1. Introduction
Grapes affected with grey mould due to infection by the fungus
Botrytis cinerea are widespread in vineyards during certain growing periods, especially during prolonged wet and humid conditions associated with excessive rainfall or irrigation. Outbreaks of infections often occur close to grape harvest resulting in a negative impact on yield and grape quality [
1,
2]. Growth of the fungus leads to the oxidisation of phenolic compounds, causing a loss of colour and the formation of a suite of off-flavours and other odours, which may impact the quality and composition of the wine and ultimately, consumer acceptance. Monitoring fungal infection levels is essential for grape quality assessment before harvest. However, fungal infections are often hidden within the interior of the grape bunch making visual inspections challenging. Image-based monitoring techniques may be useful but are also of limited value since they fail to detect
B. cinerea infections hidden in the interior of the grape bunches [
3]. A rapid and objective method suitable for assessing crop phytosanitary conditions would assist growers and winemakers as a decision support tool to decide when, or even if, to harvest. A rapid sampling and analytical method that can also be applied to monitor susceptible horticultural crops and not just grapes at post-harvest storage and transport stages would also assist in quality assurance of the supply chain.
Volatile metabolomes associated with
B. cinerea infections have recently been used to establish the severity level of infection in wine grapes [
4,
5]. Volatile compounds elicited during fungal growth may vary according to abiotic conditions, including nutrient availability, temperature, microbial interactions, and substrate [
6,
7]. Several volatile compounds have been selected as markers for
B. cinerea, including 1-octen-3-ol, 1-octen-3-one, and 3-octanone, which are widely reported to cause mouldy or mushroom odours associated with fungal infections [
8], and are therefore ideal targets for pathogen detection.
Sensitive detection and quantification of hundreds of volatile compounds in complex mixtures using mass spectrometry (MS) is possible without sample pre-labelling. It is an excellent analytical approach for studying fungal biomarkers and wine off-flavours. Current MS analysis for grape composition frequently uses solid phase microextraction (SPME) [
9,
10], to pre-concentrate the volatile compounds directly from the sample matrix prior to analysis by gas chromatography (GC) MS [
11]. Compared with distillation or solvent extraction techniques [
12], the SPME method is more time efficient, can be easily automated, and limits of detection are usually improved [
13]. Nowadays, SPME is now widely used for environmental monitoring [
14,
15], food and agricultural products analysis [
16,
17], and drug development [
18].
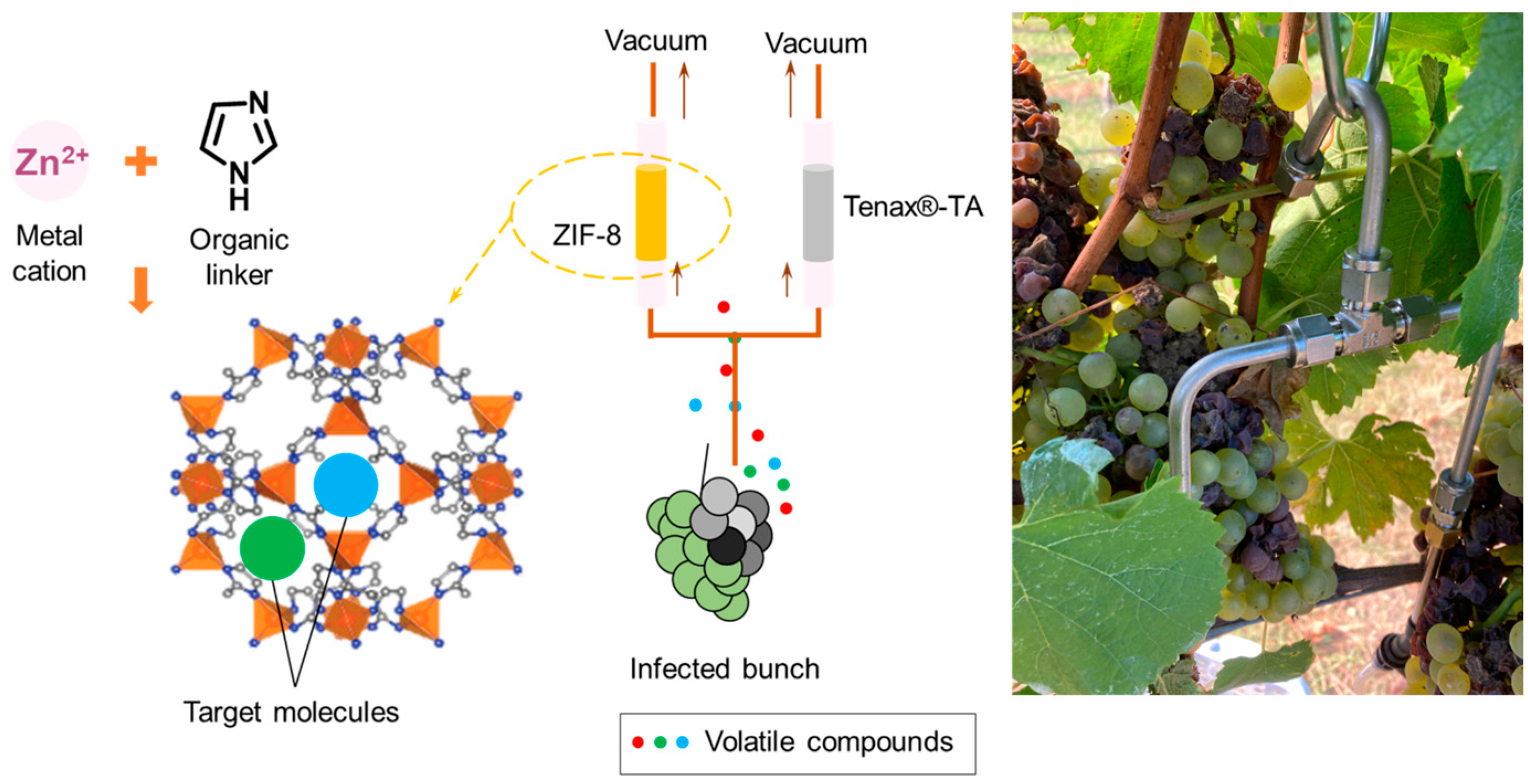
However, due to their high cost, fragility, and relatively small volume of solid phase materials, SPME fibres are more suitable for lab-based experiments than field sampling. To achieve in vivo sampling and real-time monitoring of volatile compounds in grapes, alternative absorbent materials encased in robust housing ideal for field use are desirable. Metal-organic frameworks (MOFs) have gained considerable interest due to their enormous internal surface area (> 6 000 m
2/g), ultrahigh porosity (up to 90 % free volume) and tuneable pore size [
19] and have subsequently been used for a range of analytical methods and novel applications. The unique characteristics of MOFs were used to adsorb toxic components from contaminated environments for remediation [
20,
21] and to lower detection limits for rapid and direct mass spectrometry analyses [
22]. MOF-5 [
23] has been packed in quartz tubes for the in-field collection and quantification of formaldehyde, and this procedure has shown excellent stability, adsorption efficiency and reproducibility [
24]. Mixtures of aldehydes have also been detected and quantified using different structured MOFs coupled with thermal desorption GC-MS and compared with a commercial absorbent Tenax
®-TA [
25]. These applications demonstrate the range of potential usage of MOFs extraction for GC-MS in wine characterisation.
