Submitted:
31 May 2023
Posted:
31 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Hazardous Ingredients of Spent Coffee Grounds
2.1. Environmental Problems of Disposing Spent Coffee Grounds
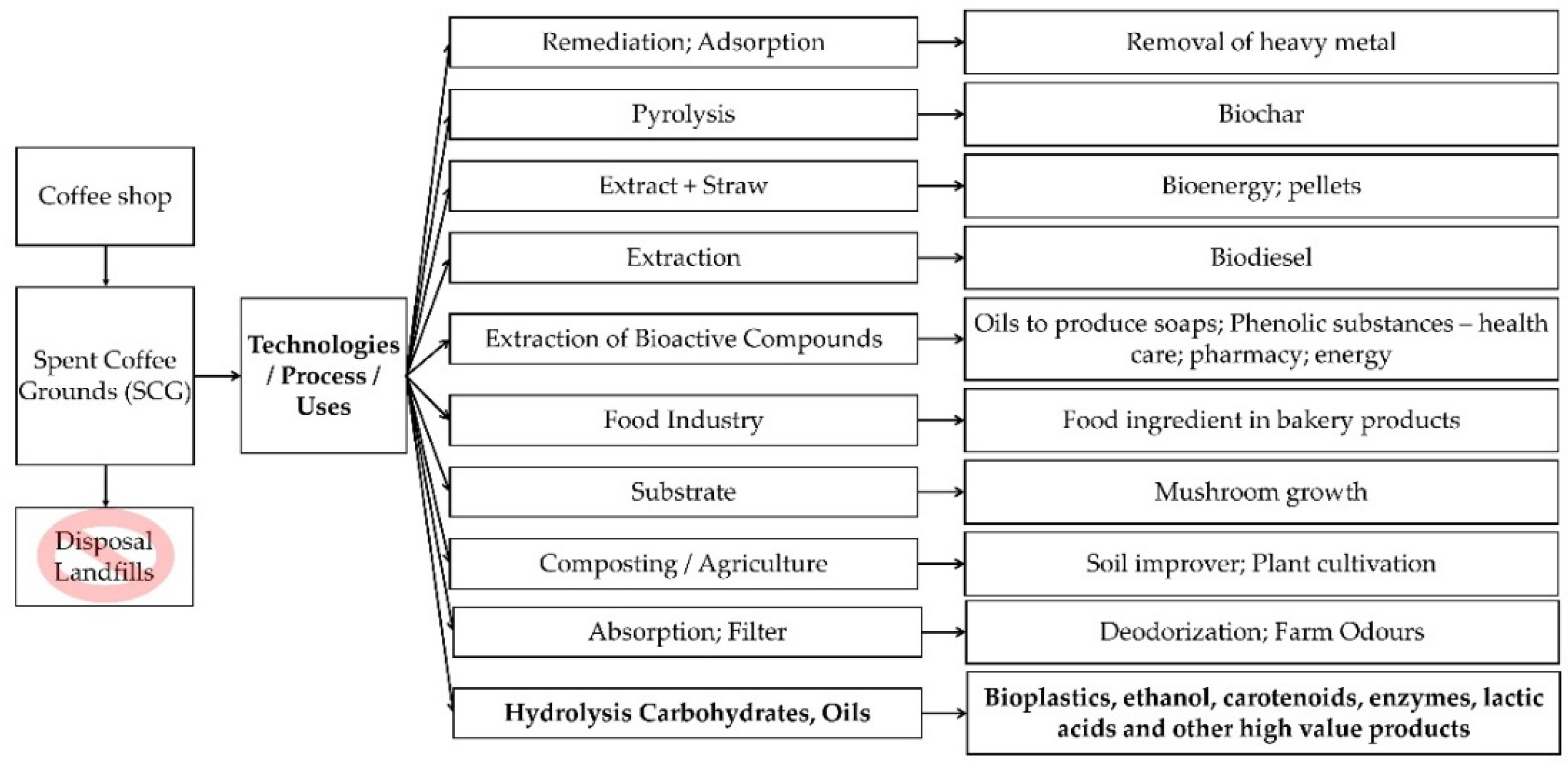
3. Properties and Potential Recycling Applications of Spent Coffee Grounds
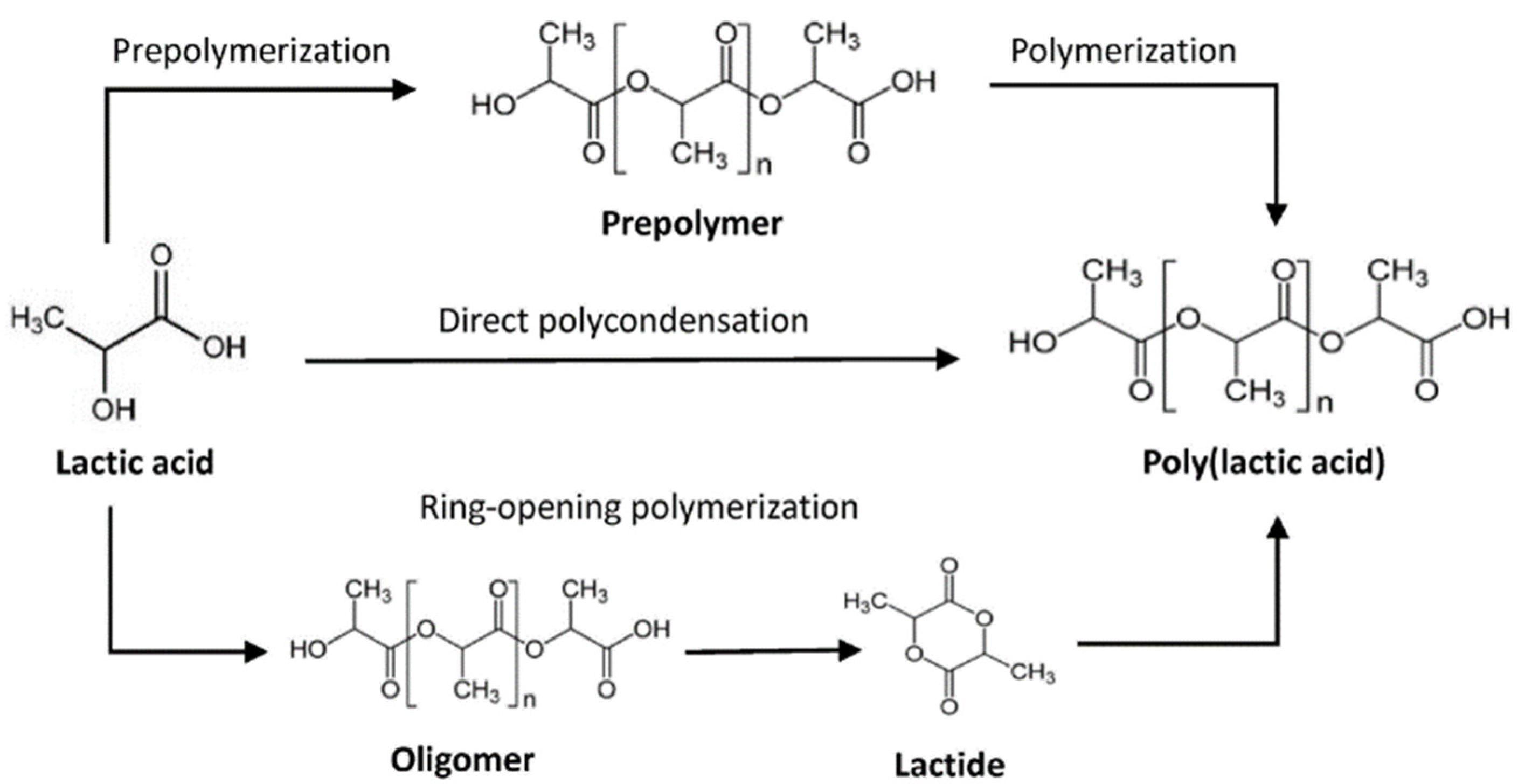
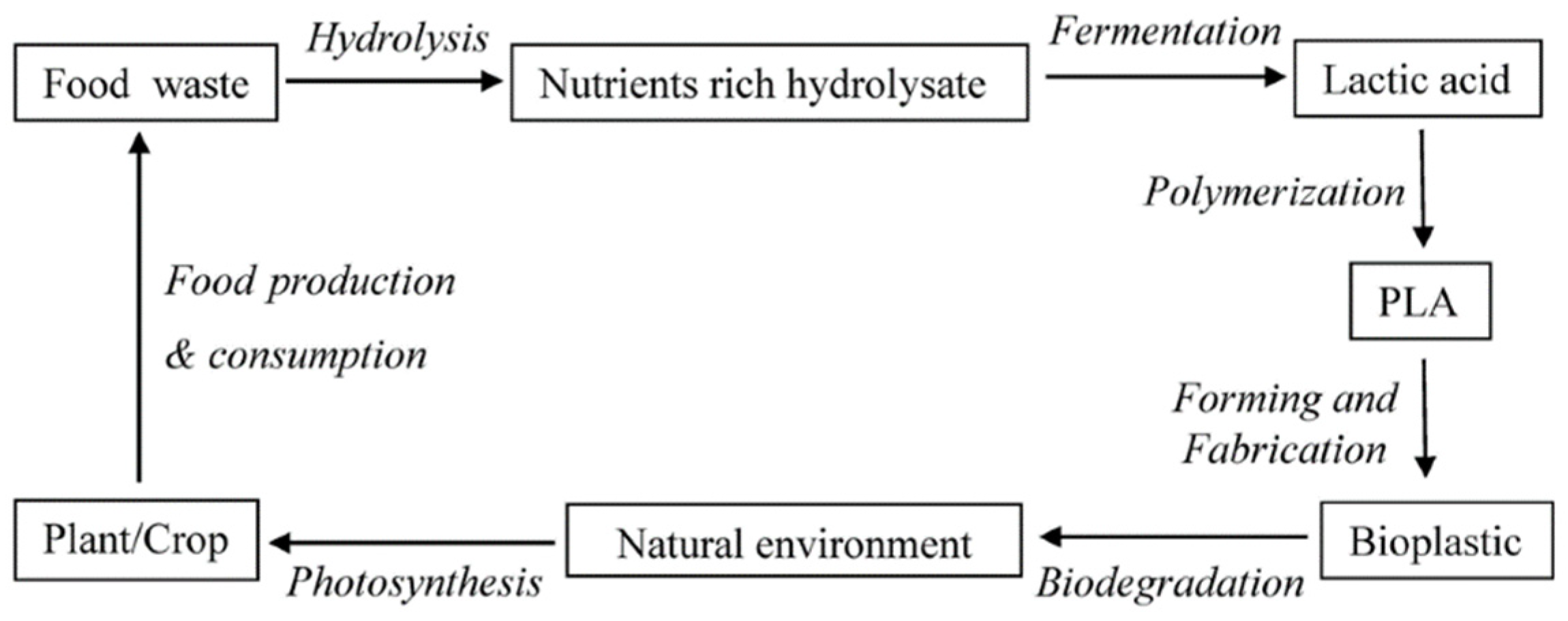
