Submitted:
31 May 2023
Posted:
01 June 2023
You are already at the latest version
Abstract
Keywords:
1. A brief introduction to APE1 biology and different functions
2. APE1 and cancer: a focus on polymorphisms and tissue expression
3. APE1 as a promising therapeutic target
3.1. Targeting the APE1 endonuclease activity
3.2. Targeting the APE1 redox activity
3.3. Targeting both the APE1 endonuclease and redox activities
4. Future perspectives from targeting the non-canonical roles of APE1 in miRNA processing
5. Secreted APE1 as a novel prognostic non-invasive biomarker of cancer development
6. Conclusive remarks and future perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Antoniali, G.; Malfatti, M.C.; Tell, G. Unveiling the Non-Repair Face of the Base Excision Repair Pathway in RNA Processing: A Missing Link between DNA Repair and Gene Expression? DNA Repair (Amst.) 2017, 56, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Malfatti, M.C.; Antoniali, G.; Codrich, M.; Burra, S.; Mangiapane, G.; Dalla, E.; Tell, G. New Perspectives in Cancer Biology from a Study of Canonical and Non-Canonical Functions of Base Excision Repair Proteins with a Focus on Early Steps. Mutagenesis 2020, 35, 129–149. [Google Scholar] [CrossRef] [PubMed]
- Malfatti, M.C.; Antoniali, G.; Codrich, M.; Tell, G. Coping with RNA Damage with a Focus on APE1, a BER Enzyme at the Crossroad between DNA Damage Repair and RNA Processing/Decay. DNA Repair 2021, 104, 103133. [Google Scholar] [CrossRef]
- Allinson, S.L.; Sleeth, K.M.; Matthewman, G.E.; Dianov, G.L. Orchestration of Base Excision Repair by Controlling the Rates of Enzymatic Activities. DNA Repair 2004, 3, 23–31. [Google Scholar] [CrossRef]
- Hurley, L.H.; Wheelhouse, R.T.; Sun, D.; Kerwin, S.M.; Salazar, M.; Fedoroff, O.Y.; Han, F.X.; Han, H.; Izbicka, E.; Von Hoff, D.D. G-Quadruplexes as Targets for Drug Design. Pharmacol. Ther. 2000, 85, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Burrows, C.J. G-Quadruplex Folds of the Human Telomere Sequence Alter the Site Reactivity and Reaction Pathway of Guanine Oxidation Compared to Duplex DNA. Chemical Research in Toxicology 2013, 26, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Zhu, J.; Ding, Y.; Visser, J.A.; Zhu, J.; Burrows, C.J. Human DNA Repair Genes Possess Potential G-Quadruplex Sequences in Their Promoters and 5’-Untranslated Regions. Biochemistry 2018, 57, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Burrows, C.J. 8-Oxo-7,8-Dihydroguanine, Friend and Foe: Epigenetic-like Regulator versus Initiator of Mutagenesis. DNA Repair (Amst.) 2017, 56, 75–83. [Google Scholar] [CrossRef]
- Fleming, A.M.; Zhu, J.; Ding, Y.; Burrows, C.J. 8-Oxo-7,8-Dihydroguanine in the Context of a Gene Promoter G-Quadruplex Is an On–Off Switch for Transcription. ACS Chemical Biology 2017, 12, 2417–2426. [Google Scholar] [CrossRef]
- Burra, S.; Marasco, D.; Malfatti, M.C.; Antoniali, G.; Virgilio, A.; Esposito, V.; Demple, B.; Galeone, A.; Tell, G. Human AP-Endonuclease (Ape1) Activity on Telomeric G4 Structures Is Modulated by Acetylatable Lysine Residues in the N-Terminal Sequence. DNA Repair (Amst.) 2019, 73, 129–143. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Pramanik, S.; Harris, H.L.; Tarpley, M.; Sarkar, A.; Spagnol, G.; Sorgen, P.L.; Chowdhury, D.; Band, V.; Klinkebiel, D.; et al. Endogenous Oxidized DNA Bases and APE1 Regulate the Formation of G-Quadruplex Structures in the Genome. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 11409–11420. [Google Scholar] [CrossRef] [PubMed]
- Antoniali, G.; Serra, F.; Lirussi, L.; Tanaka, M.; D’Ambrosio, C.; Zhang, S.; Radovic, S.; Dalla, E.; Ciani, Y.; Scaloni, A.; et al. Mammalian APE1 Controls MiRNA Processing and Its Interactome Is Linked to Cancer RNA Metabolism. Nat Commun 2017, 8, 797. [Google Scholar] [CrossRef]
- Barnes, T.; Kim, W.-C.; Mantha, A.K.; Kim, S.-E.; Izumi, T.; Mitra, S.; Lee, C.H. Identification of Apurinic/Apyrimidinic Endonuclease 1 (APE1) as the Endoribonuclease That Cleaves c-Myc MRNA. Nucleic Acids Research 2009, 37, 3946–3958. [Google Scholar] [CrossRef] [PubMed]
- Chohan, M.; Mackedenski, S.; Li, W.-M.; Lee, C.H. Human Apurinic/Apyrimidinic Endonuclease 1 (APE1) Has 3′ RNA Phosphatase and 3′ Exoribonuclease Activities. Journal of Molecular Biology 2015, 427, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Malfatti, M.C.; Balachander, S.; Antoniali, G.; Koh, K.D.; Saint-Pierre, C.; Gasparutto, D.; Chon, H.; Crouch, R.J.; Storici, F.; Tell, G. Abasic and Oxidized Ribonucleotides Embedded in DNA Are Processed by Human APE1 and Not by RNase H2. Nucleic Acids Res. 2017, 45, 11193–11212. [Google Scholar] [CrossRef] [PubMed]
- Malfatti, M.C.; Codrich, M.; Dalla, E.; D’Ambrosio, C.; Storici, F.; Scaloni, A.; Tell, G. AUF1 Recognizes 8-Oxo-Guanosine Embedded in DNA and Stimulates APE1 Endoribonuclease Activity. Antioxidants & Redox Signaling 2023, ars.2022.0105. [CrossRef]
- Tosolini, D.; Antoniali, G.; Dalla, E.; Tell, G. Role of Phase Partitioning in Coordinating DNA Damage Response: Focus on the Apurinic Apyrimidinic Endonuclease 1 Interactome. Biomolecular Concepts 2020, 11, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, H.; McMahon, A.; Yan, S. APE1 Assembles Biomolecular Condensates to Promote the ATR–Chk1 DNA Damage Response in Nucleolus. Nucleic Acids Research 2022, 50, 10503–10525. [Google Scholar] [CrossRef]
- Dall’Agnese, G.; Dall’Agnese, A.; Banani, S.F.; Codrich, M.; Malfatti, M.C.; Antoniali, G.; Tell, G. Role of Condensates in Modulating DNA Repair Pathways and Its Implication for Chemoresistance. Journal of Biological Chemistry 2023, 104800. [Google Scholar] [CrossRef]
- Mangiapane, G.; Parolini, I.; Conte, K.; Malfatti, M.C.; Corsi, J.; Sanchez, M.; Pietrantoni, A.; D’Agostino, V.G.; Tell, G. Enzymatically Active Apurinic/Apyrimidinic Endodeoxyribonuclease 1 Is Released by Mammalian Cells through Exosomes. J Biol Chem 2021, 296, 100569. [Google Scholar] [CrossRef]
- Fishel, M.L.; Kelley, M.R. The DNA Base Excision Repair Protein Ape1/Ref-1 as a Therapeutic and Chemopreventive Target. Molecular Aspects of Medicine 2007, 28, 375–395. [Google Scholar] [CrossRef]
- Shah, F.; Logsdon, D.; Messmann, R.A.; Fehrenbacher, J.C.; Fishel, M.L.; Kelley, M.R. Exploiting the Ref-1-APE1 Node in Cancer Signaling and Other Diseases: From Bench to Clinic. npj Precision Onc 2017, 1, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lirussi, L.; Antoniali, G.; D’Ambrosio, C.; Scaloni, A.; Nilsen, H.; Tell, G. APE1 Polymorphic Variants Cause Persistent Genomic Stress and Affect Cancer Cell Proliferation. Oncotarget 2016, 7, 26293–26306. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.S.; Murphy, D.L.; Sweasy, J.B. Base Excision Repair and Cancer. Cancer Lett 2012, 327, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, A.M.; Stark, W.J.; Flynn, T.S.; Freudenthal, B.D. Molecular and Structural Characterization of Disease-Associated APE1 Polymorphisms. DNA Repair (Amst) 2020, 91–92, 102867. [Google Scholar] [CrossRef]
- Kim, W.C.; Ma, C.; Li, W.-M.; Chohan, M.; Wilson III, D.M.; Lee, C.H. Altered Endoribonuclease Activity of Apurinic/Apyrimidinic Endonuclease 1 Variants Identified in the Human Population. PLoS One 2014, 9, e90837. [Google Scholar] [CrossRef]
- Wilson, D.M.; Kim, D.; Berquist, B.R.; Sigurdson, A.J. Variation in Base Excision Repair Capacity. Mutat Res 2011, 711, 100–112. [Google Scholar] [CrossRef]
- Pieretti, M.; Khattar, N.H.; Smith, S.A. Common Polymorphisms and Somatic Mutations in Human Base Excision Repair Genes in Ovarian and Endometrial Cancers. Mutat Res 2001, 432, 53–59. [Google Scholar] [CrossRef]
- Illuzzi, J.L.; Harris, N.A.; Manvilla, B.A.; Kim, D.; Li, M.; Drohat, A.C.; Iii, D.M.W. Functional Assessment of Population and Tumor-Associated APE1 Protein Variants. PLOS ONE 2013, 8, e65922. [Google Scholar] [CrossRef]
- Abyzov, A.; Uzun, A.; Strauss, P.R.; Ilyin, V.A. An AP Endonuclease 1-DNA Polymerase Beta Complex: Theoretical Prediction of Interacting Surfaces. PLoS Comput Biol 2008, 4, e1000066. [Google Scholar] [CrossRef]
