Submitted:
31 May 2023
Posted:
31 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Anise (Pimpinella anisum L.)
3. Basil (Ocimum basilicum L.)
4. Borage (Borago officinalis L.)
5. Cilantro (Coriander) (Coriandrum sativum L.)
6. Chamomile (Matricaria chamomilla L.)
7. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Fenugreek cultivation with emphasis on historical aspects and its uses in traditional medicine and modern pharmaceutical science. Mini Rev Med Chem. 2021, 21, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Natural dietary and medicinal plants with anti-obesity therapeutics activities for treatment and prevention of obesity during lock down and in post-COVID-19 era. Appl Sci. 2021, 11, 7889. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Health benefits of wolfberry (Gou Qi Zi) on the basis of ancient Chinese herbalism and Western modern medicine. Avicenna J Phytomed. 2021, 11, 109–119. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Barberry (Berberis vulgaris), a medicinal fruit and food with traditional and modern pharmaceutical uses. Isr J Plant Sci. 2021, 68, 1–11. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic potential of phenolic compounds in medicinal plants-natural health products for human health. Molecules. 2023, 28, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Different methods for molecular and rapid detection of human novel coronavirus. Curr Pharm Des. 2021, 27, 1–10. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Molecular breeding and the impacts of some important genes families on agronomic traits, a review. Genet Resour Crop Evol. 2021, 68, 1709–1730. [Google Scholar] [CrossRef]
- Das, S.; Singh, V.K.; Chaudhari, A.K.; Deepika; Dwivedy, A.K.; Dubey, N.K. Co-encapsulation of Pimpinella anisum and Coriandrum sativum essential oils based synergistic formulation through binary mixture: Physico-chemical characterization, appraisal of antifungal mechanism of action, and application as natural food preservative. Pest Biochem Physiol. 2022, 184, 105066. [Google Scholar] [CrossRef]
- Mahboubi, M.; Mahboubi, M. Pimpinella anisum and female disorders: A review. Phytomedicine Plus. 2021, 1, 100063. [Google Scholar] [CrossRef]
- Al-Bayati, F.A. Synergistic antibacterial activity between Thymus vulgaris and Pimpinella anisum essential oils and methanol extracts. J Ethnopharmacol. 2008, 116, 403–406. [Google Scholar] [CrossRef]
- Triapelli, C.R.; Andrade, C.R.D.; Cassano, A.O.; De Souza, F.A.; Ambrosio, S.R.; Costa, F.B.D.; Oliveira, A.M.D. Antispasmodic and relaxant effects of the hidroalcoholic extract of Pimpinella anisum (Apiaceae) on rat anococcygeus smooth muscle. J Ethnopharmacol. 2007, 110, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Simbar, M.; Shadipour, M.; Salamzadeh, J.; Ramezani-Tehrani, F.; Nasiri, N. The combination of Pimpinella anisum, Apium graveolens and Crocus sativus (PAC) is more effective than mefenamic acid on postpartum after-pain. J Herb Med. 2015, 5, 20–25. [Google Scholar] [CrossRef]
- Gulcin, I.; Oktay, M.; Kirecci, E.; Kufrevioglu, O.I. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003, 83, 371–382. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Canale, A.; Senthil-Nathan, S.; Maggi, F. Not just popular spices! Essential oils from Cuminum cyminum and Pimpinella anisum are toxic to insect pests and vectors without affecting non-target invertebrates. Ind Crop Prod. 2018, 124, 236–243. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Tafaghodi, M.; Abedzadeh, S.; Taghiabadi, E. Effect of aqueous and ethanolic extracts of Pimpinella anisum L. seeds on milk production in rats. J Acupunct Meridian Stud. 2014, 7, 211–216. [Google Scholar] [CrossRef]
- Kholiya, S.; Punetha, A.; Uhan, A.C.; Venkatesha, K.T.; Kumar, D.; Upadhyay, R.K.; Padalia, R.C. Essential oil yield and composition of Ocimum basilicum L. at different phenological stages, plant density and post-harvest drying methods. S Afr J Bot. 2022, 151, 919–925. [Google Scholar] [CrossRef]
- Singh, V.; Kaur, K.; Kaur, S.; Shri, R.; Singh, T.G.; Singh, M. Trimethoxyflavones from Ocimum basilicum L. leaves improves long term memory in mice by modulating multiple pathways. J Ethnopharmacol. 2022, 295, 115438. [Google Scholar] [CrossRef]
- Travadi, T.; Sharma, S.; Pandit, R.; Nakrani, M.; Joshi, C.; Joshi, M. A duplex PCR assay for authentication of Ocimum basilicum L. and Ocimum tenuiflorum L. in Tulsi churna. Food Control. 2022, 137, 108790. [Google Scholar] [CrossRef]
- Bajomo, E.M.; Aing, M.S.; Ford, L.S.; Niemeyer, E.D. Chemotyping of commercially available basil (Ocimum basilicum L.) varieties: Cultivar and morphotype influence phenolic acid composition and antioxidant properties. NFS J. 2022, 26, 1–9. [Google Scholar] [CrossRef]
- Turkay, I.; Ozturk, L. The form, dose, and method of application of vermicompost differentiate the phenylpropene biosynthesis in the peltate glandular trichomes of methylchavicol chemotype of Ocimum basilicum L. Ind Crops Prod. 2023, 198, 116688. [Google Scholar] [CrossRef]
- Qamar, F.; Sana, A.; Naveed, S.; Faizi, S. Phytochemical characterization, antioxidant activity and antihypertensive evaluation of Ocimum basilicum L. in L-NAME induced hypertensive rats and its correlation analysis. Heliyon. 2023, 9, e14644. [Google Scholar] [CrossRef]
- Junaid, A.A.; Kamarudin, M.S.; Junaid, Q.O.; Edaroyati, W.P.; Isyaka, M.S.; Dauda, A.B.; Umar, D.M.; Igoli, J.O.; Amin, S.M.N. Nutrient uptake and recovery potentials of Ocimum basilicum and Corchorus olitorius in a polyculture aquaponic system. Sci Afr. 2023, 20, e01645. [Google Scholar] [CrossRef]
- Alimi, D.; Hajri, A.; Jallouli, S.; Sebai, H. Acaricidal and anthelmintic efficacy of Ocimum basilicum essential oil and its major constituents estragole and linalool, with insights on acetylcholinesterase inhibition. Vet Parasitol. 2022, 309, 109743. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.S.; Jeronimo, G.T.; Filho, R.A.C.C.; Souza, A.I.D.; Stringhetta, G.R.; Cruz, M.G.D.; Torres, G.D.S.; Goncalves, L.U.; Povh, J.A. Ocimum basilicum essential oils as an anesthetic for tambaqui Colossoma macropomum: Hematological, biochemical, non-specific immune parameters and energy metabolism. Aquaculture. 2021, 533, 736124. [Google Scholar] [CrossRef]
- Beltran-Noboa, A.; Proano-Ojeda, J.; Guevara, M.; Gallo, B.; Berrueta, L.A.; Giampieri, F.; Perez-Castillo, Y.; Battino, M.; Alvarez-Suarez, J.M.; Tejera, E. Metabolomic profile and computational analysis for the identification of the potential anti-inflammatory mechanisms of action of the traditional medicinal plants Ocimum basilicum and Ocimum tenuiflorum. Food Chem Toxicol. 2022, 164, 113039. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Nguyen, T.N.L.; Nguyen, K.; Nguyen, H.V.T.; Tran, L.T.T.; Ngo, T.X.T.; Pham, P.T.V.; Tran, M.H. Machine learning-based screening of MCF-7 human breast cancer cells and molecular docking analysis of essential oils from Ocimum basilicum against breast cancer. J Mol Struct. 2022, 1268, 133627. [Google Scholar] [CrossRef]
- Sabouri, Z.; Oskuee, R.K.; Sabouri, S.; Moghaddas, S.S.T.H.; Samarghandian, S.; Abdulabbas, H.S.; Darroudi, M. Phytoextract-mediated synthesis of Ag-doped ZnO-MgO-CaO nanocomposite using Ocimum Basilicum L. seeds extract as a highly efficient photocatalyst and evaluation of their biological effects. Ceram Int. 2023, 49, 20989–20997. [Google Scholar] [CrossRef]
- Srivastava, S.; Lal, R.K.; Yadav, K.; Pant, Y.; Bawitlung, L.; Kumar, P.; Mishra, A.; Gupta, P.; Pal, A.; Rout, P.K.; et al. Chemical composition of phenylpropanoid rich chemotypes of Ocimum basilicum L. and their antimicrobial activities. Ind Crops Prod. 2022, 183, 114978. [Google Scholar] [CrossRef]