4. Production Processes of Polylactic Acid
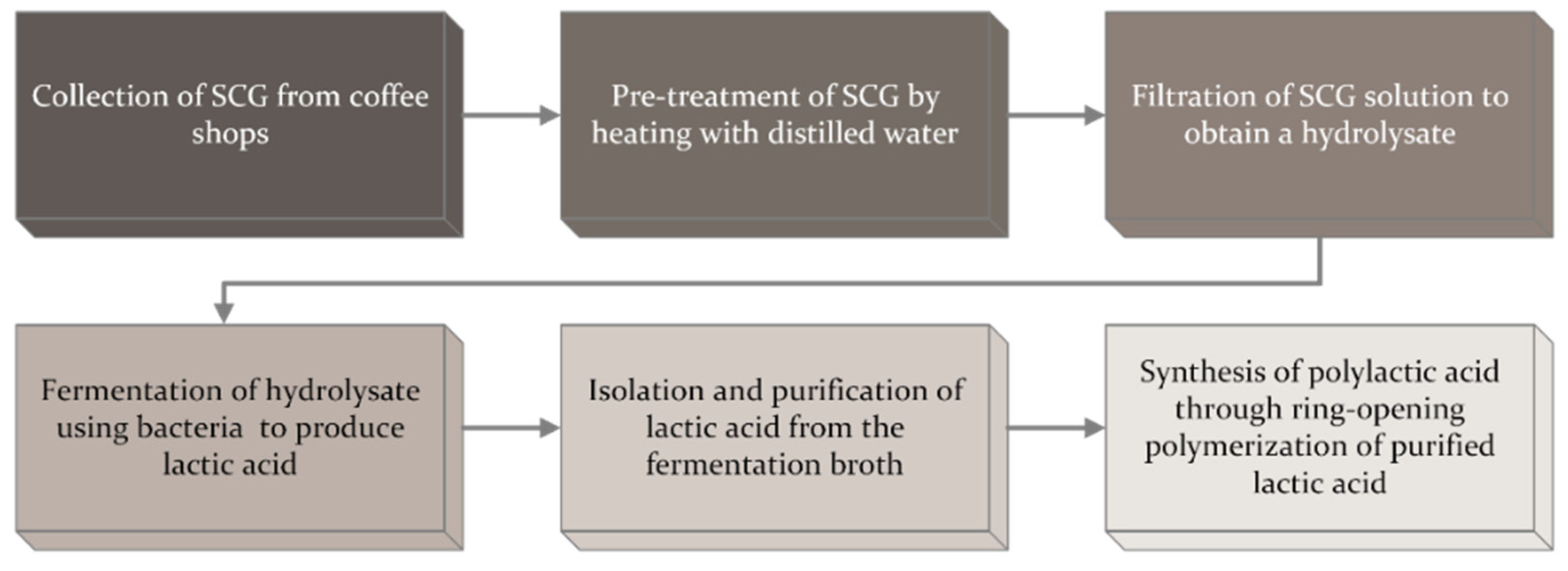
5. Method, Equipment, and Bacteria Required for Lactic Acid Production from Spent Coffee Ground
6. Discussions
6.1. Possible Ways to Improve the Yield of Polylactic Acid Production from Spent Coffee Grounds
6.2. Cost Analysis of Polylactic Acid Production from Spent Coffee Grounds
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Coffee Organization ICO - World Coffee Production; International Coffee Organization. 2021.
- International Coffee Organization ICO-World Coffee Consumption; International Coffee Organization. 2021.
- Coffee Farmers. Available online: https://www.fairtrade.org.uk/farmers-and-workers/coffee/ (accessed on 13 April 2023).
