Submitted:
01 June 2023
Posted:
01 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Search strategy
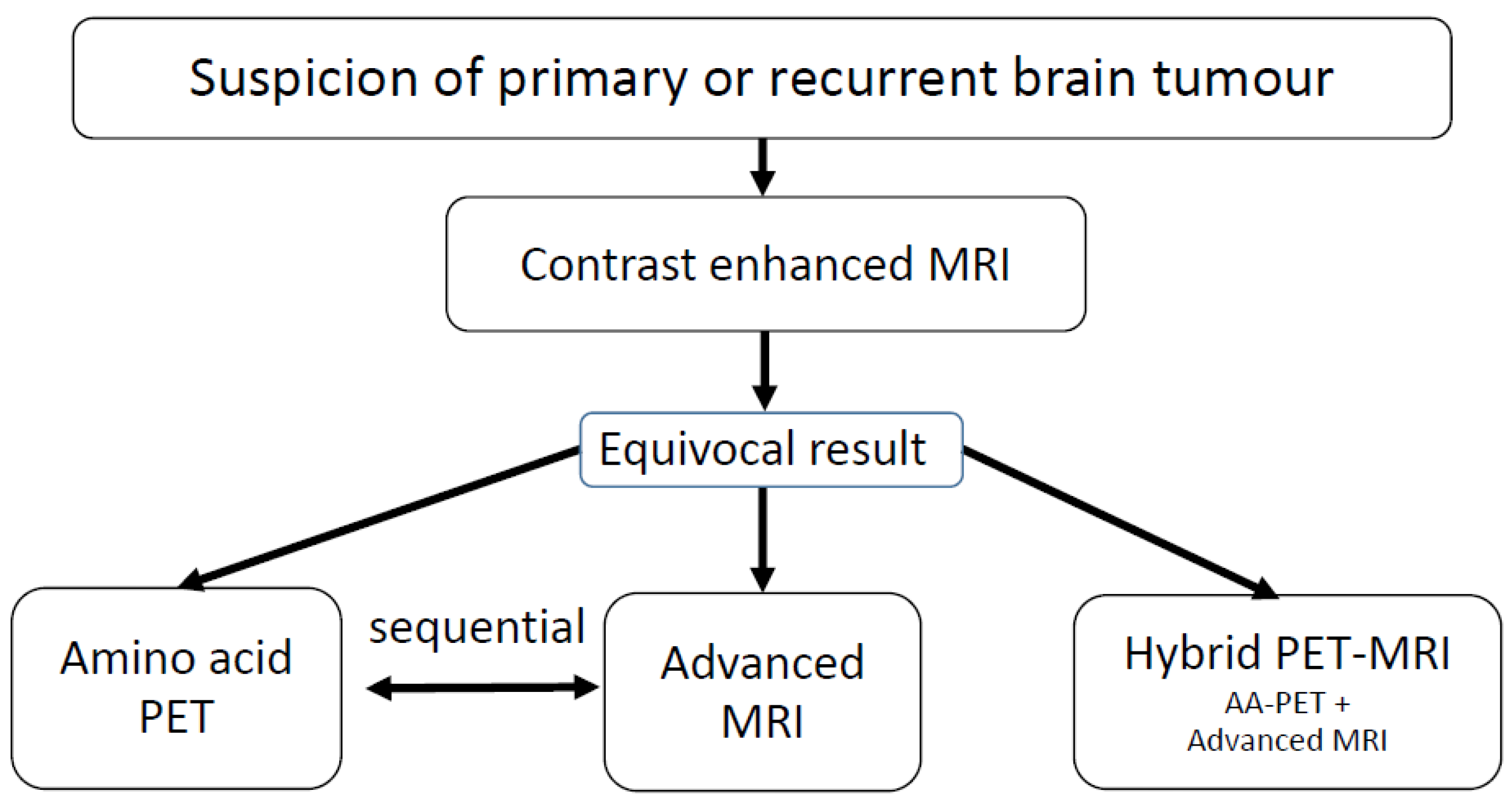
3. PET tracers for brain tumor imaging
4. Advanced MRI methods in neuro-oncology
5. Hybrid PET/MRI in animal research
6. Hybrid PET/MRI in newly diagnosed brain tumors
7. Hybrid PET/MRI in patients with recurrent gliomas
8. Hybrid PET/MRI in pediatric brain tumors
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Langen, K.-J.; Galldiks, N.; Hattingen, E.; Shah, N.J. Advances in neuro-oncology imaging. Nat. Rev. Neurol. 2017, 13, 279–289, . [CrossRef]
- Galldiks, N.; Lohmann, P.; Albert, N.L.; Tonn, J.C.; Langen, K.-J. Current status of PET imaging in neuro-oncology. Neuro-Oncology Adv. 2019, 1, vdz010, . [CrossRef]
- Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18:1199-208.