- Akhavan, N.; Parikh, K.; Salazar, G.; Arjmandi, B. The antioxidative effects of Borago officinalis in lipopolysaccharide and hydrogen peroxide-activated RAW 264.7 macrophages. Curr Dev Nutr. 2020, 4, nzaa045-001. [Google Scholar] [CrossRef]
- Miceli, A.; Aleo, A.; Corona, O.; Sardina, M.T.; Mammina, C.; Settanni, L. Antibacterial activity of Borago officinalis and Brassica juncea aqueous extracts evaluated in vitro and in situ using different food model systems. Food Control. 2014, 40, 157–164. [Google Scholar] [CrossRef]
- Avila, C.; Breakspear, I.; Hawrelak, J.; Salmond, S.; Evans, S. A systematic review and quality assessment of case reports of adverse events for borage (Borago officinalis), coltsfoot (Tussilago farfara) and comfrey (Symphytum officinale). Fitoterapia. 2020, 142, 104519. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Bashir, S.; Khan, A.-U. Pharmacological basis for the use of Borago officinalis in gastrointestinal, respiratory and cardiovascular disorders. J Ethnopharmacol. 2007, 114, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.N.; Hamed, A.N.El.-S.; Sugimoto, S.; Otsuka, H.; Kamel, M.S.; Matsunami, K. Officinlioside, a new lignan glucoside from Borago officinalis L. Nat Prod Res. 2016, 30, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.A.; Park, B.; Hwang, E.; Park, S.-Y.; Yi, T.-H. Borago officinalis L. attenuates UVB-induced skin photodamage via regulation of AP-1 and Nrf2/ARE pathway in normal human dermal fibroblasts and promotion of collagen synthesis in hairless mice. Exp Gerontol. 2018, 107, 178–186. [Google Scholar] [CrossRef]
- Mohajer, S.; Taha, R.M.; Ramli, R.B.; Mohajer, M. Phytochemical constituents and radical scavenging properties of Borago officinalis and Malva sylvestris. Ind Crop Prod. 2016, 94, 673–681. [Google Scholar] [CrossRef]
- Ramandi, N.F.; Ghassempour, A.; Najafi, N.M.; Ghasemi, E. Optimization of ultrasonic assisted extraction of fatty acids from Borago officinalis L. flower by central composite design. Arab J Chem. 2017, 10, S23–S27. [Google Scholar] [CrossRef]
- Zribi, I.; Bleton, J.; Moussa, F.; Abderrabba, M. GC-MS analysis of the volatile profile and the essential oil compositions of Tunisian Borago Officinalis L.: Regional locality and organ dependency. Ind Crops Prod. 2019, 129, 290–298. [Google Scholar] [CrossRef]
- Zemmouri, H.; Ammar, S.; Boumendjel, A.; Messarah, M.; El Fekri, A.; Bouaziz, M. Chemical composition and antioxidant activity of Borago officinalis L. leaf extract growing in Algeria. Arab J Chem. 2019, 12, 1954–1963. [Google Scholar] [CrossRef]
- Atrooz, O.; Al-Nadaf, A.; Uysal, H.; Kutlu, H.M.; Sezer, C.V. Biosynthesis of silver nanoparticles using Corianderum sativum L. extract and evaluation of their antibacterial, anti-inflammatory and antinociceptive activities. S Afr J Bot. 2023, 157, 219–227. [Google Scholar] [CrossRef]
- Munni, Y.A.; Dash, R.; Mitra, S.; Dash, N.; Shima, M.; Moon, I.S. Mechanistic study of Coriandrum sativum on neuritogenesis and synaptogenesis based on computationally guided in vitro analyses. J Ethnopharmacol. 2023, 306, 116165. [Google Scholar] [CrossRef]
- Jeyaram, S.; Geethakrishnan, T. Spectral and third-order nonlinear optical characteristics of natural pigment extracted from Coriandrum sativum. Opt Mater. 2020, 107, 110148. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, Y.; Wang, C.; Huang, S.; Lei, Y.; Huang, M.; Zhang, X. Inhibitory effect of coriander (Coriandrum sativum L.) extract marinades on the formation of polycyclic aromatic hydrocarbons in roasted duck wings. Food Sci Hum Wellness. 2023, 12, 1128–1135. [Google Scholar] [CrossRef]
- Yigit, N.O.; Kocaayan, H. Efficiency of thyme (Origanum onites) and coriander (Coriandrum sativum) essential oils on anesthesia and histopathology of rainbow trout (Oncorhynchus mykiss). Aquaculture. 2023, 562, 738813. [Google Scholar] [CrossRef]
- Tulsani, N.J.; Hamid, R.; Jacob, F.; Umretiya, N.G.; Nandha, A.K.; Tomar, R.S.; Golakiya, B.A. Transcriptome landscaping for gene mining and SSR marker development in Coriander (Coriandrum sativum L.). Genomics. 2020, 112, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.C.D.; Souza, L.Z.M.D.; Yilmaz, M.; Oliveira, M.A.D.; Bezerra, A.C.D.S.; Silva, E.F.D.; Dumont, M.R.; Machado, A.R.T. Activated carbon of Coriandrum sativum for adsorption of methylene blue: Equilibrium and kinetic modeling. Clean Mater. 2022, 3, 100052. [Google Scholar] [CrossRef]
- Wei, S.; Lyu, J.; Wei, L.; Xie, B.; Wei, J.; Zhang, G.; Li, J.; Gao, C.; Xiao, X.; Yu, J. Chemometric approaches for the optimization of headspace-solid phase microextraction to analyze volatile compounds in coriander (Coriandrum sativum L.). LWT. 2022, 167, 113842. [Google Scholar] [CrossRef]
- Shahrajabian, M.H. Medicinal herbs with anti-inflammatory activities for natural and organic healing. Curr Org Chem. 2021, 25, 1–17. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W. Asparagus (Asparagus officinalis L.) and pennyroyal (Mentha pulegium L.), impressive advantages with wondrous health-beneficial phytochemicals. Not Sci Biol. 2022, 14, 11212. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. The importance of flavonoids and phytochemicals of medicinal plants with antiviral activities. Mini Rev Org Chem. 2022, 19, 293–318. [Google Scholar] [CrossRef]
- Duan, X.; Li, J.; Cui, J.; Li, H.; Hasan, B.; Xin, X. Chemical component and in vitro protective effects of Matricaria chamomilla (L.) against lipopolysaccharide insult. J Ethnopharmacol. 2022, 296, 115471. [Google Scholar] [CrossRef]
- Karam, T.K.; Ortega, S.; Nakamura, T.U.; Auzely-Velty, R.; Nakamura, C.V. Development of chitosan nanocapsules containing essential oil of Matricaria chamomilla L. for the treatment of cutaneous leishmaniasis. Int J Biol Macromol. 2020, 162, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Heidarianpour, A.; Mohammadi, F.; Keshvari, M.; Mirazi, N. Ameliorative effects of endurance training and Matricaria chamomilla flowers hydroethanolic extract on cognitive deficit in type 2 diabetes rats. Biomed Pharmacother. 2021, 135, 111230. [Google Scholar] [CrossRef]
- Braga, A.S.; Simas, L.L.D.M.; Pires, J.G.; Souza, B.M.; Melo, F.P.D.S.R.; Saldanha, L.L.; Dokkedal, A.L.; Magalhaes, A.C. Antibiofilm and anti-caries effects of an experimental mouth rinse containing Matricaria chamomilla L. extract under microcosm biofilm on enamel. J Dent. 2020, 99, 103415. [Google Scholar] [CrossRef] [PubMed]
- Shebbo, S.; Joumaa, M.E.; Kawach, R.; Borjac, J. Hepatoprotective effect of Matricaria chamomilla aqueous extract against 1,2-Dimethylhydrazine-induced carcinogenic hepatic damage in mice. Heliyon. 2020, 6, e04082. [Google Scholar] [CrossRef]
- Ahmad, S.; Azhar, A.; Tikmani, P.; Rafique, H.; Khan, A.; Mesiya, H.; Saeed, H. A randomized clinical trial to test efficacy of chamomile and saffron for neuroprotective and anti-inflammatory responses in depressive patients. Heliyon. 2022, 8, e10774. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Ling, C.; Wang, C.; Wang, H.; Su, L.; Yang, L.; Jiang, W.; Yu, X.; Zheng, L.; Feng, Z.; et al. Analysis of terpenoid biosynthesis pathways in German chamomile (Matricara recutita) and Roman chamomle (Chamaemelum nobile) based on co-expression networks. Genomics. 2020, 112, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Habibabad, H.Z.; Afrasiabifar, A.; Mansourian, A.; Mansourian, M.; Hosseini, N. Effect of chamomile aromatherapy with and without oxygen on pain of women in post cesarean section with spinal anesthesia: A randomized clinical trial. Heliyon. 2023, 9, e15323. [Google Scholar] [CrossRef] [PubMed]
