1. Introduction
Sexual selection promotes the evolution of male traits that honestly reflect genetic quality or condition [
1] and increase reproductive success [
2]. Mating preferences based in such traits may confer females with direct benefits from parental care and territorial resources [
3], and/or indirect benefits such as the offspring inheritance of good genes for attractiveness [
4] and viability [
5].
According to the idea that sexually selected traits may facilitate female evaluation of variation in male quality [5, 6], females should prefer those characters that better reflect the quality of males. In birds, the most studied sexually selected trait has been plumage coloration [
7]. However, because birds grow feathers during moult, and moult usually occurs between breeding seasons, plumage coloration may reflect the condition of birds during moulting [8, 9] but it may not provide a good measure of current condition at the time of mating. Under this scenario, it would be advantageous for females to base their mate choice in other traits that signal current quality more accurately than plumage coloration alone.
Continuously produced traits, such as chemical compounds, can reflect more recent physiological events, thus allowing individuals to evaluate the current status of their mate. Indeed, chemical cues are accurate indicators of individual current quality because they respond rapidly to changes in condition [10, 11] and thus provide females with a more actualized information on the condition of prospective mates. For example, the uropygial gland, considered the main odour source in birds, secretes both volatile and non volatile compounds [
12] that birds spread on their feathers and play a role in updating the signal value of feathers [13, 14], not only by enhancing the visual sexual signal, but also by providing additional information about the individual. For instance, the amount and composition of this secretion has been shown to vary among seasons [15-17], sexes [16, 18], age classes [
16], diets [19, 20], hormone levels [21, 22], parasite infection [10, 23], body condition and immune status [24, 25], polymorphism [
26] and individuals [27-29], suggesting that it may convey potentially useful information during social interactions (see [
30] for a review). Furthermore, recent findings show that semiochemical profiles correlate with genetic heterozygosity [11, 31-34]. Therefore, uropygial gland secretions play a role in kin recognition [
35,
36,
37,
38] and mate choice [37, 39].
Also, it has been shown that the chemical composition of the uropygial gland is related to the body size of males [
18], suggesting that birds may use these chemical cues to assess the quality of conspecifics, which may be particularly useful in mate choice or intrasexual competition. A recent study in the house finch (
Carpodacus mexicanus) showed that when males were offered the scent of a female and a male, the difference in quality between focal and scent donor males influenced the choice of focal males: unpaired males with better body condition and immune response than scent donor males approached rival males, whereas focal males in worse condition avoid the rival male scent [
40]. From these results it can be deduced that chemical cues emitted by birds may carry information about the characteristics of birds in terms of body condition and health state that may be useful not only in assessing rivals [
40] but also in a sexual selection context.
Whereas chemical cues have been demonstrated to play a role in mate choice in other vertebrate taxa [41, 42] in which it is well known that they vary between individuals and indicate body condition, health state, parasite load and even genetic compatibility [41, 42], the role of chemical cues emitted by conspecifics to assessing quality of potential partners remains to be disentangled in birds. Here, we report the results of an experimental study in the zebra finch, Taeniopygia guttata, aimed to examine whether females use olfactory cues during breeding period to assess the quality of potential partners in terms of body condition and tarsus length. If chemical cues facilitate female evaluation of variation in male quality we expect females to choose the scent of the potential partner with greater body condition and tarsus length.
2. Methods
2.1. Study species
The zebra finch offers a good model to study the role of olfaction in assessing potential partners because the olfactory capability of this species has been previously demonstrated in social contexts [43-48]. Experimental birds come from a captive breed population located at the Foundation for the Research and the Study of Ethology and Biodiversity (Casarrubios del Monte, Toledo). Birds were housed, separated by sex, in outdoor aviaries (2.5 ×2.5 × 2.5 m). Aviaries contained bamboo branches as perches, and grass and sand on the ground. Commercial food for granivorous passerines and water were provided ad libitum. Two weeks before the beginning of the experiments, birds were individually housed in cages (60 x 40 x 40 cm) inside the aviaries. Therefore, birds were maintained at outdoor temperature and photoperiod during all the experiment. We measured birds with a digital calliper to the nearest 0.01 cm and birds were weighed with a spring balance to the nearest 0.1 g. All birds were individually banded with numbered aluminium and PVC rings. Birds were released again in the aviaries after the behavioural tests were completed. Birds maintained healthy throughout the experiments.