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; la Fougère, C.; Langen, K.-J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur. J. Nucl. Med. 2018, 46, 540–557, . [CrossRef]
- Galldiks, N.; Langen, K.-J.; Albert, N.L.; Chamberlain, M.; Soffietti, R.; Kim, M.M.; Law, I.; Le Rhun, E.; Chang, S.; Schwarting, J.; et al. PET imaging in patients with brain metastasis—report of the RANO/PET group. Neuro-Oncology 2019, 21, 585–595, . [CrossRef]
- Galldiks, N.; Langen, K.-J.; Albert, N.L.; Law, I.; Kim, M.M.; E Villanueva-Meyer, J.; Soffietti, R.; Wen, P.Y.; Weller, M.; Tonn, J.C. Investigational PET tracers in neuro-oncology—What’s on the horizon? A report of the PET/RANO group. Neuro-Oncology 2022, 24, 1815–1826, . [CrossRef]
- Piccardo, A.; Albert, N.L.; Borgwardt, L.; Fahey, F.H.; Hargrave, D.; Galldiks, N.; Jehanno, N.; Kurch, L.; Law, I.; Lim, R.; et al. Joint EANM/SIOPE/RAPNO practice guidelines/SNMMI procedure standards for imaging of paediatric gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur. J. Nucl. Med. 2022, 49, 3852–3869, . [CrossRef]
- Galldiks, N.; Niyazi, M.; Grosu, A.L.; Kocher, M.; Langen, K.-J.; Law, I.; Minniti, G.; Kim, M.M.; Tsien, C.; Dhermain, F.; et al. Contribution of PET imaging to radiotherapy planning and monitoring in glioma patients - a report of the PET/RANO group. Neuro-Oncology 2021, 23, 881–893, . [CrossRef]
- Wester, H.J.; Herz, M.; Weber, W.; Heiss, P.; Senekowitsch-Schmidtke, R.; Schwaiger, M.; Stöcklin, G. Synthesis and radiopharmacology of O-(2-[18F]fluoroethyl)-L-tyrosine for tumor imaging.. 1999, 40, 205–12.
