1. Introduction
Metastasis is a multi-step process by which a primary tumour cells migrate to a distant organ(s) in order to create secondary tumour site. It is a characteristic of cancer that leads to treatment failure and the death of many patients [
1]. As a result, the prognosis of the patient is closely related to metastasis. At diagnosis, 5–10% of breast cancer patients have metastases, and 30–40% of women with early breast cancer develop metastases during the disease [
2,
3]. Breast cancer is a clinically, pathologically, histologically, and prognostically complex disease and the classification that is important to determine treatment and the future outcome is based on analysis of the oestrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and proliferation marker (Ki67). On this basis, the most common types of breast cancer can be divided into four molecular subtypes: luminal A, luminal B, HER2-positive, and triple-negative breast cancer. These subtypes have a wide range of metastases, prognoses, and treatment options [
4]. Different gene and protein expression profiles are likely to explain the different patterns of metastasis and survival in different breast cancer subtypes [
5].
Adipose tissue is now considered to be another endocrine organ, which secretes dozens of adipokines, including leptin and adiponectin, resistin, interleukin-1 (IL-1), interleukin-6 (IL-6) and so on [
6]. By excessive release of free fatty acids, tumour necrosis factor a (TNF-α), IL-1, IL-6, and other mediators regulate endothelial function and induce endothelial damage. Thus, adipocytes are regarded as a significant source of chronic low-grade inflammation. Endothelial cells release excessive amounts of cell adhesion molecules (CAMs), such as vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and E-selectin in response to subclinical inflammation, resulting in leukocyte mobilisation and adherence to the endothelium. Leptin is a metabolic regulator with several functions, including food intake, energy expenditure, and neuroendocrine function. Leptin has been identified as an essential mediator of the pro-inflammatory state associated with obesity, which contributes to metabolic problems. As a result, the processes linked with adiponectin’s activity as an anti-inflammatory adipokine include its critical role in insulin sensitisation, and anti-inflammatory and anti-atherogenic actions. The leptin-to-adiponectin (LAR) ratio has recently gained attention as a unique predictor of cardiometabolic and other chronic disease outcomes [
7]. The LAR has been linked to insulin resistance, carotid intima-media thickness, the ‘at-risk phenotype’ in young severely obese patients, chronic kidney disease and can be used as a novel predictor of metabolic syndrome [
8,
9,
10]. To date, several studies have suggested that adiponectin, leptin, and the LAR are associated with obesity-associated malignancies (e.g., breast, colorectal and pancreatic cancer) [
7,
8,
11]. It is believed that the LAR may be considered as a predictor of the clinical response to the treatment administered [
12]. In addition, researchers have found an association between the LAR and P-selectin in obesity which showed an inverse correlation between the ratio of leptin to adiponectin and sP-selectin, suggesting that, indirectly, such a correlation may reduce platelet aggregation [
13].
CAMs are a type of protein that play an important role in the movement of primary tumour cells from the tumour mass and intravasation through the endothelium into blood vessels during the metastatic process of cancer [
14]. The interactions between cancer cells and endothelial cells play an important role in metastasis. CAMs are responsible for maintaining tissue continuity under physiological conditions by interacting with cells and the extracellular matrix. CAMs play a role in cell differentiation, proliferation, migration, and apoptosis [
15,
16]. Impaired adhesion molecule function at any stage can contribute to the loss of normal cell–cell interactions, allowing cancer cells to dedifferentiate and spread [
14]. The most recognised and studied CAMs include selectins. Leukocyte-selectin (L-selectin, CD62L), Endothelial-selectin (E-selectin, CD62E) and Platelet-selectin (P-selectin, CD62P) are the three selectin subfamily members [
17]. Their primary ligand (PSGL-1, SELPLG) on the surface of leukocytes interacts with P-, L-, and E-selectin, each with varying affinity, to mediate leukocyte adhesion and rolling along the endothelium, as well as cell–cell adhesion in pathological conditions, such as cancer metastasis [
18,
19].
The appearance of E-selectin ligands on cancer cells, for example, mucins, dead receptor-3 and a specific CD44 glycoform, is linked to increased adhesion to activated endothelial cells. The binding of E-selectin to death receptor 3 or other ligands on cancer cells has been shown to improve survival during metastasis [
20,
21]. Soluble platelet-selectin (sP-selectin) is a soluble form of P-selectin that is released by granules and is detectable in plasma [
22]. P-selectin, which allows tumour cell aggregates to adhere to the endothelium and then extravasate, is one of the proteins that mediates the close interaction between platelets and tumour cells [
23,
24]. P-selectin is expressed on stimulated endothelial cells and activated platelets and mediates leukocytes rolling on stimulated endothelial cells and heterotypic aggregation of activated platelets on leukocytes [
25]. Several studies have found that increased plasma vWF (von Willebrand factor) antigen levels in cancer cohorts are linked to disease progression and poor patient outcomes, highlighting the vWF antigen as an independent prognostic marker in specific cancer subtypes [
26].
The aim of the present study was to assess the effect of endothelial markers before and after treatment (i.e., sP-selectin, sE-selectin and vWF) on the LAR (also assessed before and after treatment) and perform an analysis of the predictive value of these parameters on the survival of patients with luminal A and B invasive breast cancer (IBrC). To determine the accuracy of our predictive model, we used Kaplan-Meier analysis, linear regression, and the receiver operating characteristic (ROC) curve in this study.
2. Materials and Methods
2.1. Patient Enrolment
The study included 70 previously untreated patients with clinically and histologically proven primary stage I–II IBrC. Between November 2015 and January 2018, the subjects were admitted to the Clinical Ward of Breast Cancer and Reconstructive Surgery, Oncology Centre, Prof F. Łukaszczyk Memorial Hospital in Bydgoszcz, Poland. All patients were of Polish descent. At admission, weight and height were taken, and the BMI was calculated and expressed as kg/m2. The patients’ median age was 54.5 years old (IQR = 49.0–59.0 years). Patients underwent a comprehensive clinicopathological and post-operative examination and were diagnosed correctly using invasive standards. The World Health Organization (WHO) classification was used to classify the histological type of breast cancer. According to the American Joint Committee on Cancer (AJCC; 7th edition), the tumour stage was determined.
Pathologically confirmed primary, invasive, unilateral, non-metastatic, early-stage (IA–IIB) breast cancer was the main inclusion criteria. The carcinoma in situ, a tumour larger than 5 cm, stage IIIA or higher, neoadjuvant treatment, locally advanced or metastatic cancer, triple-negative, non-luminal HER2 positive IBrC were the cancer-related exclusion criteria. Other exclusion criteria included morbid obesity (BMI greater than 40 kg/m
2) and type 2 diabetes. The flowchart of patients enrolled for the study is shown in
Figure 1.
2.2. Follow-Up
Patients were followed from the date of IBrC diagnosis until the date of breast cancer recurrence or death or until January 2022, whichever came first. The period from study inclusion to the date of recurrence is described as disease-free survival (DFS), and the time to the patient’s last visit or death is defined as overall survival (OS). The median follow-up was 68.5 months (IQR = 59–72 months). There were 11 events during the study, including one distant metastasis and ten deaths (recurrence rate: 15.71%).
2.3. Ethics Statement
The study was performed under the appropriate institutional ethics approvals (KB 547/2015) and in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtain from each participant.
