Submitted:
30 May 2023
Posted:
01 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- A taxonomy for computer-aided bleeding detection algorithms for capsule endoscopy was identified.
- Various color spaces and feature extraction techniques were used to boost the bleeding detection performance, which was discussed in depth.
- From the observation of existing literature, a direction to the computer-aided bleeding detection research community was provided.
2. Review Methodology
2.1. Identifying Research Questions
2.2. Database
2.3. Search Strategy
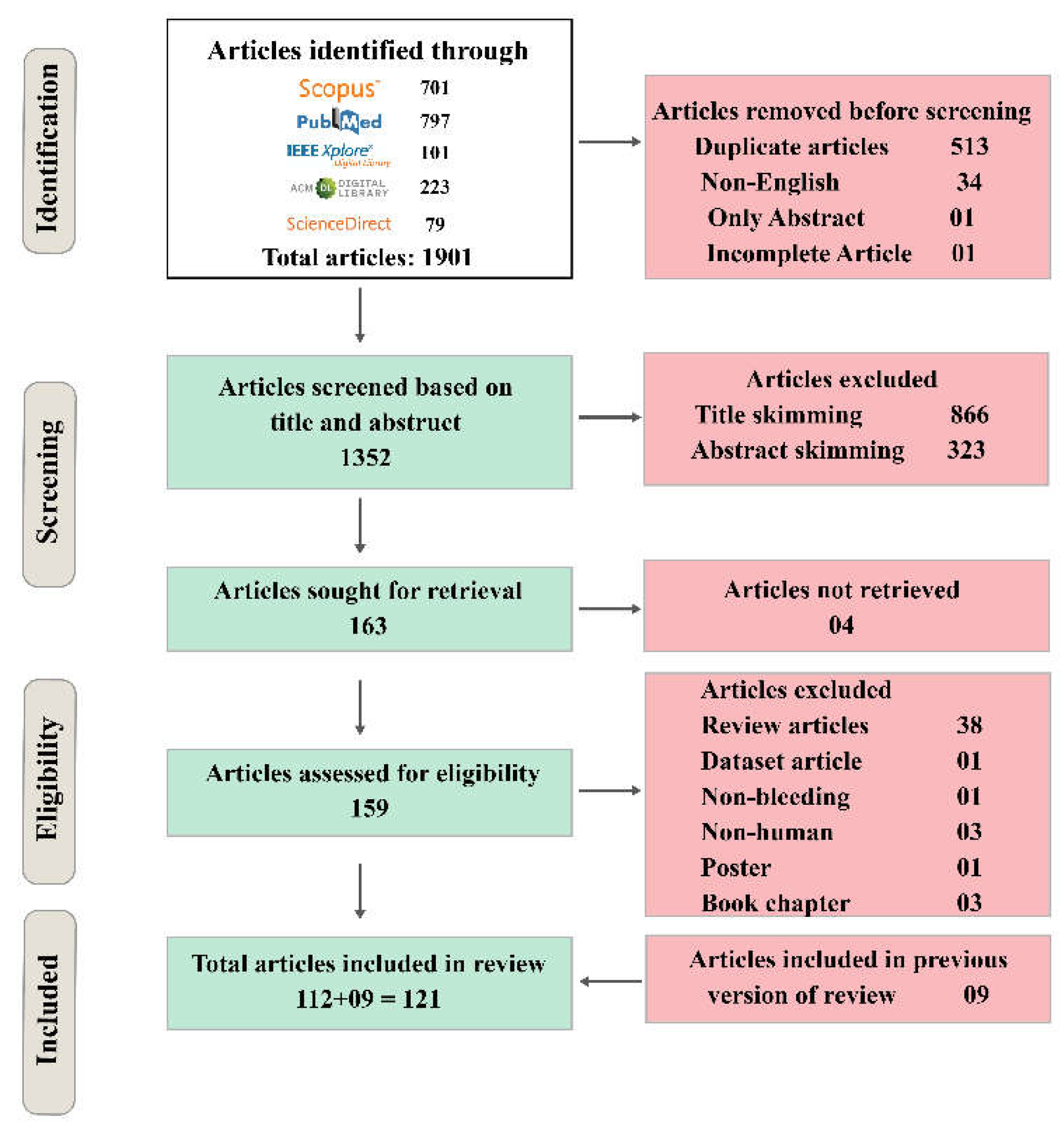
2.4. Results
3. Review Findings
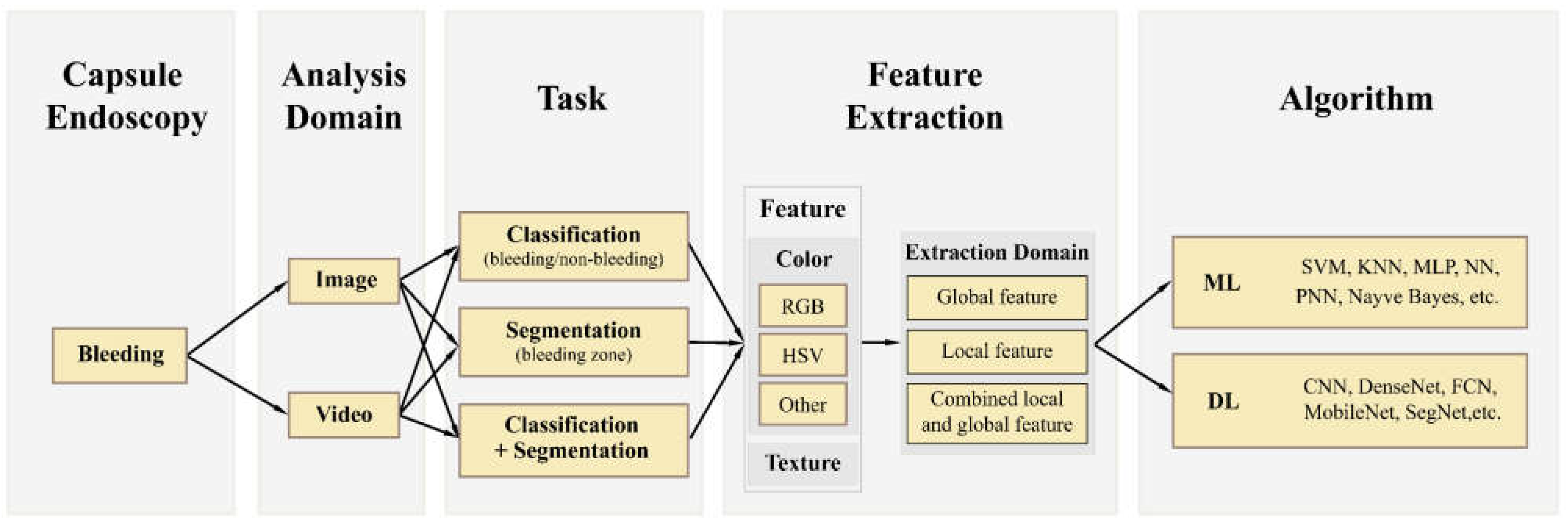
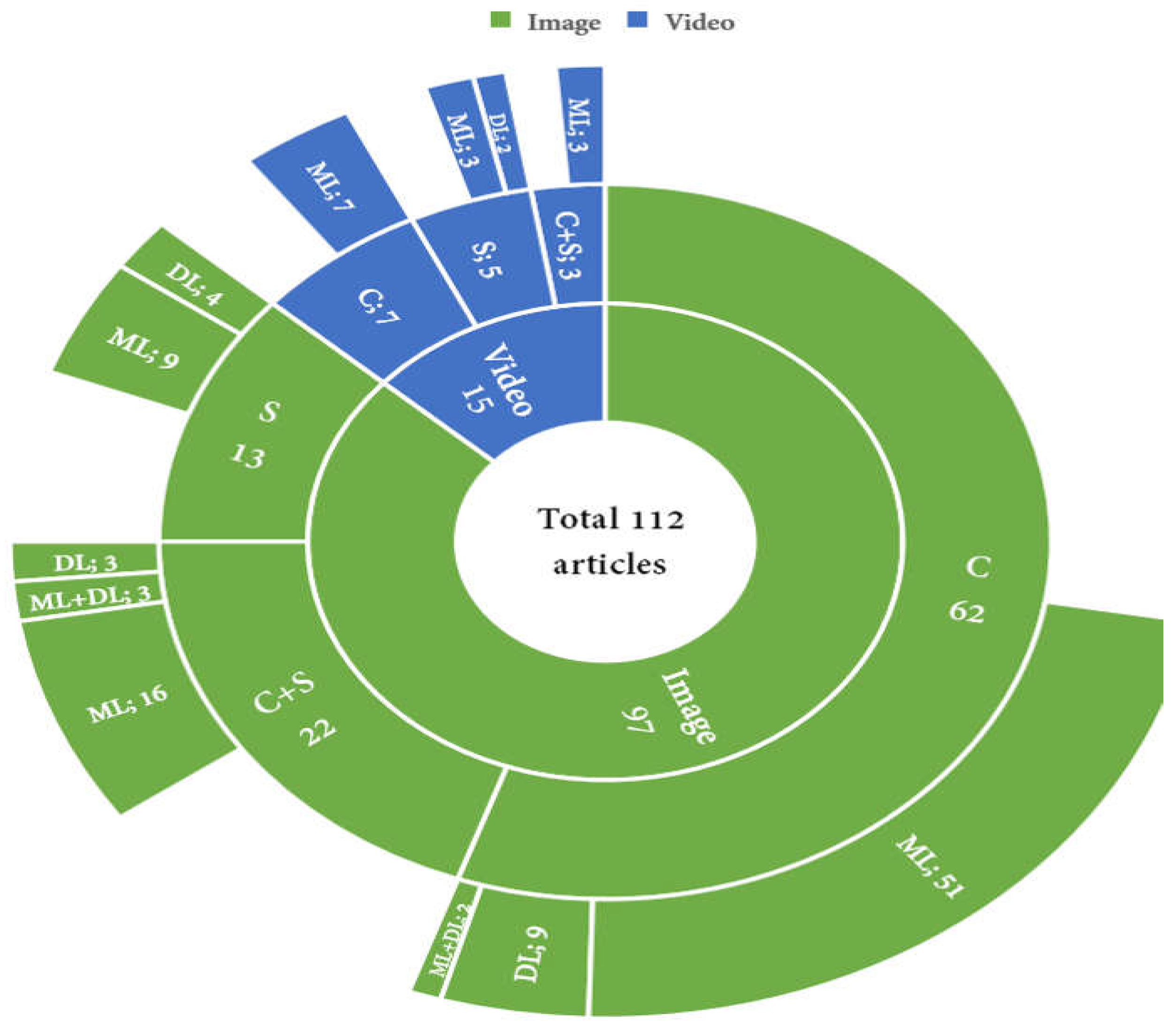
3.1. Taxonomy
3.2. Analysis Domain
3.2.1. Image
3.2.2. Video
3.2.3. Task
Classification
Segmentation
Classification + Segmentation
3. Feature Extraction
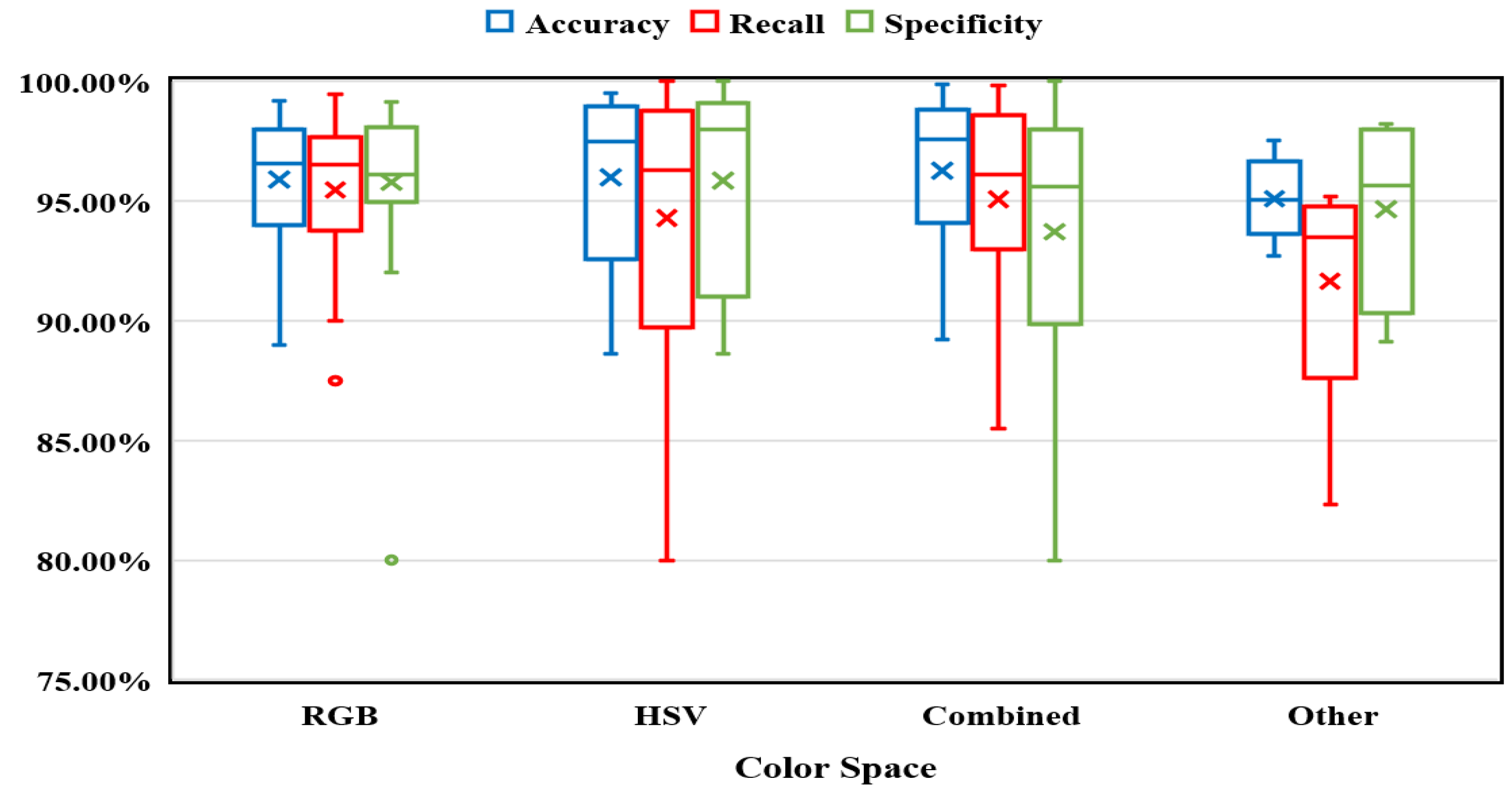
Color Space
Texture
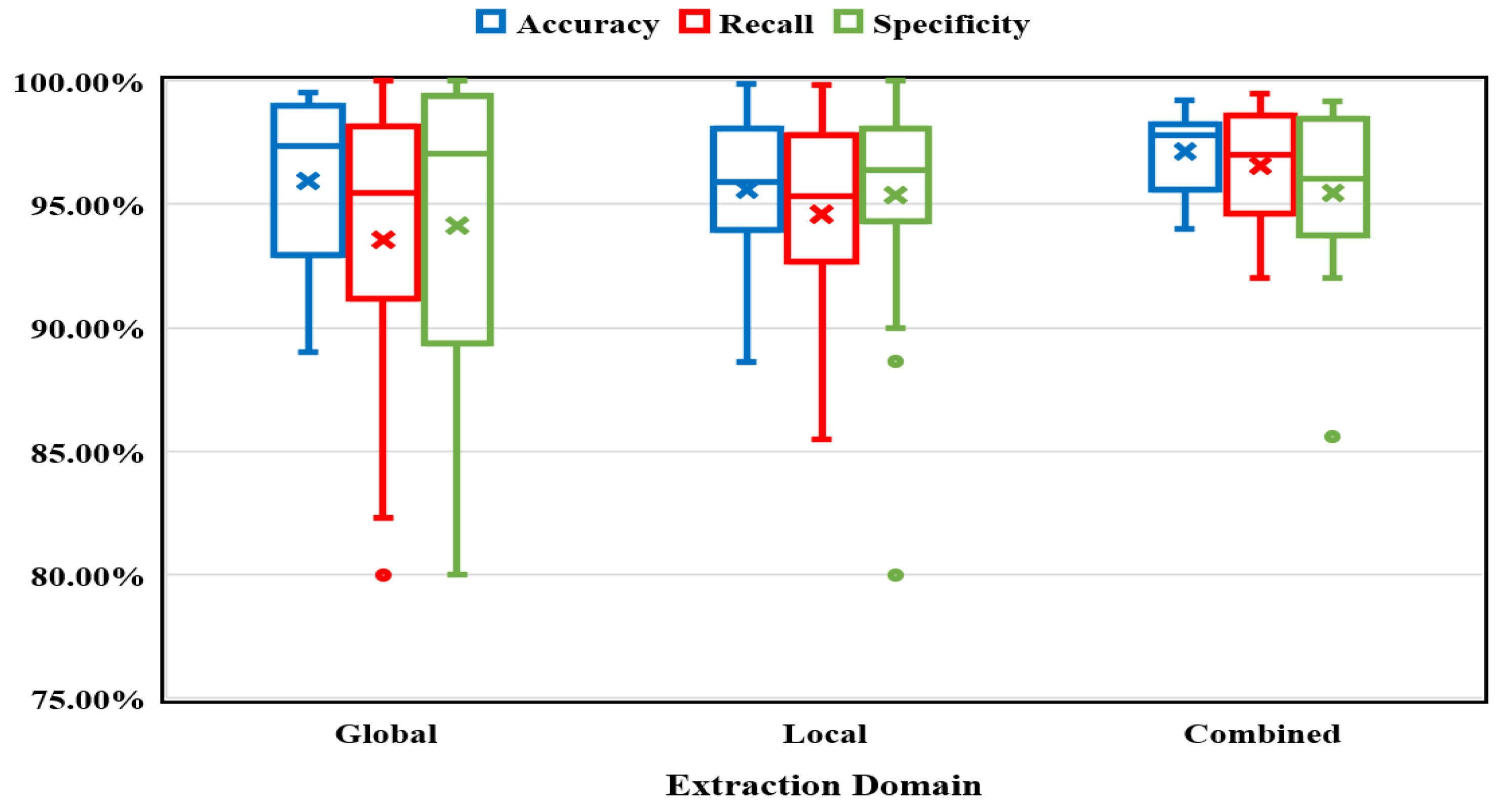
Extraction Domain
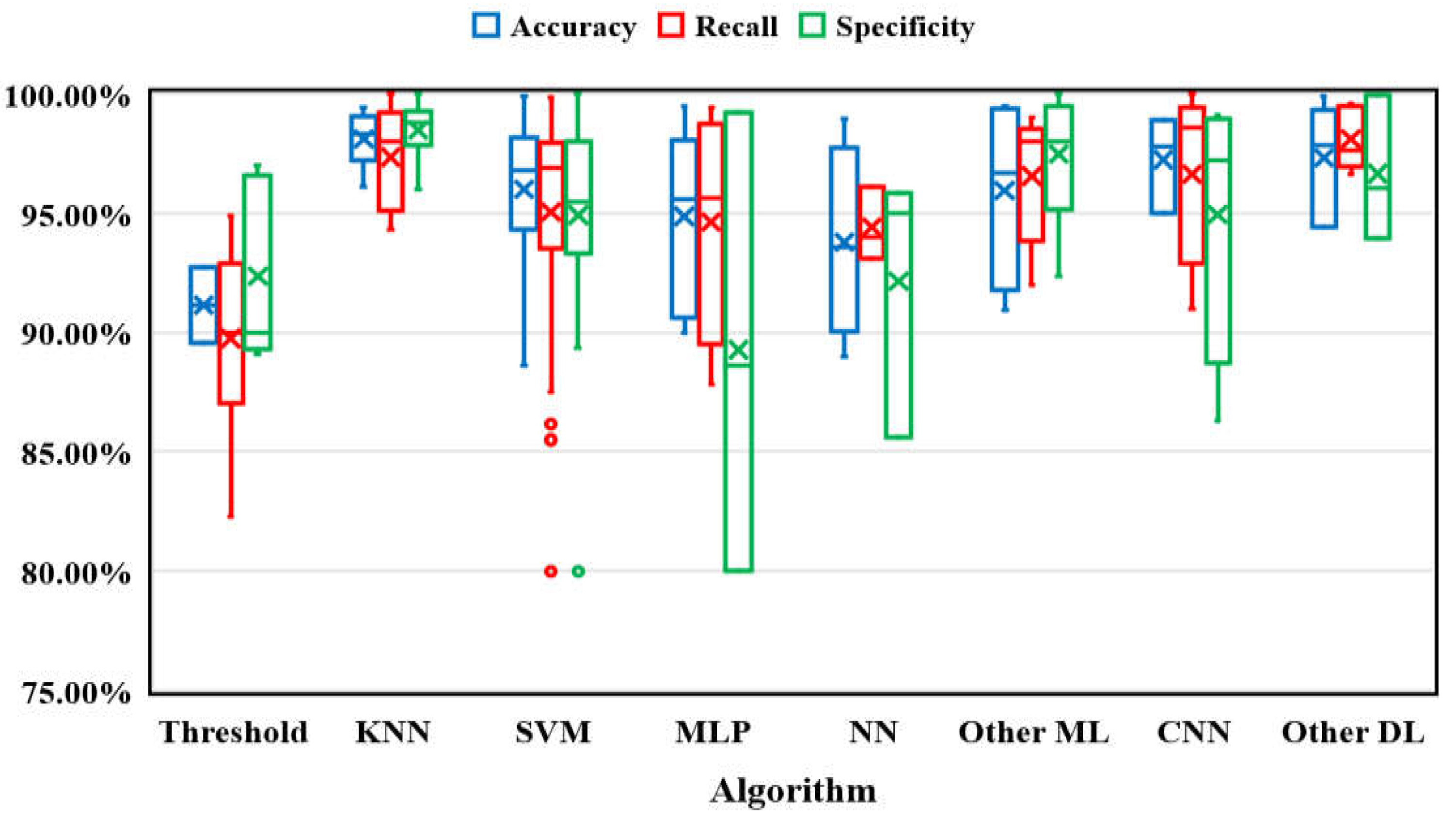
Algorithm
4. Discussion
5. Future Direction
6. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Al Mamun, P. P. Em, T. Ghosh, M. M. Hossain, M. G. Hasan, and M. G. Sadeque, “Bleeding recognition technique in wireless capsule endoscopy images using fuzzy logic and principal component analysis,” Int. J. Electr. Comput. Eng., vol. 11, no. 3, pp. 2689–2696, 2021. [CrossRef]
- S. Monteiro, F. D. De Castro, P. B. Carvalho, M. J. Moreira, B. Rosa, and J. Cotter, “PillCam® SB3 capsule: Does the increased frame rate eliminate the risk of missing lesions?,” World J. Gastroenterol., vol. 22, no. 10, pp. 3066–3068, Mar. 2016. [CrossRef]
- S. Fan, L. Xu, Y. Fan, K. Wei, and L. Li, “Computer-aided detection of small intestinal ulcer and erosion in wireless capsule endoscopy images,” Phys. Med. Biol., vol. 63, no. 16, Aug. 2018. [CrossRef]
- K. Pogorelov et al., “Bleeding detection in wireless capsule endoscopy videos — Color versus texture features,” J. Appl. Clin. Med. Phys., vol. 20, no. 8, pp. 141–154, Aug. 2019. [CrossRef]
- Karargyris and N. Bourbakis, “Detection of small bowel polyps and ulcers in wireless capsule endoscopy videos,” IEEE Trans. Biomed. Eng., vol. 58, no. 10 PART 1, pp. 2777–2786, Oct. 2011. [CrossRef]
- G. R. Zuckerman, C. Prakash, M. P. Askin, and B. S. Lewis, “AGA technical review on the evaluation and management of occult and obscure gastrointestinal bleeding,” Gastroenterology, vol. 118, no. 1, pp. 201–221, 2000. [CrossRef]
- Y. Lee, G. Yoon, and A. O. Architecture, “for Capsule Endoscopy,” pp. 672–677, 2011.
- S. C. Park et al., “Sensitivity of the suspected blood indicator: An experimental study,” World J. Gastroenterol., vol. 18, no. 31, pp. 4169–4174, 2012. [CrossRef]
- Liaqat, M. A. Khan, J. H. Shah, M. Sharif, M. Yasmin, and S. L. Fernandes, “AUTOMATED ULCER and BLEEDING CLASSIFICATION from WCE IMAGES USING MULTIPLE FEATURES FUSION and SELECTION,” J. Mech. Med. Biol., vol. 18, no. 4, Jun. 2018. [CrossRef]
- Al Mamun, M. S. Hossain, P. P. Em, A. Tahabilder, R. Sultana, and M. A. Islam, “Small intestine bleeding detection using color threshold and morphological operation in WCE images,” Int. J. Electr. Comput. Eng., vol. 11, no. 4, pp. 3040–3048, Aug. 2021. [CrossRef]
- Al Mamun, M. S. Hossain, M. M. Hossain, and M. G. Hasan, “Discretion Way for Bleeding Detection in Wireless Capsule Endoscopy Images,” May 2019. [CrossRef]
- H. Neumann, L. C. Fry, A. Nägel, and M. F. Neurath, “Wireless capsule endoscopy of the small intestine: a review with future directions.,” Curr. Opin. Gastroenterol., vol. 30, no. 5, pp. 463–471, 2014. [CrossRef]
- Karargyris and N. Bourbakis, “Wireless Capsule Endoscopy and Endoscopic Imaging: A Survey on Various Methodologies Presented,” IEEE Eng. Med. Biol. Mag., vol. 29, no. 1, pp. 72–83, 2010. [CrossRef]