In-field static volatile sampling from plants material has been performed using SPME fibres for monitoring volatiles from single grape berries and bunches of grapes in which the sampling environment was protected using glass enclosures or polyvinyl fluoride plastic bags for the duration of sampling [
26]. Enclosed environments used with SPME sampling have also been created using inverted glass funnels placed over flowers to enable monitoring of volatile emission during flower development. Static headspace sampling is an easy and non-destructive volatile collection method suitable for in situ fingerprint profiling and quantitative analysis of VOCs. However, the required sampling time with enclosed samples is typically very long, up to 1 day each, which may substantially increase confounding impacts of airspace humidity and heat accumulation upon volatile expression from the sample and absorption efficiency. Rapid volatile sampling methods are appropriate for high throughput sample analysis. Reduction of sampling time can be achieved using low vacuum devices to draw air through a sampling tube packed with solid phase material, and such devices have been deployed for a volatile sampling of Meyer lemon citrus trees and plant VOCs, enabling detection of α–pinene, limonene, linalool and α–terpineol with as little as a 5 min sampling duration [
27]. In another study, a commercially available SPME syringe coupled with an air sampling pump was used to collect volatiles from lavender plants,
Lavandula angustifolia and
Lavandula × intermedia, thereby enabling species discrimination followed by a portable GC MS for in-field volatile analysis with a 5-min sampling time and 3-min chromatographic separation [
28]. An advantage of air sampling pumps is that they produce a constant low flow rate at around 50 mL/min, allowing enough time for absorption materials to trap volatile compounds. These dynamic, volatile sampling methods allow rapid sample extraction and analysis demonstrating potential application for in-field or point-of-sample volatile monitoring for fruit quality assessment.
The aim of the current work is to develop a rapid, volatile sampling method for wine grapes that could be used prior to harvest to detect and measure B. cinerea infection. Sampling tubes with zeolitic imidazolate framework-8 (ZIF-8) crystal were applied for volatile in-field sampling and then analysed using thermal desorption GC-MS. Using a low-flow rate pump, volatile metabolites of interest were collected in 15 min for each sample, and commercially available material, Tenax®-TA, was used for extraction simultaneously as a comparison.
2. Results and Discussion
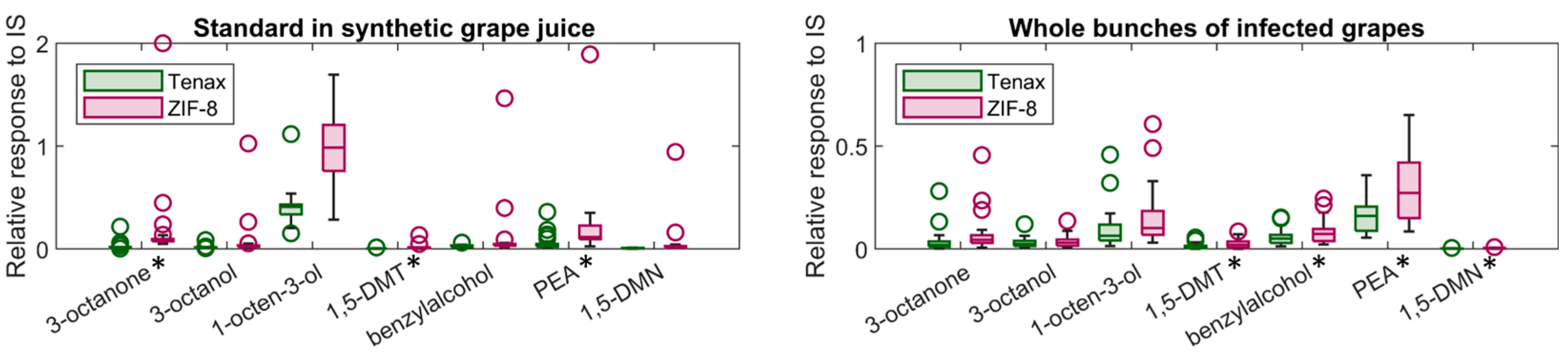
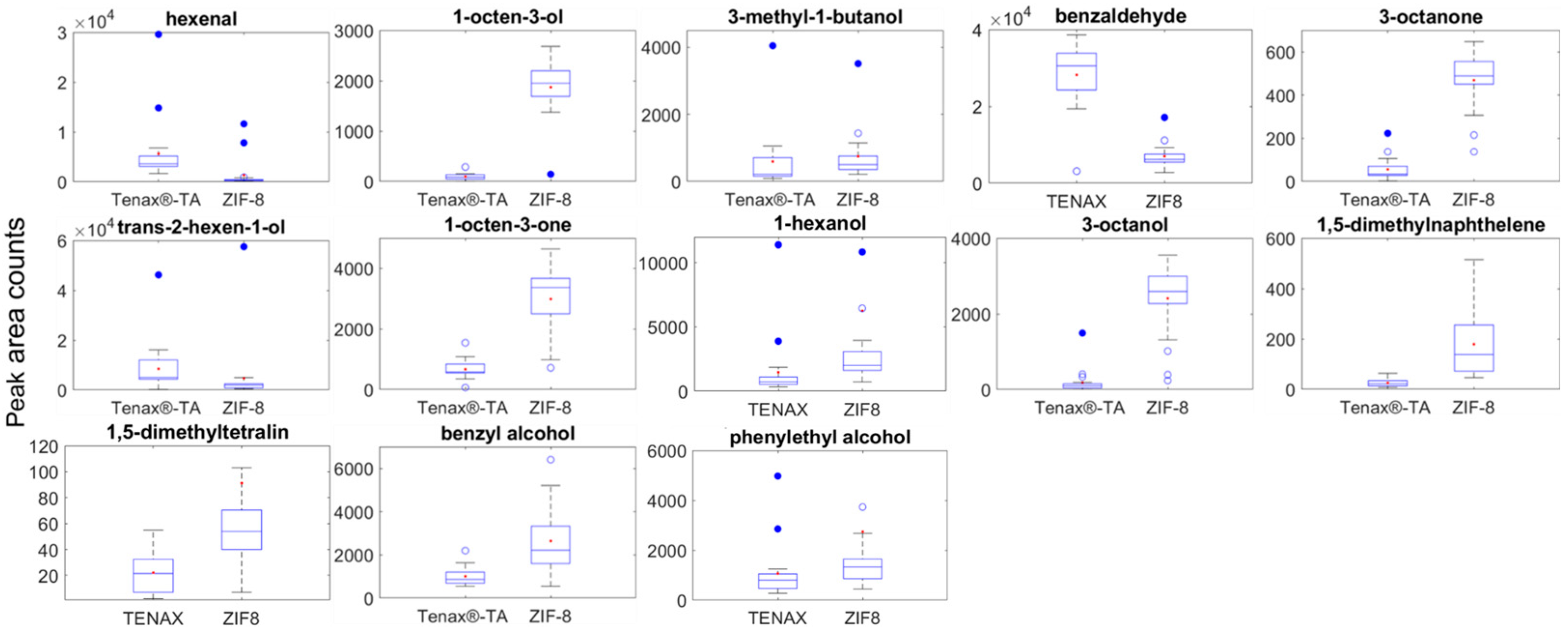
2.1. Controlled Envoronmental Condition for Gas Sampling
Key volatile compounds related to
B. cinerea infection in grapes including 3-octanone, 3-octanol, 1-octen-3-ol, 1,5-diemthyltetralin, benzyl alcohol, phenylethyl alcohol and 1,5-dimethylnaphthalene were detected from a controlled environment using ZIF-8 and Tenax
®-TA (
Figure 1). In this experiment, 2-methyl-4-pentanol was applied on a filter paper as an internal standard (IS) to enable consistent volatilisation and avoid interference from grape contamination and potential absorption into the waxy berry layer, thereby confounding analysis. The controlled environment ensured relatively stable conditions of temperature, humidity, and limited air movement, so the partitioning of volatile from solution to the air within the chamber was consistent throughout the experiment. The relative response rate for most compounds using ZIF-8 was compatible with Tenax
®-TA.