- Cerino-Córdova, F.J.; Dávila-Guzmán, N.E.; León, A.M.G.; Salazar-Rabago, J.J.; Soto-Regalado, E. Revalorization of Coffee Waste. In Coffee - Production and Research; 2020.
- British Coffee Association Coffee Consumption. Coffee Consumption. 2021.
- Santos, C.; Fonseca, J.; Aires, A.; Coutinho, J.; Trindade, H. Effect of Different Rates of Spent Coffee Grounds (SCG) on Composting Process, Gaseous Emissions and Quality of End-Product. Waste Management 2017, 59, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Blinová, L.; Sirotiak, M.; Pastierova, A.; Soldán, M. Review: Utilization of Waste From Coffee Production. Research Papers Faculty of Materials Science and Technology Slovak University of Technology 2017, 25. [Google Scholar] [CrossRef]
- Colantoni, A.; Paris, E.; Bianchini, L.; Ferri, S.; Marcantonio, V.; Carnevale, M.; Palma, A.; Civitarese, V.; Gallucci, F. Spent Coffee Ground Characterization, Pelletization Test and Emissions Assessment in the Combustion Process. Sci Rep 2021, 11, 5119. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.Y.; Krishnan, U.G.; Sabaratnam, V.; Tan, J.B.L. Assessment of Coffee Waste in Formulation of Substrate for Oyster Mushrooms Pleurotus Pulmonarius and Pleurotus Floridanus. Future Foods 2021, 4, 100075. [Google Scholar] [CrossRef]
- Sarno, M.; Iuliano, M. Active Biocatalyst for Biodiesel Production from Spent Coffee Ground. Bioresource Technology 2018, 266. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, A.S.C. de; de Oliveira, D.M.; Walling, E.; Babin, A.; Hersant, G.; Vaneeckhaute, C.; Dumont, M.-J.; Rodrigue, D. Spent Coffee Grounds Characterization and Reuse in Composting and Soil Amendment. Waste 2023, 1, 2–20. [Google Scholar] [CrossRef]
- Atelge, M.R.; Atabani, A.E.; Abut, S.; Kaya, M.; Eskicioglu, C.; Semaan, G.; Lee, C.; Yildiz, Y.Ş.; Unalan, S.; Mohanasundaram, R.; et al. Anaerobic Co-Digestion of Oil-Extracted Spent Coffee Grounds with Various Wastes: Experimental and Kinetic Modeling Studies. Bioresource Technology 2021, 322, 124470. [Google Scholar] [CrossRef] [PubMed]
- Kanniah, J.C. What Happens To Coffee Grounds After They’re Used? Perfect Daily Grind 2020.
- Planet Ark Coffee Grounds. Available online: https://businessrecycling.com.au/pages/coffee-grounds.cfm? (accessed on 22 April 2022).
- Ahmed, J.; Varshney, S.K. Polylactides—Chemistry, Properties and Green Packaging Technology: A Review. International Journal of Food Properties 2011, 14, 37–58. [Google Scholar] [CrossRef]
- Davis, A.P.; Chadburn, H.; Moat, J.; O’Sullivan, R.; Hargreaves, S.; Nic Lughadha, E. High Extinction Risk for Wild Coffee Species and Implications for Coffee Sector Sustainability. Sci Adv 2019, 5, eaav3473. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, W.; Azmat, A.; Ahmed, M. Comparative Effect of Coffee Robusta and Coffee Arabica (Qahwa) on Memory and Attention. Metabolic Brain Disease 2018, 33. [Google Scholar] [CrossRef]
- Ahmed, S.; Brinkley, S.; Smith, E.; Sela, A.; Theisen, M.; Thibodeau, C.; Warne, T.; Anderson, E.; Van Dusen, N.; Giuliano, P.; et al. Climate Change and Coffee Quality: Systematic Review on the Effects of Environmental and Management Variation on Secondary Metabolites and Sensory Attributes of Coffea Arabica and Coffea Canephora. Frontiers in Plant Science 2021, 12. [Google Scholar] [CrossRef]
- Volsi, B.; Telles, T.S.; Caldarelli, C.E.; da Camara, M.R.G. The Dynamics of Coffee Production in Brazil. PLoS One 2019, 14, e0219742. [Google Scholar] [CrossRef]
- Weyesa, G.W.; Tilahun, R. Documentation of Traditional Knowledge on “Coffee” (Coffea Arabica) in Jimma, Ilubabor and Wollega Zones. European Journal of Biophysics 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Starbucks at Home Why Arabica Coffee? Available online: https://www.starbucksathome.com/hk/en-hk/story/why-arabica-coffee (accessed on 29 March 2022).
- Dimitrakaki, I. STRATEGIC GROWTH CASE STUDY: STARBUCKS. Journal of Economics 2021, 2, 342–356. [Google Scholar]
- Pacific Coffee Our Coffee Beans. Available online: https://www.pacificcoffee.com/en/coffee-culture/our-bean/ (accessed on 29 March 2022).
- Planet Ark Coffee Grounds. Available online: https://businessrecycling.com.au/pages/coffee-grounds.cfm? (accessed on 22 April 2022).