- H. P. Brito et al., “Video capsule endoscopy vs double-balloon enteroscopy in the diagnosis of small bowel bleeding: A systematic review and meta-analysis,” World J. Gastrointest. Endosc., vol. 10, no. 12, pp. 400–421, 2018. [CrossRef]
- Koulaouzidis, E. Rondonotti, and A. Karargyris, “Small-bowel capsule endoscopy: A ten-point contemporary review,” World J. Gastroenterol., vol. 19, no. 24, pp. 3726–3746, 2013. [CrossRef]
- D. K. Iakovidis and A. Koulaouzidis, “Software for enhanced video capsule endoscopy: challenges for essential progress.,” Nat. Rev. Gastroenterol. Hepatol., vol. 12, no. 3, pp. 172–186, 2015. [CrossRef]
- Y. Hwang, J. Park, Y. J. Lim, and H. J. Chun, “Application of artificial intelligence in capsule endoscopy: Where are we now?,” Clin. Endosc., vol. 51, no. 6, pp. 547–551, 2018. [CrossRef]
- Y. Chen and J. Lee, “A review of machine-vision-based analysis of wireless capsule endoscopy video,” Diagn. Ther. Endosc., vol. 2012, 2012. [CrossRef]
- N. Shah et al., “Video capsule endoscopy for upper gastrointestinal hemorrhage in the emergency department: A systematic review and meta-analysis,” Am. J. Emerg. Med., vol. 38, no. 6, pp. 1245–1252, 2020. [CrossRef]
- S. Soffer et al., “Deep learning for wireless capsule endoscopy: a systematic review and meta-analysis,” Gastrointest. Endosc., vol. 92, no. 4, pp. 831-839.e8, 2020. [CrossRef]
- S. Suman, F. A. B. Hussin, N. Walter, A. S. Malik, S. H. Ho, and K. L. Goh, “Detection and classification of bleeding using statistical color features for wireless capsule endoscopy images,” 2017. [CrossRef]
- Z. Amiri, H. Hassanpour, and A. Beghdadi, “Feature Selection for Bleeding Detection in Capsule Endoscopy Images using Genetic Algorithm,” 2019. [CrossRef]
- Caroppo, A. Leone, and P. Siciliano, “Deep transfer learning approaches for bleeding detection in endoscopy images.,” Comput. Med. Imaging Graph., vol. 88, p. 101852, 2021. [CrossRef]
- X. Xing, Y. Yuan, X. Jia, and Q. H. M. Max, “A saliency-aware hybrid dense network for bleeding detection in wireless capsule endoscopy images,” in Proceedings - International Symposium on Biomedical Imaging, 2019, vol. 2019-April, pp. 104–107. [CrossRef]
- Y. Fu, M. Mandal, and G. Guo, “Bleeding region detection in WCE images based on color features and neural network,” 2011. [CrossRef]
- Y. Xiong, Y. Zhu, Z. Pang, Y. Ma, D. Chen, and X. Wang, “Bleeding detection in wireless capsule endoscopy based on MST clustering and SVM,” in IEEE Workshop on Signal Processing Systems, SiPS: Design and Implementation, 2015, vol. 2015-Decem. [CrossRef]
- X. Jia and M. Q.-H. Meng, “A study on automated segmentation of blood regions in Wireless Capsule Endoscopy images using fully convolutional networks,” in Proceedings - International Symposium on Biomedical Imaging, 2017, pp. 179–182. [CrossRef]
- P. Coelho, A. Pereira, A. Leite, M. Salgado, and A. Cunha, “A Deep Learning Approach for Red Lesions Detection in Video Capsule Endoscopies,” Lect. Notes Comput. Sci. (including Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinformatics), vol. 10882 LNCS, pp. 553–561, 2018. [CrossRef]
- M. A. Usman, G. B. Satrya, M. R. Usman, and S. Y. Shin, “Detection of small colon bleeding in wireless capsule endoscopy videos.,” Comput. Med. Imaging Graph., vol. 54, pp. 16–26, 2016. [CrossRef]
- M. A. Usman et al., “QI-BRiCE: Quality index for bleeding regions in capsule endoscopy videos,” Comput. Mater. Contin., vol. 67, no. 2, pp. 1697–1712, 2021. [CrossRef]
- Novozámský, J. Flusser, I. Tachecí, L. Sulík, J. Bureš, and O. Krejcar, “Automatic blood detection in capsule endoscopy video.,” J. Biomed. Opt., vol. 21, no. 12, p. 126007, 2016. [CrossRef]
- S. Sainju, F. M. Bui, and K. A. Wahid, “Automated bleeding detection in capsule endoscopy videos using statistical features and region growing.,” J. Med. Syst., vol. 38, no. 4, p. 25, 2014. [CrossRef]