The whole bunches of
B. cinerea infected grapes were placed into the same glass container system for the headspace volatile analysis as shown in
Figure 1 (right). The variance for some compounds such as phenylethyl alcohol may be caused by the biological diversity of grapes with
B. cinerea infection of different severities.
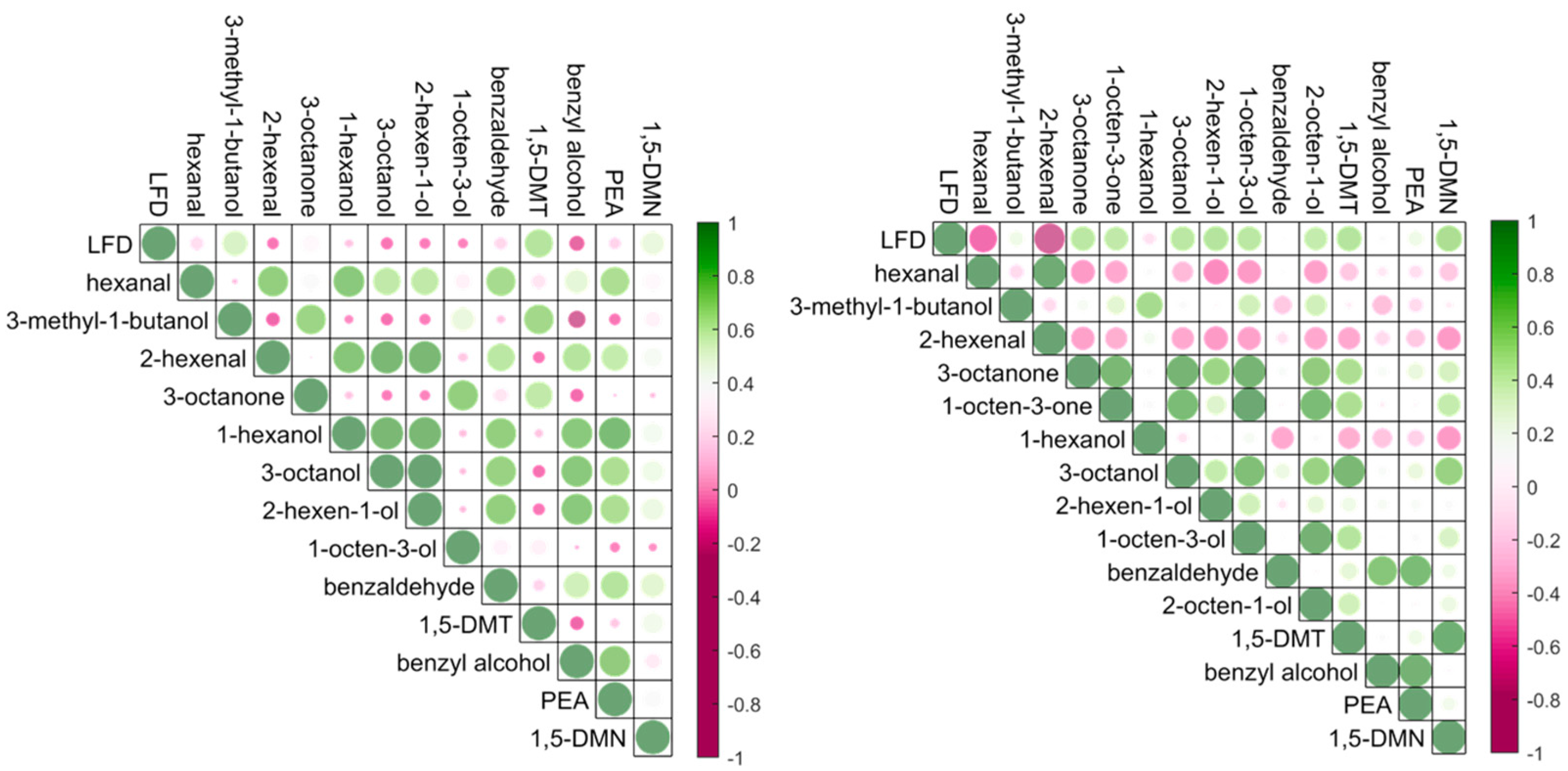
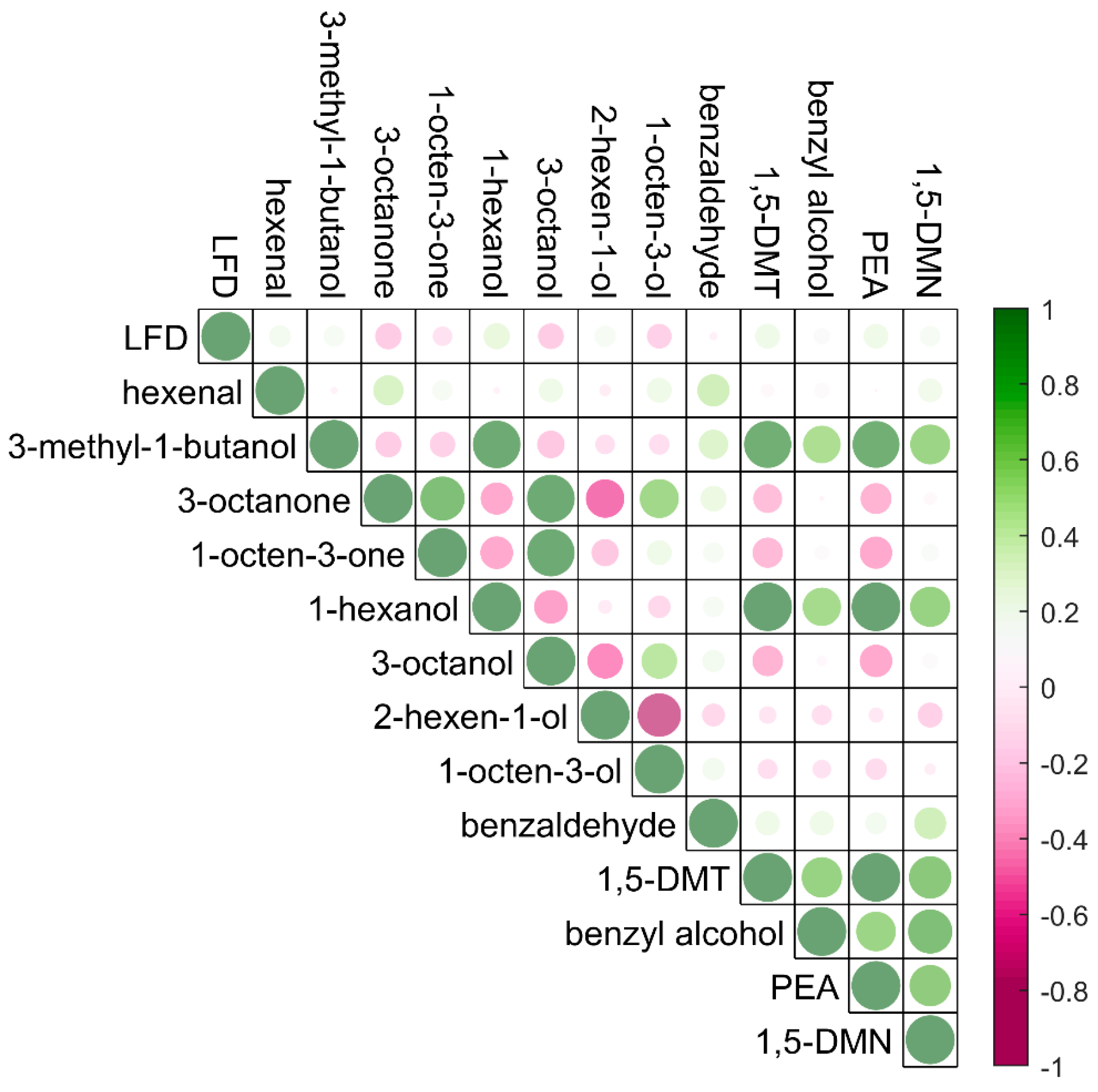
2.2. Correlation between Volatile Detection and B. cinerea Infection Severities
Correlation between the
B. cinerea infection levels and the targeted compounds detected using thermal desorption GC-MS with ZIF-8 as absorbent materials in a controlled environmental condition are shown in
Figure 2 (left).
