1. Introduction
Immobilization of microalgal cells on various synthetic and natural carriers attracted increasing interest in the field of wastewater treatment [
1,
2]. The main advantages of immobilized (attached) microalgal cultures are higher resistance to toxicants and overall robustness, increased rate of biomass and value-added molecule accumulation as compared to suspended cultures [
3]. Moreover, attached cultivation eliminates the need for costly cell-harvesting procedures such as centrifugation; attached cultures can be also used through several production cycles without significant loss of their activity [
1,
4]. Immobilized microalgae cultivation was shown to be a promising technology for removal of antibiotics from wastewater [
3,
5,
6]. Microalgae can effectively remove antibiotics from wastewaters (see [
5,
7] and references therein). For example, a high-rate algal pond (HRAP) system dominated by
Coelastrum sp. removed 33 antibiotics (with an average concentration of 223 µg/L) from municipal wastewater with average antibiotic removal rates being up to 50% higher than those of the conventional activated sludge process [
8]. The immobilized microalgal biomass grown in suitable wastewater and enriched with nutrients, lipids, and carotenoids can be used as biofertilizer, for production of biofuel and value-added compounds [
9].
The successful application of immobilized microalgae in wastewater treatment depends on the informed choice of the optimal carrier and immobilization technique [
2]. Current progress in material science made available a broad spectrum of natural and synthetic materials for microalgae immobilization [
1]. Among them, synthetic polymers (acrylamide resins, polyurethanes, polyvinyl alcohol, polypropylene) feature a high mechanical and chemical stability, but using of synthetic polymers entails the problem of the utilization of used cell carriers [
2]. The key advantages of the natural carriers (loofa, sphagnum, turf, cellulose, carrageenan, alginate) are hydrophilicity, biocompatibility, and safety for the environment should be balanced against their low porosity and mechanical stability in wastewater [
2,
4]. Particularly, the cross-linked chitosan-based polymeric materials as biocompatible, cost-effective, and non-toxic carriers are prospective for immobilization of microalgae [
2,
10]. These polymers have a variety of potential applications as biosorbents for water biotreatment applications due to a high potential for sorption of heavy metal ions, dyes, phenol, polychlorinated biphenyls [
11].
A serious concern is that of leakage of antibiotics into the environment from wastewater treatment plants [
5,
12]. Most antibiotics are hardly removed by the bacteria of activated sewage sludge since the antibiotics are purposedly designed to inhibit bacterial growth and metabolism [
13]. Incomplete, slow degradation of the antibiotics leads to their increased persistence in the environment and enrichment of natural communities and wastewater treatment plants with pathogenic and opportunistic microorganisms resistant to antibiotics and hence dangerous to human health [
12,
14]. Although concentrations of hazardous micropollutants including antibiotics currently encountered in waste streams are low in comparison with their total organics content, these micropollutants impose a serious threat to the environment due to their high biotoxicity. Although environmental concentrations of antibiotics are seldom reported to have acute toxicity to aquatic organisms, long-term negative effects on individual organisms and populations have been documented, including the impact on microbial consortia [
6].
As noted above, microalgae-based technology has been reported as an effective method to remove and degrade antibiotics with added benefits of CO
2 fixation, accumulation of value-added products, nutrients, and other organic pollutants removal [
6,
15]. At the same time, little is known about the influence of antibiotics on the biology of immobilized microalgal cells. To bridge this gap, at least in part, we studied the influence of a widespread antibiotic ceftriaxone (CTA) on the accumulation of arachidonic by suspended and immobilized on the cross-linked chitosan carrier microalgae
Lobosphaera sp. strain IPPAS C-2047. Representatives of the genus
Lobosphaera including the studied strain are the richest plant source of the polyunsaturated ω-6 arachidonic acid (ARA) [
16]. Arachidonic acid is an essential, structural, and functional constituent of cell membranes, it is used as a nutraceutical food and feed additive [
17,
18].
The removal of the antibiotic by different cultures of the Lobosphaera sp. was also studied. Results of this study would augment the development of the technologies with chitosan-immobilized microalgae intended for antibiotics removal from wastewater coupled with the production of valuable polyunsaturated fatty acids by attached microalgal cultures.
