Submitted:
31 May 2023
Posted:
01 June 2023
You are already at the latest version
Abstract
Keywords:
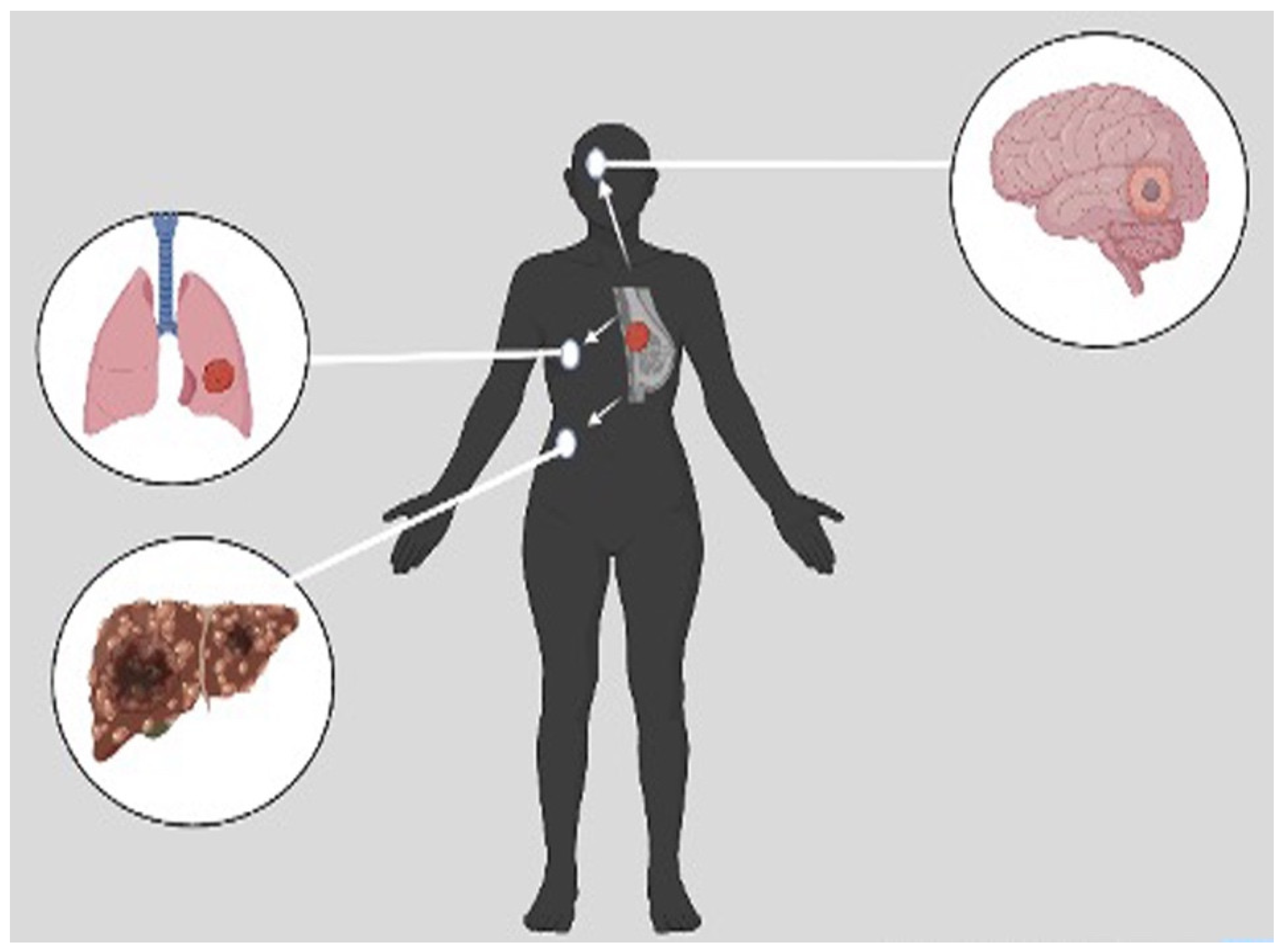
1. Introduction
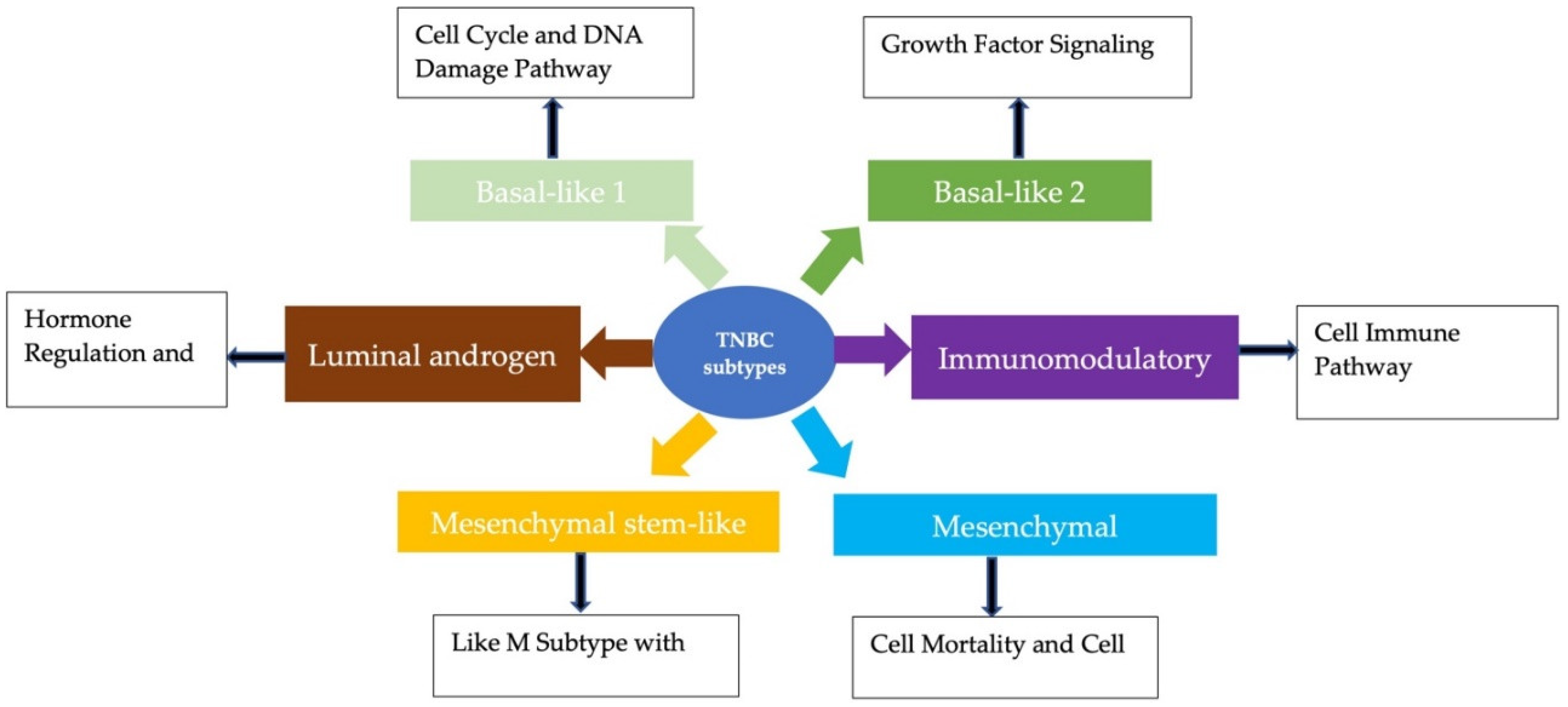
2. Subtypes of TNBC
2.1. Basal-like 1 and 2 Subtypes
2.2. Luminal Androgen Receptor Subtype
2.3. Mesenchymal and Mesenchymal Stem-Like Subtypes
2.4. Immunomodulatory Subtype
3. Prognostic Biomarkers
| No. | Prognostic Biomarkers | Ref. |
|---|---|---|
| 1 | Estrogen receptor (ER) and progesterone receptor (PR) status | [47] |
| 2 | Cathepsin D | [8] |
| 3 | Mutations of p53 | [45] |
| 4 | Epidermal growth factor receptor (EGFR) | [14] |
| 5 | BRCA 1/2 germline mutation | [39] |
| 6 | Vascular endothelial growth factor (VEGF) | [48] |
| 7 | Notch signaling pathway | [49] |
| 8 | Tumor-infiltrating lymphocytes (TILs) | [28] |
| 9 | Androgen receptor | [50] |
| 10 | Ki67 index | [51] |
4. Treatment Strategies for TNBC
4.1. Surgery
4.2. Radiation
4.3. Chemotherapy
4.3.1. Anthracyclines
4.3.2. Taxanes
4.3.3. Platinum
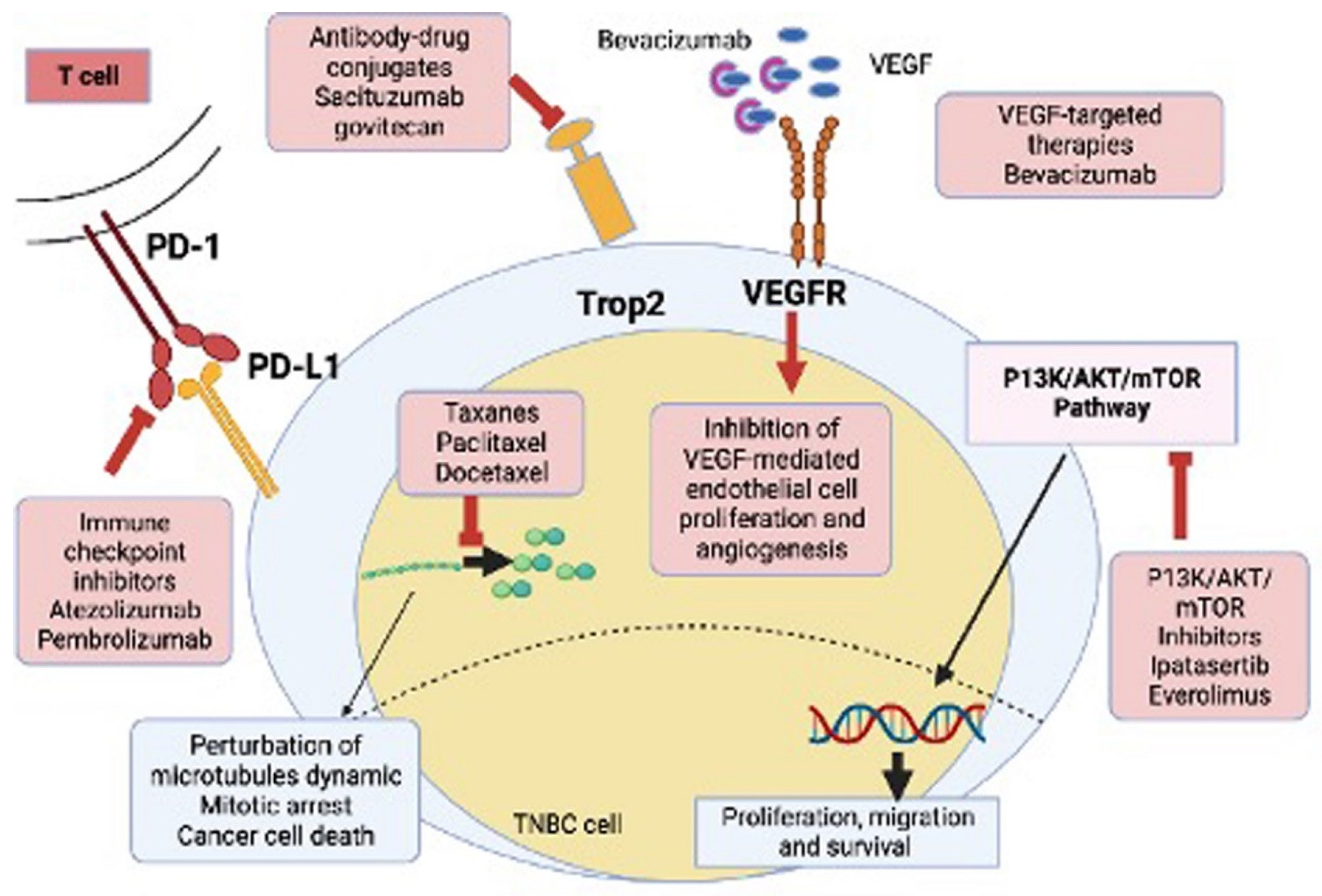
4.4. Targeted Therapy
4.4.1. PARP Inhibitors
4.4.2. Androgen Receptor Antagonists
4.4.3. EGFR Inhibitors
4.4.4. VEGF Inhibitors
4.5. Immunotherapy
4.5.1. Immune Check Point Inhibitors (ICIs)
4.5.2. Antibody-Drug Conjugates
4.5.3. P13/AKT/mTOR Inhibitors
4.6. Combination Therapies
5. Challenges in TNBC Treatment
6. Future Perspective
6.1. Drugs in Clinical Trial
6.2. Natural Agents
6.3. Nanotechnology-Based Outlook
6.3.1. Nanotechnology-Based Drug Targeting
Active Targeting
Passive Targeting
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody-Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. MCR 2020, 18, 3–19. https://doi.org/10.1158/1541-7786.MCR-19-0582. [CrossRef]
- Jusu, S.M.; Obayemi, J.D.; Salifu, A.A.; Nwazojie, C.C.; Uzonwanne, V.; Odusanya, O.S.; Soboyejo, W.O. Drug-Encapsulated Blend of PLGA-PEG Microspheres: In Vitro and in Vivo Study of the Effects of Localized/Targeted Drug Delivery on the Treatment of Triple-Negative Breast Cancer. Sci. Rep. 2020, 10. https://doi.org/10.1038/s41598-020-71129-0. [CrossRef]
- Polley, M.-Y.C.; Leon-Ferre, R.A.; Leung, S.; Cheng, A.; Gao, D.; Sinnwell, J.; Liu, H.; Hillman, D.W.; Eyman-Casey, A.; Gilbert, J.A.; et al. A Clinical Calculator to Predict Disease Outcomes in Women with Triple-Negative Breast Cancer. Breast Cancer Res. Treat. 2021, 185, 557–566. https://doi.org/10.1007/s10549-020-06030-5. [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. https://doi.org/10.3322/caac.21660. [CrossRef]
- Schick, J.; Ritchie, R.P.; Restini, C. Breast Cancer Therapeutics and Biomarkers: Past, Present, and Future Approaches. Breast Cancer Basic Clin. Res. 2021, 15, 1178223421995854. https://doi.org/10.1177/1178223421995854. [CrossRef]
- Palomeras, S.; Ruiz-Martínez, S.; Puig, T. Targeting Breast Cancer Stem Cells to Overcome Treatment Resistance. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 2193. https://doi.org/10.3390/molecules23092193. [CrossRef]
- Zhang, L.; Mu, C.; Zhang, T.; Yang, D.; Wang, C.; Chen, Q.; Tang, L.; Fan, L.; Liu, C.; Shen, J.; et al. Development of Targeted Therapy Therapeutics to Sensitize Triple-Negative Breast Cancer Chemosensitivity Utilizing Bacteriophage Phi29 Derived Packaging RNA. J. Nanobiotechnology 2021, 19. https://doi.org/10.1186/s12951-020-00758-4. [CrossRef]
