1. Introduction
Bone is a dynamic connective tissue that protects internal organs, helps in locomotion and in the maintenance of homeostasis. A common finding in various systemic and dental disorders, bone disorders as a result of trauma are characterized by disturbed natural healing, resulting in functional and structural oddness [
1]
. Periodontitis is an infectious disease of the tooth-supporting structures affecting about 20-50% of the world’s population [
2]. The rationale for periodontal treatment is bifold- to relieve symptoms as well as regenerate the destroyed tissues. Alternative regenerative procedures take the forefront when clinical periodontal therapy fails to regenerate lost periodontal tissues [
3]. Current research and developments in dentistry have revolutionized the approach to managing periodontal disease [
4]. While tissue engineering of periodontal structures is quite challenging due to its complex anatomy of soft tissue interspersed between two distinct hard tissue structures [
5], an interplay among materials, cells, and bioactive signals could initiate regeneration. Different grafts, materials, barrier membranes, and bone substitutes can restore osseous defects [
6]. Effective, yet limited scope due to additional surgery, inadequate bone supply, inappropriate biodegradation, immune response, and low tissue compatibility has shifted the focus to the fabrication & convention of natural biomaterials [
6].
Natural biomaterials and polymers have gained significant interest in the field of regeneration due to their superior biological properties. Preformed scaffolds or hydrogels, primary components of bone tissue engineering, have been widely researched and proved as promising bone regenerative materials. They are three-dimensional hydrophilic polymer chains which exhibit excellent mechanical strength, maximum penetration, and mimics extracellular matrix. However, certain limitations like poor integration, limited penetration and cost have paved the way for the use of injectable hydrogels in bone regeneration [
1].
Chitosan, a deacetylated chitin, is a natural polymer exhibiting exceptional biological properties like non-toxicity, anti-inflammatory, adaptability, biocompatibility, biodegradability, and bio-adhesion, thus stimulating osteoconductive functions and playing a pivotal role in tissue engineering, drug delivery, and wound healing [
7] . Its main drawbacks like lack of bioactivity and degradation have it cross-linked with other polymers such as alginate, gelatin, hyaluronic acid, amylopectin, carbon nanotubes, poly (methyl methacrylate), polylactic acid, growth factors and calcium phosphate to enhance mechanical and osteoconductive properties of chitosan [
8]. Yet, cross-linked chitosan alone fails to improve osteogenic potential completely [
9]. Hence with this interest, a wider literature search revealed the application of Fucoidan brown seaweed for biomedical purposes.
Fucoidan is a sulfated polysaccharide in brown seaweeds, like heparin and characterized by most biological activities, including cell proliferation and differentiation. Recent research shows fucoidan’s biomedical applications, which include anti-inflammatory, antiviral, antibacterial, anti-coagulant, anti-obesity, and anticancer properties [
10]. Fucoidan for bone tissue regeneration has been comprehensively reported, presenting both osteoclast and osteogenic potential. Fucoidan is known to promote bone markers confirmed by ALP activity, osteogenic gene expressions such as osteopontin, Runx2, and mineral deposition, and increase the proliferation of osteoblast MG-63 cells, human alveolar bone marrow-derived mesenchymal stem cells, and human amniotic fluid stem cells [
11,
12,
13]
Fucoidan containing chitosan hydrogel is now being researched to study its combined properties and has shown superior wound healing in animals, with faster dermal papillary formation and the closure of the wound after 14 days treatment [
14]. Similarly, chitosan- alginate- fucoidan scaffold has shown to be a promising material for bone regeneration [
15]. Fucoidan-induced osteogenesis studied through cellular mechanisms shows activation of mitogen-activated protein kinases (MAPKs), including JNK and extracellular signal-related kinase (ERK), bone morphogenetic protein-2 (BMP-2), and Smad 1/5/8 signaling. Currently, fucoidan encourages PI3K/Akt signaling pathways for osteoblastic differentiation in stem cells and angiogenesis during bone repair. In addition, various studies have been reported on the role of the Wnt/β catenin pathway in bone biology.
The extent of influence of fucoidan on the osteogenic potential of periodontal cells is unexplored and hence, we aimed to compare and evaluate the osteogenic potential of fucoidan-containing chitosan hydrogel and concentrated growth factor in managing periodontal intra-bony defects.