B. cinerea infection severities were determined by the concentration of species-specific antigens using a lateral flow device (LFD). Lateral flow devices are reported to have excellent performance for detection and quantification of infection levels in naturally infected grapes compared with other measures of infection such as measurement of ergosterol [
5].
In this experiment, positive correlation coefficients were observed between the LFD scores and the concentrations of 3-methyl-1-butanol, 1,5-dimethyltetralin and 1,5-dimethylnaphthalene. 1,5-dimethyltetralin and 1,5-dimethylnaphthalene are reported as key markers for
B. cinerea infection in grapes [
5] and are thought to arise from the breakdown of berry carotenoids [
29]. 3-Methyl-1-butanol was reported as a key volatile organic compound for the discrimination of bunch rot and noble rot detected in the progression of grey mould disease in grape berries [
30]. In some cases, noble rot caused by
B. cinerea is desirable as the growth of fungi may weaken the grape skin, accelerate water evaporation and lead to high concentrations of sugars and acids, especially for dessert wines. However, with high humidity environments, the growth of grey mould results in the reduction of sugar accumulation and the increase of acetic acid in the juice of the grape berries which may significantly impact the wine quality. Thus, the detection of 3-methyl-1-butanol is important for harvest decisions, and volatile analysis on berries before sample crushing using a non-invasive sampling technique directly from the gas space around intact bunches provides a potential pathway for more rapid and thorough analytical support tools.
Apart from the correlations between the grape
B. cinerea infection levels and the detection of the volatile compounds,
Figure 2 (left) demonstrates correlations among the metabolites. For example, the concentration of 3-octanone was positively correlated with the concentrations of 3-methyl-1-butanol, 1-octen-3-ol, and 1,5-dimethyltetralin. Eight-carbon oxylipin compounds such as 3-octanone, 1-octen-3-ol are common compounds related to fungal infection in grapes possessing a typical mushroom or earthy odours [
31,
32,
33]. They are significant metabolites produced predominantly by fungi through the enzymatic oxidation and cleavage of linoleic acid by lipoxygenase and hydroperoxide [
30], and could be considered as markers for fungal infection on grapes or other horticultural crops.
SPME GC-MS was used for the analysis of the grape homogenates from the same sample set as a comparison and a benchmark method.
Figure 2 (right) demonstrates the correlations between the LFD scores and the volatile metabolites detected using SPME GC-MS. Volatile metabolites including 3-octanone, 3-octanol, 1-octen-3-ol, and 1-octen-3-one were detected from
B. cinerea infected grapes, which supports the positive correlation by thermal desorption GC-MS for the detection of 3-octanone and 1-octen-3-ol. C6 compounds hexanal and 2-henenal were negatively correlated with the
B. cinerea grape infections and are important volatile components in grape skins derived from unsaturated fatty acids via the lipoxygenase hydroperoxide lyase enzymatic pathway, which is usually activated immediately following wounding [
34,
35]. Different performance of headspace volatile sampling using SPME fiber and ZIF-8 may depend on the physical or chemical properties or the detection range of the absorbent materials.
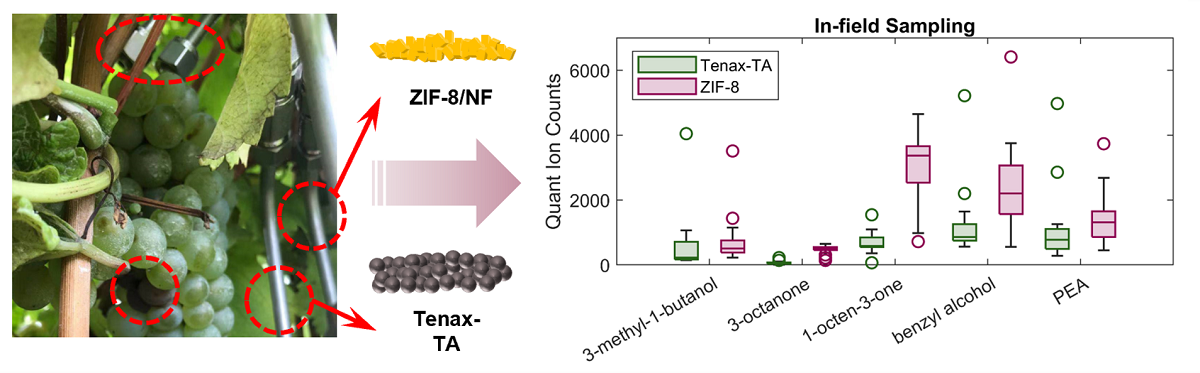
2.3. In-Field Volatile Sampling
Thermal desorption GC-MS was applied for the in-field volatile sampling using ZIF-8 as absorption materials, and a commercially available material Tenax®-TA was used simultaneously as comparison. In this experiment, the absolute abundances of peak area counts were integrated to compare the extraction efficiency of the two materials, due to the restriction of the IS application in the field environment. This sampling experiment was conducted at a local commercial vineyard in an open-air condition with variant temperatures, humidities and random air movement, compared to the lab-based volatile extraction experiment within a glass container. The purpose of this experiment is to demonstrate the extraction ability of fugal-related metabolites using ZIF-8 and the proof of concept for the correlations between the in-field volatile detection and the B. cinerea infection severities in the grapes.
Volatiles related to fungal infection, hexenal, 1-octen-3-ol, 3-methyl-1-butanol, benzaldehyde, 3-octenone,
trans-2-hexen-1-ol, 1-octen-1-one, 1-hexanol, 3-octanol, 1,5-dimethylnaphthalene, 1-5-dimethyltetralin, benzyl alcohol and phenylethyl alcohol were readily detected using ZIF-8 and Tenax
®-TA as absorbent materials (
Figure 3). A range of volatile concentration are evident for the in-field sample results which reflect the range of
B. cinerea infection severities encountered. Generally, ZIF-8 performed better with higher peak area counts for alcohol compounds, 1-octen-3-ol, 3-octanol, benzyl alcohol and phenyl alcohol. With the formula of zinc(2-methylimidazolate)
2, ZIF-8 is highly hydrophobic, and repels adsorption of water vapour before the onset of capillary condensation and such material was reported to be suitable for use in humid environments [
36]. The selective properties of ZIF8 hydrophobic channels playing a significant role for improved affinity for bio-alcohols was demonstrated with analysis of isobutanol [
37]. Our results demonstrating increased sensitivity of ZIF-8 compared to Tenax
®-TA for the absorption of low molecular weight alcohols including 1-octen-3-ol, 1-hexanol, 3-octanol, benzyl alcohol and phenylethyl alcohol (
Figure 3) are supported by prior reports of ZIF-8 selectivity. An exceptionally high capacity of ZIF8 for some alcohols is reported, which is thought to arise from the flexible structural properties of the solid phase material thereby increasing selectivity for this chemical class [
37]. Another observation is the higher sensitivities of C8 compounds using ZIF-8 as absorbent materials, which are crucial markers for fungal infection. In this experiment, the overall peak areas detected using ZIF-8 were 8.29, 4.65, 13.13 and 18.52 times higher than Tenax
®-TA for the detection of 3-octenone, 1-octen-3-one, 3-octanol and 1-octen-3-ol, respectively.