2.4. Treatment Requirements
All individuals were treated in accordance with the National Comprehensive Cancer Network (NCCN) Guidelines for Practice. Fifty-six patients received breast-conserving surgery (BCS), seven had a conventional mastectomy, and seven underwent a modified radical mastectomy (MRM). All surgical operations were performed under normal procedures. Adjuvant therapy was given to 68 women. Surgery was the initial treatment for all research participants, followed by adjuvant therapy that included radiation, brachytherapy, hormone therapy, chemotherapy, and immunotherapy. Post-operative radiation was mostly given to patients who had undergone BCS. In the study group, post-operative radiation was delivered in 17–20 fractions over 4–6 weeks using X photons with energy of 6/15 MeV and a dosage of 42.5 gray (Gy). Moreover, brachytherapy at a dosage of 10 Gy was administered to the tumour bed in half of the women. Adjuvant chemotherapies included anthracycline-containing (n = 23) and non-anthracycline-containing (n = 4) medicines administered in three to six cycles. Menopausal status determined the type of endocrine treatment; 40 (57%) received tamoxifen (Egis Pharmaceuticals, Budapest, Hungary), 17 (24%) received aromatase inhibitors (AIs) (Arimidex [anastrozole], AstraZeneca, Cambridge, UK), and seven (10%) received a combination of tamoxifen and AIs. Adjuvant immunotherapy was necessary for four HER2-positive individuals (6%) (trastuzumab).
2.5. Sample Collection and Analysis
Venous blood samples were taken twice into 4.5 ml tubes (BD Vacutainer® Plus Plastic Serum Tubes; Franklin Lakes, NJ, USA) without anticoagulant to determine adiponectin, leptin, and soluble forms of E-selectin (sE-selectin) and P-selectin (sP-selectin) concentrations. In tubes (BDTM VacutainerTM Citrate Tube) containing an anticoagulant additive equivalent to 3.2% trisodium citrate for coagulation investigations, 4.5 ml for von Willebrand factor analysis were collected.
The first blood sample was taken 24 hours before the surgical procedure (I – pre-treatment values). To avoid the direct effects of chemotherapy or surgical wounds, the second blood specimen (II - post-treatment values) was collected a maximum of three months after the last cytotoxic infusion and generally nine months (IQR=6.0-10.0) after the tumour removal procedure.
Material was gathered under strict conditions, such as fasting (after a 12-hour fast) and resting for 30 minutes. After mixing the samples, they were centrifuged at 3000xg at +4 °C for 15 minutes, aliquoted, and stored at -80 °C (as recommended by the manufacturer) until assayed, but not for more than six months. Aliquots were limited to one freeze-thaw cycle during the bath analysis, and storage conditions were maintained.
2.5.1. Leptin-to-Adiponectin Ratio
The Human Leptin Enzyme-Linked Immunosorbent Assay (ELISA) Clinical Range (BioVendor Research and Diagnostic Products, Brno, Czech Republic; catalogue number: RD191001100) were used to measure baseline serum pre- and post-treatment leptin concentrations. The detection limit for leptin was 0.2 ng/mL. The intra-assay coefficient of variation (within run) was 5.9%, with a run-to-run coefficient of variation of 5.6%.
A human adiponectin ELISA high-sensitivity ELISA kit was used to measure pre- and post-treatment serum adiponectin levels (BioVendor Research and Diagnostic Products, Brno, Czech Republic; catalogue number: RD191023100). The detection limit for adiponectin was 0.47 ng/mL, with a 3.9% intra-assay coefficient of variation (within-run) and a 6.0% inter-assay coefficient of variation (run-to-run).
The leptin-to-adiponectin ratio was assessed using the following formula:
2.5.2. Serum sE-Selectin Measurements
Serum pre- and post-treatment sE-selectin level were determined by the Diaclone CD62E/ELAM-1 ELISA Set (catalogue number: 851.580). The detection limit was 0.5 ng/mL with an assay range of 1 ng/ml to 32 ng/ml.
2.5.3. Serum sP-Selectin Analysis
Serum pre- and post- treatment sP-selectin level were measured using a commercially available kit, Enzyme-linked Immunosorbent Assay (ELISA) Kit for sP-selectin (SELP) (Cloud-Clone Corp., CCC, USA, catalogue number: SEA569Hu). The sP-selectin detection limit was 27 pg/mL. The intra-assay coefficient of variation (within-run) was 10%, with an inter-assay coefficient of variation (run-to-run) of 12%.
2.5.4. von Willebrand Factor Antigen Measurements
Plasma pre- and post-treatment concentrations of von Willebrand factor were determined using the Imubind® vWF ELISA ref: 828 (BioMedica Diagnostics, Stamford, CT, USA) test. The vWF detection limit was 0.1 mU/mL, with an assay range of 0–10 mU/mL.
2.5.5. Immunohistochemistry (IHC) Analysis
The evaluation of ER and PR status, expression of HER2, and Ki67 was done using IHC. ER and PR status were evaluated using SP1 and 1E2 primary antibodies (Ventana Medical Systems, Tucson, Arizona, USA) in line with ASCO and CAP standards. Hormone receptor status was characterised as positive if there was at least 1% of tumour cells with nuclear staining and negative if there was no nuclear staining at all. The rabbit monoclonal primary antibody VENTANA anti-HER2/neu (4B5) was used with a VENTANA aperture to stain the IHC microscope slide (Benchmark Ultra, Roche-Ventana) for semi-quantitative identification of HER2. On a scale of 0, 1+, 2+, and 3+, HER2 scores were calculated using the usual ASCO/CAP guideline reporting method. Tumours with a score of 0 or 1+ were classified as HER2-negative, whereas those with a value of 3+ were labelled HER2-positive. Tumours with 2+ scores were deemed ambiguous and subjected to fluorescence in situ hybridization (FISH) with a dual HER2/Cep17 probe. Using a monoclonal mouse antibody (Auto-stainer Link 48, Agilent Technologies, USA), the Ki67 antigen was scored as a percentage of nuclei-stained cells in all cancer cells. We utilised a 20% threshold to designate high or low proliferative instances in the Ki67 proliferation index.
2.6. Statistical Analysis
Statistica version 13.1 (StatStoft
®, Cracow, Poland) was used for statistical analysis. The Shapiro-Wilk test was used to ensure that the data distribution was normal. The Student’s t-test (normal distribution) or the Mann-Whitney U test were used to compare two groups of continuous data (non-normal distribution). Univariate ANOVA analysis with normal distributions or the Kruskal-Wallis ANOVA analysis with non-normal distributions were used to compare more than two groups of continuous data. As appropriate, patient data are presented as mean and standard deviation or median and interquartile range (IQR). In brackets ‘()‘, we have given the standard deviation and the values separated by a slash ‘/’ are Q1 and Q3. Furthermore, for two dependent variables, the data were compared using a non-parametric Wilcoxon signed rank test. The ROC curves, AUC (area under a curve), and Youden’s index were also used in the analysis (see
Supplementary Materials). Cut-off values were determined based on the ROC and median. Survival times were expressed using Kaplan-Meier curves, and the log-rank test was utilised to compare survival times (statistically non-significant results have been moved to the
Supplementary Materials section). The term OS refers to the time between the start of randomisation or treatment and death. The time from randomisation or treatment initiation to the incidence of disease progression or death is referred to as DFS. Multiple linear regression models with adjustments for BMI, age, parity, menopausal status, smoking status, tumour stage, tumour diameter, histological type, intrinsic type, and nodal involvement included the LAR and endothelial markers. The Cox proportional hazards model was used for multivariate and univariate regression analysis. To assess the independent impact of selected factors at the time of diagnosis on breast cancer survival, a multivariate Cox regression model included all variables with a significant effect in the univariate analysis. All analyses performed were summarised and reported in tables and figures. The statistical significance cut-off value was set at a
p-value < 0.05.
3. Results
3.1. Baseline Characteristics
Seventy women who met the study’s inclusion and exclusion criteria were enrolled.