- Al-Mahmood, S.; Sapiezynski, J.; Garbuzenko, O.B.; Minko, T. Metastatic and Triple-Negative Breast Cancer: Challenges and Treatment Options. Drug Deliv. Transl. Res. 2018, 8, 1483–1507. https://doi.org/10.1007/s13346-018-0551-3. [CrossRef]
- Kwapisz, D. Pembrolizumab and Atezolizumab in Triple-Negative Breast Cancer. Cancer Immunol. Immunother. CII 2021, 70, 607–617. https://doi.org/10.1007/s00262-020-02736-z. [CrossRef]
- Jing, X.; Shao, S.; Zhang, Y.; Luo, A.; Zhao, L.; Zhang, L.; Gu, S.; Zhao, X. BRD4 Inhibition Suppresses PD-L1 Expression in Triple-Negative Breast Cancer. Exp. Cell Res. 2020, 392, 112034. https://doi.org/10.1016/j.yexcr.2020.112034. [CrossRef]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S. Triple-Negative Breast Cancer Molecular Subtyping and Treatment Progress. Breast Cancer Res. BCR 2020, 22, 61. https://doi.org/10.1186/s13058-020-01296-5. [CrossRef]
- Vagia, E.; Mahalingam, D.; Cristofanilli, M. The Landscape of Targeted Therapies in TNBC. Cancers 2020, 12, E916. https://doi.org/10.3390/cancers12040916. [CrossRef]
- Lv, Y.; Ma, X.; Du, Y.; Feng, J. Understanding Patterns of Brain Metastasis in Triple-Negative Breast Cancer and Exploring Potential Therapeutic Targets. OncoTargets Ther. 2021, 14, 589–607. https://doi.org/10.2147/OTT.S293685. [CrossRef]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-C. Triple-Negative Breast Cancer Molecular Subtyping and Treatment Progress. Breast Cancer Res. BCR 2020, 22, 61. https://doi.org/10.1186/s13058-020-01296-5. [CrossRef]
- Miller-Kleinhenz, J.M.; Bozeman, E.N.; Yang, L. Targeted Nanoparticles for Image-Guided Treatment of Triple Negative Breast Cancer: Clinical Significance and Technological Advances. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 797–816. https://doi.org/10.1002/wnan.1343. [CrossRef]
- Mehanna, J.; Haddad, F.G.; Eid, R.; Lambertini, M.; Kourie, H.R. <p>Triple-Negative Breast Cancer: Current Perspective on the Evolving Therapeutic Landscape</P>. Int. J. Womens Health 2019, Volume 11, 431–437. https://doi.org/10.2147/ijwh.s178349. [CrossRef]
- Dass, S.A.; Tan, K.L.; Selva Rajan, R.; Mokhtar, N.F.; Mohd Adzmi, E.R.; Wan Abdul Rahman, W.F.; Tengku Din, T.A.D.A.-A.; Balakrishnan, V. Triple Negative Breast Cancer: A Review of Present and Future Diagnostic Modalities. Medicina (Mex.) 2021, 57, 62. https://doi.org/10.3390/medicina57010062. [CrossRef]
- Heimes, A.-S.; Schmidt, M. Atezolizumab for the Treatment of Triple-Negative Breast Cancer. Expert Opin. Investig. Drugs 2019, 28, 1–5. https://doi.org/10.1080/13543784.2019.1552255. [CrossRef]
- Rigiracciolo, D.C.; Nohata, N.; Lappano, R.; Cirillo, F.; Talia, M.; Scordamaglia, D.; Gutkind, J.S.; Maggiolini, M. IGF-1/IGF-1R/FAK/YAP Transduction Signaling Prompts Growth Effects in Triple-Negative Breast Cancer (TNBC) Cells. Cells 2020, 9, E1010. https://doi.org/10.3390/cells9041010. [CrossRef]
- Das, D.; Koirala, N.; Li, X.; Khan, N.; Dong, F.; Zhang, W.; Mulay, P.; Shrikhande, G.; Puskas, J.; Drazba, J.; et al. Screening of Polymer-Based Drug Delivery Vehicles Targeting Folate Receptors in Triple-Negative Breast Cancer. J. Vasc. Interv. Radiol. 2020, 31, 1866-1873.e2. https://doi.org/10.1016/j.jvir.2020.05.010. [CrossRef]
- Cheng, Y.; Lin, L.; Li, X.; Lu, A.; Hou, C.; Wu, Q.; Hu, X.; Zhou, Z.; Chen, Z.; Tang, F. ADAM10 Is Involved in the Oncogenic Process and Chemo-Resistance of Triple-Negative Breast Cancer via Regulating Notch1 Signaling Pathway, CD44 and PrPc. Cancer Cell Int. 2021, 21, 32. https://doi.org/10.1186/s12935-020-01727-5. [CrossRef]