2.2. Behavioural experiments
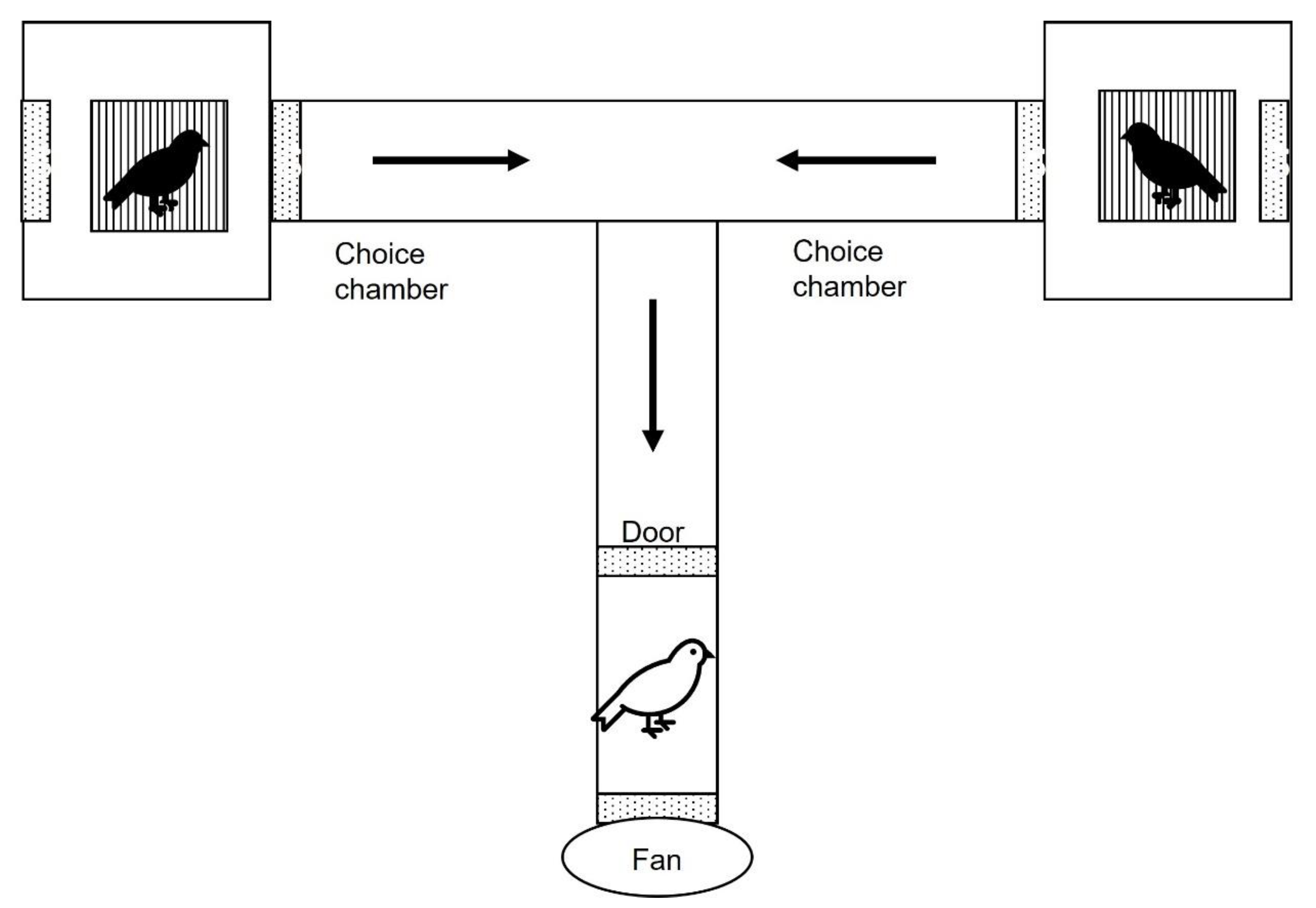
The experiments were performed in May, during the breeding period, in an olfactometry chamber in indoor conditions. The device was T shaped (Fig. 1) and built with PVC tubes (40 m diameter). It was composed by a central tube (25 cm length) where the experimental bird was introduced. The central tube had a door located at 15 cm from the entrance. The door was built with methacrylate and had small holes to allow airflow. The central tube was connected to two lateral tubes (25 cm length) referred to as choice tubes. The choice tubes were connected to plastic opaque boxes (30 x 25 x 25 cm) that contained two little cages (13.4 x 23.5 x 19.8 cm) where the scent donor birds were situated. Overall, the device was sealed and only openings at the farthest walls of the plastic boxes allowed air flow. The central tube contained a small 12 V PC fan at the entrance door that extracted the air from the device creating a controlled low-noise airflow. The fan created two constant air flows, each one entering across the openings located at the farthest walls of each plastic boxes containing the scent donor birds, passing by the donor birds, and crossing the central tube, and going outside from the device through the fan. Thus, the focal bird received two separate air flows, each carrying the scent of the corresponding donor bird. Donor birds were kept in darkness (opaque boxes) for the entire trial duration and reduced space (scent donor cages), preventing them from moving or calling. The experimental bird only perceived the scent of the donor birds without visual or acoustics contact with donor birds. The experimental room was sealed from exterior noise, enabling the experimenter to perceive any acoustic signals from focal and scent donor birds in the device. The experimenter was present during the entire trial period, but not visible/audible to the focal bird. Similar device and methodology have been successfully used in social context studies before [16, 38, 40,49,50].
Figure 2.
Olfactometry chamber. The solid arrows indicate the direction of air flow within the chamber. Scent donor birds (black) were located at the father parts of the choice chambers, inside cages. The focal bird (white) was introduced in the chamber and kept at the entrance for 5 minutes. After that time, the door was opened, and the focal bird was allowed to move to one of the two choice chambers.
Figure 2.