Table 1 shows the baseline characteristics of each study group. The median age was 54.5 years (IQR = 49.0–59.0 years), the median BMI was 25.06 kg/m
2 (IQR = 22.6–28.8 kg/m
2), and the median tumour size was 1.5 cm (IQR = 1.1–2.1 cm). There were 26 premenopausal women and 44 postmenopausal women among the 70 women. There were 48 T1 patients and 22 T2 patients in the TNM classification of breast cancer. When the histological type of breast cancer was examined, it was discovered that 61 patients had invasive ductal carcinoma and nine had invasive lobular carcinoma. Only 17 of the 70 patients had local lymph node metastases. In terms of molecular type, 50 patients had luminal A cancer, 16 had luminal B HER2 (negative) cancer, and four had luminal B HER (positive) cancer. PR and E-cadherin positivity was found in 65 patients. The Ki-67 proliferation index was lower than 20% in 50 patients. BCS was performed on 56 patients, and 14 had a mastectomy. Forty-three patients with breast cancer did not require chemotherapy, and 68 women received endocrine therapy.
3.2. LAR Levels Prior to and after Treatment in Relation to the Types of Therapy
Table 2 presents the LAR regarding the types of therapy. Regardless of the treatment pattern, the LAR increased after treatment.
3.3. Patients’ Treatment in Relation to Their Pre- and Post-Treatment sE-Selectin Concentrations
Table 3 presents the sE-selectin concentrations related to treatment strategy. Regardless of the treatment pattern, the sE-selectin concentration increased after treatment. The pre-treatment sE-selectin concentrations were higher in patients who received breast-conserving therapy with a trend towards statistical significance (
p = 0.0840). Considering the types of chemotherapy, pre-treatment sE-selectin concentrations were higher in patients treated with non-anthracycline chemotherapy (
p = 0.0125). Surprisingly, post-treatment sE-selectin concentrations were higher in patients who had not been treated with chemotherapy (
p = 0.0081).
3.4. Relationship between sP-Selectin Concentrations before and After Treatment
Table 4 presents the sP-selectin concentrations with regard to the types of therapy. Regardless of the treatment pattern, the sP-selectin concentration increased after treatment. Considering the types of endocrine therapy, post-treatment sP-selectin concentrations were higher in patients who had not been treated with endocrine therapy (
p = 0.0015), but this observation needs to be confirmed in a group with larger numbers.
3.5. vWF Concentrations Prior to and after Treatment in Relation to the Types of Therapy
Table 5 shows the vWF concentrations in relation to therapy type. The vWF concentration increased after treatment regardless of treatment pattern. Post-treatment vWF concentrations were higher in patients who received chemotherapy based on anthracycline (
p = 0.0486).
3.6. Association between Endothelial Markers and Pre-Treatment LAR Concentrations
The next step in the statistical analysis (
Table 6) was to test the pre-treatment and post-treatment concentrations of sE-selectin, sP-selectin and vWF against the pre-treatment concentration of the LAR. We divided the breast cancer patients into three subgroups – those with low (< 0.27), moderate (0.27–0.65) and high (> 0.65) pre-treatment LAR concentrations. No statistically significant data were observed in this analysis.
3.7. Association between Endothelial Markers and Post-Treatment LAR Concentrations
The pre-treatment and post-treatment concentrations of sE-selectin, sP-selectin, and vWF were then tested against the post-treatment concentration of the LAR in the statistical analysis (
Table 7). We divided the breast cancer patients into three subgroups based on post-treatment LAR concentrations: low (< 0.60), moderate (0.60–1.04), and high (> 1.04). With a trend towards statistical significance, the higher the post-treatment LAR level in breast cancer patients, the higher the pre-treatment sP-selectin as a result (
p = 0.0528).
3.8. Association of the Analysed Parameters with DFS in Linear Regression
The next step in the statistical analysis (
Table 8) was to determine the associations of pre- and post-treatment LAR and endothelial markers and DFS by multiple linear regression. In Model 3, adjusted for age, BMI, parity, menopausal status, and smoking status, the result showed that the lower post-treatment sP-selectin concentration was correlated with a higher risk of breast cancer relapse with tendency to significance (Beta = -0.2576,
p = 0.0504). Similarly, in Model 4, adjusted for age, BMI, parity, menopausal status, smoking status, tumour stage, tumour diameter, intrinsic type, histological type, and nodal involvement, the outcome demonstrated a tendency towards significance, with a higher risk of breast cancer relapse associated with a lower post-treatment sP-selectin concentration (Beta = -0.2437,
p = 0.0583).
3.9. Survival Analysis Regarding Pre- and Post-Treatment LAR and Endothelial Markers
The cut-off points from the ROC curve for the LAR and endothelial markers before and after treatment were used in the statistical analysis, and the cut-off point based on the median was calculated (
Tables S2 and S3 in the
Supplementary Materials). The patients were then divided into two groups: those who were below and those who were above the cut-off point. Each group’s OS and DFS were evaluated. The median duration of follow-up was 68.5 months (IQR = 59–72 months). During the study, ten patients died from systemic metastatic disease, with only one case having a recurrence. The recurrence rate was 15.71%. Subsequently, we performed Kaplan-Meier curves. Those that are not statistically significant were transferred to the
Supplementary Materials (pre-treatment LAR concentration (
Figure S1), pre-treatment sE-selectin concentration (
Figure S2), post-treatment sE-selectin concentration (
Figure S3), post-treatment vWF concentration (
Figure S4)).
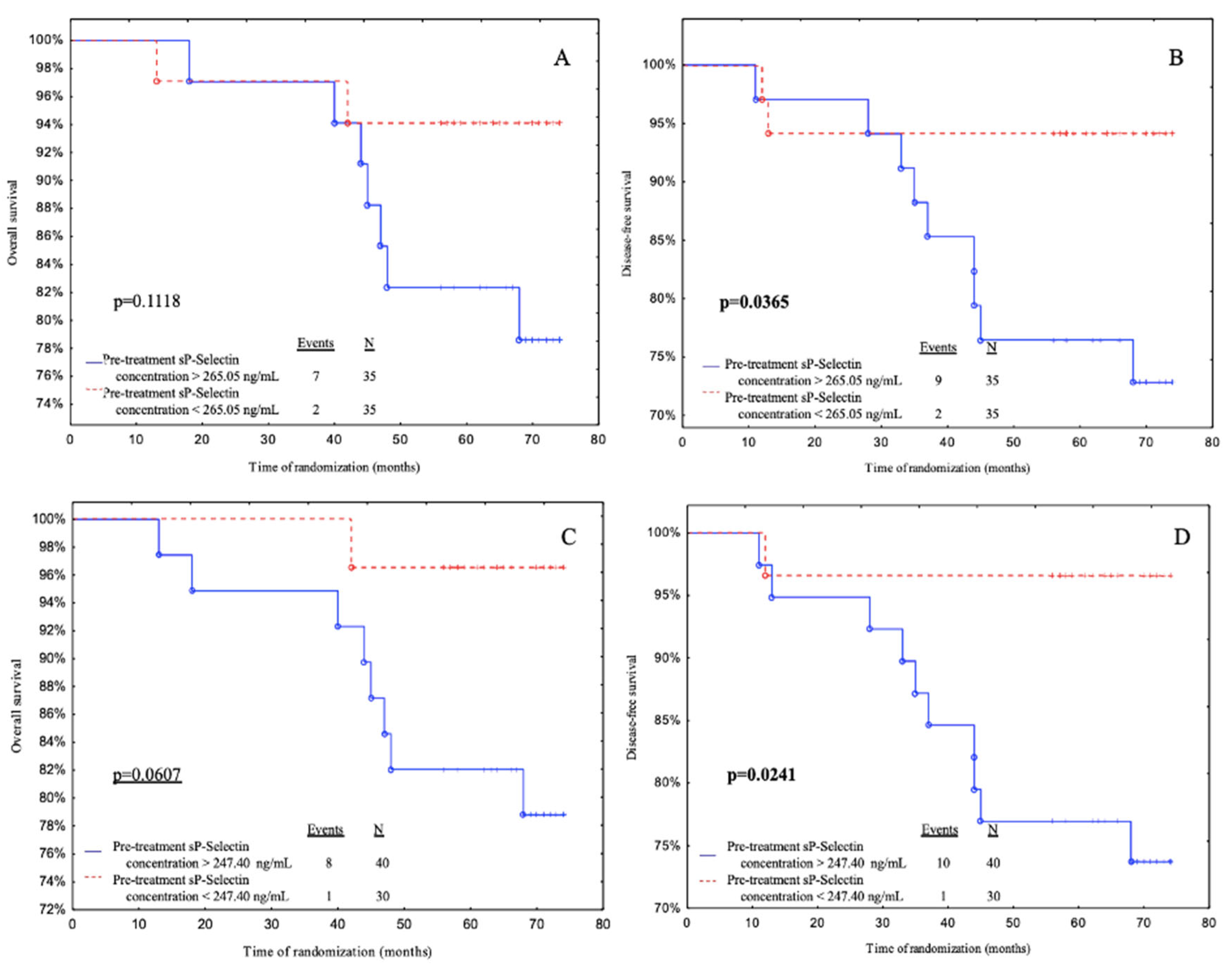
Subjects with pre-treatment sP-selectin levels below 265.05 ng/mL had a significantly better DFS than patients with pre-treatment sP-selectin levels above 265.05 ng/mL according to median value cut- off (
p = 0.0365) (
Figure 2B). Also, patients with pre-treatment sP-selectin levels below 247.40 ng/mL (ROC cut-off) had a better OS (with a tendency to significance
p = 0.0607) and DFS than patients with pre-treatment sP-Selectin levels above 247.40 ng/mL (
p = 0.0241) (
Figure 2C and 2D).
