1. Introduction
Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) are the most widely used imaging modalities for imaging the brain. Patients who present with suspected stroke most commonly receive a CT scan for initial evaluation, due to its lower cost and shorter acquisition time compared to MRI. However, MRI has been shown to be more accurate than CT for diagnosing stroke, and can provide additional information that can be useful for diagnosis, treatment, and prognosis of stroke and other brain disorders. Additionally, MRI can be used to detect other abnormalities in the brain that may contribute to stroke risk, such as aneurysms, arteriovenous malformations, or tumours.
MRI is more sensitive than CT at detecting lesions in conditions such as stroke due to its superior soft tissue contrast and ability to provide detailed anatomical information [
1,
2,
3,
4]. Once medical images are obtained, analysis is necessary to facilitate diagnosis. Comparing the patient’s brain scans to a brain atlas can be helpful in understanding the location of lesions or abnormalities with respect to the anatomical and functional structures of the brain. Many commonly used brain atlases, including the MNI152 T1-weighted MRI brain template [
5], Talairach atlas [
6], Brodmann atlas [
7], and JHU DTI-based white-matter atlases [
8], are derived from MRIs. However, cross-modality image registration between CT and MRI is challenging due to the high variability of tissue or organ appearance caused by the different imaging mechanisms. The pixel intensity, voxel size, image orientation, and field of view are also different between CT and MRI images, making multi-modality registration less straightforward than mono-modality alignment [
9].
While MRI is more accurate than CT in diagnosing stroke, not all emergency rooms are equipped with MRI scanners, especially in smaller or poorer areas. In such cases, it may be beneficial to use deep learning techniques to generate a synthetic MRI from the patient’s CT scan. Deep learning techniques are being increasingly applied to various areas of medicine, including stroke diagnosis and treatment. One of the primary uses of deep learning in this field is medical image segmentation, where an algorithm is trained to automatically identify and segment lesions from a brain scan. This can help to improve accuracy and efficiency in diagnosis and treatment planning for stroke patients.
Previous studies have primarily focused on using MRI to CT generation [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19], often for the purpose of MRI-only radiotherapy. Some studies have investigated CT to MRI generation using GANs [
20,
21,
22,
23]; of these, only one study [
23] investigated MRI generation for stroke patients.
The purpose of this study is to investigate the use of different deep learning algorithms to generate realistic and accurate MRIs of a patient from their CT scan, while considering the niche aspects of this problem in translating stroke lesions between the scans. This study uses multiple versions of a Unet model adapted for image synthesis, as well as Unet++ and attention based UNets, and compares 2D, 3D, and patch-based models. These paired architectures are compared to the unpaired CycleGAN. The results are evaluated, and the performance of the different models is compared using a combination of qualitative and quantitative methods.