Olfactometry chamber. The solid arrows indicate the direction of air flow within the chamber. Scent donor birds (black) were located at the father parts of the choice chambers, inside cages. The focal bird (white) was introduced in the chamber and kept at the entrance for 5 minutes. After that time, the door was opened, and the focal bird was allowed to move to one of the two choice chambers.
In each test, a bird was introduced into the central tube and maintained in the dark for 5 min before the door was opened by the experimenter. We noted down the choice tube that was first approached by each tested focal bird after opening the central tube. As the device was opaque and the experimental room was in darkness and silence, the experimenter scored by hearing the choice of the focal bird. Immediately after hearing the movement of the bird, the experimenter opened the tubes to ensure the bird was in the choice tube where it was heard.
We offered focal birds (N = 28 females), the scent of two potential partners of different body condition and body size. We used 28 different scent donor males, in 14 pairs of scent-donor birds, and pairs were used twice. The location of the scent donor birds within the olfactometry device was randomized between trials (14 times in the left and 14 times in the right side). As soon as birds were tested, they were returned to their cages. The olfactometry device was cleaned with alcohol between trials.
2.3. Data analysis
To test whether there were significant differences in the body condition and tarsus length between the two scent donor birds (good quality vs bad quality), we performed two repeated measures ANOVA including the pair as within measures factor.
To analyse whether females could detect the quality of potential partners by using chemical cues alone, we performed a generalized linear model with binomial errors and a logit link function (GLMM). We modelled the probability that females chose the side of the chamber containing the good quality male (as a dichotomous variable: left (yes) vs. right (not)). We included the side of the chamber where the good quality male was located. Analyses were performed using Statistica 8.0.
3. Results
There were significant differences in the body condition of scent donor birds (good quality (mean ± SE = 18.40 ± 0.35) vs bad quality (mean ± SE = 13.64 ± 0.24), repeated measured ANOVA, F1,13 = 229.91, p < 0.001) and in the tarsus length (good quality (mean ± SE = 15.63 ± 0.22) vs bad quality (mean ± SE = 14.55 ± 0.15), repeated measured ANOVA, F1,13 = 25.65, p = 0.0002) in relation to the type of donor bird.