3. Discussion
Immobilization of the cells on biocompatible cell carriers is frequently employed in microalgal biotechnology to facilitate the biomass harvesting as well as to increase the stress resilience of microalgae and hence the culture robustness [
1,
4]. Chitosan-based biopolymers are highly promising cell carriers for immobilization of microalgal cells [
11]. However, they can also be a source of nutrients interfering with the culture manipulation by controlled stress such as nitrogen deprivation for induction of lipids harboring valuable fatty acids. The using of attached cultivation and increased stress resilience ensued by is especially relevant for growing of microalgae in waste- and sidestreams as a source of nutrients. Many types of wastewaters (e.g., farming and municipal waste streams) contain sizeable amounts of hazardous micropollutant such as antibiotics [
2,
11,
19]. At the same time, the information on possible effects of the presence of antibiotics on the biology of immobilized microalgal cell is scarce. In view of this, we attempted to reveal interactive effects of immobilization of the cells and a widespread antibiotic CTA on the production of a valuable polyunsaturated fatty acid ARA by its producer
Lobosphaera sp. IPPAS C-2047. As a provocative test, we deliberately employed a high (20 mg/L) CTA concentration since it was shown that the
Lobosphaera sp. is tolerant of CTA in the environmentally relevant concentrations around 1–3 mg/L [
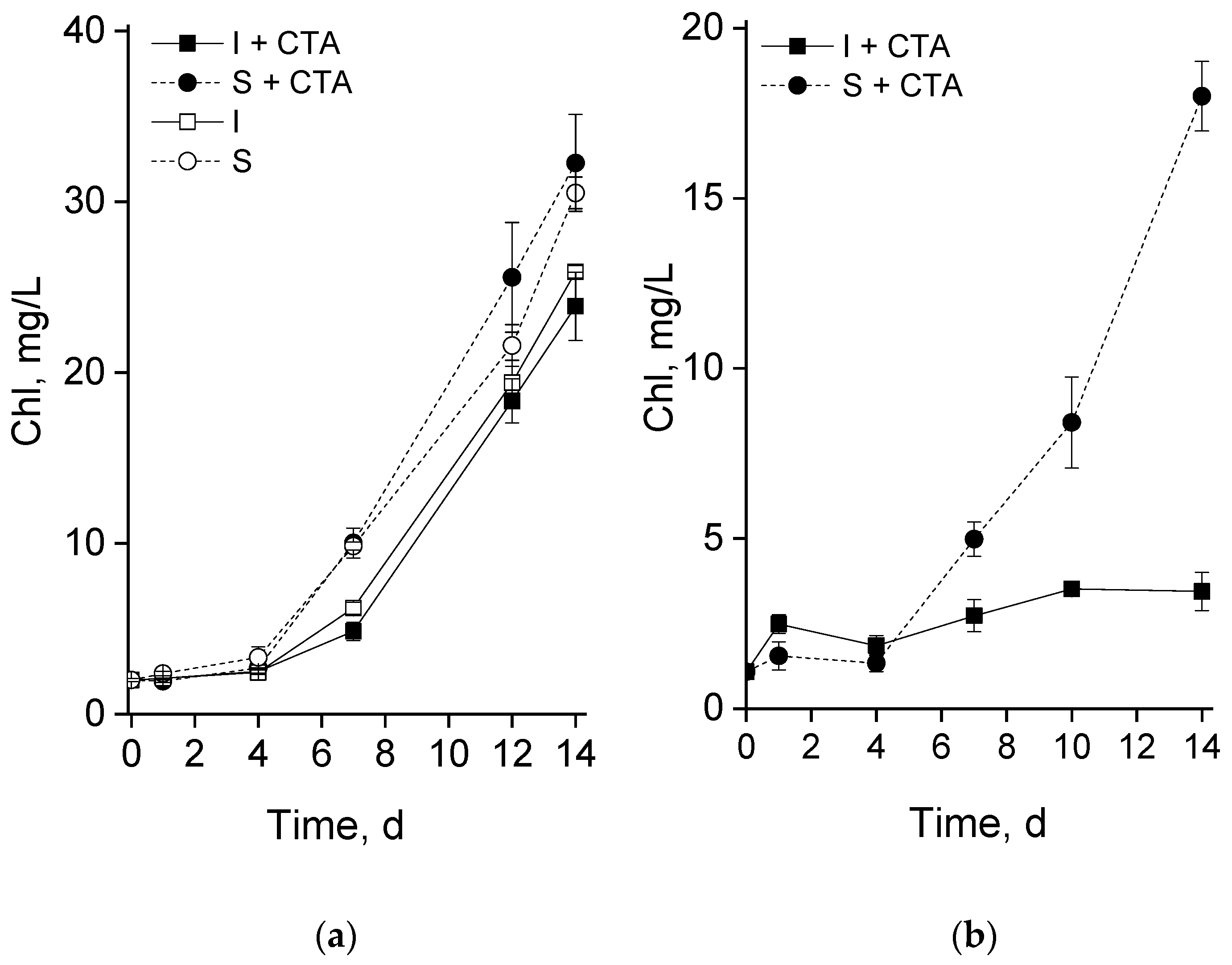
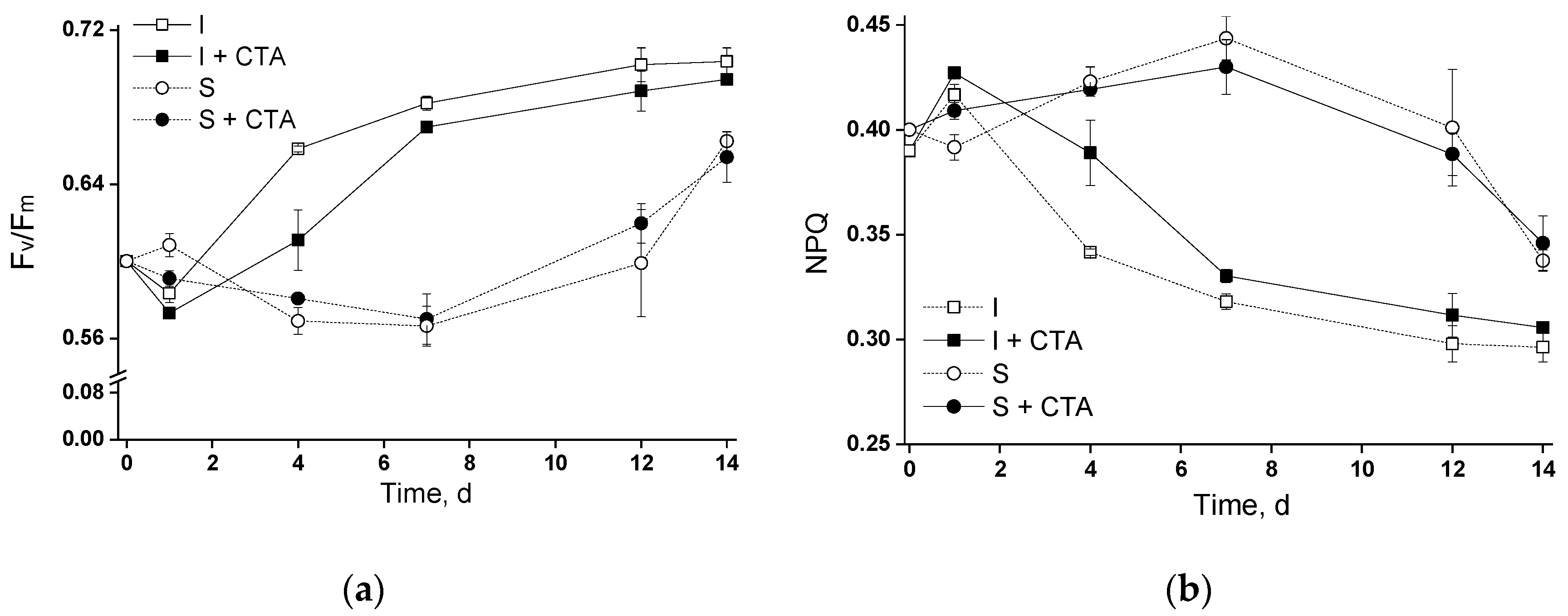
20]. Therefore, the first (pilot) phase of the study has been dedicated to testing the potential acute toxicity of CTA to the selected microalgal strain. Judging from the obtained results (Figures 2a, and 3), 20 mg/L CTA did not induce a profound deteriorative effect on the cells of
Lobosphaera sp. cultivated in flasks regardless of the immobilization, only a slight inhibition of photosynthetic activity has been recorded in the immobilized cells at day 4 (
Figure 3).