- Habibzadeh, S.; Zohalinezhad, M.E. Antiviral activity of Matricaria chamomilla in the treatment of COVID-19: Molecular docking study. Eur J Integr Med. 2021, 48, 101975. [Google Scholar] [CrossRef]
- Altiparmaki, G.; Vasileiadou, M.A.; Vakalis, S. The effect of excess water on the hydrothermal carbonization of anise waste from ouzo production on Lesvos island. Sustain Chem Pharm. 2022, 29, 100831. [Google Scholar] [CrossRef]
- Ashry, A.M.; Habiba, M.M.; El-Zayat, A.M.; Hassan, A.M.; Moonmanee, T.; Doan, H.V.; Shadrack, R.S.; Dawood, M.A.O. Dietary anise (Pimpinella anisum L.) enhances growth performance and serum immunity of European sea bass (Dicentrarchus labrax). Aquac Rep. 2022, 23, 101083. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Bohorquez, A.R.R.; Stashenko, E.E. Three-component imino Diels-Alder reaction with essential oil and seeds of anise: Generation of new tetrahydroquinolines. Tetrahedron Lett. 2007, 48, 8855–8860. [Google Scholar] [CrossRef]
- Shori, A.B. Proteolytic activity, antioxidant, and α-Amylase inhibitory activity of yogurt enriched with coriander and cumin seeds. LWT. 2020, 133, 109912. [Google Scholar] [CrossRef]
- Iannarelli, R.; Marinelli, O.; Morelli, M.B.; Santoni, G.; Amantini, C.; Nabissi, M.; Maggi, F. Aniseed (Pimpinella anisum L.) essential oil reduces pro-inflammatory cytokines and stimulates mucus secretion in primary airway bronchial and tracheal epithelial cell lines. Ind Crop Prod. 2018, 114, 81–86. [Google Scholar] [CrossRef]
- Samojlik, I.; Mijatovic, V.; Petkovic, S.; Skrbic, B.; Bozin, B. The influence of essential oil of aniseed (Pimpinella anisum L.) on drug effects on the central nervous system. Fitoterapia, 2012, 83, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Nikoletta, N.; Despoina, Z.; Maria, A.D.; Efimia, P.M.; Urania, M.-S.; Nikolaos, M. Anise, parsley and rocket as nematicidal soil amendments and their impact on non-target soil organisms. Appl Soil Ecol. 2019, 143, 17–25. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Anise (Pimpinella anisum L.), a dominant spice and traditional medicinal herb for both food and medicinal purposes. Cogent Biol, 2019, 5, 1673688. [Google Scholar] [CrossRef]
- Ghazy, O.A.; Fouad, M.T.; Saleh, H.H.; Kholif, A.E.; Morsy, T.A. Ultrasound-assisted preparation of anise extract nanoemulsion and its bioactivity against different pathogenic bacteria. Food Chem. 2021, 341, 128259. [Google Scholar] [CrossRef] [PubMed]
- Goksen, G.; Ekiz, H.I. Use of aniseed cold-pressed by-product as a food ingredient in muffin formulation. LWT, 2021, 148, 111722. [Google Scholar] [CrossRef]
- Willocx, M.; Beeten, I.V.D.; Asselman, P.; Delgat, L.; Baert, W.; Janssens, S.B.; Leliaert, F.; Picron, J.-F.; Vahee, C. Sorting out the plants responsible for a contamination with pyrrolizidine alkaloids in spice seeds by means of LC-MS/MS and DNA barcoding: Proof of principle with cumin and anise spice seeds. Food Chem Mol Sci. 2022, 4, 100070. [Google Scholar] [CrossRef]
- Luna, C.; Chavez, V.H.G.; Barriga-Castro, E.D.; Nunez, N.O.; Mendoza-Resendez, R. Biosynthesis of silver fine particles and particles decorated with nanoparticles using the extract of Illicium verum (star anise) seeds. Spectrochim Acta A Mol Biomol Spectrosc. 2015, 141, 43–50. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Cohen, S.M.; Eisenbrand, G.; Fukushima, S.; Gooderham, N.J.; Guengerich, F.P.; Hecht, S.S.; Rosol, T.J.; Davidsen, J.M.; Harman, C.L.; et al. FEMA GRAS assessment of natural flavor complexes: Allspice, anise, fennel-derived and related flavoring ingredients. Food Chem Toxicol. 2023, 174, 113643. [Google Scholar] [CrossRef] [PubMed]
- Kreydiyyeh, S.I.; Usta, J.; Knio, K.; Markossian, S.; Dagher, S. Aniseed oil increases glucose absorption and reduces urine output in the rat. Life Sci. 2003, 74, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Jafari, R.; Zandi, M.; Ganjloo, A. Effect of ultrasound and microwave pretreatments on extraction of anise (Pimpinella anisum L.) seed essential oil by ohmic-assisted hydrodistillation. J Appl Res Med Aromat Plants. 2022, 31, 100418. [Google Scholar] [CrossRef]
- Balbino, S.; Repajic, M.; Obranovic, M.; Medved, A.M.; Tonkovic, P.; Dragovic-Uzelac, V. Characterization of lipid fraction of Apiaceae family seed spices: Impact of species and extraction method. J Appl Res Med Aromat Plants. 2021, 25, 100326. [Google Scholar] [CrossRef]
- Lee, H.-S. Food protective effect of acaricidal components isolated from anise seeds against the stored food mite, Tyrophagus putrescentiae (Schrank). J Food Protect. 2005, 68, 1208–1210. [Google Scholar] [CrossRef]
- Yazdi, F.F.; Ghalamkari, G.; Toghiani, M.; Modaresi, M.; Landy, N. Anise seed (Pimpinella anisum L.) as an alternative to antibiotic growth promoters on performance, carcass traits and immune responses in broiler chicks. Asian Pac J Trop Dis. 2014, 4, 447–451. [Google Scholar] [CrossRef]
- Canto-Tejero, M.; Pascual-Villalobos, M.J.; Guirao, P. Aniseed essential oil botanical insecticides for the management of the currant-lettuce aphid. Ind Crop Prod. 2022, 181, 114804. [Google Scholar] [CrossRef]
- Gamberini, M.T.; Rodrigues, D.S.; Rodrigues, D.; Pontes, V.B. Effects of the aqueous extract of Pimpinella anisum L. seeds on exploratory activity and emotional behavior in rats using the open field and elevated plus maze tests. J Ethnopharmacol. 2015, 168, 45–49. [Google Scholar] [CrossRef]
- Lodhi, B.A.; Hussain, M.A.; Ashraf, M.U.; Haseeb, M.T.; Muhammad, G.; Farid-ul-Haq, M.; Naeem-ul-Hassan, M. Basil (Ocimum basilicum L.) seeds engender a smart material for intelligent drug delivery: On-Off switching and real-time swelling, in vivo transit detection, and mechanistic studies. Ind Crops Prod. 2020, 155, 112780. [Google Scholar] [CrossRef]
- Kolvari, E.; Koukabi, N.; Ozmaei, Z.; Khoshkho, H.; Seidi, F. Synthesis of 2-amino-4H-pyran and 2-benzylidene malononitrile derivatives using a basil seed as a cheap, natural and biodegradable catalyst. Curr Res Green Sustain Chem. 2022, 5, 100327. [Google Scholar] [CrossRef]
- Mahdavi, H.; Marandi, A.; Karami, M.; Heidari, A.A. Efficient dye rejection using a mixed matrix polyphenylsulfone/polysulfone membrane containing basil seed mucilage hydrogel. J Environ Chem Engin. 2022, 10, 108767. [Google Scholar] [CrossRef]
- Nazir, S.; Wani, I.A. Fractionation and characterization of mucilage from Basil (Ocimum basilicum L.) seed. J Appl Res Med Aromat Plants. 2022, 31, 100429. [Google Scholar] [CrossRef]
- Nosouhian, E.; Hojjatoleslamy, M.; Goli, M.; Jafari, M.; Kiani, H. The effect of periodate oxidation of basil seed gum and its addition on protein binding. Int J Biol Macromol. 2023, 240, 124298. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.; Singhal, R.S.; Kulkarni, P.R. Ocimum basilicum: A new non-conventional source of fiber. Food Chem. 1993, 47, 399–401. [Google Scholar] [CrossRef]
- Osano, J.P.; Hosseini-Parvar, S.H.; Matia-Merino, L.; Golding, M. Emulsifying properties of a novel polysaccharide extracted from basil seed (Ocimum bacilicum L.): Effect of polysaccharide and protein content. Food Hydrocoll. 2014, 37, 40–48. [Google Scholar] [CrossRef]
- Kurd, F.; Fathi, M.; Shekarchizadeh, H. Nanoencapsulation of hesperetin using basil seed mucilage nanofibers: Characterization and release modeling. Food Biosci. 2019, 32, 100475. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Eskandari, M.H.; Meeren, P.V.D.; Hosseini, S.M.H. Study on hydrophobic modification of basil seed gum-based (BSG) films by octenyl succinate anhydride (OSA). Carbohydr Polym. 2019, 219, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Hyeonbin, O.; Lee, P.; Kim, Y.-S. The quality characteristics, antioxidant activity, and sensory evaluation of reduced-fast yogurt and nonfat yogurt supplemented with basil seed gum as a fat substitute. J Dairy Sci. 2020, 103, 1324–1336. [Google Scholar] [CrossRef]