- Ahmed, J.; Varshney, S.K. Polylactides—Chemistry, Properties and Green Packaging Technology: A Review. International Journal of Food Properties 2011, 14, 37–58. [Google Scholar] [CrossRef]
- Davis, A.P.; Chadburn, H.; Moat, J.; O’Sullivan, R.; Hargreaves, S.; Nic Lughadha, E. High Extinction Risk for Wild Coffee Species and Implications for Coffee Sector Sustainability. Sci Adv 2019, 5, eaav3473. [Google Scholar] [CrossRef]
- Obruca, S.; Benesova, P.; Kucera, D.; Petrik, S.; Marova, I. Biotechnological Conversion of Spent Coffee Grounds into Polyhydroxyalkanoates and Carotenoids. New Biotechnology 2015, 32, 569–574. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A Study on Chemical Constituents and Sugars Extraction from Spent Coffee Grounds. Carbohydrate Polymers 2011, 83, 368–374. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent Coffee Grounds: A Review on Current Research and Future Prospects. Trends in Food Science & Technology 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Obruca, S.; Petrik, S.; Benesova, P.; Svoboda, Z.; Eremka, L.; Marova, I. Utilization of Oil Extracted from Spent Coffee Grounds for Sustainable Production of Polyhydroxyalkanoates. Appl Microbiol Biotechnol 2014, 98, 5883–5890. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.V.; Kim, Y.-T. Spent Coffee Grounds and Coffee Silverskin as Potential Materials for Packaging: A Review. J Polym Environ 2021, 29, 2372–2384. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A Study on Chemical Constituents and Sugars Extraction from Spent Coffee Grounds. Carbohydrate Polymers 2011, 83, 368–374. [Google Scholar] [CrossRef]
- Stylianou, M.; Agapiou, A.; Omirou, M.; Vyrides, I.; Ioannides, I.M.; Maratheftis, G.; Fasoula, D. Converting Environmental Risks to Benefits by Using Spent Coffee Grounds (SCG) as a Valuable Resource. Environ Sci Pollut Res 2018, 25, 35776–35790. [Google Scholar] [CrossRef] [PubMed]
- Yamane, K.; Kono, M.; Fukunaga, T.; Iwai, K.; Sekine, R.; Watanabe, Y.; Iijima, M. Field Evaluation of Coffee Grounds Application for Crop Growth Enhancement, Weed Control, and Soil Improvement. Plant Production Science 2014, 17, 93–102. [Google Scholar] [CrossRef]
- Silva, M.A.; Nebra, S.A.; Silva, M.J.M.; Sanchez, C.G. THE USE OF BIOMASS RESIDUES IN THE BRAZILIAN SOLUBLE COFFEE INDUSTRY. Biomass & Bioenergy 1998, 14, 457–467. [Google Scholar]
- Block, I.; Günter, C.; Duarte Rodrigues, A.; Paasch, S.; Hesemann, P.; Taubert, A. Carbon Adsorbents from Spent Coffee for Removal of Methylene Blue and Methyl Orange from Water. Materials 2021, 14, 3996. [Google Scholar] [CrossRef]
- Muñoz Velasco, P.; Mendívil, M.A.; Morales, M.P.; Muñoz, L. Eco-Fired Clay Bricks Made by Adding Spent Coffee Grounds: A Sustainable Way to Improve Buildings Insulation. Mater Struct 2016, 49, 641–650. [Google Scholar] [CrossRef]
- Chung, L.L.P.; Wong, Y.C.; Arulrajah, A. The Application of Spent Coffee Grounds and Tea Wastes as Additives in Alkali-Activated Bricks. Waste Biomass Valor 2021, 12, 6273–6291. [Google Scholar] [CrossRef]
- Arulrajah, A.; Kua, T.-A.; Suksiripattanapong, C.; Horpibulsuk, S.; Shen, J.S. Compressive Strength and Microstructural Properties of Spent Coffee Grounds-Bagasse Ash Based Geopolymers with Slag Supplements. Journal of Cleaner Production 2017, 162, 1491–1501. [Google Scholar] [CrossRef]
- Starbucks Japan Finds New Ways to Recycle Coffee Grounds. Available online: https://stories.starbucks.com/stories/2016/starbucks-japan-coffee-grounds/ (accessed on 20 April 2023).
- Katayama, A. Recycle Coffee Grounds With Zero Impact: Japanese Koji Turns Waste Into Coffee Bars At Low Cost. Available online: https://www.forbes.com/sites/akikokatayama/2022/03/28/recycle-coffee-grounds-with-zero-impact-japanese-koji-turns-the-waste-into-delicious-coffee-bars-at-low-cost/ (accessed on 20 April 2023).
- UCL Business Founded by UCL Graduate Is Now World’s Largest Recycler of Coffee Grounds. Available online: https://www.ucl.ac.uk/enterprise/case-studies/2020/sep/business-founded-ucl-graduate-now-worlds-largest-recycler-coffee-grounds (accessed on 20 April 2023).