- Langen, K.-J.; Hamacher, K.; Weckesser, M.; Floeth, F.; Stoffels, G.; Bauer, D.; Coenen, H.H.; Pauleit, D. O-(2-[18F]fluoroethyl)-l-tyrosine: uptake mechanisms and clinical applications. Nucl. Med. Biol. 2006, 33, 287–294, . [CrossRef]
- Hamacher, K.; Coenen, H. Efficient routine production of the 18F-labelled amino acid O-(2-[18F]fluoroethyl)-l-tyrosine. Appl. Radiat. Isot. 2002, 57, 853–856, . [CrossRef]
- Langen, K.-J.; Stoffels, G.; Filss, C.; Heinzel, A.; Stegmayr, C.; Lohmann, P.; Willuweit, A.; Neumaier, B.; Mottaghy, F.M.; Galldiks, N. Imaging of amino acid transport in brain tumours: Positron emission tomography with O-(2-[ 18 F]fluoroethyl)- L -tyrosine (FET). Methods 2017, 130, 124–134, . [CrossRef]
- Stegmayr, C.; Willuweit, A.; Lohmann, P.; Langen, K.-J. O-(2-[18F]-Fluoroethyl)-L-Tyrosine (FET) in Neurooncology: A Review of Experimental Results. Curr. Radiopharm. 2019, 12, 201–210, . [CrossRef]
- Heinzel, A.; Dedic, D.; Galldiks, N.; Lohmann, P.; Stoffels, G.; Filss, C.P.; Kocher, M.; Migliorini, F.; Dillen, K.N.H.; Geisler, S.; et al. Two Decades of Brain Tumour Imaging with O-(2-[18F]fluoroethyl)-L-tyrosine PET: The Forschungszentrum Jülich Experience. Cancers 2022, 14, 3336, . [CrossRef]
- Poeppel, T.; Krause, B.; Heusner, T.; Boy, C.; Bockisch, A.; Antoch, G. PET/CT for the staging and follow-up of patients with malignancies. Eur. J. Radiol. 2009, 70, 382–392, . [CrossRef]
- Czernin, J.; Allen-Auerbach, M.; Schelbert, H.R. Improvements in cancer staging with PET/CT: literature-based evidence as of September 2006.. 2007, 78S–88S.
- Pietrzyk, U.; Herholz, K.; Schuster, A.; Stockhausen, H.-M.V.; Lucht, H.; Heiss, W.-D. Clinical applications of registration and fusion of multimodality brain images from PET, SPECT, CT, and MRI. Eur. J. Radiol. 1996, 21, 174–182, . [CrossRef]
- Marner L, Henriksen OM, Lundemann M, Larsen VA, Law I. Clinical PET/MRI in neurooncology: opportunities and challenges from a single-institution perspective. Clin Transl Imaging. 2017;5:135-49.
- Catana, C.; Drzezga, A.; Heiss, W.-D.; Rosen, B.R. PET/MRI for Neurologic Applications. J. Nucl. Med. 2012, 53, 1916–1925, . [CrossRef]
- Ziegenfeuter, J.; Delbridge, C.; Bernhardt, D.; Gempt, J.; Schmidt-Graf, F.; Griessmair, M.; Thomas, M.; Meyer, H.S.; Zimmer, C.; Meyer, B.; et al. Sequential and Hybrid PET/MRI Acquisition in Follow-Up Examination of Glioblastoma Show Similar Diagnostic Performance. Cancers 2022, 15, 83, . [CrossRef]
- Herzog, H.; Langen, K.-J.; Weirich, C.; Rota Kops, E.; Kaffanke, J.; Tellmann, L.; Scheins, J.; Neuner, I.; Stoffels, G.; Fischer, K.; et al. High resolution BrainPET combined with simultaneous MRI. Nuklearmedizin 2011, 50, 74–82, doi:10.3413/nukmed-0347-10-09.
- Almansory, K.; Fraioli, F. Combined PET/MRI in brain glioma imaging. Br. J. Hosp. Med. 2019, 80, 380–386, . [CrossRef]
- Yang, Z.L.; Zhang, L.J. PET/MRI of central nervous system: current status and future perspective. Eur. Radiol. 2016, 26, 3534–3541, . [CrossRef]
- Werner, P.; Barthel, H.; Drzezga, A.; Sabri, O. Current status and future role of brain PET/MRI in clinical and research settings. Eur. J. Nucl. Med. 2015, 42, 512–526, . [CrossRef]
- Albano, D.; Tomasini, D.; Bonù, M.; Giubbini, R.; Bertagna, F. 18F-Fluciclovine (18F-FACBC) PET/CT or PET/MRI in gliomas/glioblastomas. Ann. Nucl. Med. 2020, 34, 81–86, . [CrossRef]
- Tsuyuguchi N, Terakawa Y, Uda T, Nakajo K, Kanemura Y. Diagnosis of Brain Tumors Using Amino Acid Transport PET Imaging with (18)F-fluciclovine: A Comparative Study with L-methyl-(11)C-methionine PET Imaging. Asia Ocean J Nucl Med Biol. 2017;5:85-94.