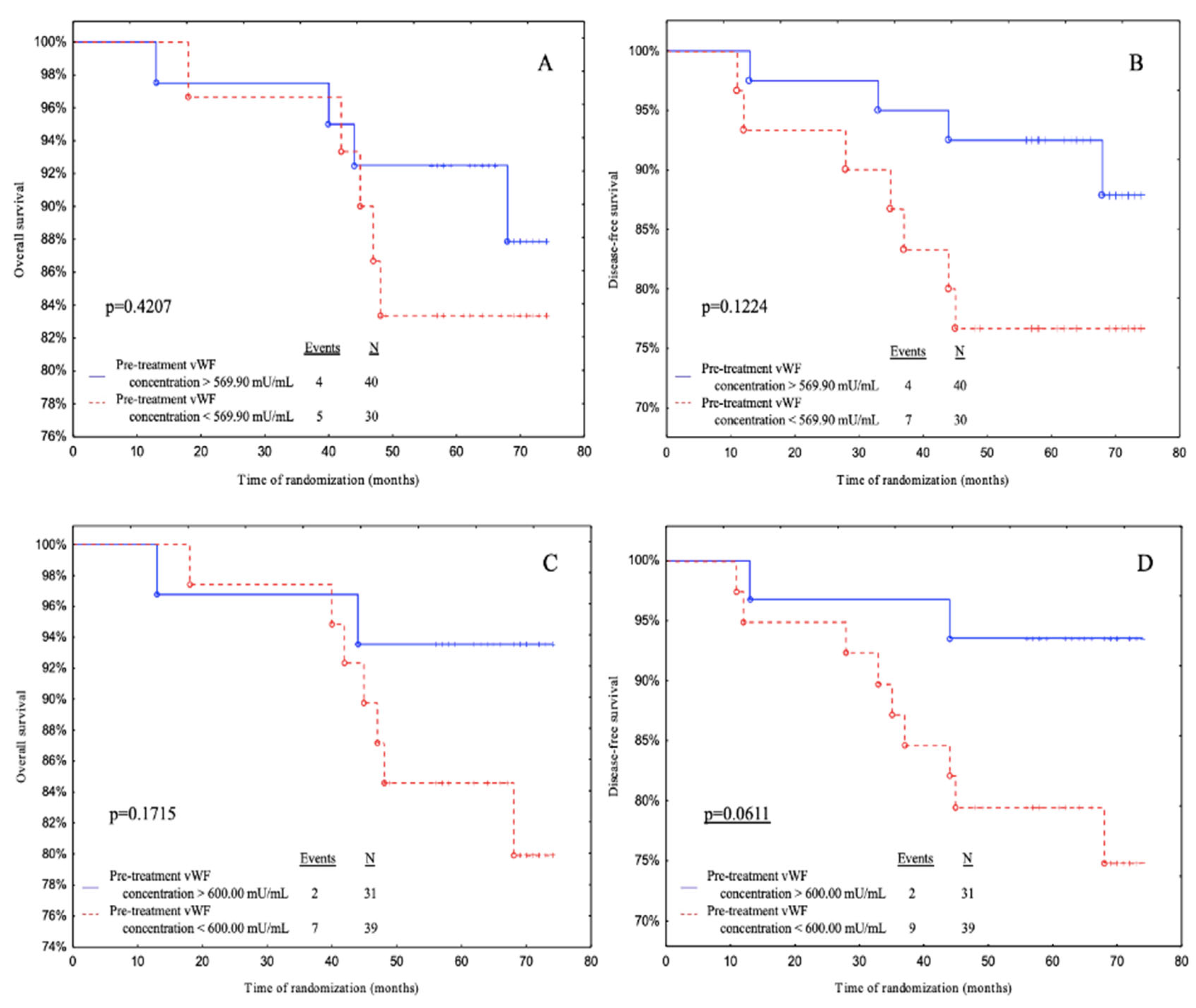
Subjects with pre-treatment vWF levels above 600.00 mU/mL (ROC cut-off) had a significantly better DFS than patients with pre-treatment vWF levels below 600.00 mU/mL with a tendency to significance (
p = 0.0611) (
Figure 3D).