- F. Deeba, M. Islam, F. M. Bui, and K. A. Wahid, “Performance assessment of a bleeding detection algorithm for endoscopic video based on classifier fusion method and exhaustive feature selection,” Biomed. Signal Process. Control, vol. 40, pp. 415–424, 2018. [CrossRef]
- M. Mackiewicz, M. Fisher, and C. Jamieson, “Bleeding detection in wireless capsule endoscopy using adaptive colour histogram model and support vector classification,” in Progress in Biomedical Optics and Imaging - Proceedings of SPIE, 2008, vol. 6914. [CrossRef]
- T. Ghosh, S. A. Fattah, and K. A. Wahid, “Automatic Computer Aided Bleeding Detection Scheme for Wireless Capsule Endoscopy (WCE) Video Based on Higher and Lower Order Statistical Features in a Composite Color,” J. Med. Biol. Eng., vol. 38, no. 3, pp. 482–496, 2018. [CrossRef]
- T. Ghosh, S. A. Fattah, and K. A. Wahid, “CHOBS: Color Histogram of Block Statistics for Automatic Bleeding Detection in Wireless Capsule Endoscopy Video.,” IEEE J. Transl. Eng. Heal. Med., vol. 6, p. 1800112, 2018. [CrossRef]
- Q. Zhao, M. Q.-H. Meng, and B. Li, “WCE video clips segmentation based on abnormality,” in 2010 IEEE International Conference on Robotics and Biomimetics, ROBIO 2010, 2010, pp. 442–447. [CrossRef]
- T. Ghosh, S. A. Fattah, K. A. Wahid, W.-P. Zhu, and M. O. Ahmad, “Cluster based statistical feature extraction method for automatic bleeding detection in wireless capsule endoscopy video.,” Comput. Biol. Med., vol. 94, pp. 41–54, 2018. [CrossRef]
- W. Shi, J. Chen, H. Chen, Q. Peng, and T. Gan, “Bleeding fragment localization using time domain information for WCE videos,” in Proceedings - 2015 8th International Conference on BioMedical Engineering and Informatics, BMEI 2015, 2016, pp. 73–78. [CrossRef]
- R. Leenhardt et al., “A neural network algorithm for detection of GI angiectasia during small-bowel capsule endoscopy.,” Gastrointest. Endosc., vol. 89, no. 1, pp. 189–194, 2019. [CrossRef]
- F. Rustam et al., “Wireless Capsule Endoscopy Bleeding Images Classification Using CNN Based Model,” IEEE Access, vol. 9, pp. 33675–33688, 2021. [CrossRef]
- E. Tuba, M. Tuba, and R. Jovanovic, “An algorithm for automated segmentation for bleeding detection in endoscopic images,” in Proceedings of the International Joint Conference on Neural Networks, 2017, vol. 2017-May, pp. 4579–4586. [CrossRef]
- S. Li, J. Zhang, C. Ruan, and Y. Zhang, “Multi-Stage Attention-Unet for Wireless Capsule Endoscopy Image Bleeding Area Segmentation,” in Proceedings - 2019 IEEE International Conference on Bioinformatics and Biomedicine, BIBM 2019, 2019, pp. 818–825. [CrossRef]
- S. Rathnamala and S. Jenicka, “Automated bleeding detection in wireless capsule endoscopy images based on color feature extraction from Gaussian mixture model superpixels.,” Med. Biol. Eng. Comput., 2021. [CrossRef]
- T. Ghosh and J. Chakareski, “Deep Transfer Learning for Automated Intestinal Bleeding Detection in Capsule Endoscopy Imaging.,” J. Digit. Imaging, 2021. [CrossRef]
- S. Suman et al., “Detection and classification of bleeding region in WCE images using color feature,” in ACM International Conference Proceeding Series, 2017, vol. Part F1301. [CrossRef]
- J. Liu and X. Yuan, “Obscure bleeding detection in endoscopy images using support vector machines,” Optim. Eng., vol. 10, no. 2, pp. 289–299, 2009. [CrossRef]
- J. Li, J. Ma, T. Tillo, B. Zhang, and E. G. Lim, “A training based Support Vector Machine technique for blood detection in wireless capsule endoscopy images,” in 2012 IEEE-EMBS Conference on Biomedical Engineering and Sciences, IECBES 2012, 2012, pp. 826–830. [CrossRef]
- S. Sainju, F. M. Bui, and K. Wahid, “Bleeding detection in wireless capsule endoscopy based on color features from histogram probability,” 2013. [CrossRef]
- G. Pan, F. Xu, and J. Chen, “Bleeding detection in wireless capsule endoscopy using color similarity coefficient,” Appl. Mech. Mater., vol. 195–196, pp. 307–312, 2012. [CrossRef]