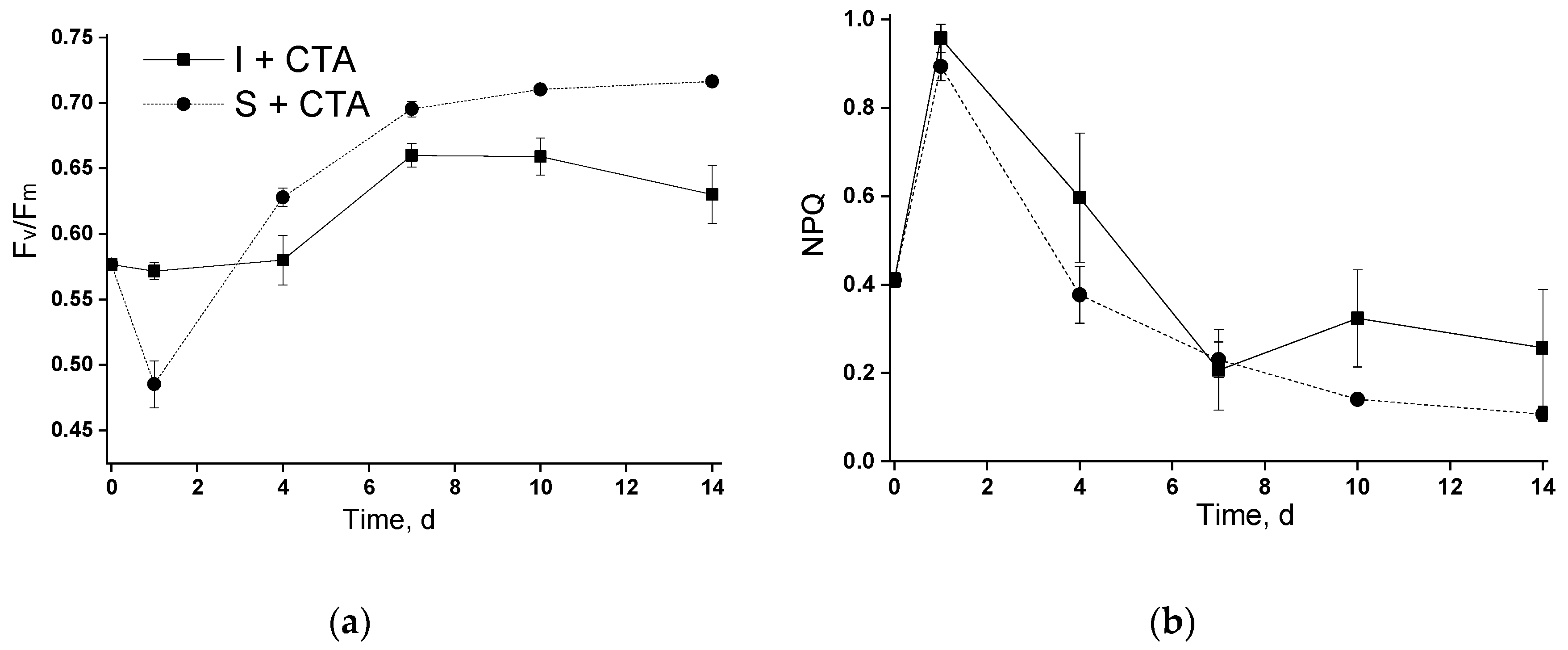
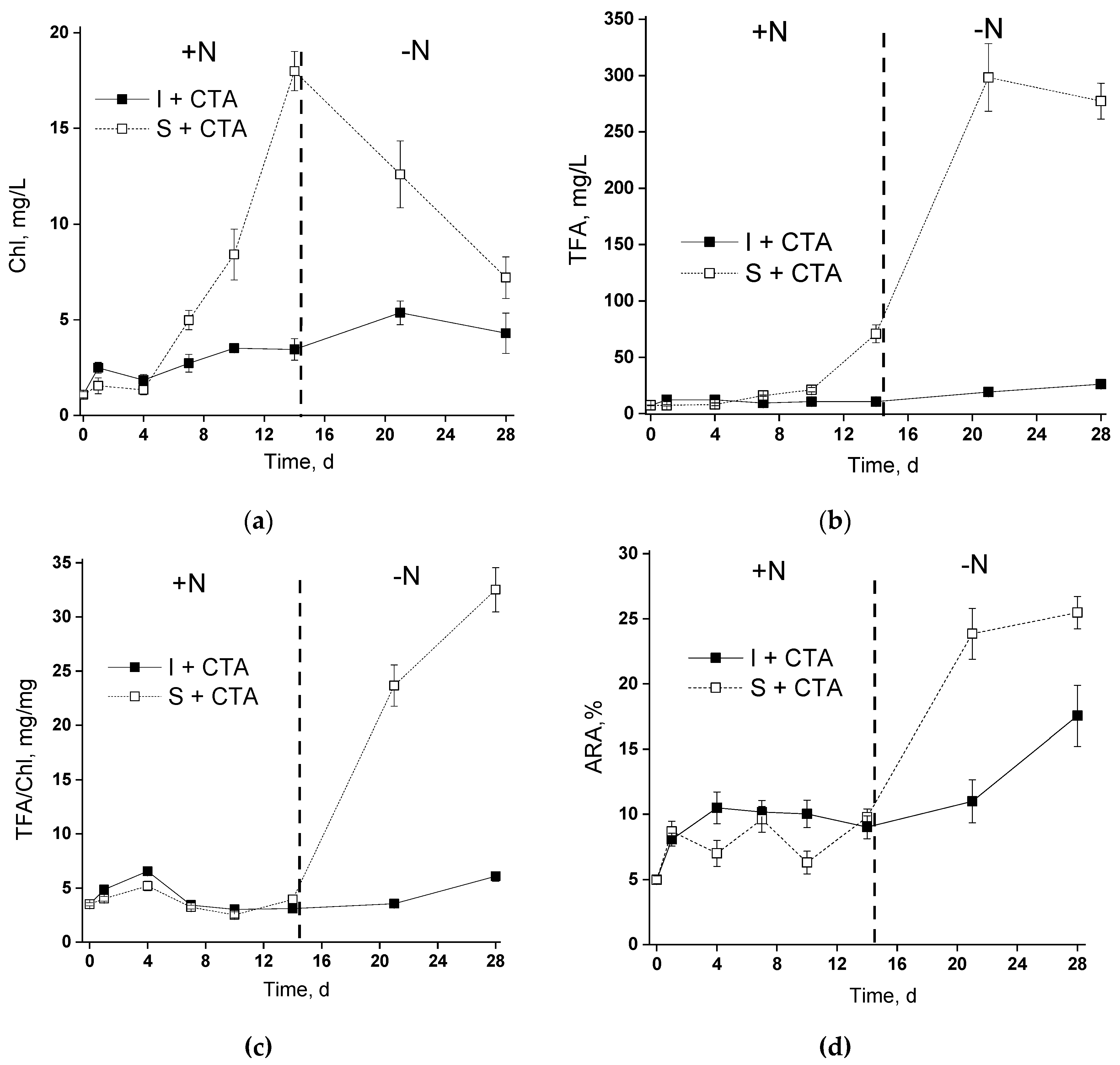
An unexpected result was represented by a severe retardation of the growth (as manifested by chlorophyll accumulation) of the chitosan-immobilized culture in the presence of CTA. Remarkably, this effect was not accompanied by severe disorders of the photosynthetic apparatus (
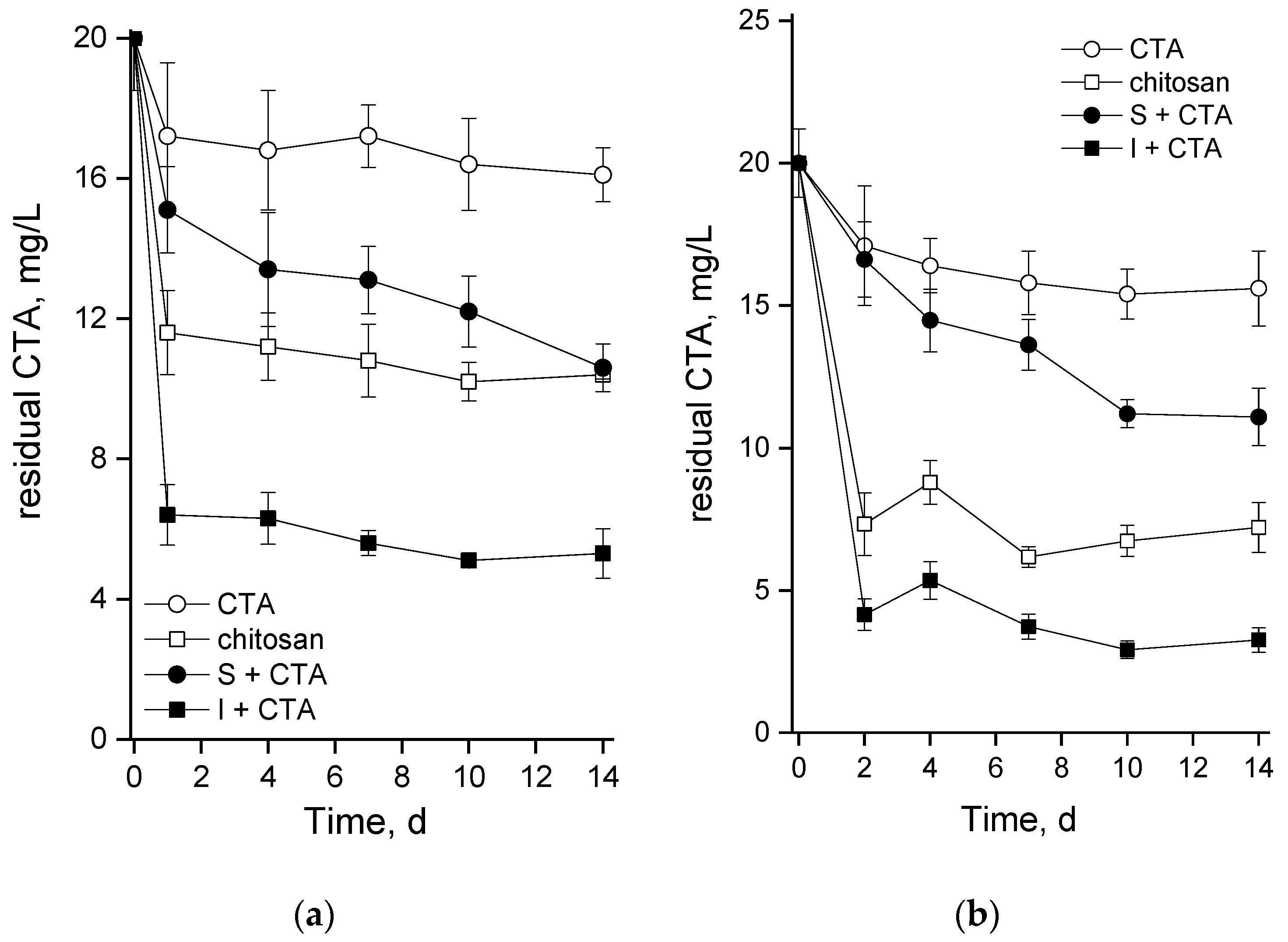
Figure 4). A plausible explanation can be related with an increase in the concentration of CTA due to its sorption on chitosan. This effect may be not so pronounced during incubation in flasks in the absence of vigorous mixing while in the bubbled columns the rate of CTA adsorption (and hence the rate of the increase in its effective concentration in the vicinity of microalgal cells) was ca. 30% higher (open squares in Figures 6a and 6b). It seems that a sub-population of
Lobosphaera sp. cells which was more resilient to increased CTA concentration was effectively selected on the chitosan-based cell carrier. These cells might have retained a functional photosynthetic apparatus and the essential responses of their lipid metabolism to nitrogen deprivation manifested as an induction of lipid accumulation.