2. Results and Discussion
The mean age of the participants was 43.75 (5.638) years, with a higher percentage of females (60%, n=24). Statistically significantly lower plaque index scores were observed in both groups at 6 and 9 months as compared to baseline (p<.05), while significantly lower scores in all other clinical parameters were observed at all three-time intervals when compared to baseline in both groups. Intergroup analyses showed significantly lower scores were observed in the GCF group at 3 and 6 months, while both groups were comparable at 9 months (
Table 2). A statistically significant reduction was observed in radiographic parameters at 9 months in both groups (p<.05). A higher defect fill was seen in the fucoidan-chitosan group at 9 months, with lower CEJ-base measurement (
Table 3). The clinical and radiographic improvement is shown in
Figure 1.
Table 1.
Age and gender distribution:.
Table 1.
Age and gender distribution:.
| Age Group |
N=40 |
% |
| 31-40 years |
12 |
30 |
| 41-50 years |
23 |
57.5 |
| >50 years |
5 |
12.5 |
| Age |
Mean |
SD |
| Mean Age (years) |
43.75 |
5.638 |
| Gender |
N=40 |
% |
| Males |
16 |
40 |
| Females |
24 |
60 |
Table 2.
Comparison of clinical parameters between study groups.
Table 2.
Comparison of clinical parameters between study groups.
| Clinical Parameters |
Fucoidan-Chitosan Hydrogel |
CGF |
t- statistic |
p-Value$
Group 1 vs 2 |
| Mean |
SD |
Mean |
SD |
| N |
20 |
20 |
|
|
| Plaque Index |
| Baseline |
1.515 |
.2641 |
1.465 |
.2681 |
|
|
| 3 months |
1.265 |
.2581 |
1.445 |
.3379 |
2.192 |
0.037$ |
| p-value* |
<0.001* |
1.000
|
|
|
| 6 months |
1.140 |
.2479 |
.930 |
.1081 |
-2.297 |
0.027$ |