- S. J. Yun, H. K. Young, H. L. Dong, H. L. Sang, J. S. Jeong, and H. K. Jong, “Automatic patient-adaptive bleeding detection in a capsule endoscopy,” in Progress in Biomedical Optics and Imaging - Proceedings of SPIE, 2009, vol. 7260. [CrossRef]
- T. Ghosh, S. A. Fattah, C. Shahnaz, and K. A. Wahid, “An automatic bleeding detection scheme in wireless capsule endoscopy based on histogram of an RGB-indexed image.,” Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Int. Conf., vol. 2014, pp. 4683–4686, 2014. [CrossRef]
- P. Sivakumar and B. M. Kumar, “A novel method to detect bleeding frame and region in wireless capsule endoscopy video,” Cluster Comput., vol. 22, pp. 12219–12225, 2019. [CrossRef]
- J. Ma, T. Tillo, B. Zhang, Z. Wang, and E. G. Lim, “Novel training and comparison method for blood detection in wireless capsule endoscopy images,” in International Symposium on Medical Information and Communication Technology, ISMICT, 2013, pp. 56–60. [CrossRef]
- T. Ghosh, S. K. Bashar, M. S. Alam, K. Wahid, and S. A. Fattah, “A statistical feature based novel method to detect bleeding in wireless capsule endoscopy images,” 2014. [CrossRef]
- T. Ghosh, S. A. Fattah, C. Shahnaz, A. K. Kundu, and M. N. Rizve, “Block based histogram feature extraction method for bleeding detection in wireless capsule endoscopy,” in IEEE Region 10 Annual International Conference, Proceedings/TENCON, 2016, vol. 2016-Janua. [CrossRef]
- T. Ghosh, S. A. Fattah, and K. A. Wahid, “Automatic bleeding detection in wireless capsule endoscopy based on RGB pixel intensity ratio,” 2014. [CrossRef]
- Y. Fu, W. Zhang, M. Mandal, and M. Q.-H. Meng, “Computer-aided bleeding detection in WCE video.,” IEEE J. Biomed. Heal. informatics, vol. 18, no. 2, pp. 636–642, 2014. [CrossRef]
- Z. Liu, C. Hu, and Z. Shen, “Research on a new feature detection algorithm for wireless capsule endoscope bleeding images based on super-pixel segmentation,” in IEEE International Conference on Robotics and Biomimetics, ROBIO 2019, 2019, pp. 1744–1749. [CrossRef]
- S. Kumar, I. N. Figueiredo, C. Graca, and G. Falcao, “A GPU accelerated algorithm for blood detection inwireless capsule endoscopy images,” Lect. Notes Comput. Vis. Biomech., vol. 19, pp. 55–71, 2015. [CrossRef]
- K. Kundu, M. N. Rizve, T. Ghosh, and S. A. Fattah, “A segmented color plane histogram based feature extraction scheme for automatic bleeding detection in wireless capsule endoscopy,” in 2016 IEEE Students’ Technology Symposium, TechSym 2016, 2017, pp. 245–249. [CrossRef]
- Y. Yuan and M. Q. H. Meng, “Automatic bleeding frame detection in the wireless capsule endoscopy images,” in Proceedings - IEEE International Conference on Robotics and Automation, 2015, vol. 2015-June, no. June, pp. 1310–1315. [CrossRef]
- X. Jia, L. Cai, J. Liu, W. Dai, and M. Q.-H. Meng, “GI bleeding detection in wireless capsule endoscopy images based on pattern recognition and a MapReduce framework,” in 2016 IEEE International Conference on Real-Time Computing and Robotics, RCAR 2016, 2016, pp. 266–271. [CrossRef]
- S. Hwang, J. Oh, J. Cox, S. J. Tang, and H. F. Tibbals, “Blood detection in wireless capsule endoscopy using expectation maximization clustering,” in Progress in Biomedical Optics and Imaging - Proceedings of SPIE, 2006, vol. 6144 I. [CrossRef]
- K. Poh et al., “Multi-level local feature classification for bleeding detection in Wireless Capsule Endoscopy images,” in 2010 IEEE Conference on Cybernetics and Intelligent Systems, CIS 2010, 2010, pp. 76–81. [CrossRef]
- P. Pons, R. Noorda, A. Nevárez, A. Colomer, V. P. Beltrán, and V. Naranjo, “Design and Development of an Automatic Blood Detection System for Capsule Endoscopy Images,” Lect. Notes Comput. Sci. (including Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinformatics), vol. 11872 LNCS, pp. 105–113, 2019. [CrossRef]