- Imam, H.; Lian, S.; Kasimu, R.; Rakhmanberdyeva, R.K.; Aisa, H.A. Extraction of an antidiabetic polysaccharide from seeds of Ocimum basilicum and determination of the monosaccharide composition by precolumn high-efficiency capillary electrophoresisa. Chem Nat Compd. 2012, 48, 653–654. [Google Scholar] [CrossRef]
- Gajendiran, A.; Abraham, J.; Thangaraman, V.; Thangamani, S.; Ravi, D. Antimicroial, antioxidant and anticancer screening of Ocimum basilicum seeds. Bull Pharm Res. 2016, 6, 114–119. [Google Scholar]
- Simon, J.E.; Morales, M.R.; Phippen, W.B.; Vieira, R.F.; Hao, Z. Basil: A source of aroma compounds and a popular culinary and ornamental herb. In Perspectives on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; pp. 499–505. [Google Scholar]
- Amini, G.; Salehi, F.; Rasouli, M. Color changes and drying kinetics modeling of basil seed mucilage during infrared drying process. Inform Process Agric. 2022, 9, 397–405. [Google Scholar] [CrossRef]
- Salehi, F.; Kashaninejad, M. Statis rheological study of Ocimum basilicum seed gum. Int J Food Eng, 2015, 11, 97–103. [Google Scholar] [CrossRef]
- Zhong, H.; Gao, X.; Zhang, X.; Chen, A.; Qiu, Z.; Kong, X.; Huang, W. Minimizing the filtration loss of water-based drilling fluid with sustaiable basil seed powder. Petroleum, 2022, 8, 39–52. [Google Scholar] [CrossRef]
- Hosseini-Parvar, S.; Matia-Merino, L.; Goh, K.K.; Razavi, S.; Mortazavi, S.A. Steady shear flow behavior of gum extracted from Ocimum basilicum L. seed: Effect of concentration and temperature. J Food Eng. 2010, 101, 236–243. [Google Scholar] [CrossRef]
- Razavi, S.M.; Bostan, A.; Rezaie, M. Image processing and physico-mechanical properties of basil seed (Ocimum basilicum). J Food Process Eng. 2010, 33, 51–64. [Google Scholar] [CrossRef]
- Bhadange, Y.A.; Saharan, V.K. Optimization and kinetic studies of D-galacturonic acid extraction from basil seed using different extraction techniques. Sustain Chem Pharm. 2023, 33, 101080. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A. New studies on basil (Ocimum bacilicum L.) seed gum: Part II-Emulsifying and foaming characterization. Carbohydr Polym. 2016, 149, 140–150. [Google Scholar] [CrossRef]
- Munir, M.; Qayyum, A.; Raza, S.; Siddiqui, N.R.; Mumtaz, A.; Safdar, N.; Shible, S.; Afzal, S.; Bashir, S. Nutritional assessment of basil seeds and its utilization in development of value added beverage. Pak J Agric Res. 2017, 30, 266–271. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A. New studies on basil (Ocimum bacilicum L.) seed gum: Part III- Steady and dynamic shear rheology. Food Hydrocoll. 2017, 67, 243–250. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Asnaashari, M.; Salahi, M.R.; khosravi Rad, T. Effects of basil seed gum, cress seed gum and quince seed gum on the physical, textural and rheological properties of whipped cream. Int J Biol Macromol. 2017, 98, 820–828. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.-R.; Du, Y.-N.; Chen, H.-Q. Heat-induced arachin and basil seed gum composite gels improved by NaCl and microbial transglutaminase: Gelling properties and structure. Food Hydrocoll. 2023, 135, 108200. [Google Scholar] [CrossRef]
- Hosseini-Parvar, S.; Matia-Merino, L.; Golding, M. Effect of basil seed gum (BSG) on textural rheological and microstructural properties of model processed cheese. Food Hydrocoll. 2015, 43, 557–567. [Google Scholar] [CrossRef]
- Movahedi, H.; Farahani, M.V.; Jamshidi, S. Application of hydrated basil seeds (HBS) as the herbal fiber on hole cleaning and filtration control. J Petrol Sci Engin. 2017, 152, 212–228. [Google Scholar] [CrossRef]
- Mirabolhassani, S.E.; Rafe, A.; Razavi, S.M.A. The influence of temperature, sucrose and lactose on dilute solution properties of basil (Ocimum basilicum) seed gum. Int J Biol Macromol. 2016, 93, 623–629. [Google Scholar] [CrossRef]
- Razi, S.M.; Motamedzadegan, A.; Matia-Merino, L.; Shahidi, S.-A.; Rashidinejad, A. The effect of pH and high-pressure processing (HPP) on the rheological properties of egg whie albumin and basil seed gum mixtures. Food Hydrocoll. 2019, 94, 399–410. [Google Scholar] [CrossRef]
- Maqsood, H.; Uroos, M.; Muazzam, R.; Naz, S.; Muhammad, N. Extraction of basil seed mucilage using ionic liquid and preparation of AuNps/mucilage nanocomposite for catalytic degradation of dye. Int J Biol Macromol. 2020, 164, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Nazir, S.; Wani, I.A. Functional characterization of basil (Ocimum basilicum L.) seed mucilage. Bioact Carbohydr Diet Fibre. 2021, 25, 100261. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A.; Mohebbi, M.; Malaekeh-Nikouei, B. New studies on basil (Ocimum bacilicum L.) seed gum: Part I- Fractionation, physicochemical and surface activity characterization. Food Hydrocoll. 2016, 52, 350–358. [Google Scholar] [CrossRef]
- Khazaei, N.; Esmaiili, M.; Emam-Djomeh, Z. Effect of active edible coatings made by basil seed gum and thymol on oil uptake and oxidation in shrimp during deep-fat frying. Carbohydr Polym. 2016, 137, 249–254. [Google Scholar] [CrossRef]
- Rayegan, A.; Allafchian, A.; Sarsari, I.A.; Kameli, P. Synthesis and characterization of basil seed mucilage coated Fe3O4 magnetic nanoparticles as a drug carrier for the controlled delivery of cephalexin. Int J Biol Macromol. 2018, 113, 317–328. [Google Scholar] [CrossRef]
- Lodhi, B.A.; Abbas, A.; Hussain, M.A.; Hussan, S.Z.; Sher, M.; Hussain, I. Design, characterization and appraisal of chemically modified polysaccharide based mucilage from Ocimum basilicum (basil) seeds for the removal of Cd(II) from spiked high-hardness ground water. J Mol Liq. 2019, 274, 15–24. [Google Scholar] [CrossRef]
- Ziemichod, A.; Wojcik, M.; Rozylo, R. Ocimum tenuiflorum seeds and Salvia hispanica seeds: Mineral and amino acid composition, physical properties, and use in gluten-free bread. CyTA J Food. 2019, 17, 804–813. [Google Scholar] [CrossRef]
- Gao, X.; Zhong, H.-Y.; Zhang, X.-B.; Chen, A.-L.; Qiu, Z.-S.; Huang, W.-A. Application of sustainable basil seed as an eco-friendly multifunctional additive for water-based drilling fluids. Pet Sci. 2021, 18, 1163–1181. [Google Scholar] [CrossRef]
- Uematsu, Y.; Ogata, F.; Nagai, N.; Saenjum, C.; Nakamura, T.; Kawasaki, N. In vitro removal of paraquat and diquat from aqueous media using raw and calcined basil seed. Heliyon. 2021, 7, e07644. [Google Scholar] [CrossRef] [PubMed]
- Bejeshk, M.A.; Aminizadeh, A.H.; Rajizadeh, M.A.; Khaksari, M.; Lashkarizadeh, M.; Shahrokhi, N.; Zahedi, M.J.; Azimi, M. The effect of combining basil seeds and gum Arabic on the healing process of experimental acetic acid-induced ulcerative colitis in rats. J Tradit Complement Med. 2022, 12, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Semwal, A.; Kumar, H.; Verma, H.C.; Kumar, A. In-vivo study for anti-hyperglycemic potential of aqueous extract of Basil seeds (Ocimum basilicum Linn) and its influence on biochemical parameters, serum electrolytes and haematological indices. Biomed Pharmacother. 2016, 84, 2008–2013. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, H.; Amid, B.T. A review study on chemical composition and molecular structure of newly plant gum exudates and seed gums. Food Res Int. 2012, 46, 387–398. [Google Scholar] [CrossRef]