2. Materials and Methods
The dataset used in this research consists of 190 patients who were treated for stroke at Auckland City Hospital between 2009 and 2020. All patients received both a CT and T1-weighted MRI scan which were available on file. The dataset was formed by combining multiple datasets from previous research studies, in each of these patients were required to be at least 18 years old and gave written informed consent. Patients with Ischemic and Haemorrhagic strokes were included.
2.1. Pre-Processing
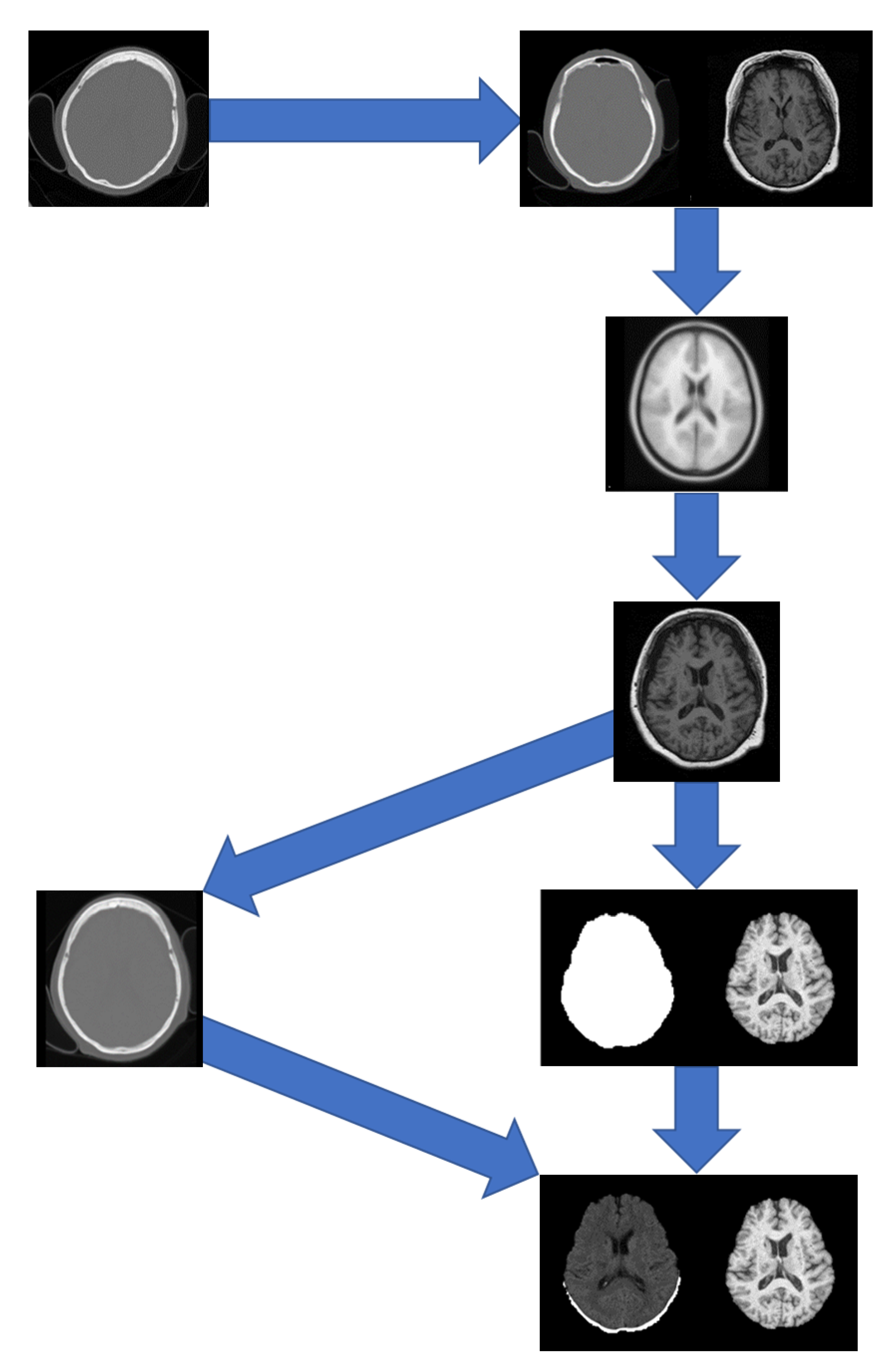
A pipeline was developed to pre-process the data which included registration to a brain atlas and brain extraction. The following steps detail the pre-processing pipeline (
Figure 1):
Registration of the CT to the MRI using FSL Flirt [
24,
25,
26] with 12 degrees of freedom and a mutual information cost function.
Registration of the MRI to the ICBM152 T1w linear atlas using FSL Flirt [
24,
25,
26] with 12 degrees of freedom and a correlation ratio cost function, and saving the transformation matrix.
Applying the transformation matrix from step 2 to the resultant CT from step 1.
Apply the runhdbet function of HD-BET [
27] to the resultant MRI from step 2. The resultant extraction brain and brain mask are then saved.
Using pixelwise multiplication between the resultant CT scan from step 3 and the brain mask from step 4 to extract the brain from the CT.
After the data were processed through the pipeline, the data underwent manual quality control by overlaying the CT and MRI of each patient and visually inspecting the alignment over multiple slices. Data that could not be successfully registered from CT to MRI in FSL Flirt were attempted to be registered in 3D Slicer [
28,
29,
30,
31]. Only 9 pairs could not be registered to a suitable accuracy, resulting in a dataset of 181 patients. To ensure consistency between the images, all MRI images were set to a data range of [0, 255] by dividing the intensities of each image by its maximum intensity and multiplying by 255. CT scans were kept in their original intensity range as they were in Hounsfield Units.
2.2. Model Architectures
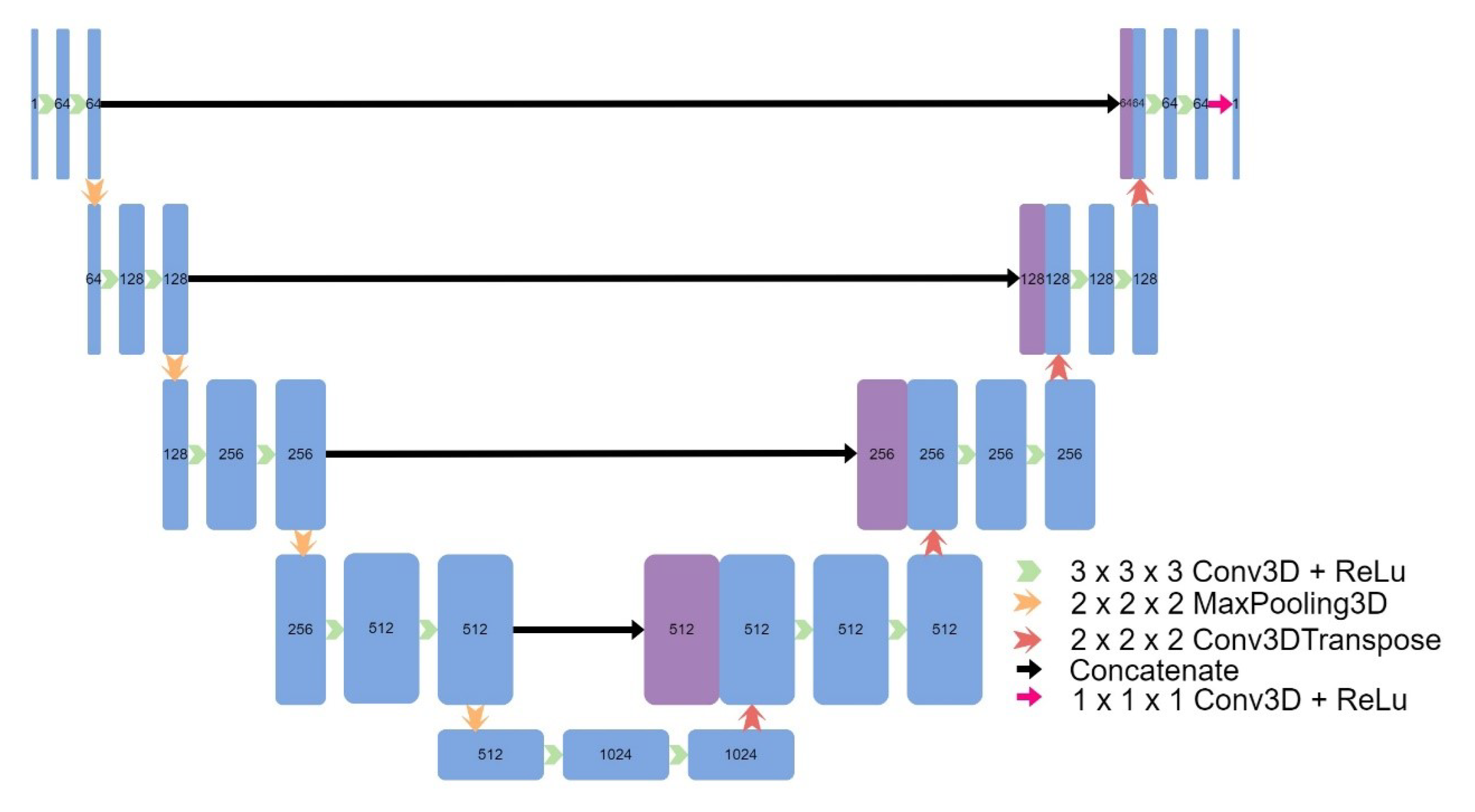
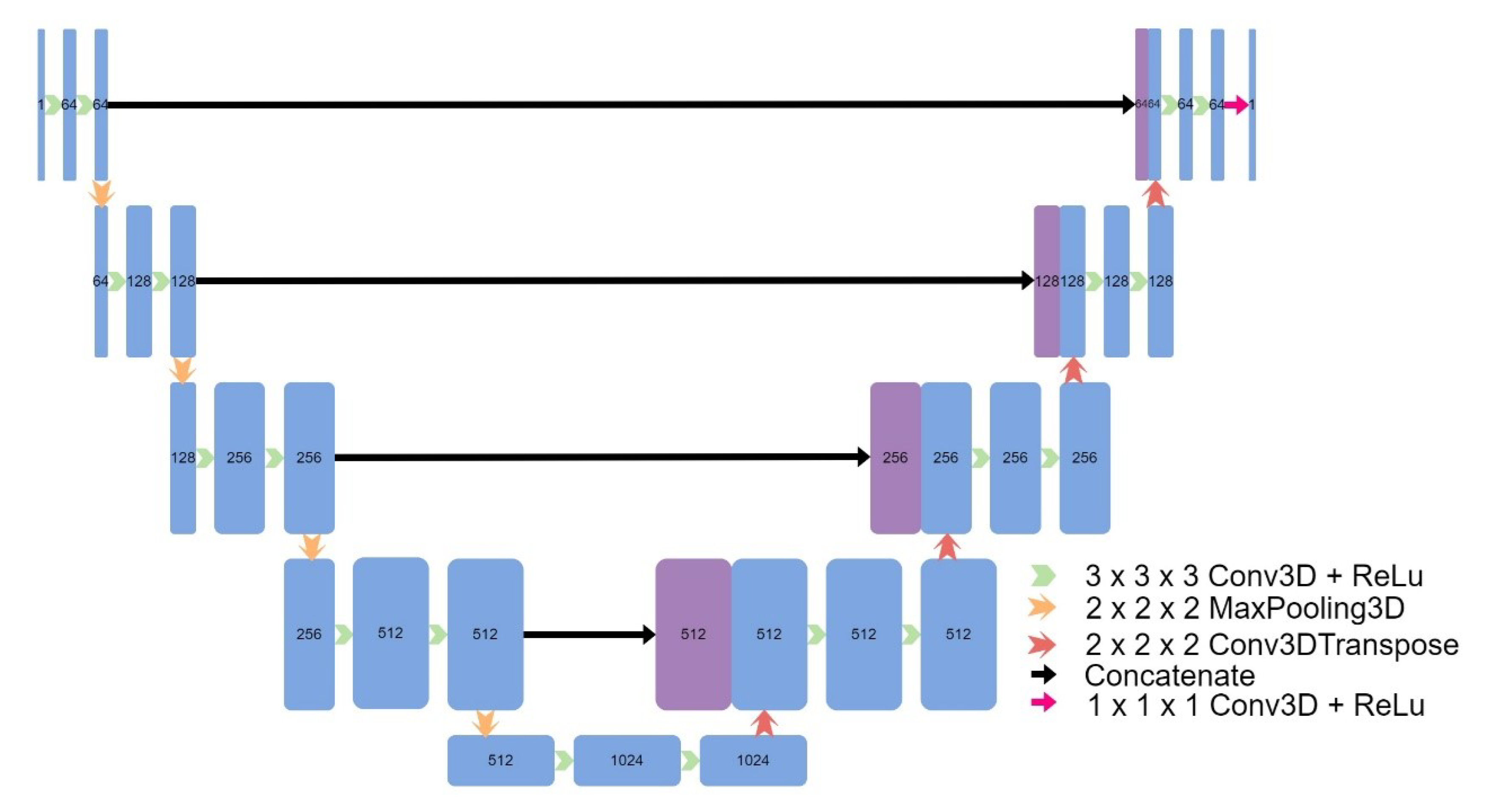
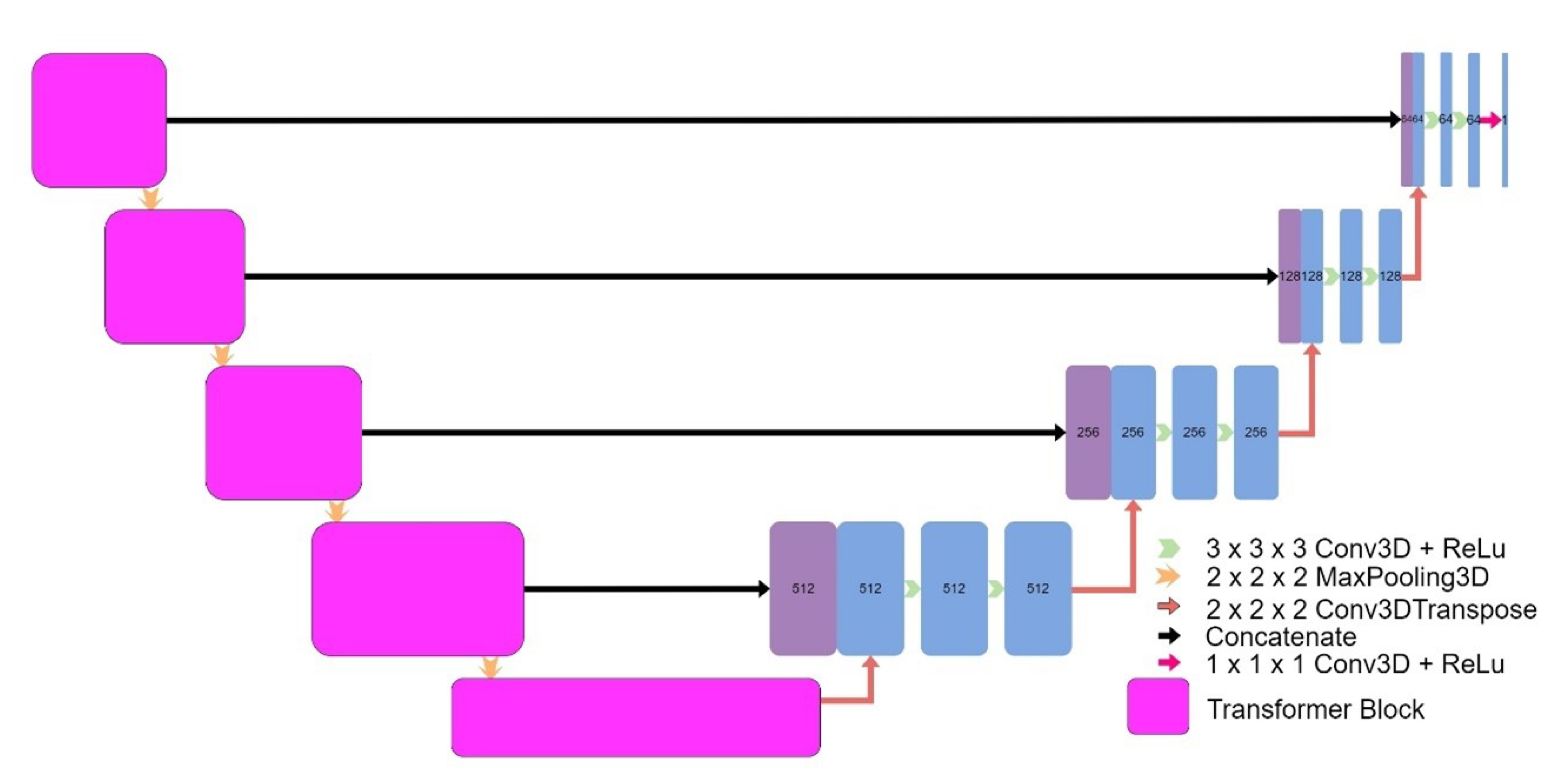
Eight models were implemented, including four models based on the original UNet: a 3D UNet (
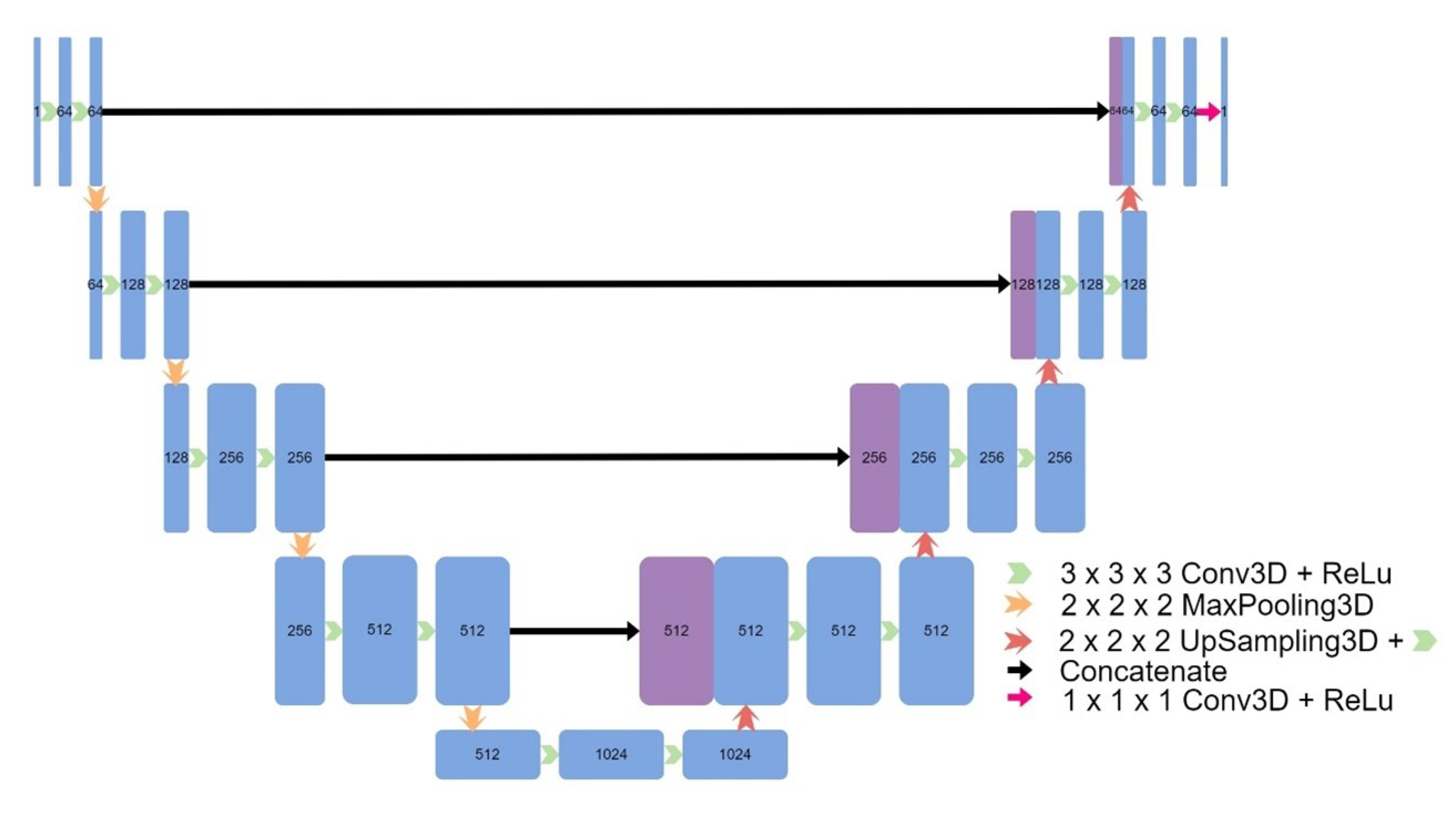
Figure 2), a 3D UNet which used upsampling and convolutions instead of transpose convolutions (
Figure 3), a 3D patch-based UNet (
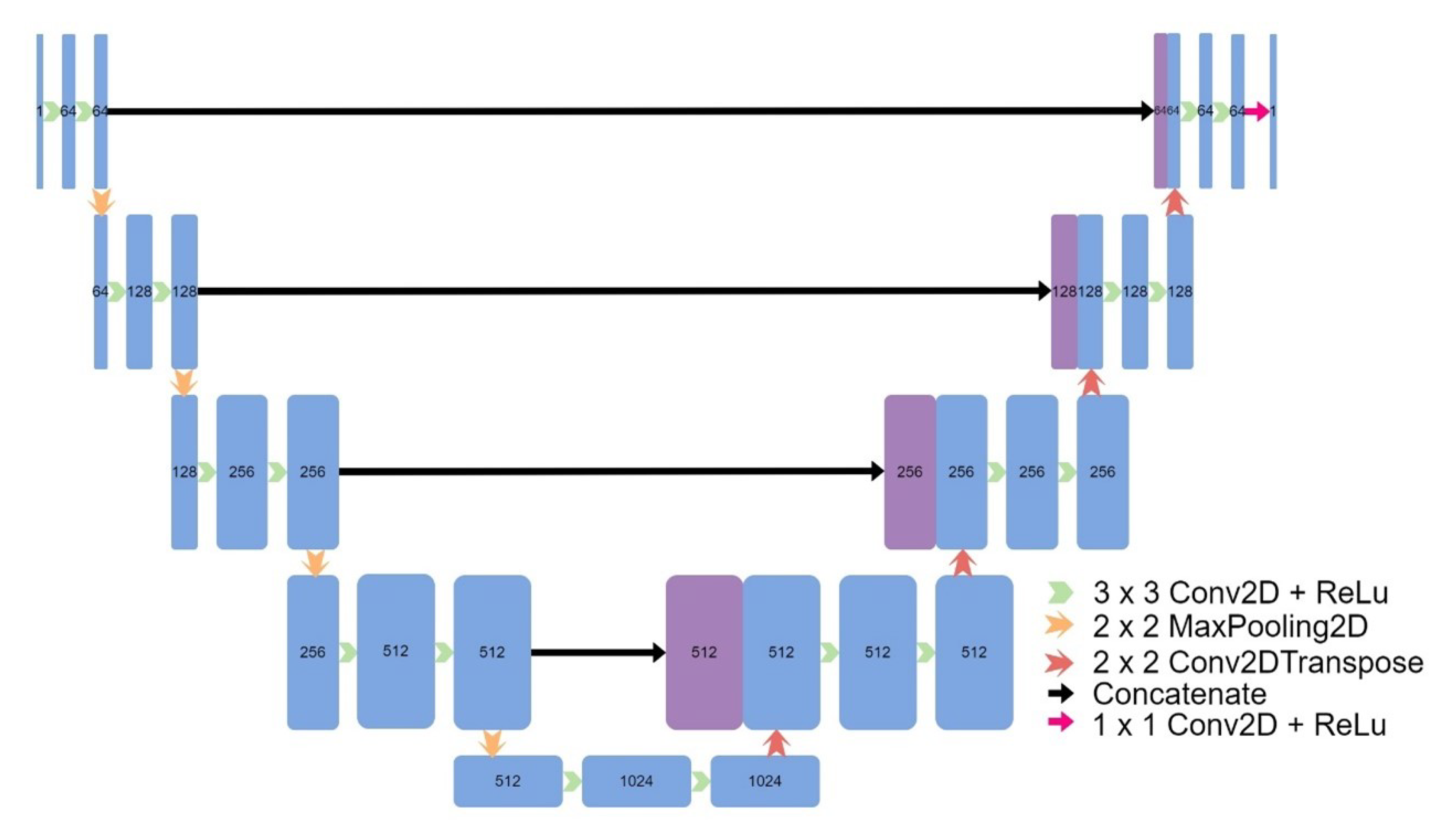
Figure 4), and a 2D UNet (
Figure 5). The other models included a UNet++ model (
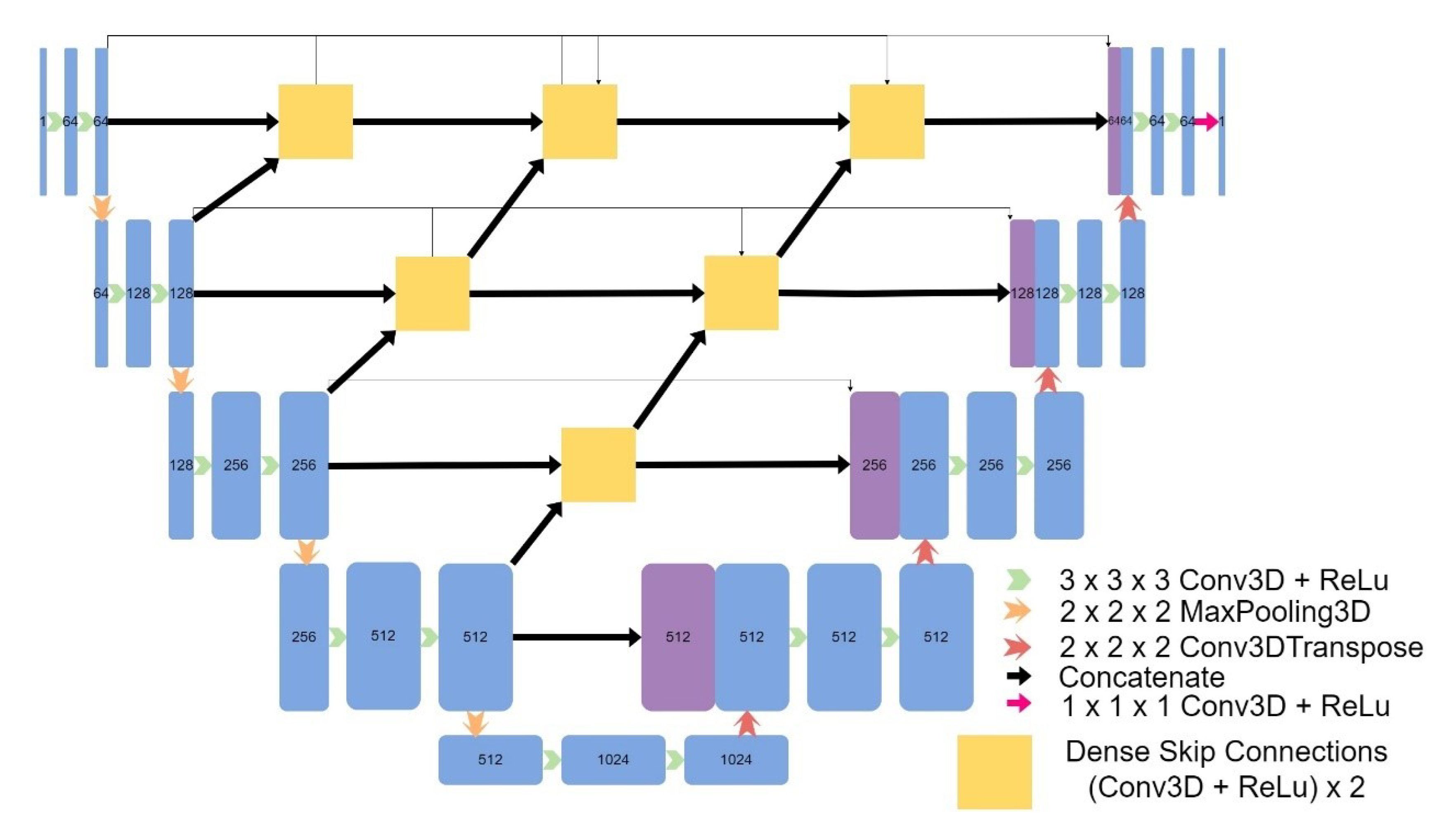
Figure 6) and an Attention UNet model (
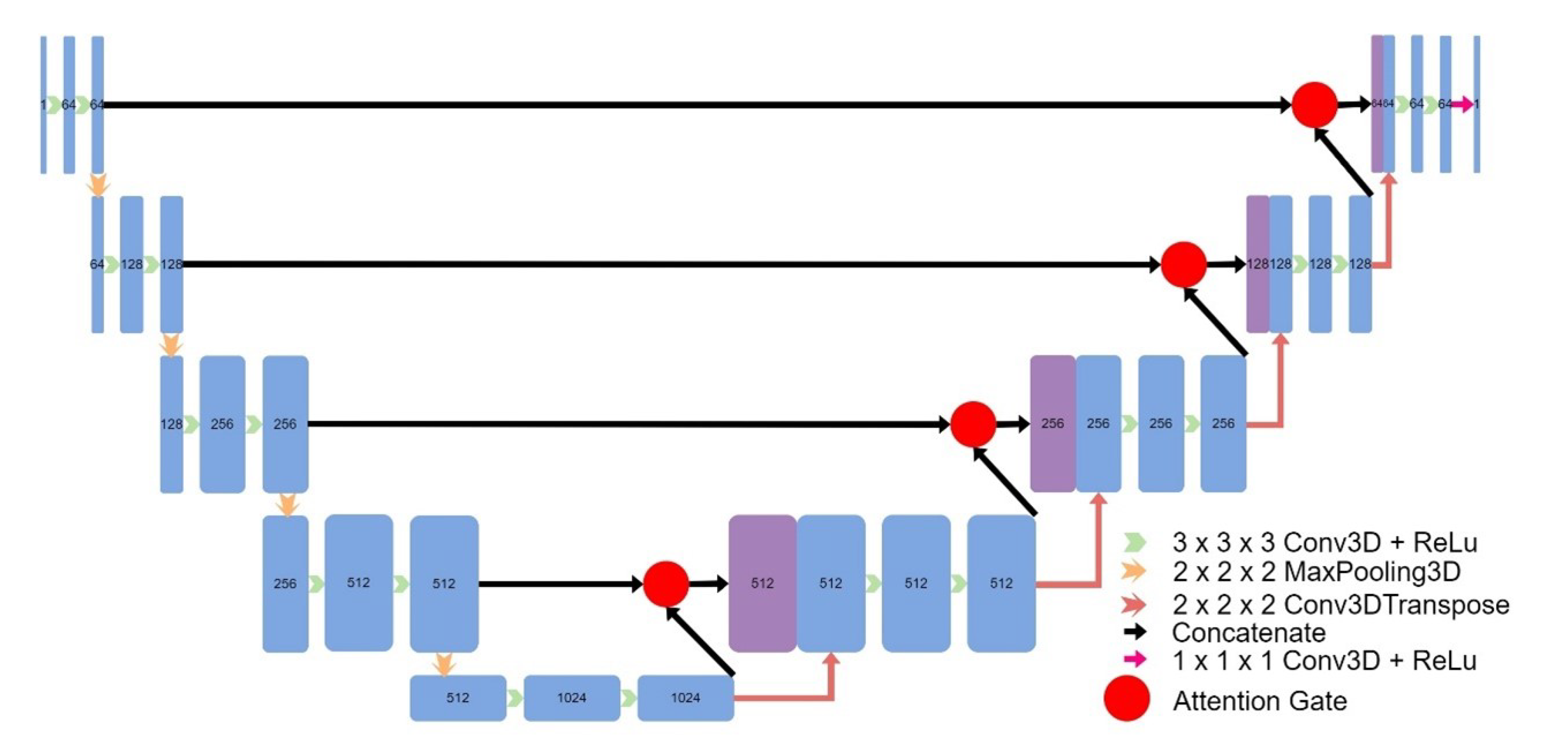
Figure 7), and an implementation of CycleGAN by David Iommi [
32]. Finally, transformers were implemented into the encoder of a UNet model to form Transformer UNet (
Figure 8). UNet++, Attention UNet, and Transformer UNet were patch-based models due to memory limitations. Code for these models is publicly available [
33].