Taking into account the results of our previous experiments on immobilization of
Lobosphaera sp. on chitosan-based cell carriers one can conclude that the immobilization itself does not affect the FA profile (
Tables S1 and S2), degree of lipid and, specifically, ARA induction profoundly (
Figure S1, see also [
19]). Typical ARA percentage of TFA of immobilized was in the range 20%–25%. Therefore, the decline in the amount TFA and ARA observed in the presence of CTA is attributable mostly to the effect of the antibiotic and not to the effect of the attachment to the carrier.
An intriguing question to be answered by this study was that about the capacity of suspended and chitosan-immobilized cultures of green microalgae such as
Lobosphaera sp. to remove CTA from the medium. Comparison of the abiotic degradation of CTA and its adsorption to the chitosan-based carrier with the bulk CTA removal (
Figure 6) made it clear that the removal of CTA in our system occurred mainly due to these two processes and contribution of the microalgal cells to CTA removal was minor. It is difficult to distinguish between the adsorption of CTA on the cell surface and uptake of the antibiotic by the cells. Still, one cannot rule out at least partial metabolization of CTA. Importantly, the cultures at the stage of nitrogen starvation were also affected by CTA despite replacement of the medium (see Methods) since a considerable amount of the antibiotic was adsorbed to the cell carrier (see
Figure 6); likely, there was also some adsorption of CTA to the microalgal cell surface as well.
Diverse processes such as adsorption, advanced oxidation, and photocatalysis have been studied to remove antibiotics. As was summarized recently [
5], the antibiotic removal efficiency by adsorption is highly adsorbent-dependent, and most of the adsorbents are expensive. Advanced oxidation and photocatalysis-based processes may be effective, but they need expensive catalysts; these processes can generate dangerous secondary pollutants. Advantages of the microalgae-based wastewater treatment process as a biological process are the absence of the need for expensive reagents and a low risk of secondary pollution [
21]. Immobilization of the microalgal cells on biopolymer carriers increases efficiency of the process, also due to the sorption capacity of the carrier itself. In our experiments the CTA level was taken own to 6 mg/L or 4 mg/L.
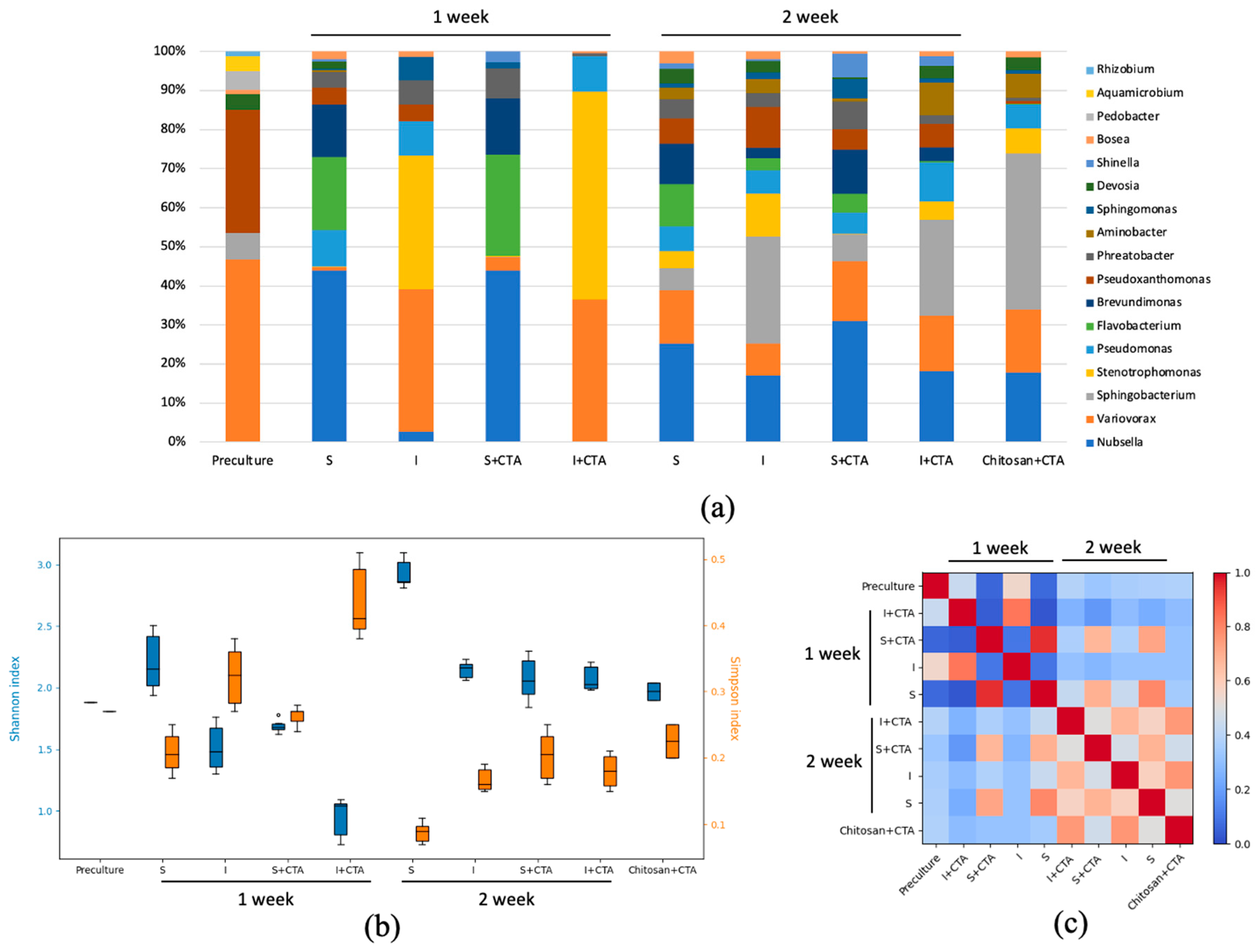
To elucidate possible trends of changes of the
Lobosphaera sp. culture microbiome, it was essential to evaluate the most represented microbial taxa and the effect of immobilization and CTA on them (
Figure 5a). Notably, that all the bacterial genera discovered are typical for microalgal cultures under different cultivation conditions. Many representatives of these genera belong to the PGPB (plant growth promoting bacteria) group, such as
Variovorax,
Stenotrophomonas,
Shinella,
Devosia,
Sphingomonas, etc. Representatives of microalgal parasites were not discovered. Importantly, some representatives of
Variovorax,
Stenotrophomonas,
Flavobacterium are known to be resistant to a wide range of antibiotics, including CTA ceftriaxone, having the ability to degrade them [
22,
23].
Ample representation of the genera
Variovorax and