| p-value* |
<0.001* |
<0.001* |
|
|
| 9 months |
.945 |
.1234 |
.940 |
.1231 |
0.665 |
0.510 |
| p-value* |
<0.001* |
<0.001* |
|
|
| Gingival Index |
| Baseline |
1.550 |
.3426 |
1.620 |
.3270 |
|
|
| 3 months |
1.375 |
.3596 |
1.220 |
.2726 |
-2.239 |
0.036$ |
| p-value* |
<0.001* |
0.004* |
|
|
| 6 months |
1.320 |
.3238 |
1.370 |
.3045 |
-0.483 |
0.632 |
| p-value* |
<0.001* |
<0.001* |
|
|
| 9 months |
1.010 |
.1119 |
1.055 |
.1605 |
-0.291 |
0.773 |
| p-value* |
<0.001* |
<0.001* |
|
|
| Periodontal Probing Depth |
| Baseline |
7.55 |
.887 |
7.80 |
.894 |
|
|
| 3 months |
5.50 |
.946 |
6.80 |
.523 |
4.098 |
<0.001$ |
| p-value* |
<0.001* |
<0.001* |
|
|
| 6 months |
5.50 |
.946 |
6.35 |
.489 |
2.214 |
0.033$ |
| p-value* |
<0.001* |
<0.001* |
|
|
| 9 months |
4.50 |
.513 |
5.05 |
.224 |
0.993 |
0.327 |
| p-value* |
<0.001* |
<0.001* |
|
|
| Clinical Attachment Level |
| Baseline |
8.40 |
.598 |
8.45 |
.510 |
|
|
| 3 months |
7.30 |
1.031 |
6.80 |
.523 |
-2.377 |
0.023$ |
| p-value* |
<0.001* |
<0.001* |
|
|
| 6 months |
6.85 |
1.461 |
6.35 |
1.387 |
0.948 |
0.234 |
| p-value* |
0.001* |
<0.001* |
|
|
| 9 months |
5.15 |
.489 |
5.15 |
.366 |
-0.261 |
0.796 |
| p-value* |
<0.001* |
<0.001* |
|
|
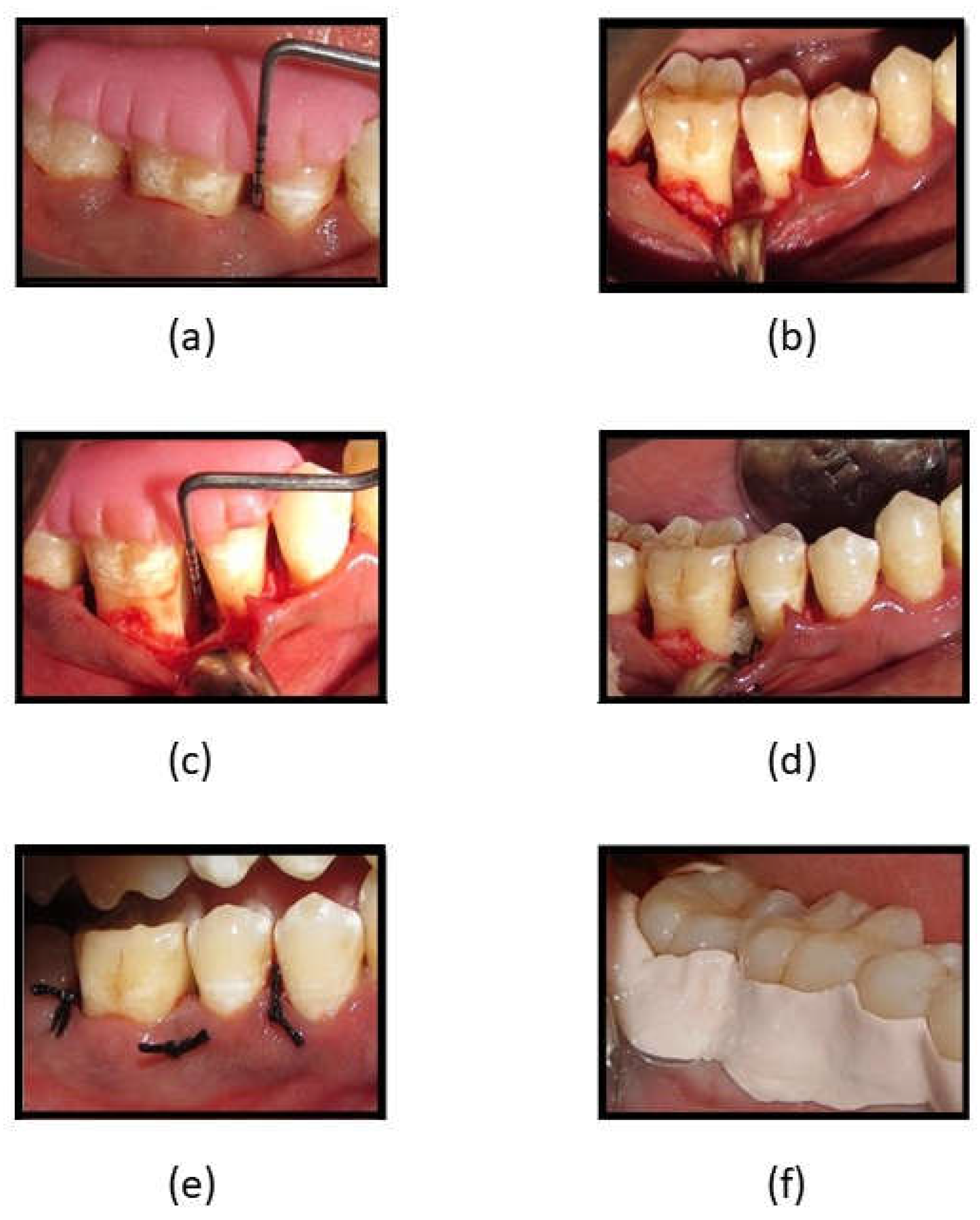
Figure 1.
(a) Reduction in PPD at 3 months in Fucoidan-Chitosan group; (b) Reduction in PPD at 6 months in Fucoidan-Chitosan group; (c) RVG image of intrabony defect at baseline in Fucoidan-Chitosan group; (d) RVG image of improvement in intrabony defect at 9 months in Fucoidan-Chitosan group; (e) RVG image of intrabony defect at baseline in CGF group; (f) RVG image of intrabony defect at 9 months in CGF group.