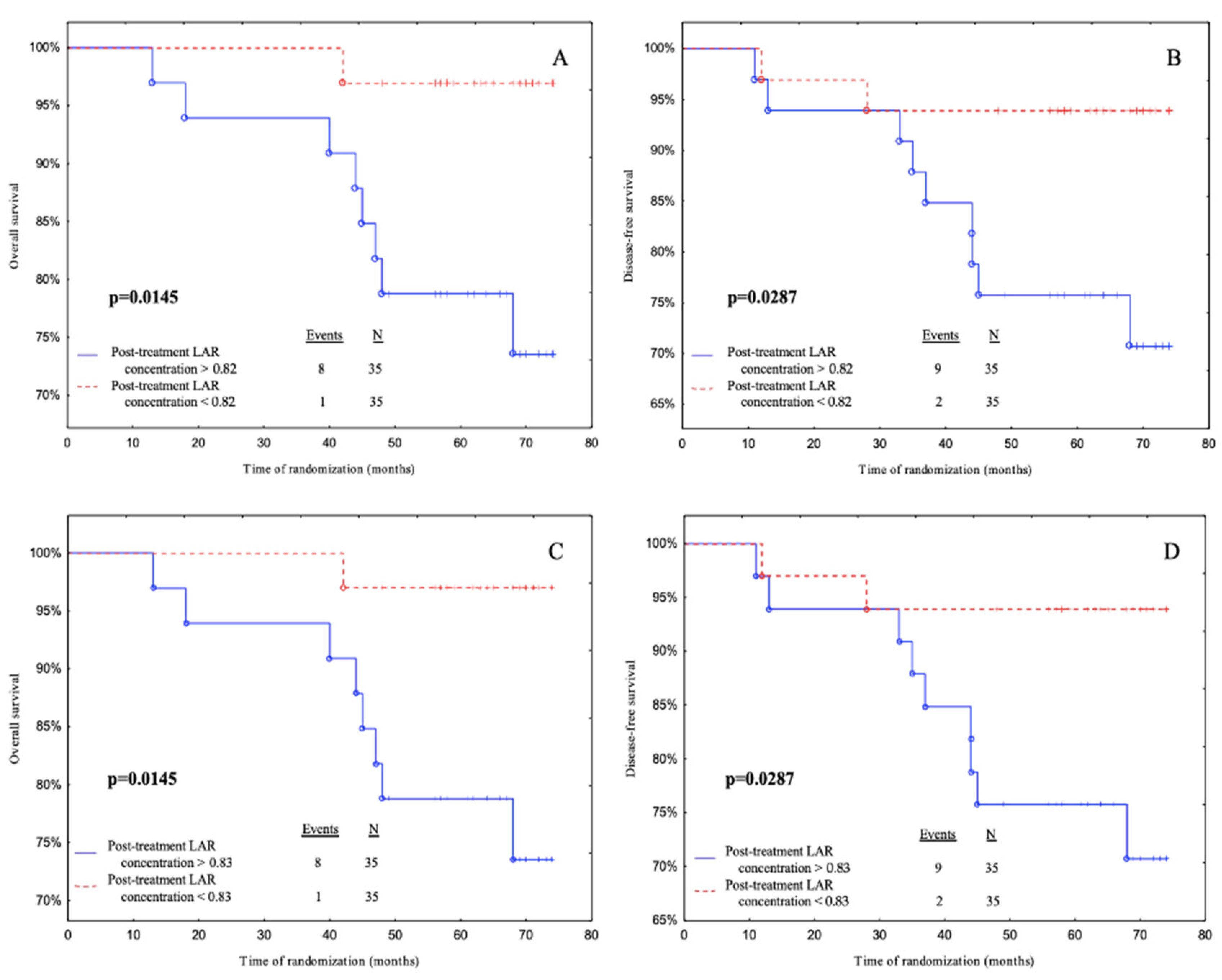
Subjects with post-treatment LAR levels below 0.82 had a significantly better OS and DFS than patients with post-treatment LAR levels above 0.82 according to median cut-off (
p = 0.0145,
p = 0.0287 respectively) (
Figure 4A and 4B). Also, patients with post-treatment LAR levels below 0.83 had a better OS and DFS than patients with post-treatment LAR levels above 0.83 according to the ROC cut-off (
p = 0.0145,
p = 0.0287 respectively) (
Figure 4C and 4D).
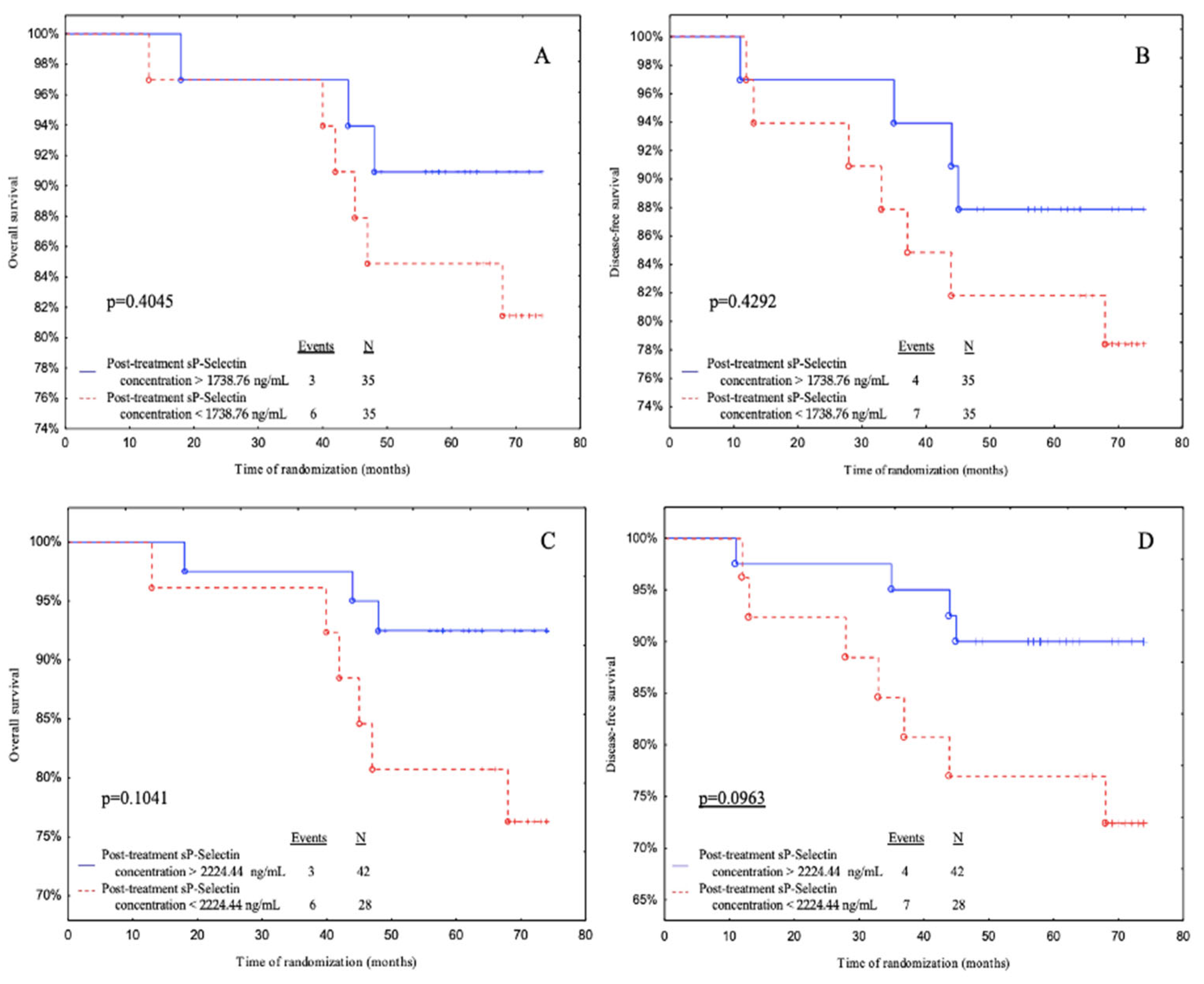
Subjects with post-treatment sP-selectin levels above 2224.44 ng/mL (ROC cut-off) had a significantly better DFS than patients with post-treatment sP-selectin levels below 2224.44 ng/mL with a tendency to significance (
p = 0.0963) (
Figure 5D).
3.10. Analysis of Investigated Parameters as Prognostic Markers Using Univariate and Multivariate Cox Proportional Hazards Regression Models
In this study, we also examined predictive markers for DFS, which included a function of time, using univariate and multivariate Cox regression. BMI, age at diagnosis, smoking status, tumour stage, intrinsic type, histological type, nodal involvement, and tumour diameter were among the prognostic characteristics that were considered when creating the multivariate Cox regression model (
Table 10).