- Ihnen, A.C.; Petrock, A.M.; Chou, T.; Samuels, P.J.; Fuchs, B.E.; Lee, W.Y. Crystal Morphology Variation in Inkjet-Printed Organic Materials. Applied Surface Science 2011, 258, 827–833. [Google Scholar] [CrossRef]
- Fischer, S.; Thümmler, K.; Volkert, B.; Hettrich, K.; Schmidt, I.; Fischer, K. Properties and Applications of Cellulose Acetate. Macromolecular Symposia 2008, 262, 89–96. [Google Scholar] [CrossRef]
- Elida, F.; Azizah; Wiyono, H.; Muzakhar, K. Efficiency of Cellulase Production Using Coffee Pulp Waste under Solid State Fermentation by Aspergillus Sp. VT12.; November 16 2020; Vol. 2296, p. 020020. 16 November.
- Efficiency of Cellulase Production Using Coffee Pulp Waste under Solid State Fermentation by Aspergillus Sp. Available online: https://aip.scitation.org/doi/epdf/10.1063/5.0030482?src=getftr (accessed on 12 April 2023).
- Cellulose Nanofibers from Coffee Grounds Could Be Used to Make Bioplastics. Available online: https://www.azonano.com/news.aspx?newsID=37223 (accessed on 12 April 2023).
- Battista, F.; Zuliani, L.; Rizzioli, F.; Fusco, S.; Bolzonella, D. Biodiesel, Biogas and Fermentable Sugars Production from Spent Coffee Grounds: A Cascade Biorefinery Approach. Bioresource Technology 2021, 342, 125952. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef]
- Chen, C.; Xi, Y.; Weng, Y. Recent Advances in Cellulose-Based Hydrogels for Tissue Engineering Applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef]
- Kueh, A.B.H. Spent Ground Coffee – Awaking the Sustainability Prospects. Environmental and Toxicology Management 2021, 1, 1–6. [Google Scholar] [CrossRef]
- Ciesielczuk, T.; Rosik-Dulewska, C.; Poluszyńska, J.; Miłek, D.; Szewczyk, A.; Sławińska, I. Acute Toxicity of Experimental Fertilizers Made of Spent Coffee Grounds. Waste Biomass Valor 2018, 9, 2157–2164. [Google Scholar] [CrossRef]
- International Coffee Organization - What’s New. Available online: https://www.ico.org/ (accessed on 13 April 2023).
- Beyene, A.; Kebede, Y.; Addis, T.; Assefa, F.; Amsalu, A.; Mulat, W.; Kloos, H.; Triest, L. The Impact of Traditional Coffee Processing on River Water Quality in Ethiopia and the Urgency of Adopting Sound Environmental Practices. Environmental monitoring and assessment 2011, 184, 7053–7063. [Google Scholar] [CrossRef] [PubMed]
- Blinová, L.; Sirotiak, M. Utilization of Spent Coffee Grounds for Removal of Hazardous Substances from Water: A Review. Research Papers Faculty of Materials Science and Technology Slovak University of Technology 2019, 27, 145–152. [Google Scholar] [CrossRef]
- Sarghini, F.; Marra, F.; De Vivo, A.; Vitaglione, P.; Mauriello, G.; Maresca, D.; Troise, A.D.; Echeverria-Jaramillo, E. Acid Hydrolysis of Spent Coffee Grounds: Effects on Possible Prebiotic Activity of Oligosaccharides. Chemical and Biological Technologies in Agriculture 2021, 8, 67. [Google Scholar] [CrossRef]
- Herpin, U.; Gloaguen, T.V.; da Fonseca, A.F.; Montes, C.R.; Mendonça, F.C.; Piveli, R.P.; Breulmann, G.; Forti, M.C.; Melfi, A.J. Chemical Effects on the Soil–Plant System in a Secondary Treated Wastewater Irrigated Coffee Plantation—A Pilot Field Study in Brazil. Agricultural Water Management 2007, 89, 105–115. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Delgado, G.; Fernández-Arteaga, A.; Fornasier, F.; Mondini, C. Spent Coffee Grounds By-Products and Their Influence on Soil C–N Dynamics. Journal of Environmental Management 2022, 302, 114075. [Google Scholar] [CrossRef] [PubMed]
- Lirio-Paredes, J.; Ogata-Gutiérrez, K.; Zúñiga-Dávila, D. Effects of Rhizobia Isolated from Coffee Fields in the High Jungle Peruvian Region, Tested on Phaseolus Vulgaris L. Var. Red Kidney. Microorganisms 2022, 10, 823. [Google Scholar] [CrossRef] [PubMed]
- Gollapalli, M.; Kota, S.H. Methane Emissions from a Landfill in North-East India: Performance of Various Landfill Gas Emission Models. Environmental Pollution 2018, 234, 174–180. [Google Scholar] [CrossRef]
- Mahmoud, E.; Atabani, A.E.; Badruddin, I.A. Valorization of Spent Coffee Grounds for Biogas Production: A Circular Bioeconomy Approach for a Biorefinery. Fuel 2022, 328, 125296. [Google Scholar] [CrossRef]
- Talalaj, I.A.; Biedka, P. Use of the Landfill Water Pollution Index (LWPI) for Groundwater Quality Assessment near the Landfill Sites. Environ Sci Pollut Res 2016, 23, 24601–24613. [Google Scholar] [CrossRef]
- Thenepalli, T.; Chilakala, R.; Ahn, J.-W. Environmental Effect of the Coffee Waste and Anti-Microbial Property of Oyster Shell Waste Treatment. Journal of Energy Engineering 2017, 26, 39–49. [Google Scholar] [CrossRef]
- Methane and Other Gases: A Range of Greenhouse Gases Contribute to Climate Change - Document - Gale OneFile: High School Edition. Available online: https://go-gale-com.ezproxy.lib.hkmu.edu.hk/ps/retrieve.do?tabID=T003&resultListType=RESULT_LIST&searchResultsType=SingleTab&retrievalId=f45e87da-bed2-4a15-8161-86c40dc4b3f4&hitCount=1&searchType=AdvancedSearchForm¤tPosition=1&docId=GALE%7CA172597067&docType=Article&sort=RELEVANCE&contentSegment=ZTSF-198501P&prodId=STOM&pageNum=1&contentSet=GALE%7CA172597067&searchId=R1&userGroupName=hkmu&inPS=true (accessed on 12 April 2023).