Figure 1.
(a) Reduction in PPD at 3 months in Fucoidan-Chitosan group; (b) Reduction in PPD at 6 months in Fucoidan-Chitosan group; (c) RVG image of intrabony defect at baseline in Fucoidan-Chitosan group; (d) RVG image of improvement in intrabony defect at 9 months in Fucoidan-Chitosan group; (e) RVG image of intrabony defect at baseline in CGF group; (f) RVG image of intrabony defect at 9 months in CGF group.
Table 3.
Comparison of radiographic parameters between study groups:.
Table 3.
Comparison of radiographic parameters between study groups:.
| Clinical Parameters |
Fucoidan-Chitosan |
CGF |
t statistic |
p-Value$
Group 1 vs 2 |
|
| Mean |
SD |
Mean |
SD |
|
| N |
20 |
20 |
|
|
|
| |
Cementoenamel Junction to Base |
|
| Baseline |
6.70 |
1.302 |
6.60 |
1.353 |
|
|
|
| 9 months |
4.95 |
.759 |
6.10 |
.718 |
|
|
|
| p-value* |
<0.001* |
0.008* |
3.817 |
<0.001$ |
|
| |
Cementoenamel Junction to Bone Level |
| Baseline |
3.55 |
.510 |
5.85 |
.745 |
|
|
|
| 9 months |
2.90 |
.447 |
4.90 |
.553 |
|
|
|
| p-value* |
<0.001* |
<0.001* |
-1.485 |
0.146 |
|
| |
Intrabony Defects |
| Baseline |
3.35 |
.489 |
3.45 |
.510 |
|
|
|
| 9 months |
2.10 |
.308 |
2.05 |
.224 |
|
|
|
| p-value* |
<0.001* |
<0.001* |
-0.900 |
0.374 |
|
| |
Defect Fill |
| 9 months |
1.20 |
.410 |
.20 |
.410 |
7.706 |
<0.001$ |
|
Tissue engineering is an upcoming interdisciplinary field, which focuses on replacing lost tissues and organs. Regeneration encompasses the use of four approaches - cells, scaffolds, signaling molecules and a genetic matrix. Since its inception, this scientific innovation has stimulated research on various materials to accomplish bone regeneration. Ideal features like biocompatibility, biodegradability, ease of application, adaptability to the tissues and bone repair are essential to successful regeneration. In this regard, natural polymers stand out and are researched globally, owing to their excellent biological properties, especially in the replacement of body parts. Thus, thrust areas in tissue engineering investigations are directed towards the repair of many different tissues like bone, cartilage, cornea, skin, liver, heart, and periodontal tissue.
In this study, we attempted to develop and compare the effectiveness of a thermosensitive injectable fucoidan-chitosan hydrogel with concentrated growth factor in the regeneration of periodontal intra-bony defects. The hydrogel was prepared using the sol gel method and its physical, chemical, and biological properties with stringently scrutinized to assess its efficacy in inducing bone formation [
16]. Fucoidan containing Chitosan hydrogel was light brown in colour, uniformly dispersed with no flakes seen. Semi-crystalline in nature, it had good solubility, interconnectivity, biocompatibility, degradation, and adequate water uptake and porosity. The particle size as measured using scanning electron microscopy was found to be 6.000nm, with X –ray diffraction analysis indicating the crystalline nature of the polymer [
16]. Biological characterization with MG-63 osteoblasts cell line showed good biocompatibility, biodegradability, slow and sustained drug release, increased cell proliferation, and enhanced alkaline phosphatase secretion, with greater mineralization properties [
16]. Chitosan, when combined with other cells and synthetic polymers, has shown to be osteo-promotive and osteo-inductive. Thus, in combination with Fucoidan, it takes on an osteogenic role [
15,
17,
18]. Histologically, this hydrogel has proven to be an accelerator of bone regeneration in rat tibia [
19].