- Salehi, F.; Kashaninejad, M.; Tadayyon, A.; Arabameri, F. Modeling of extraction process of crude polysaccharides from Basil seeds (Ocimum basilicum L.) as affected by process variables. J Food Sci Technol. 2014, 52, 5220–5227. [Google Scholar] [CrossRef]
- Javanmardi, J.; Stushnoff, C.; Locke, E.; Vivanco, J.M. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- Mabood, F.; Gilani, S.A.; Hussain, J.; Alshidani, S.; Alghawi, S.; Albroumi, M.; Alameri, S.; Jabeen, F.; Hussain, Z.; Al-Harrasi, A.; et al. New design of experiment combined with UV-Vis spectroscopy for extraction and estimation of polyphenols from Basil seeds, Red seeds, Sesame seeds and Ajwan seeds. Spectrochim Acta Part A Mol Biomol Spectrosc. 2017, 178, 14–18. [Google Scholar] [CrossRef]
- Vieira, R.F.; Simon, J.E. Chemical characterization of basil (Ocimum spp.) found in the markets and used in traditional medicine in Brazil. Econ Bot. 2000, 54, 207–216. [Google Scholar] [CrossRef]
- Galle, A.M.; Joseph, M.; Demandre, C.; Guerche, P.; Dubacq, J.P.; Qursel, A.; Mazliak, P.; Pelletier, G.; Kader, J.C. Biosynthesis of γ-linolenic acid in developing seeds of boragae (Borago officinalis L.). Biochim Biophys Acta Gen Subj. 1993, 1158, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.M.; Ossa, E.M.D.I. Quality of borage seed oil extracted by liquid and supercritical carbon dioxide. Chem Engin J. 2002, 88, 103–109. [Google Scholar] [CrossRef]
- Feghhenabi, F.; Hadi, H.; Khodaverdiloo, H.; Genuchten, M.T.V. Borage (Borago officinalis L.) response to salinity at early growth stages as influenced by seed pre-treatment. Agric Water Manag. 2021, 253, 106925. [Google Scholar] [CrossRef]
- Soto, C.; Concha, J.; Zuniga, M.E. Antioxidant content of oil and defatted meal obtained from borage seeds by an enzymatic-aided cold pressing process. Process Biochem. 2008, 43, 696–699. [Google Scholar] [CrossRef]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Korma, S.A.; Khan, I.M.; Mohsin, A.; Manzoor, M.F.; Ashraf, W.; Mushtaq, B.S.; Zainab, S.; et al. Spray dried nanoemulsions loaded with curcumin, resveratrol, and borage seed oil: The role of two different modified starches as encapsulating materials. Int J Biol Macromol. 2021, 186, 820–828. [Google Scholar] [CrossRef]
- Sattler, M.; Muller, V.; Bunzel, D.; Kulling, S.E.; Soukup, S.T. Pyrrolizidine alkaloids in borage (Borago officinalis): Comprehensive profiling and development of a validated LC-MS/MS method for quantification. Talanta. 2023, 258, 124425. [Google Scholar] [CrossRef]
- Galle, A.-M.; Oursel, A.; Joseph, M.; Kader, J.-C. Solubilization of membrane bound Δ12-and Δ6-fatty acid desaturases from borage seeds. Phytochemistry. 1997, 45, 1587–1590. [Google Scholar] [CrossRef]
- Hafid, R.E.; Blade, S.F.; Hoyano, Y. Seeding date and nitrogen fertilization effects on the performance of borage (Borago officinalis L.). Ind Crop Prod. 2002, 16, 193–199. [Google Scholar] [CrossRef]
- Gilbertson, P.K.; Berti, M.T.; Johnson, B.L. Borage cardinal germination temperatures and seed development. Ind Crop Prod. 2014, 59, 202–209. [Google Scholar] [CrossRef]
- Morteza, E.; Akbari, G.-A.; Moaveni, P.; Alahdadi, I.; Bihamta, M.-R.; Hasanloo, T.; Joorabloo, A. Compositions of the seed oil of the Borago officinalis from Iran. Nat Prod Res. 2015, 29, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Kawish, S.M.; Hasan, N.; Beg, S.; Qadir, A.; Jain, G.K.; Aqil, M.; Ahmad, F.J. Docetaxel-loaded borage seed oil nanoemulsion with improved antitumor activity for solid tumor treatment: Formulation development, in vitro, in silico and in vivo evaluation. J Drug Deliv Sci Technol. 2022, 75, 103693. [Google Scholar] [CrossRef]
- Rehman, A.; Jafari, S.M.; Tong, Q.; Karim, A.; Mahdi, A.A.; Iqbal, M.W.; Aadil, R.M.; Ali, A.; Manzoor, M.F. Role of peppermint oil in improving the oxidative stability and antioxidant capacity of borage oil-loaded nanoemulsions fabricated by modified starch. Int J Biol Macromol. 2020, 153, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Wettasinghe, M.; Shahidi, F. Antioxidant and free radical-scavenging properties of ethanolic extracts of defatted borage (Borago officinalis L.) seeds. Food Chem. 1999, 67, 399–414. [Google Scholar] [CrossRef]
- Lopez-Martinez, J.C.; Campra-Madrid, P.; Guil-Guuerrero, J.L. γ-Linolenic acid enrichment from Borago officinalis and Echium fastuosum seed oils and fatty acids by low temperature. J Biosci Bioeng. 2004, 97, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Dauksas, E.; Venskutonis, P.R.; Sivik, B. Supercritical fluid extraction of borage (Borago officinalis L.) seeds with pure CO2 and its mixture with caprylic acid methyl ester. J Supercrit Fluid. 2002, 22, 211–219. [Google Scholar] [CrossRef]
- Lu, T.; Gaspar, F.; Marriott, R.; Mellor, S.; Watkinson, C.; Al-Duri, B.; Seville, J.; Santos, R. Extraction of borage seed oil by compressed CO2: Effect of extraction parameters and modelling. J Supercrit Fluid. 2007, 41, 68–73. [Google Scholar] [CrossRef]
- Wettasinghe, M.; Shahidi, F.; Amarowicz, R.; Abou-Zaid, M.M. Phenolic acids in defatted seeds of borage (Borago officinalis L.). Food Chem. 2001, 75, 49–56. [Google Scholar] [CrossRef]
- Mhamdi, B.; Wannes, W.A.; Sriti, J.; Jellali, I.; Ksouri, R.; Marzouk, B. Effect of harvesting time on phenolic compounds and antiradical scavening activity of Borago officinalis seed extracts. Ind Crop Prod. 2010, 31, e1–e4. [Google Scholar] [CrossRef]
- Abdel-Salam, A.M.; Al Hemaid, W.A.; Afifi, A.A.; Othman, A.I.; Farrag, A.R.H.; Zeitoun, M.M. Consolidating probiotic with dandelion, coriander and date palm seeds extracts against mercury neurotoxicity and for maintaining normal testosterone levels in male rats. Toxicol Rep. 2018, 5, 1069–1077. [Google Scholar] [CrossRef]
- Grosso, C.; Ferraro, V.; Figueiredo, A.C.; Barroso, J.G.; Coelho, J.A.; Palavra, A.M. Supercritical carbon dioxide extraction of volatile oil from Italian coriander seeds. Food Chem. 2008, 111, 197–203. [Google Scholar] [CrossRef]
- Illes, V.; Daood, H.G.; Perneczki, S.; Szokonya, L.; Then, M. Extraction of coriander seed oil by CO2 and propane at super- and subcritical conditions. J Supercrit Fluid. 2000, 17, 177–186. [Google Scholar] [CrossRef]
- Moser, B.P.; Vaughn, S.F. Corianer seed oil methyl esters as biodiesel fuel: Unique fatty acid composition and excellent oxidative stability. Biomass Bioenergy. 2010, 34, 550–558. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Pickett, K.M.; Caldwell, C.D.; Pincock, J.A.; Roberts, J.C.; Mapplebeck, L. Cultivar and sowing date effects on seed yield and oil composition of coriander in Atlantic Canada. Ind Crops Prod. 2008, 28, 88–94. [Google Scholar] [CrossRef]
- Sekhon, J.K.; Maness, N.O.; Jones, C.L. Effect of compressed propane extraction on storage stability of dried cilantro (Coriandrum sativum L.). J Food Eng. 2016, 178, 159–169. [Google Scholar] [CrossRef]
- Siddappa, S.; Basrur, V.; Rai, V.R.; Marathe, G.K. Biochemical and functional characterization of an atypical plant L-arginase from Cilantro (Coriandrum sativum L.). Int J Biol Macromol. 2018, 118, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Z.; Ding, X.; Khan, S.; Ayaz, T.; Fidel, R.; Khan, M.A. Biochar efficacy for reduing heavy metals uptake by Cilantro (Coriandrum sativum) and spinach (Spinaccia oleracea) to minimize human health risk. Chmosphere. 2020, 244, 125543. [Google Scholar] [CrossRef] [PubMed]
- Bardsley, C.A.; Boyer, R.R.; Rideout, S.L.; Strawn, L.K. Survival of Listeria monocytogenes on the surface of basil, cilantro, dill, and parsley plants. Food Control 2019, 95, 90–94. [Google Scholar] [CrossRef]