- Dilna and V. P. Gopi, “A novel method for bleeding detection in Wireless Capsule Endoscopic images,” in 2015 International Conference on Computing and Network Communications, CoCoNet 2015, 2016, pp. 854–858. [CrossRef]
- K. R. Reeha, K. Shailaja, and V. P. Gopi, “Undecimated Complex Wavelet Transform based bleeding detection for endoscopic images,” in Proceedings - 2016 2nd International Conference on Cognitive Computing and Information Processing, CCIP 2016, 2016. [CrossRef]
- S. Charfi and M. El Ansari, “Gastrointestinal tract bleeding detection from wireless capsule endoscopy videos,” in ACM International Conference Proceeding Series, 2017. [CrossRef]
- Giritharan, X. Yuan, J. Liu, B. Buckles, J. Oh, and S. J. Tang, “Bleeding detection from capsule endoscopy videos.,” Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Int. Conf., vol. 2008, pp. 4780–4783, 2008. [CrossRef]
- Bchir, M. M. Ben Ismail, and N. AlZahrani, “Multiple bleeding detection in wireless capsule endoscopy,” Signal, Image Video Process., vol. 13, no. 1, pp. 121–126, 2019. [CrossRef]
- P. Y. Lau and P. L. Correia, “Detection of bleeding patterns in WCE video using multiple features.,” Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Int. Conf., vol. 2007, pp. 5601–5604, 2007. [CrossRef]
- Kukushkin et al., “Recognition of hemorrhage in the images of wireless capsule endoscopy,” in Proceedings of the Mediterranean Electrotechnical Conference - MELECON, 2012, pp. 899–902. [CrossRef]
- K. Poh, Z. Zhang, Z. Y. Liang, L. Li, and J. Liu, “Feature selection and classification for wireless capsule endoscopic frames,” 2009. [CrossRef]
- K. Poh, Z. Zhang, Z. Y. Liang, L. Li, and J. Liu, “Feature selection and classification for wireless capsule endoscopic frames,” 2009. [CrossRef]
- T. Ghosh, S. K. Bashar, S. A. Fattah, C. Shahnaz, and K. A. Wahid, “An automatic bleeding detection scheme in wireless capsule endoscopy based on statistical features in hue space,” in 2014 17th International Conference on Computer and Information Technology, ICCIT 2014, 2003, pp. 354–357. [CrossRef]
- K. Timotius, S.-G. Miaou, E. B. Valdeavilla, and Y.-H. Liu, “Abnormality detection for capsule endoscope images based on support vector machines,” Biomed. Eng. - Appl. Basis Commun., vol. 24, no. 1, pp. 71–83, 2012. [CrossRef]
- S. Zhou, X. Song, M. A. Siddique, J. Xu, and P. Zhou, “Bleeding detection in wireless capsule endoscopy images based on binary feature vector,” in 5th International Conference on Intelligent Control and Information Processing, ICICIP 2014 - Proceedings, 2015, pp. 29–33. [CrossRef]
- L. Cui, C. Hu, Y. Zou, and M. Q.-H. Meng, “Bleeding detection in wireless capsule endoscopy images by support vector classifier,” in 2010 IEEE International Conference on Information and Automation, ICIA 2010, 2010, pp. 1746–1751. [CrossRef]
- T. Ghosh et al., “An automatic bleeding detection technique in wireless capsule endoscopy from region of interest,” in International Conference on Digital Signal Processing, DSP, 2015, vol. 2015-Septe, pp. 1293–1297. [CrossRef]
- K. Kundu, M. N. Rizve, T. Ghosh, S. A. Fattah, and C. Shahnaz, “A histogram based scheme in YIQ domain for automatic bleeding image detection from wireless capsule endoscopy,” in 2015 IEEE International WIE Conference on Electrical and Computer Engineering, WIECON-ECE 2015, 2016, pp. 455–458. [CrossRef]
- D.-Y. Liu et al., “Identification of lesion images from gastrointestinal endoscope based on feature extraction of combinational methods with and without learning process.,” Med. Image Anal., vol. 32, pp. 281–294, 2016. [CrossRef]
- Y. Yuan, B. Li, and M. Q.-H. Meng, “Bleeding Frame and Region Detection in the Wireless Capsule Endoscopy Video.,” IEEE J. Biomed. Heal. informatics, vol. 20, no. 2, pp. 624–630, 2016. [CrossRef]
- N. Figueiredo, S. Kumar, C. Leal, and P. N. Figueiredo, “Computer-assisted bleeding detection in wireless capsule endoscopy images,” Comput. Methods Biomech. Biomed. Eng. Imaging Vis., vol. 1, no. 4, pp. 198–210, 2013. [CrossRef]