- Hinz, J.M.; Mao, P.; McNeill, D.R.; Wilson, D.M. Reduced Nuclease Activity of Apurinic/Apyrimidinic Endonuclease (APE1) Variants on Nucleosomes: IDENTIFICATION OF ACCESS RESIDUES. J Biol Chem 2015, 290, 21067–21075. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the CBioPortal. Sci Signal 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The CBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Reva, B.; Antipin, Y.; Sander, C. Predicting the Functional Impact of Protein Mutations: Application to Cancer Genomics. Nucleic Acids Research 2011, 39, e118. [Google Scholar] [CrossRef] [PubMed]
- Reva, B.; Antipin, Y.; Sander, C. Determinants of Protein Function Revealed by Combinatorial Entropy Optimization. Genome Biol 2007, 8, R232. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Shin, J.H.; Lee, Y.R.; Joo, H.K.; Song, K.H.; Na, Y.G.; Chang, S.J.; Lim, J.S.; Jeon, B.H. Urinary APE1/Ref-1: A Potential Bladder Cancer Biomarker. Dis Markers 2016, 2016, 7276502. [Google Scholar] [CrossRef]
- Fishel, M.L.; Xia, H.; McGeown, J.; McIlwain, D.W.; Elbanna, M.; Craft, A.A.; Kaimakliotis, H.Z.; Sandusky, G.E.; Zhang, C.; Pili, R.; et al. Antitumor Activity and Mechanistic Characterization of APE1/Ref-1 Inhibitors in Bladder Cancer. Mol Cancer Ther 2019, 18, 1947–1960. [Google Scholar] [CrossRef]
- Shin, J.H.; Choi, S.; Lee, Y.R.; Park, M.S.; Na, Y.G.; Irani, K.; Lee, S.D.; Park, J.B.; Kim, J.M.; Lim, J.S.; et al. APE1/Ref-1 as a Serological Biomarker for the Detection of Bladder Cancer. Cancer Res Treat 2015, 47, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zeng, J.; Lele, S.; LaGrange, C.A.; Bhakat, K.K. APE1 and SSRP1 Is Overexpressed in Muscle Invasive Bladder Cancer and Associated with Poor Survival. Heliyon 2021, 7, e06756. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-A.; Yang, B.; Tang, T.; Yang, Y.; Zhang, D.; Xiao, H.; Xu, J.; Wang, L.; Lin, L.; Jiang, J. Correlation of APE1 with VEGFA and CD163+ Macrophage Infiltration in Bladder Cancer and Their Prognostic Significance. Oncol Lett 2020, 20, 2881–2887. [Google Scholar] [CrossRef]
- Güllü Amuran, G.; Tinay, I.; Filinte, D.; Ilgin, C.; Peker Eyüboğlu, I.; Akkiprik, M. Urinary Micro-RNA Expressions and Protein Concentrations May Differentiate Bladder Cancer Patients from Healthy Controls. Int Urol Nephrol 2020, 52, 461–468. [Google Scholar] [CrossRef]
- Kumar, M.; Shukla, V.K.; Misra, P.K.; Raman, M.J. Dysregulated Expression and Subcellular Localization of Base Excision Repair (BER) Pathway Enzymes in Gallbladder Cancer. Int J Mol Cell Med 2018, 7, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, C.; Lu, H.; Yin, M.; Shao, C.; Hu, X.; Wu, J.; Wang, Y. The Expression of APE1 in Triple-Negative Breast Cancer and Its Effect on Drug Sensitivity of Olaparib. Tumour Biol 2017, 39, 1010428317713390. [Google Scholar] [CrossRef]
- Woo, J.; Park, H.; Sung, S.H.; Moon, B.-I.; Suh, H.; Lim, W. Prognostic Value of Human Apurinic/Apyrimidinic Endonuclease 1 (APE1) Expression in Breast Cancer. PLoS One 2014, 9, e99528. [Google Scholar] [CrossRef] [PubMed]
- Jian, D.; Li, X.-M.; Dai, N.; Liang, D.-D.; Zhang, G.; Mao, C.-Y.; Wang, D.; Song, G.-B.; Li, M.-X.; Luo, H. Inhibition of APE1 Expression Enhances the Antitumor Activity of Olaparib in Triple-Negative Breast Cancer. Evid Based Complement Alternat Med 2022, 2022, 6048017. [Google Scholar] [CrossRef] [PubMed]
- Poletto, M.; Di Loreto, C.; Marasco, D.; Poletto, E.; Puglisi, F.; Damante, G.; Tell, G. Acetylation on Critical Lysine Residues of Apurinic/Apyrimidinic Endonuclease 1 (APE1) in Triple Negative Breast Cancers. Biochem Biophys Res Commun 2012, 424, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Malfatti, M.C.; Gerratana, L.; Dalla, E.; Isola, M.; Damante, G.; Di Loreto, C.; Puglisi, F.; Tell, G. APE1 and NPM1 Protect Cancer Cells from Platinum Compounds Cytotoxicity and Their Expression Pattern Has a Prognostic Value in TNBC. J Exp Clin Cancer Res 2019, 38, 309. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fatah, T.M.A.; Perry, C.; Moseley, P.; Johnson, K.; Arora, A.; Chan, S.; Ellis, I.O.; Madhusudan, S. Clinicopathological Significance of Human Apurinic/Apyrimidinic Endonuclease 1 (APE1) Expression in Oestrogen-Receptor-Positive Breast Cancer. Breast Cancer Res Treat 2014, 143, 411–421. [Google Scholar] [CrossRef]
- Herring, C.J.; West, C.M.L.; Wilks, D.P.; Davidson, S.E.; Hunter, R.D.; Berry, P.; Forster, G.; MacKinnon, J.; Rafferty, J.A.; Elder, R.H.; et al. Levels of the DNA Repair Enzyme Human Apurinic/Apyrimidinic Endonuclease (APE1, APEX, Ref-1) Are Associated with the Intrinsic Radiosensitivity of Cervical Cancers. Br J Cancer 1998, 78, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Schindl, M.; Oberhuber, G.; Pichlbauer, E.G.; Obermair, A.; Birner, P.; Kelley, M.R. DNA Repair-Redox Enzyme Apurinic Endonuclease in Cervical Cancer: Evaluation of Redox Control of HIF-1alpha and Prognostic Significance. Int J Oncol 2001, 19, 799–802. [Google Scholar] [CrossRef]
- Li, Q.; Wei, X.; Zhou, Z.-W.; Wang, S.-N.; Jin, H.; Chen, K.-J.; Luo, J.; Westover, K.D.; Wang, J.-M.; Wang, D.; et al. GADD45α Sensitizes Cervical Cancer Cells to Radiotherapy via Increasing Cytoplasmic APE1 Level. Cell Death Dis 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Z.-W.; Duan, W.; Qian, C.-Y.; Wang, S.-N.; Deng, M.-S.; Zi, D.; Wang, J.-M.; Mao, C.-Y.; Song, G.; et al. Inhibiting the Redox Function of APE1 Suppresses Cervical Cancer Metastasis via Disengagement of ZEB1 from E-Cadherin in EMT. J Exp Clin Cancer Res 2021, 40, 220. [Google Scholar] [CrossRef]
- Bhakat, K.K.; Sengupta, S.; Adeniyi, V.F.; Roychoudhury, S.; Nath, S.; Bellot, L.J.; Feng, D.; Mantha, A.K.; Sinha, M.; Qiu, S.; et al. Regulation of Limited N-Terminal Proteolysis of APE1 in Tumor via Acetylation and Its Role in Cell Proliferation. Oncotarget 2016, 7, 22590–22604. [Google Scholar] [CrossRef] [PubMed]
- Kakolyris, S.; Kaklamanis, L.; Engels, K.; Turley, H.; Hickson, I.D.; Gatter, K.C.; Harris, A.L. Human Apurinic Endonuclease 1 Expression in a Colorectal Adenoma-Carcinoma Sequence. Cancer Res 1997, 57, 1794–1797. [Google Scholar] [PubMed]
- Lou, D.; Zhu, L.; Ding, H.; Dai, H.-Y.; Zou, G.-M. Aberrant Expression of Redox Protein Ape1 in Colon Cancer Stem Cells. Oncol Lett 2014, 7, 1078–1082. [Google Scholar] [CrossRef]
- Codrich, M.; Comelli, M.; Malfatti, M.C.; Mio, C.; Ayyildiz, D.; Zhang, C.; Kelley, M.R.; Terrosu, G.; Pucillo, C.E.M.; Tell, G. Inhibition of APE1-Endonuclease Activity Affects Cell Metabolism in Colon Cancer Cells via a P53-Dependent Pathway. DNA Repair (Amst) 2019, 82, 102675. [Google Scholar] [CrossRef]
- Kühl Svoboda Baldin, R.; Austrália Paredes Marcondes Ribas, C.; de Noronha, L.; Veloso da Silva-Camargo, C.C.; Santos Sotomaior, V.; Martins Sebastião, A.P.; Vasconcelos de Castilho, A.P.; Rodrigues Montemor Netto, M. Expression of Parkin, APC, APE1, and Bcl-XL in Colorectal Polyps. J Histochem Cytochem 2021, 69, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Noike, T.; Miwa, S.; Soeda, J.; Kobayashi, A.; Miyagawa, S. Increased Expression of Thioredoxin-1, Vascular Endothelial Growth Factor, and Redox Factor-1 Is Associated with Poor Prognosis in Patients with Liver Metastasis from Colorectal Cancer. Hum Pathol 2008, 39, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zeng, J.; Roychoudhury, S.; Biswas, P.; Mohapatra, B.; Ray, S.; Dowlatshahi, K.; Wang, J.; Band, V.; Talmon, G.; et al. Targeting Histone Chaperone FACT Complex Overcomes 5-Fluorouracil Resistance in Colon Cancer. Mol Cancer Ther 2020, 19, 258–269. [Google Scholar] [CrossRef]
- Huajun, W.; Ying, F.; Hongxing, Z.; Weifeng, S.; Pingyang, S.; Mingde, H.; Guoguang, L. Clinical Value of Combined Detection of Serum APE1-Aabs and CEACAM-1 in the Diagnosis of Colorectal Cancer. Eur Rev Med Pharmacol Sci 2018, 22, 1286–1289. [Google Scholar] [CrossRef]
- Deng, X.; Zhen, P.; Niu, X.; Dai, Y.; Wang, Y.; Zhou, M. APE1 Promotes Proliferation and Migration of Cutaneous Squamous Cell Carcinoma. J Dermatol Sci 2020, 100, 67–74. [Google Scholar] [CrossRef]
- Ajucarmelprecilla, A.; Pandi, J.; Dhandapani, R.; Ramanathan, S.; Chinnappan, J.; Paramasivam, R.; Thangavelu, S.; Mohammed Ghilan, A.-K.; Aljohani, S.A.S.; Oyouni, A.A.A.; et al. In Silico Identification of Hub Genes as Observing Biomarkers for Gastric Cancer Metastasis. Evid Based Complement Alternat Med 2022, 2022, 6316158. [Google Scholar] [CrossRef] [PubMed]
- Manoel-Caetano, F.S.; Rossi, A.F.T.; Calvet de Morais, G.; Severino, F.E.; Silva, A.E. Upregulation of the APE1 and H2AX Genes and MiRNAs Involved in DNA Damage Response and Repair in Gastric Cancer. Genes Dis 2019, 6, 176–184. [Google Scholar] [CrossRef]