The difference in body condition between the two scent donor birds influenced the choice of focal bird (Wald stat = 4.84, df = 1, p = 0.03). Most females (20/28) avoided the scent of the potential partner with better body condition (Fig. 2). The choice of females was not affected by the side of the chamber where the better male was located (Wald stat = 0.69, df = 1, p = 0.41).
Figure 2.
First choice of female zebra finches when exposed to the scent of two males differing in quality, in terms of body condition and tarsus length. Most females (N = 20/28) chose the side of the chamber containing the scent of the male of worse quality (lower body condition and smaller tarsus length).
Figure 2.
First choice of female zebra finches when exposed to the scent of two males differing in quality, in terms of body condition and tarsus length. Most females (N = 20/28) chose the side of the chamber containing the scent of the male of worse quality (lower body condition and smaller tarsus length).
4. Discussion
Our results show that zebra finch females use olfaction to assess the body condition of potential partners. However, although the study was performed during the reproductive period of the species when we would expect a preference for the scent of the best potential partner, our results show that females avoided the scent of males with greater body condition and body size. Our results are difficult to explain in a sexual context because males with better body condition may have a greater reproductive success [
51]. Furthermore, previous evidence has found an overall preference of females for males of better body condition and size [
52], although other studies have found assortative mating in this species [53, 54]. However, interpreting scent preferences in a choice test is challenging because odour preferences may be related to other behaviours that also take place during the mating period, such as aggressive interactions. Zebra finches are gregarious and are known to establish dominance hierarchies [
55], with larger males being more aggressive than smaller ones [
55]. The preference for the scent of the potential partner with worse body condition and smaller tarsus length suggest that our results instead can be interpreted as a social preference more than as a mating preference, independent of breeding times.
Conespecific chemical cues are known to be useful in many species to evaluate the quality of conspecifics in social contexts [56, 57]. In a context of aggression, asymmetries between opponents are used to decide whether to get involved in, or to what extent to escalate a fight [
58]. Therefore, the ability to assess the quality of the rival is useful for individuals to reduce the costs of aggression. Previous studies examining avian olfactory capabilities have found evidence that birds use chemical cues to assess the characteristics of potential rivals in social contexts. For example, Whittaker et al. [
18] and Amo et al. [
16] exposed male and female dark-eyed juncos (
Junco hyemalis), and spotless starlings (
Sturnus unicolor), respectively, to the scent of male and female conspecifics and found that both sexes exhibit a preference for the scent of males. In both studies, that were performed during the mating period, the attraction of males to male scent was explained in terms of intraspecific aggression because during the mating period other interactions between conspecifics take places, such as intrasexual competition for breeding areas or access to partners. In birds, the chemical composition of the uropygial gland is related to the body size of males [
18], and results of a previous study suggest that house finches can assess the quality of rivals, in terms of body condition and T-cell mediated immune responses thanks to olfactory cues [
40]. Our results add new evidence with zebra finches that chemical cues seem to be useful in social interactions.
First choice is a good proxy of the spontaneous interest of an animal in a particular cue, but time spent close to the stimulus [
54] may be related to the behaviour that takes place later on in the series of events triggered by the exposure to the scent. Indeed, birds exposed to the scent of two potential partners differing in body condition in an olfactometer may first avoid an encounter with the bird in better body condition trying to avoid an aggressive interaction, and, only when they realize that the bird is not prone to be involved in an aggressive encounter, they may spend more time close to that potential partner. We used living birds as scent donors, and therefore, the first choice was a valid measure of the response of birds to the scents in our study. However, more studies are needed to assess the subsequent response of birds to the scent of potential partners to analyse whether the preferences for the scent of the potential partner with lower body condition is maintained over the time or if it may change and reflect a mate choice instead of a social choice.
Due to expected differences in the volatile profile of feathers and uropygial gland secretions [
59] we used live birds as scent sources as opposed to merely uropygial gland secretions to increase the robustness of our study approach. However, our results are in line with results of a previous study that showed that
Junco hyemalis females spent more time with the odour of males with smaller body size [
18]. Uropygial gland size, a proxy of gland activity, has been found to differ between males and males during the reproductive period [
60]. Therefore, differences in the secretory activity of the uropygial gland or in the composition of the uropygial gland secretion may signal body condition to potential partners. Further research may determine whether uropygial gland composition is related to body condition and body size in zebra finches.