Our multivariate analysis showed an increase in the risk of disease relapse with an increase in the pre-treatment LAR concentration according to the median, and post-treatment LAR concentration according to the median and ROC cut-off (HR = 11.32, 95% CI = 1.16–110.81, p = 0.0371; HR = 10.32, 95% CI = 1.30–82.12, p = 0.0274, respectively) for DFS. Thus, subjects with a pre-treatment LAR concentration higher than 0.46 appear to have a 11.32-times-higher risk of disease recurrence; also, patients with post-treatment LAR concentrations higher than 0.82 and 0.83 (median and ROC cut-off, respectively) have a 10.32-times-higher risk of disease relapse. In addition, we obtained a result with a trend towards statistical significance; as the post-treatment vWF concentration increased according to the cut-off point from the ROC curve, there was an increase in the risk of relapse (HR = 6.26, 95% CI = 0.87–44.94, p = 0.0681). Subjects with a post-treatment vWF concentration higher than 2621.25 mU/mL appear to have a 6.26-times-higher risk of disease recurrence. However, the concentration of pre-treatment sE-selectin and vWF concentration with ROC cut-off indicated opposite associations for DFS (HR = 0.18, 95% CI = 0.04-0.88, p = 0.0334; HR = 0.22, 95% CI = 0.04–1.26, p = 0.0880, respectively). Subjects with a pre-treatment sE-selectin concentration lower than 25.04 ng/mL appear to have a 0.18-times-higher risk of disease recurrence; also, patients with pre-treatment vWF concentration lower than 600.00 mU/mL have a 0.22-times-higher risk of disease relapse.
The univariate Cox regression model also showed an increase in the risk of disease relapse with an increase in the post-treatment LAR concentration (HR = 4.70, 95% CI = 1.02–21.78, p = 0.0477). Subjects with a post-treatment LAR concentration higher than 0.82 and 0.83 (median and ROC cut-off, respectively) appear to have a 4.70-times-higher risk of disease recurrence. In addition, we obtained a result with a trend towards statistical significance; as the pre-treatment sP-selectin concentration increased according to the median and ROC cut-off point, there was an increase in the risk of relapse (HR = 4.48, 95% CI = 0.97–20.82, p = 0.0555; HR = 7.55, 95% CI = 0.96–59.19, p = 0.0543, respectively). Subject with pre-treatment sP-selectin higher than 265.05 ng/mL and 247.40 ng/mL appear to have 4.48- and 7.55-times-higher risk of disease recurrence, respectively. However, the pre-treatment vWF concentration with the ROC cut-off indicated opposite associations for DFS (HR = 0.26, 95% CI = 0.06–1.20, p = 0.0841). Subjects with a pre-treatment vWF concentration lower than 25.04 ng/mL appear to have a 0.18-times-higher risk of disease recurrence; also, patients with a circulating pre-treatment vWF concentration lower than 600.00 mU/mL have a 0.26-times-higher risk of disease relapse.
4. Discussion
Breast cancer poses a danger to the health of women worldwide. In 2020, there were approximately 2.26 million new cases and 680,000 new deaths from breast cancer worldwide [
27]. Metastasis is a highly complex process that involves multiple cellular mechanisms such as tumour division, invasion, immune evasion, and tissue microenvironment regulation [
28]. Despite recent advances in medicine, metastasis is still the leading cause of death in breast cancer patients [
29].
4.1. Endothelial Markers before and after Treatment Depending on the Type of Treatment
In the first stage of our study, we compared endothelial markers with different types of treatment. In our investigation, the LAR increased after treatment despite the treatment pattern. Our results seem to support those of Sun et al. who observed a raised concentration of leptin and adiponectin in the bone marrow of patients with acute leukaemia after treatment with dexamethasone. They reasoned that hypothalamic leptin resistance should be considered in obese patients with acute lymphocytic leukemia (ALL) [
30]. Furthermore, in our research we observed that pre-treatment sE-selectin concentrations were higher in patients with breast-conserving therapy (with a tendency to significance) and treated with non-anthracycline chemotherapy. Interestingly, post-treatment sE-selectin concentrations were higher regardless of the treatment pattern and in patients not receiving chemotherapy. Considering that sE-selectin is involved in leukocyte and cancer cell extravasation, homing, adhesion, proliferation, cellular dormancy, and drug resistance, it is a potentially promising target for researchers. According to Muz et al., as a specific E-selectin antagonist, Uproleselan (also known as GMI-1271) disrupts the tumour microenvironment by affecting two major steps in metastasis – extrinsic and adhesion – so that blocking E-selectin reduces tumour spread. Furthermore, Uproleselan moves tumour cells from the protective vascular niche into the circulation, making them more vulnerable to chemotherapy [
31]. In our study, we also discovered that patients, despite the treatment pattern, had higher post-treatment sP-selectin concentrations. Adjuvant treatment causes platelet activation which leads to prothrombotic activation. This observation is consistent with Mills et al. who showed higher P-selectin levels after chemotherapy treatment [
32]. Moreover, in our investigation, we observed that post-treatment vWF concentrations were higher in patients, regardless of treatment pattern, who were treated with anthracycline chemotherapy. The concentration of vWF is higher after adjuvant treatment because it causes vascular endothelial damage and increases its prothrombotic nature. vWF is considered a marker of vascular endothelial cell damage. Our results corroborate the study by Gil-Bazo et al. who showed increased vWF levels after surgery, which may be due to secondary tissue damage [
33]. In their study, Giri et al. observed higher levels of vWF in patients undergoing chemotherapy, which may mean that chemotherapy predisposes patients to develop thrombosis and endothelial dysfunction [
34].
4.2. Endothelial Markers’ Relationship with LAR Concentrations before and after Treatment
The next step of the analysis was to demonstrate the relationship between endothelial markers and LAR levels. We observed that the higher the LAR level was after treatment in breast cancer patients, the higher the sP-selectin score was before treatment (with a trend towards statistical significance). This may be because the higher the LAR, the higher the inflammation in the body. In inflammation, platelets are activated, which secrete various compounds, for example, P-selectin. This is responsible for the formation of leukocyte-platelet complexes and the migration of leukocytes across the endothelium [
35]. In particular, the soluble form of P-selectin may exhibit proatherogenic and prothrombotic effects. Therefore, it is involved, among other things, in the pathogenesis of thromboembolic complications in breast cancer patients [
36].
4.3. Regression Model Analysis of Research Variables as Prognostic Indicators
The following stage of analysis was to test the regression model analysis of the variables as predictors. We performed a linear regression in which the lower post-treatment sP-selectin concentration was correlated with a shorter DFS with a tendency to significance in Model 3, which was adjusted for age, BMI, parity, menopausal status, and smoking status. Likewise, when age, BMI, parity, menopausal status, smoking status, tumour stage, tumour diameter, intrinsic type, histological type, and nodal involvement were all controlled for, the outcome showed a trend towards significance, with a shorter DFS associated with a lower post-treatment sP-selectin concentration. Furthermore, we produced ROC and Kaplan-Meier curves as an additional analysis. We showed that the post-treatment sP-selectin concentration based on the cut-off point according to the ROC curve is a predictor of breast cancer risk in DFS (a result with a trend towards statistical significance), and confirms the result we obtained by performing a linear regression. Furthermore, in our ROC and Kaplan-Meier analysis, we demonstrated that the higher the sP-selectin concentration before treatment, the worse the prognosis. Our findings appear to be supported by the Cox regression; higher pre-treatment sP-selectin concentrations in the univariate Cox regression model according to the median and ROC cut-off points were associated with a higher risk of disease recurrence with a tendency to significance. It is interesting to note that high levels of sP-Selectin before treatment prognosticate poorly and after treatment prognosticate well. Rhone et al. in their study analysed the levels of circulating bone marrow-derived endothelial progenitor cells (EPCs) in patients with IBrC. They noted that EPCs concentrations were high before treatment and increased further after treatment, which was associated with a good prognosis for patients. They cited studies by various authors in their analysis [
37]. Tanaka et al. contend that the absence of an increase in circulating EPCs after a cycle of chemotherapy is an independent negative prognostic factor and that changes in the number of circulating EPCs are associated with the treatment response, as the activity of EPCs may be related to the body’s adaptation to chemotherapeutics [
38].