The good performance of ZIF-8 for the extraction of B. cinerea related volatiles from in field samples, including 3-methyl-1-butanol, 3-octanone, 1-octen-3-one, 3-octanol, 1-octen-3-ol and phenylethyl alcohol demonstrates the ability to apply this sorbent material to in-situ volatile collection and detection of B. cinerea infection in grapes prior to harvest. This non-destructive sampling method can also apply to other agricultural products and horticultural products that may be subject to fungal during prolonged storage or transport periods.
A correlation coefficient plot to show the relationship between the grape
B. cinerea infection and the detections of volatile compounds is presented in
Figure 4. Strong positive correlations were observed between the detections of 1-octen-3-one, 3-octanone, 3-octanol and 1-octen-3-ol, as well as the detections of 3-methyl-1-butanol, 1-hexanol, 1,5-dimethyltetralin, benzyl alcohol, phenylethyl alcohol and 1,5-dimethylnaphthalene for in field results. These volatiles were important for
B. cinerea grape infection, and the correlations may be explained by the growth progression of the pathogen. For example, 3-methyl-1-butanol and phenylethyl alcohol were reported as key volatiles for noble rot and grey mould discrimination [
30], and 3-octanol, phenylethyl alcohol and 1,5-dimethylnaphthalene were regarded as key biomarkers for the detection and prediction of
B. cinerea infection in grapes [
5]. Compared to the sampling in controlled conditions (
Figure 2, left), relatively weak correlations were observed between the LFD scores and the detection of 3-methyl-1-butanol, 1,5-dimethyltetralin, phenylethyl alcohol and 1,5-dimethylnaphthalene from in-field sampling. The apparent difference between sampling strategies is likely to be related to the uncontrolled environmental conditions encountered during in field sampling of grape bunches. Whilst every effort was made to position extraction tubes closely and evenly to grape bunches, changing conditions during the day will alter the relative volatility of the target molecules. Variances in sample extraction efficacy with controlled environments are corrected through use of the IS [
38], however their incorporation into a suite of target compounds is not possible in the field.
Key volatile metabolites were able to be extracted from in-field sampling using ZIF-8 as absorbent materials demonstrating a proof of concept for in field sampling for the detection of Botrytis cinerea infection. The use of a hermetically sealed controlled environment such as a glass container to eliminate air movement and maintain standard sampling conditions enabled a higher level of sensitivity for some volatiles to be achieved for ZIF8 compared to Tenax®-TA.
3. Materials and Methods
3.1. Chemical and Materials
Analytical-grade chemicals and reagents for preparing standard solutions for GC-MS analysis were used without further purification. Standard 1-octen-3-one (96 %), 1-octen-3-ol (≥98 %), 3-octanone (≥98 %), benzaldehyde (≥99.5 %), trans-2-hexen-1-ol (96 %), benzyl alcohol (99 %+), 1,5-dimethyl naphthalene (98 %), 3-methyl-1-butanol (99 %+), 1,5-dimethyltetralin (≥90 %), phenylethyl alcohol (≥99 %), 4-methyl-2-pentanol (98%), glucose (≥99.5 %), fructose (≥99 %), tartaric acid (99 %), naphthalene-d8 (99 atom % D, ≥98% (CP)), 3-octanol (99 %), 2-octanol (≥99.5 %), zinc nitrate hexahydrate (98 %), 2-methylimidazole (98 %) and glass wool were purchased from Sigma-Aldrich (Sydney, NSW, Australia). Hexanal (98 %), 1-hexanol (99 %), menthol (HPLC) and N,N-dimethylformamide (DMF) (99 %) were purchased from Alfa Aesar (Scoresby, VIC, Australia). Hexanal-d12 (98.5 atom % D), 2,4,6-tribromoanisol-d5 (99 atom % D) were purchased from CDN Isotopes (Pointe-Claire, Quebec, Canada). Milli-Q™ water (18 MΩ·cm) was collected through a Millipore™ water purification system (Merk, Bayswater, VIC, Australia). Synthetical grape juice was made using 100 g glucose, 100 g fructose and 4 g tartaric acid. All solutions were prepared in Milli-Q™ water, with pH adjusted to 7.0.
3.2. ZIF-8 Synthesis and Sampling Tube Preparation
ZIF-8 were synthesised based on a previous reported study [
39]. In brief, 0.67 g (2 mmol) of zinc nitrate hexahydrate, 0.167 g (2 mmol) of 2-methylimidazole and 50 mL
N,N-dimethylformamide were mixed with 5 min of sonication in a 100 mL screw-thread vial. The mixture was then heated at 140 °C for 24 h in a convection oven, and then filtered and washed with DMF several times, kept in methanol for 3 days and dried at room temperature.
The volatile sampling tube was prepared in a pre-treated empty thermal desorption tube (O.D. × L 1/4 in. × 3 ½, LECO Australia Pty. Ltd., Castle Hill, NSW, Australia) with 250 mg dried ZIF-8, making it a comparable weight to a commercially available sorbent tube, Markes Tenax®-TA (Kinesis Australia, Redland Bay, QLD, Australia). Each tube was packed in the following sequence: stainless-steel gauze disc, ZIF-8, glass wool and stainless-steel G-Clip (Kinesis Australia, Redland Bay, QLD, Australia). Prior to sampling, the ZIF-8 and Tenax®-TA sorbent tubes were conditioned using a Markes TC-20 tube conditioner system (Kinesis Australia, Redland Bay, QLD, Australia), with a 40 mL/min nitrogen flow at 300 °C for 2 h.
3.3. VOCs Extraction Using ZIF-8
3.3.1. Volatile Extraction in a Closed Environment
Volatile metabolites from the headspace of whole bunch of the same grapes harvested from the in-field sampling experiment, 10 Chardonnay and 10 Riesling grape bunches collected from a local vineyard in Tumbarumba, NSW, Australia, were in the glass container with the same procedure.