2.3. Hyperparameters
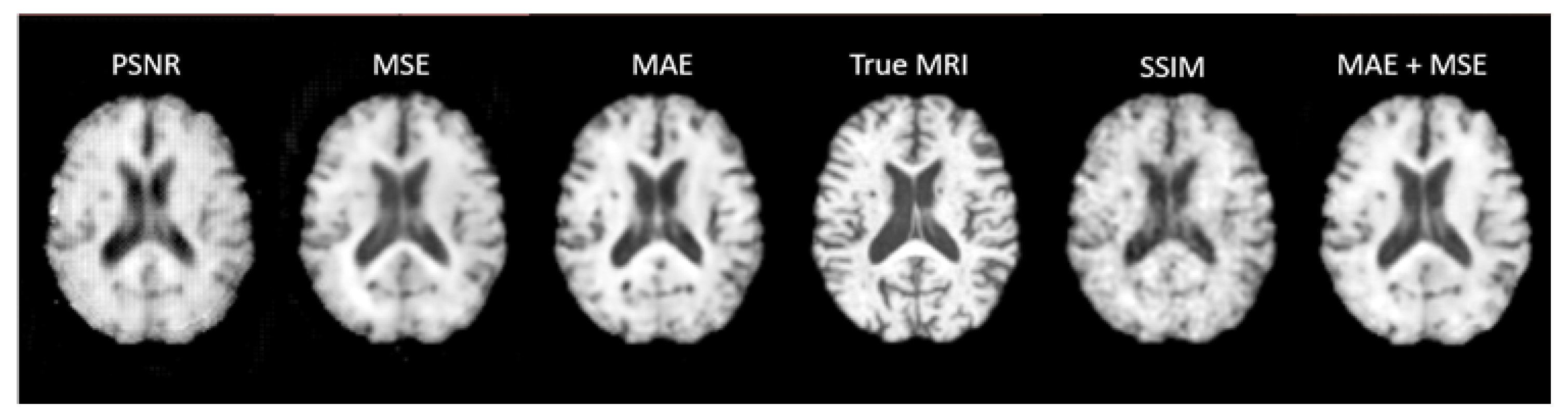
A learning rate of 5e-5 was used with an Adam optimiser in all of the UNet based models. In a preliminary implementation of UNet, five loss functions were trialled (Mean Absolute Error, Mean Squared Error, Peak Signal to Noise Ratio, Structural Similarity Index, and Mean Absolute Error + Mean Squared Error) with the model being trained through 300 epochs each time.
UNet, UNet V2, and Transformer UNet were run on one 80GB GPU with a batch size of 1. Patch Based UNet, UNet++, and Attention UNet were run on four 32GB GPUs with a batch size of 4. 2D UNet was run on four 32GB GPUs with a batch size of 16. All seven models were trained with Mean Absolute Error as the loss function.
For the CycleGAN model, a learning rate of 2e-4 was used with a patch size of 112 x 112 x 112. The model was trained through 400 epochs, however the results at 200 epochs were superior and are presented. A batch size of 6 was used and the model was trained using six 32GB GPUs.
2.4. Training and Evaluation
The dataset was split into training, validation, and test sets with 144 patients in the training set, 18 patients in the validation set, and 19 patients in the test set. CycleGAN did not use a validation set and instead had 162 patients in the training set and 19 patients in the test set.
Each model was evaluated on the data from the 19 test subjects. The model was run with CT scans as inputs and the outputs were compared to the true MRIs through quantitative metrics: PSNR, SSIM, MAE, and MSE. The results were visually compared, and the appearance of any lesions was analysed.
The quantitative metrics were calculated only over the voxels within the brain – excluding the background of the image. To calculate the SSIM just over the brain, the mean was taken of only the voxels within the brain.
To ensure that the synthetic MRIs accurately portrayed lesions, we tested if the true lesion(s) in the original MRI were present in the synthetic MRIs. In research settings, lesion segmentation is often performed manually by medical professionals, but many studies have aimed to automate this task using machine learning, however generally not for T1w MRIs at the acute stage. As obtaining a well-trained lesion segmentation model can be challenging, a pre-trained model from the ATLAS dataset [
34] was used in this study. To assess the performance of the 8 models in generating synthetic MRIs that accurately portrayed lesions, the pre-trained segmentation model was applied to the synthetic MRIs generated by each model and the original MRI scans of two patients in the test set.
One of the main advantages of MRIs over CT scans for stroke diagnosis is the higher contrast they provide between different types of tissue. To evaluate the ability of synthetic MRIs to replicate this feature, tissue segmentation of white matter, grey matter, and cerebral spinal fluid was performed using FSL FAST [
35].
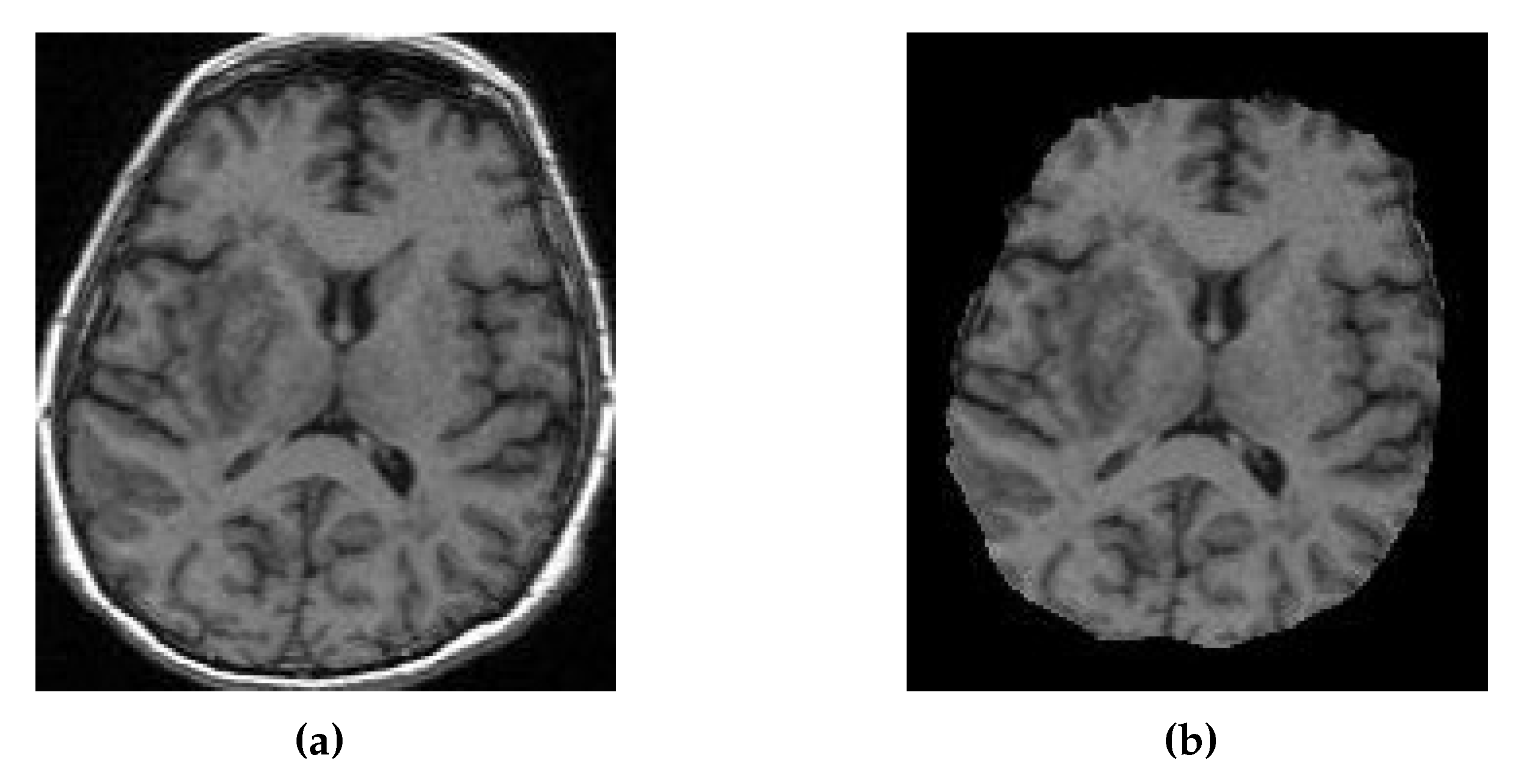
Registration of the CT, synthetic MRI, and true MRI was performed using the registration module in 3D Slicer to evaluate the usefulness of synthetic MRIs in reducing cross-modal registration to mono-modal registration. A randomly selected patient (Patient Alpha) from the training set, whose original MRI was not registered to the MNI152 atlas during the pre-processing, was chosen as the target registration space (
Figure 9). Brain extraction using the SwissSkullStripper module of 3DSlicer was performed on Patient Alpha’s MRI to prepare it for registration. Patient H’s true CT, true MRI, and synthetic MRIs from each of the eight models were individually registered to Patient Alpha’s MRI using the BRAINS Registration in 3D Slicer, with the transformation matrices and registered volumes saved. All the transforms were applied to one volume to compare registration results without being affected by differences between synthetic and true MRIs. The CT underwent all nine saved transformations, and the CT transformation matrix from the true MRI was taken to be the optimal registration that all others were compared to. Matte’s Mutual Information (MMI) was calculated using the BRAINS metrics module in 3D Slicer to compare the nine other transformations.
3. Results
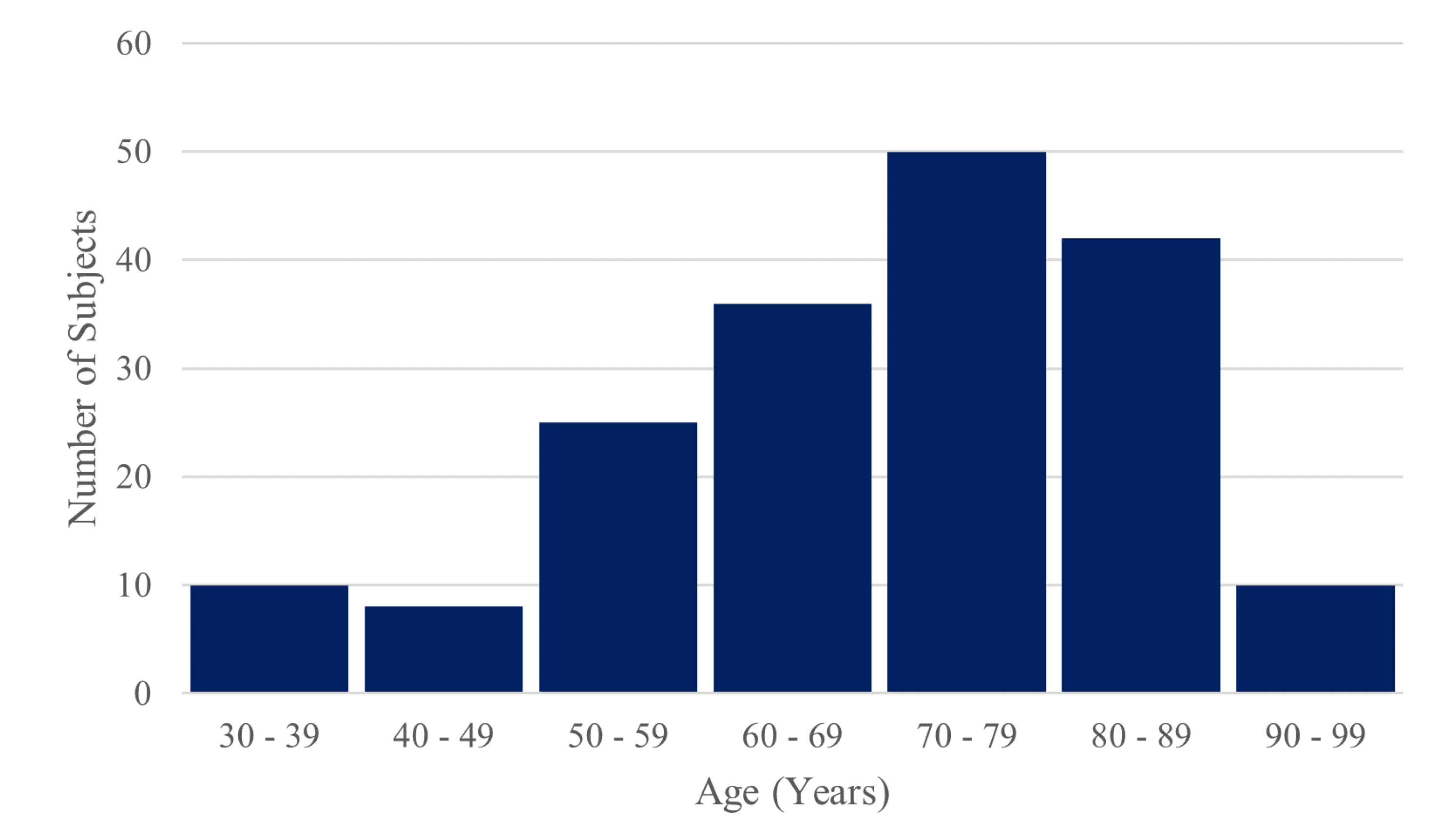
The dataset of 181 stroke patients consisted of 89 females and 92 males. The age range was 31 – 97 years with a mean of 70 years, a median of 71 years and a standard deviation of 15 years. 18 subjects suffered a haemorrhagic stroke, and 163 suffered an ischemic stroke. 99 patients had a stroke in the right hemisphere and 82 had in left hemisphere.
Table 1 gives the MRI acquisition parameters for the included studies. All MRIs were acquired with 1.0 x 1.0 x 1.0 mm resolution.
*n – Sample Size, TR – Repetition Time, TE – Echo Time, TI – Inversion Time, Flip – Flip Angle.
Figure 10.
Histogram of the ages of included subjects.
Figure 10.
Histogram of the ages of included subjects.
Preliminary training of 3D UNet concluded that a Mean Absolute Error loss yielded the best results on the validation and test sets both visually (
Figure 11) and across the other four metrics trialled as loss functions. Therefore, this was chosen as the loss function for all the UNet based models.
