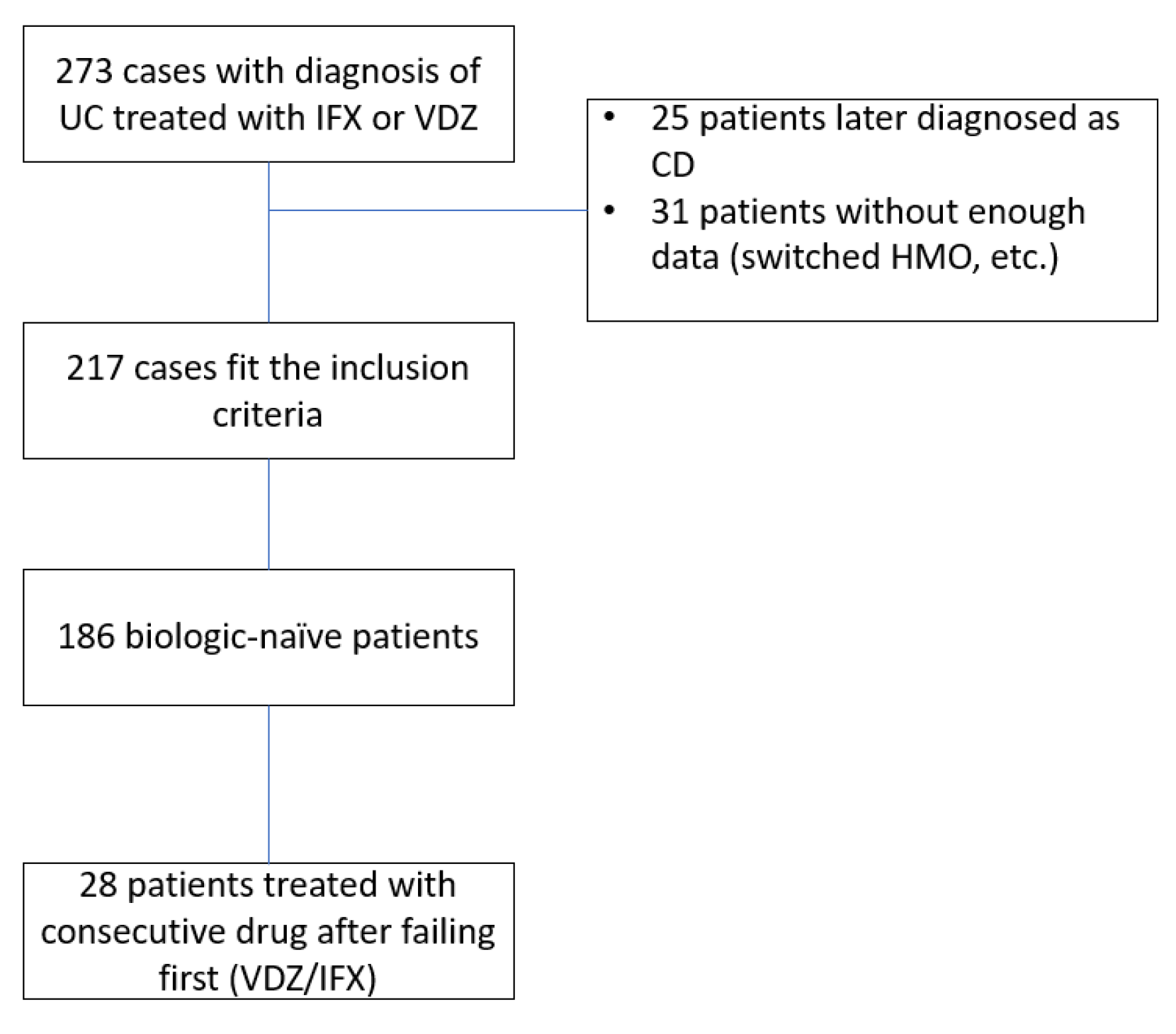
A total of 273 patients were treated with IFX or VDZ at our center within the study time frame. Twenty-five patients were excluded due to a change of diagnosis, and 31 were excluded due to insufficient data (usually after switching HMO or treatment site or traveling abroad). The study design scheme is shown in
Figure 1. A final 217 patients were included in the analysis, with a total follow-up period of 29686 patient weeks. Of those, 186 were treatment-(biologic)-naïve, and of whom 28 were consequently treated with the second agent investigated after failing the first. One hundred nine patients were treated with VDZ as the first drug, and 77 (41.3%) patients were treated with IFX as the first drug.