Literature evidences the efficacy of chitosan for regenerating periodontal structures and concludes the superior potential of natural polymers combined with bio composites, growth factors, gene-activated matrix and signaling molecules in increasing bioactivity, thereby, enhancing bone regeneration [
20]. As chitosan’s excellent biocompatibility and degradation are coupled with low bioactivity, a combination with fucoidan was experimented with and showed promising results in treating periodontal intrabony defects. To the best of our knowledge, this is the first study on fucoidan-chitosan hydrogel for periodontal intra-bony defect correction; no studies showing the effectiveness of fucoidan natural-based polymer for periodontal bone regeneration in humans are available as research in this domain is still at the level of Phase I trials.
However, there are three reported studies on the effectiveness of chitosan gel in treating intra-bony defects. Boynuegri D, et al., 2009 [
21] reported a pocket depth reduction of 2.60 ± 0.17 mm, gain in the attachment of 1.80 ± 0.12 mm and intra-bony defect depth was 1.40 ± 0.08 mm at the end of 6 months with 1% chitosan gel with allograft, while Babrawala I, et al., 2019 [
22] reported that the use of 15% chitosan gel for 3 walled defects compared with open flap debridement as a control measure exhibited a significant PPD reduction of 5.30 ± 0.822 mm, CAL gain of 5.80 ± 0.499 mm, reduction in IBD depth of 3.00 ± 0.497 mm and defect resolution of 78.32 ± 5.80% at 9 months. The effectiveness of chitosan nano hydrogel as a bone regenerative material showed a mean reduction in PPD & CAL in the chitosan group when compared with allograft as reported by Meenakshi SS, et al., 2021 [
23]. The results we obtained for the fucoidan-chitosan combination have only strengthened the evidence upholding its regenerative potential. This positive effect can be attributed to the ability of fucoidan in inducing osteogenic differentiation in human-derived stem cells, increasing ALP activity, calcium accumulation, increasing osteocalcin, osteopontin, BMP and RUN x2. Fucoidan is known to increase phosphorylation (e (PI3K) isoforms, p110α and p110γ isoforms), and increase expression of β-catenin while chitosan structurally resembles the glycosaminoglycan hyaluronic acid which is found in extracellular matrices of many tissues.
This study is not without limitations. While the sample size used in this study was within the range adopted by most clinical regenerative studies in humans, scope exists to reproduce this protocol with a larger sample size to revalidate the present study findings. The limited sample size limits the external validity or generalizability. Another limiting factor was ethical restraints, due to which surgical re-entry and histologic investigation could not be performed to assess the nature of the regenerated bone.
3. Conclusions
Fucoidan- Chitosan hydrogel exhibited statistically significantly lower PPD values as compared to the CGF group at the end of 3 and 6 months. The mean reduction in clinical attachment level with the combination was significantly higher than CGF, with an increased bone defect fill in the combination at 9 months, thus proving the efficacy of Fucoidan–chitosan hydrogel as a promising material for tissue engineering applications.
4. Materials and Methods
A randomized controlled trial clinical trial, following CONSORT guidelines, was conducted among subjects visiting the outpatient services in the Department of Periodontics, KLE Society’s Institute of Dental Sciences, Bangalore, India. The sample size was estimated considering a small effect size (0.2) for the two-tailed hypothesis, keeping the power at 80% and the margin of the alpha error at 5% using GPower software v. 3.1.9.2. (Heinrich-Heine University) [
24]. 40 subjects with 20 in each study group were recruited using the convenience sampling method.
The study was followed up for nine months to evaluate the effectiveness of Fucoidan - Chitosan nano hydrogel and concentrated growth factor as a bone regenerative material in periodontal intra-bony defects. Before commencing, clearance for the study was obtained from the institutional ethics committee and written informed consent was obtained from every participant. Subjects aged between 25 to 50 years, without the presence of systemic diseases, non-smokers with chronic periodontitis and a pocket depth of 5 - 7 mm [
25] accompanied by two wall intra-bony defects and less than <25 defect angle were recruited. Subjects who have undertaken the treatment within a year, pregnant women & having mobile furcation-involved teeth were excluded from the study.