- Qing, Y.; Li, Q.; Ren, T.; Xia, W.; Peng, Y.; Liu, G.-L.; Luo, H.; Yang, Y.-X.; Dai, X.-Y.; Zhou, S.-F.; et al. Upregulation of PD-L1 and APE1 Is Associated with Tumorigenesis and Poor Prognosis of Gastric Cancer. Drug Des Devel Ther 2015, 9, 901–909. [Google Scholar] [CrossRef]
- Wei, X.; Li, Y.-B.; Li, Y.; Lin, B.-C.; Shen, X.-M.; Cui, R.-L.; Gu, Y.-J.; Gao, M.; Li, Y.-G.; Zhang, S. Prediction of Lymph Node Metastases in Gastric Cancer by Serum APE1 Expression. J Cancer 2017, 8, 1492–1497. [Google Scholar] [CrossRef]
- Bobola, M.S.; Blank, A.; Berger, M.S.; Stevens, B.A.; Silber, J.R. Apurinic/Apyrimidinic Endonuclease Activity Is Elevated in Human Adult Gliomas. Clin Cancer Res 2001, 7, 3510–3518. [Google Scholar] [PubMed]
- Scott, T.L.; Wicker, C.A.; Suganya, R.; Dhar, B.; Pittman, T.; Horbinski, C.; Izumi, T. Polyubiquitination of Apurinic/Apyrimidinic Endonuclease 1 by Parkin. Mol Carcinog 2017, 56, 325–336. [Google Scholar] [CrossRef]
- Naidu, M.D.; Mason, J.M.; Pica, R.V.; Fung, H.; Peña, L.A. Radiation Resistance in Glioma Cells Determined by DNA Damage Repair Activity of Ape1/Ref-1. J Radiat Res 2010, 51, 393–404. [Google Scholar] [CrossRef]
- Hudson, A.L.; Parker, N.R.; Khong, P.; Parkinson, J.F.; Dwight, T.; Ikin, R.J.; Zhu, Y.; Chen, J.; Wheeler, H.R.; Howell, V.M. Glioblastoma Recurrence Correlates With Increased APE1 and Polarization Toward an Immuno-Suppressive Microenvironment. Front Oncol 2018, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xiao, H.; Luo, Q.; Li, M.; Wei, S.; Zhu, X.; Xiao, H.; Chen, L. Low APE1/Ref-1 Expression Significantly Correlates with MGMT Promoter Methylation in Patients with High-Grade Gliomas. Int J Clin Exp Pathol 2016, 9, 9562–9568. [Google Scholar]
- Perry, C.; Agarwal, D.; Abdel-Fatah, T.M.A.; Lourdusamy, A.; Grundy, R.; Auer, D.T.; Walker, D.; Lakhani, R.; Scott, I.S.; Chan, S.; et al. Dissecting DNA Repair in Adult High Grade Gliomas for Patient Stratification in the Post-Genomic Era. Oncotarget 2014, 5, 5764–5781. [Google Scholar] [CrossRef]
- Hsia, K.-T.; Liu, C.-J.; Mar, K.; Lin, L.-H.; Lin, C.-S.; Cheng, M.-F.; Lee, H.-S.; Chiu, S.-Y. Impact of Apurinic/Apyrimidinic Endonuclease 1/Redox Factor-1 on Treatment Response and Survival in Oral Squamous Cell Carcinoma. Head Neck 2016, 38, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Wicker, C.A.; Takiar, V.; Suganya, R.; Arnold, S.M.; Brill, Y.M.; Chen, L.; Horbinski, C.M.; Napier, D.; Valentino, J.; Kudrimoti, M.R.; et al. Evaluation of Antioxidant Network Proteins as Novel Prognostic Biomarkers for Head and Neck Cancer Patients. Oral Oncol 2020, 111, 104949. [Google Scholar] [CrossRef] [PubMed]
- Santana, T.; Sá, M.C.; de Moura Santos, E.; Galvão, H.C.; Coletta, R.D.; Freitas, R. de A. DNA Base Excision Repair Proteins APE-1 and XRCC-1 Are Overexpressed in Oral Tongue Squamous Cell Carcinoma. J Oral Pathol Med 2017, 46, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, Y.; Kong, J.; Li, C. Apurinic/Apyrimidinic Endonuclease 1/Redox Factor-1 Could Serve as a Potential Serological Biomarker for the Diagnosis and Prognosis of Oral Squamous Cell Carcinoma. J Oral Maxillofac Surg 2019, 77, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lun, L.; Jiang, X.; Wang, Y.; Li, X.; Du, G.; Wang, J. APE1 Facilitates PD-L1-Mediated Progression of Laryngeal and Hypopharyngeal Squamous Cell Carcinoma. Int Immunopharmacol 2021, 97, 107675. [Google Scholar] [CrossRef]
- Lee, J.W.; Jin, J.; Rha, K.-S.; Kim, Y.M. Expression Pattern of Apurinic/Apyrimidinic Endonuclease in Sinonasal Squamous Cell Carcinoma. Otolaryngol Head Neck Surg 2012, 147, 788–795. [Google Scholar] [CrossRef]
- Souza, L.R.; Fonseca-Silva, T.; Pereira, C.S.; Santos, E.P.; Lima, L.C.; Carvalho, H.A.; Gomez, R.S.; Guimarães, A.L.S.; De Paula, A.M.B. Immunohistochemical Analysis of P53, APE1, HMSH2 and ERCC1 Proteins in Actinic Cheilitis and Lip Squamous Cell Carcinoma. Histopathology 2011, 58, 352–360. [Google Scholar] [CrossRef]
- Di Maso, V.; Mediavilla, M.G.; Vascotto, C.; Lupo, F.; Baccarani, U.; Avellini, C.; Tell, G.; Tiribelli, C.; Crocè, L.S. Transcriptional Up-Regulation of APE1/Ref-1 in Hepatic Tumor: Role in Hepatocytes Resistance to Oxidative Stress and Apoptosis. PLoS One 2015, 10, e0143289. [Google Scholar] [CrossRef]
- Sun, Z.; Zhu, Y.; Aminbuhe; Fan, Q.; Peng, J.; Zhang, N. Differential Expression of APE1 in Hepatocellular Carcinoma and the Effects on Proliferation and Apoptosis of Cancer Cells. BST 2018, 12, 456–462. [CrossRef]
- Lu, X.; Zhao, H.; Yuan, H.; Chu, Y.; Zhu, X. High Nuclear Expression of APE1 Correlates with Unfavorable Prognosis and Promotes Tumor Growth in Hepatocellular Carcinoma. J Mol Histol 2021, 52, 219–231. [Google Scholar] [CrossRef]
- Pascut, D.; Sukowati, C.H.C.; Antoniali, G.; Mangiapane, G.; Burra, S.; Mascaretti, L.G.; Buonocore, M.R.; Crocè, L.S.; Tiribelli, C.; Tell, G. Serum AP-Endonuclease 1 (SAPE1) as Novel Biomarker for Hepatocellular Carcinoma. Oncotarget 2019, 10, 383–394. [Google Scholar] [CrossRef]
- Di Maso, V.; Avellini, C.; Crocè, L.S.; Rosso, N.; Quadrifoglio, F.; Cesaratto, L.; Codarin, E.; Bedogni, G.; Beltrami, C.A.; Tell, G.; et al. Subcellular Localization of APE1/Ref-1 in Human Hepatocellular Carcinoma: Possible Prognostic Significance. Mol Med 2007, 13, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Bazzani, V.; Barchiesi, A.; Radecka, D.; Pravisani, R.; Guadagno, A.; Di Loreto, C.; Baccarani, U.; Vascotto, C. Mitochondrial Apurinic/Apyrimidinic Endonuclease 1 Enhances MtDNA Repair Contributing to Cell Proliferation and Mitochondrial Integrity in Early Stages of Hepatocellular Carcinoma. BMC Cancer 2020, 20, 969. [Google Scholar] [CrossRef]
- Sengupta, S.; Mantha, A.K.; Song, H.; Roychoudhury, S.; Nath, S.; Ray, S.; Bhakat, K.K. Elevated Level of Acetylation of APE1 in Tumor Cells Modulates DNA Damage Repair. Oncotarget 2016, 7, 75197–75209. [Google Scholar] [CrossRef] [PubMed]
- Long, K.; Gu, L.; Li, L.; Zhang, Z.; Li, E.; Zhang, Y.; He, L.; Pan, F.; Guo, Z.; Hu, Z. Small-Molecule Inhibition of APE1 Induces Apoptosis, Pyroptosis, and Necroptosis in Non-Small Cell Lung Cancer. Cell Death Dis 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Yoo, D.G.; Song, Y.J.; Cho, E.J.; Lee, S.K.; Park, J.B.; Yu, J.H.; Lim, S.P.; Kim, J.M.; Jeon, B.H. Alteration of APE1/Ref-1 Expression in Non-Small Cell Lung Cancer: The Implications of Impaired Extracellular Superoxide Dismutase and Catalase Antioxidant Systems. Lung Cancer 2008, 60, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, Q.; Li, Y.; Duan, W.; Huang, C.; Zheng, X.; Sun, L.; Luo, J.; Wang, D.; Zhang, S.; et al. Prediction of Survival Prognosis of Non-Small Cell Lung Cancer by APE1 through Regulation of Epithelial-Mesenchymal Transition. Oncotarget 2016, 7, 28523–28539. [Google Scholar] [CrossRef]
- Gu, X.; Cun, Y.; Li, M.; Qing, Y.; Jin, F.; Zhong, Z.; Dai, N.; Qian, C.; Sui, J.; Wang, D. Human Apurinic/Apyrimidinic Endonuclease SiRNA Inhibits the Angiogenesis Induced by X-Ray Irradiation in Lung Cancer Cells. Int J Med Sci 2013, 10, 870–882. [Google Scholar] [CrossRef]
- Zhang, S.; He, L.; Dai, N.; Guan, W.; Shan, J.; Yang, X.; Zhong, Z.; Qing, Y.; Jin, F.; Chen, C.; et al. Serum APE1 as a Predictive Marker for Platinum-Based Chemotherapy of Non-Small Cell Lung Cancer Patients. Oncotarget 2016, 7, 77482–77494. [Google Scholar] [CrossRef]
- Kakolyris, S.; Giatromanolaki, A.; Koukourakis, M.; Kaklamanis, L.; Kanavaros, P.; Hickson, I.D.; Barzilay, G.; Georgoulias, V.; Gatter, K.C.; Harris, A.L. Nuclear Localization of Human AP Endonuclease 1 (HAP1/Ref-1) Associates with Prognosis in Early Operable Non-Small Cell Lung Cancer (NSCLC). J Pathol 1999, 189, 351–357. [Google Scholar] [CrossRef]
- Puglisi, F.; Aprile, G.; Minisini, A.M.; Barbone, F.; Cataldi, P.; Tell, G.; Kelley, M.R.; Damante, G.; Beltrami, C.A.; Di Loreto, C. Prognostic Significance of Ape1/Ref-1 Subcellular Localization in Non-Small Cell Lung Carcinomas. Anticancer Res 2001, 21, 4041–4049. [Google Scholar] [PubMed]
- Wu, H.-H.; Chu, Y.-C.; Wang, L.; Tsai, L.-H.; Lee, M.-C.; Chen, C.-Y.; Shieh, S.-H.; Cheng, Y.-W.; Lee, H. Cytoplasmic Ape1 Expression Elevated by P53 Aberration May Predict Survival and Relapse in Resected Non-Small Cell Lung Cancer. Ann Surg Oncol 2013, 20 Suppl 3, S336–347. [Google Scholar] [CrossRef]