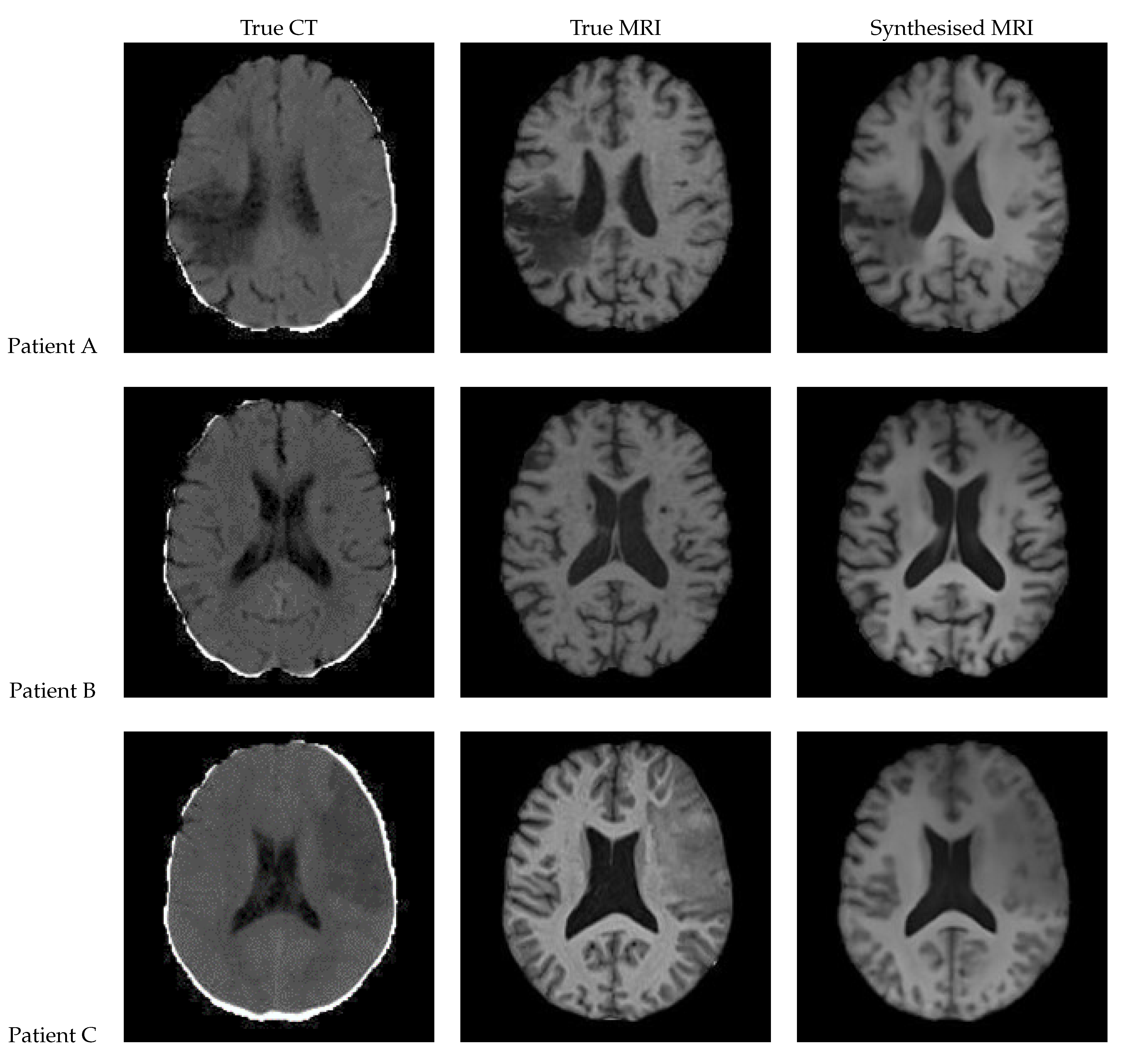
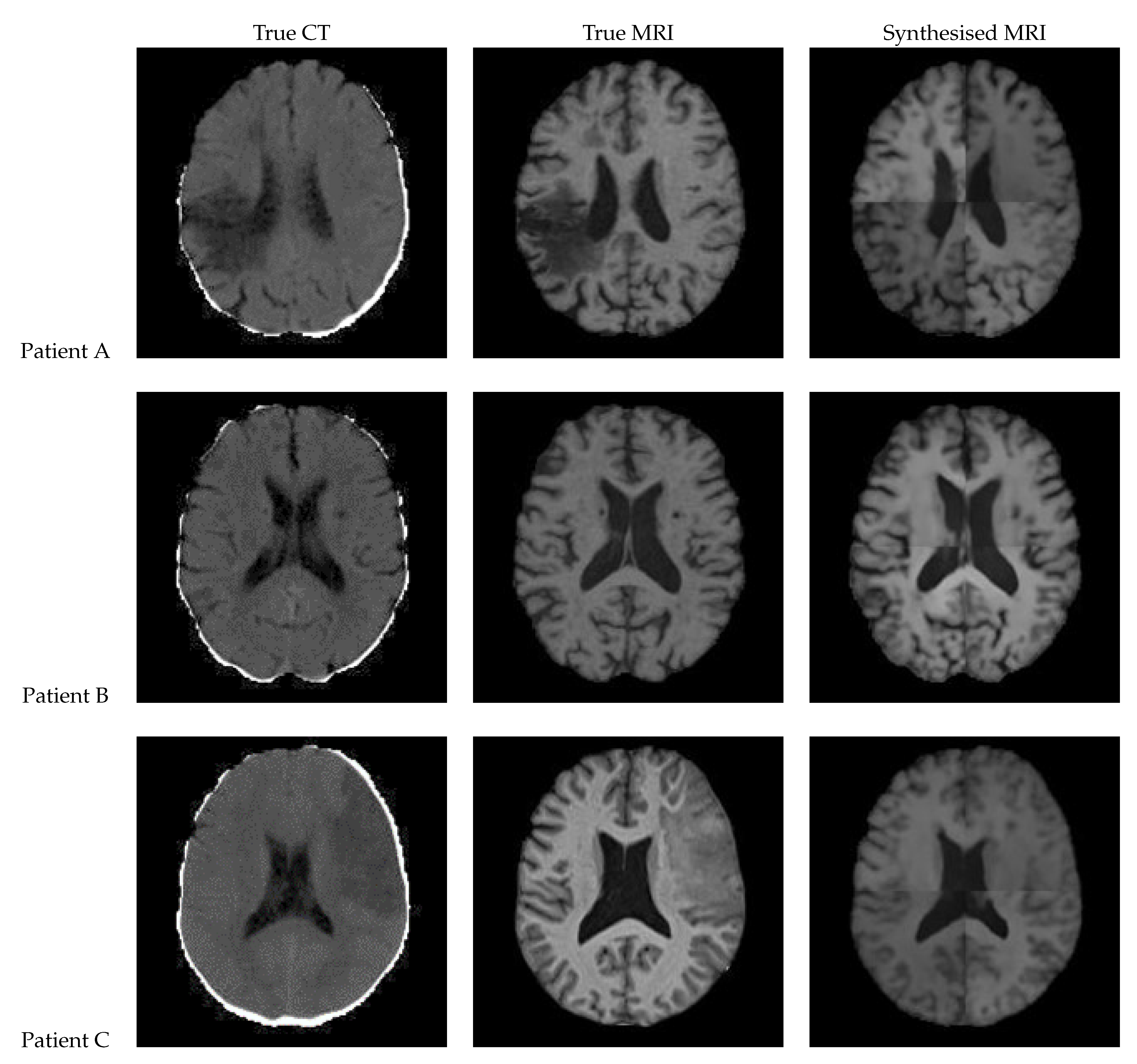
3.1. UNet
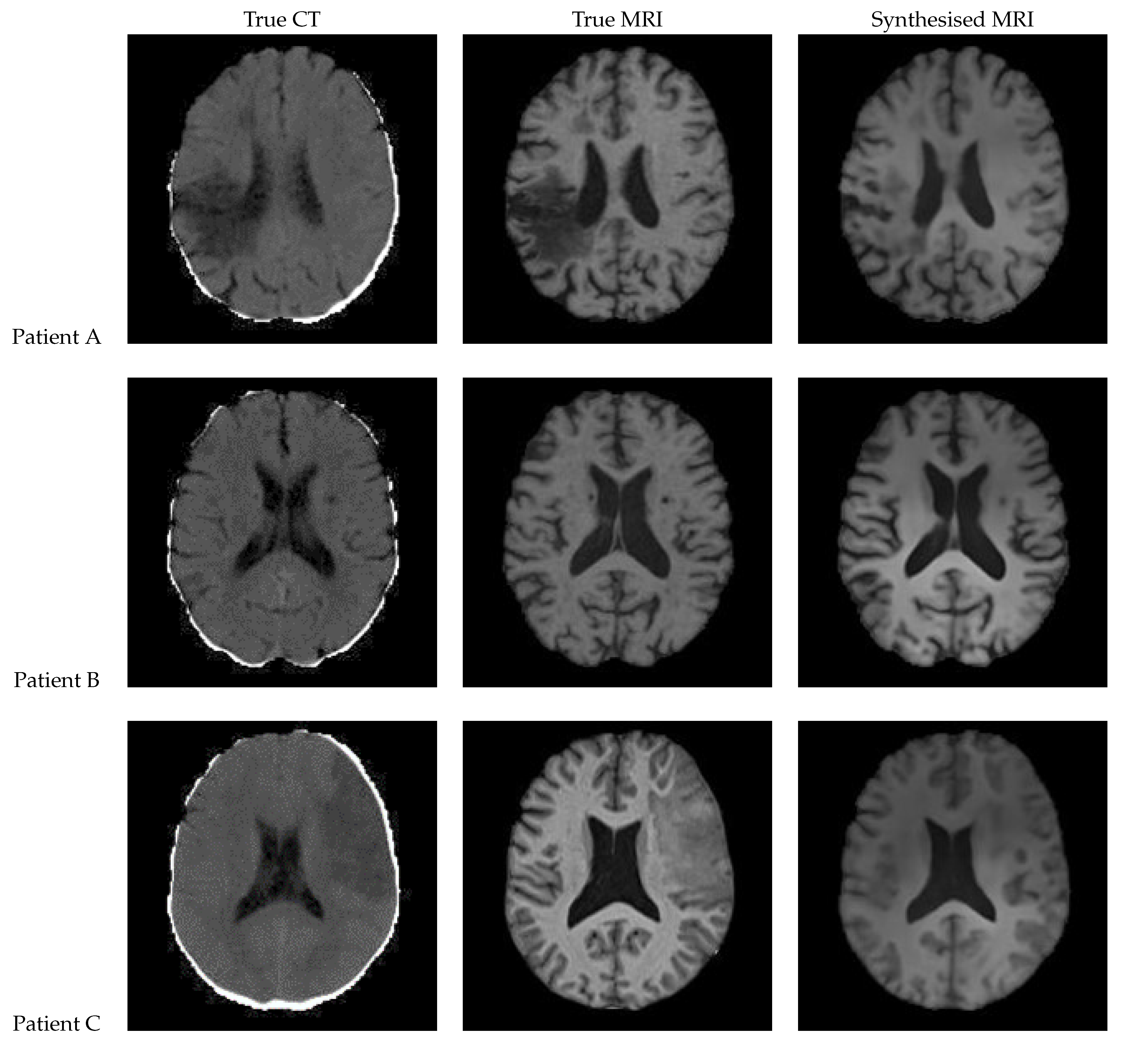
Figure 12 shows a sample slice of the synthetic MRI generated by a 3D UNet for three patients in the test set, along with the corresponding true MRI and CT slices. Patient A presents a large lesion, which can also be seen in the synthesised MRI, but with reduced contrast and less precise borders. Patient B shows a similar overall contrast to the true MRI. Patient C displays a very large, faint lesion that is not clearly visible in the synthetic MRI.
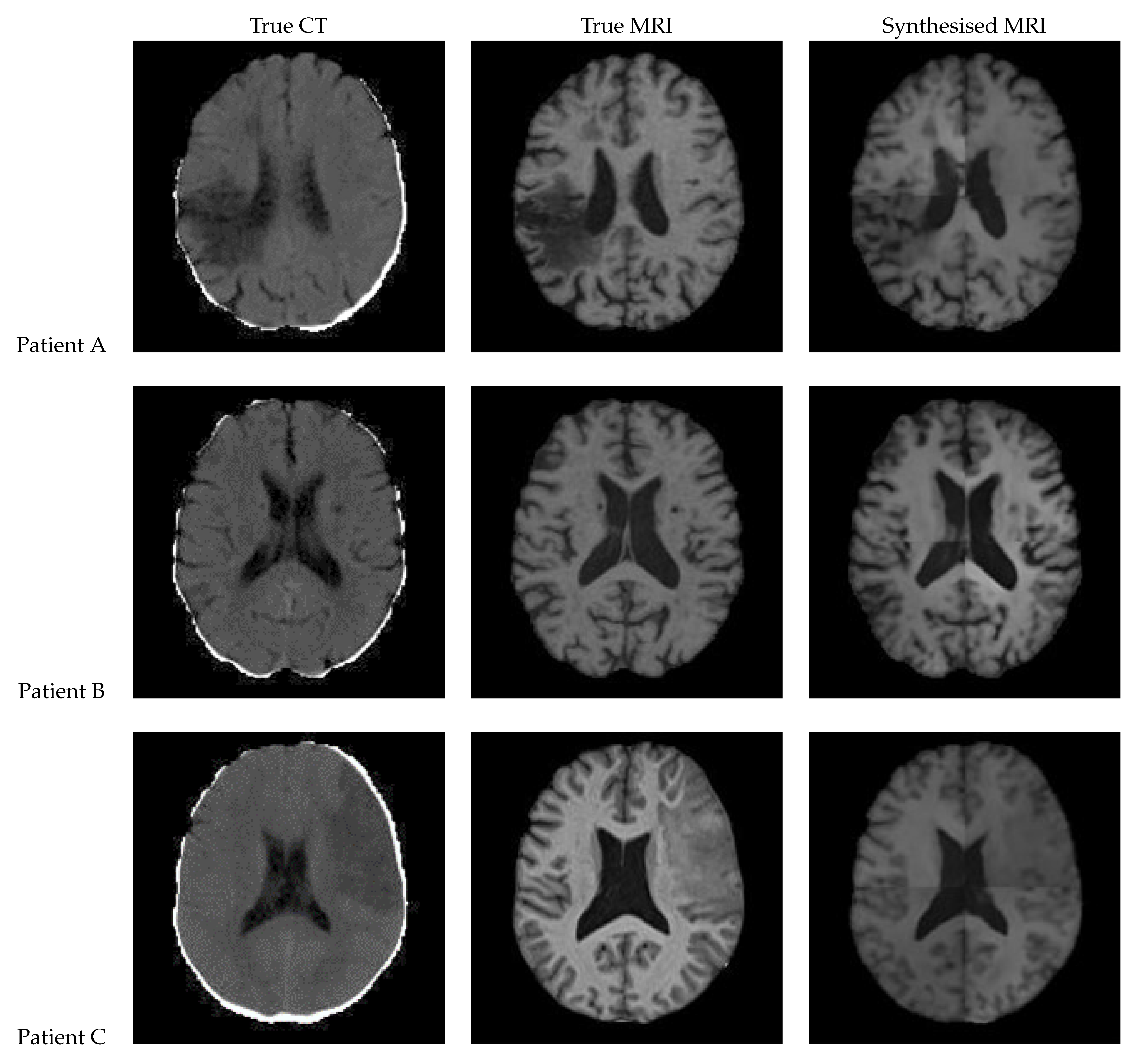
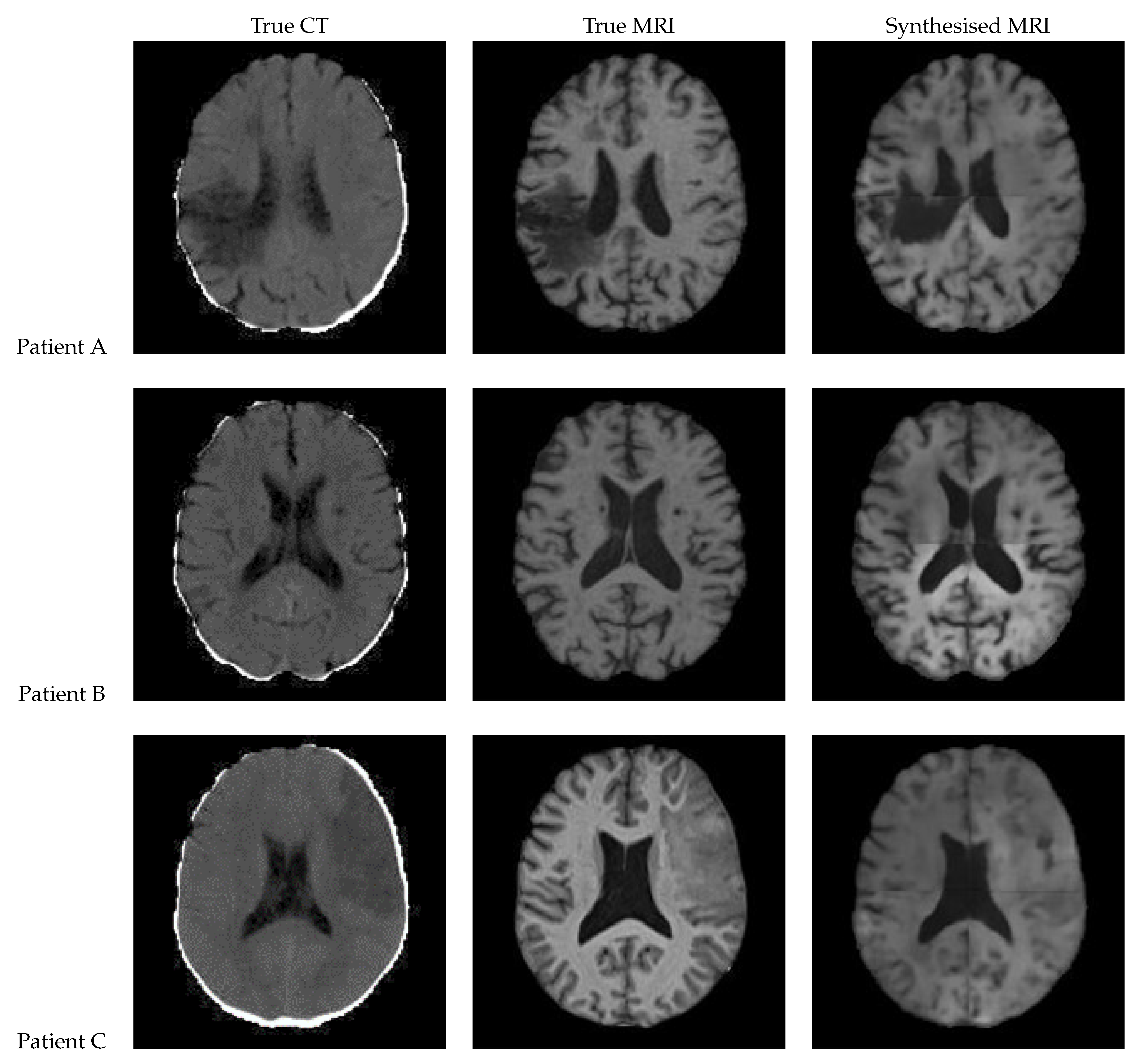
3.2. UNet V2
Figure 13 shows a slice of the synthetic MRI generated by a 3D UNet using an UpSampling layer and a Convolution layer instead of the Conv3DTranspose layer. The lesion for Patient A is clearly visible and appears similar in shape to the true MRI. The lesion in the Patient C is also discernible, albeit faint and lacking a clear outline. All three synthetic images show good contrast and likeness to the original image.