- Wakabayashi, T.; Hirose, Y.; Miyake, K.; Arakawa, Y.; Kagawa, N.; Nariai, T.; Narita, Y.; Nishikawa, R.; Tsuyuguchi, N.; Fukami, T.; et al. Determining the extent of tumor resection at surgical planning with 18F-fluciclovine PET/CT in patients with suspected glioma: multicenter phase III trials. Ann. Nucl. Med. 2021, 35, 1279–1292, . [CrossRef]
- Collet, S.; Valable, S.; Constans, J.; Lechapt-Zalcman, E.; Roussel, S.; Delcroix, N.; Abbas, A.; Ibazizene, M.; Bernaudin, M.; Barré, L.; et al. [ 18 F]-fluoro- l -thymidine PET and advanced MRI for preoperative grading of gliomas. NeuroImage: Clin. 2015, 8, 448–454, . [CrossRef]
- Mitamura, K.; Yamamoto, Y.; Kudomi, N.; Maeda, Y.; Norikane, T.; Miyake, K.; Nishiyama, Y. Intratumoral heterogeneity of 18F-FLT uptake predicts proliferation and survival in patients with newly diagnosed gliomas. Ann. Nucl. Med. 2017, 31, 46–52, . [CrossRef]
- Nowosielski, M.; DiFranco, M.D.; Putzer, D.; Seiz, M.; Recheis, W.; Jacobs, A.H.; Stockhammer, G.; Hutterer, M. An Intra-Individual Comparison of MRI, [18F]-FET and [18F]-FLT PET in Patients with High-Grade Gliomas. PLOS ONE 2014, 9, e95830, . [CrossRef]
- Sollini, M.; Sghedoni, R.; Erba, P.A.; Cavuto, S.; Froio, A.; De Berti, G.; Pisanello, A.; Fraternali, A.; Iori, M.; Iaccarino, C.; et al. Diagnostic performances of [18F]fluorocholine positron emission tomography in brain tumors. Q. J. Nucl. Med. Mol. Imaging 2015, 62, 209–219, . [CrossRef]
- Kwee, S.A.; Ko, J.P.; Jiang, C.S.; Watters, M.R.; Coel, M.N. Solitary Brain Lesions Enhancing at MR Imaging: Evaluation with Fluorine 18–Fluorocholine PET. Radiology 2007, 244, 557–565, . [CrossRef]
- Ohtani, T.; Kurihara, H.; Ishiuchi, S.; Saito, N.; Oriuchi, N.; Inoue, T.; Sasaki, T. Brain tumour imaging with carbon-11 choline: comparison with FDG PET and gadolinium-enhanced MR imaging. Eur. J. Nucl. Med. 2001, 28, 1664–1670, . [CrossRef]
- Calabria, F.F.; Barbarisi, M.; Gangemi, V.; Grillea, G.; Cascini, G.L. Molecular imaging of brain tumors with radiolabeled choline PET. Neurosurg. Rev. 2018, 41, 67–76, . [CrossRef]
- Van Waarde, A.; Jager, P.L.; Ishiwata, K.; A Dierckx, R.; Elsinga, P.H. Comparison of sigma-ligands and metabolic PET tracers for differentiating tumor from inflammation.. 2006, 47, 150–4.
- Huang, Z.; Zuo, C.; Guan, Y.; Zhang, Z.; Liu, P.; Xue, F.; Lin, X. Misdiagnoses of 11C-choline combined with 18F-FDG PET imaging in brain tumours. Nucl. Med. Commun. 2008, 29, 354–358, . [CrossRef]
- Gerstner ER, Zhang Z, Fink JR, Muzi M, Hanna L, Greco E, et al. ACRIN 6684: Assessment of Tumor Hypoxia in Newly Diagnosed Glioblastoma Using 18F-FMISO PET and MRI. Clin Cancer Res. 2016;22:5079-86.