Preparation of hydrogel containing fucoidan–chitosan: Chitosan (Everest Biotech Pharma, Bangalore) was subjected to purification by dissolution-precipitation, dialysis, and deacetylation up to an 85% degree to produce gels at 37◦C following simple neutralization with sodium hydroxide. On exposure to sodium hydroxide, the hydroxide ions (OH-) react with the amino groups (-NH2) present in chitosan to removal of protons (H+) from the amino groups, resulting in their deprotonation and conversion into amino groups (-NH). The gelation was slow with a weak increase in viscosity to produce a stable formulation for a medical application [
26]. Fucoidan from brown seaweed species,
Sargassum wightii, was collected, dried overnight in an oven, milled, and strained. 1g/mg of seaweed was mixed with 10 ml solvent, stored for 2 days, and centrifuged at 10000 rpm for 15 minutes of which 20 mg powder was treated with ethanol, stirred for 12 hours, and centrifuged for 20 mins [
27]. To prepare the hydrogel, Gelatin was dissolved homogeneously in 100 ml of water by stirring for 1 hour, to which 100 mg of fucoidan was added with the help of a dropper while the stirring continued for another hour to dissolve the entire polymer in gelatin. Chitosan was then added to the mixture solution and was continuously stirred for 2 hours until it attained homogeneity. 0.1% Methyl paraben was added to the gel as a preservative and the hydrogel was sterilized by autoclaving (121 c for 15 mins).
Preparation of concentrated growth factor: CGF was prepared by collecting 10 ml venous blood which was centrifuged for 4 mins at 2700 rpm. After centrifugation, the layer with growth factor was carried with the instrument and adapted to the membrane [
28]. The gels were loaded in 2ml short needle syringes for ease of placement in the defect site during periodontal surgery. The surgical operator and the subjects were blinded to the study groups and the test products.
Study Method- Before the commencement of the study, personalized acrylic stents were designed for each patient to fit the selected teeth. At the defect site on the stent, vertical grooves were made to guide the probe penetration in the plane. Detailed history and periodontal examination were performed for all the study participants, following which phase I therapy consisting of scaling, root planning, and oral hygiene instructions were completed. Clinical measurements recorded included Plaque Index (PI) by Silness and Loe (1964)[
29], Gingival Index (GI) by Loe and Silness (1963)[
30], Periodontal Probing Depth (PPD) and Clinical attachment level (CAL). The former two indices were evaluated as 0-3, based on the range of plaque and gingivitis present. An overall score was then estimated for each participant. The latter two are manifestations of periodontitis where PPD is measured from the gingival margin, that usually changes across time while CAL relies on a fixed reference point like the cementoenamel junction, until the base of the sulcus. The measurements (in mm) were recorded using a graduated periodontal probe. Participants were then randomized in 1:1 allocation randomization sequence using a computer-generated table of random numbers into Group 1 receiving the Fucoidan-chitosan hydrogel and Group 2 receiving concentrated growth factor. The respective surgical procedure was performed and assessed for the outcome. All the clinical, and radiographic measurements and surgeries were performed by one examiner who was blinded to the study groups. Patients were blinded for allocation to a particular group and treatment. The study design and participant flow are provided in the
supplemental information.
Surgical procedure-Before the surgical procedure, the defect site was anesthetized by administering 2% lignocaine and an epinephrine concentration of 1:80,000, following which sulcular incisions were made on the buccal and lingual aspects of the involved sites and full-thickness mucoperiosteal flaps was raised. Area-specific curettes and ultrasonic scalers were used for thorough debridement, after which, the defect site in participants allocated to Group 1 was filled with Fucoidan containing chitosan hydrogel as a bone regenerative material, and in Group 2 had CGF for bone regeneration. Simple interrupted sutures were placed to achieve primary wound closure followed by the placement of periodontal dressing.
Postoperative wound management: Immediately after surgery, 500 mg of amoxicillin every 6 h for 5 days, 400 mg of Ibuprofen every 8 hours, and 0.2% chlorhexidine digluconate mouthwash twice daily for 4 weeks were prescribed to all the participants. They were re-evaluated for pain, sensitivity and discomfort and sutures were removed after 7 days. Clinical parameters, radiographic assessments using the stent and bone fill and alveolar crest height using linear measurement analysis of radiovisiography and intraoral periapical radiographs were performed at intervals of 3, 6, and 9 months post-surgically. The periodontal status was recorded considering the hard and soft tissue measurements 9 months post-surgically (
Figure 2).