- Kutty, R.V.; Feng, S.-S. Cetuximab Conjugated Vitamin E TPGS Micelles for Targeted Delivery of Docetaxel for Treatment of Triple Negative Breast Cancers. Biomaterials 2013, 34, 10160–10171. https://doi.org/10.1016/j.biomaterials.2013.09.043. [CrossRef]
- Haffty, B.G.; Yang, Q.; Reiss, M.; Kearney, T.; Higgins, S.A.; Weidhaas, J.; Harris, L.; Hait, W.; Toppmeyer, D. Locoregional Relapse and Distant Metastasis in Conservatively Managed Triple Negative Early-Stage Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 5652–5657. https://doi.org/10.1200/JCO.2006.06.5664. [CrossRef]
- Bauer, K.R.; Brown, M.; Cress, R.D.; Parise, C.A.; Caggiano, V. Descriptive Analysis of Estrogen Receptor (ER)-Negative, Progesterone Receptor (PR)-Negative, and HER2-Negative Invasive Breast Cancer, the so-Called Triple-Negative Phenotype: A Population-Based Study from the California Cancer Registry. Cancer 2007, 109, 1721–1728. https://doi.org/10.1002/cncr.22618. [CrossRef]
- Plasilova, M.L.; Hayse, B.; Killelea, B.K.; Horowitz, N.R.; Chagpar, A.B.; Lannin, D.R. Features of Triple-Negative Breast Cancer: Analysis of 38,813 Cases from the National Cancer Database. Medicine (Baltimore) 2016, 95, e4614. https://doi.org/10.1097/MD.0000000000004614. [CrossRef]
- Prakash, O.; Hossain, F.; Danos, D.; Lassak, A.; Scribner, R.; Miele, L. Racial Disparities in Triple Negative Breast Cancer: A Review of the Role of Biologic and Non-Biologic Factors. Front. Public Health 2020, 8.
- Cho, B.; Han, Y.; Lian, M.; Colditz, G.A.; Weber, J.D.; Ma, C.; Liu, Y. Evaluation of Racial/Ethnic Differences in Treatment and Mortality Among Women With Triple-Negative Breast Cancer. JAMA Oncol. 2021, 7, 1016–1023. https://doi.org/10.1001/jamaoncol.2021.1254. [CrossRef]
- Maqbool, M.; Bekele, F.; Fekadu, G. Treatment Strategies Against Triple-Negative Breast Cancer: An Updated Review. Breast Cancer Targets Ther. 2022, Volume 14, 15–24. https://doi.org/10.2147/bctt.s348060. [CrossRef]
- Yu, S.; Bi, X.; Yang, L.; Wu, S.; Yu, Y.; Jiang, B.; Zhang, A.; Lan, K.; Duan, S. Co-Delivery of Paclitaxel and PLK1-Targeted SiRNA Using Aptamer-Functionalized Cationic Liposome for Synergistic Anti-Breast Cancer Effects In Vivo. J. Biomed. Nanotechnol. 2019, 15, 1135–1148. https://doi.org/10.1166/jbn.2019.2751. [CrossRef]
- Sun, Y.; Wang, Z.; Na, L.; Dong, D.; Wang, W.; Zhao, C. FZD5 Contributes to TNBC Proliferation, DNA Damage Repair and Stemness. Cell Death Dis. 2020, 11, 1060. https://doi.org/10.1038/s41419-020-03282-3. [CrossRef]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S. Triple-Negative Breast Cancer Molecular Subtyping and Treatment Progress. Breast Cancer Res. BCR 2020, 22, 61. https://doi.org/10.1186/s13058-020-01296-5. [CrossRef]
- LIU, J.; XIAO, Y.; WEI, W.; GUO, J.-X.; LIU, Y.-C.; HUANG, X.-H.; ZHANG, R.-X.; WU, Y.-J.; ZHOU, J. Clinical Efficacy of Administering Oxaliplatin Combined with S-1 in the Treatment of Advanced Triple-Negative Breast Cancer. Exp. Ther. Med. 2015, 10, 379–385. https://doi.org/10.3892/etm.2015.2489. [CrossRef]
- Small-Molecule Drug Discovery in Triple Negative Breast Cancer: Current Situation and Future Directions | Journal of Medicinal Chemistry Available online: https://pubs.acs.org/doi/10.1021/acs.jmedchem.0c01180 (accessed on 9 May 2023).