When comparing the two groups, more patients with proctitis were treated with VDZ (22% vs. 10.3%, p=0.048). IFX was used more often with an immunomodulatory agent (14.2% vs. 2.7%, p=0.004). There were also differences between the groups in baseline clinical features. Specifically, patients treated with IFX had lower baseline albumin (3.4 vs. 4.3, p=0.001) and total protein (6.2 vs. 7.2, p=<.001) as well as a higher baseline calprotectin (4425 vs. 529, p=0.001), baseline CRP (1.3 vs. 0.5, p=0.002) and higher baseline neutrophil-to-lymphocyte ratio (4.2 vs. 2.7, p=0.000). Patients treated with IFX also had lower baseline hemoglobin (median levels 11 vs. 13, p= 0.001) and baseline hematocrit levels (median 33.8 vs. 40.9, p=,.001); the hemoglobin-to-hematocrit ratio was not different between the groups (p=0.640). Baseline characteristics are shown in
Table 1.
2.2. DS
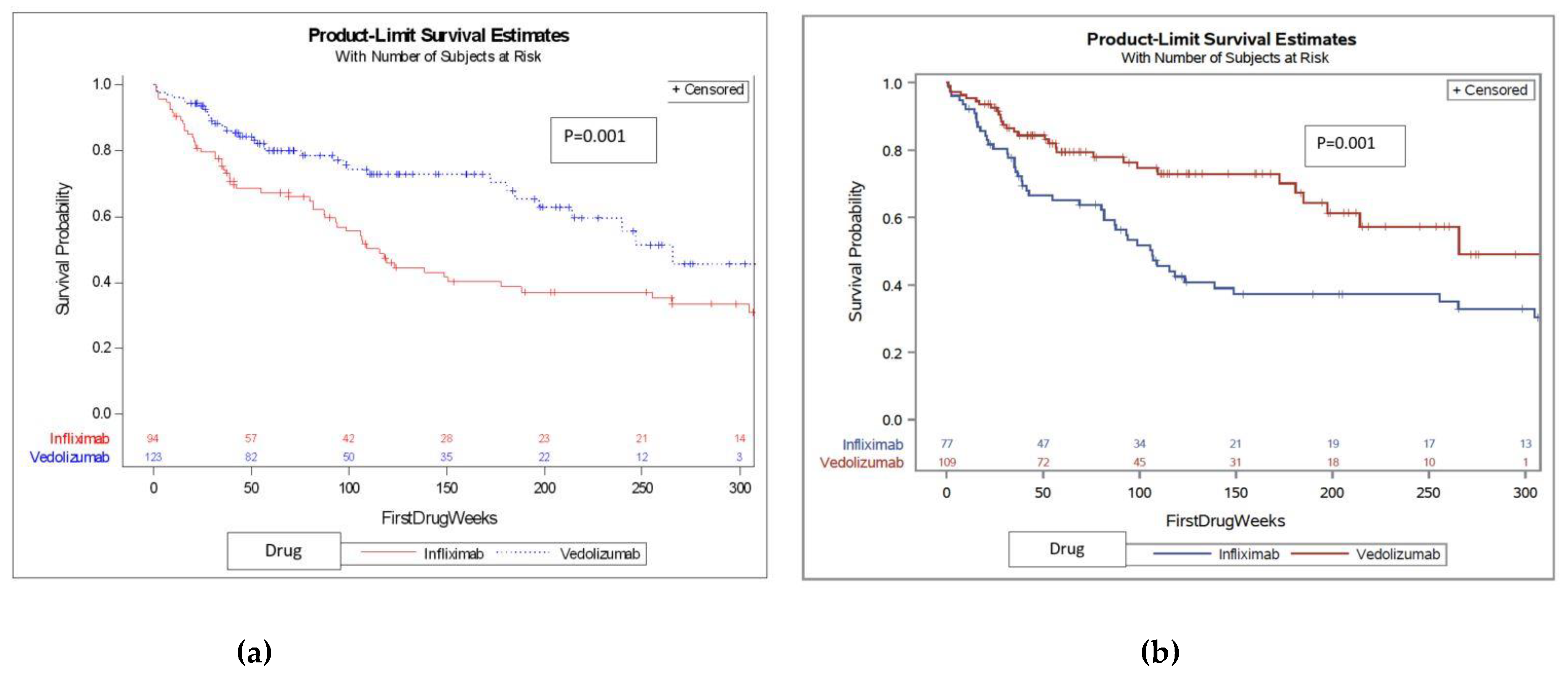
We first compared treatment naïve patients and found that VDZ was associated with a median survival time of 265.6 weeks (121.3-273.1), whereas IFX was associated with a significantly shorter median survival time of 106.5 (82.9-135) weeks; see KM survival curve shown in
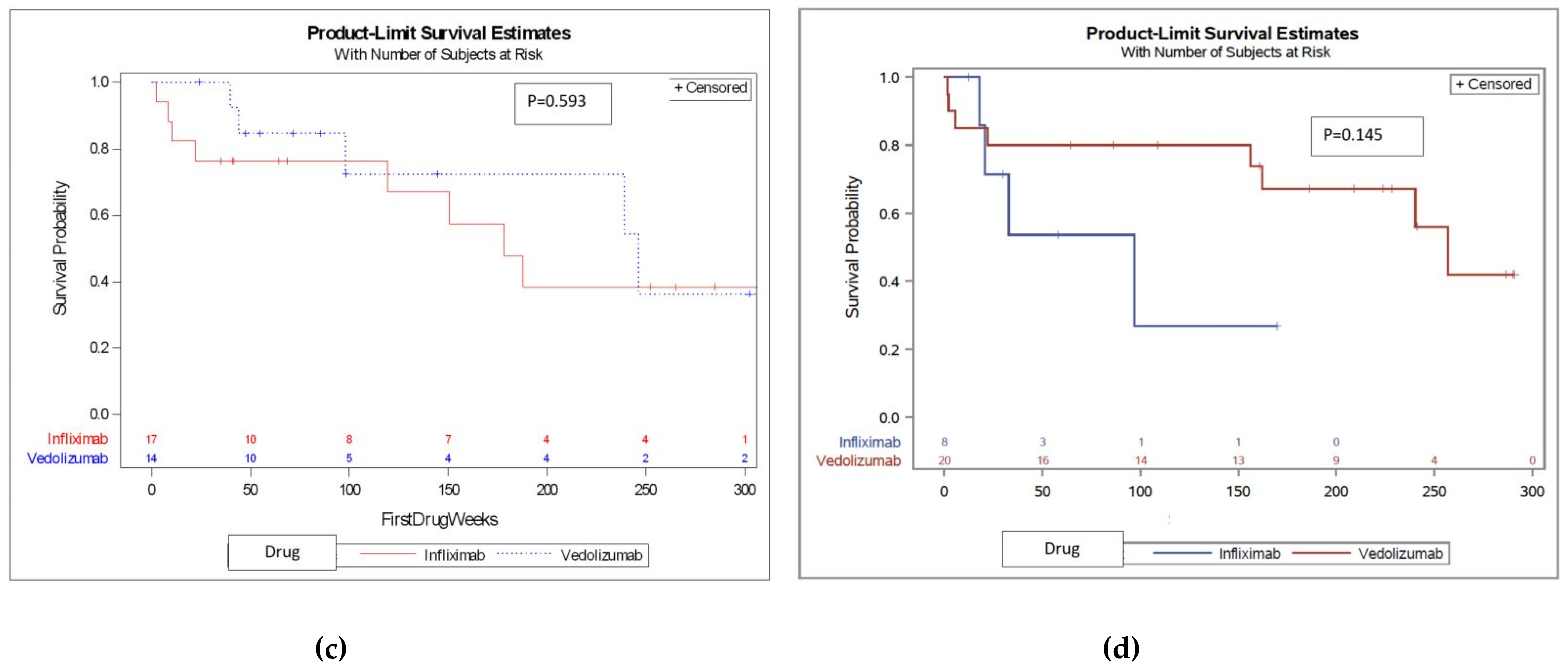
Figure 2, p=0.001. We then compared treatment-experienced patients and found no difference in DS between IFX and VDZ (KM survival curve is shown in
Figure 2 and Figure 3, p=0.593). When combining the whole cohort (treatment naïve and experienced), VDZ was again associated with significantly longer drug survival (
Figure 2, p=0.001).