Statistical Analysis:
Descriptive statistics were computed for all study parameters. Data were normally distributed as assessed by the Shapiro-Wilk test. The difference in the mean measurements of hard and soft tissue parameters between the two groups at all time points was assessed using the Independent Samples t-test, while intragroup comparisons across the time intervals were assessed using Paired t-test and Repeated Measures ANOVA, followed by Bonferroni post- hoc analysis. The difference in the values was significant at a p-value of less than 0.05.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
Conceptualization, S.E., K.K.; methodology, S.E, K.K.; formal analysis, A.B., V.J.; investigation, S.E., S.M.; resources, S.E., S.M.,S.S.; data curation, S.E.; writing—original draft preparation, S.E., A.B.; writing—review and editing, V.J.; visualization, A.B., V.J.; supervision, K.K..; project administration, S.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of KLE Society’s Institute of Dental Sciences, Bengaluru, India (Protocol code: KIDS/IEC/Nov-2017/8; date of approval: 21.11.2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical reasons and confidentiality.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saravanan S, Vimalraj S, Thanikaivelan P, Banudevi S, Manivasagam G. A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. Int J Biol Macromol. 2019 Jan;121:38–54. [CrossRef]
- Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci. 2017;11(2):72–80. PMID: 28539867; PMCID: PMC5426403.
- Chen FM, Jin Y. Periodontal Tissue Engineering and Regeneration: Current Approaches and Expanding Opportunities. Tissue Eng Part B Rev. 2010 Apr;16(2):219–55. [CrossRef]
- Chen FM, An Y, Zhang R, Zhang M. New insights into and novel applications of release technology for periodontal reconstructive therapies. J Controlled Release. 2011 Jan;149(2):92–110. [CrossRef]
- Changotade SIT, Korb G, Bassil J, Barroukh B, Willig C, Colliec-Jouault S, et al. Potential effects of a low-molecular-weight fucoidan extracted from brown algae on bone biomaterial osteoconductive properties. J Biomed Mater Res A. 2008 Dec 1;87A(3):666–75. [CrossRef]
- Cho YS, Jung WK, Kim JA, Choi IW, Kim SK. Beneficial effects of fucoidan on osteoblastic MG-63 cell differentiation. Food Chem. 2009 Oct;116(4):990–4. [CrossRef]
- İkinci G, Şenel S, Akıncıbay H, Kaş S, Erciş S, Wilson CG, et al. Effect of chitosan on a periodontal pathogen Porphyromonas gingivalis. Int J Pharm. 2002 Mar;235(1–2):121–7. [CrossRef]
- Kasperk C, Wergedal J, Strong D, Farley J, Wangerin K, Gropp H, et al. Human bone cell phenotypes differ depending on their skeletal site of origin. J Clin Endocrinol Metab. 1995 Aug;80(8):2511–7. [CrossRef]
- Klokkevold PR, Vandemark L, Kenney EB, Bernard GW. Osteogenesis Enhanced by Chitosan (Poly-N-Acetyl Glucosaminoglycan) In Vitro. J Periodontol. 1996 Nov;67(11):1170–5. [CrossRef]
- Li B, Lu F, Wei X, Zhao R. Fucoidan: Structure and Bioactivity. Molecules. 2008 Aug 12;13(8):1671–95. [CrossRef]
- Mao JS, Zhao LG, Yin YJ, Yao KD. Structure and properties of bilayer chitosan–gelatin scaffolds. Biomaterials. 2003 Mar;24(6):1067–74. [CrossRef]
- Park SJ, Lee KW, Lim DS, Lee S. The Sulfated Polysaccharide Fucoidan Stimulates Osteogenic Differentiation of Human Adipose-Derived Stem Cells. Stem Cells Dev. 2012 Aug 10;21(12):2204–11. [CrossRef]
- Kim BS, Kang HJ, Park JY, Lee J. Fucoidan promotes osteoblast differentiation via JNK- and ERK-dependent BMP2–Smad 1/5/8 signaling in human mesenchymal stem cells. Exp Mol Med. 2015 Jan 9;47(1):e128–e128. [CrossRef]