- Kobayashi, H.; Hirata, K.; Yamaguchi, S.; Terasaka, S.; Shiga, T.; Houkin, K. Usefulness of FMISO—PET for Glioma Analysis. Neurol. medico-chirurgica 2013, 53, 773–778, . [CrossRef]
- Choudhary, G.; Langen, K.-J.; Galldiks, N.; McConathy, J. Investigational PET tracers for high-grade gliomas. Q. J. Nucl. Med. Mol. Imaging 2018, 62, 281–294, . [CrossRef]
- Hirata, K.; Yamaguchi, S.; Shiga, T.; Kuge, Y.; Tamaki, N. The Roles of Hypoxia Imaging Using 18F-Fluoromisonidazole Positron Emission Tomography in Glioma Treatment. J. Clin. Med. 2019, 8, 1088, . [CrossRef]
- Laudicella R, Quartuccio N, Alongi P, Albano D, Gazzilli M, Durmo R, et al. F-18-FMISO PET imaging: insights over MRI in patients with glioma (vol 8, pg 3, 2020). Clinical and Translational Imaging. 2020;8:123-.
- Kanoto, M.; Kirii, K.; Hiraka, T.; Toyoguchi, Y.; Sugai, Y.; Matsuda, K.; Sakurada, K.; Sonoda, Y.; Hatazawa, J.; Hosoya, T. Correlation between hypoxic area in primary brain tumors and WHO grade: differentiation from malignancy using 18F-fluoromisonidazole positron emission tomography. Acta Radiol. 2018, 59, 229–235, . [CrossRef]
- Gérard, M.; Corroyer-Dulmont, A.; Lesueur, P.; Collet, S.; Chérel, M.; Bourgeois, M.; Stefan, D.; Limkin, E.J.; Perrio, C.; Guillamo, J.-S.; et al. Hypoxia Imaging and Adaptive Radiotherapy: A State-of-the-Art Approach in the Management of Glioma. Front. Med. 2019, 6, 117, . [CrossRef]
- Werner, J.-M.; Lohmann, P.; Fink, G.R.; Langen, K.-J.; Galldiks, N. Current Landscape and Emerging Fields of PET Imaging in Patients with Brain Tumors. Molecules 2020, 25, 1471, . [CrossRef]
- Ammer, L.-M.; Vollmann-Zwerenz, A.; Ruf, V.; Wetzel, C.H.; Riemenschneider, M.J.; Albert, N.L.; Beckhove, P.; Hau, P. The Role of Translocator Protein TSPO in Hallmarks of Glioblastoma. Cancers 2020, 12, 2973, . [CrossRef]
- Albert, N.L.; Unterrainer, M.; Fleischmann, D.F.; Lindner, S.; Vettermann, F.; Brunegraf, A.; Vomacka, L.; Brendel, M.; Wenter, V.; Wetzel, C.; et al. TSPO PET for glioma imaging using the novel ligand 18F-GE-180: first results in patients with glioblastoma. Eur. J. Nucl. Med. 2017, 44, 2230–2238, . [CrossRef]
- Langen KJ, Willuweit A. TSPO PET using 18F-GE-180: a new perspective in neurooncology? Eur J Nucl Med Mol Imaging. 2017;44:2227-9.
- Roncaroli F, Su Z, Herholz K, Gerhard A, Turkheimer FE. TSPO expression in brain tumours: is TSPO a target for brain tumour imaging? Clin Transl Imaging. 2016;4:145-56.