- Wu, H.-H.; Cheng, Y.-W.; Chang, J.T.; Wu, T.-C.; Liu, W.-S.; Chen, C.-Y.; Lee, H. Subcellular Localization of Apurinic Endonuclease 1 Promotes Lung Tumor Aggressiveness via NF-KappaB Activation. Oncogene 2010, 29, 4330–4340. [Google Scholar] [CrossRef]
- Abbotts, R.; Jewell, R.; Nsengimana, J.; Maloney, D.J.; Simeonov, A.; Seedhouse, C.; Elliott, F.; Laye, J.; Walker, C.; Jadhav, A.; et al. Targeting Human Apurinic/Apyrimidinic Endonuclease 1 (APE1) in Phosphatase and Tensin Homolog (PTEN) Deficient Melanoma Cells for Personalized Therapy. Oncotarget 2014, 5, 3273–3286. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.Z.; Vyjayanti, V.N.; Laughton, C.A.; Dekker, L.V.; Fischer, P.M.; Wilson, D.M.; Abbotts, R.; Shah, S.; Patel, P.M.; Hickson, I.D.; et al. Development and Evaluation of Human AP Endonuclease Inhibitors in Melanoma and Glioma Cell Lines. Br J Cancer 2011, 104, 653–663. [Google Scholar] [CrossRef]
- Yang, S.; Irani, K.; Heffron, S.E.; Jurnak, F.; Meyskens, F.L. Alterations in the Expression of the Apurinic/Apyrimidinic Endonuclease-1/Redox Factor-1 (APE/Ref-1) in Human Melanoma and Identification of the Therapeutic Potential of Resveratrol as an APE/Ref-1 Inhibitor. Mol Cancer Ther 2005, 4, 1923–1935. [Google Scholar] [CrossRef] [PubMed]
- Guida, M.; Tommasi, S.; Strippoli, S.; Natalicchio, M.I.; De Summa, S.; Pinto, R.; Cramarossa, A.; Albano, A.; Pisconti, S.; Aieta, M.; et al. The Search for a Melanoma-Tailored Chemotherapy in the New Era of Personalized Therapy: A Phase II Study of Chemo-Modulating Temozolomide Followed by Fotemustine and a Cooperative Study of GOIM (Gruppo Oncologico Italia Meridionale). BMC Cancer 2018, 18, 552. [Google Scholar] [CrossRef]
- Al-Attar, A.; Gossage, L.; Fareed, K.R.; Shehata, M.; Mohammed, M.; Zaitoun, A.M.; Soomro, I.; Lobo, D.N.; Abbotts, R.; Chan, S.; et al. Human Apurinic/Apyrimidinic Endonuclease (APE1) Is a Prognostic Factor in Ovarian, Gastro-Oesophageal and Pancreatico-Biliary Cancers. Br J Cancer 2010, 102, 704–709. [Google Scholar] [CrossRef]
- Hong, J.; Chen, Z.; Peng, D.; Zaika, A.; Revetta, F.; Washington, M.K.; Belkhiri, A.; El-Rifai, W. APE1-Mediated DNA Damage Repair Provides Survival Advantage for Esophageal Adenocarcinoma Cells in Response to Acidic Bile Salts. Oncotarget 2016, 7, 16688–16702. [Google Scholar] [CrossRef]
- Bhat, A.A.; Lu, H.; Soutto, M.; Capobianco, A.; Rai, P.; Zaika, A.; El-Rifai, W. Exposure of Barrett’s and Esophageal Adenocarcinoma Cells to Bile Acids Activates EGFR-STAT3 Signaling Axis via Induction of APE1. Oncogene 2018, 37, 6011–6024. [Google Scholar] [CrossRef]
- Sriramajayam, K.; Peng, D.; Lu, H.; Zhou, S.; Bhat, N.; McDonald, O.G.; Que, J.; Zaika, A.; El-Rifai, W. Activation of NRF2 by APE1/REF1 Is Redox-Dependent in Barrett’s Related Esophageal Adenocarcinoma Cells. Redox Biol 2021, 43, 101970. [Google Scholar] [CrossRef]
- Han, G.; Tian, Y.; Duan, B.; Sheng, H.; Gao, H.; Huang, J. Association of Nuclear Annexin A1 with Prognosis of Patients with Esophageal Squamous Cell Carcinoma. Int J Clin Exp Pathol 2014, 7, 751–759. [Google Scholar]
- Song, J.; Futagami, S.; Nagoya, H.; Kawagoe, T.; Yamawaki, H.; Kodaka, Y.; Tatsuguchi, A.; Gudis, K.; Wakabayashi, T.; Yonezawa, M.; et al. Apurinic/Apyrimidinic Endonuclease-1 (APE-1) Is Overexpressed via the Activation of NF-ΚB-P65 in MCP-1-Positive Esophageal Squamous Cell Carcinoma Tissue. J Clin Biochem Nutr 2013, 52, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Z.; Li, Q.; Zhang, L.; Cheng, Y.; Zhong, Z. Mitochondrial APE1 Promotes Cisplatin Resistance by Downregulating ROS in Osteosarcoma. Oncol Rep 2020, 44, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Z.; Zhang, L.; Zhong, Z. Cytoplasmic APE1 Promotes Resistance Response in Osteosarcoma Patients with Cisplatin Treatment. Cell Biochemistry and Function 2020, 38, 195–203. [Google Scholar] [CrossRef]
- Wang, D.; Luo, M.; Kelley, M.R. Human Apurinic Endonuclease 1 (APE1) Expression and Prognostic Significance in Osteosarcoma: Enhanced Sensitivity of Osteosarcoma to DNA Damaging Agents Using Silencing RNA APE1 Expression Inhibition. Mol Cancer Ther 2004, 3, 679–686. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Yuan, Z.; Wang, C.; Shi, Y. Predicting Chemosensitivity in Osteosarcoma Prior to Chemotherapy: An Investigational Study of Biomarkers with Immunohistochemistry. Oncology Letters 2012, 3, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Qing, Y.; Dai, N.; Li, M.; Qian, C.; Yang, Y.; Cheng, Y.; Li, Z.; Zhang, S.; Zhong, Z.; et al. Apurinic/Apyrimidinic Endonuclease 1 Induced Upregulation of Fibroblast Growth Factor 2 and Its Receptor 3 Induces Angiogenesis in Human Osteosarcoma Cells. Cancer Sci 2014, 105, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Shan, J.; Dai, N.; Zhong, Z.; Qing, Y.; Yang, Y.; Zhang, S.; Li, C.; Sui, J.; Ren, T.; et al. Apurinic/Apyrimidinic Endonuclease 1 Regulates Angiogenesis in a Transforming Growth Factor β-Dependent Manner in Human Osteosarcoma. Cancer Sci 2015, 106, 1394–1401. [Google Scholar] [CrossRef]
- Dai, N.; Qing, Y.; Cun, Y.; Zhong, Z.; Li, C.; Zhang, S.; Shan, J.; Yang, X.; Dai, X.; Cheng, Y.; et al. MiR-513a-5p Regulates Radiosensitivity of Osteosarcoma by Targeting Human Apurinic/Apyrimidinic Endonuclease. Oncotarget 2016, 9, 25414–25426. [Google Scholar] [CrossRef]
- Londero, A.P.; Orsaria, M.; Tell, G.; Marzinotto, S.; Capodicasa, V.; Poletto, M.; Vascotto, C.; Sacco, C.; Mariuzzi, L. Expression and Prognostic Significance of APE1/Ref-1 and NPM1 Proteins in High-Grade Ovarian Serous Cancer. Am J Clin Pathol 2014, 141, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Lu, R.; Xie, S.; Zheng, H.; Wang, H.; Wang, Y.; Sun, J.; Gao, X.; Guo, L. APE1 Overexpression Promotes the Progression of Ovarian Cancer and Serves as a Potential Therapeutic Target. Cancer Biomark 2016, 17, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Xiang, D.; Wang, D.; Xin, X. Alterations in the Expression of the Apurinic/Apyrimidinic Endonuclease-1/Redox Factor-1 (APE1/Ref-1) in Human Ovarian Cancer and Indentification of the Therapeutic Potential of APE1/Ref-1 Inhibitor. Int J Oncol 2009, 35, 1069–1079. [Google Scholar] [PubMed]
- Fan, X.; Wen, L.; Li, Y.; Lou, L.; Liu, W.; Zhang, J. The Expression Profile and Prognostic Value of APE/Ref-1 and NPM1 in High-Grade Serous Ovarian Adenocarcinoma. APMIS 2017, 125, 857–862. [Google Scholar] [CrossRef]
- Poletto, M.; Malfatti, M.C.; Dorjsuren, D.; Scognamiglio, P.L.; Marasco, D.; Vascotto, C.; Jadhav, A.; Maloney, D.J.; Wilson, D.M.; Simeonov, A.; et al. Inhibitors of the Apurinic/Apyrimidinic Endonuclease 1 (APE1)/Nucleophosmin (NPM1) Interaction That Display Anti-Tumor Properties. Mol Carcinog 2016, 55, 688–704. [Google Scholar] [CrossRef]
- Sheng, Q.; Zhang, Y.; Wang, R.; Zhang, J.; Chen, B.; Wang, J.; Zhang, W.; Xin, X. Prognostic Significance of APE1 Cytoplasmic Localization in Human Epithelial Ovarian Cancer. Med Oncol 2012, 29, 1265–1271. [Google Scholar] [CrossRef]
- Moore, D.H.; Michael, H.; Tritt, R.; Parsons, S.H.; Kelley, M.R. Alterations in the Expression of the DNA Repair/Redox Enzyme APE/Ref-1 in Epithelial Ovarian Cancers. Clin Cancer Res 2000, 6, 602–609. [Google Scholar]
- Tanner, B.; Grimme, S.; Schiffer, I.; Heimerdinger, C.; Schmidt, M.; Dutkowski, P.; Neubert, S.; Oesch, F.; Franzen, A.; Kölbl, H.; et al. Nuclear Expression of Apurinic/Apyrimidinic Endonuclease Increases with Progression of Ovarian Carcinomas. Gynecologic Oncology 2004, 92, 568–577. [Google Scholar] [CrossRef]
- Pramanik, S.; Chen, Y.; Song, H.; Khutsishvili, I.; Marky, L.A.; Ray, S.; Natarajan, A.; Singh, P.K.; Bhakat, K.K. The Human AP-Endonuclease 1 (APE1) Is a DNA G-Quadruplex Structure Binding Protein and Regulates KRAS Expression in Pancreatic Ductal Adenocarcinoma Cells. Nucleic Acids Res 2022, 50, 3394–3412. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, S.; Sandusky, G.E.; Kelley, M.R.; Fishel, M.L. Reduced Expression of DNA Repair and Redox Signaling Protein APE1/Ref-1 Impairs Human Pancreatic Cancer Cell Survival, Proliferation, and Cell Cycle Progression. Cancer Invest 2010, 28, 885–895. [Google Scholar] [CrossRef]