- M. Mathew and V. P. Gopi, “Transform based bleeding detection technique for endoscopic images,” in 2nd International Conference on Electronics and Communication Systems, ICECS 2015, 2015, pp. 1730–1734. [CrossRef]
- Karargyris and N. Bourbakis, “A methodology for detecting blood-based abnormalities in wireless capsule endoscopy videos,” 2008. [CrossRef]
- X. Liu, J. Gu, Y. Xie, J. Xiong, and W. Qin, “A new approach to detecting ulcer and bleeding in wireless capsule endoscopy images,” in Proceedings - IEEE-EMBS International Conference on Biomedical and Health Informatics: Global Grand Challenge of Health Informatics, BHI 2012, 2012, pp. 737–740. [CrossRef]
- Priyadharshini and T. Gomathi, “Navie bayes classifier for wireless capsule endoscopy video to detect bleeding frames,” Int. J. Sci. Technol. Res., vol. 9, no. 1, pp. 3286–3291, 2020, [Online]. Available: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85093909545&partnerID=40&md5=6215a2e2a1ae9c0f862eb0f35c40631c.
- T. Ghosh, S. K. Bashar, S. A. Fattah, C. Shahnaz, and K. A. Wahid, “A feature extraction scheme from region of interest of wireless capsule endoscopy images for automatic bleeding detection,” 2015. [CrossRef]
- S. K. Mohammed, F. Deeba, F. M. Bui, and K. A. Wahid, “Application of modified ant colony optimization for computer aided bleeding detection system,” in Proceedings of the International Joint Conference on Neural Networks, 2016, vol. 2016-Octob, pp. 4317–4324. [CrossRef]
- X. Xing, X. Jia, and M.-H. Q. Meng, “Bleeding Detection in Wireless Capsule Endoscopy Image Video Using Superpixel-Color Histogram and a Subspace KNN Classifier.,” Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Int. Conf., vol. 2018, pp. 1–4, 2018. [CrossRef]
- Y. S. Jung, Y. H. Kim, D. H. Lee, and J. H. Kim, “Active blood detection in a high resolution capsule endoscopy using color spectrum transformation,” in BioMedical Engineering and Informatics: New Development and the Future - Proceedings of the 1st International Conference on BioMedical Engineering and Informatics, BMEI 2008, 2008, vol. 1, pp. 859–862. [CrossRef]
- G. Lv, G. Yan, and Z. Wang, “Bleeding detection in wireless capsule endoscopy images based on color invariants and spatial pyramids using support vector machines.,” Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Int. Conf., vol. 2011, pp. 6643–6646, 2011. [CrossRef]
- G. Pan, G. Yan, X. Song, and X. Qiu, “BP neural network classification for bleeding detection in wireless capsule endoscopy.,” J. Med. Eng. Technol., vol. 33, no. 7, pp. 575–581, 2009. [CrossRef]
- G. Pan, G. Yan, X. Qiu, and J. Cui, “Bleeding detection in Wireless Capsule Endoscopy based on Probabilistic Neural Network.,” J. Med. Syst., vol. 35, no. 6, pp. 1477–1484, 2010. [CrossRef]
- S. K. Mohammed, F. Deeba, F. M. Bui, and K. A. Wahid, “Feature selection using modified ant colony optimization for wireless capsule endoscopy,” in 2016 IEEE 7th Annual Ubiquitous Computing, Electronics and Mobile Communication Conference, UEMCON 2016, 2016. [CrossRef]
- K. Iakovidis, D. Chatzis, P. Chrysanthopoulos, and A. Koulaouzidis, “Blood detection in wireless capsule endoscope images based on salient superpixels.,” Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Int. Conf., vol. 2015, pp. 731–734, 2015. [CrossRef]
- N. Obukhova, A. Motyko, B. Timofeev, and A. Pozdeev, “Method of endoscopic images analysis for automatic bleeding detection and segmentation,” in Conference of Open Innovation Association, FRUCT, 2019, vol. 2019-April, pp. 285–290. [CrossRef]
- S. Yi et al., “A clinically viable Capsule Endoscopy video analysis platform for automatic bleeding detection,” in Proceedings of SPIE - The International Society for Optical Engineering, 2013, vol. 8670. [CrossRef]