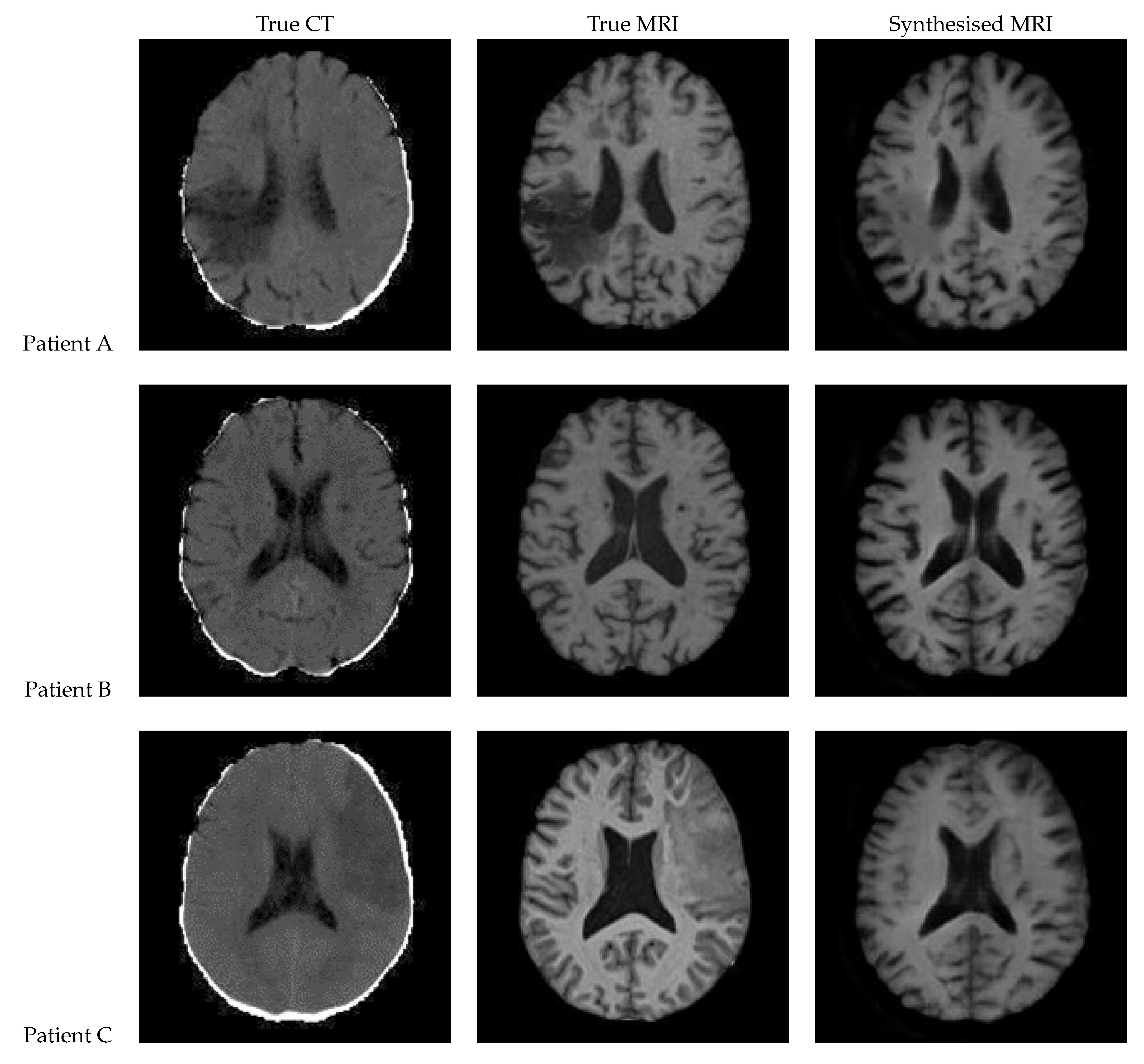
3.3. Patch Based UNet
Figure 14 presents the results of a patch-based 3D UNet. The edges of the patches are clearly visible, and the patches do align smoothly but still differ in intensity. The lesion can be clearly seen in the synthetic image for Patient A, appearing on both of the left-hand side patches. It is difficult to tell whether the lesion is present for Patient C, although the general region appears hypointense.
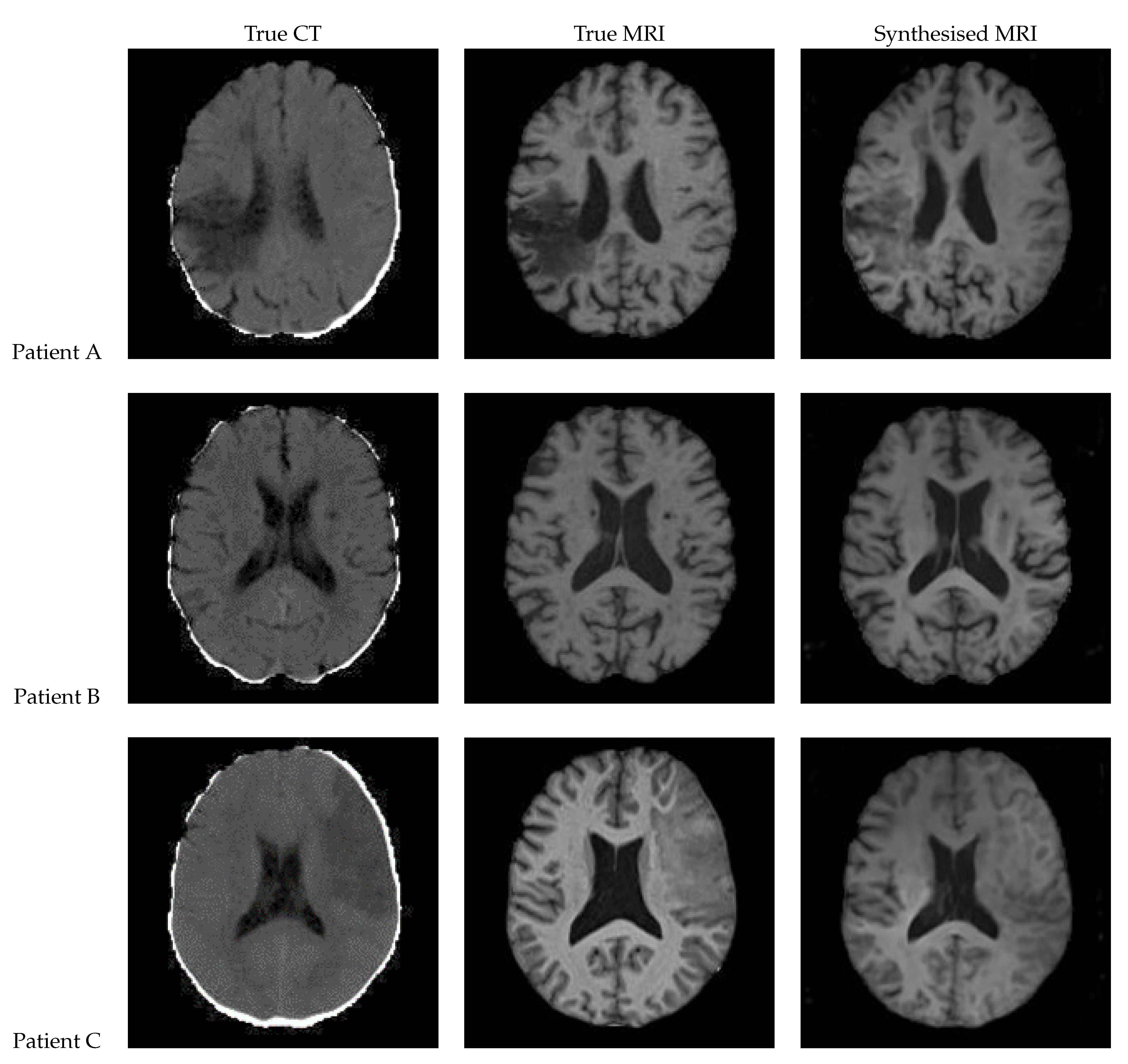
3.4. 2D UNet
Figure 15 shows the results of a 2D UNet trained on the axial slices of the dataset. All three of the synthetic MRIs show a wide range of shades and detail across the brain while maintaining suitable contrast. For Patient A, the lesion is clearly visible with an accurate boundary – however, it is quite faint, and the intensity is not consistent throughout. The synthetic MRI for Patient C does not have a clearly visible lesion, but there appears to be a boundary with similar shape and size to the true lesion.
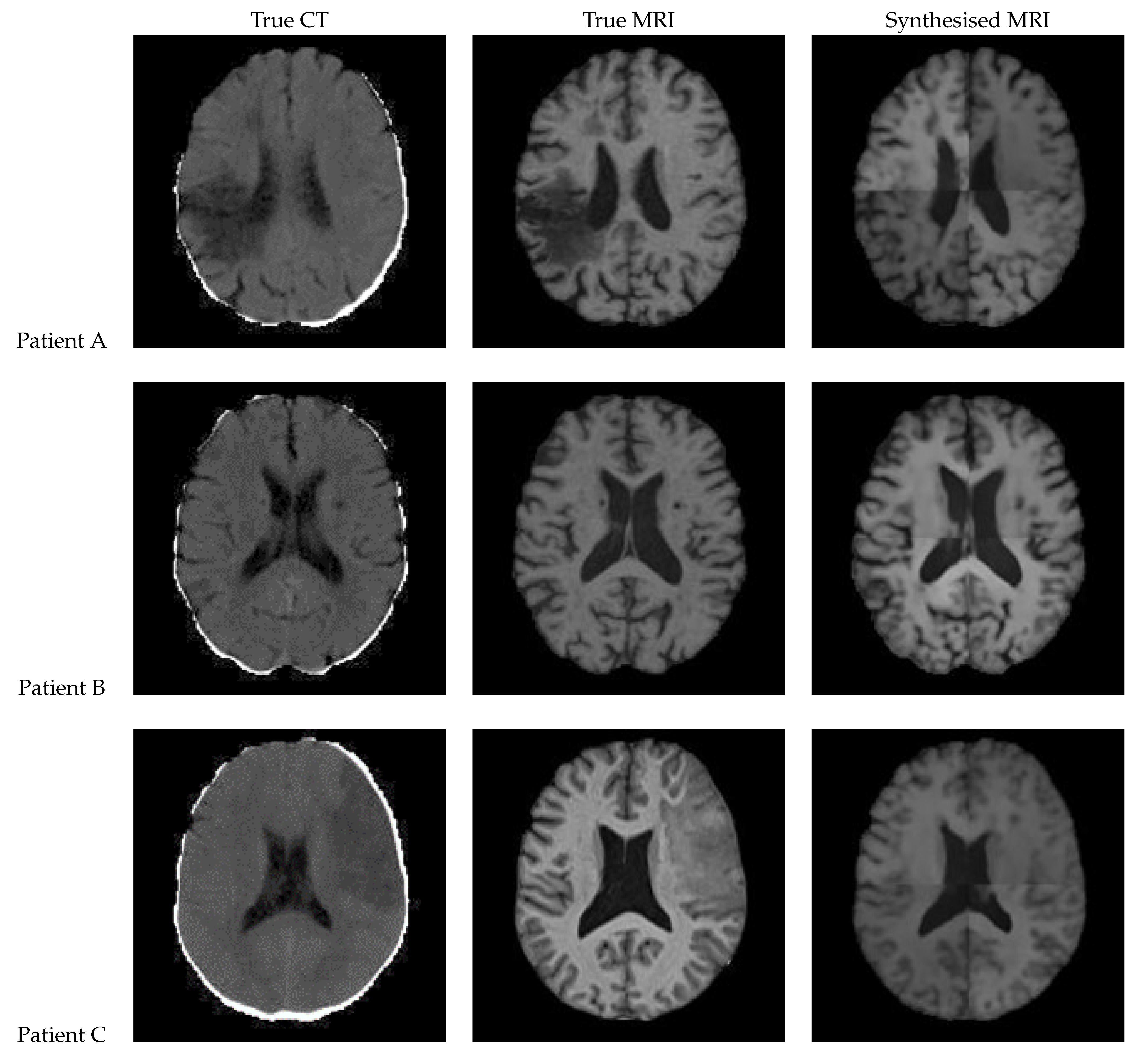
3.5. UNet++
Figure 16 shows the results from a 3D patch based UNet++ model. In the synthesised MRI for Patient A, the intensity varies between patches, making the patch edges very visible, and the presence of a lesion uncertain. On the other hand, Patient B’s synthesised MRI presents an excellent and realistic level of contrast and detailing of the grey matter structures. Patient C’s MRI lacks contrast and the lesion seems to be absent.
3.6. Attention UNet
Figure 17 shows the results of a patch based UNet with attention gates built into the skip connections. In the synthesised MRI for Patient A, the intensity again varies between the patches, making the patch edges very visible. Patient B displays good contrast however some of the grey matter structures are blurry. The lesions do not appear to be present for Patients A and C.
3.7. Transformer UNet
Figure 18 presents the results of a patch based UNet using transformer blocks in the encoder. In the synthetic MRI for Patient A, the lesion appears extremely dark with a sharp, but inaccurate boundary. Patient B’s synthetic MRI displays good contrast and accurately captures many regions of grey matter. In the synthetic MRI for Patient C, the lesion is not clearly visible.
3.8. CycleGAN
Figure 19 shows the results of 3D CycleGAN [
32] run with a patch size of 112 x 112 x 112. The images display very good contrast; however, the shape of the sulci is not always accurate. The lesion for Patient A is faint and there is no lesion visible in Patient C. The images also show a checkerboard pattern.