- Fishel, M.L.; Jiang, Y.; Rajeshkumar, N.V.; Scandura, G.; Sinn, A.L.; He, Y.; Shen, C.; Jones, D.R.; Pollok, K.E.; Ivan, M.; et al. Impact of APE1/Ref-1 Redox Inhibition on Pancreatic Tumor Growth. Mol Cancer Ther 2011, 10, 1698–1708. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.R.; Cheng, L.; Foster, R.; Tritt, R.; Jiang, J.; Broshears, J.; Koch, M. Elevated and Altered Expression of the Multifunctional DNA Base Excision Repair and Redox Enzyme Ape1/Ref-1 in Prostate Cancer. Clin Cancer Res 2001, 7, 824–830. [Google Scholar]
- Juhnke, M.; Heumann, A.; Chirico, V.; Höflmayer, D.; Menz, A.; Hinsch, A.; Hube-Magg, C.; Kluth, M.; Lang, D.S.; Möller-Koop, C.; et al. Apurinic/Apyrimidinic Endonuclease 1 (APE1/Ref-1) Overexpression Is an Independent Prognostic Marker in Prostate Cancer without TMPRSS2:ERG Fusion. Mol Carcinog 2017, 56, 2135–2145. [Google Scholar] [CrossRef]
- Silva, L.P.; Santana, T.; Sedassari, B.T.; de Sousa, S.M.; Sobral, A.P.V.; Freitas, R. de A.; Barboza, C.A.G.; de Souza, L.B. Apurinic/Apyrimidinic Endonuclease 1 (APE1) Is Overexpressed in Malignant Transformation of Salivary Gland Pleomorphic Adenoma. Eur Arch Otorhinolaryngol 2017, 274, 3203–3209. [Google Scholar] [CrossRef]
- Felix, F.A.; da Silva, L.P.; Lopes, M.L.D. de S.; Sobral, A.P.V.; Freitas, R. de A.; de Souza, L.B.; Barboza, C.A.G. DNA Base Excision Repair and Nucleotide Excision Repair Proteins in Malignant Salivary Gland Tumors. Arch Oral Biol 2021, 121, 104987. [Google Scholar] [CrossRef]
- Lee, Y.R.; Lim, J.S.; Shin, J.H.; Choi, S.; Joo, H.K.; Jeon, B.H. Altered Secretory Activity of APE1/Ref-1 D148E Variants Identified in Human Patients With Bladder Cancer. Int Neurourol J 2016, 20, S30–37. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wilson, D.M. Human Apurinic/Apyrimidinic Endonuclease 1. Antioxid. Redox Signal. 2014, 20, 678–707. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Acosta, V.M.; Ruiz-Perez, L.M.; Yang, W.; Gonzalez-Pacanowska, D.; Vidal, A.E. Identification of a Residue Critical for the Excision of 3’-Blocking Ends in Apurinic/Apyrimidinic Endonucleases of the Xth Family. Nucleic Acids Research 2009, 37, 1829–1842. [Google Scholar] [CrossRef] [PubMed]
- Timofeyeva, N.A.; Koval, V.V.; Ishchenko, A.A.; Saparbaev, M.K.; Fedorova, O.S. Lys98 Substitution in Human AP Endonuclease 1 Affects the Kinetic Mechanism of Enzyme Action in Base Excision and Nucleotide Incision Repair Pathways. PLoS ONE 2011, 6, e24063. [Google Scholar] [CrossRef]
- Whitaker, A.M.; Flynn, T.S.; Freudenthal, B.D. Molecular Snapshots of APE1 Proofreading Mismatches and Removing DNA Damage. Nature Communications 2018, 9. [Google Scholar] [CrossRef]
- Wilson, D.M.; Simeonov, A. Small Molecule Inhibitors of DNA Repair Nuclease Activities of APE1. Cellular and Molecular Life Sciences 2010, 67, 3621–3631. [Google Scholar] [CrossRef] [PubMed]
- Al-Safi, R.I.; Odde, S.; Shabaik, Y.; Neamati, N. Small-Molecule Inhibitors of APE1 DNA Repair Function: An Overview. Curr Mol Pharmacol. 2012, 5, 14–35. [Google Scholar] [CrossRef]
- Laev, S.S.; Salakhutdinov, N.F.; Lavrik, O.I. Inhibitors of Nuclease and Redox Activity of Apurinic/Apyrimidinic Endonuclease 1/Redox Effector Factor 1 (APE1/Ref-1). Bioorg Med Chem 2017, 25, 2531–2544. [Google Scholar] [CrossRef]
- Caston, R.A.; Gampala, S.; Armstrong, L.; Messmann, R.A.; Fishel, M.L.; Kelley, M.R. The Multifunctional APE1 DNA Repair-Redox Signaling Protein as a Drug Target in Human Disease. Drug Discov Today 2021, 26, 218–228. [Google Scholar] [CrossRef]
- Xue, Z.; Demple, B. Knockout and Inhibition of Ape1: Roles of Ape1 in Base Excision DNA Repair and Modulation of Gene Expression. Antioxidants (Basel) 2022, 11, 1817. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, M.; Weinfeld, M.; Paterson, M.C. Selective Inhibition by Methoxyamine of the Apurinic/Apyrimidinic Endonuclease Activity Associated with Pyrimidine Dimer-DNA Glycosylases from Micrococcus Luteus and Bacteriophage T4. Biochemistry 1987, 26, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gerson, S.L. Therapeutic Impact of Methoxyamine: Blocking Repair of Abasic Sites in the Base Excision Repair Pathway. Curr Opin Investig Drugs 2004, 5, 623–627. [Google Scholar] [PubMed]
- Liu, L.; Nakatsuru, Y.; Gerson, S.L. Base Excision Repair as a Therapeutic Target in Colon Cancer. Clin Cancer Res 2002, 8, 2985–2991. [Google Scholar] [PubMed]
- Fishel, M.L.; He, Y.; Smith, M.L.; Kelley, M.R. Manipulation of Base Excision Repair to Sensitize Ovarian Cancer Cells to Alkylating Agent Temozolomide. Clin Cancer Res 2007, 13, 260–267. [Google Scholar] [CrossRef]
- Bases, R. Enhancement of X-Ray Damage in HeLa Cells by Exposure to Lucanthone (Miracil D) Following Radiation. Cancer Res 1970, 30, 2007–2011. [Google Scholar]
- Luo, M.; Kelley, M.R. Inhibition of the Human Apurinic/Apyrimidinic Endonuclease (Ape1) Repair Activity and Sensitization of Breast Cancer Cells to DNA Alkylating Agents with Lucanthone. Anticancer Research 2004, 24, 2127–2134. [Google Scholar]
- Naidu, M.D.; Agarwal, R.; Pena, L.A.; Cunha, L.; Mezei, M.; Shen, M.; Wilson, D.M.; Liu, Y.; Sanchez, Z.; Chaudhary, P.; et al. Lucanthone and Its Derivative Hycanthone Inhibit Apurinic Endonuclease-1 (APE1) by Direct Protein Binding. PLoS ONE 2011, 6, e23679. [Google Scholar] [CrossRef] [PubMed]
- Madhusudan, S.; Smart, F.; Shrimpton, P.; Parsons, J.L.; Gardiner, L.; Houlbrook, S.; Talbot, D.C.; Hammonds, T.; Freemont, P.A.; Sternberg, M.J.E.; et al. Isolation of a Small Molecule Inhibitor of DNA Base Excision Repair. Nucleic Acids Research 2005, 33, 4711–4724. [Google Scholar] [CrossRef] [PubMed]
- Simeonov, A.; Kulkarni, A.; Dorjsuren, D.; Jadhav, A.; Shen, M.; McNeill, D.R.; Austin, C.P.; Wilson, D.M. Identification and Characterization of Inhibitors of Human Apurinic/Apyrimidinic Endonuclease APE1. PLoS ONE 2009, 4, e5740. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Fu, D.; Xu, Y.; Wang, X.; Deng, X.; Zhou, S.; Du, F.; Cui, X.; Deng, Y.; Tang, Z. Pt(IV) Prodrug as a Potential Antitumor Agent with APE1 Inhibitory Activity. J Med Chem 2022, 65, 15344–15357. [Google Scholar] [CrossRef] [PubMed]
- Seiple, L.A.; Cardellina, J.H.; Akee, R.; Stivers, J.T. Potent Inhibition of Human Apurinic/Apyrimidinic Endonuclease 1 by Arylstibonic Acids. Mol Pharmacol 2008, 73, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Zawahir, Z.; Dayam, R.; Deng, J.; Pereira, C.; Neamati, N. Pharmacophore Guided Discovery of Small-Molecule Human Apurinic/Apyrimidinic Endonuclease 1 Inhibitors. J Med Chem 2009, 52, 20–32. [Google Scholar] [CrossRef]
- Bapat, A.; Glass, L.S.; Luo, M.; Fishel, M.L.; Long, E.C.; Georgiadis, M.M.; Kelley, M.R. Novel Small-Molecule Inhibitor of Apurinic/Apyrimidinic Endonuclease 1 Blocks Proliferation and Reduces Viability of Glioblastoma Cells. Journal of Pharmacology and Experimental Therapeutics 2010, 334, 988–998. [Google Scholar] [CrossRef]
- Aiello, F.; Shabaik, Y.; Esqueda, A.; Sanchez, T.W.; Grande, F.; Garofalo, A.; Neamati, N. Design and Synthesis of 3-Carbamoylbenzoic Acid Derivatives as Inhibitors of Human Apurinic/Apyrimidinic Endonuclease 1 (APE1). ChemMedChem 2012, 7, 1825–1839. [Google Scholar] [CrossRef]
- Ruiz, F.M.; Francis, S.M.; Tintoré, M.; Ferreira, R.; Gil-Redondo, R.; Morreale, A.; Ortiz, Á.R.; Eritja, R.; Fàbrega, C. Receptor-Based Virtual Screening and Biological Characterization of Human Apurinic/Apyrimidinic Endonuclease (Ape1) Inhibitors. ChemMedChem 2012, 7, 2168–2178. [Google Scholar] [CrossRef]
- Dorjsuren, D.; Kim, D.; Vyjayanti, V.N.; Maloney, D.J.; Jadhav, A.; Wilson, D.M.; Simeonov, A. Diverse Small Molecule Inhibitors of Human Apurinic/Apyrimidinic Endonuclease APE1 Identified from a Screen of a Large Public Collection. PLoS ONE 2012, 7, e47974. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.; Vyjayanti, V.N.; Dorjsuren, D.; Simeonov, A.; Jadhav, A.; Wilson, D.M.; Maloney, D.J. Synthesis, Biological Evaluation, and Structure–Activity Relationships of a Novel Class of Apurinic/Apyrimidinic Endonuclease 1 Inhibitors. Journal of Medicinal Chemistry 2012, 55, 3101–3112. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Wang, L.; Cline, C.J.; Xie, Z.; Sobol, R.W.; Xie, X.-Q.; Gold, B. Identification and Characterization of Human Apurinic/Apyrimidinic Endonuclease-1 Inhibitors; American Chemical Society, 2012; Vol. 51; ISBN 00062960.