- Zinnhardt, B.; Roncaroli, F.; Foray, C.; Agushi, E.; Osrah, B.; Hugon, G.; Jacobs, A.H.; Winkeler, A. Imaging of the glioma microenvironment by TSPO PET. Eur. J. Nucl. Med. 2021, 49, 174–185, . [CrossRef]
- Unterrainer, M.; Fleischmann, D.F.; Diekmann, C.; Vomacka, L.; Lindner, S.; Vettermann, F.; Brendel, M.; Wenter, V.; Ertl-Wagner, B.; Herms, J.; et al. Comparison of 18F-GE-180 and dynamic 18F-FET PET in high grade glioma: a double-tracer pilot study. Eur. J. Nucl. Med. 2019, 46, 580–590, . [CrossRef]
- Laudicella, R.; Quartuccio, N.; Argiroffi, G.; Alongi, P.; Baratto, L.; Califaretti, E.; Frantellizzi, V.; De Vincentis, G.; Del Sole, A.; Evangelista, L.; et al. Unconventional non-amino acidic PET radiotracers for molecular imaging in gliomas. Eur. J. Nucl. Med. 2021, 48, 3925–3939, . [CrossRef]
- Herholz, K.; Coope, D.; Jackson, A. Metabolic and molecular imaging in neuro-oncology. Lancet Neurol. 2007, 6, 711–724, . [CrossRef]
- Lohmann, P.; Werner, J.-M.; Shah, N.J.; Fink, G.R.; Langen, K.-J.; Galldiks, N. Combined Amino Acid Positron Emission Tomography and Advanced Magnetic Resonance Imaging in Glioma Patients. Cancers 2019, 11, 153, . [CrossRef]
- Warmuth, C.; Günther, M.; Zimmer, C. Quantification of Blood Flow in Brain Tumors: Comparison of Arterial Spin Labeling and Dynamic Susceptibility-weighted Contrast-enhanced MR Imaging. 2003, 228, 523–532, . [CrossRef]
- Patel, P.; Baradaran, H.; Delgado, D.; Askin, G.; Christos, P.; Tsiouris, A.J.; Gupta, A. MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: a systematic review and meta-analysis. Neuro-Oncology 2016, 19, 118–127, . [CrossRef]
- Aseel, A.; McCarthy, P.; Mohammed, A. Brain magnetic resonance spectroscopy to differentiate recurrent neoplasm from radiation necrosis: A systematic review and meta-analysis. J. Neuroimaging 2023, 33, 189–201, . [CrossRef]
- Koh, D.-M.; Padhani, A.R. Diffusion-weighted MRI: a new functional clinical technique for tumour imaging. Br. J. Radiol. 2006, 79, 633–635, . [CrossRef]
- Jensen, J.H.; Helpern, J.A.; Ramani, A.; Lu, H.; Kaczynski, K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 2005, 53, 1432–1440, . [CrossRef]
- Duyn, J.H. The future of ultra-high field MRI and fMRI for study of the human brain. NeuroImage 2012, 62, 1241–1248, . [CrossRef]
- Ward, K.; Aletras, A.; Balaban, R. A New Class of Contrast Agents for MRI Based on Proton Chemical Exchange Dependent Saturation Transfer (CEST). J. Magn. Reson. 2000, 143, 79–87, . [CrossRef]
- Shah, N.J.; Worthoff, W.A.; Langen, K. Imaging of sodium in the brain: a brief review. NMR Biomed. 2016, 29, 162–174, . [CrossRef]
- Ouwerkerk, R.; Bleich, K.B.; Gillen, J.S.; Pomper, M.G.; Bottomley, P.A. Tissue Sodium Concentration in Human Brain Tumors as Measured with23Na MR Imaging. Radiology 2003, 227, 529–537, . [CrossRef]
- Thulborn, K.R.; Lu, A.; Atkinson, I.C.; Damen, F.; Villano, J.L. Quantitative Sodium MR Imaging and Sodium Bioscales for the Management of Brain Tumors. Neuroimaging Clin. North Am. 2009, 19, 615–624, . [CrossRef]