- Nielsen, T.O.; Hsu, F.D.; Jensen, K.; Cheang, M.; Karaca, G.; Hu, Z.; Hernandez-Boussard, T.; Livasy, C.; Cowan, D.; Dressler, L.; et al. Immunohistochemical and Clinical Characterization of the Basal-like Subtype of Invasive Breast Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 5367–5374. https://doi.org/10.1158/1078-0432.CCR-04-0220. [CrossRef]
- Liao, M.; Zhang, J.; Wang, G.; Wang, L.; Liu, J.; Ouyang, L.; Liu, B. Small-Molecule Drug Discovery in Triple Negative Breast Cancer: Current Situation and Future Directions. J. Med. Chem. 2021, 64, 2382–2418. https://doi.org/10.1021/acs.jmedchem.0c01180. [CrossRef]
- Anders, C.K.; Abramson, V.; Tan, T.; Dent, R. The Evolution of Triple-Negative Breast Cancer: From Biology to Novel Therapeutics. Am. Soc. Clin. Oncol. Educ. Book 2016, 34–42. https://doi.org/10.1200/EDBK_159135. [CrossRef]
- Yadav, B.S.; Sharma, S.C.; Chanana, P.; Jhamb, S. Systemic Treatment Strategies for Triple-Negative Breast Cancer. World J. Clin. Oncol. 2014, 5, 125–133. https://doi.org/10.5306/wjco.v5.i2.125. [CrossRef]
- Lynce, F.; Nunes, R. Role of Platinums in Triple-Negative Breast Cancer. Curr. Oncol. Rep. 2021, 23, 50. https://doi.org/10.1007/s11912-021-01041-x. [CrossRef]
- Geenen, J.J.J.; Linn, S.C.; Beijnen, J.H.; Schellens, J.H.M. PARP Inhibitors in the Treatment of Triple-Negative Breast Cancer. Clin. Pharmacokinet. 2018, 57, 427–437. https://doi.org/10.1007/s40262-017-0587-4. [CrossRef]
- Coussy, F.; Lavigne, M.; de Koning, L.; Botty, R.E.; Nemati, F.; Naguez, A.; Bataillon, G.; Ouine, B.; Dahmani, A.; Montaudon, E.; et al. Response to MTOR and PI3K Inhibitors in Enzalutamide-Resistant Luminal Androgen Receptor Triple-Negative Breast Cancer Patient-Derived Xenografts. Theranostics 2020, 10, 1531–1543. https://doi.org/10.7150/thno.36182. [CrossRef]
- O’Reilly, D.; Sendi, M.A.; Kelly, C.M. Overview of Recent Advances in Metastatic Triple Negative Breast Cancer. World J. Clin. Oncol. 2021, 12, 164–182. https://doi.org/10.5306/wjco.v12.i3.164. [CrossRef]
- Ibrahim, E.M.; Al-Foheidi, M.E.; Al-Mansour, M.M.; Kazkaz, G.A. The Prognostic Value of Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: A Meta-Analysis. Breast Cancer Res. Treat. 2014, 148, 467–476. https://doi.org/10.1007/s10549-014-3185-2. [CrossRef]
- O’Donnell, J.S.; Hoefsmit, E.P.; Smyth, M.J.; Blank, C.U.; Teng, M.W.L. The Promise of Neoadjuvant Immunotherapy and Surgery for Cancer Treatment. Clin. Cancer Res. 2019, 25, 5743–5751. https://doi.org/10.1158/1078-0432.CCR-18-2641. [CrossRef]
- Furlanetto, J.; Loibl, S. Optimal Systemic Treatment for Early Triple-Negative Breast Cancer. Breast Care 2020, 15, 217–226. https://doi.org/10.1159/000508759. [CrossRef]
- da Silva, J.L.; Cardoso Nunes, N.C.; Izetti, P.; de Mesquita, G.G.; de Melo, A.C. Triple Negative Breast Cancer: A Thorough Review of Biomarkers. Crit. Rev. Oncol. Hematol. 2020, 145, 102855. https://doi.org/10.1016/j.critrevonc.2019.102855. [CrossRef]
- Alli, E.; Sharma, V.B.; Hartman, A.-R.; Lin, P.S.; McPherson, L.; Ford, J.M. Enhanced Sensitivity to Cisplatin and Gemcitabine in Brca1-Deficient Murine Mammary Epithelial Cells. BMC Pharmacol. 2011, 11, 7. https://doi.org/10.1186/1471-2210-11-7. [CrossRef]
- Wathoni, N.; Puluhulawa, L.E.; Joni, I.M.; Muchtaridi, M.; Mohammed, A.F.A.; Elamin, K.M.; Milanda, T.; Gozali, D. Monoclonal Antibody as a Targeting Mediator for Nanoparticle Targeted Delivery System for Lung Cancer. Drug Deliv. 2022, 29, 2959–2970. https://doi.org/10.1080/10717544.2022.2120566. [CrossRef]
- Sukumar, J.; Gast, K.; Quiroga, D.; Lustberg, M.; Williams, N. Triple-Negative Breast Cancer: Promising Prognostic Biomarkers Currently in Development. Expert Rev. Anticancer Ther. 2021, 21, 135–148. https://doi.org/10.1080/14737140.2021.1840984. [CrossRef]
- Jamdade, V.S.; Sethi, N.; Mundhe, N.A.; Kumar, P.; Lahkar, M.; Sinha, N. Therapeutic Targets of Triple-Negative Breast Cancer: A Review. Br. J. Pharmacol. 2015, 172, 4228–4237. https://doi.org/10.1111/bph.13211. [CrossRef]
- Sporikova, Z.; Koudelakova, V.; Trojanec, R.; Hajduch, M. Genetic Markers in Triple-Negative Breast Cancer. Clin. Breast Cancer 2018, 18, e841–e850. https://doi.org/10.1016/j.clbc.2018.07.023. [CrossRef]
- Schneider, B.P.; Winer, E.P.; Foulkes, W.D.; Garber, J.; Perou, C.M.; Richardson, A.; Sledge, G.W.; Carey, L.A. Triple-Negative Breast Cancer: Risk Factors to Potential Targets. Clin. Cancer Res. 2008, 14, 8010–8018. https://doi.org/10.1158/1078-0432.CCR-08-1208. [CrossRef]
- Keihan Shokooh, M.; Emami, F.; Jeong, J.-H.; Yook, S. Bio-Inspired and Smart Nanoparticles for Triple Negative Breast Cancer Microenvironment. Pharmaceutics 2021, 13, 287. https://doi.org/10.3390/pharmaceutics13020287. [CrossRef]
- Rouzier, R.; Perou, C.M.; Symmans, W.F.; Ibrahim, N.; Cristofanilli, M.; Anderson, K.; Hess, K.R.; Stec, J.; Ayers, M.; Wagner, P.; et al. Breast Cancer Molecular Subtypes Respond Differently to Preoperative Chemotherapy. Clin. Cancer Res. 2005, 11, 5678–5685. https://doi.org/10.1158/1078-0432.CCR-04-2421. [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Mariconda, A.; Rosano, C.; Scumaci, D.; Saturnino, C.; Longo, P.; Sinicropi, M.S. New Achievements for the Treatment of Triple-Negative Breast Cancer. Appl. Sci. 2022, 12, 5554. https://doi.org/10.3390/app12115554. [CrossRef]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorganic Chem. 2019, 88, 102925. https://doi.org/10.1016/j.bioorg.2019.102925. [CrossRef]
- Mallipeddi, H.; Thyagarajan, A.; Sahu, R.P. Implications of Withaferin-A for Triple-Negative Breast Cancer Chemoprevention. Biomed. Pharmacother. 2021, 134, 111124. https://doi.org/10.1016/j.biopha.2020.111124. [CrossRef]
- Gupta, G.K.; Collier, A.L.; Lee, D.; Hoefer, R.A.; Zheleva, V.; Siewertsz van Reesema, L.L.; Tang-Tan, A.M.; Guye, M.L.; Chang, D.Z.; Winston, J.S.; et al. Perspectives on Triple-Negative Breast Cancer: Current Treatment Strategies, Unmet Needs, and Potential Targets for Future Therapies. Cancers 2020, 12, 2392. https://doi.org/10.3390/cancers12092392. [CrossRef]
- Park, J.H.; Ahn, J.-H.; Kim, S.-B. How Shall We Treat Early Triple-Negative Breast Cancer (TNBC): From the Current Standard to Upcoming Immuno-Molecular Strategies. ESMO Open 2018, 3, e000357. https://doi.org/10.1136/esmoopen-2018-000357. [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-Infiltrating Lymphocytes and Prognosis in Different Subtypes of Breast Cancer: A Pooled Analysis of 3771 Patients Treated with Neoadjuvant Therapy. Lancet Oncol. 2018, 19, 40–50. https://doi.org/10.1016/S1470-2045(17)30904-X. [CrossRef]
- Oner, G.; Altintas, S.; Canturk, Z.; Tjalma, W.; Verhoeven, Y.; Van Berckelaer, C.; Berneman, Z.; Peeters, M.; Pauwels, P.; van Dam, P.A. Triple-Negative Breast Cancer-Role of Immunology: A Systemic Review. Breast J. 2020, 26, 995–999. https://doi.org/10.1111/tbj.13696. [CrossRef]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8, E957. https://doi.org/10.3390/cells8090957. [CrossRef]
- Shen, M.; Pan, H.; Chen, Y.; Xu, Y.H.; Yang, W.; Wu, Z. A Review of Current Progress in Triple-Negative Breast Cancer Therapy. Open Med. Wars. Pol. 2020, 15, 1143–1149. https://doi.org/10.1515/med-2020-0138. [CrossRef]
- Berko, Y.A.; Akala, E.O. Computer Optimization of Stealth Biodegradable Polymeric Dual-Loaded Nanoparticles for Cancer Therapy Using Central Composite Face-Centered Design. Pharm. Nanotechnol. 2020, 8, 108–132. https://doi.org/10.2174/2211738508666200224110410. [CrossRef]
- Li, S.; Goins, B.; Hrycushko, B.A.; Phillips, W.T.; Bao, A. Feasibility of Eradication of Breast Cancer Cells Remaining in Postlumpectomy Cavity and Draining Lymph Nodes Following Intracavitary Injection of Radioactive Immunoliposomes. Mol. Pharm. 2012, 9, 2513–2522. https://doi.org/10.1021/mp300132f. [CrossRef]