- Pullagurala, V.L.R.; Adisa, I.O.; Rawat, S.; Kalagara, S.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. ZnO nanoparticles increase photosynthetic pigments and decrease lipid peroxidation in soil grown cilantro (Coriandrum sativum). Plant Physiol Biochem. 2018, 132, 120–127. [Google Scholar] [CrossRef]
- Sekhon, J.K.; Maness, N.O.; Jones, C.L. Effect of preprocessing and compressed propane extraction on quality of cilantro (Coriandrum sativum L.). Food Chem. 2015, 175, 322–328. [Google Scholar] [CrossRef]
- Song, E.-J.; Ko, M.-J. Extraction of monoterpenes from coriander (Coriandrum sativum L.) seeds using subcritical water extraction (SWE) technique. J Supercrit Fluids. 2022, 188, 105668. [Google Scholar] [CrossRef]
- Wong, P.Y.Y.; Kitts, D.D. Studies on the dal antioxidant and antibacterial properties of parsely (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006, 97, 505–515. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Wahdan, K.M.M. Blending of corn oil with black cumin (Nigella sativa) and coriander (Coriandrum sativum) seed oils: Impact on functionality, stability and radical scavenging activity. Food Chem. 2012, 132, 873–879. [Google Scholar] [CrossRef]
- Beyzi, E.; Karaman, K.; Gunes, A.; Beyzi, S.B. Change in some biochemical and bioactive properties and essential oil composition of coriander seed (Coriandrum sativum L.) varieties from Turkey. Ind Crop Prod. 2017, 109, 74–78. [Google Scholar] [CrossRef]
- Marmitt, D.; Shahrajabian, M.H. Plant species used in Brazil and Asia regions with toxic properties. Phytother Res. 2021, 2021, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zekovic, Z.; Pavlic, B.; Cvetanovic, A.; Durovic, S. Supercritical fluid extraction of coriander seeds: Process optimization, chemical profile and antioxidant activity of lipid extracts. Ind Crops Prod. 2016, 94, 353–362. [Google Scholar] [CrossRef]
- Coskuner, Y.; Karababa, E. Physical properties of coriander seeds (Coriandrum sativum L.). J Food Engin. 2007, 80, 408–416. [Google Scholar] [CrossRef]
- Mhemdi, H.; Rodier, E.; Kechaou, N.; Fages, J. A supercritical tuneable process for the selective extraction of fats and essential oil from coriander seeds. J Food Engin. 2011, 105, 609–616. [Google Scholar] [CrossRef]
- Carrubba, A.; Lombardo, A. Plant structure as a determinant of coriander (Coriandrum sativum L.) seed and straw yield. Eur J Agron. 2020, 113, 125969. [Google Scholar] [CrossRef]
- Donega, M.A.; Mello, S.C.; Moraes, R.M.; Cantrell, C.L. Nutrient uptake, biomass yield and quantitative analysis of aliphatic aldehydes in cilantro plants. Ind Crop Prod. 2013, 44, 127–131. [Google Scholar] [CrossRef]
- Lasram, S.; Zemni, H.; Hamdi, Z.; Chenenaoui, S.; Houissa, H.; Tounsi, M.S.; Ghorbel, A. Antifungal and antiaflatoxinogenic activities of Carum carvi L., Coriandrum sativum L. seed essential oils and their major terpene component against Aspergillus flavus. Ind Crop Prod. 2019, 134, 11–18. [Google Scholar] [CrossRef]
- Zekovic, Z.; Busic, A.; Komes, D.; Vadic, J.; Adamovic, D.; Pavlic, B. Coriander seeds processing: Sequential extraction of non-polar and polar fractions using supercritical carbon dioxide extraction and ultrasound-assisted extraction. Food Bioprod Process. 2015, 95, 218–227. [Google Scholar] [CrossRef]
- Delaquis, P.J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int J Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Upadhyay, N.; Singh, P.; Sharma, S.; Dubey, N.K. Encapsulation in chitosan-based nanomatrix as an efficienct green technology to boost the antimicrobial, antioxidant and in situ efficacy of Coriandrum sativum essential oil. Int J Biol Macromol. 2019, 133, 294–305. [Google Scholar] [CrossRef]
- Eikani, M.H.; Golmohammad, F.; Rowshanzamir, S. Subcritical water extraction of essential oils from coriander seeds (Coriandrum sativum L.). J Food Engin. 2007, 80, 735–740. [Google Scholar] [CrossRef]
- Zekovic, Z.; Vidovic, S.; Vladic, J.; Radosavljevic, R.; Cvejin, A.; Elgndi, M.A.; Pavlic, B. Optimization of subcritical water extraction of antioxidants from Coriandrum sativum seeds by response surface methodology. J Supercrit Fluid. 2014, 95, 560–566. [Google Scholar] [CrossRef]
- Shokri, A.; Hatami, T.; Khamforoush, M. Near critical carbon dioxide extraction of anise (Pimpinella anisum L.) seed: Mathematical and artificial neural network modeling. J Supercrit Fluid. 2011, 58, 49–57. [Google Scholar] [CrossRef]
- Ghazanfari, N.; Yazdi, F.T.; Mortazavi, S.A.; Mohammadi, M. Using pulsed electric field pre-treatment to optimize coriander seeds essential oil extraction and evaluate antimicrobial properties, antioxidant activity, and essential oil compositions. LWT 2023, 182, 114852. [Google Scholar] [CrossRef]
- Mandal, Sh.; Mandal, M. Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pac J Tropical Biomed. 2015, 5, 421–428. [Google Scholar] [CrossRef]
- Laribi, B.; Kouki, K.; M/Hamdi, M.; Bettaieb, T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia. 2015, 103, 9–26. [Google Scholar] [CrossRef]
- Ghazanfari, N.; Mortazavi, S.A.; Yazdi, F.T.; Mohammadi, M. Microwave-assisted hydrodistillation extraction of essential oil from coriander seeds evaluation of their composition, antioxidant and antimicrobial activity. Heliyon. 2020, 6, e04893. [Google Scholar] [CrossRef]
- Sahoo, S.; Brijesh, S. Anxiolytic activity of Coriandrum sativum seeds aqueous extract on chronic restraint stressed mice and effect on brain neurotransmitters. J Funct Food. 2020, 68, 103884. [Google Scholar] [CrossRef]
- Mavandi, P.; Zarifi, E. Karyomorphological study and its correlation with the quantity and quality of essential oil in Iranian chamomile accessions (Matricaria chamomilla L.). Biocatal Agric Biotechnol. 2022, 41, 102320. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. The insight and survey on medicinal properties and nutritive components of shallot. J Med Plant Res. 2019, 13, 452–457. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Lin, M. Research progress of fermented functional foods and protein factory-microbial fermentation technology. Fermentation. 2022, 8, 688. [Google Scholar] [CrossRef]
- Rosol, T.J.; Cohen, S.M.; Eisenbrand, G.; Fukushima, S.; Gooderham, N.J.; Guengerich, F.P.; Hecht, S.S.; Rietjens, I.M.C.M.; Davidsen, J.M.; Harman, C.L.; et al. FEMA GRAS assessment of natural flavor complexes: Lemongrass oil, chamomile oils, citronella oil and related flavoring ingredients. Food Chem Toxicol. 2023, 175, 113697. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Soleymani, A.; Cheng, Q. Traditional herbal medicines to overcome stress, anxiety and improve mental health in outbreaks of human coronaviruses. Phytother Res. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of Basil (Ocimum basilicum): A review. Int J Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Traditional herbal medicine for the prevention and treatment of cold and flu in the autumn of 2020, overlapped with Covid-19. Nat Prod Commun. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Pandey, M.; Gupta, S. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sci. 2009, 85, 663–669. [Google Scholar] [CrossRef]
- Katsoulis, G.I.; Kimbaris, A.C.; Anastasaki, E.; Damalas, C.A.; Kyriazopoulos, A.P. Chamomile and anise cultivation in olive agroforestry systems. Forests. 2022, 13, 128. [Google Scholar] [CrossRef]
- Almeida, D.J.S.; Alberton, O.; Otenio, J.K.; Carrenho, R. Growth of chamomile (Matricaria chamomilla L.) and production of essential oil stimulated by arbuscular mycorrhizal symbiosis. Rhizosphere. 2020, 15, 100208. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.). Ind Crop Prod. 2013, 44, 437–445. [Google Scholar] [CrossRef]