5. Conclusions
In summary, our results suggest that female zebra finches can assess the body condition and body size of males. Females avoided the scent of the male with greater body condition and body size. Therefore, despite performing the study during breeding period, our results suggest that social interactions may be mediating the avoidance of the scent of the conspecific of the opposite sex with better body condition and body size in this gregarious species.
Author Contributions
"Conceptualization, L.A.; Methodology, L.A., I.L.R.; Investigation, I.L.R.; Writing – Original Draft Preparation, L.A.; Writing – Review & Editing, L.A. & I.L.R.; Funding Acquisition, L.A..”
Funding
LA was supported by the Ramón y Cajal program (RYC-2012-11353). The study was funded by the Volkswagen Foundation (85 994-1). Authors declare no conflict of interest.
Institutional Review Board Statement
Experiments were carried out under license of the Ethical Committee of the CSIC (563/2017) and the Animal Experimental Committee of the Junta de Castilla la Mancha (19-2017).
Data Availability Statement
All data are reported in the manuscript.
Acknowledgements
LA is grateful to the Ramón y Cajal program and the Volkswagen Foundation.
Conflicts of Interest
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
References
- Grafen, A. Biological signals as handicaps. J. Theor. Biol. 1990, 144, 517–546. [Google Scholar] [CrossRef] [PubMed]
- Kokko, H.; Jennions, M.D.; Brooks, R. Unifying and testing models of sexual selection. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 43–66. [Google Scholar] [CrossRef]
- Trivers, R. Parental investment and sexual selection. In Sexual Selection and the Descent of Man; Campbell, B., Ed.; Aldine: Chicago, 1972; pp. 136–179. [Google Scholar]
- Fisher, R.A. The Genetical Theory of Natural Selection; Oxford University Press: Oxford, 1930. [Google Scholar]
- Zahavi, A. Mate selection—a selection for a handicap. J. Theor. Biol. 1975, 67, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.D.; Zuk, M. Heritable true fitness and bright birds: a role for parasites? Science 1982, 218, 384–387. [Google Scholar] [CrossRef]
- Hill, G.E. A Red Bird in a Brown Bag. The Function and Evolution of Colourful Plumage in the House Finch; Oxford University Press: Oxford, 2002. [Google Scholar]
- McGraw, K.J.; Nolan, P.M.; Crino, O.L. Carotenoid accumulation strategies for becoming a colourful House Finch: analyses of plasma and liver pigments in wild moulting birds. Funct. Ecol. 2006, 20, 678–688. [Google Scholar] [CrossRef]
- Laucht, S.; Dale, J. Development of Badges of Status in Captive Male House Sparrows (Passer domesticus) in Relation to the Relative Ornamentation of Flock-Mates. Ethology 2012, 118, 644–653. [Google Scholar] [CrossRef]
- Grieves, L.A.; Kelly, T.R.; Bernards, M.A.; MacDougall-Shackleton, E.A. Malarial infection alters wax ester composition of preen oil in songbirds: results of an experimental study. Auk 2018, 135, 767–776. [Google Scholar] [CrossRef]
- Whittaker DJ, , Slowinski SP, Greenberg JM, Alian O, Winters AD, Ahmad MM, Burrell MJE, Soini HA, Novotny MV, Ketterson ED, Theis KR. Experimental evidence that symbiotic bacteria produce chemical cues in a songbird. J. Exp. Biol. 2019, 222, jeb202978. [CrossRef]
- Jacob J, Zisweiler V. The uropygial gland. In Avian Biology; Farner, D. S., King, J. R., Parkes, K. C., Eds.; Academic Press: New York, 1982; vol. 6, pp. 199–314. [Google Scholar]