- Shymanskaya, A.; Worthoff, W.A.; Stoffels, G.; Lindemeyer, J.; Neumaier, B.; Lohmann, P.; Galldiks, N.; Langen, K.-J.; Shah, N.J. Comparison of [18F]Fluoroethyltyrosine PET and Sodium MRI in Cerebral Gliomas: a Pilot Study. Mol. Imaging Biol. 2020, 22, 198–207, . [CrossRef]
- Ionescu, T.M.; Amend, M.; Hafiz, R.; Biswal, B.B.; Maurer, A.; Pichler, B.J.; Wehrl, H.F.; Herfert, K. Striatal and prefrontal D2R and SERT distributions contrastingly correlate with default-mode connectivity. NeuroImage 2021, 243, 118501, . [CrossRef]
- Ionescu, T.M.; Amend, M.; Hafiz, R.; Biswal, B.B.; Wehrl, H.F.; Herfert, K.; Pichler, B.J. Elucidating the complementarity of resting-state networks derived from dynamic [18F]FDG and hemodynamic fluctuations using simultaneous small-animal PET/MRI. NeuroImage 2021, 236, 118045–118045, . [CrossRef]
- Ionescu, T.M.; Amend, M.; Watabe, T.; Hatazawa, J.; Maurer, A.; Reischl, G.; Pichler, B.J.; Wehrl, H.F.; Herfert, K. Neurovascular Uncoupling: Multimodal Imaging Delineates the Acute Effects of 3,4-Methylenedioxymethamphetamine. J. Nucl. Med. 2023, 64, 466–471, . [CrossRef]
- Vidal, B.; Fieux, S.; Redouté, J.; Villien, M.; Bonnefoi, F.; Le Bars, D.; Newman-Tancredi, A.; Costes, N.; Zimmer, L. In vivo biased agonism at 5-HT1A receptors: characterisation by simultaneous PET/MR imaging. Neuropsychopharmacology 2018, 43, 2310–2319, . [CrossRef]
- Stegmayr, C.; Bandelow, U.; Oliveira, D.; Lohmann, P.; Willuweit, A.; Filss, C.; Galldiks, N.; Lübke, J.H.R.; Shah, N.J.; Ermert, J.; et al. Influence of blood-brain barrier permeability on O-(2-18F-fluoroethyl)-L-tyrosine uptake in rat gliomas. Eur. J. Nucl. Med. 2017, 44, 408–416, . [CrossRef]
- Stegmayr, C.; Oliveira, D.; Niemietz, N.; Willuweit, A.; Lohmann, P.; Galldiks, N.; Shah, N.J.; Ermert, J.; Langen, K.-J. Influence of Bevacizumab on Blood–Brain Barrier Permeability and O-(2-18F-Fluoroethyl)-l-Tyrosine Uptake in Rat Gliomas. J. Nucl. Med. 2017, 58, 700–705, . [CrossRef]
- Stegmayr C SM, Oliveira D, Willuweit A, Filß C, Galldiks N, Shah NJ, Neumaier B, Langen KJ. . O-(2-[F-18]fluoroethyl)-L-tyrosine uptake and blood-brain barrier permeability under anti-angiogenic therapy in rat glioma models. J Nucl Med 2017;accepted 26.12.2016.
- Jackson, L.R.; Masi, M.R.; Selman, B.M.; Sandusky, G.E.; Zarrinmayeh, H.; Das, S.K.; Maharjan, S.; Wang, N.; Zheng, Q.-H.; Pollok, K.E.; et al. Use of multimodality imaging, histology, and treatment feasibility to characterize a transgenic Rag2-null rat model of glioblastoma. Front. Oncol. 2022, 12, 939260, . [CrossRef]
- Verhoeven, J.; Baguet, T.; Piron, S.; Pauwelyn, G.; Bouckaert, C.; Descamps, B.; Raedt, R.; Vanhove, C.; De Vos, F.; Goethals, I. 2-[18F]FELP, a novel LAT1-specific PET tracer, for the discrimination between glioblastoma, radiation necrosis and inflammation. Nucl. Med. Biol. 2020, 82-83, 9–16, . [CrossRef]
- Wehrl, H.F.; Wiehr, S.; Divine, M.R.; Gatidis, S.; Gullberg, G.T.; Maier, F.C.; Rolle, A.-M.; Schwenck, J.; Thaiss, W.M.; Pichler, B.J. Preclinical and Translational PET/MR Imaging. J. Nucl. Med. 2014, 55, 11S–18S, . [CrossRef]