- K. Kundu and S. A. Fattah, “Probability density function based modeling of spatial feature variation in capsule endoscopy data for automatic bleeding detection.,” Comput. Biol. Med., vol. 115, p. 103478, 2019. [CrossRef]
- Z. Amiri, H. Hassanpour, and A. Beghdadi, “A Computer- Aided Method to Detect Bleeding Frames in Capsule Endoscopy Images,” in Proceedings - European Workshop on Visual Information Processing, EUVIP, 2019, vol. 2019-Octob, pp. 217–221. [CrossRef]
- P. Szczypiński, A. Klepaczko, M. Pazurek, and P. Daniel, “Texture and color based image segmentation and pathology detection in capsule endoscopy videos,” Comput. Methods Programs Biomed., vol. 113, no. 1, pp. 396–411, 2014. [CrossRef]
- K. Kundu, S. A. Fattah, and K. A. Wahid, “Least Square Saliency Transformation of Capsule Endoscopy Images for PDF Model Based Multiple Gastrointestinal Disease Classification,” IEEE Access, vol. 8, pp. 58509–58521, 2020. [CrossRef]
- Tuba, S. Tomic, M. Beko, D. Zivkovic, and M. Tuba, “Bleeding Detection in Wireless Capsule Endoscopy Images Using Texture and Color Features,” in 2018 26th Telecommunications Forum (TELFOR), 2018, pp. 1–4. [CrossRef]
- Li and M. Q.-H. Meng, “Computer aided detection of bleeding in capsule endoscopy images,” in Canadian Conference on Electrical and Computer Engineering, 2008, pp. 1963–1966. [CrossRef]
- Pogorelov et al., “Bleeding detection in wireless capsule endoscopy videos - Color versus texture features.,” J. Appl. Clin. Med. Phys., vol. 20, no. 8, pp. 141–154, 2019. [CrossRef]
- R. Ponnusamy and S. Sathiamoorthy, “An efficient gastrointestinal hemorrhage detection and diagnosis model for wireless capsule endoscopy,” Int. J. Recent Technol. Eng., vol. 8, no. 3, pp. 7549–7554, 2019. [CrossRef]
- R. Hassan and M. A. Haque, “Computer-aided gastrointestinal hemorrhage detection in wireless capsule endoscopy videos.,” Comput. Methods Programs Biomed., vol. 122, no. 3, pp. 341–353, 2015. [CrossRef]
- H. Maghsoudi, M. Alizadeh, and M. Mirmomen, “A computer aided method to detect bleeding, tumor, and disease regions in Wireless Capsule Endoscopy,” 2017. [CrossRef]
- Patel, K. Rani, S. Kumar, I. N. Figueiredo, and P. N. Figueiredo, “Automated bleeding detection in wireless capsule endoscopy images based on sparse coding,” Multimed. Tools Appl., 2020. [CrossRef]
- Joshi, S. Kumar, and I. N. Figueiredo, “Bag of visual words approach for bleeding detection in wireless capsule endoscopy images,” Lect. Notes Comput. Sci. (including Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinformatics), vol. 9730, pp. 575–582, 2016. [CrossRef]
- Li and M. Q.-H. Meng, “Computer-aided detection of bleeding regions for capsule endoscopy images.,” IEEE Trans. Biomed. Eng., vol. 56, no. 4, pp. 1032–1039, 2009. [CrossRef]
- Li and M. Q.-H. Meng, “Computer-based detection of bleeding and ulcer in wireless capsule endoscopy images by chromaticity moments.,” Comput. Biol. Med., vol. 39, no. 2, pp. 141–147, 2009. [CrossRef]
- X. Jia and M. Q.-H. Meng, “Gastrointestinal bleeding detection in wireless capsule endoscopy images using handcrafted and CNN features.,” Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Int. Conf., vol. 2017, pp. 3154–3157, 2017. [CrossRef]
- M. D. Vasilakakis, D. Diamantis, E. Spyrou, A. Koulaouzidis, and D. K. Iakovidis, “Weakly supervised multilabel classification for semantic interpretation of endoscopy video frames,” Evol. Syst., vol. 11, no. 3, pp. 409–421, 2020. [CrossRef]
- M. Hajabdollahi, R. Esfandiarpoor, K. Najarian, N. Karimi, S. Samavi, and S. M. Reza Soroushmehr, “Low Complexity CNN Structure for Automatic Bleeding Zone Detection in Wireless Capsule Endoscopy Imaging.,” Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Int. Conf., vol. 2019, pp. 7227–7230, 2019. [CrossRef]
- M. Hajabdollahi, R. Esfandiarpoor, E. Sabeti, N. Karimi, S. M. R. Soroushmehr, and S. Samavi, “Multiple abnormality detection for automatic medical image diagnosis using bifurcated convolutional neural network,” Biomed. Signal Process. Control, vol. 57, 2020. [CrossRef]