We also conclude that the post-treatment LAR concentration according to the median and cut-off point of the ROC curve is a predictor of breast cancer risk in OS and DFS. ROC curves were used for additional analysis. The study’s findings revealed that the post-treatment LAR was the best predictor of disease relapse. We also performed Kaplan-Meier curves in which we found that patients with higher LAR levels had worse OS and DFS. Additionally, according to a multivariate and univariate Cox regression model, we showed an increase in the risk of disease relapse with an increase in the post-treatment LAR concentration. Diaz et al. hypothesised in their study that the LAR has an impact on the survival of patients with epithelial ovarian cancer. They performed a Kaplan-Meier survival analysis in which women with a low LAR showed a statistically longer disease-specific survival (57 months) compared to those with a median or high level. However, when they performed a Cox regression analysis, the LAR did not prove to be a statistically significant prognostic factor [
39].
Furthermore, pre-treatment vWF concentration according to the ROC cut-off point is a predictor of breast cancer risk in DFS with a trend towards significance. This result is confirmed by the Kaplan-Meier curves and multivariate and univariate Cox regression; lower pre-treatment vWF levels according to ROC cut-off points were associated with an increase in the risk of disease relapse with a tendency to significance. Terraube et al. achieved similar results in their investigation examining whether vWF is involved in metastatic development. They discovered a substantial increase in the frequency of lung metastatic foci in vWF-null mice compared to wild-type mice in an experimental model. They discovered that greater metastasis was caused by higher survival of tumour cells in the lung during the first 24 hours in the absence of vWF [
40].
4.4. Strengths and Limitations of the Study
We would like to highlight some of the study’s limitations. We enrolled a small number of patients and recruited them from only one study centre, which itself limits the number of patients. The sample size was determined by obtaining patients’ permission to participate and having them meet very strict inclusion criteria. Because we only looked at women of Polish descent, our findings may not be applicable to other ethnic groups. As we recruited patients at an early stage of breast cancer with no metastases, we cannot say what the prognostic value would be for larger and more advanced tumours. The fact that this study was based on a six-year follow-up of patients with complete clinicopathological characteristics is its strength. All blood samples were collected prospectively from fasting patients one day before surgery, reducing any variations that might have occurred if the samples had been collected at different times.
5. Conclusions
Despite the small number of luminal IBrC patients included in the study, our findings reveal a few key points: (1) Regardless of treatment pattern, adjuvant IBrC therapy most likely boosted the LAR and endothelial marker concentrations; (2) The post-treatment LAR levels appear to have been associated with future outcomes in patients with luminal IBrC, as LAR levels higher than 0.82 (median cut-off) and 0.83 (ROC cut-off) have been shown to promote the probability of relapse and mortality in the IBrC cohort; (3) The pre-treatment sP-selectin levels appear to be related to future outcomes in patients with luminal IBrC, with LAR levels more than 265.05 ng/mL (median cut-off) and 247.40 ng/mL (ROC cut-off) increasing the likelihood of recurrence and death in the IBrC cohort; (4) Regardless of the linear regression model used, only the pre-treatment sP-selectin levels showed prognostic implications; (5) Higher pre-treatment sP-selectin and post-treatment LAR levels were mainly associated with poorer future outcomes for patients with luminal IBrC.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
Conceptualization, B.R.-C., K.K.; methodology, B.R.-C., K.K., P.K; soft-ware, K.K.; validation, K.K., B.R.-C., D.F.; formal analysis, K.K.; investigation, K.K., B.R.-C.; resources, B.R-C., P.R.; data curation, B.R.-C., P.R.; writing—original draft, K.K., B.R.-C.; writing—review & editing, B.R.-C.; visualization, K.K., D.F.; supervision, B.R.-C.; project administration, B.R.-C., P.R.; funding acquisition, B.R.-C., P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was undertaken in accordance with the Declaration of Helsinki, following the guidelines and approval of the local Ethics Committee (permission number: KB 547/2015). Informed consent was obtained from all participants included in the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
We would like to thank all the patients for participating in our study. Many thanks to the biomedical laboratory scientists Barbara Góralczyk, Małgorzata Michalska for their contribution in samples preparation and laboratory assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- van Denderen, B.J.; Thompson, E.W. Cancer: The to and fro of tumour spread. Nature 2013, 493, 487–488. [Google Scholar] [CrossRef]
- Xu, B.; Hu, X.; Feng, J.; Geng, C.; Jin, F.; Li, H.; Li, M.; Li, Q.; Liao, N.; Liu, D.; et al. Chinese expert consensus on the clinical diagnosis and treatment of advanced breast cancer (2018). Cancer 2020, 126, 3867–3882. [Google Scholar] [CrossRef]
- O’Shaughnessy, J. Extending Survival with Chemotherapy in Metastatic Breast Cancer. Oncologist 2005, 10, 20–29. [Google Scholar] [CrossRef]
- Jin, X.; Mu, P. Targeting Breast Cancer Metastasis. Breast Cancer (Auckl.) 2015, 9 (Suppl. 1), 23–34. [Google Scholar] [CrossRef]
- Guo, Y.; Arciero, C.A.; Jiang, R.; Behera, M.; Peng, L.; Li, X. Different Breast Cancer Subtypes Show Different Metastatic Patterns: A Study from A Large Public Database. Asian Pacific Journal of Cancer Prevention. 2020;21(12):3587-3593. [CrossRef]
- Gong, T.-T.; Wu, Q.-J.; Wang, Y.-L.; Ma, X.-X. Circulating adiponectin, leptin and adiponectin-leptin ratio and endometrial cancer risk: Evidence from a meta-analysis of epidemiologic studies. International Journal of Cancer 2015;137(8), 1967–1978. [CrossRef]
- Agostinis-Sobrinho, C. , Vicente, S. E. C. F., Norkiene, S., Rauckienė-Michaelsson, A., Kievisienė, J., Dubey, V. P., Razbadauskas, A., Lopes, L., & Santos, R. Is the Leptin/Adiponectin Ratio a Better Diagnostic Biomarker for Insulin Resistance than Leptin or Adiponectin Alone in Adolescents? Children (Basel, Switzerland), 2022, 9 (8), 1193. [Google Scholar] [CrossRef]
- Lopez-Jaramillo, P. SSA 04-3 leptin/adiponectin in cardiometabolic disease. Journal of Hypertension 2016;34(Suppl 1-ISH 2016 Abstract Book):e7.