Stenotrophomonas in the chitosan-immobilized cultures can be due to the fact that the presence of the polymer creates favorable conditions for the development of these microorganisms. Thus, representatives of the genus
Variovorax are known to be able to actively form biofilms, also on the surface of carriers [
24]. Representatives of the genus
Stenotrophomonas are known for the presence of chitinase and the ability to degrade chitosan [
25]. At the same time, it was shown that some forms chitosan also have antimicrobial activity, particularly suppressing the growth of certain representatives of the genus
Flavobacterium [
26].
The decline of the difference between the microbiome in different experimental treatments can be explained by (i) decline in the CTA residual content and (ii) by acclimation and selection of the microorganisms favored by the presence of chitosan and CTA. This hypothesis is supported by gradual proliferation of the microorganisms with modest resistance to ceftriaxone (
Pseudoxanthomonas,
Devosia,
Bosea) in the samples containing CTA. The potential inhibitory effects of chitosan for certain bacteria (e.g. from the genus
Flavobacterium) was also gradually overcame, likely via formation of biofilm frequently observed on the surface of the carrier [
19].
To conclude, the cultures of Lobosphaera sp., especially those immobilized on the chitosan-based cell carriers, turned to be suitable for removal of the antibiotics, especially taking into account that the CTA concentration in this study (20 mg/L) is higher than that typically encountered in the environment and even in the wastewater treatment plants. However, the attempts to couple the removal of hazardous pollutants such as antibiotics with production of valuable microalgal metabolites should use caution since toxic effects can emerge unexpectedly due to concentrating of the pollutants on the carrier. One should also be aware of the possible enrichment of the microbiome of the attached microalgal cultures with antibiotic-resistant bacteria.
4. Materials and Methods
4.1. Synthesis of the Cross-Linked Chitosan-Based Polymer
The cross-linked chitosan polymers were synthesized in the Laboratory of Polymer Materials of the Kurchatov Institute Research Center. Two mg of chitosan ChitoClear HQG 800 (Primex, Island; molecular weight of 600 kDa) were dissolved in 98 mL of 2% aqueous solution of acetic acid (LLC “Component-Reactiv”, Russia). The mixture was homogenized using a magnetic stirrer (Heidolph MR Hei-Tec, USA) for 24 h at 23 °C. The chitosan-based carrier polymers were synthesized from the solution of chitosan and glutaraldehyde as cross-linking agent using lyophilization technique. An aliquot of 0.76 mL of 2.5% aqueous solution of glutaraldehyde was added to the chitosan mixture. After 15-min stirring with a magnetic stirrer the mixture was placed in 12-well plates (2.7 mL per well), incubated in a freezer (24 h at –25 °C), and lyophilized using ALPHA 1-4LSC lyophilizer (Martin Christ, Germany) at 0.250 mbar for 24 h [
27].
The resulting carriers in the form of porous discs (14 mm diameter, 10 mm thickness) were used in the experiments.
4.2. Strain and Cultivation Conditions
Lobosphaera sp. IPPAS C-2047 used in the presented investigation as the model microalga was obtained from the Culture Collection of K.A. Timiryazev Institute of Plant Physiology (IPPAS, Russian Academy of Science).