- López-Rull, I.; Pagán, I.; Macías Garcia, C. Cosmetic enhancement of signal coloration: experimental evidence in the house finch. Behav. Ecol. 2010, 21, 781–787. [Google Scholar] [CrossRef]
- Moreno-Rueda, G. Uropygial gland and bib colouration in the house sparrow. Peer J 2016, 4, e2102. [Google Scholar] [CrossRef]
- Reneerkens, J.; Piersma, T.; Sinninghe Damsté, J.S. Sandpipers (Scolopacidae) switch from monoester to diester preen waxes during courtship and incubation, but why? Proc. R. Soc. Lond. B 2002, 269, 2135–2139. [Google Scholar] [CrossRef]
- Amo, L.; Avilés, J.M.; Parejo, D.; Peña, A.; Rodríguez, J.; Tomás, G. Sex recognition by odour and variation in the uropygial gland secretion in starlings. J. Anim. Ecol. 2012, 81, 695–613. [Google Scholar] [CrossRef] [PubMed]
- Grieves, L.A.; Bernards, M.A.; MacDougall-Shackleton, E.A. Wax ester composition of songbird preen oil varies seasonally and differs between sexes, ages, and populations. J. Chem. Ecol. 2019, 45, 37–45. [Google Scholar] [CrossRef]
- Whittaker, D.J.; Richmond, K.M.; Miller, A.K.; Kiley, R.; Burns, C.B.; Atwell, J.W.; Ketterson, E.D. Intraspecific preen oil odor preferences in dark-eyed juncos (Junco hyemalis). Behav. Ecol. 2011, 22, 1256–1263. [Google Scholar] [CrossRef]
- Sandilands, V.; Powell, K.; Keeling, L.; Savory, C.J. Preen gland function in layer fowls: factors affecting preen oil fatty acid composition. British Poultry Sci. 2004, 45, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, V.; Savory, J.; Powell, K. Preen gland function in layer fowls: factors affecting morphology and feather lipid levels. Comp. Biochem. Physiol. A 2004, 137, 217–225. [Google Scholar] [CrossRef]
- Whelan, R.J.; Levin, T.C.; Owen, J.C.; Garvin, M.C. Short-chain carboxylic acids from gray catbird (Dumetella carolinensis) uropygial secretions vary with testosterone levels and photoperiod. Comp. Biochem. Physiol. B 2010, 156, 183–188. [Google Scholar] [CrossRef]
- Whittaker, D.J.; Rosvall, K.A.; Slowinski, S.P.; Soini, H.A.; Novotny, M.V.; Ketterson, E.D. Songbird chemical signals reflect uropygial gland androgen sensitivity and predict aggression: implications for the role of the periphery in chemosignaling. J. Comp. Physiol. A 2018, 204, 5–15. [Google Scholar] [CrossRef]
- Pap, P.L.; Vágási, C.I.; Osváth, G.; Mureçan, C.; Barta, Z. Seasonality in the uropygial gland size and feather mite abundance in house sparrows Passer domesticus: natural covariation and an experiment. J. Avian Biol. 2010, 41, 653–661. [Google Scholar] [CrossRef]
- Moreno-Rueda, G. Uropygial gland size correlates with feather holes, body condition and wingbar size in the house sparrow Passer domesticus. J. Avian Biol. 2010, 41, 229–236. [Google Scholar] [CrossRef]
- Moreno-Rueda, G. Body-mass-dependent trade-off between immune response and uropygial gland size in house sparrows Passer domesticus. J. Avian Biol. 2015, 46, 40–45. [Google Scholar] [CrossRef]
- Tuttle, E.M.; Sebastian, P.J.; Posto, A.L.; Soini, H.A.; Novotny, M.V.; Gonser, R.A. Variation in preen oil composition pertaining to season, sex, and genotype in the polymorphic white-throated sparrow. J. Chem. Ecol. 2014, 40, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Mardon, J.; Saunders, S.M.; Anderson, M.J.; Couchoux, C.; Bonadonna, F. Species, gender, and identity: cracking petrels’ sociochemical code. Chem. Senses 2010, 35, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, D.J.; Soini, H.A.; Atwell, J.W.; Hollars, C.; Novotny, M.V.; Ketterson, E.D. Songbird chemosignals: volatile compounds in preen gland secretions vary among individuals, sexes, and populations. Behav. Ecol. 2010, 21, 608–614. [Google Scholar] [CrossRef]