- Schmidt Rivera, X.C.; Gallego-Schmid, A.; Najdanovic-Visak, V.; Azapagic, A. Life Cycle Environmental Sustainability of Valorisation Routes for Spent Coffee Grounds: From Waste to Resources. Resources, Conservation and Recycling 2020, 157, 104751. [Google Scholar] [CrossRef]
- Forcina, A.; Petrillo, A.; Travaglioni, M.; di Chiara, S.; De Felice, F. A Comparative Life Cycle Assessment of Different Spent Coffee Ground Reuse Strategies and a Sensitivity Analysis for Verifying the Environmental Convenience Based on the Location of Sites. Journal of Cleaner Production 2023, 385, 135727. [Google Scholar] [CrossRef]
- Vítězová, M.; Jančiková, S.; Dordević, D.; Vítěz, T.; Elbl, J.; Hanišáková, N.; Jampílek, J.; Kushkevych, I. The Possibility of Using Spent Coffee Grounds to Improve Wastewater Treatment Due to Respiration Activity of Microorganisms. Applied Sciences 2019, 9, 3155. [Google Scholar] [CrossRef]
- Solomakou, N.; Tsafrakidou, P.; Goula, A.M. Valorization of SCG through Extraction of Phenolic Compounds and Synthesis of New Biosorbent. Sustainability 2022, 14, 9358. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent Coffee Grounds: A Review on Current Research and Future Prospects. Trends in Food Science & Technology 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Hu, Y.; Daoud, W.A.; Cheuk, K.K.L.; Lin, C.S.K. Newly Developed Techniques on Polycondensation, Ring-Opening Polymerization and Polymer Modification: Focus on Poly(Lactic Acid). 2016, 14.
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W.A. 2 - Synthesis and Production of Poly(Lactic Acid). In Polylactic Acid; Sin, L.T., Rahmat, A.R., Rahman, W.A.W.A., Eds.; Plastics Design Library; William Andrew Publishing: Oxford, 2013; pp. 71–107. ISBN 978-1-4377-4459-0. [Google Scholar]
- Hudeckova, H.; Neureiter, M.; Obruca, S.; Frühauf, S.; Marova, I. Biotechnological Conversion of Spent Coffee Grounds into Lactic Acid. Lett Appl Microbiol 2018, 66, 306–312. [Google Scholar] [CrossRef]
- Bomfim, A.S.C. de; Oliveira, D.M. de; Voorwald, H.J.C.; Benini, K.C.C. de C.; Dumont, M.-J.; Rodrigue, D. Valorization of Spent Coffee Grounds as Precursors for Biopolymers and Composite Production. Polymers 2022, 14, 437. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.S.; Fahim, I.; Hosny, M.; El-Badry, M.A. Optimization of Lactic Acid Production from Agro-Industrial Wastes Produced by Kosakonia Cowanii. Current Research in Green and Sustainable Chemistry 2022, 5, 100228. [Google Scholar] [CrossRef]
- Breton Toral, A.; Trejo Estrada, S.R.; McDonald, A.G. Lactic Acid Production from Potato Peel Waste, Spent Coffee Grounds and Almond Shells with Undefined Mixed Cultures Isolated from Coffee Mucilage from Coatepec Mexico. Ferment Technol 2016, 06. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Foerster, S.; Hartmann, F.; Kröger, M.; Kaltschmitt, M. Oil Extracted from Spent Coffee Grounds as a Renewable Source for Fatty Acid Methyl Ester Manufacturing. Fuel 2012, 96, 70–76. [Google Scholar] [CrossRef]
- Bomfim, A.S.C. de; Oliveira, D.M. de; Voorwald, H.J.C.; Benini, K.C.C. de C.; Dumont, M.-J.; Rodrigue, D. Valorization of Spent Coffee Grounds as Precursors for Biopolymers and Composite Production. Polymers 2022, 14, 437. [Google Scholar] [CrossRef] [PubMed]
- Chiyanzy, I.; Brienzo, M.; García-Aparicio, M.; Agudelo, R.; Görgens, J. Spent Coffee Ground Mass Solubilisation by Steam Explosion and Enzymatic Hydrolysis. Journal of Chemical Technology & Biotechnology 2015, 90, 449–458. [Google Scholar] [CrossRef]
- Kasai, N.; Konishi, A.; Iwai, K.; Maeda, G. Efficient Digestion and Structural Characteristics of Cell Walls of Coffee Beans. J. Agric. Food Chem. 2006, 54, 6336–6342. [Google Scholar] [CrossRef]
- Silver, R.S.; Whalen-Pedersen, E.; Perkins, D.E.; Plumb, S.; Ceriali, S.; Wragg, A. Enzyme-Assisted Soluble Coffee Production 2007.