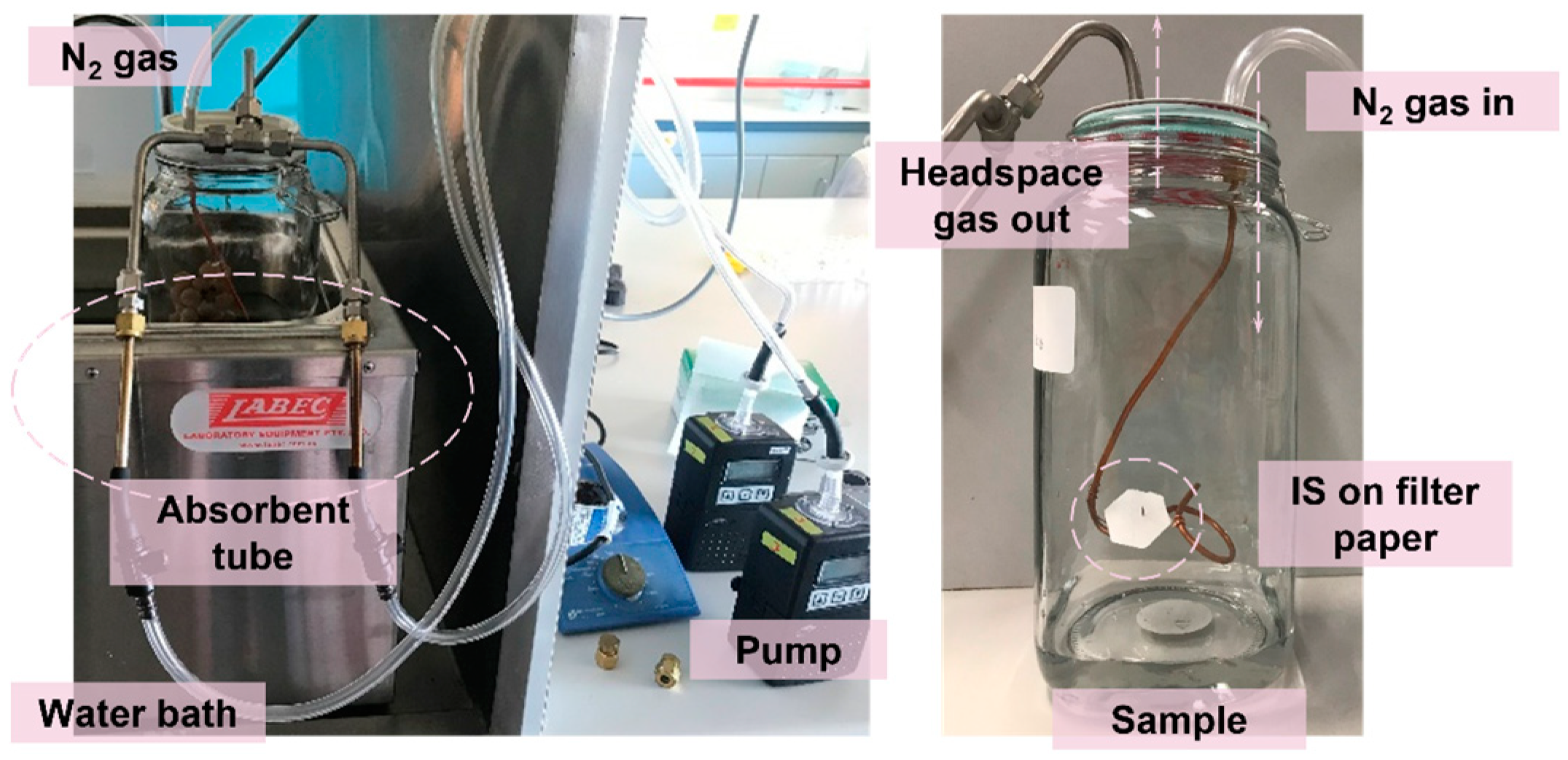
Sampling for volatiles from a standard mix of target volatile compounds and from naturally infected bunches of grapes, assessed for visible signs of grey mould, was undertaken a 2.5 L glass container to control the temperature and air movement. Synthetic grape juice (100 mL, as described previously) spiked with 50 μL mixed standard in methanol to a final concentration of 7.2 μg/L 1-octen-3-one, 93.6 7.2 μg/L 3-octanol, 32.8 μg/L 1-octen-3-ol, 2.39 μg/L 1,5-diemthyltetralin, 1690 μg/L benzyl alcohol, 247 μg/L phenylethyl alcohol and 3.39 μg/L 1,5-dimethylnaphthalene in the container, or individual grape bunches were placed into the container prior to insertion of the IS. An IS comprising 10 μL 40 mg/L 2-methyl-4-pentanol in methanol was applied on a cut filter paper disc (200 mm, Whatman #1, Sigma-Aldrich, Sydney, NSW, Australia) fixed onto a copper wire inside the glass container (
Figure 5). The paper discs were heated at 120 °C for 2 h before used to remove any impregnated volatiles via volatilization. Prior to sampling, the glass container was placed in a water bath for pre-heating at 30 °C for 5 min to simulate the field environment. During the headspace sampling process, a ZIF-8 tube, and a Tenax
®-TA sorbent tube were connected in parallel for simultaneous sampling. As shown in
Figure 5, a SKC pump was connected to the absorbent tube for a 15 min extraction at 100 mL/min and a nitrogen gas was applied at 200 mL/min for pressure equivalent.
3.3.2. In-Field Volatile Extraction
In-field sampling was performed at a local vineyard in Tumbarumba, NSW, Australia. Volatile metabolites were extracted from 10
Chardonnay bunches and 10
Riesling bunches of different
B. cinerea infection severities. During in-field sampling, a ‘T’ shape stainless-steel tube was used to connect a ZIF-8 tube and a Tenax
®-TA sorbent tube, allowing the two tubes to be used simultaneously. The other side of the ‘T’ tube was placed just next to a grape bunch (
Figure 6). A low flow-rate air sampling pump (AirCheck XR5000 Pump, SKC Ltd., Blandford, UK) was connected to each tube to create an air flow of 100 mL/min for 15 min dynamic extraction. The whole grape bunches for this experiment were harvested and stored at -20 °C until further analysis.
3.4. VOCs Detection Using GC-MS
3.4.1. Thermal Desorption Procedure
Thermal desorption was processed with a Markes UNITY-xr single-tube thermal desorption unit (Kinesis Australia, Redland Bay, QLD, Australia) connected to a GC-MS system by a transfer line maintained at 150 °C with a constant nitrogen flow at 10 mL/min. The sampling tubes were pre-desorbed for 1 min with a trap flow of 50 mL/min and then thermally desorbed at 250 °C for 10 min with the same flow rate and then re- trapped at 30 °C for 1 min by a Markes focusing (cold) trap made of quartz and 60 mm graphitised carbon (General Purpose, Kinesis Australia, Redland Bay, QLD, Australia). The direction of the gas flow was always reversed during the desorption process, enabling higher boiling compounds to backflash from the sampling end of the tube. After analyte concentration, the trap was flash heated to 300 °C for 3 min at with the split flow of 20 mL/min. A moisture control system was applied to remove water vapour before sample injection to the GC-MS system.
3.4.2. SPME Extraction Parameter
Individual grape berries were carefully removed from bunches after whole bunch volatile extraction and homogenised using a high-speed grinder (UltraTurrax T25 IKA Dispersers, Staufen, Germany). The grape homogenates were stored at -20°C until immediately prior to analysis. During SPME analysis, 2.5 mL of grape homogenate was mixed with 7.5 mL Milli-Q water, 3 g sodium chloride and 10 μL of the IS solution (Table S1) in a 20 mL headspace glass vial (Agilent Technologies Ltd., Mulgrave, VIC, Australia). All samples were analysed in randomised order and the vials were vortexed for 10 seconds before analysis.
During SPME extraction, a DVB/CAR/PDMS fibre (Stableflex 23 Ga fibre; Sigma-Aldrich, Sydney, NSW, Australia) was used with an MPS2XL Multipurpose Sampler (Gerstel Inc., Linthicum, MD, USA), a Peltier sample cooler tray, and a heated agitator stirrer. Sample vials were placed into the Peltier sample cooler (4 °C) before analysis. Vials were subsequently transferred into the heated (65 °C), where they were incubated for 2 min before insertion of the SPME fibre for 64 min under agitation at 250 rpm. Injection of the SPME fibre into an ultra-inert straight SPME liner (0.75 mm; Agilent Technologies Ltd., Mulgrave, VIC, Australia) was performed at an inlet temperature of 260 °C in split-less mode for 1 min. The fibre was then baked for 10 min at 270 °C in a secondary injector.
The calibration curves for SPME GC-MS were established using mixed standard solutions prepared in synthetic grape juice at six concentrations in triplicate. The calibration curves and specific GC parameters were previously reported [
5]. A mix of internal standards was added to each sample/standard vial to achieve final concentration of: hexanal-d12 (6.25 μg/L), 2-octanol (0.22 μg/L), naphehalene-d8 (0.10 μg/L), 2,4,6-trichloroanisole-d5 (0.15 μg/L) and phenol-d6 (3.00 μg/L).