Due to differences in baseline parameters (described in
Table 1 for treatment-naïve patients) between patients receiving IFX versus VDZ, we further analyzed the cohort after adjusting for factors associated with disease severity, specifically for hemoglobin, hematocrit, neutrophil-to-lymphocyte ratio, albumin, CRP, and total protein, as well as other important treatment-related factors such as age and combination therapy. This difference in DS between drugs (with vedolizumab having longer DS) was preserved after adjusting for these factors (HR 0.55 95 CI 0.3-0.98, p=0.042). After adjustment, the type of drug (VDZ or IFX) was the only statistically significant factor associated with DS over time, as shown in
Table 2.
2.3. Subsequent Treatment with a Second Drug
A total of 28 patients that failed either IFX (n=8) or VDZ (n=20) went on to be treated with the other drug (baseline characteristics of the two groups are found in
Table S1 in the supplementary material). When analyzing these patients, there was no significant difference in DS between IFX and VDZ (median 96.9 vs. 156.1, P=0.145. KM survival curve shown in Figure 3). See
Table S2 (supplementary material) for factors associated with DS in this subgroup.
4. Discussion
In this retrospective cohort study with detailed patient-level data, we aimed to assess the difference in DS between IFX and VDZ in the treatment of patients with UC.
We found that VDZ was associated with longer DS than IFX, even after correcting for multiple parameters associated with disease severity. In the absence of head-to-head comparisons, the retrospective evaluation of real-world data is an accepted approach for comparing drug efficacy. DS has been increasingly used as a surrogate for treatment efficacy when evaluating different therapeutic agents in a real-world setting6-8. It measures continuing medication prescription based on ongoing therapeutic efficacy. Importantly, it reflects real-world practice that allows for treatment optimization, including dose adjustments. The rate of failure and duration of DS for various drugs are different and provide important comparative efficacy data given the paucity of head-to-head trials of advanced therapies in UC6,8. DS data, in some respects, may be even more informative than head-to-head trials by more closely resembling real-world practice. Our study's strengths included the large amount of patient-level data that we had for each patient, thus allowing assessment of baseline disease severity and the ability to control for these factors when comparing the two drugs. We also had a strict definition of DS, defined as long as patients were corticosteroid, hospitalization, and surgery-free.
Most previous real-world studies comparing IFX and VDZ have used a myriad of clinical and endoscopic endpoints, usually with a follow-up time of up to 1 year, and have shown conflicting results. Two meta-analyses of several randomized control studies accumulating to over 2700 patients did not find a significant efficacy difference between VDZ and IFX for induction and maintenance of endoscopic remission at 52 weeks (¬1 year) of follow-up in treatment-naïve patients9,10. Conversely, in a large multi-center observational study, patients treated with VDZ were more likely to achieve and maintain steroid-free remission7 during a similar time frame. In another, smaller, real-world study of 97 patients, the efficacy of VDZ and IFX were comparable in achieving clinical remission at 1 year. Still, VDZ was associated with a higher chance of steroid-free remission11. In our cohort, the follow-up period was significantly longer compared to these previously published studies, and we assessed DS as the end-point.