- Srikanthan, K.; Feyh, A.; Visweshwar, H.; Shapiro, J.I.; Sodhi, K. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the West Virginian population. International Journal of Medical Sciences 2016, 13(1), 25–38. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.T.; O’Dea, K.; Piera, K.; Barzi, F.; Cass, A.; Hoy, W.E. et al. Associations of serum adiponectin with markers of cardio-metabolic disease risk in indigenous Australian adults with good health, diabetes and chronic kidney disease. Obesity Research & Clinical Practice. 2016;10(6):659–72. [CrossRef]
- Akyol, M.; Demir, L.; Alacacioglu, A.; Ellidokuz, H.; Kucukzeybek, Y.; Yildiz, Y.; Gumus, Z.; Bayoglu, V.; Yildiz, I.; Salman, T.; Varol, U.; Kucukzeybek, B.; Demir, L.; Dirican, A.; Sutcu, R.; Tarhan, M. O. The Effects of Adjuvant Endocrine Treatment on Serum Leptin, Serum Adiponectin and Body Composition in Patients with Breast Cancer: The Izmir Oncology Group (IZOG) Study. Chemotherapy 2016; 61(2), 57–64. [CrossRef]
- Słomian, G. J., Nowak, D., Buczkowska, M., Głogowska-Gruszka, A., Słomian, S. P., Roczniak, W., Janyga, S., & Nowak, P. The role of adiponectin and leptin in the treatment of ovarian cancer patients. Endokrynologia Polska 2019;70(1), 57–63. [CrossRef]
- Iwan-Zietek, I.; Ruszkowska-Ciastek, B.; Michalska, M.; Overskaug, E.; Goralczyk, K.; Dabrowiecki, S.; Rosc, D. Association of adiponectin and leptin-to-adiponectin ratio with the function of platelets in morbidly obese patients. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society 2016, 67(4), 555–561. [Google Scholar] [PubMed]
- Borsig, L.; Laubli, H. Cell Adhesion during Tumorigenesis and Metastasis. In Encyclopedia of Cancer, 3rd ed.; Boffetta, P., Hainaut, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 307–314. [Google Scholar]
- Drnasfield, I.; Stocks, S.C.; Haslett, C. Regulation of cell adhesion molecule expression and function associated with neutrophil apoptosis. Blood 1995, 85, 3264–3273. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Lee, S.; YChoi, H.; Cho, S.G. The Impact of Adhesion Molecules on the In Vitro Culture and Differentiation of Stem Cells. Biotechnology Journal. 2018, 13, 1700575. [Google Scholar] [CrossRef] [PubMed]
- Ley, K. Functions of selectins. Mammalian Carbohydate Recognition System. 2001, 33, 177–200. [Google Scholar] [CrossRef]
- Tinoco, R.; Otero, D.C.; Takahashi, A.A.; Bradley, L.M. PSGL-1: A new player in the immune checkpoint landscape. Trends in Immunology 2017, 38, 323–335. [Google Scholar] [CrossRef]
- Alves, C.S.; Burdick, M.M.; Thomas, S.N.; Pawar, P.; Konstantopoulos, K. The dual role of CD44 as a functional P-selectin ligand and fibrin receptor in colon carcinoma cell adhesion American Journal of Physiology-Cell Physiology 2008, 294, C907–C916. [CrossRef]
- Mannori, G.; Crottet, P.; Cecconi, O.; Hanasaki, K.; Aruffo, A.; Nelson, R.M. et al. Differential colon cancer cell adhesion to E-, P-, and L-selectin: role of mucin-type glycoproteins. Cancer Research 1995, 55, 4425–4431. [Google Scholar]
- Gout, S.; Morin, C.; Houle, F.; Huot, J. Death receptor-3, a new E-Selectin counter-receptor that confers migration and survival advantages to colon carcinoma cells by triggering p38 and ERK MAPK activation. Cancer Research 2006; 66:9117–24. [CrossRef]
- Schrijver, I.T.; Kemperman, H.; Roest, M.; Kesecioglu, J.; de Lange, D.W. Soluble P-selectin as a Biomarker for Infection and Survival in Patients with a Systemic Inflammatory Response Syndrome on the Intensive Care Unit. Biomarker insights 2017; 12, 1177271916684823. [CrossRef]
- Pang, J.H.; Coupland, L.A.; Freeman, C.; Chong, B.H.; Parish, C.R. Activation of tumour cell ECM degradation by thrombin-activated platelet membranes: Potentially a P-selectin and GPIIb/IIIa-dependent process. Clinical & Experimental Metastasis 2015, 32, 495–505. [Google Scholar] [CrossRef]
- Coupland, L.A.; Chong, B.H.; Parish, C.R. Platelets and P-selectin control tumor cell metastasis in an organ-specific manner and independently of NK cells. Cancer Research 2012, 72, 4662–4671. [Google Scholar] [CrossRef]
- Geng, J. G., Chen, M., & Chou, K. C. P-selectin cell adhesion molecule in inflammation, thrombosis, cancer growth and metastasis. Current medicinal chemistry 2004; 11(16), 2153–2160. [CrossRef]
- Dhami, S.P.S.; Patmore, S.; Comerford, C. et al. Breast cancer cells mediate endothelial cell activation, promoting von Willebrand factor release, tumor adhesion, and transendothelial migration. Journal of Thrombosis and Haemostasis. 2022;20(10):2350-2365. [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Williams, E.D.; Gao, D.; Redfern, A.; Thompson, E.W. Controversies around epithelial-mesenchymal plasticity in cancer metastasis. Nature Reviews Cancer 2019, 19, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Kim, D.; Ko, S.; Kim, A.; Mo, K.; Yoon, H. Breast Cancer Metastasis: Mechanisms and Therapeutic Implications. International Journal of Molecular Sciences 2022, 23(12), 6806. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, R.; Tang, J.; Wu, X.; Zhu, L.; Huang, H.; Chen, H.; Xiao, M.; Luo, H.; Zheng, H.; Chen, J. Prognostic Observational Analysis of BMI, Leptin, and Adiponectin in Children With Acute Lymphocytic Leukemia Undergoing Remission-Induction Chemotherapy. Frontiers in Pediatrics 2022, 10, 797836. [Google Scholar] [CrossRef]
- Muz, B.; Abdelghafer, A.; Markovic, M.; Yavner, J.; Melam, A.; Salama, N.N.; Azab, A.K. Targeting E-selectin to Tackle Cancer Using Uproleselan. Cancers 2021, 13(2), 335. [Google Scholar] [CrossRef]
- Mills, P.J.; Ancoli-Israel, S.; Parker, B.; Natarajan, L.; Hong, S.; Jain, S.; Sadler, G.R.; von Känel, R. Predictors of inflammation in response to anthracycline-based chemotherapy for breast cancer. Brain, behavior, and immunity 2008, 22(1), 98–104. [Google Scholar] [CrossRef] [PubMed]
- Gil-Bazo, I.; Catalán Goni, V.; Alonso Gutiérrez, A.; Rodríguez Rodríguez, J.; Páramo Fernández, J.A.; de la Cámara Gómez, J.; Hernández Lizoain, J. L.; García-Foncillas López, J. Impact of surgery and chemotherapy on von Willebrand factor and vascular endothelial growth factor levels in colorectal cancer. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico 2005, 7(4), 150–155. [Google Scholar] [CrossRef]
- Giri, S.; Bose, J.C.; Chandrasekar, A.; Tiwary, B.K.; Gajalakshmi, P.; Chatterjee, S. Increased Plasma Nitrite and von Willebrand Factor Indicates Early Diagnosis of Vascular Diseases in Chemotherapy Treated Cancer Patients. Cardiovascular toxicology 2019, 19(1), 36–47. [Google Scholar] [CrossRef]
- Maślanka, K. The role of platelets in inflammatory processes Journal of Transfusion Medicine 2014, tom 7, nr 3, 102–109 Copyright © 2014 Via Medica ISSN 1689–6017.
- Woollard, K.J.; Chin-Dusting, J. P-selectin antagonism in inflammatory disease. Current pharmaceutical design 2010, 16(37), 4113–4118. [Google Scholar] [CrossRef]
- Rhone, P.; Bielawski, K.; Ziołkowska, K.; Rość, D.; Ruszkowska-Ciastek, B. Low Pre-Treatment Count of Circulating Endothelial Progenitors as a Prognostic Biomarker of the High Risk of Breast Cancer Recurrence. Journal of clinical medicine 2019, 8(11), 1984. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Ueno, T.; Ishiguro, H.; Morita, S.; Toi, M. The lack of increases in circulating endothelial progenitor cell as a negative predictor for pathological response to neoadjuvant chemotherapy in breast cancer patients. NPJ Precision Oncology 2017, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Diaz, E.S.; Karlan, B. Y.; Li, A.J. Obesity-associated adipokines correlate with survival in epithelial ovarian cancer. Gynecologic oncology 2013, 129(2), 353–357. [Google Scholar] [CrossRef] [PubMed]
- Terraube, V.; Pendu, R.; Baruch, D.; Gebbink, M.F.; Meyer, D.; Lenting, P. J.; Denis, C.V. Increased metastatic potential of tumor cells in von Willebrand factor-deficient mice. Journal of thrombosis and haemostasis : JTH 2006, 4(3), 519–526. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).