3.9. Qualitative Assessment
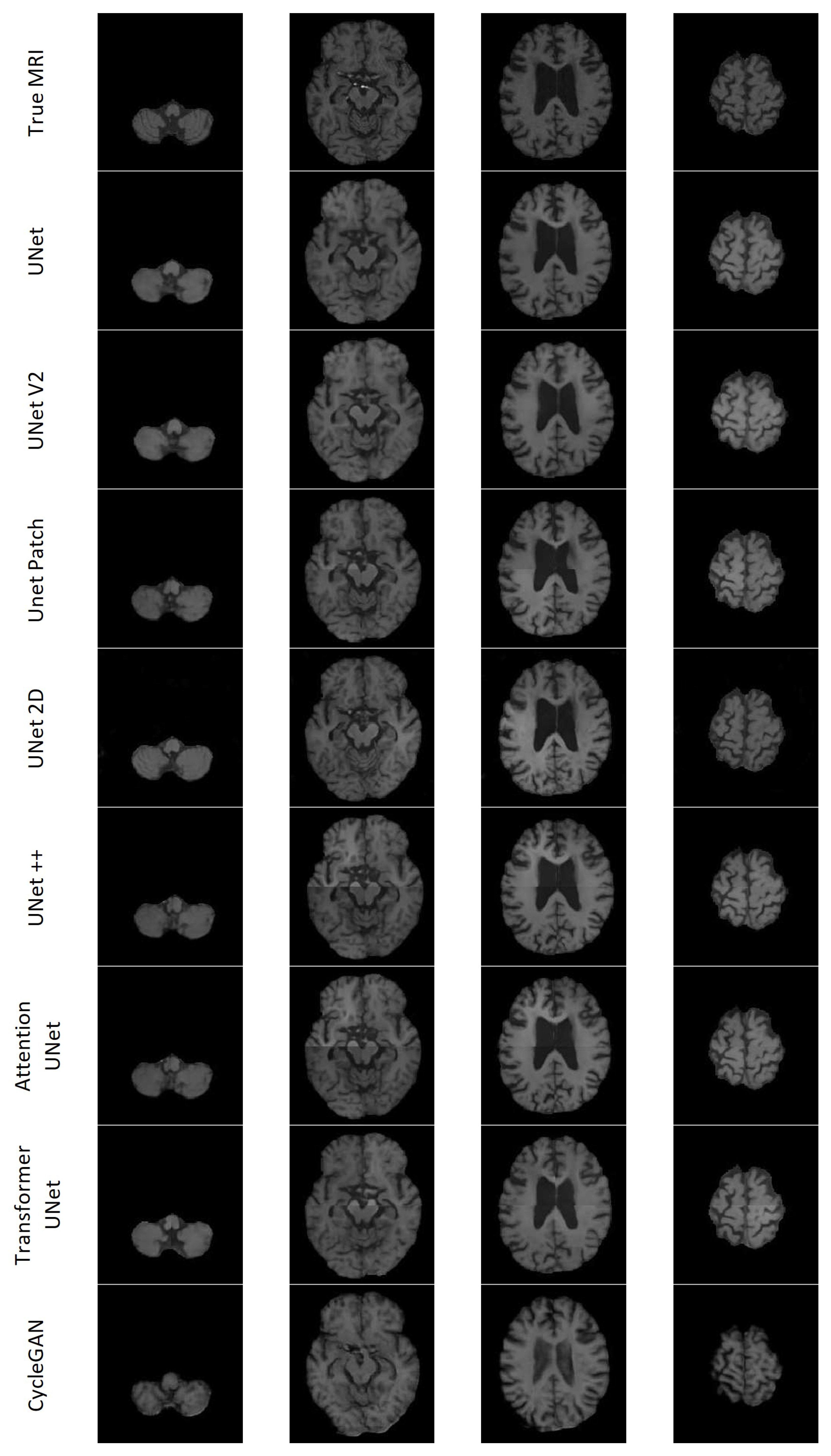
All the models except UNet++ and Attention UNet clearly depict the lesion for Patient A; however, most of the lesions in the synthesised MRIs lacked contrast. CycleGAN appeared to have the least contrast between the lesion and surrounding area. Transformer UNet exhibited considerably higher contrast for the lesion; however, it failed to accurately capture the lesion’s boundary. 2D UNet gave rise to a mottled effect over the lesion area. Patient C presented with a notably large lesion; however, only UNet V2 and 2D UNet managed to retain any indication of the lesion. Nevertheless, the presence of the lesion remains subject to debate in both cases. The models encountered difficulties in accurately reproducing other features of Patient C’s dataset. Additionally, with the exception of CycleGAN, they lacked the necessary contrast between the various types of brain tissue. The inability to generate sufficient contrast was observed across multiple synthetic MRIs, particularly in cases where the true MRI exhibited lower levels of contrast. CycleGAN demonstrated good contrast across all synthetic MRIs; however, it often failed to replicate the brain structures found on the true MRI. Specifically, CycleGAN seemed to have learned the presence of sulci along the brain’s edge, but generated them in a semi-random manner rather than reproducing the patterns observed in the CT. The 2D UNet model exhibited notable accuracy in modelling gyri and sulci in the synthesised MRI, displaying good contrast. On the other hand, the patch-based models faced challenges in accurately predicting the edges of the patches, resulting in misalignment issues.
Figure 20 shows seven axial slices of the true MRI and the synthetic MRIs from each of the eight models for Patient D.
3.10. Quantitative Assessment
Table 2 shows the four common image evaluation metrics (MAE, MSE, PSNR, SSIM) calculated between the synthetic MRIs and true MRIs averaged over the 19 patients in the test set. Only the voxels in the brain were used to compute these metrics, due to the complexities of calculating SSIM over only the brain voxels. Total SSIM (calculated over the entire volume) is also listed. UNet achieved the best scores across all metrics, while CycleGAN achieved the worst scores across all metrics.
3.11. Performance at Clinically Relevant Tasks
3.11.1. Lesion Segmentation
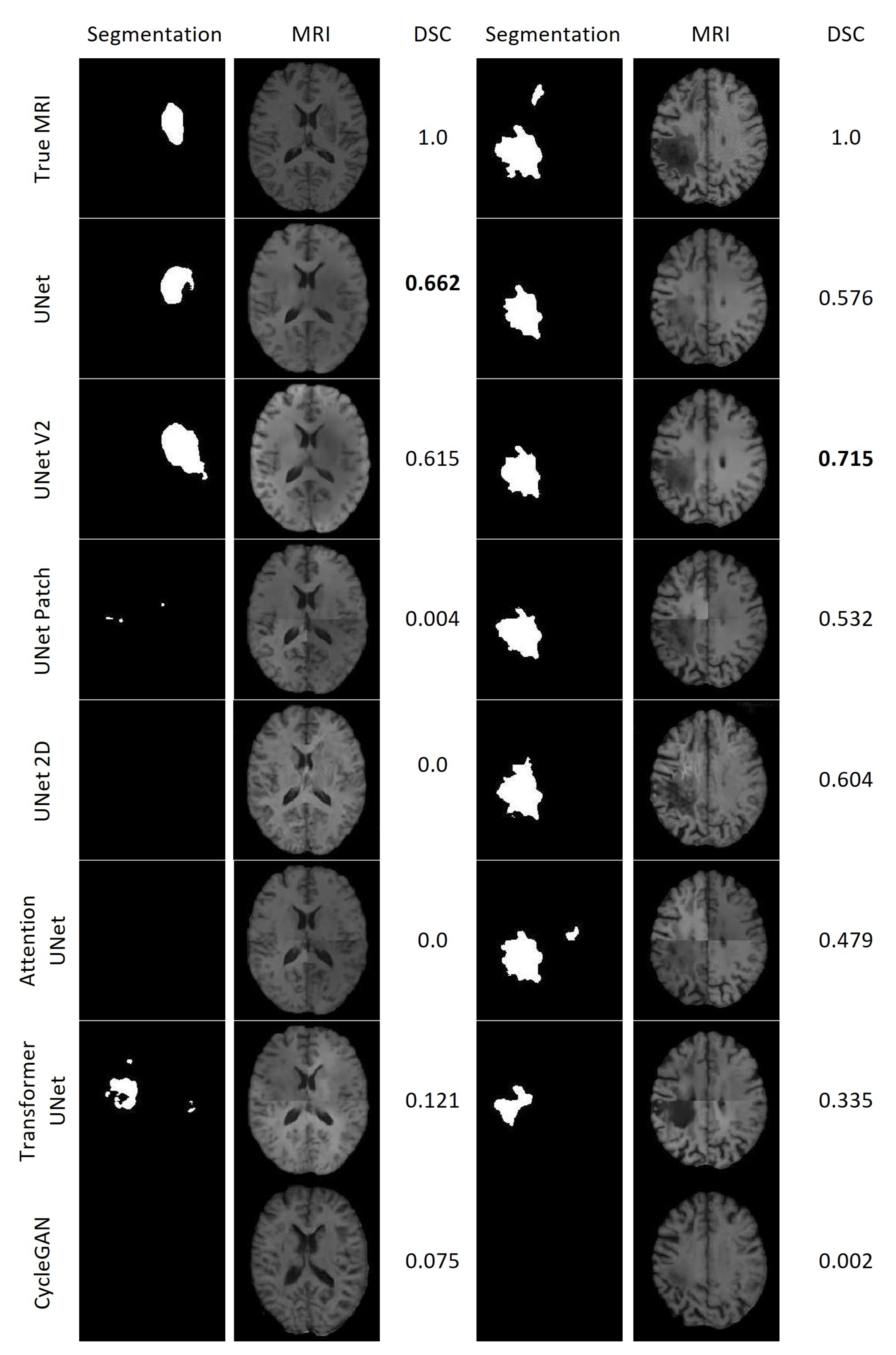
Figure 21 displays the results of the lesion segmentation algorithm applied to the synthetic and true MRIs of two patients within the test set. Patient E exhibited a smaller lesion that lacked significant contrast in any of the synthetic MRIs. Among the synthetic MRIs, only those generated by UNet and UNet V2 produced lesion segmentations resembling the original MRI for Patient E, with Dice Scores (DSC) of 0.662 and 0.615, respectively. The next best performing model was Transformer UNet, achieving a DSC of 0.121. For Patient A, all synthetic MRIs, except those generated by CycleGAN, yielded lesion segmentations resembling the true MRI. The most accurate lesion segmentation for Patient E was obtained from the UNet V2 synthetic MRI, with a DSC of 0.715.
3.11.2. Brain Tissue Segmentation
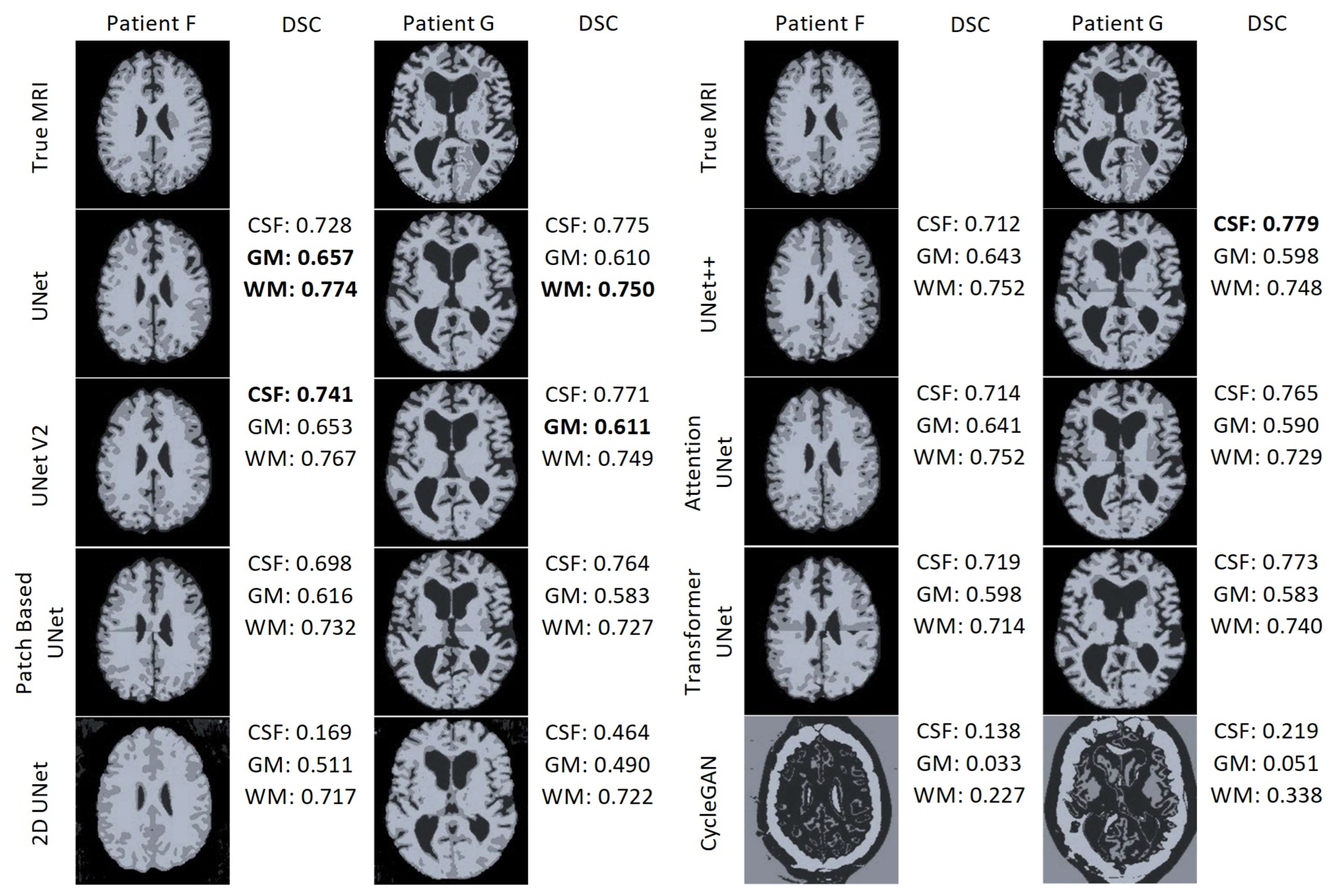
Figure 22 displays the results of the FAST algorithm applied to each MRI synthesis model and the true MRI for two patients. FAST did not perform well when applied to the CycleGAN results. Interestingly, the output classified most voxels as cerebrospinal fluid (CSF) for both patients, despite and unknown reason for this behaviour . Furthermore, in the patch-based models, it is evident that the FAST algorithm struggles to classify the regions around the edges of the patches. In the case of the 2D UNet synthetic MRI, the FAST model fails to identify CSF around the edge of the brain and instead misclassifies some areas outside the brain as CSF. Overall, UNet and UNet V2 demonstrate the best performance. UNet achieved the highest DSC for the GM and WM for Patient F, as well as the best WM DSC for Patient G. On the other hand, UNet V2 achieved the best WM DSC for Patient F, and the best GM DSC for patient G. UNet++ attained the highest DSC CSF score for Patient G.