- Fancellu, A.; Houssami, N.; Sanna, V.; Porcu, A.; Ninniri, C.; Marinovich, M.L. Outcomes after Breast-Conserving Surgery or Mastectomy in Patients with Triple-Negative Breast Cancer: Meta-Analysis. Br. J. Surg. 2021, 108, 760–768. https://doi.org/10.1093/bjs/znab145. [CrossRef]
- Chen, Q.-X.; Wang, X.-X.; Lin, P.-Y.; Zhang, J.; Li, J.-J.; Song, C.-G.; Shao, Z.-M. The Different Outcomes between Breast-Conserving Surgery and Mastectomy in Triple-Negative Breast Cancer: A Population-Based Study from the SEER 18 Database. Oncotarget 2016, 8, 4773–4780. https://doi.org/10.18632/oncotarget.13976. [CrossRef]
- Yao, Y.; Chu, Y.; Xu, B.; Hu, Q.; Song, Q. Radiotherapy after Surgery Has Significant Survival Benefits for Patients with Triple-Negative Breast Cancer. Cancer Med. 2019, 8, 554–563. https://doi.org/10.1002/cam4.1954. [CrossRef]
- Azoury, F.; Misra, S.; Barry, A.; Helou, J. Role of Radiation Therapy in Triple Negative Breast Cancer: Current State and Future Directions—A Narrative Review. Precis. Cancer Med. 2022, 5. https://doi.org/10.21037/pcm-21-9. [CrossRef]
- Ellmark, P.; Mangsbo, S.M.; Furebring, C.; Norlén, P.; Tötterman, T.H. Tumor-Directed Immunotherapy Can Generate Tumor-Specific T Cell Responses through Localized Co-Stimulation. Cancer Immunol. Immunother. 2017, 66, 1–7. https://doi.org/10.1007/s00262-016-1909-3. [CrossRef]
- Connolly, E. Effects of MK-3475 (Pembrolizumab) on the Breast Tumor Microenvironment in Triple Negative Breast Cancer With and Without Intra-Operative RT: A Window of Opportunity Study; clinicaltrials.gov, 2022;
- Medhi, H.; Khumukcham, S.S.; Manavathi, B.; Paik, P. Effective in Vitro Delivery of Paclitaxel by Nanocargo of Mesoporous Polycaprolactone against Triple Negative Breast Cancer Cells by Minimalizing Drug Dose. RSC Adv. 2020, 10, 24095–24107. https://doi.org/10.1039/D0RA04505E. [CrossRef]
- Núñez Abad, M.; Calabuig-Fariñas, S.; Lobo de Mena, M.; José Godes Sanz de Bremond, M.; García González, C.; Torres Martínez, S.; García-García, J.Á.; Iranzo González-Cruz, V.; Camps Herrero, C. Update on Systemic Treatment in Early Triple Negative Breast Cancer. Ther. Adv. Med. Oncol. 2021, 13, 1758835920986749. https://doi.org/10.1177/1758835920986749. [CrossRef]
- Vaz-Luis, I.; Ottesen, R.A.; Hughes, M.E.; Mamet, R.; Burstein, H.J.; Edge, S.B.; Gonzalez-Angulo, A.M.; Moy, B.; Rugo, H.S.; Theriault, R.L.; et al. Outcomes by Tumor Subtype and Treatment Pattern in Women With Small, Node-Negative Breast Cancer: A Multi-Institutional Study. J. Clin. Oncol. 2014, 32, 2142–2150. https://doi.org/10.1200/JCO.2013.53.1608. [CrossRef]
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.-U.; Grischke, E.-M.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A Randomised Phase II Study Investigating Durvalumab in Addition to an Anthracycline Taxane-Based Neoadjuvant Therapy in Early Triple-Negative Breast Cancer: Clinical Results and Biomarker Analysis of GeparNuevo Study. Ann. Oncol. 2019, 30, 1279–1288. https://doi.org/10.1093/annonc/mdz158. [CrossRef]
- Katz, H.; Alsharedi, M. Immunotherapy in Triple-Negative Breast Cancer. Med. Oncol. 2017, 35, 13. https://doi.org/10.1007/s12032-017-1071-6. [CrossRef]
- Chowdhury, P.; Ghosh, U.; Samanta, K.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Bioactive Nanotherapeutic Trends to Combat Triple Negative Breast Cancer. Bioact. Mater. 2021, 6, 3269–3287. https://doi.org/10.1016/j.bioactmat.2021.02.037. [CrossRef]
- Mosca, L.; Ilari, A.; Fazi, F.; Assaraf, Y.G.; Colotti, G. Taxanes in Cancer Treatment: Activity, Chemoresistance and Its Overcoming. Drug Resist. Updat. 2021, 54, 100742. https://doi.org/10.1016/j.drup.2020.100742. [CrossRef]
- Khan, M.; Alam, Z.; Siddiqui, S.A.; Akram, M.; Alam, M.S. Outcomes of Triple-Negative Breast Cancer Patients in Response to Taxane and Nontaxane-Based Neoadjuvant Chemotherapies. Asian J. Oncol. 2022. https://doi.org/10.1055/s-0042-1750086. [CrossRef]
- Blanchard, Z.; Paul, B.T.; Craft, B.; ElShamy, W.M. BRCA1-IRIS Inactivation Overcomes Paclitaxel Resistance in Triple Negative Breast Cancers. Breast Cancer Res. BCR 2015, 17, 5. https://doi.org/10.1186/s13058-014-0512-9. [CrossRef]
- Reddy, L.H.; Bazile, D. Drug Delivery Design for Intravenous Route with Integrated Physicochemistry, Pharmacokinetics and Pharmacodynamics: Illustration with the Case of Taxane Therapeutics. Adv. Drug Deliv. Rev. 2014, 71, 34–57. https://doi.org/10.1016/j.addr.2013.10.007. [CrossRef]
- Lee, J.; Cho, Y.J.; Lee, J.-W.; Ahn, H.J. KSP SiRNA/Paclitaxel-Loaded PEGylated Cationic Liposomes for Overcoming Resistance to KSP Inhibitors: Synergistic Antitumor Effects in Drug-Resistant Ovarian Cancer. J. Control. Release Off. J. Control. Release Soc. 2020, 321, 184–197. https://doi.org/10.1016/j.jconrel.2020.02.013. [CrossRef]
- Futamura, M.; Oba, M.; Masuda, N.; Bando, H.; Okada, M.; Yamamoto, Y.; Kin, T.; Saeki, T.; Nagashima, T.; Kuwayama, T.; et al. Meta-Analysis of Nanoparticle Albumin-Bound Paclitaxel Used as Neoadjuvant Chemotherapy for Operable Breast Cancer Based on Individual Patient Data (JBCRG-S01 Study). Breast Cancer Tokyo Jpn. 2021, 28, 1023–1037. https://doi.org/10.1007/s12282-021-01238-9. [CrossRef]
- Yardley, D.A.; Coleman, R.; Conte, P.; Cortes, J.; Brufsky, A.; Shtivelband, M.; Young, R.; Bengala, C.; Ali, H.; Eakel, J.; et al. Nab-Paclitaxel plus Carboplatin or Gemcitabine versus Gemcitabine plus Carboplatin as First-Line Treatment of Patients with Triple-Negative Metastatic Breast Cancer: Results from the TnAcity Trial. Ann. Oncol. 2018, 29, 1763–1770. https://doi.org/10.1093/annonc/mdy201. [CrossRef]
- Yu, K.-D.; Ye, F.-G.; He, M.; Fan, L.; Ma, D.; Mo, M.; Wu, J.; Liu, G.-Y.; Di, G.-H.; Zeng, X.-H.; et al. Effect of Adjuvant Paclitaxel and Carboplatin on Survival in Women With Triple-Negative Breast Cancer. JAMA Oncol. 2020, 6, 1–8. https://doi.org/10.1001/jamaoncol.2020.2965. [CrossRef]
- Brown, S.D.; Nativo, P.; Smith, J.-A.; Stirling, D.; Edwards, P.R.; Venugopal, B.; Flint, D.J.; Plumb, J.A.; Graham, D.; Wheate, N.J. Gold Nanoparticles for the Improved Anticancer Drug Delivery of the Active Component of Oxaliplatin. J. Am. Chem. Soc. 2010, 132, 4678–4684. https://doi.org/10.1021/ja908117a. [CrossRef]
- Tutt, A.; Tovey, H.; Cheang, M.C.U.; Kernaghan, S.; Kilburn, L.; Gazinska, P.; Owen, J.; Abraham, J.; Barrett, S.; Barrett-Lee, P.; et al. Carboplatin in BRCA1/2-Mutated and Triple-Negative Breast Cancer BRCAness Subgroups: The TNT Trial. Nat. Med. 2018, 24, 628–637. https://doi.org/10.1038/s41591-018-0009-7. [CrossRef]
- Leon-Ferre, R.A.; Hieken, T.J.; Boughey, J.C. The Landmark Series: Neoadjuvant Chemotherapy for Triple-Negative and HER2-Positive Breast Cancer. Ann. Surg. Oncol. 2021, 28, 2111–2119. https://doi.org/10.1245/s10434-020-09480-9. [CrossRef]
- Anders, C.K.; Zagar, T.M.; Carey, L.A. The Management of Early-Stage and Metastatic Triple-Negative Breast Cancer: A Review. Hematol. Oncol. Clin. North Am. 2013, 27, 737–749, viii. https://doi.org/10.1016/j.hoc.2013.05.003. [CrossRef]
- Cipriano, É.; Mesquita, A. Emerging Therapeutic Drugs in Metastatic Triple-Negative Breast Cancer. Breast Cancer Basic Clin. Res. 2021, 15, 11782234211002492. https://doi.org/10.1177/11782234211002491. [CrossRef]
- Platinum-Based Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer: A Systematic Review and Meta-Analysis - Annals of Oncology Available online: https://www.annalsofoncology.org/article/S0923-7534(19)32098-8/fulltext (accessed on 14 May 2023).