- Feng, Z.; Kochanek, S.; Close, D.; Wang, L.; Srinivasan, A.; Almehizia, A.A.; Iyer, P.; Xie, X.-Q.; Johnston, P.A.; Gold, B. Design and Activity of AP Endonuclease-1 Inhibitors. J Chem Biol 2015, 8, 79–93. [Google Scholar] [CrossRef]
- Trilles, R.; Beglov, D.; Chen, Q.; He, H.; Wireman, R.; Reed, A.; Chennamadhavuni, S.; Panek, J.S.; Brown, L.E.; Vajda, S.; et al. Discovery of Macrocyclic Inhibitors of Apurinic/Apyrimidinic Endonuclease 1. J Med Chem 2019, 62, 1971–1988. [Google Scholar] [CrossRef] [PubMed]
- Pidugu, L.S.; Servius, H.W.; Sevdalis, S.E.; Cook, M.E.; Varney, K.M.; Pozharski, E.; Drohat, A.C. Characterizing Inhibitors of Human AP Endonuclease 1. PLoS One 2023, 18, e0280526. [Google Scholar] [CrossRef]
- Masani, S.; Han, L.; Yu, K. Apurinic/Apyrimidinic Endonuclease 1 Is the Essential Nuclease during Immunoglobulin Class Switch Recombination. Molecular and Cellular Biology 2013, 33, 1468–1473. [Google Scholar] [CrossRef]
- Georgiadis, M.M.; Luo, M.; Gaur, R.K.; Delaplane, S.; Li, X.; Kelley, M.R. Evolution of the Redox Function in Mammalian Apurinic/Apyrimidinic Endonuclease. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2008, 643, 54–63. [Google Scholar] [CrossRef]
- Ando, K.; Hirao, S.; Kabe, Y.; Ogura, Y.; Sato, I.; Yamaguchi, Y.; Wada, T.; Handa, H. A New APE1/Ref-1-Dependent Pathway Leading to Reduction of NF- B and AP-1, and Activation of Their DNA-Binding Activity. Nucleic Acids Research 2008, 36, 4327–4336. [Google Scholar] [CrossRef]
- Logsdon, D.P.; Grimard, M.; Luo, M.; Shahda, S.; Jiang, Y.; Tong, Y.; Yu, Z.; Zyromski, N.; Schipani, E.; Carta, F.; et al. Regulation of HIF1α under Hypoxia by APE1/Ref-1 Impacts CA9 Expression: Dual Targeting in Patient-Derived 3D Pancreatic Cancer Models. Mol Cancer Ther 2016, 15, 2722–2732. [Google Scholar] [CrossRef]
- Ray, S.; Lee, C.; Hou, T.; Bhakat, K.K.; Brasier, A.R. Regulation of Signal Transducer and Activator of Transcription 3 Enhanceosome Formation by Apurinic/Apyrimidinic Endonuclease 1 in Hepatic Acute Phase Response. Molecular Endocrinology 2010, 24, 391–401. [Google Scholar] [CrossRef]
- Cardoso, A.A.; Jiang, Y.; Luo, M.; Reed, A.M.; Shahda, S.; He, Y.; Maitra, A.; Kelley, M.R.; Fishel, M.L. APE1/Ref-1 Regulates STAT3 Transcriptional Activity and APE1/Ref-1–STAT3 Dual-Targeting Effectively Inhibits Pancreatic Cancer Cell Survival. PLoS ONE 2012, 7, e47462. [Google Scholar] [CrossRef]
- Jayaraman, L.; Murthy, K.G.; Zhu, C.; Curran, T.; Xanthoudakis, S.; Prives, C. Identification of Redox/Repair Protein Ref-1 as a Potent Activator of P53. Genes Dev. 1997, 11, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Fishel, M.L.; Wu, X.; Devlin, C.M.; Logsdon, D.P.; Jiang, Y.; Luo, M.; He, Y.; Yu, Z.; Tong, Y.; Lipking, K.P.; et al. Apurinic/Apyrimidinic Endonuclease/Redox Factor-1 (APE1/Ref-1) Redox Function Negatively Regulates NRF2. Journal of Biological Chemistry 2015, 290, 3057–3068. [Google Scholar] [CrossRef]
- Tell, G.; Scaloni, A.; Pellizzari, L.; Formisano, S.; Pucillo, C.; Damante, G. Redox Potential Controls the Structure and DNA Binding Activity of the Paired Domain. Journal of Biological Chemistry 1998, 273, 25062–25072. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.R.; Limp-Foster, M.; Kelley, M.R. Going APE over Ref-1. Mutation Research/DNA Repair 2000, 461, 83–108. [Google Scholar] [CrossRef] [PubMed]
- Xanthoudakis, S.; Curran, T. Identification and Characterization of Ref-1, a Nuclear Protein That Facilitates AP-1 DNA-Binding Activity. The EMBO Journal 1992, 11, 653–665. [Google Scholar] [CrossRef]
- Xanthoudakis, S.; Miao, G.; Wang, F.; Pan, Y.C.; Curran, T. Redox Activation of Fos-Jun DNA Binding Activity Is Mediated by a DNA Repair Enzyme. The EMBO Journal 1992, 11, 3323–3335. [Google Scholar] [CrossRef]
- Arlt, A.; Vorndamm, J.; Breitenbroich, M.; Fölsch, U.R.; Kalthoff, H.; Schmidt, W.E.; Schäfer, H. Inhibition of NF-ΚB Sensitizes Human Pancreatic Carcinoma Cells to Apoptosis Induced by Etoposide (VP16) or Doxorubicin. Oncogene 2001, 20, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Arlt, A.; Gehrz, A.; Müerköster, S.; Vorndamm, J.; Kruse, M.-L.; Fölsch, U.R.; Schäfer, H. Role of NF-ΚB and Akt/PI3K in the Resistance of Pancreatic Carcinoma Cell Lines against Gemcitabine-Induced Cell Death. Oncogene 2003, 22, 3243–3251. [Google Scholar] [CrossRef]
- Raffoul, J.J.; Heydari, A.R.; Hillman, G.G. DNA Repair and Cancer Therapy: Targeting APE1/Ref-1 Using Dietary Agents. Journal of Oncology 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhong, C.; Wang, Q.; Chen, W.; Yuan, Y. Curcumin Is an APE1 Redox Inhibitor and Exhibits an Antiviral Activity against KSHV Replication and Pathogenesis. Antiviral Research 2019, 167, 98–103. [Google Scholar] [CrossRef]
- Escobar, I.; Xu, J.; Jackson, C.W.; Stegelmann, S.D.; Fagerli, E.A.; Dave, K.R.; Perez-Pinzon, M.A. Resveratrol Preconditioning Protects Against Ischemia-Induced Synaptic Dysfunction and Cofilin Hyperactivation in the Mouse Hippocampal Slice. Neurotherapeutics 2023. [Google Scholar] [CrossRef]
- Raffoul, J.J.; Banerjee, S.; Singh-Gupta, V.; Knoll, Z.E.; Fite, A.; Zhang, H.; Abrams, J.; Sarkar, F.H.; Hillman, G.G. Down-Regulation of Apurinic/Apyrimidinic Endonuclease 1/Redox Factor-1 Expression by Soy Isoflavones Enhances Prostate Cancer Radiotherapy In Vitro and In Vivo. Cancer Research 2007, 67, 2141–2149. [Google Scholar] [CrossRef]
- Kelley, M.R.; Wikel, J.H.; Guo, C.; Pollok, K.E.; Bailey, B.J.; Wireman, R.; Fishel, M.L.; Vasko, M.R. Identification and Characterization of New Chemical Entities Targeting Apurinic/Apyrimidinic Endonuclease 1 for the Prevention of Chemotherapy-Induced Peripheral Neuropathy. J Pharmacol Exp Ther 2016, 359, 300–309. [Google Scholar] [CrossRef]
- Shimizu, N.; Sugimoto, K.; Tang, J.; Nishi, T.; Sato, I.; Hiramoto, M.; Aizawa, S.; Hatakeyama, M.; Ohba, R.; Hatori, H.; et al. High-Performance Affinity Beads for Identifying Drug Receptors. Nat Biotechnol 2000, 18, 877–881. [Google Scholar] [CrossRef]
- Zou, G.-M.; Karikari, C.; Kabe, Y.; Handa, H.; Anders, R.A.; Maitra, A. The Ape-1/Ref-1 Redox Antagonist E3330 Inhibits the Growth of Tumor Endothelium and Endothelial Progenitor Cells: Therapeutic Implications in Tumor Angiogenesis. J. Cell. Physiol. 2009, 219, 209–218. [Google Scholar] [CrossRef]