- Sezer AD, Cevher E, Hatıpoğlu F, Oğurtan Z, Baş AL, Akbuğa J. Preparation of Fucoidan-Chitosan Hydrogel and Its Application as Burn Healing Accelerator on Rabbits. Biol Pharm Bull. 2008;31(12):2326–33. [CrossRef]
- Venkatesan J, Bhatnagar I, Kim SK. Chitosan-Alginate Biocomposite Containing Fucoidan for Bone Tissue Engineering. Mar Drugs. 2014 Jan 16;12(1):300–16. [CrossRef]
- Shruthi Eshwar, Kranthi K, Supriya Manvi, Kalavathy D J. Development, and in vitro evaluation of Fucoidan Injectable Hydrogel Containing gelatine and chitosan in bone regeneration. Int J Res Pharm Sci 2020;11(4): 7309- 16. [CrossRef]
- Lowe B, Venkatesan J, Anil S, Shim MS, Kim SK. Preparation and characterization of chitosan-natural nano hydroxyapatite-fucoidan nanocomposites for bone tissue engineering. Int J Biol Macromol. 2016 Dec;93:1479–87. [CrossRef]
- Lu HT, Huang GY, Chang WJ, Lu TW, Huang TW, Ho MH, et al. Modification of chitosan nanofibers with CuS and fucoidan for antibacterial and bone tissue engineering applications. Carbohydr Polym. 2022 Apr;281:119035. [CrossRef]
- Eshwar S, Kranthi K, Manvi S, Ashok P, Surana YS, Sangeetha R, et al. Histological Assessment of Fucoidan Gelatine Chitosan Compound Injectable Hydrogel for Bone Regeneration in Wistar Rats. Indian J Pharm Sci [Internet]. 2021 [cited 2023 May 26];83(6). [CrossRef]
- Ribeiro JCV, Vieira RS, Melo IM, Araújo VMA, Lima V. Versatility of Chitosan-Based Biomaterials and Their Use as Scaffolds for Tissue Regeneration. Sci World J. 2017;2017:1–25. [CrossRef]
- Boynueğri D, Özcan G, Şenel S, Uç D, Uraz A, Öğüş E, et al. Clinical and radiographic evaluations of chitosan gel in periodontal intraosseous defects: A pilot study. J Biomed Mater Res B Appl Biomater. 2009 Jan 14;90B(1):461–6. [CrossRef]
- Babrawala I, Prabhuji ML, Karthikeyan BV. Using a Composite Graft of Natural 15% Chitosan Gel in the Management of Intrabony Defects: A Case Series. J Int Acad Periodontol. 2019 Jan 31;21(1):4–10. PMID: 31522157.
- Meenakshi SS, Sankari M. Effectiveness of Chitosan Nanohydrogel as a Bone Regenerative Material in Intrabony Defects in Patients with Chronic Periodontitis: A Randomized Clinical Trial. J Adv Oral Res. 2021 Nov;12(2):222–8. [CrossRef]
- Kang H. Sample size determination and power analysis using the G*Power software. J Educ Eval Health Prof. 2021 Jul 30;18:17. [CrossRef]
- Armitage GC. Development of a Classification System for Periodontal Diseases and Conditions. Ann Periodontol. 1999 Dec;4(1):1–6. [CrossRef]
- Younes I, Rinaudo M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar Drugs. 2015 Mar 2;13(3):1133–74. [CrossRef]
- Immanuel G, Sivagnanavelmurugan M, Marudhupandi T, Radhakrishnan S, Palavesam A. The effect of fucoidan from brown seaweed Sargassum wightii on WSSV resistance and immune activity in shrimp Penaeus monodon (Fab). Fish Shellfish Immunol. 2012 Apr;32(4):551–64. [CrossRef]
- Kumar Y P. Role of CGF (Concentrated Growth Factor) in periodontal regeneration. J Dent Health Oral Disord Ther 2018;9(5):350-2. [CrossRef]
- Silness J, Löe H. Periodontal Disease in Pregnancy II. Correlation Between Oral Hygiene and Periodontal Condition. Acta Odontol Scand. 1964 Jan;22(1):121–35. [CrossRef]
- Löe H, Silness J. Periodontal Disease in Pregnancy I. Prevalence and Severity. Acta Odontol Scand. 1963 Jan;21(6):533–51. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).