- Pandy, J.G.P.; Balolong-Garcia, J.C.; Cruz-Ordinario, M.V.B.; Que, F.V.F. Triple Negative Breast Cancer and Platinum-Based Systemic Treatment: A Meta-Analysis and Systematic Review. BMC Cancer 2019, 19, 1065. https://doi.org/10.1186/s12885-019-6253-5. [CrossRef]
- Addition of the PARP Inhibitor Veliparib plus Carboplatin or Carboplatin Alone to Standard Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer (BrighTNess): A Randomised, Phase 3 Trial - ClinicalKey Available online: https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S1470204518301116?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1470204518301116%3Fshowall%3Dtrue&referrer=https:%2F%2Fpubmed.ncbi.nlm.nih.gov%2F (accessed on 2 May 2023).
- Medina, M.A.; Oza, G.; Sharma, A.; Arriaga, L.G.; Hernández Hernández, J.M.; Rotello, V.M.; Ramirez, J.T. Triple-Negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies. Int. J. Environ. Res. Public. Health 2020, 17, 2078. https://doi.org/10.3390/ijerph17062078. [CrossRef]
- Stringer-Reasor, E.M.; May, J.E.; Olariu, E.; Caterinicchia, V.; Li, Y.; Chen, D.; Della Manna, D.L.; Rocque, G.B.; Vaklavas, C.; Falkson, C.I.; et al. An Open-Label, Pilot Study of Veliparib and Lapatinib in Patients with Metastatic, Triple-Negative Breast Cancer. Breast Cancer Res. BCR 2021, 23, 30. https://doi.org/10.1186/s13058-021-01408-9. [CrossRef]
- Taylor, A.M.; Chan, D.L.H.; Tio, M.; Patil, S.M.; Traina, T.A.; Robson, M.E.; Khasraw, M. PARP (Poly ADP-Ribose Polymerase) Inhibitors for Locally Advanced or Metastatic Breast Cancer. Cochrane Database Syst. Rev. 2021, 2021, CD011395. https://doi.org/10.1002/14651858.CD011395.pub2. [CrossRef]
- Lord, C.J.; Ashworth, A. BRCAness Revisited. Nat. Rev. Cancer 2016, 16, 110–120. https://doi.org/10.1038/nrc.2015.21. [CrossRef]
- Sharma, P.; López-Tarruella, S.; García-Saenz, J.A.; Khan, Q.J.; Gómez, H.L.; Prat, A.; Moreno, F.; Jerez-Gilarranz, Y.; Barnadas, A.; Picornell, A.C.; et al. Pathological Response and Survival in Triple-Negative Breast Cancer Following Neoadjuvant Carboplatin plus Docetaxel. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 5820–5829. https://doi.org/10.1158/1078-0432.CCR-18-0585. [CrossRef]
- Han, Y.; Yu, X.; Li, S.; Tian, Y.; Liu, C. New Perspectives for Resistance to PARP Inhibitors in Triple-Negative Breast Cancer. Front. Oncol. 2020, 10.
- Barchiesi, G.; Roberto, M.; Verrico, M.; Vici, P.; Tomao, S.; Tomao, F. Emerging Role of PARP Inhibitors in Metastatic Triple Negative Breast Cancer. Current Scenario and Future Perspectives. Front. Oncol. 2021, 11.
- Guney Eskiler, G.; Ozturk, M. Therapeutic Potential of the PI3K Inhibitor LY294002 and PARP Inhibitor Talazoparib Combination in BRCA-Deficient Triple Negative Breast Cancer Cells. Cell. Signal. 2022, 91, 110229. https://doi.org/10.1016/j.cellsig.2021.110229. [CrossRef]
- Misra, S.; Zhang, X.; Wani, N.A.; Sizemore, S.; Ray, A. Both BRCA1-Wild Type and -Mutant Triple-Negative Breast Cancers Show Sensitivity to the NAE Inhibitor MLN4924 Which Is Enhanced upon MLN4924 and Cisplatin Combination Treatment. Oncotarget 2020, 11, 784–800. https://doi.org/10.18632/oncotarget.27485. [CrossRef]
- Barton, V.N.; D’Amato, N.C.; Gordon, M.A.; Lind, H.T.; Spoelstra, N.S.; Babbs, B.L.; Heinz, R.E.; Elias, A.; Jedlicka, P.; Jacobsen, B.M.; et al. Multiple Molecular Subtypes of Triple-Negative Breast Cancer Critically Rely on Androgen Receptor and Respond to Enzalutamide In Vivo. Mol. Cancer Ther. 2015, 14, 769–778. https://doi.org/10.1158/1535-7163.MCT-14-0926. [CrossRef]
- Rampurwala, M.; Wisinski, K.B.; O’Regan, R. Role of the Androgen Receptor in Triple-Negative Breast Cancer. Clin. Adv. Hematol. Oncol. HO 2016, 14, 186–193.