- Leclaire, S.; Merkling, T.; Raynaud, C.; Giacinti, G.; Bessière, J.M.; Hatch, S.A.; Danchin, E. An individual and a sex odor signature in kittiwakes? Study of the semiochemical composition of preen secretion and preen down feathers. Naturwissenschaften 2011, 98, 615–624. [Google Scholar] [CrossRef]
- Campagna, S.; Mardon, J.A.; Celerier, A.; Bonadonna, F. Potential semiochemical molecules from birds: a practical and comprehensive compilation of the last 20 years studies. Chem. Senses 2012, 37, 3–25. [Google Scholar] [CrossRef]
- Leclaire, S.; Merkling, T.; Raynaud, C.; Mulard, H.; Bessière, J.M.; Lhuillier, E.; Hatch, S.A.; Danchin, E. Semiochemical compounds of preen secretion reflect genetic make-up in a seabird species. Proc. R. Soc. Lond. B 2011, 279, 1185–1193. [Google Scholar] [CrossRef]
- Leclaire S, van Dongen WFD, Voccia S, Merkling T, Ducamp C, Hatch SA, Blanchard P, Danchin É, Wagner RH. Preen secretions encode information on MHC similarity in certain sex-dyads in a monogamous seabird. Sci Rep 2014, 4, 6920. [CrossRef]
- Strandh, M.; Westerdahl, H.; Pontarp, M.; Canbäck, B.; Dubois, M.P.; Miquel, C.; Taberlet, P.; Bonadonna, F. Major histocompatibility complex class II compatibility, but not class I, predicts mate choice in a bird with highly developed olfaction. Proc. R. Soc. Lond. B 2012, 279, 4457–4463. [Google Scholar] [CrossRef]
- Slade, J.W.G.; Watson, M.J.; Kelly, T.R.; Gloor, G.B.; Bernards, M.A.; MacDougall-Shackleton, E.A. Chemical composition of preen wax reflects major histocompatibility complex similarity in songbirds. Proc. R. Soc. Lond. B 2016, 283, 20161966. [Google Scholar] [CrossRef]
- Coffin, H.; Watters, J.; Mateo, J. Odor-based recognition of familiar and related conspecifics: A first test conducted on captive Humboldt penguins (Spheniscus humboldti). PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Krause, E.T.; Krüger, O.; Kohlmeier, P.; Caspers, B.A. Olfactory kin recognition in a songbird. Biol. Let. 2012, 8, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, F.; Sanz-Aguilar, A. Kin recognition and inbreeding avoidance in wild birds: the first evidence for individual kin-related odour recognition. Anim. Behav. 2012, 84, 509–513. [Google Scholar] [CrossRef]
- Leclaire, S.; Strandh, M.; Mardon, J.; Westerdahl, H.; Bonadonna, F. Odour-based discrimination of similarity at the major histocompatibility complex in birds. Proc. R. Soc. B 2017, 284, 20162466. [Google Scholar] [CrossRef] [PubMed]
- Grieves, L.A.; Gloor, G.B.; Bernards, M.A.; MacDougall-Shackleton, E.A. Songbirds show odour-based discrimination of similarity and diversity at the major histocompatibility complex. Anim. Behav. 2019, 158, 131–138. [Google Scholar] [CrossRef]
- Amo, L.; López-Rull, I.; Pagán, I.; Macías Garcia, C. Male quality and conspecific scent preferences in the house finch Carpodacus mexicanus. Anim. Behav. 2012, 84, 1483–1489. [Google Scholar] [CrossRef]
- Johansson, B.G.; Jones, T.M. The role of chemical communication in mate choice. Biol. Rev. 2007, 82, 265–289. [Google Scholar] [CrossRef]
- Thomas, M.L. Detection of female mating status using chemical signals and cues. Biol. Rev. 2011, 86, 1–13. [Google Scholar] [CrossRef]