3.4.3. GC-MS Parameters and Statistics Processing
An agilent 7890A gas chromatography coupled with an Agilent 5975C triple axis mass selective detector (Agilent Technologies Ltd., Mulgrave, VIC, Australia) was used for MS detection with a DB-Waxetr column (60 m × 250 μm inner diameter × 0.25 μm film thickness; Agilent Technologies Ltd., Mulgrave, VIC, Australia). The GC method was based on a published study for volatiles detection from fungal infected grapes [
9]. In brief, helium gas flow rate was held at 3°C/min, with an initial oven temperature of 40°C, held for 3 min before heating at 5°C/min until 115°C; increased at 2°C/min until 166°C; then increased at 8°C/min until 240°C was reached. The final temperature was held for 15 min, resulting in a total run time of 67.75 min. Source, quadrupole and transfer line temperatures were set to 230°C, 150°C and 280°C, respectively. After a solvent delay of 8 min, the mass detector recorded a range between mass ion ratios (
m/
z) 35-550 using selective ion monitoring/scan mode according to Table S1 at -70 eV. The mass spectral data were acquired using the Enhanced MSD Chemstation software (version E.02.00.493, Agilent Technologies Ltd., Mulgrave, VIC Australia) and assessed using NIST MS search (version 2.0).
Raw data MS data were analysed using Agilent MassHunter Quantitative Analysis (MS, version 8.0, Agilent Technologies Ltd., Mulgrave, VIC, Australia). The limit of detection (LOD) and limit of quantification (LOQ) were calculated with concentrations for signal to noise ratio of three and ten, respectively. Peak areas, relative responses, and calculated concentrations of each analytes detected from grape samples were imported into Matlab (version R2022a, The Mathworks Inc., MI USA).
3.5. Botrytis Cinerea Quantificaiton
B. cinerea infection severities were determined by
B. cinerea antigen measurement using a lateral flow device (LFD; Global Access Diagnostics (GADX), Thurleigh, Bedfordshire, UK) with the
B. cinerea monoclonal antibody, BC-12.CA4 as the target, as previously reported [
5]. In brief, 2 mL of grape homogenates were centrifuged at 3000
g for 10 minutes followed by 1/1000 dilution with a 0.1 % (v/v) Tween 20 solution in phosphate-buffered saline (PBS; Sigma-Aldrich, Sydney, NSW, Australia). After that, a 200 µL aliquot of the diluted homogenates were applied into a 2 mL Eppendorf tubes at room temperature. A
B. cinerea test strip (GADX, Thurleigh, Bedfordshire, UK) was placed into each tube and read using a cube reader (GADX, Thurleigh, Bedfordshire, UK) after 10 minutes. The signal intensities were recorded as LFD scores described in the manufacturer’s instructions.
4. Conclusions
In this experiment, volatile metabolites related to B. cinerea grape infection were readily detected using ZIF-8 as absorbent materials for thermal desorption GC-MS from in-field volatile sampling experiments. When used with stable environmental conditions incorporating an internal standard to improve sampling efficacy, ZIF8 absorbent resulted in excellent correlations between the target volatile compounds B. cinerea grape infection antigen LFD scores. The performance of key markers for fungal infection 3-octanone, 3-ocatnol, 1-octen-3-one and 1-octen-ol using ZIF-8 demonstrated improved sensitivity than a commercially available material, Tenax®-TA. Positive correlations between the detection of key B. cinerea grape infection markers, such as 1,5-dimethyltetralin and the LFD scores were observed, indicating that this sampling collection methods may be potentially applied for the detection and prediction of grape B. cinerea infection in field. This series of experiments has demonstrated a proof of concept for the use of tunable and selective absorbent solid phase material for in filed sampling of targeted compounds.
Author Contributions
Liang Jiang: Conceptualisation, Formal analysis, Investigation, Methodology, Visualisation, Writing—original draft, Writing—review & editing. Morphy C. Dumlao: Formal analysis, Investigation, Methodology, Conceptualisation, Supervision. William A Donald: Conceptualisation, Supervision, Writing—review & editing. Christopher C. Steel: Conceptualisation, Supervision, Resources, Writing—review & editing. Leigh M. Schmidtke: Conceptualisation, Visualisation, Funding acquisition, Project administration, Resources, Supervision, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was conducted by the Australian Research Council Training Centre for Innovative Wine Production (www.ARC- winecentre.org.au; project number IC170100008), funded by the Australian Government with additional support from Faculty of Science, Charles Sturt University, and industry partners.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
The raw data is available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to acknowledge the generous donation of grapes from commercial vineyards in the Riverina. We thank Global Access Diagnostics, UK for generously providing the LFD test kit and strips.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Steel, C.C.; Blackman, J.W.; Schmidtke, L.M. Grapevine Bunch Rots: Impacts on Wine Composition, Quality, and Potential Procedures for the Removal of Wine Faults. Journal of Agricultural and Food Chemistry 2013, 61, 5189-5206. [CrossRef]
- Armijo, G.; Schlechter, R.; Agurto, M.; Muñoz, D.; Nuñez, C.; Arce-Johnson, P. Grapevine Pathogenic Microorganisms: Understanding Infection Strategies and Host Response Scenarios. Frontiers in Plant Science 2016, 7. [CrossRef]
- Zhou, Y.J.; Zhang, J.; Wang, X.D.; Yang, L.; Jiang, D.H.; Li, G.Q.; Hsiang, T.; Zhuang, W.Y. Morphological and phylogenetic identification of Botrytis sinoviticola, a novel cryptic species causing gray mold disease of table grapes (Vitis vinifera) in China. Mycologia 2014, 106, 43-56. [CrossRef]
- Santos, H.; Augusto, C.; Reis, P.; Rego, C.; Figueiredo, A.C.; Fortes, A.M. Volatile Metabolism of Wine Grape Trincadeira: Impact of Infection with Botrytis cinerea. Plants 2022, 11, 141. [CrossRef]
- Jiang, L.; Qiu, Y.; Dumlao, M.C.; Donald, W.A.; Steel, C.C.; Schmidtke, L.M. Detection and prediction of Botrytis cinerea infection levels in wine grapes using volatile analysis. Food Chemistry 2023, 421, 136120. [CrossRef]
- Piechulla, B.; Lemfack, M.C.; Kai, M. Effects of discrete bioactive microbial volatiles on plants and fungi. Plant, Cell & Environment 2017, 40, 2042-2067. [CrossRef]
- Elhamouly, N.A.; Hewedy, O.A.; Zaitoon, A.; Miraples, A.; Elshorbagy, O.T.; Hussien, S.; El-Tahan, A.; Peng, D. The hidden power of secondary metabolites in plant-fungi interactions and sustainable phytoremediation. Front Plant Sci 2022, 13, 1044896. [CrossRef]
- Ky, I.; Lorrain, B.; Jourdes, M.; Pasquier, G.; Fermaud, M.; Geny, L.; Rey, P.; Doneche, B.; Teissedre, P.-L. Assessment of grey mould (Botrytis cinerea) impact on phenolic and sensory quality of Bordeaux grapes, musts and wines for two consecutive vintages. Australian Journal of Grape and Wine Research 2012, 18, 215-226. [CrossRef]