Preculture of
Lobosphaera sp. IPPAS C-2047 was grown at 20 °C or 23 °C in a shaker incubator (New Brunswick, Innova-44R, N.Y., USA) at 120 rpm in 0.75 L flasks with 0.3 L of P-enriched modified BG-11 [
28] medium, BG-11
M (g/L: NaNO
3 = 0.74, KNO
3 = 0.9, K
2HPO
4 = 0.181, KH
2PO
4 = 0.089, MgSO
4·7H
2O = 0.075, CaCl
2·2H
2O = 0.036, citric acid = 0.006, ferric ammonium citrate = 0.006, Na
2EDTA·2H
2O = 0.001, Na
2CO
3 = 0.02, BG–11 trace metal solution) at 40 μmol PAR (photons/m
2/s) provided by daylight fluorescent tubes (Philips TL-D 36W/54-765) as measured with a LiCor 850 quantum sensor (LiCor, Lincoln, NE, USA) and the atmospheric CO
2 level. Culture pH was measured aseptically with a bench-top pH-meter (Hanna Instruments, Ann Arbor MI, USA).
To obtain the suspended cultures for the first phase (pilot) experiments, equal aliquots of the Lobosphaera sp. pre-culture were pelleted by centrifugation (5 min, 1000× g) and resuspended to ca. 1.5 mg chlorophyll/L in BG-11M medium or in the same medium supplemented with CTA (LLC “Biosintez,” Penza, Russia) to the final concentration of 20 mg/L and placed in 100-mL vented cultivation flasks (TPP, Switzerland).
In the second phase (bioreactor tests), the microalgal cell suspension was placed into the glass columns (600 mL; 4 cm i.d.;
Figure 1) containing 400 mL of the cell suspension. The columns were incubated in a temperature-controlled water bath at 27 °C with constant bubbling with air passed through a 0.22 μm bacterial filter (Merck-Millipore, Billerica, MA, USA) and delivered at a rate of 300 mL/min (STP) at 40 μmol PAR (photons/m
2/s).
Immobilization of the cells has been carried out as follows. A group of the pre-culture aliquots was gently re-suspended in BG-11
M and pipetted evenly on the surface of the chitosan discs to carry out the immobilization of the cells (the final concentration of chlorophyll was ca. 24 µg/disc). For the pilot experiments, the chitosan discs with firmly attached
Lobosphaera sp. cells were placed into the 100-mL vented-cap cultivation flasks (TPP, Switzerland) containing BG-11
M medium supplemented with 20 mg/L of CTA or BG-11
M lacking the antibiotic. Fir the second phase experiments (photobioreactor tests), the chitosan discs with attached
Lobosphaera sp. cells were fixed on the in-house made stainless steel spikes within the bioreactor column (
Figure 1) containing BG-11
M medium pr the same medium supplemented with CTA to the final concentration of 20 mg/L.
At the first phase (pilot experiments), the flasks with the suspended or immobilized Lobosphaera sp. cells were cultured for 14 days on the Innova-44R incubator shaker at 120 rpm under the condition specified above. At the second phase, the columns were incubated in the bioreactor under the conditions described above.
To induce nitrogen starvation for lipid accumulation, the complete BG-11M medium in the columns of the photobioreactor was replaced with nitrogen-free medium (BG-11M0) after 14 d of cultivation. Specifically, the suspended cells were gently harvested by centrifugation (2 min, 1000× g), washed with BG-11M–N medium and re-suspended in the same medium. The immobilized cultures were transferred into the identical columns with BG-11M–N medium. All the cultures were incubated in the photobioreactor for another 14 days at 200 μmol PAR (photons/m2/s); all the other conditions were as specified above.
4.3. Chlorophyll and Nutrients Assay
Growth estimation was based on the chlorophyll content measurements. For chlorophyll assay determination, an aliquot of the cell suspension was sampled and harvested by centrifugation for 5 min at 3000 g. Total Chl were extracted by heating the cell pellet with 2 mL of dimethyl sulfoxide (DMSO) for 10 min at 70 °C. From the cells attached to the carrier, chlorophyll was extracted by adding 2 mL of DMSO to the carrier with the cells and heating for 10 min at 70 °C. Concentration of chlorophyll was determined in the DMSO extracts with an Agilent Cary 300 spectrophotometer (Walnut Creek, CA, USA) using equations reported in [
29].
The residual phosphate and nitrate contents were checked using Thermo Dionex ICS 1600 HPLC (Sunnyvale, CA, USA) with a conductivity detector and IonPac AS12A anionic analytical column (5μm; 2 × 50 mm). The column temperature was maintained at 30 °C. The ions were eluted isocratically with 2.7 mM sodium carbonate/0.3 mM sodium bicarbonate buffer (flow rate of 0.3 mL/min).
4.4. Photosynthetic Activity and Photoprotective Mechanism Probing
Viability of the immobilized and suspended
Lobosphaera cells was assumed to be reflected by photosynthetic activity of the cells. were monitored with a FluorCam FC 800-C (PSI, Drasov, Czech Republic) kinetic fluorescence imager. The chitosan carriers with immobilized cells or 5-mL aliquots of suspended culture were transferred into well plates and sealed aseptically. Estimations of the photosynthetic activity of the microalgal cells were obtained by recording Chl
a fluorescence induction curves The recorded curves were processed by the built-in software of the fluorometer, and the following parameters indicative of the functional condition of the photosynthetic apparatus of the microalgal cells were calculated: potential maximum photochemical quantum yield of photosystem II, Q
y = (Fm – Fo)/Fm = Fv/Fm, and non–photochemical quenching of the electron excitation energy in the light-harvesting antenna (parameter NPQ, NPQ = Fm/Fm' – 1) [
30]. The PAM measurements were carried out after 10-min dark adaptation. Chlorophyll fluorescence was excited at 650 nm and recorded in the red region of the spectrum (λ > 680 nm). After the measurements, the samples were returned to the corresponding cultivation flacks or the bioreactor column under aseptic conditions. This procedure allowed repeated assessments of the attached and suspended cells under our experimental conditions.