3.11.3. Registration
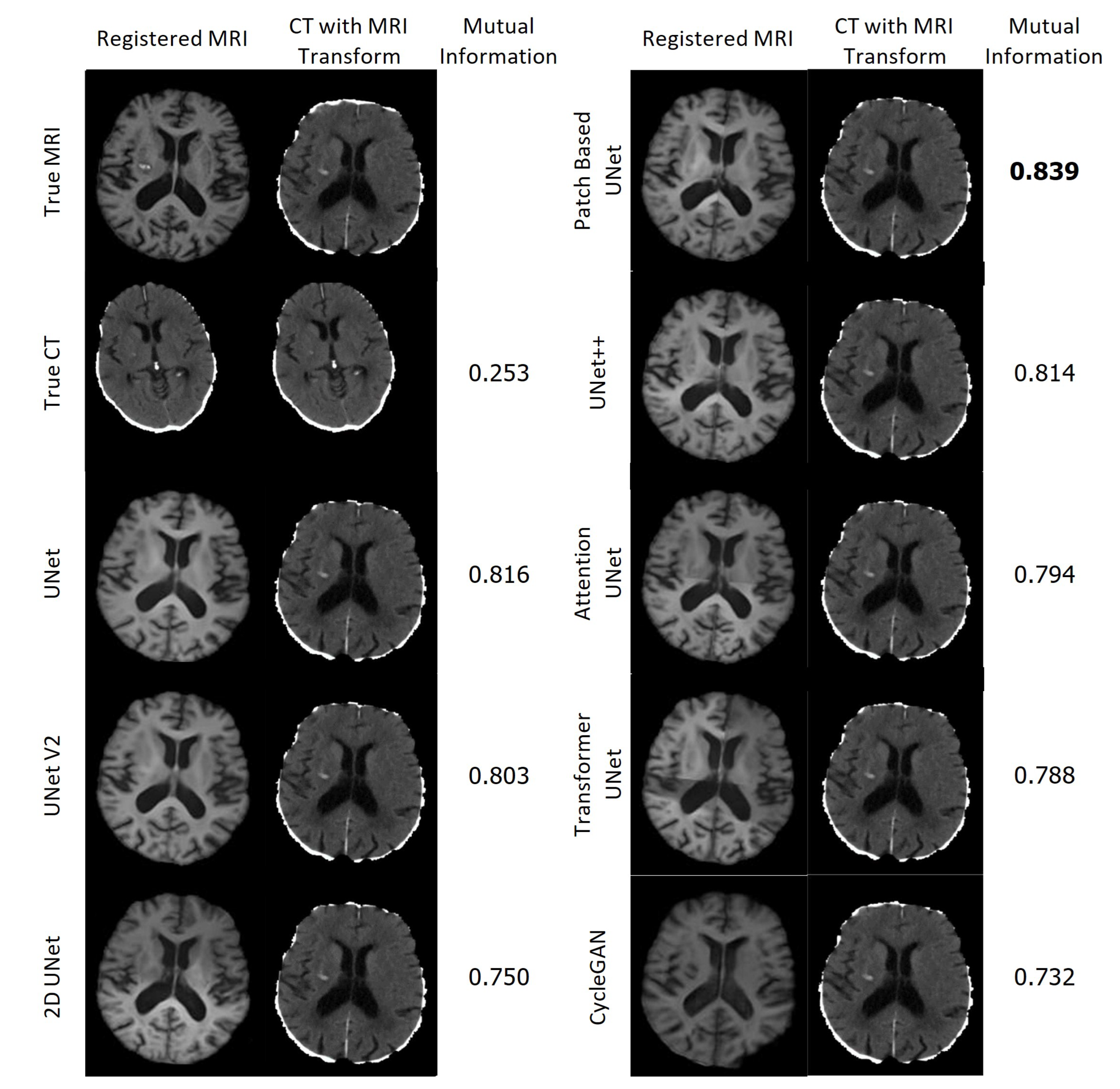
Figure 23 displays the resulting registrations for each MRI and the corresponding CT in the first column, along with the CT that underwent the associated transformation in the second column. The Mutual Information between the presented CT and the CT which underwent the True MRI transformation is provided for each case. Remarkably, all of the synthetic MRIs achieve a high degree of accuracy in aligning with the target MRI. In contrast, the CT scans were not aligned accurately and required significantly more time for calculating the transformation.
4. Discussion
This study evaluated the synthetic MRIs generated from CT scans using eight different models. The synthetic MRIs, along with their lesion segmentation, tissue segmentation, and registration outputs, were assessed and compared using various metrics. Across all metrics, UNet consistently outperformed the other models, while CycleGAN performed the poorest. Visually, the results from CycleGAN appeared blurry with a noticeable checkerboard effect, and the presence of lesions were barely discernible, if present at all. The registration process of the CT scans was notably less successful, as it required a considerable amount of time and resulted in incorrect alignment. However, the findings demonstrate that synthetic MRIs generated through the methods employed in this study can be used to guide the cross-modal registration of CT to MRI scans.
Overall, the generation of synthetic MRIs from CT scans using the methods described in this paper produces realistic MRIs that can aid in registering CT scans to an MRI atlas. The synthetic MRIs enable the segmentation of white matter, gray matter, and CSF using algorithms designed for MRIs, exhibiting a high degree of similarity to true MRIs. UNet and UNet V2 consistently demonstrated superior performance across all tasks, surpassing the 2D and patch-based UNet implementations.
4.1. Different Architectures
CNNs require paired datasets to perform regression tasks, including image synthesis. One attractive feature of GANs is their ability to produce high quality images even when trained on small unpaired datasets. This is attributed to the unique architecture of GANs, which generally uses a CNN as the generator, which indirectly learns through the discriminator. The discriminator enforces close matching between the generated output and the data distribution of the training data, resulting in high detail and contrast in the generated images, and visual similarity between the generated and target images.
One issue with GANs is that they may overlook important relationships between the input and target, in an individual case basis. The impact of distribution statistics on the output is significant, leading GANs to potentially include or exclude important structures, such as lesions, which may be present at the individual level but are not adequately reflected in the groupwise distribution. This is discussed at length in Cohen et al. [
36].
GANs excel in cases where there is not a single correct answer – such as text-to-image generation or translating photographs into different art styles. However, in scenarios like MRI generation from a CT scan, where the focus lies not on the overall visual appearance but on contrast and the presence of specific structures, it becomes imperative to retain the necessary structures in the generated image.
Paired GANs, such as Pix2Pix, attempt to address these issues by incorportating terms that compare the generated image to the true target. However, these are still susceptible to the aforementioned challenges due to the tendency of GANs to fit to the distribution of the training data. Training GANs are notoriously difficult, which could partly explain why CycleGAN performed poorly in this study and did not achieve a similar level of performance as observed in a previous similar study [
22].
For the task of CT to MRI synthesis for stroke patients, accurately representing the lesion(s) and surrounding structures is more important than image quality and fidelity, making CNNs potentially more suitable. However, CNNs tend to exhibit worse image quality than GANs due to the absence of a discriminator. Nevertheless, CNNs are easier to train and do not suffer from the issues outlined above. The limited use of CNNs in the literature is surprising, and this study demonstrates the value of exploring this approach further.
4.2. Limitations
The main limitation encountered during the development and implementation of the models was memory issues. The MNI152 atlas used in pre-processing had dimensions of 181 x 217 x 181 voxels. To meet the requirements of the UNet model, the pre-processed dataset had to be appropriately cropped and padded to ensure each dimension was a multiple of 16. Inputting these to the 5-layer 3D UNet model with a batch size of 1 exceeded the memory limit of the 32GB GPUs. One potential solution was downsampling the data, but this resulted in a loss of information and introduced checkerboard artifacts during model training. The downsampling process disrupted the data distribution of the training data, ultimately leading to lower quality and contrast in the generated images. Another workaround involved cropping the background of the images as much as possible. By reducing the size to 176 x 192 x 176, the 3D UNet model could run with a batch size of 1 on the 80GB GPUs, yielding better results compared to using downsampled images. However, the architectures of UNet++ and Attention UNet had more parameters than UNet, which still caused memory errors, even with the cropped images on the 80GB GPUs.
To address the issue of large images, two options were considered. The first option involved using a 2D UNet model on 2D slices of the data. While this allowed for higher resolution images to fit on the GPU and be input into the model, it introduced a potential bias in the output in the slice direction since the network does not consider spatial relationships in that direction. The second solution was to feed smaller patched of the original data by using a patch-based model, enabling the use of 3D models. The patchify library was used in this study to create non-overlapping patches, resulting in clearly defined patch edges in the synthetic MRIs. However, using overlapping patches and averaging the overlapping areas would produce smoother final images and may help the model capture brain structures more accurately.
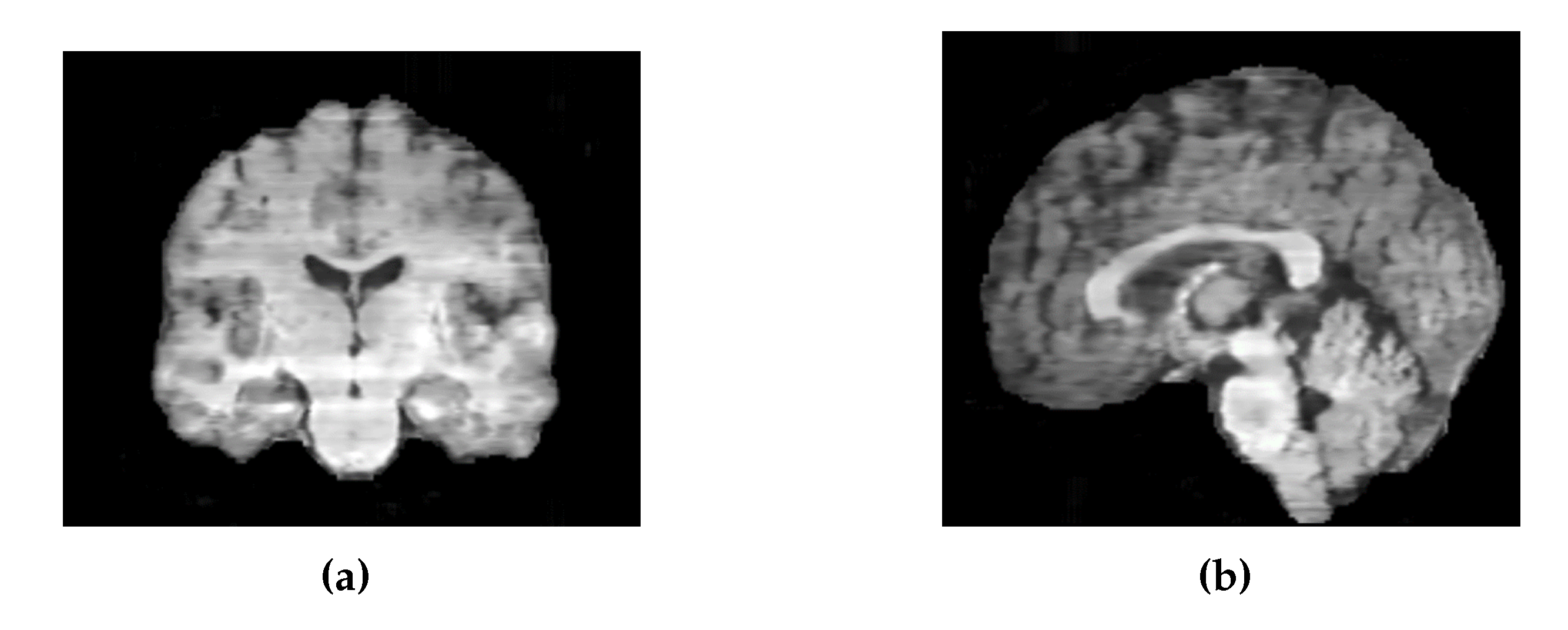
Similarly, the images generated by the 2D UNet model exhibited intensity variations between slices along the sagittal and coronal axes (
Figure 24). To address this, it would be preferable to train the model on axial, coronal, and sagittal slices, and then average the results across all three dimensions.
Another limitation of the study was the evaluation through clinically relevant tasks, which were only performed on one or two of the patients in the test set. To obtain a more reliable comparison of the performance of the synthetic medical images, it would be beneficial to use synthetic MRIs from a larger number of patients in the test set. Furthermore, the presence of errors in the lesion segmentation used on the true MRI may have resulted in errors in the synthetic MRI lesion segmentations, further emphasising the importance of accurately comparing them to the true lesion segmentations.
4.3. Input Data Quality
Small misalignments between the MRI and CT could potentially contribute to blurriness and inaccuracies in the synthetic MRIs. A previous study [
22] attempted to address this issue by implementing a perceptual loss using the VGG network. However, it was found that this approach did not have a positive impact on model performance. Moreover, employing the perceptual loss requires significant computational power as UNet results need to be fed through a second network to calculate the loss before adjusting the network again. Alternatively, investing more time and effort into the pre-processing pipeline may be a more effective approach to improving image clarity.
The UNet model exhibited extreme sensitivity to the input data used. Depending on the order and nature of the pre-processing steps, the. Model frequently got stuck in the first epoch, with the loss and other metrics remaining unchanged throughout the training process. In such cases, the model would often predict completely black volumes for every CT scan. Furthermore, the inclusion or exclusion of normalisation and regularisation layers had a significant impact on the stability of the model. When batch normalisation layers were included, a problem arose where the background was predicted as grey, resulting in significantly higher loss during the testing phase, even when evaluated on the training data. This discrepancy occurred because batch normalisation operates differently during training and testing phases. Due to the encountered issues and small batch sizes used, batch normalisation was not used in the final models.
4.4. Metrics
During the adaptation of the different models, especially during the implementation of the base UNet model, it was observed that accuracy metrics did not effectively represent the performance of the model. The model could produce significantly different image outputs, even when exhibiting similar accuracy metrics on the test and validation set. This observation was also noted by Kalantar et al. [
22], where they concluded that their best performing model did not have the highest scores on commonly used quantitative metrics. Furthermore, there are no currently established benchmarks for quantifying the accuracy of synthesised MRIs. The commonly used quantitative metrics are strongly influenced by the background of the image, which spuriously inflates accuracy when calculated over the entire synthesised image. One possible solution is to extract the brain region and calculate the metrics only for the voxels within the brain. Without employing such an approach, it becomes challenging to compare accuracies between different studies and datasets.
As the SSIM employs a sliding window of 11 x 11 x 11 voxels, the voxels up to 11 voxels away from the perimeter of the brain contribute some information from the both the brain and background. It could be argued that including these voxels in the average might provide a more accurate representation, but it also introduces background information into the SSIM calculation. A previous study also calculated SSIM over a specific region of interest [
22], but did not report the methodology used for their calculation, making it difficult to draw direct comparisons with the results.
4.5. Other Datasets
To enhance the diversity of the training dataset, it would be helpful to include a larger amount of patient data, especially from patients with stroke mimics and healthy individuals. When developing a model for use in clinical settings, it is important to train it on a diverse range of inputs, rather than solely relying on data from patients who were ultimately diagnosed with strokes. This becomes particularly important when training GANs since they aim to match the distribution of the training data. However, even though CycleGAN was trained exclusively on a dataset of stroke patients, it did not perform well at translating lesions into the synthetic MRIs it generated.
4.6. Further Research
Introducing a term in the loss function that penalises gradients of intensities could address the lack of clarity in the synthesised images. Such a term would reward sharp intensity changes (boundary lines) or regions with similar intensity, and promote increased contrast. This approach could prove particularly helpful in making the outlines of lesions and other brain structures more distinct. Furthermore, an appealing direction for further research could be to incorporate a lesion segmentation model into the loss function that would encourage the model to accurately model the lesion with improved accuracy and contrast in the synthetic MRI.
Further investigation into the benchmarks that synthetic MRIs should aim to achieve before considering the integration of CT-to-MRI synthesis into the clinical workflow for stroke diagnosis and treatment is highly recommended. This area presents several open-ended questions, such as what the optimal methods are for assessing the accuracy of MRI generation models and establishing appropriate benchmarks for evaluation.
Author Contributions
Conceptualization, A.W.; methodology, J.M. and A.W.; validation, J.M.; formal analysis, J.M.; investigation, J.M.; resources, B.C.; data curation, B.C.; writing—original draft preparation, J.M.; writing—review and editing, S.H., B.C., V.S., J.F.; supervision, A.W.; funding acquisition, A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the Health Research Council of New Zealand grant number 21/144.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| CNN |
Convolutional Neural Network |
| CSF |
Cerebrospinal Fluid |
| CT |
Computed Tomography |
| DSC |
Dice Score |
| GAN |
Generative Adversarial Network |
| GM |
Gray Matter |
| MAE |
Mean Absolute Error |
| MRI |
Magnetic Resonance Imaging |
| MSE |
Mean Squared Error |
| PSNR |
Peak Signal to Noise Ratio |
| SSIM |
Structural Similarity Index Measure |
| WM |
White Matter |
References
- Chalela, J.A.; Kidwell, C.S.; Nentwich, L.M.; Luby, M.; Butman, J.A.; Demchuk, A.M.; Hill, M.D.; Patronas, N.; Latour, L.; Warach, S. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. The Lancet 2007, 369, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Moreau, F.; Asdaghi, N.; Modi, J.; Goyal, M.; Coutts, S.B. Magnetic Resonance Imaging versus Computed Tomography in Transient Ischemic Attack and Minor Stroke: The More you See the More You Know. Cerebrovascular Diseases Extra 2013, 3, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Provost, C.; Soudant, M.; Legrand, L.; Ben Hassen, W.; Xie, Y.; Soize, S.; Bourcier, R.; Benzakoun, J.; Edjlali, M.; Boulouis, G.; Raoult, H.; Guillemin, F.; Naggara, O.; Bracard, S.; Oppenheim, C. Magnetic Resonance Imaging or Computed Tomography Before Treatment in Acute Ischemic Stroke. Stroke 2019, 50, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Birenbaum, D.; Bancroft, L.W.; Felsberg, G.J. Imaging in acute stroke. The western journal of emergency medicine. 2011, 12, 67–76. [Google Scholar] [PubMed]
- Wu, J.; Ngo, G.H.; Greve, D.; Li, J.; He, T.; Fischl, B.; Eickhoff, S.B.; Yeo, B.T. Accurate nonlinear mapping between MNI volumetric and FreeSurfer surface coordinate systems. Human Brain Mapping 2018, 39, 3793–3808. [Google Scholar] [CrossRef] [PubMed]
- Talairach, J.; Tournoux, P. Co-planar Stereotaxic Atlas of the Human Brain: 3-dimensional Proportional System : an Approach to Cerebral Imaging; Thieme Publishers Series, G. Thieme, 1988.