- Sadoughi, N.; Schmidtke, L.M.; Antalick, G.; Blackman, J.W.; Steel, C.C. Gas Chromatography–Mass Spectrometry Method Optimized Using Response Surface Modeling for the Quantitation of Fungal Off-Flavors in Grapes and Wine. Journal of Agricultural and Food Chemistry 2015, 63, 2877-2885. [CrossRef]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite Profiling of Grape: Flavonols and Anthocyanins. Journal of Agricultural and Food Chemistry 2006, 54, 7692-7702. [CrossRef]
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Applications of solid-phase microextraction in food analysis. Journal of Chromatography A 2000, 880, 35-62. [CrossRef]
- Boschfuste, J.; Riuaumatell, M.; Guadayol, J.; Caixach, J.; Lopeztamames, E.; Buxaderas, S. Volatile profiles of sparkling wines obtained by three extraction methods and gas chromatography–mass spectrometry (GC–MS) analysis. Food Chemistry 2007, 105, 428-435. [CrossRef]
- Vas, G.; Vékey, K. Solid-phase microextraction: a powerful sample preparation tool prior to mass spectrometric analysis. Journal of Mass Spectrometry 2004, 39, 233-254. [CrossRef]
- De Fátima Alpendurada, M. Solid-phase microextraction: a promising technique for sample preparation in environmental analysis. Journal of Chromatography A 2000, 889, 3-14. [CrossRef]
- Llop, A.; Borrull, F.; Pocurull, E. Fully automated determination of N -nitrosamines in environmental waters by headspace solid-phase microextraction followed by GC-MS-MS. Journal of Separation Science 2010, 33, 3692-3700. [CrossRef]
- Siebert, T.E.; Smyth, H.E.; Capone, D.L.; Neuw Hner, C.; Pardon, K.H.; Skouroumounis, G.K.; Herderich, M.J.; Sefton, M.A.; Pollnitz, A.P. Stable isotope dilution analysis of wine fermentation products by HS-SPME-GC-MS. Analytical and Bioanalytical Chemistry 2005, 381, 937-947. [CrossRef]
- Ziółkowska, A.; Wąsowicz, E.; Jeleń, H.H. Differentiation of wines according to grape variety and geographical origin based on volatiles profiling using SPME-MS and SPME-GC/MS methods. Food Chemistry 2016, 213, 714-720. [CrossRef]
- Brown, S.D.; Rhodes, D.J.; Pritchard, B.J. A validated SPME-GC–MS method for simultaneous quantification of club drugs in human urine. Forensic Science International 2007, 171, 142-150. [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. Journal of Hazardous Materials 2013, 244-245, 444-456. [CrossRef]
- Padial, N.M.; Quartapelle Procopio, E.; Montoro, C.; López, E.; Oltra, J.E.; Colombo, V.; Maspero, A.; Masciocchi, N.; Galli, S.; Senkovska, I.; et al. Highly Hydrophobic Isoreticular Porous Metal-Organic Frameworks for the Capture of Harmful Volatile Organic Compounds. Angewandte Chemie International Edition 2013, 52, 8290-8294. [CrossRef]
- Yang, K.; Sun, Q.; Xue, F.; Lin, D. Adsorption of volatile organic compounds by metal–organic frameworks MIL-101: Influence of molecular size and shape. Journal of Hazardous Materials 2011, 195, 124-131. [CrossRef]
- Suwannakot, P.; Lisi, F.; Ahmed, E.; Liang, K.; Babarao, R.; Gooding, J.J.; Donald, W.A. Metal–Organic Framework-Enhanced Solid-Phase Microextraction Mass Spectrometry for the Direct and Rapid Detection of Perfluorooctanoic Acid in Environmental Water Samples. Analytical Chemistry 2020, 92, 6900-6908. [CrossRef]
- Gu, Z.-Y.; Yang, C.-X.; Chang, N.; Yan, X.-P. Metal–Organic Frameworks for Analytical Chemistry: From Sample Collection to Chromatographic Separation. Accounts of Chemical Research 2012, 45, 734-745. [CrossRef]
- Kim, Y.-H.; Kumar, P.; Kwon, E.E.; Kim, K.-H. Metal-organic frameworks as superior media for thermal desorption-gas chromatography application: A critical assessment of MOF-5 for the quantitation of airborne formaldehyde. Microchemical Journal 2017, 132, 219-226. [CrossRef]
- Gu, Z.-Y.; Wang, G.; Yan, X.-P. MOF-5 Metal−Organic Framework as Sorbent for In-Field Sampling and Preconcentration in Combination with Thermal Desorption GC/MS for Determination of Atmospheric Formaldehyde. Analytical Chemistry 2010, 82, 1365-1370. [CrossRef]
- Rice, S.; Maurer, D.; Fennell, A.; Dharmadhikari, M.; Koziel, J. Evaluation of Volatile Metabolites Emitted In-Vivo from Cold-Hardy Grapes during Ripening Using SPME and GC-MS: A Proof-of-Concept. Molecules 2019, 24, 536. [CrossRef]
- Fung, A.G.; Yamaguchi, M.S.; McCartney, M.M.; Aksenov, A.A.; Pasamontes, A.; Davis, C.E. SPME-based mobile field device for active sampling of volatiles. Microchemical Journal 2019, 146, 407-413. [CrossRef]
- Stierlin, É.; Michel, T.; Fernandez, X. Field analyses of lavender volatile organic compounds: performance evaluation of a portable gas chromatography–mass spectrometry device. Phytochemical Analysis 2020, 31, 778-785. [CrossRef]
- Schueuermann, C.; Steel, C.C.; Blackman, J.W.; Clark, A.C.; Schwarz, L.J.; Moraga, J.; Collado, I.G.; Schmidtke, L.M. A GC–MS untargeted metabolomics approach for the classification of chemical differences in grape juices based on fungal pathogen. Food Chemistry 2019, 270, 375-384. [CrossRef]
- Dankó, T.; Szelényi, M.; Janda, T.; Molnár, B.P.; Pogány, M. Distinct volatile signatures of bunch rot and noble rot. Physiological and Molecular Plant Pathology 2021, 114, 101626. [CrossRef]
- Callejón, R.M.; Ubeda, C.; Ríos-Reina, R.; Morales, M.L.; Troncoso, A.M. Recent developments in the analysis of musty odour compounds in water and wine: A review. Journal of Chromatography A 2016, 1428, 72-85. [CrossRef]
- Darriet, P.; Pons, M.; Henry, R.; Dumont, O.; Findeling, V.; Cartolaro, P.; Calonnec, A.; Dubourdieu, D. Impact Odorants Contributing to the Fungus Type Aroma from Grape Berries Contaminated by Powdery Mildew (Uncinula necator); Incidence of Enzymatic Activities of the Yeast Saccharomyces cerevisiae. Journal of Agricultural and Food Chemistry 2002, 50, 3277-3282. [CrossRef]
- Hung, R.; Lee, S.; Rodriguez-Saona, C.; Bennett, J.W. Common gas phase molecules from fungi affect seed germination and plant health in Arabidopsis thaliana. AMB Express 2014, 4, 53. [CrossRef]
- Archbold, D.D.; Hamilton-Kemp, T.R.; Barth, M.M.; Langlois, B.E. Identifying Natural Volatile Compounds That Control Gray Mold (Botrytis cinerea) during Postharvest Storage of Strawberry, Blackberry, and Grape. Journal of Agricultural and Food Chemistry 1997, 45, 4032-4037. [CrossRef]
- Lan, Y.-B.; Xiang, X.-F.; Yang, W.-X.; Zhu, B.-Q.; Pu, H.-T.; Duan, C.-Q. Characterization of free and glycosidically bound volatile compounds, fatty acids, and amino acids in Vitis davidii Foex grape species native to China. Food Science and Biotechnology 2020, 29, 1641-1653. [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proceedings of the National Academy of Sciences 2006, 103, 10186-10191. [CrossRef]
- Liu, X.-L.; Li, Y.-S.; Zhu, G.-Q.; Ban, Y.-J.; Xu, L.-Y.; Yang, W.-S. An Organophilic Pervaporation Membrane Derived from Metal-Organic Framework Nanoparticles for Efficient Recovery of Bio-Alcohols. Angewandte Chemie International Edition 2011, 50, 10636-10639. [CrossRef]
- Yalage Don, S.M.; Schmidtke, L.M.; Gambetta, J.M.; Steel, C.C. Aureobasidium pullulans volatilome identified by a novel, quantitative approach employing SPME-GC-MS, suppressed Botrytis cinerea and Alternaria alternata in vitro. Scientific Reports 2020, 10. [CrossRef]
- Lee, Y.-R.; Jang, M.-S.; Cho, H.-Y.; Kwon, H.-J.; Kim, S.; Ahn, W.-S. ZIF-8: A comparison of synthesis methods. Chemical Engineering Journal 2015, 271, 276-280. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).