- Method of Producing Coffee Extract 1973.
- Evangelista, S.R.; Silva, C.F.; Miguel, M.G.P. da C.; Cordeiro, C. de S.; Pinheiro, A.C.M.; Duarte, W.F.; Schwan, R.F. Improvement of Coffee Beverage Quality by Using Selected Yeasts Strains during the Fermentation in Dry Process. Food Research International 2014, 61, 183–195. [Google Scholar] [CrossRef]
- Ruta, L.L.; Farcasanu, I.C. Coffee and Yeasts: From Flavor to Biotechnology. Fermentation 2021, 7, 9. [Google Scholar] [CrossRef]
- Burniol-Figols, A.; Cenian, K.; Skiadas, I.; Gavala, H. Integration of Chlorogenic Acid Recovery and Bioethanol Production from Spent Coffee Grounds. Biochemical Engineering Journal 2016, 116. [Google Scholar] [CrossRef]
- Lee, J.W.; In, J.H.; Park, J.-B.; Shin, J.; Park, J.H.; Sung, B.H.; Sohn, J.-H.; Seo, J.-H.; Park, J.-B.; Kim, S.R.; et al. Co-Expression of Two Heterologous Lactate Dehydrogenases Genes in Kluyveromyces Marxianus for l-Lactic Acid Production. J Biotechnol 2017, 241, 81–86. [Google Scholar] [CrossRef]
- Manandhar, A. ; Shah Techno-Economic Analysis of Bio-Based Lactic Acid Production Utilizing Corn Grain as Feedstock. Processes 2020, 8, 199. [Google Scholar] [CrossRef]
- Sanaei, S.; Stuart, P.R. Systematic Assessment of Triticale-Based Biorefinery Strategies: Techno-Economic Analysis to Identify Investment Opportunities. Biofuels, Bioproducts and Biorefining 2018, 12, S46–S59. [Google Scholar] [CrossRef]
- Wellenreuther, C.; Wolf, A.; Zander, N. Cost Competitiveness of Sustainable Bioplastic Feedstocks – A Monte Carlo Analysis for Polylactic Acid. Cleaner Engineering and Technology 2022, 6, 100411. [Google Scholar] [CrossRef]
- Chiarakorn, S.; Permpoonwiwat, C.K.; Nanthachatchavankul, P. Cost Benefit Analysis of Bioplastic Production in Thailand Workplaces as Learning Spaces-Conceptual and Empirical Insights View Project Sustainable Transport Policy in ASEAN View Project COST BENEFIT ANALYSIS OF BIOPLASTIC PRODUCTION IN THAILAND 1 57 COST BENEFIT ANALYSIS OF BIOPLASTIC PRODUCTION IN THAILAND. 2011.
- Kwan, T.H.; Hu, Y.; Lin, C.S.K. Techno-Economic Analysis of a Food Waste Valorisation Process for Lactic Acid, Lactide and Poly(Lactic Acid) Production. Journal of Cleaner Production 2018, 181, 72–87. [Google Scholar] [CrossRef]
- British Coffee Association Coffee Consumption. Coffee Consumption 2021.
- International Coffee Organization - What’s New. Available online: https://www.ico.org/ (accessed on 13 April 2023).
- Jim &associates, L.; Center, T.C.H.S. The Business Case for Commercial Production of Bioplastics in Maine Sustainable Bioplastics Council of Maine. 2010.
| Project | Feedstock | Min cost per ton (USD) | Max cost per ton (USD) |
| Manandhar & Shah (2020) | Cron grains | 844 | 1,251 |
| Sanaei & Stuart (2018) | Triticale | 911 | 1,496 |
| Wellenreuther et al, (2022) | Corn grain & stover | 1,004 | 1,374 |
| Chiarakorn et al., (2014) | Cassava roots | 2,410 | 2,620 |
| Kwan et al., (2018) | Food waste powder | 3558 | 3558 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