- Brodmann, K.; Garey, L. Brodmann’s: Localisation in the Cerebral Cortex; Springer US, 2007.
- Mori, S.; Wakana, S.; van Zijl, P.; Nagae-Poetscher, L. MRI Atlas of Human White Matter; Elsevier Science, 2005.
- Zachiu, C.; de Senneville, B.D.; Moonen, C.T.W.; Raaymakers, B.W.; Ries, M. Anatomically plausible models and quality assurance criteria for online mono- and multi-modal medical image registration. Phys Med Biol 2018, 63, 155016. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Johansson, A.; Cao, Y.; Dow, J.; Lawrence, T.S.; Balter, J.M. Abdominal synthetic CT generation from MR Dixon images using a U-net trained with ’semi-synthetic’ CT data. Phys Med Biol 2020, 65, 125001. [Google Scholar] [CrossRef]
- Dinkla, A.; Florkow, M.; Maspero, M.; Savenije, M.; Zijlstra, F.; Doornaert, P.; van Stralen, M.; Philippens, M.; van den Berg, C.; Seevinck, P. Dosimetric evaluation of synthetic CT for head and neck radiotherapy generated by a patch-based three-dimensional convolutional neural network; Vol. 46, 2019; pp. 4095–4104. [CrossRef]
- Brou Boni, K.N.D.; Klein, J.; Vanquin, L.; Wagner, A.; Lacornerie, T.; Pasquier, D.; Reynaert, N. MR to CT synthesis with multicenter data in the pelvic area using a conditional generative adversarial network. Phys Med Biol 2020, 65, 075002. [Google Scholar] [CrossRef]
- Han, X. MR-based synthetic CT generation using a deep convolutional neural network method. Med Phys 2017, 44, 1408–1419. [Google Scholar] [CrossRef]
- Chen, S.; Peng, Y.; Qin, A.; Liu, Y.; Zhao, C.; Deng, X.; Deraniyagala, R.; Stevens, C.; Ding, X. MR-based synthetic CT image for intensity-modulated proton treatment planning of nasopharyngeal carcinoma patients. Acta Oncol 2022, 61, 1417–1424. [Google Scholar] [CrossRef]
- Florkow, M.C.; Willemsen, K.; Zijlstra, F.; Foppen, W.; van der Wal, B.C.H.; van der Voort van Zyp, J.R.N.; Viergever, M.A.; Castelein, R.M.; Weinans, H.; van Stralen, M.; Sakkers, R.J.B.; Seevinck, P.R. MRI-based synthetic CT shows equivalence to conventional CT for the morphological assessment of the hip joint. J Orthop Res 2022, 40, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lei, Y.; Wang, T.; Kayode, O.; Tian, S.; Liu, T.; Patel, P.; Curran, W.J.; Ren, L.; Yang, X. MRI-based treatment planning for liver stereotactic body radiotherapy: validation of a deep learning-based synthetic CT generation method. Br J Radiol 2019, 92, 20190067. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Harms, J.; Wang, T.; Liu, Y.; Shu, H.K.; Jani, A.B.; Curran, W.J.; Mao, H.; Liu, T.; Yang, X. MRI-only based synthetic CT generation using dense cycle consistent generative adversarial networks. Med Phys 2019, 46, 3565–3581. [Google Scholar] [CrossRef]
- Kazemifar, S.; McGuire, S.; Timmerman, R.; Wardak, Z.; Nguyen, D.; Park, Y.; Jiang, S.; Owrangi, A. MRI-only brain radiotherapy: Assessing the dosimetric accuracy of synthetic CT images generated using a deep learning approach. Radiother Oncol 2019, 136, 56–63. [Google Scholar] [CrossRef]
- Qi, M.; Li, Y.; Wu, A.; Jia, Q.; Li, B.; Sun, W.; Dai, Z.; Lu, X.; Zhou, L.; Deng, X.; Song, T. Multi-sequence MR image-based synthetic CT generation using a generative adversarial network for head and neck MRI-only radiotherapy. Med Phys 2020, 47, 1880–1894. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Qin, W.; Liang, X.; Xu, J.; Xiong, J.; Xie, Y. Magnetic resonance image (MRI) synthesis from brain computed tomography (CT) images based on deep learning methods for magnetic resonance (MR)-guided radiotherapy. Quant Imaging Med Surg 2020, 10, 1223–1236. [Google Scholar] [CrossRef]
- Dong, X.; Lei, Y.; Tian, S.; Wang, T.; Patel, P.; Curran, W.J.; Jani, A.B.; Liu, T.; Yang, X. Synthetic MRI-aided multi-organ segmentation on male pelvic CT using cycle consistent deep attention network. Radiother Oncol 2019, 141, 192–199. [Google Scholar] [CrossRef]
- Kalantar, R.; Messiou, C.; Winfield, J.M.; Renn, A.; Latifoltojar, A.; Downey, K.; Sohaib, A.; Lalondrelle, S.; Koh, D.M.; Blackledge, M.D. CT-Based Pelvic T(1)-Weighted MR Image Synthesis Using UNet, UNet++ and Cycle-Consistent Generative Adversarial Network (Cycle-GAN). Front Oncol 2021, 11, 665807. [Google Scholar] [CrossRef] [PubMed]
- Feng, E.; Qin, P.; Chai, R.; Zeng, J.; Wang, Q.; Meng, Y.; Wang, P. MRI Generated From CT for Acute Ischemic Stroke Combining Radiomics and Generative Adversarial Networks. IEEE Journal of Biomedical and Health Informatics 2022, 26, 6047–6057. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Smith, S. A global optimisation method for robust affine registration of brain images. Med Image Anal 2001, 5, 143–56. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage. 2002, 17, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Greve, D.N.; Fischl, B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage 2009, 48, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Isensee, F.; Schell, M.; Pflueger, I.; Brugnara, G.; Bonekamp, D.; Neuberger, U.; Wick, A.; Schlemmer, H.; Heiland, S.; Wick, W.; Bendszus, M.; Maier-Hein, K.H.; Kickingereder, P. Automated brain extraction of multisequence MRI using artificial neural networks. Human Brain Mapping 2019, 40, 4952–4964. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.; Harris, G.; Williams, K. BRAINSFit: Mutual Information Registrations of Whole-Brain 3D Images, Using the Insight Toolkit. The Insight Journal 2007, 180. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; Buatti, J.; Aylward, S.; Miller, J.V.; Pieper, S.; Kikinis, R. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magnetic Resonance Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Kikinis, R.; Pieper, S.D.; Vosburgh, K.G. , 3D Slicer: A Platform for Subject-Specific Image Analysis, Visualization, and Clinical Support. In Intraoperative Imaging and Image-Guided Therapy; Springer New York, 2014; pp. 277–289. [CrossRef]
- Kapur, T.; Pieper, S.; Fedorov, A.; Fillion-Robin, J.C.; Halle, M.; O’Donnell, L.; Lasso, A.; Ungi, T.; Pinter, C.; Finet, J.; Pujol, S.; Jagadeesan, J.; Tokuda, J.; Norton, I.; Estepar, R.S.J.; Gering, D.; Aerts, H.J.; Jakab, M.; Hata, N.; Ibanez, L.; Blezek, D.; Miller, J.; Aylward, S.; Grimson, W.E.L.; Fichtinger, G.; Wells, W.M.; Lorensen, W.E.; Schroeder, W.; Kikinis, R. Increasing the impact of medical image computing using community-based open-access hackathons: The NA-MIC and 3D Slicer experience. Medical Image Analysis 2016, 33, 176–180. [Google Scholar] [CrossRef]
- Iommi, D. 3D-CycleGan-Pytorch-MedImaging, 2021.
- McNaughton, J. CT-to-Synthetic-MRI-Generation, 2023.
- Brudfors, M.; Chalcroft, L. ATLAS_UNET, 2022.
- Zhang, Y.; Brady, M.; Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging 2001, 20, 45–57. [Google Scholar] [CrossRef]
- Cohen, J.P.; Luck, M.; Honari, S. Distribution Matching Losses Can Hallucinate Features in Medical Image Translation. Medical Image Computing and Computer Assisted Intervention – MICCAI 2018; Frangi, A.F., Schnabel, J.A., Davatzikos, C., Alberola-López, C., Fichtinger, G., Eds.; Springer International Publishing: Cham, 2018; pp. 529–536. [Google Scholar]
Figure 1.
Preprocessing pipeline showing registrations of the CT to MRI and the MRI to MNI152 followed by brain extraction.
Figure 1.
Preprocessing pipeline showing registrations of the CT to MRI and the MRI to MNI152 followed by brain extraction.
Figure 2.
Architecture of UNet.
Figure 2.
Architecture of UNet.
Figure 3.
Architecture of UNet V2.
Figure 3.
Architecture of UNet V2.
Figure 4.
Architecture of Patch Based UNet.
Figure 4.
Architecture of Patch Based UNet.
Figure 5.
Architecture of 2D UNet.
Figure 5.
Architecture of 2D UNet.
Figure 6.
Architecture of UNet++.
Figure 6.
Architecture of UNet++.
Figure 7.
Architecture of Attention UNet.
Figure 7.
Architecture of Attention UNet.
Figure 8.
Architecture of Transformer UNet.
Figure 8.
Architecture of Transformer UNet.
Figure 9.
(a) MRI of Patient Alpha. (b) Target MRI for the registration task.
Figure 9.
(a) MRI of Patient Alpha. (b) Target MRI for the registration task.
Figure 11.
Slices of a Synthetic MRI produced from a preliminary UNet trained with five different loss functions compared to the true MRI. The Synthetic MRI generated by the model trained with Mean Absolute Error (MAE) as the loss functions appears the most visually similar to the true MRI. PSNR – Peak Signal to Noise Ratio, MSE – Mean Squared Error, MAE – Mean Absolute Error, SSIM - Structural Similarity Index Measurement.
Figure 11.
Slices of a Synthetic MRI produced from a preliminary UNet trained with five different loss functions compared to the true MRI. The Synthetic MRI generated by the model trained with Mean Absolute Error (MAE) as the loss functions appears the most visually similar to the true MRI. PSNR – Peak Signal to Noise Ratio, MSE – Mean Squared Error, MAE – Mean Absolute Error, SSIM - Structural Similarity Index Measurement.
Figure 12.
Axial slices of CT Scans (Left), True MRIs (Centre), and Synthetic MRIs generated by UNet (Right).
Figure 12.
Axial slices of CT Scans (Left), True MRIs (Centre), and Synthetic MRIs generated by UNet (Right).
| |
True CT |
True MRI |
Synthesised MRI |
| Patient A |
|
|
|
| |
| Patient B |
|
|
|
| |
| Patient C |
|
|
|
Figure 13.
Axial slices of CT Scans (Left), True MRIs (Center), and Synthetic MRIs generated by UNet V2 (Right).
Figure 13.
Axial slices of CT Scans (Left), True MRIs (Center), and Synthetic MRIs generated by UNet V2 (Right).
| |
True CT |
True MRI |
Synthesised MRI |
| Patient A |
|
|
|
| |
| Patient B |
|
|
|
| |
| Patient C |
|
|
|
Figure 14.
Axial slices of CT Scans (Left), True MRIs (Centre), and Synthetic MRIs generated by Patch Based UNet (Right).
Figure 14.
Axial slices of CT Scans (Left), True MRIs (Centre), and Synthetic MRIs generated by Patch Based UNet (Right).
| |
True CT |
True MRI |
Synthesised MRI |
| Patient A |
|
|
|
| |
| Patient B |
|
|
|
| |
| Patient C |
|
|
|
Figure 15.
Axial slices of CT Scans (Left), True MRIs (Centre), and Synthetic MRIs generated by 2D UNet (Right).
Figure 15.
Axial slices of CT Scans (Left), True MRIs (Centre), and Synthetic MRIs generated by 2D UNet (Right).
| |
True CT |
True MRI |
Synthesised MRI |
| Patient A |
|
|
|
| |
| Patient B |
|
|
|
| |
| Patient C |
|
|
|
Figure 16.
Axial slices of CT Scans (Left), True MRIs (Centre), and Synthetic MRIs generated by UNet++ (Right).
Figure 16.
Axial slices of CT Scans (Left), True MRIs (Centre), and Synthetic MRIs generated by UNet++ (Right).
| |
True CT |
True MRI |
Synthesised MRI |
| Patient A |
|
|
|
| |
| Patient B |
|
|
|
| |
| Patient C |
|
|
|
Figure 17.
Axial slices of CT Scans (Left), True MRIs (Centre), and Synthetic MRIs generated by Attention UNet (Right).
Figure 17.
Axial slices of CT Scans (Left), True MRIs (Centre), and Synthetic MRIs generated by Attention UNet (Right).
| |
True CT |
True MRI |
Synthesised MRI |
| Patient A |
|
|
|
| |
| Patient B |
|
|
|
| |
| Patient C |
|
|
|
Figure 18.
Axial slices of CT Scans (Left), True MRIs (Centre), and Synthetic MRIs generated by Transformer UNet (Right).
Figure 18.
Axial slices of CT Scans (Left), True MRIs (Centre), and Synthetic MRIs generated by Transformer UNet (Right).
| |
True CT |
True MRI |
Synthesised MRI |
| Patient A |
|
|
|
| |
| Patient B |
|
|
|
| |
| Patient C |
|
|
|
Figure 19.
Axial slices of CT Scans (Left), True MRIs (Centre), and Synthetic MRIs generated by CycleGAN (Right).
Figure 19.
Axial slices of CT Scans (Left), True MRIs (Centre), and Synthetic MRIs generated by CycleGAN (Right).
| |
True CT |
True MRI |
Synthesised MRI |
| Patient A |
|
|
|
| |
| Patient B |
|
|
|
| |
| Patient C |
|
|
|
Figure 20.
Four slices of an MRI of Patient D generated by each of the eight models along with the True MRI.
Figure 20.
Four slices of an MRI of Patient D generated by each of the eight models along with the True MRI.
Figure 21.
Lesion segmentations of the True MRI and Synthetic MRIs for Patient E (Left) and Patient A (Right).
Figure 21.
Lesion segmentations of the True MRI and Synthetic MRIs for Patient E (Left) and Patient A (Right).
Figure 22.
Segmentation maps of the True MRI and Synthetic MRIs of Patient F and Patient G.
Figure 22.
Segmentation maps of the True MRI and Synthetic MRIs of Patient F and Patient G.
Figure 23.
Registration of the True MRI and Synthetic MRIs of Patient H and the associated registrations of the CT of Patient H.
Figure 23.
Registration of the True MRI and Synthetic MRIs of Patient H and the associated registrations of the CT of Patient H.
Figure 24.
(a) Coronal slices of an MRI generated by 2D UNet. (b) Sagittal slices of an MRI generated by 2D UNet.
Figure 24.
(a) Coronal slices of an MRI generated by 2D UNet. (b) Sagittal slices of an MRI generated by 2D UNet.
Table 1.
MRI acquisition parameters for included studies.
Table 1.
MRI acquisition parameters for included studies.
| Study |
n |
Scanner |
TR (ms) |
TE (ms) |
TI (ms) |
Flip (°) |
Sequence*
|
| 1 |
55 |
Avanto 1.5T |
11 |
4.94 |
n/a |
15 |
FLASH3D |
| 2 |
47 |
Avanto 1.5T |
13 |
4.76 |
n/a |
25 |
FLASH3D |
| 3 |
8 |
Skyra 3T |
23 |
2.46 |
n/a |
23 |
FLASH3D |
| 4 |
18 |
Skyra 3T |
1900 |
2.07 |
900 |
9 |
FLASH3D, MPRAGE |
| 5 |
53 |
Avanto 1.5T |
2200 |
2.97 |
900 |
8 |
FLASH3D, MPRAGE |
Table 2.
Evaluation metrics for each model averaged over the test set along with standard deviations.
Table 2.
Evaluation metrics for each model averaged over the test set along with standard deviations.
| Model |
MAE ↓ |
MSE ↓ |
SSIM ↑ |
PSNR ↑ |
Total SSIM ↑*
|
| UNet |
|
|
|
|
|
| UNet V2 |
|
|
|
|
|
| 2D UNet |
|
|
|
|
|
| Patch Based UNet |
|
|
|
|
|
| Attention UNet |
|
|
|
|
|
| UNet++ |
|
|
|
|
|
| Transformer UNet |
|
|
|
|
|
| CycleGAN |
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).