- Zou, G.-M.; Maitra, A. Small-Molecule Inhibitor of the AP Endonuclease 1/REF-1 E3330 Inhibits Pancreatic Cancer Cell Growth and Migration. Molecular Cancer Therapeutics 2008, 7, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Gao, H.; Kelley, M.R.; Qiao, X. Inhibition of APE1/Ref-1 Redox Activity with APX3330 Blocks Retinal Angiogenesis in Vitro and in Vivo. Vision Research 2011, 51, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Manguinhas, R.; Fernandes, A.S.; Costa, J.G.; Saraiva, N.; Camões, S.P.; Gil, N.; Rosell, R.; Castro, M.; Miranda, J.P.; Oliveira, N.G. Impact of the APE1 Redox Function Inhibitor E3330 in Non-Small Cell Lung Cancer Cells Exposed to Cisplatin: Increased Cytotoxicity and Impairment of Cell Migration and Invasion. Antioxidants 2020, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, P.S.; Corvacho, E.; Costa, J.G.; Saraiva, N.; Fernandes, A.S.; Castro, M.; Miranda, J.P.; Oliveira, N.G. The APE1 Redox Inhibitor E3330 Reduces Collective Cell Migration of Human Breast Cancer Cells and Decreases Chemoinvasion and Colony Formation When Combined with Docetaxel. Chem Biol Drug Des 2017, 90, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Zhou, T.; Kelley, M.R.; Edwards, P.A.; Gao, H.; Qiao, X. Suppression of Choroidal Neovascularization Through Inhibition of APE1/Ref-1 Redox Activity. Invest. Ophthalmol. Vis. Sci. 2014, 55, 4461. [Google Scholar] [CrossRef]
- Hartman, G.D.; Lambert-Cheatham, N.A.; Kelley, M.R.; Corson, T.W. Inhibition of APE1/Ref-1 for Neovascular Eye Diseases: From Biology to Therapy. IJMS 2021, 22, 10279. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Yuan, Y. Inhibitors of APE1 Redox Function Effectively Inhibit γ-Herpesvirus Replication in Vitro and in Vivo. Antiviral Research 2021, 185, 104985. [Google Scholar] [CrossRef] [PubMed]
- Shahda, S.; Lakhani, N.J.; O’Neil, B.; Rasco, D.W.; Wan, J.; Mosley, A.L.; Liu, H.; Kelley, M.R.; Messmann, R.A. A Phase I Study of the APE1 Protein Inhibitor APX3330 in Patients with Advanced Solid Tumors. JCO 2019, 37, 3097–3097. [Google Scholar] [CrossRef]
- Kelley, M.R.; Luo, M.; Reed, A.; Su, D.; Delaplane, S.; Borch, R.F.; Nyland, R.L.; Gross, M.L.; Georgiadis, M.M. Functional Analysis of Novel Analogues of E3330 That Block the Redox Signaling Activity of the Multifunctional AP Endonuclease/Redox Signaling Enzyme APE1/Ref-1. Antioxidants & Redox Signaling 2011, 14, 1387–1401. [Google Scholar] [CrossRef]
- Nyland, R.L.; Luo, M.; Kelley, M.R.; Borch, R.F. Design and Synthesis of Novel Quinone Inhibitors Targeted to the Redox Function of Apurinic/Apyrimidinic Endonuclease 1/Redox Enhancing Factor-1 (Ape1/Ref-1). Journal of Medicinal Chemistry 2010, 53, 1200–1210. [Google Scholar] [CrossRef]
- Qian, C.; Li, M.; Sui, J.; Ren, T.; Li, Z.; Zhang, L.; Zhou, L.; Cheng, Y.; Wang, D. Identification of a Novel Potential Antitumor Activity of Gossypol as an APE1/Ref-1 Inhibitor. Drug Des Devel Ther 2014, 8, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Zhang, L.; Li, M.; Dai, N.; Luo, H.; Shan, J.; Yang, X.; Xu, M.; Feng, Y.; et al. A Randomized, Double-Blind, Placebo-Controlled Study of B-Cell Lymphoma 2 Homology 3 Mimetic Gossypol Combined with Docetaxel and Cisplatin for Advanced Non-Small Cell Lung Cancer with High Expression of Apurinic/Apyrimidinic Endonuclease 1. Invest New Drugs 2020, 38, 1862–1871. [Google Scholar] [CrossRef]
- Ren, T.; Shan, J.; Qing, Y.; Qian, C.; Li, Q.; Lu, G.; Li, M.; Li, C.; Peng, Y.; Luo, H.; et al. Sequential Treatment with AT-101 Enhances Cisplatin Chemosensitivity in Human Non-Small Cell Lung Cancer Cells through Inhibition of Apurinic/Apyrimidinic Endonuclease 1-Activated IL-6/STAT3 Signaling Pathway. Drug Des Devel Ther 2014, 8, 2517–2529. [Google Scholar] [CrossRef]
- Wei, X.; Duan, W.; Li, Y.; Zhang, S.; Xin, X.; Sun, L.; Gao, M.; Li, Q.; Wang, D. AT101 Exerts a Synergetic Efficacy in Gastric Cancer Patients with 5-FU Based Treatment through Promoting Apoptosis and Autophagy. Oncotarget 2016, 7, 34430–34441. [Google Scholar] [CrossRef]
- Poletto, M.; Vascotto, C.; Scognamiglio, P.L.; Lirussi, L.; Marasco, D.; Tell, G. Role of the Unstructured N-Terminal Domain of the HAPE1 (Human Apurinic/Apyrimidinic Endonuclease 1) in the Modulation of Its Interaction with Nucleic Acids and NPM1 (Nucleophosmin). Biochem. J. 2013, 452, 545–557. [Google Scholar] [CrossRef]
- Antoniali, G.; Dalla, E.; Mangiapane, G.; Zhao, X.; Jing, X.; Cheng, Y.; De Sanctis, V.; Ayyildiz, D.; Piazza, S.; Li, M.; et al. APE1 Controls DICER1 Expression in NSCLC through MiR-33a and MiR-130b. Cell. Mol. Life Sci. 2022, 79, 446. [Google Scholar] [CrossRef] [PubMed]
- Vascotto, C.; Cesaratto, L.; Zeef, L.A.H.; Deganuto, M.; D’Ambrosio, C.; Scaloni, A.; Romanello, M.; Damante, G.; Taglialatela, G.; Delneri, D.; et al. Genome-Wide Analysis and Proteomic Studies Reveal APE1/Ref-1 Multifunctional Role in Mammalian Cells. PROTEOMICS 2009, 9, 1058–1074. [Google Scholar] [CrossRef]
- Ayyildiz, D.; Antoniali, G.; D’Ambrosio, C.; Mangiapane, G.; Dalla, E.; Scaloni, A.; Tell, G.; Piazza, S. Architecture of The Human Ape1 Interactome Defines Novel Cancers Signatures. Sci Rep 2020, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; Deacon, A.M.; Duncton, M.A.J.; Pellicena, P.; Georgiadis, M.M.; Yeh, A.P.; Arvai, A.S.; Moiani, D.; Tainer, J.A.; Das, D. Fragment- and Structure-Based Drug Discovery for Developing Therapeutic Agents Targeting the DNA Damage Response. Prog Biophys Mol Biol 2021, 163, 130–142. [Google Scholar] [CrossRef]
- Codrich, M.; Degrassi, M.; Malfatti, M.C.; Antoniali, G.; Gorassini, A.; Ayyildiz, D.; De Marco, R.; Verardo, G.; Tell, G. APE1 Interacts with the Nuclear Exosome Complex Protein MTR4 and Is Involved in Cisplatin- and 5-Fluorouracil-Induced RNA Damage Response. The FEBS Journal 2022, febs.16671. [CrossRef]
- Garutti, M.; Pelizzari, G.; Bartoletti, M.; Malfatti, M.C.; Gerratana, L.; Tell, G.; Puglisi, F. Platinum Salts in Patients with Breast Cancer: A Focus on Predictive Factors. IJMS 2019, 20, 3390. [Google Scholar] [CrossRef]
- Dumas, L.; Herviou, P.; Dassi, E.; Cammas, A.; Millevoi, S. G-Quadruplexes in RNA Biology: Recent Advances and Future Directions. Trends in Biochemical Sciences 2021, 46, 270–283. [Google Scholar] [CrossRef]
- Kwok, C.K.; Sahakyan, A.B.; Balasubramanian, S. Structural Analysis Using SHALiPE to Reveal RNA G-Quadruplex Formation in Human Precursor MicroRNA. Angew. Chem. Int. Ed. 2016, 55, 8958–8961. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, J.; Santos, T.; Miranda, A.; Alexandre, D.; Teixeira, B.; Simões, P.; Lopes-Nunes, J.; Cruz, C. Ligands as Stabilizers of G-Quadruplexes in Non-Coding RNAs. Molecules 2021, 26, 6164. [Google Scholar] [CrossRef]
- Koralewska, N.; Szczepanska, A.; Ciechanowska, K.; Wojnicka, M.; Pokornowska, M.; Milewski, M.C.; Gudanis, D.; Baranowski, D.; Nithin, C.; Bujnicki, J.M.; et al. RNA and DNA G-Quadruplexes Bind to Human Dicer and Inhibit Its Activity. Cell. Mol. Life Sci. 2021, 78, 3709–3724. [Google Scholar] [CrossRef] [PubMed]
- Asamitsu, S.; Shioda, N. Potential Roles of G-Quadruplex Structures in RNA Granules for Physiological and Pathological Phase Separation. The Journal of Biochemistry 2021, 169, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, M.; Duncan, S.; Yang, X.; Abdelhamid, M.A.S.; Huang, L.; Zhang, H.; Benfey, P.N.; Waller, Z.A.E.; Ding, Y. G-Quadruplex Structures Trigger RNA Phase Separation. Nucleic Acids Research 2019, 47, 11746–11754. [Google Scholar] [CrossRef] [PubMed]
- Mirihana Arachchilage, G.; Dassanayake, A.C.; Basu, S. A Potassium Ion-Dependent RNA Structural Switch Regulates Human Pre-MiRNA 92b Maturation. Chemistry & Biology 2015, 22, 262–272. [Google Scholar] [CrossRef]