Studies using DS as an efficacy endpoint are fewer in number. A nationwide Swedish register-based study assessing DS for 2nd line treatment with IFX and VDZ showed similar DS between these medications12. Based on a claims database, a second study from the USA described drug persistence (sustainability) tendencies with slightly longer drug persistence for IFX, without direct comparison between the drugs and no adjustment to different factors that might affect DS13. In a nationwide Korean register-based study, IFX drug persistence was assessed and was longer than other drugs investigated, but the study did not include VDZ14. On the other hand, other studies have shown longer DS with VDZ compared to IFX15,16, similar to our study results.
Several key factors might explain the different results between studies. First, our cohort found that patients treated with IFX and VDZ differed significantly in baseline characteristics, including age and patient-level clinical data indicative of disease severity; this may be unintentionally translated into erroneous results in registry-based and real-world studies lacking sufficient patient-level clinical data. Even propensity score adjustment may be inaccurate without detailed patient-level clinical data. The wide range of clinical data, including key lab data, in our study adjusted for disease severity, age, and sex and is a further strength contributing to the confidence of our findings.
In our cohort, in contrast to patients naïve to biologic therapies, there was no difference between the two drugs in biologic-treatment-experienced patients. This was also true for patients treated with VDZ and switched to IFX or vice versa. There is limited data regarding efficacy among biologic experienced patients, and data, if available, is mainly for anti-TNF experienced patients, and results are conflicting. In a multi-center study, VDZ was superior to IFX in achieving steroid-free remission in both treatment-naïve and treatment-experienced patients7. In contrast, in the nationwide Swedish register-based study assessing 2nd line treatment mentioned above, the efficacy of IFX and VDZ at 1 year appeared largely similar12. In a recently published post hoc analysis of several UC clinical trials by Narula N et al., IFX had higher rates than VDZ of clinical and endoscopic remission at 1 year17. It’s difficult comparing previous studies and ours. Very few studies or meta-analyses compare IFX and VDZ in this specific subgroup in terms of sustainability. Previous studies' follow-up length is mostly limited to 1 year, whereas our study evaluates a much longer follow-up period (up to 300 weeks).
Our study has some limitations. First, our study includes a relatively small number of patients and is a single-center study. Adding to the study's retrospective nature, this carries a risk of bias. Nevertheless, the large amount of data per patient (including detailed lab parameters) enabled us to overcome these limitations to a certain extent by adjusting according to the different parameters, especially for disease severity. The main limitation, as expected from real-world data, is the lack of a controlled environment. Our end-point was defined as steroid-free, surgical-free, hospitalization-free treatment duration without specific documentation of mucosal healing. This necessitates a degree of confidence that the clinical management is following acceptable international guidelines.
5. Conclusions
In conclusion, in this population-based study, VDZ showed significantly longer DS in treatment-naïve patients compared to IFX, also following adjustments for disease severity. There was no difference in DS between VDZ and IFX in treatment-experienced patients and patients switching from one drug to the other. This supports prescribing VDZ as a first-line biologic choice in moderate-to-severe UC.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org; Table S1: Baseline characteristics of those treated with subsequent second drug; Table S2: Parameters associated with DS of subsequent treatment.
Author Contributions
Conceptualization, T.K. and J.E.O.; methodology, T.K and J.E.O.; data acquisition – T.K, I.AB, Y.S, H.B, L.B; data curation, D.L.; writing—original draft preparation, T.K.; writing—review and editing, I.D., H.Y and J.E.O.; supervision, I.D. All authors have read and agreed to the published version of the manuscript. Please turn to the CRediT taxonomy for the term explanation.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Rabin Medical Center (0706-13-RMC 8.11.21).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
For data requestes please contact Jacob E. Ollech (jacobel@clalit.org.il) Data will be provided only under authorization of Rabin Medical Center according to local patient privacy regulations.
Acknowledgments
The authors would like to thank Tzipi Shochat for the contribution to the statistical analysis
Conflicts of Interest
The authors declare no relevant conflict of interest.