- H. S. Pannu, S. Ahuja, N. Dang, S. Soni, and A. K. Malhi, “Deep learning based image classification for intestinal hemorrhage,” Multimed. Tools Appl., vol. 79, no. 29–30, pp. 21941–21966, 2020. [CrossRef]
- E. Diamantis, D. K. Iakovidis, and A. Koulaouzidis, “Look-behind fully convolutional neural network for computer-aided endoscopy,” Biomed. Signal Process. Control, vol. 49, pp. 192–201, 2019. [CrossRef]
- P. Li, Z. Li, F. Gao, L. Wan, and J. Yu, “Convolutional neural networks for intestinal hemorrhage detection in wireless capsule endoscopy images,” in Proceedings - IEEE International Conference on Multimedia and Expo, 2017, pp. 1518–1523. [CrossRef]
- T. Ghosh, L. Li, and J. Chakareski, “Effective Deep Learning for Semantic Segmentation Based Bleeding Zone Detection in Capsule Endoscopy Images,” in Proceedings - International Conference on Image Processing, ICIP, 2018, pp. 3034–3038. [CrossRef]
- T. Aoki et al., “Automatic detection of blood content in capsule endoscopy images based on a deep convolutional neural network.,” J. Gastroenterol. Hepatol., vol. 35, no. 7, pp. 1196–1200, 2020. [CrossRef]
- Y. Hwang et al., “Improved classification and localization approach to small bowel capsule endoscopy using convolutional neural network.,” Dig. Endosc., 2020. [CrossRef]
- Tsuboi et al., “Artificial intelligence using a convolutional neural network for automatic detection of small-bowel angioectasia in capsule endoscopy images.,” Dig. Endosc., vol. 32, no. 3, pp. 382–390, 2020. [CrossRef]
- M. Sharif, M. Attique Khan, M. Rashid, M. Yasmin, F. Afza, and U. J. Tanik, “Deep CNN and geometric features-based gastrointestinal tract diseases detection and classification from wireless capsule endoscopy images,” J. Exp. Theor. Artif. Intell., 2019. [CrossRef]
- M. Hajabdollahi et al., “Segmentation of bleeding regions in wireless capsule endoscopy for detection of informative frames,” Biomed. Signal Process. Control, vol. 53, 2019. [CrossRef]
- M. A. Khan, M. Sharif, T. Akram, M. Yasmin, and R. S. Nayak, “Stomach Deformities Recognition Using Rank-Based Deep Features Selection.,” J. Med. Syst., vol. 43, no. 12, p. 329, 2019. [CrossRef]
- X. Jia and M. Q.-H. Meng, “A deep convolutional neural network for bleeding detection in Wireless Capsule Endoscopy images.,” Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Int. Conf., vol. 2016, pp. 639–642, 2016. [CrossRef]
- P. Zhuang, A. G. Schwing, and O. Koyejo, “FMRI data augmentation via synthesis,” Proc. - Int. Symp. Biomed. Imaging, vol. 2019-April, pp. 1783–1787, 2019. [CrossRef]
- H. Zhao, H. Li, S. Maurer-Stroh, and L. Cheng, “Synthesizing retinal and neuronal images with generative adversarial nets,” Med. Image Anal., vol. 49, pp. 14–26, 2018. [CrossRef]
- H. Zhao, H. Li, and L. Cheng, “Synthesizing Filamentary Structured Images with GANs,” pp. 1–10.
- V. Bellemo, P. Burlina, L. Yong, T. Y. Wong, and D. S. W. Ting, “Generative Adversarial Networks (GANs) for Retinal Fundus Image Synthesis,” Lect. Notes Comput. Sci. (including Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinformatics), vol. 11367 LNCS, no. March 2020, pp. 289–302, 2019. [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Articles published in peer-reviewed venues. | Articles that do not involve bleeding, lesion, or hemorrhage. |
| Articles published in the year: 2001-2021 | Articles not written in English. |
| Articles must address a set of keywords: (Bleeding OR Hemorrhage OR blood) AND (Detection OR Segmentation OR Recognition OR Classification) AND (Capsule Endoscopy) | Exclude articles for non-human. |
| Articles that describe an automatic computer-aided bleeding detection for capsule endoscopy. | Exclude poster and book chapter. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