- Traina, T.A.; Miller, K.; Yardley, D.A.; Eakle, J.; Schwartzberg, L.S.; O’Shaughnessy, J.; Gradishar, W.; Schmid, P.; Winer, E.; Kelly, C.; et al. Enzalutamide for the Treatment of Androgen Receptor–Expressing Triple-Negative Breast Cancer. J. Clin. Oncol. 2018, 36, 884–890. https://doi.org/10.1200/JCO.2016.71.3495. [CrossRef]
- Masuda, H.; Zhang, D.; Bartholomeusz, C.; Doihara, H.; Hortobagyi, G.N.; Ueno, N.T. Role of Epidermal Growth Factor Receptor in Breast Cancer. Breast Cancer Res. Treat. 2012, 136, 331–345. https://doi.org/10.1007/s10549-012-2289-9. [CrossRef]
- Song, X.; Liu, Z.; Yu, Z. EGFR Promotes the Development of Triple Negative Breast Cancer Through JAK/STAT3 Signaling. Cancer Manag. Res. 2020, 12, 703–717. https://doi.org/10.2147/CMAR.S225376. [CrossRef]
- Yang, M.; Li, J.; Gu, P.; Fan, X. The Application of Nanoparticles in Cancer Immunotherapy: Targeting Tumor Microenvironment. Bioact. Mater. 2020, 6, 1973–1987. https://doi.org/10.1016/j.bioactmat.2020.12.010. [CrossRef]
- Darvishi, B.; Farahmand, L.; Majidzadeh-A, K. Stimuli-Responsive Mesoporous Silica NPs as Non-Viral Dual SiRNA/Chemotherapy Carriers for Triple Negative Breast Cancer. Mol. Ther. Nucleic Acids 2017, 7, 164–180. https://doi.org/10.1016/j.omtn.2017.03.007. [CrossRef]
- Mamot, C.; Ritschard, R.; Wicki, A.; Küng, W.; Schuller, J.; Herrmann, R.; Rochlitz, C. Immunoliposomal Delivery of Doxorubicin Can Overcome Multidrug Resistance Mechanisms in EGFR-Overexpressing Tumor Cells. J. Drug Target. 2012, 20, 422–432. https://doi.org/10.3109/1061186X.2012.680960. [CrossRef]
- Corkery, B.; Crown, J.; Clynes, M.; O’Donovan, N. Epidermal Growth Factor Receptor as a Potential Therapeutic Target in Triple-Negative Breast Cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2009, 20, 862–867. https://doi.org/10.1093/annonc/mdn710. [CrossRef]
- Yang, R.; Li, Y.; Wang, H.; Qin, T.; Yin, X.; Ma, X. Therapeutic Progress and Challenges for Triple Negative Breast Cancer: Targeted Therapy and Immunotherapy. Mol. Biomed. 2022, 3, 8. https://doi.org/10.1186/s43556-022-00071-6. [CrossRef]
- Lembo, R.R.; Manna, L.; Froechlich, G.; Sasso, E.; Passariello, M.; De Lorenzo, C. New Insights on the Role of Anti-PD-L1 and Anti-CTLA-4 MAbs on Different Lymphocytes Subpopulations in TNBC. Cancers 2022, 14, 5289. https://doi.org/10.3390/cancers14215289. [CrossRef]
- Luo, C.; Wang, P.; He, S.; Zhu, J.; Shi, Y.; Wang, J. Progress and Prospect of Immunotherapy for Triple-Negative Breast Cancer. Front. Oncol. 2022, 12, 919072. https://doi.org/10.3389/fonc.2022.919072. [CrossRef]
- Liu, J.; Liu, Q.; Li, Y.; Li, Q.; Su, F.; Yao, H.; Su, S.; Wang, Q.; Jin, L.; Wang, Y.; et al. Efficacy and Safety of Camrelizumab Combined with Apatinib in Advanced Triple-Negative Breast Cancer: An Open-Label Phase II Trial. J. Immunother. Cancer 2020, 8, e000696. https://doi.org/10.1136/jitc-2020-000696. [CrossRef]
- Wu, S.-Y.; Xu, Y.; Chen, L.; Fan, L.; Ma, X.-Y.; Zhao, S.; Song, X.-Q.; Hu, X.; Yang, W.-T.; Chai, W.-J.; et al. Combined Angiogenesis and PD-1 Inhibition for Immunomodulatory TNBC: Concept Exploration and Biomarker Analysis in the FUTURE-C-Plus Trial. Mol. Cancer 2022, 21, 84. https://doi.org/10.1186/s12943-022-01536-6. [CrossRef]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab Monotherapy for Previously Treated Metastatic Triple-Negative Breast Cancer: Cohort A of the Phase II KEYNOTE-086 Study. Ann. Oncol. 2019, 30, 397–404. https://doi.org/10.1093/annonc/mdy517. [CrossRef]
- Schmid, P.; Salgado, R.; Park, Y.H.; Muñoz-Couselo, E.; Kim, S.B.; Sohn, J.; Im, S.-A.; Foukakis, T.; Kuemmel, S.; Dent, R.; et al. Pembrolizumab plus Chemotherapy as Neoadjuvant Treatment of High-Risk, Early-Stage Triple-Negative Breast Cancer: Results from the Phase 1b Open-Label, Multicohort KEYNOTE-173 Study. Ann. Oncol. 2020, 31, 569–581. https://doi.org/10.1016/j.annonc.2020.01.072. [CrossRef]
- Liu, J.; Pandya, P.; Afshar, S. Therapeutic Advances in Oncology. Int. J. Mol. Sci. 2021, 22, 2008. https://doi.org/10.3390/ijms22042008. [CrossRef]
- Fisusi, F.; Brandy, N.; Wu, J.; Akala, E.O. Studies on Polyethylene Glycol-Monoclonal Antibody Conjugates for Fabrication of Nanoparticles for Biomedical Applications. J. Nanosci. Nanomedicine 2020, 4, 1–9.
- Liu, T.; Song, P.; Märcher, A.; Kjems, J.; Yang, C.; Gothelf, K.V. Selective Delivery of Doxorubicin to EGFR+ Cancer Cells by Cetuximab-DNA Conjugates. Chembiochem Eur. J. Chem. Biol. 2019, 20, 1014–1018. https://doi.org/10.1002/cbic.201800685. [CrossRef]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecan-Hziy in Refractory Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2019, 380, 741–751. https://doi.org/10.1056/NEJMoa1814213. [CrossRef]
- Analysis of Patients without and with an Initial Triple-Negative Breast Cancer Diagnosis in the Phase 3 Randomized ASCENT Study of Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer - PMC Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9374646/ (accessed on 14 May 2023).
- Stemline Therapeutics, Inc. A Phase 1b/2, Open-Label Umbrella Study To Evaluate Safety And Efficacy Of Elacestrant In Various Combination In Patients With Metastatic Breast Cancer; clinicaltrials.gov, 2023;
- Alzahrani, A.S. PI3K/Akt/MTOR Inhibitors in Cancer: At the Bench and Bedside. Semin. Cancer Biol. 2019, 59, 125–132. https://doi.org/10.1016/j.semcancer.2019.07.009. [CrossRef]
- Chan, J.J.; Tan, T.J.Y.; Dent, R.A. Novel Therapeutic Avenues in Triple-Negative Breast Cancer: PI3K/AKT Inhibition, Androgen Receptor Blockade, and Beyond. Ther. Adv. Med. Oncol. 2019, 11, 175883591988042. https://doi.org/10.1177/1758835919880429. [CrossRef]
- Khan, M.A.; Jain, V.K.; Rizwanullah, Md.; Ahmad, J.; Jain, K. PI3K/AKT/MTOR Pathway Inhibitors in Triple-Negative Breast Cancer: A Review on Drug Discovery and Future Challenges. Drug Discov. Today 2019, 24, 2181–2191. https://doi.org/10.1016/j.drudis.2019.09.001. [CrossRef]
- Ghebeh, H.; Al-Sayed, A.; Eiada, R.; Cabangon, L.; Ajarim, D.; Suleman, K.; Tulbah, A.; Al-Tweigeri, T. Weekly Paclitaxel given Concurrently with Durvalumab Has a Favorable Safety Profile in Triple-Negative Metastatic Breast Cancer. Sci. Rep. 2021, 11, 19154. https://doi.org/10.1038/s41598-021-98113-6. [CrossRef]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2018, 61, 5108–5121. https://doi.org/10.1021/acs.jmedchem.7b01457. [CrossRef]
- Wan, X.; Beaudoin, J.J.; Vinod, N.; Min, Y.; Makita, N.; Bludau, H.; Jordan, R.; Wang, A.; Sokolsky, M.; Kabanov, A.V. Co-Delivery of Paclitaxel and Cisplatin in Poly(2-Oxazoline) Polymeric Micelles: Implications for Drug Loading, Release, Pharmacokinetics and Outcome of Ovarian and Breast Cancer Treatments. Biomaterials 2019, 192, 1–14. https://doi.org/10.1016/j.biomaterials.2018.10.032. [CrossRef]
- Berko, Y.A.; Funmilola, A.F.; Akala, E.O. Fabrication of Paclitaxel and 17AAG-Loaded Poly-ε-Caprolactone Nanoparticles for Breast Cancer Treatment. J. Pharm. Drug Deliv. Res. 2021, 10, 196.