References
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Danese, S.; Argollo, M.; et al. Loss of Response to VDZ and Ability of Dose Intensification to Restore Response in Patients With Crohn's Disease or Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2019, 17, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Jharap, B.; Neeraj, N.; Colombel, J.F. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin Transl Gastroenterol 2016, 7, e135. [Google Scholar] [CrossRef] [PubMed]
- Allez, M.; Karmiris, K.; Louis, E.; et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: Definitions, frequency and pharmacological aspects. J Crohns Colitis 2010, 4, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, F.S.; Maida, M.; Grova, M.; et al. Head-to-head comparison of biological drugs for inflammatory bowel disease: From randomized controlled trials to real-world experience. Therap Adv Gastroenterol 2021, 14, 17562848211010668. [Google Scholar] [CrossRef] [PubMed]
- Baer, P.A.; Aumais, G.; Ewara, E.M.; et al. Patterns and predictors of long-term retention of inflammatory bowel or rheumatoid disease patients on innovator IFX: An analysis of a Canadian prescriptions claims database. Patient Prefer Adherence 2018, 12, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Lukin, D.; Faleck, D.; Xu, R.; et al. Comparative Safety and Effectiveness of VDZ to Tumor Necrosis Factor Antagonist Therapy for Ulcerative Colitis. Clin Gastroenterol Hepatol 2020. [Google Scholar] [CrossRef]
- Visuri, I.; Eriksson, C.; Olen, O.; et al. Predictors of drug survival: A cohort study comparing anti-tumour necrosis factor agents using the Swedish inflammatory bowel disease quality register. Aliment Pharmacol Ther 2021, 54, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Cholapranee, A.; Hazlewood, G.S.; Kaplan, G.G.; Peyrin-Biroulet, L.; Ananthakrishnan, A.N. Systematic review with meta-analysis: Comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn's disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther 2017, 45, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Fumery, M.; Sandborn, W.J.; Murad, M.H. Systematic review with network meta-analysis: First- and second-line pharmacotherapy for moderate-severe ulcerative colitis. Aliment Pharmacol Ther 2018, 47, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; McParland, P.; Dodd, S.; et al. Comparative effectiveness of antitumour necrosis factor agents and VDZ in ulcerative colitis. Eur J Gastroenterol Hepatol 2019, 31, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Rundquist, S.; Sachs, M.C.; Eriksson, C.; et al. Drug survival of anti-TNF agents compared with VDZ as a second-line biological treatment in inflammatory bowel disease: Results from nationwide Swedish registers. Aliment Pharmacol Ther 2021, 53, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hartzema, A.G.; Xiao, H.; et al. Real-world Pattern of Biologic Use in Patients With Inflammatory Bowel Disease: Treatment Persistence, Switching, and Importance of Concurrent Immunosuppressive Therapy. Inflamm Bowel Dis 2019, 25, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Han, M.; Park, S.; Cheon, J.H. Biologic Use Patterns and Predictors for Non-persistence and Switching of Biologics in Patients with Inflammatory Bowel Disease: A Nationwide Population-Based Study. Dig Dis Sci 2020, 65, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Bressler, B.; Yarur, A.; Silverberg, M.S.; et al. VDZ and Anti-Tumour Necrosis Factor alpha Real-World Outcomes in Biologic-Naive Inflammatory Bowel Disease Patients: Results from the EVOLVE Study. J Crohns Colitis 2021, 15, 1694–1706. [Google Scholar] [CrossRef] [PubMed]
- Pudipeddi, A.; Ko, Y.; Paramsothy, S.; Leong, R.W. VDZ has longer persistence than IFX as a first-line biological agent but not as a second-line biological agent in moderate-to-severe ulcerative colitis: Real-world registry data from the Persistence Australian National IBD Cohort (PANIC) study. Therap Adv Gastroenterol 2022, 15, 17562848221080793. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Wong, E.C.L.; Marshall, J.K.; Colombel, J.F.; Dulai, P.S.; Reinisch, W. Comparative Efficacy for IFX Vs VDZ in Biologic Naive Ulcerative Colitis. Clin Gastroenterol Hepatol 2021. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).