- Rafii, F.; Ameri, F.; Haghani, H.; Ghobadi, A. The effect of aromatherapy massage with lavender and chamomile oil on anxiety and sleep quality of patients with burns. Burns. 2020, 46, 164–171. [Google Scholar] [CrossRef]
- Ubessi, C.; Tedesco, S.B.; Silva, C.D.B.D.; Baldoni, M.; Krysczun, D.K.; Heinzmann, B.M.; Rosa, I.A.; Mori, N.C. Antiproliferative potential and phenolic compounds of infusions and essential oil of chamomile cultivated with homeopathy. J Ethnopharmacol. 2019, 239, 111907. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Riedl, K.M.; Schwartz, S.J. Effects of food formulation and thermal processing on flavones in celery and chamomile. Food Chem. 2013, 141, 1406–1411. [Google Scholar] [CrossRef]
- Salehi, A.; Tasdighi, H.; Gholamhoseini, M. Evaluation of proline, chlorophyll, soluble sugar content and uptake of nutrients in the German chamomile (Matricaria chamomilla L.) under drought stress and organic fertilizer treatments. Asia Pac J Trop Biomed. 2016, 6, 886–891. [Google Scholar] [CrossRef]
- Mezaka, I.; Kronberga, A.; Nakurte, I.; Taskova, I.; Jakovels, D.; Primavera, A. Genetic, chemical and morphological variability of chamomile (Chamomilla recutita L.) populations of Latvia. Ind Crop Prod. 2020, 154, 112614. [Google Scholar] [CrossRef]
- Nasr, A.M.; Ela, S.E.-D.S.A.; Ismail, I.E.; Aldhahrani, A.; Soliman, M.M.; Alotaibi, S.S.; Bassiony, S.S.; El-Hack, M.E.A. A comparative study among dietary supplementations of antibiotic, grape seed and chamomile oils on growth performance and carcass properties of growing rabbits. Saudi J Biol Sci. 2022, 29, 2483–2488. [Google Scholar] [CrossRef]
- Cvetanovic, A.; Zekovic, Z.; Zengin, G.; Maskovic, P.; Petronijevic, M.; Radojkovic, M. Multidirectional approaches on autofermented chamomile ligulate flowers: Antioxidant, antimicrobial, cytotoxic and enzyme inhibitory effects. S Afr J Bot. 2019, 120, 112–118. [Google Scholar] [CrossRef]
- Petrulova-Poracka, V.; Repcak, M.; Vilkova, M.; Imrich, J. Coumarins of Matricaria chamomilla L.: Aglycones and glycosides. Food Chem. 2013, 141, 54–59. [Google Scholar] [CrossRef]
- Dadashpour, M.; Firouzi-Amandi, A.; Pourhassan-Moghaddam, M.; Maleki, M.J.; Soozangar, N.; Jeddi, F.; Nouri, M.; Zarghami, N.; Pilehvar-Soltanahmadi, Y. Biomimetic synthesis of silver nanoparticles using Matricaria chamomilla extract and their potential anticancer activity against human lung cancer cells. Mater Sci Eng C. 2018, 92, 902–912. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.R.; Ali, F.S.; Turky, A.F. Control of Tetranychus urticae Koch by extracts of three essential oils of chamomile, marjoram and Eucalyptus. Asian Pac J Trop Biomed. 2012, 2, 24–30. [Google Scholar] [CrossRef]
- Baranauskiene, R.; Venskutonis, P.R.; Ragazinskiene, O. Valorisation of Roman chamomile (Chamaemelum nobile L.) herb by comprehensive evaluation of hydrodistilled aroma and residual non-volatile fractions. Food Res Int. 2022, 160, 111715. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Zheng, L.; Yu, X.; Wang, H.; Wang, C.; Wu, H.; Zhang, J.; Yao, P.; Tai, Y.; Yuan, Y. Cloning and functional analysis of three aphid alarm pheromone genes from German chamomile (Matricaria chamomilla L.). Plant Sci. 2020, 294, 110463. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Kumar, R. Agronomic interventions affect the growth, yield, and essential oil composition of German chamomile (Matricaria chamomilla L.) in the western Himalaya. Ind Crop Prod. 2021, 171, 113873. [Google Scholar] [CrossRef]
- Bozo, I.C.M.; Urzua, B.R.; Rojas, G.A.; Lefimil, C.A.; Manriquez, J.M.; Ortega, A.V.; Aitken, J.P.; Salinas, J.O. Evaluation of the efficacy of a chamomile (Matricaria chamomilla) and flax seed (Linum usitatissimum) based salvia substitute formulated for the relief of Xerostomia: A preliminary report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015, 119, e169. [Google Scholar] [CrossRef]
- Cicco, P.D.; Ercolano, G.; Sirignano, C.; Rubino, V.; Rigano, D.; Ianaro, A.; Formisano, C. Chamomile essential oil exert anti-inflammatory effects involving human and murine macrophages: Evidence to support a therapeutic action. J Ethnopharmacol. 2023, 311, 116391. [Google Scholar] [CrossRef]
- Madadi, E.; Fallah, S.; Sadeghbpour, A.; Barani-Beiranvand, H.B. Exploring the use of chamomile (Matricaria chamomilla L.) bioactive compounds to control flixweed (Descurainia sophia L.) in bread wheat (Triticum aestivum L.): Implication for reducing chemical herbicide pollution. Saudi J Biol Sci. 2022, 29, 103421. [Google Scholar] [CrossRef]
- Nemati, S.; Yousefbeyk, F.; Ebrahimi, S.M.; FaghihHabibi, A.F.; Shakiba, M.; Ramezani, H. Effects of chamomile extract nasal drop on chronic rhinosinusitis treatment: A randomized double blind study. Am J Otolaryngol. 2021, 42, 102743. [Google Scholar] [CrossRef]
- Banjaw, D.T.; Wolde, T.G. Effect of seed storage duration and seedling raising method on chamomile seedling establishment. Int J Adv Biol Biomed Res. 2017, 5, 16–18. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W. Importance of thymoquinone, sulforaphane, phloretin, and epigallocatechin and their health benefits. Lett Drug Des Discov. 2023, 19. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W. Survey on medicinal plants and herbs in traditional Iranian medicine with anti-oxidant, anti-viral, anti-microbial, and anti-inflammation properties. Lett Drug Des Discov. 2023, 19. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W. Assessment of wine quality, traceability and detection of grapes wine, detection of harmful substances in alcohol and liquor composition analysis. Lett Drug Des Discov. 2023, 20. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Petropoulos, S.A.; Sun, W. Survey of the influences of microbial biostimulants on horticultural crops: Case studies and successful paradigms. Horticulturae. 2023, 9, 1–24. [Google Scholar] [CrossRef]
- Shakya, P.; Thakur, R.; Sharan, H.; Yadav, N.; Kumar, M.; Chauhan, R.; Kumar, D.; Kumar, A.; Singh, S.; Singh, S. GGE biplot and regression based multi-environment investigations for higher yield and essential oil content in German chamomile (Matricaria chamomilla L.). Ind Crop Prod. 2023, 193, 116145. [Google Scholar] [CrossRef]


| *Anise seed consists of fixed oil, volatile oil, mucilage, proteins and starch. |
| *Essential oil of Anise seeds consists of eugenol trans-anethole, coumarins, anisaldehyde, estragole, scopoletin, umbelliferon, polyacetylenes, estrolterpene, and methyl chavicol anisaldehyde. |
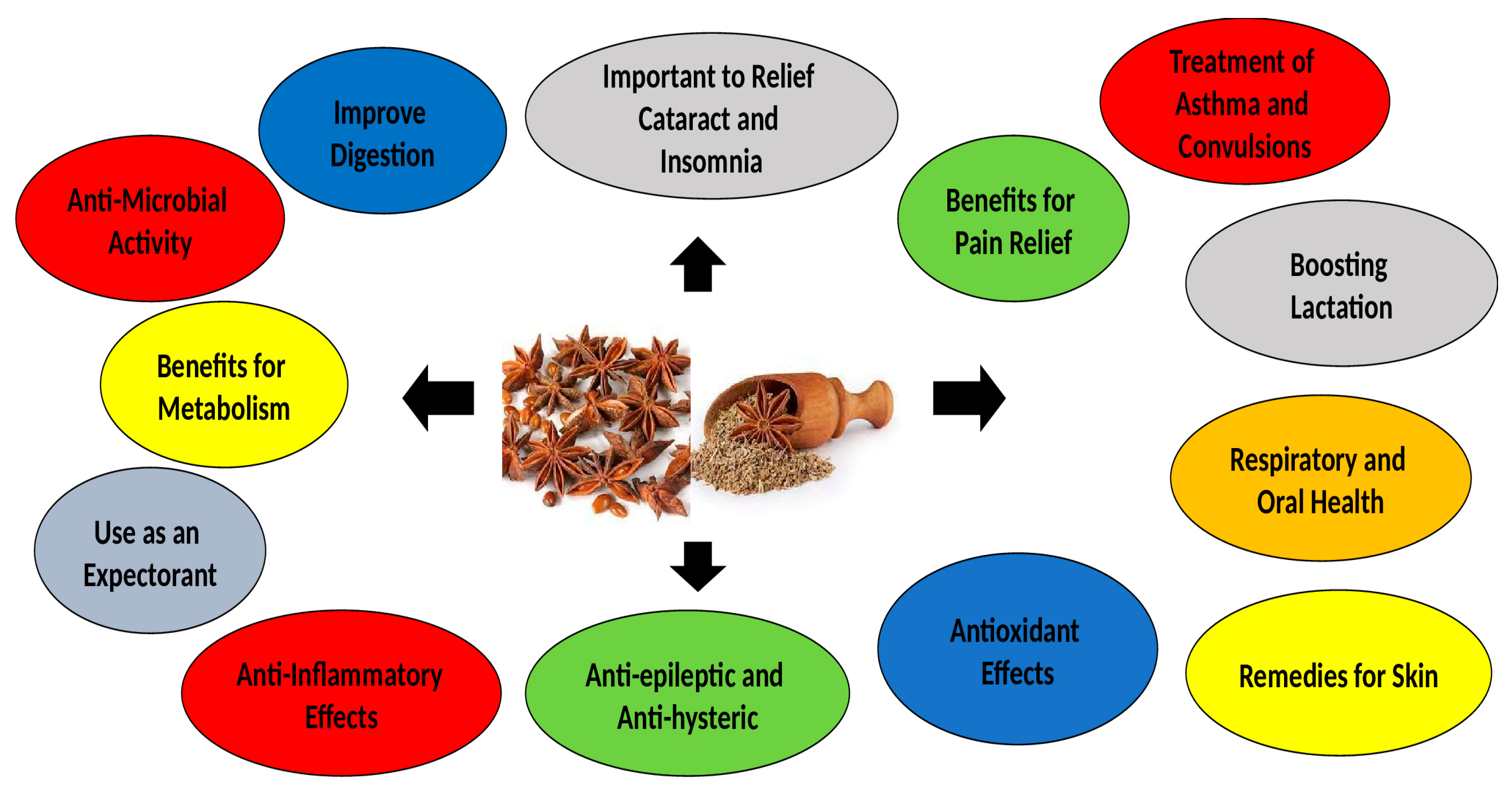
| *Aniseeds contain 1.5-5% essential oil and utilized as flavouring, carminative, digestive, and relief of gastrointestinal spasms. |
| *Aniseeds have different characteristics like as antimicrobial, antiviral, antifungal, antioxidant, and insecticidal effects. |
| *Aniseeds can cause muscle relaxant, gastric protection, and influence digestive system. |
| *In diabetic patients, it has hypolipidemic and hypoglycemic impacts and decreases lipid peroxidation. |
| *Aniseed also has significant impacts on dysmenorrhea and menopausal hot flashes in women. |
| *The most notable compounds of aniseeds essential oil were trans-anethole, γ-hymachalen, estragole, p-anisaldehyde, and methyl chavicol. |
| *It is commonly called sweet basil or basil, which belongs to the Lamiaceae family. |
| *Its name comes from the Greek word Basileus meaning Royal or king, and it is usually known as king of the herbs because of its different applications in cosmetic, medicine, food and pharmaceutical industries. |
| *Its seeds are utilized to enrich fruit-based beverages for functional and visual goals. |
| *Basil seeds are high in dietary fiber which has made it unique as a functional ingredient. |