- Caspers, B.A.; Hagelin, J.C.; Paul, M.; Bock, S.; Willeke, S.; Krause, E.T. Zebra finch chicks recognize parental scent, and retain chemosensory knowledge of their genetic mother, even after egg cross-fostering. Sci. Rep. 2017, 7, 12859. [Google Scholar] [CrossRef]
- Caspers, B.A.; Gagliardo, A.; Krause, E.T. Impact of kin odour on reproduction in zebra finches. Behav. Ecol. Sociobiol. 2015, 69, 1827–1833. [Google Scholar] [CrossRef]
- Caspers, B.A.; Hagelin, J.; Bock, S.; Krause, E.T. An Easy Method to Test Odour Recognition in Songbird Hatchlings. Ethology 2015, 121, 882–887. [Google Scholar] [CrossRef]
- Golüke, S.; Bischof, H.J.; Caspers, B.A. Nestling odour modulates behavioural response in male, but not in female zebra finches. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Krause, E.T.; Brummel, C.; Kohlwey, S.; Baier, M.C.; Müller, C.; Bonadonna, F.; Caspers, B.A. Differences in olfactory species recognition in the females of two Australian songbird species. Behav. Ecol. Sociobiol. 2014, 68, 1819–1827. [Google Scholar] [CrossRef]
- Krause, E.T.; Bischof, H.J.; Engel, K.; Golüke, S.; Maraci, O.; Mayer, U.; Sauer, J.; Caspers, B.A. Olfaction in the Zebra Finch (Taeniopygia guttata): What Is Known and Further Perspectives. In Advances in the Study of Behavior; Naguib, M., Barrett, L., Healy, S.D., Eds.; 2018. [Google Scholar]
- Bonadonna, F.; Nevitt, G.A. Partner-specific odor recognition in an Antarctic seabird. Science 2004, 306, 835. [Google Scholar] [CrossRef]
- Bonadonna, F.; Caro, S.; Jouventin, P.; Nevitt, G.A. Evidence that blue petrel, Halobaena caerulea, fledglings can detect and orient to dimethyl sulfide. J. Exper. Biol. 2006, 209, 2165–2169. [Google Scholar] [CrossRef]
- Crino, O.L.; Klaassen van Oorschot, B.; Crandell, K.E.; Breuner, C.W.; Tobalske, B.W. Flight performance in the altricial zebra finch: Developmental effects and reproductive consequences. Ecol Evol. 2017, 7, 2316–2326. [Google Scholar] [CrossRef]
- Kogel CH de, Prijs HJ. Effects of brood size manipulations on sexual attractiveness of offspring in the zebra finch. Anim. Behav. 1996, 51, 699–708. [Google Scholar] [CrossRef]
- Holveck, M.J.; Riebel, K. Low-quality females prefer low-quality males when choosing a mate. Proc. R. Soc. B 2010, 277, 153–160. [Google Scholar] [CrossRef]
- Holveck, M.J.; Geberzahn, N.; Riebel, K. An experimental test of condition-dependent male and female mate choice in zebra finches. PLoS ONE 2011, 6, e23974. [Google Scholar] [CrossRef]
- Bolund, E.; Schielzeth, H.; Forstmeier, W. Intrasexual competition in zebra finches, the role of beak colour and body size. Anim. Behav. 2007, 74, 715–724. [Google Scholar] [CrossRef]
- Arakawa, H.; Blanchard, D.C.; Arakawa, K.; Dunlap, C.; Blanchard, R.J. Scent marking behavior as an odorant communication in mice. Neurosci. Biobehav. Rev. 2008, 32, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.T.; Parker, M.R. Social behavior and pheromonal communication in reptiles. J. Comp. Physiol. A 2010, 196, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Maynard Smith, J.; Parker, G.A. The logic of asymmetrical contests. Anim. Behav. 1976, 32, 564–578. [Google Scholar] [CrossRef]
- Mardon, J.; Saunders, S.M.; Bonadonna, F. From preen secretions to plumage: the chemical trajectory of blue petrels' Halobaena caerulea social scent. J. Avian Biol. 2011, 42, 29–38. [Google Scholar] [CrossRef]
- Golüke, S.; Caspers, B.A. Sex-specific differences in preen gland size of Zebra Finches during the course of breeding. Auk 2017, 134, 821–831. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).