- Ban, H.; Kim, K.-S.; Oh, I.-J.; Yoon, S.-H.; Lee, B.; Yu, J.; Kim, S.; Lee, H.-S.; Shin, H.-J.; Park, C.-K.; et al. Efficacy and Safety of Docetaxel plus Oxaliplatin as a First-Line Chemotherapy in Patients with Advanced or Metastatic Non-Small Cell Lung Cancer. Thorac. Cancer 2014, 5, 525–529. https://doi.org/10.1111/1759-7714.12123. [CrossRef]
- Andreopoulou, E.; Schweber, S.J.; Sparano, J.A.; McDaid, H.M. Therapies for Triple Negative Breast Cancer. Expert Opin. Pharmacother. 2015, 16, 983–998. https://doi.org/10.1517/14656566.2015.1032246. [CrossRef]
- O’Reilly, E.A.; Gubbins, L.; Sharma, S.; Tully, R.; Guang, M.H.Z.; Weiner-Gorzel, K.; McCaffrey, J.; Harrison, M.; Furlong, F.; Kell, M.; et al. The Fate of Chemoresistance in Triple Negative Breast Cancer (TNBC). BBA Clin. 2015, 3. https://doi.org/10.1016/j.bbacli.2015.03.003. [CrossRef]
- Chen, Z.; Wang, X.; Li, X.; Zhou, Y.; Chen, K. Deep Exploration of PARP Inhibitors in Breast Cancer: Monotherapy and Combination Therapy. J. Int. Med. Res. 2021, 49, 0300060521991019. https://doi.org/10.1177/0300060521991019. [CrossRef]
- Oualla, K.; El-Zawahry, H.M.; Arun, B.; Reuben, J.M.; Woodward, W.A.; Gamal El-Din, H.; Lim, B.; Mellas, N.; Ueno, N.T.; Fouad, T.M. Novel Therapeutic Strategies in the Treatment of Triple-Negative Breast Cancer. Ther. Adv. Med. Oncol. 2017, 9, 493–511. https://doi.org/10.1177/1758834017711380. [CrossRef]
- Yagata, H.; Kajiura, Y.; Yamauchi, H. Current Strategy for Triple-Negative Breast Cancer: Appropriate Combination of Surgery, Radiation, and Chemotherapy. Breast Cancer 2011, 18, 165–173. https://doi.org/10.1007/s12282-011-0254-9. [CrossRef]
- Divgi, C.; Carrasquillo, J.A.; Meredith, R.; Seo, Y.; Frey, E.C.; Bolch, W.E.; Zimmerman, B.E.; Akabani, G.; Jacobson, D.A.; Brown, B.; et al. Overcoming Barriers to Radiopharmaceutical Therapy (RPT): An Overview From the NRG-NCI Working Group on Dosimetry of Radiopharmaceutical Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 905–912. https://doi.org/10.1016/j.ijrobp.2020.12.002. [CrossRef]
- Shah, A.N.; Gradishar, W.J. Adjuvant Anthracyclines in Breast Cancer: What Is Their Role? The Oncologist 2018, 23, 1153–1161. https://doi.org/10.1634/theoncologist.2017-0672. [CrossRef]
- Yang, R.; Shi, Y.-Y.; Han, X.-H.; Liu, S. The Impact of Platinum-Containing Chemotherapies in Advanced Triple-Negative Breast Cancer: Meta-Analytical Approach to Evaluating Its Efficacy and Safety. Oncol. Res. Treat. 2021, 44, 333–343. https://doi.org/10.1159/000515353. [CrossRef]
- Mizrahi, D.; Park, S.B.; Li, T.; Timmins, H.C.; Trinh, T.; Au, K.; Battaglini, E.; Wyld, D.; Henderson, R.D.; Grimison, P.; et al. Hemoglobin, Body Mass Index, and Age as Risk Factors for Paclitaxel- and Oxaliplatin-Induced Peripheral Neuropathy. JAMA Netw. Open 2021, 4, e2036695. https://doi.org/10.1001/jamanetworkopen.2020.36695. [CrossRef]
- Eikesdal, H.P.; Yndestad, S.; Elzawahry, A.; Llop-Guevara, A.; Gilje, B.; Blix, E.S.; Espelid, H.; Lundgren, S.; Geisler, J.; Vagstad, G.; et al. Olaparib Monotherapy as Primary Treatment in Unselected Triple Negative Breast Cancer☆. Ann. Oncol. 2021, 32, 240–249. https://doi.org/10.1016/j.annonc.2020.11.009. [CrossRef]
- Wang, X.; Wang, S.-S.; Huang, H.; Cai, L.; Zhao, L.; Peng, R.-J.; Lin, Y.; Tang, J.; Zeng, J.; Zhang, L.-H.; et al. Effect of Capecitabine Maintenance Therapy Using Lower Dosage and Higher Frequency vs Observation on Disease-Free Survival Among Patients With Early-Stage Triple-Negative Breast Cancer Who Had Received Standard Treatment. JAMA 2021, 325, 1–9. https://doi.org/10.1001/jama.2020.23370. [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. https://doi.org/10.3390/molecules27238367. [CrossRef]
- Dessale, M.; Mengistu, G.; Mengist, H.M. Nanotechnology: A Promising Approach for Cancer Diagnosis, Therapeutics and Theragnosis. Int. J. Nanomedicine 2022, 17, 3735–3749. https://doi.org/10.2147/IJN.S378074. [CrossRef]
- Browning, R.J.; Reardon, P.J.T.; Parhizkar, M.; Pedley, R.B.; Edirisinghe, M.; Knowles, J.C.; Stride, E. Drug Delivery Strategies for Platinum-Based Chemotherapy. ACS Nano 2017, 11, 8560–8578. https://doi.org/10.1021/acsnano.7b04092. [CrossRef]
- Esim, O.; Bakirhan, N.K.; Yildirim, N.; Sarper, M.; Savaser, A.; Ozkan, S.A.; Ozkan, Y. Development, Optimization and in Vitro Evaluation of Oxaliplatin Loaded Nanoparticles in Non-Small Cell Lung Cancer. DARU J. Pharm. Sci. 2020, 28, 673–684. https://doi.org/10.1007/s40199-020-00374-5. [CrossRef]
- Setyawati, M.I.; Kutty, R.V.; Leong, D.T. DNA Nanostructures Carrying Stoichiometrically Definable Antibodies. Small Weinh. Bergstr. Ger. 2016, 12, 5601–5611. https://doi.org/10.1002/smll.201601669. [CrossRef]
- Narum, S.M.; Le, T.; Le, D.P.; Lee, J.C.; Donahue, N.D.; Yang, W.; Wilhelm, S. Chapter 4 - Passive Targeting in Nanomedicine: Fundamental Concepts, Body Interactions, and Clinical Potential. In Nanoparticles for Biomedical Applications; Chung, E.J., Leon, L., Rinaldi, C., Eds.; Micro and Nano Technologies; Elsevier, 2020; pp. 37–53 ISBN 978-0-12-816662-8.
- Yang, T.; Zhai, J.; Hu, D.; Yang, R.; Wang, G.; Li, Y.; Liang, G. “Targeting Design” of Nanoparticles in Tumor Therapy. Pharmaceutics 2022, 14, 1919. https://doi.org/10.3390/pharmaceutics14091919. [CrossRef]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors. Chem. Rev. 2021, 121, 1746–1803. https://doi.org/10.1021/acs.chemrev.0c00779. [CrossRef]
- Ikeda-Imafuku, M.; Wang, L.L.-W.; Rodrigues, D.; Shaha, S.; Zhao, Z.; Mitragotri, S. Strategies to Improve the EPR Effect: A Mechanistic Perspective and Clinical Translation. J. Controlled Release 2022, 345, 512–536. https://doi.org/10.1016/j.jconrel.2022.03.043. [CrossRef]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392.
- Patel, J.K.; Patel, A.P. Passive Targeting of Nanoparticles to Cancer. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Pathak, Y.V., Ed.; Springer International Publishing: Cham, 2019; pp. 125–143 ISBN 978-3-030-06115-9.
- Ejigah, V.; Owoseni, O.; Bataille-Backer, P.; Ogundipe, O.D.; Fisusi, F.A.; Adesina, S.K. Approaches to Improve Macromolecule and Nanoparticle Accumulation in the Tumor Microenvironment by the Enhanced Permeability and Retention Effect. Polymers 2022, 14, 2601. https://doi.org/10.3390/polym14132601. [CrossRef]
- Behera, A.; Padhi, S. Passive and Active Targeting Strategies for the Delivery of the Camptothecin Anticancer Drug: A Review. Environ. Chem. Lett. 2020, 18, 1557–1567. https://doi.org/10.1007/s10311-020-01022-9. [CrossRef]
- Bazak, R.; Houri, M.; El Achy, S.; Hussein, W.; Refaat, T. Passive Targeting of Nanoparticles to Cancer: A Comprehensive Review of the Literature. Mol. Clin. Oncol. 2014, 2, 904–908. https://doi.org/10.3892/mco.2014.356. [CrossRef]
| Class | Examples | Limitation/Toxicities | Ref. |
|---|---|---|---|
| Surgery | Lumpectomy Mastectomy |
Residual disease that recurs or metastasizes Limited effectiveness in advanced stagesSurgical complications/impact on cosmesis |
[136] |
| Radiation | External beam radiation therapy IORT |
Myelosuppression Skin reactions Radiation pneumonitis Lymphedema |
[137] |
| Anthracyclines | Doxorubicin Epirubicin |
Cardiotoxicity Bone marrow suppression Cumulative dose limitations Drug Resistance Limited efficacy in metastatic disease Risk of secondary malignancies |
[41,76,138] |
| Taxanes | Paclitaxel Docetaxel Cabazitaxel |
Drug resistance Bone marrow suppression Peripheral neuropathy Gastrointestinal issues Hypersensitivity reactions |
[54,77,81] |
| Platinum | Cisplatin Carboplatin Oxaliplatin |
Bone marrow suppression Kidney damage Peripheral neuropathy Allergic reactions Gastrointestinal issues |
[139,140] |
| PARP Inhibitors | Olaparib Talazoparib |
Immunosuppression-induced sepsis Myelodysplastic syndrome Acute myeloid leukemia Fatigue Anemia Drug resistance |
[98,141] |
|
P13/AKT /mTOR Inhibitors |
Ipatasertib Everolimus |
Pulmonary toxicity Elevated liver enzyme Mucositis Hyperglycemia |
[124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