| *Basil seeds has not only high nutritious value, but also they have used due to their high and notable health benefits such as anticancer, antioxidant, antidiabetic and antimicrobial activities. |
| *The important of physical and morphological characterization of seeds are because of the relationship between size and the shape of seeds, and the design of tools for agricultural activities such as production, storage and its potency for food application. |
| *The area in which they are planted and their origin are important factors which influence of seeds changes. |
| *The correlation with moisture may influence size of seeds. |
| *The seeds can vary bioactive components and nutritional composition on the basis of environmental conditions, agronomic management, altitude, geographical location, origin of the seeds, soil properties and the degree of water absorption. |
| *The basil seeds are important source of carbohydrate which vary between 43.9 and 63.8 g/100 g of seed. |
| *The seeds contain non-starchy polysaccharides in the form of lignin, hemicellulose and cellulose. |
| *The basil seeds contain mucilage and the content is about 17-20%. |
| *The basil seed gum is also applied for different purposes like a disintegrant, good source of fiber, a suspending agent, a pharmaceutical excipient, an anti-diabetic agent and a notable biodegradable edible film. |
| *The main non-essential amino acids of basil seeds are aspartic and glutamic acid. |
| *All essential amino acids except tryptophan and S-containing types can be found in basil seeds. |
| *The amino acid composition of basil seeds are aspartic acid, serine, glutamic acid, glycine, histidine, arginine, threonine, tyrosine, valine, lysine, alanine, proline, isoleucine, leucine, phenylalanine, cysteic acid, methionine sulfone and tryptophan. |
| *Basil seeds have a fat content vary between 9.7% and 33.0%. |
| *The most important fatty acid composition of basil seeds are palmitic acid, stearic acid, oleic acid, linoleic acid, and linolenic acid. |
| *The main macronutrients minerals that are needed in higher amounts include phosphorus, calcium, sulfur, potassium, magnesium, sodium and chloride. |
| *The major micronutrients are iron, zinc, cooper, manganese, silicon, iodine, fluoride and chromium. |
| *Basil seeds contain high antioxidant potential, and the total phenolic content and antioxidant capacity of basil seeds are determined by DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) and Folin-Ciocalteu methods. |
| *The antimocrivial activity of basil seed oil again both Gram-negative and Gram-positive bacteria is reported. |
| *In traditional medicine, basil seeds applied as natural remedy for the treatment of ulcers, indigestion, kidney disorders, sore throats and diarrhea. |
| *The basil extracts also demonstrated antioxidant, anti-inflammatory, antidiarrheal, antiulcer and chemo-preventive impacts. |
| *Borage is an oilseed with a high gamma-linolenic acid content. |
| *The major producers of borage seeds are the USA, Canada, England and Chile. |
| *Borage seed is one of the most notable sensitive agronomic seeds as significant deterioration happens after only one year of storage. |
| *The main reasons of membrane disruption are both increased free fatty acid levels and free radical productivity by lipid per oxidation. |
| *The seeds also contain palmitic, linoleic, stearic, α-linolenic, oleic, erucic, and erucic acids |
| *Gamma-linolenic acid showed the potential to relieve the symptoms and signs of various chronic inflammatory diseases such as atopic dermatitis and rheumatoid arthritis. |
| *Gamma-linolenic acid can also be appropriate in respiratory, gastrointestinal and cardiovascular disorders. |
| *Borage seeds also have different volatile compounds with antimicrobial activities. |
| *Methods and units of measurement of antioxidant activity of borage seeds are Folin-Ciocalteu (mg polyphenols.g-1 seeds d.w.), FRAP (μmol Fe II.g-1 seeds d.w.), DPPH (DPPH rem, %), and AE (dm3.[μmol s-1]). |
| *The average oil content of the borage seed is 30-40% by weight. |
| *Borage seed oil is used to treat various skin disorders such seborrheic dermatitis, atopic dermatitis, and neurodermatitis. |
| *Cilantro is the seed of an annual small plant, which is commonly refers to the spice and belongs to the Apiaceae family (Umbelliferae). |
| *Cilantro also known as coriander, Mexican parsley and Chinese parsley. |
| *The largest producer of cilantro is India. |
| *The seeds of cilantro are nearly ovate globular and there are several longitudinal ridges on the surface. |
| *The length of the seed is 3-5 mm, color. |
| *Cilantro seeds have a sweet, mild, slight pungent like citrus flavor with a hint of sage. |
| *Fatty oil and essential oil are two major components of coriander seeds. |
| *Cilantro can be grown from transplanted or seed. |
| *The fatty oil content changes between 9.9% and 27.7%, and the essential oil content of dried cilantro seeds changes between 0.03% and 2.6%. |
| *Linalool is the most important essential oil in seeds of coriander. |
| * Linalool, which a terpene alcohol identified in cilantro has important function in many therapeutic benefits and possesses anxiolytic, analgesic, anticonvulsant and neuroprotective effects. |
| *Its essential oil is an important component of detergents, emulsifiers, creams, surfactants, perfumes and lotions. |
| *Moisture content is one of principle factor which influences the physical properties of seeds. |
| *The seeds showed the presence of different compounds such as glucosides, monoterpenoid, monoterpenoid glycosides and aromatic constituent glycosides like norcarotenoid glucoside. |
| *Different methods such as solvent extraction such as water, methanol and n-hexane, hydrodistillation, sonication and microwave-assisted extraction use to extract the chemical components of cilantro. |
| *The extracts and essential oil of coriander seeds contains sedative-hypnotic activity. |
| *In traditional medicine, its seeds were consumed to relieve pain, inflammation and rheumatoid arthritis. |
| *In traditional Iranian medicine, cilantro seeds has been used for relief of insomnia. |
| *The seeds have been consumed to relieve different gastrointestinal disorders like diarrhea, flatulence, nausea and indigestion. |
| *In Morocco, Saudi Arabia and Jordan, they are used to lower blood glucose levels. |
| *The most important health benefits of cilantro are hypolipidemic activity, anti-atherogenic and antioxidant properties, antihypertensive potential, and antiarrhythmic activity. |
| *Two main and popular kinds of chamomile are German chamomile and Roman chamomile which belongs to Asteraceae (Compositae) family. |
| *Chamomile contains terpenes, volatile oils, organic acids, coumarins, flavonoids, sterols and polysaccharides. |
| *Chamomile is mainly cultivated by seeds, but keeping seed viability for long time is very important factor as well as seed germination. |
| *Chamomile has anti-inflammatory, antioxidant, anticancer, neuroprotective, anti-diarrheal, antibacterial and anti-allergic activities. |
| *Chamomile is widely utilized herb in traditional medicine of China, Rome, Greece, Germany and West of Asia. |
| *In traditional medicine, it is used to treat ulcers, wounds, gout, eczema, bruises, skin irritations, canker sores, burns, sciatica, neuralgia, hemorrhoids, rheumatic pain, mastitis and hemorrhoids. |
| *It has been used to treat croup, colic and fevers in children, and also applied as an emmenagogue and an uterine tonic in women. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