4.5. Assay of Cell Lipid Fatty Acid Composition
Total cell lipids were extracted from these samples according to Folch [
31]: the cell pellets or the cells on the carrier were homogenized in a mixture of chloroform and methanol (2:1, by volume). Distilled water was added to the homogenate in the amount of 20% of the homogenate volume, the mixture was dark incubated overnight at 4 °C, and centrifuged (10 min, 3000 g) until phase separation. The chloroform phase of the extract was evaporated to dryness on a Heidolph Laborota 4000 rotary evaporator (Heidolph, Schwabach, Germany) at 30 °C. As an internal standard, 50 micrograms of margaric acid (C17:0) were added to the samples. The samples were transmethylated by incubation with 2% sulfuric acid in methanol for 1.5 h at 80 °C. Fatty acid (FA) methyl esters were extracted with 2 ml of n-hexane. In the case of immobilized cells, the carriers with the cells were ground and extracted as described above.
The fatty acid (FA) profile of total cell lipids was analyzed using an Agilent 7890A gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with a 30-meter HP-5MS UI capillary column (30 m × 0.25 mm × 0.25 microns; Agilent, USA). Helium with a flow rate of 1 mL/min was used as a carrier gas (for more details, see [
32]).
The proportion of individual FA in the total FA of the Lobosphaera sp. cell lipids was inferred from the corre-sponding peak area. The absolute FA contents were calculated relative to the internal standard (C17:0) peak area.
4.6. UPLC-ESI-MS Assay of Ceftriaxone
The lyophilized culture broth samples were dissolved in methanol-water mixture (1:1, by vol.) and centrifuged (15 min, 15 000 rpm; centrifuge ELMI CM-50, Latvia). The samples were chromatographed on an ACQUITY UPLC H-Class PLUS (Waters, USA) equipped with a time-of-flight mass-selective detector Xevo G2-XS TOF (Waters, USA). An 0.5 µL sample aliquot was injected on a Titan C18 (100 × 2.1 mm, 1.9 µm; Supelco, Bellefonte, CA, USA) column maintained at 50°С and eluted with a gradient of 10 mM solution of ammonium acetate in deionized (Simplicity UV, Millipore, France) water (solvent A) and LC-MS grade (Panreac, Spain) acetonitrile (solvent B) delivered at a rate of 0.5 mL/min. The following gradient program was used (vol. % of the solvent B): 0–1 min, from 5% to 15%; 1–2 min, from 15% to 65%; 2–3 min, from 65% to 85%; 3–5 min, from 85% to 95% (see also [
33]). The analysis was carried out in the negative ion detection mode (m/z range of 100–1900), the ion source parameters were: ion source temperature—150 °С, desolvation temperature—650°С; capillary voltage—3.0 kV; sample injection cone voltage—30 V, nitrogen (desolvation gas) flow rate—1101 L/h. The recorded data were processed with MassLynx software (Waters, USA) and CTA was quantified using external calibration method.
4.7. Taxonomical Profiling of the Culture Microbiome
Cell pellet was collected from 2–5 mL of each freshly taken sample by centrifugation (8000 × g) for 10 min and immediately frozen at –80°C and stored until analysis. For metagenomic DNA (mgDNA) extraction, the cell pellets were frozen in liquid nitrogen and ground to a fine powder using homogenizing pestles (SSIBio Corp., Lodi, CA, USA) in a 1.5 ml microcentrifuge tube. The freezing-homogenization procedure was repeated three times for each sample. mgDNA was isolated from the obtained homogenates using the DNeasy Plant Pro kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions, replacing the sample mixing procedure on the Vortex with manual mixing by shaking the tube.
Amplification of the 16S rRNA gene fragment with the V4 hypervariable region and preparation of libraries for sequencing was carried out as described previously [
34] using oligonucleotide primers F515 (5'-gtgccagcmgccgcggtaa-3') and R806 (5'-ggactacvsgggtatctaat-3 ′) [
35]. Sequencing was performed on a MiSeq instrument (Illumina, USA) using a MiSeq Reagent Kit v3 (600-cycle) (part number MS-102-3003, Illumina, USA) for paired-end read (2 × 300 bp). Initial data processing—sample demultiplexing and removal of adapters—was carried out using the Illumina software (Illumina, USA). Further denoising, sequence merging, restoration of original phylotypes (ASV, Amplicon sequence variant), deletion of chimeric reads, and taxonomic classification of the resulting ASVs were performed in the R software environment using the dada2 [
36], phyloseq [
37], and DECIPHER [
38] packages, as well as the SSU 16s rRNA SILVA database (release 132) [
39]. When analyzing the results of DNA metabarcoding, unclassified readings were removed from the samples, as well as those taxonomic groups that corresponded to one unpaired reading. For the number of readings, the average value was calculated for two biological replicates, for each of which two technical replicates were made. The Shannon-Weaver a-diversity (H) and Morishita β-diversity indices were calculated [
40]. Morishita index values were visualized using an algorithm in the Python programming language (version 3.7.1) using the Matplotlib library.
4.8. Statistical Treatment
Under the specified conditions, three independent experiments were carried out for each treatment repeated in triplicate. The average values (n = 9) and corresponding standard deviation are shown unless stated otherwise. The significance of difference if the average values were analyzed using Student’s t test.
Author Contributions
Conceptualization, S.V. and A.S.; methodology, T.G., C.A., and S.V.; validation, O.C. and O.G.; formal analysis, S.V., D.K., and P.Z.; investigation, S.V., A.L., P.Z., O.G., C.A., T.F., P.S., O.C., and A.S.; writing—original draft preparation, A.S. and S.V.; writing—review and editing, A.S.; visualization, S.V. and P.Z.; supervision, E.L.; project administration, A.S. and E.L.; funding acquisition, A.S. and S.V. All authors have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.