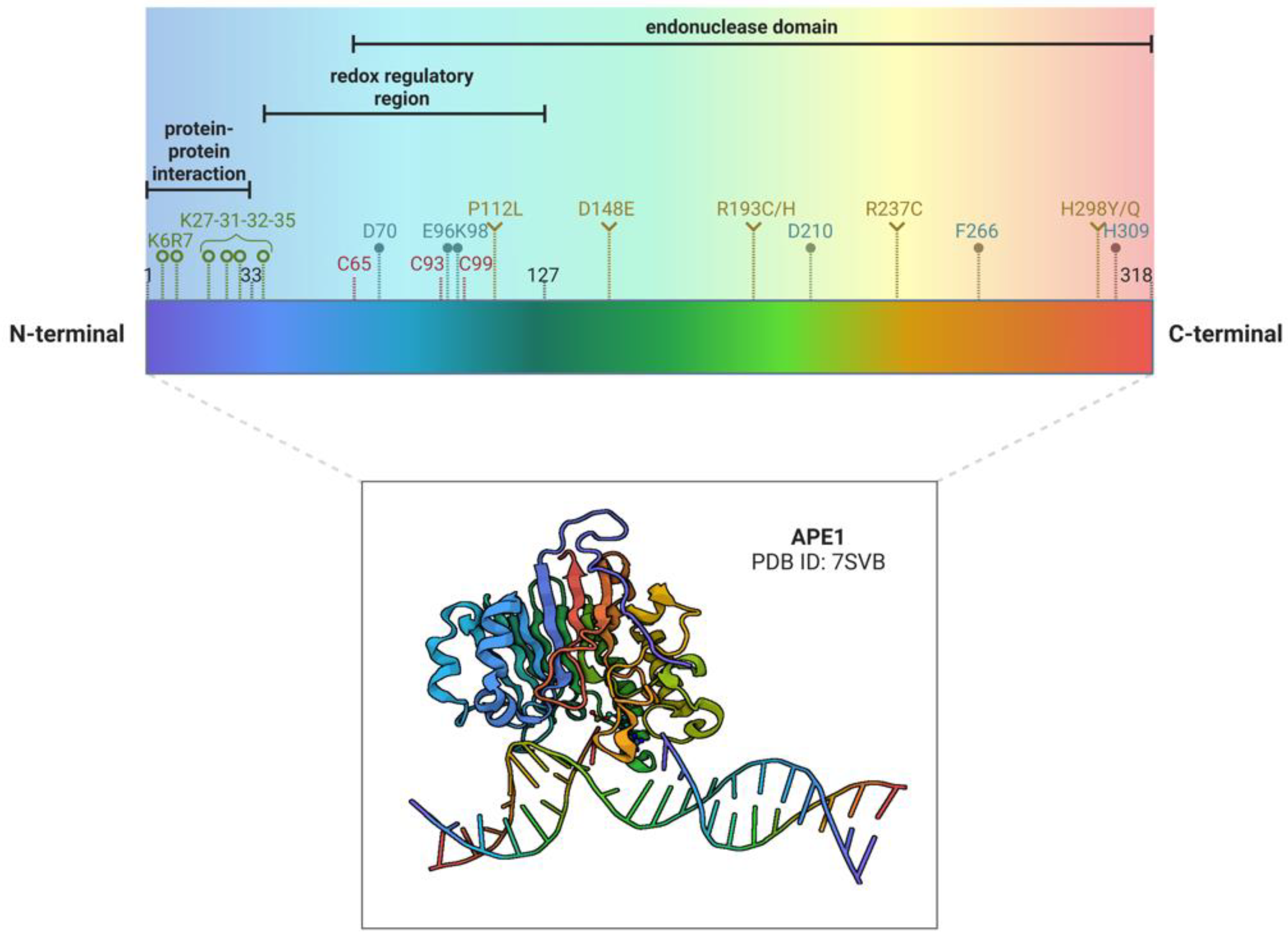
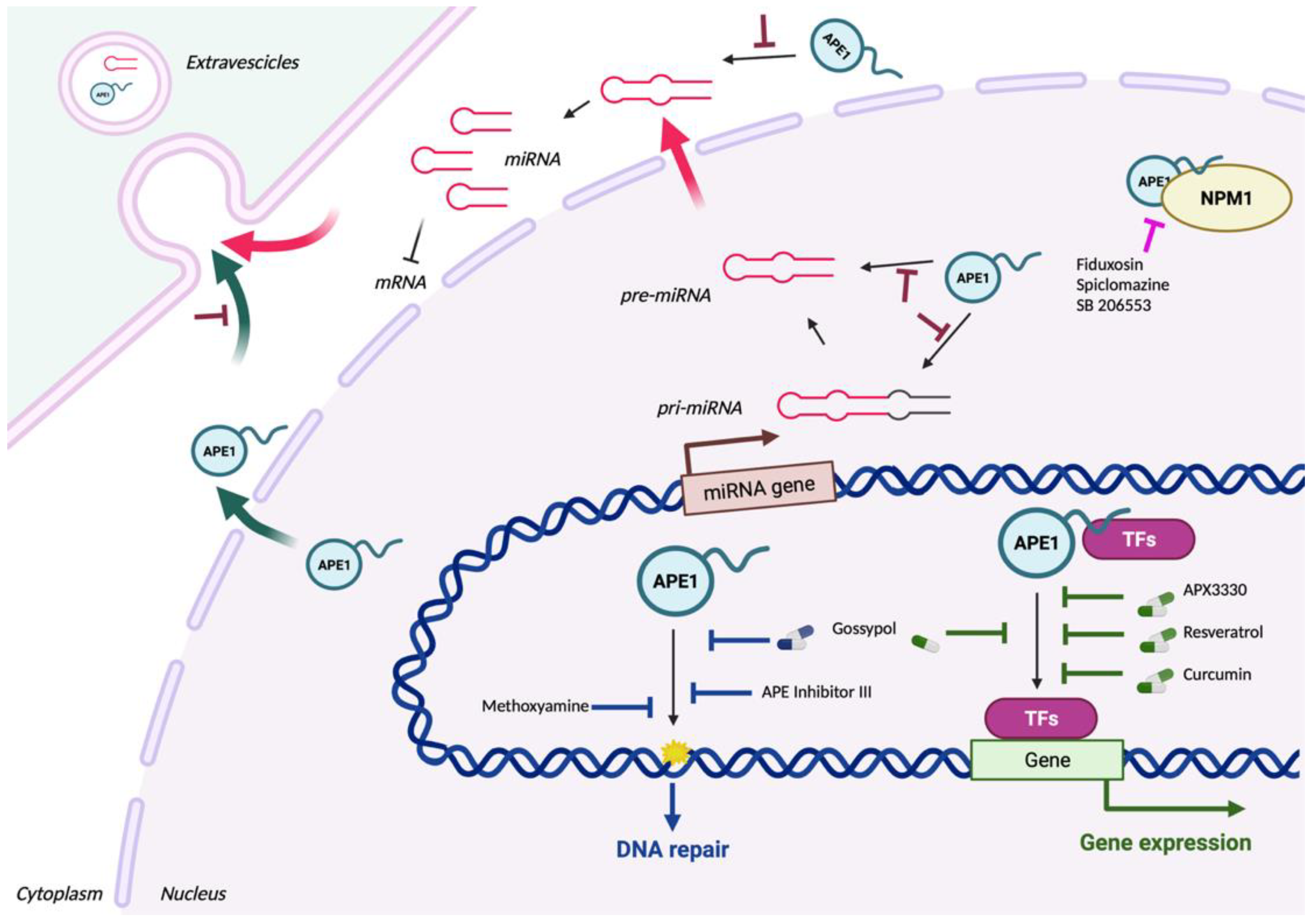
| Cancer | Polymorphisms |
|---|---|
| Adrenocortical carcinoma | D283H; X20_splice |
| Ampullary cancer | M271T |
| Bladder cancer | D15H; S146L; Q109E; E217* |
| Bone cancer | V131M |
| Breast cancer | L244Tfs*8; Q51*; H289Y; G5A; K7Rfs*75; R185W; A60G |
| Cervical cancer | R281C; K7R |
| Colorectal cancer | A273T; R193C; R221H; G130D; R247Q; A230D; M271I; R221H; R181*; E242D; R274*; P293S; P49Qfs*33; L220I; T233M; N226Efs*26; V131M; L291Vfs*6; R221C; K63E; D210N; R281H; X82_splice; E101D; K77N; P331H; G306S; G132D |
| Endometrial cancer | R193H; S164L; R221H; A170V; P223L; V84I; V278D; R281H; K228T |
| Gastric cancer | R281C; P122A; K27N |
| Glioma | H289Q; Q245R; G80E; D142N |
| Head and Neck cancer | P48H |
| Leukaemia and Lymphoma | R181Q; L17P; K7R; R187H |
| Liver cancer | G5E; W280S; L220P; W280R; D50Rfs*28; G8R; H289Y, N226Efs*26; Y264_G279del |
| Lung cancer | G41C; V206Cfs*11; V142Sfs*8; E16Q; P331T; E149Q; X147_splice; R177*; D90H; Q51*;X237_splice; R193C; R28S; M271del; V206Cfs*11; S115F; G8R; I146V; D148E |
| Melanoma | G241W; E16K; G127V; R136S; P122T; D148E; A263V; K7Rfs*75; L108F; V69L |
| Oesophageal cancer | E46D; D251N; M270Nfs*14; F165V; N102I; L291Vfs*6; K3R; G145D |
| Ovarian cancer | Q95*, V168I; R193H; L291Vfs*6 |
| Pancreatic cancer | R193C; M271del |
| Prostate cancer | P139Q; A30T; R187H |
| Renal cancer | E149* |
| Sarcoma | R187L; K35Rfs*11; K35Q |
| Skin cancer | P89S |
| Cancer | Expression | Diagnostic value | Localization | Refs |
|---|---|---|---|---|
| Bladder cancer (BCa) |
Protein overexpression, associated with poor survival and invasion. | Serum and urine levels as a diagnostic biomarker. |
|
[36,37,38,39,40,41,42] |
| Breast cancer |
|
n.d. | Nuclear localization. | [43,44,45,46,47,48] |
| Cervical cancer |
High protein expression associated with lymph node metastasis, EMT and decreasing radiosensitivity. | n.d. |
|
[49,50,51,52] |
| Colorectal cancer (CRC) |
|
Serum APE1-autoantibodies levels as a diagnostic biomarker. |
|
[53,54,55,56,57,58,59,60] |
| Cutaneous Squamous Cell carcinoma (cSCC) |
Protein overexpression in tumour tissues, which promotes proliferation of cancer cells and migration by EMT. | n.d. | n.d. | [61] |
| Gastric carcinoma |
mRNA and protein upregulation, correlated with lymph node metastasis, depth of invasion and poor prognosis. | Serum levels as a diagnostic biomarker for metastasis prediction. | Nuclear and cytoplasmatic localization. | [62,63,64,65] |
| Glioma |
|
n.d. | Nuclear localization. | [66,67,68,69,70,71] |
| Head and Neck Squamous Cell carcinoma (HNSCC) |
|
In oral SCC (oSCC): Serum levels as a diagnostic biomarker, with high levels correlated with late TNM stages, lymph node metastasis and worse pathologic differentiation. |
higher cytoplasmatic staining in sSCC, associated with T-stage and histological grade.
|
[72,73,74,75,76,77,78] |
| Liver cancer |
|
Serum levels as a diagnostic biomarker. |
|
[79,80,81,82,83,84] |
| Lung cancer |
|
High post-treatment serum levels are associated with lower overall survival. |
|
[53,85,86,87,88,89,90,91,92,93,94] |
| Melanoma | mRNA and protein overexpression associated with vascular invasion, high mitotic rate, lower response to therapy and poor prognosis. | n.d. | Nuclear localization. | [95,96,97,98] |
| Oesophageal carcinoma (EAC) | Protein overexpression in tumour tissues, associated with worse overall survival. | n.d. | Mainly nuclear localization. | [99,100,101,102,103,104] |
| Osteosarcoma |
|
n.d. |
|
[105,106,107,108,109,110,111] |
| Ovarian cancer |
Protein overexpression in tumour tissues, associated with advanced stages, platinum resistance and poor chemosensitivity, decreased overall survival and lymph node metastasis. | n.d. |
|
[99,112,113,114,115,116,117,118,119] |
| Pancreatic adenocarcinoma (PDAC) |
|
n.d. | Strong nuclear staining in tumour tissues, with cytosol staining only in advanced stages. | [53,85,99,120,121,122] |
| Prostate carcinoma (PCa) |
Protein overexpression in cancer samples. | n.d. | Nuclear and cytoplasmatic localization. | [123,124] |
| Salivary gland carcinoma |
|
n.d. | Mainly nuclear staining, with nuclear and cytosolic staining in some malignant forms. | [125,126] |
| APE1 function inhibited | Name | IUPAC name | PubChem CID | Molecular Formula | Molecular Weight (g/mol) | Structure | Refs |
|---|---|---|---|---|---|---|---|
| Endonuclease | Methoxyamine | O-methylhydroxylamine | 4113 | CH5NO | 47.057 | 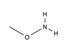 |
[137,138,139,140] |
| Lucanthone | 1-[2-(diethylamino)ethylamino]-4-methylthioxanthen-9-one | 10180 | C20H24N2OS | 340.5 | 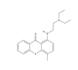 |
[141,142,143] | |
| CRT0044867 | 7-Nitroindole-2-carboxylic acid | 81409 | C9H6N2O4 | 206.15 | 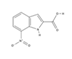 |
[21,144,145,146] | |
| Myricetin | 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one | 5281672 | C15H10O8 | 318.23 | 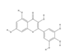 |
[145] | |
| AR03 | 2,4,9-Trimethylbenzo[b][1,8]naphthyridin-5-amine | 698490 | C15H15N3 | 237.30 |  |
[149] | |
| APE Inhibitor III | N-[3-(1,3-benzothiazol-2-yl)-6-isopropyl-4,5,6,7-tetrahydrothieno [2,3-c]pyridin-2-yl]acetamide | 3581333 | C19H21N3OS2 | 371.5 | 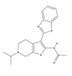 |
[153,154] | |
| Redox | Curcumin | (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione | 969516 | C21H20O6 | 368.4 |  |
[173,174] |
| Resveratrol | 5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol | 445154 | C14H12O3 | 228.24 | 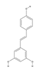 |
[97,175] | |
| APX3330 | (2E)-2-[(4,5-dimethoxy-2-methyl-3,6-dioxocyclohexa-1,4-dien-1-yl)methylidene]undecanoic acid | 6439397 | C21H30O6 | 378.5 | 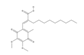 |
[22,177,178,179,180,181,182,183,184,185,186,187] | |
| APX2009 | (2E)-N,N-diethyl-2-[(3-methoxy-1,4-dioxonaphthalen-2-yl)methylidene]pentanamide | 71618575 | C21H25NO4 | 355.4 | 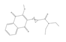 |
[177] | |
| Endonuclease Redox | Gossypol | 7-(8-formyl-1,6,7-trihydroxy-3-methyl-5-propan-2-ylnaphthalen-2-yl)-2,3,8-trihydroxy-6-methyl-4-propan-2-ylnaphthalene-1-carbaldehyde | 3503 | C30H30O8 | 518.6 |  |
[190,191] |
| protein-protein interaction | Fiduxosin | 5-[4-[(3aR,9bR)-9-methoxy-3,3a,4,9b-tetrahydro-1H-chromeno[3,4-c]pyrrol-2-yl]butyl]-12-phenyl-8-thia-3,5,10,13-tetrazatricyclo[7.4.0.02,7]trideca-1(13),2(7),9,11-tetraene-4,6-dione | 172307 | C30H29N5O4S | 555.6 |  |
[47,116,200] |
| Spiclomazine | 8-[3-(2-chlorophenothiazin-10-yl)propyl]-1-thia-4,8-diazaspiro[4.5]decan-3-one | 65714 | C22H24ClN3OS2 | 446.0 | 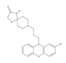 |
[47,116,200] | |
| SB 206553 | 1-methyl-N-pyridin-3-yl-6,7-dihydropyrrolo[2,3-f]indole-5-carboxamide | 5163 | C17H16N4O | 292.33 | 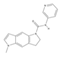 |
[47,116,200] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