- Choi, C.-H.; Stegmayr, C.; Shymanskaya, A.; Worthoff, W.A.; da Silva, N.A.; Felder, J.; Langen, K.-J.; Shah, N.J. An in vivo multimodal feasibility study in a rat brain tumour model using flexible multinuclear MR and PET systems. EJNMMI Phys. 2020, 7, 1–11, . [CrossRef]
- Pyka, T.; Krzyzanowska, I.; Rominger, A.; Delbridge, C.; Meyer, B.; Boeckh-Behrens, T.; Zimmer, C.; Gempt, J. Multiparametric Characterization of Intracranial Gliomas Using Dynamic [18F]FET-PET and Magnetic Resonance Spectroscopy. Diagnostics 2022, 12, 2331, . [CrossRef]
- Song, S.; Wang, L.; Yang, H.; Shan, Y.; Cheng, Y.; Xu, L.; Dong, C.; Zhao, G.; Lu, J. Static 18F-FET PET and DSC-PWI based on hybrid PET/MR for the prediction of gliomas defined by IDH and 1p/19q status. Eur. Radiol. 2021, 31, 4087–4096, . [CrossRef]
- Haubold, J.; Demircioglu, A.; Gratz, M.; Glas, M.; Wrede, K.; Sure, U.; Antoch, G.; Keyvani, K.; Nittka, M.; Kannengiesser, S.; et al. Non-invasive tumor decoding and phenotyping of cerebral gliomas utilizing multiparametric 18F-FET PET-MRI and MR Fingerprinting. Eur. J. Nucl. Med. 2020, 47, 1435–1445, . [CrossRef]
- Pauleit, D.; Floeth, F.; Hamacher, K.; Riemenschneider, M.J.; Reifenberger, G.; Müller, H.-W.; Zilles, K.; Coenen, H.H.; Langen, K.-J. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 2005, 128, 678–687, . [CrossRef]
- Mosskin, M.; Ericson, K.; Hindmarsh, T.; Von Holst, H.; Collins, V.P.; Bergström, M.; Eriksson, L.; Johnström, P. Positron emission tomography compared with magnetic resonance imaging and computed tomography in supratentorial gliomas using multiple stereotactic biopsies as reference.. 1989, 30, 225–32.
- Song, S.; Cheng, Y.; Ma, J.; Wang, L.; Dong, C.; Wei, Y.; Xu, G.; An, Y.; Qi, Z.; Lin, Q.; et al. Simultaneous FET-PET and contrast-enhanced MRI based on hybrid PET/MR improves delineation of tumor spatial biodistribution in gliomas: a biopsy validation study. Eur. J. Nucl. Med. 2020, 47, 1458–1467, . [CrossRef]
- Kracht LW, Miletic H, Busch S, Jacobs AH, Voges J, Hoevels M, et al. Delineation of brain tumor extent with [11C]L-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res. 2004;10:7163-70.
- Pafundi, D.H.; Laack, N.N.; Youland, R.S.; Parney, I.F.; Lowe, V.J.; Giannini, C.; Kemp, B.J.; Grams, M.P.; Morris, J.M.; Hoover, J.M.; et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro-Oncology 2013, 15, 1058–1067, . [CrossRef]
- Lopez, W.O.C.; Cordeiro, J.G.; Albicker, U.; Doostkam, S.; Nikkhah, G.; Kirch, R.D.; Trippel, M.; Reithmeier, T. Correlation of 18F-fluoroethyl tyrosine positron-emission tomography uptake values and histomorphological findings by stereotactic serial biopsy in newly diagnosed brain tumors using a refined software tool. OncoTargets Ther. 2015, 8, 3803–3815, . [CrossRef]
- Roodakker, K.R.; Alhuseinalkhudhur, A.; Al-Jaff, M.; Georganaki, M.; Zetterling, M.; Berntsson, S.G.; Danfors, T.; Strand, R.; Edqvist, P.-H.; Dimberg, A.; et al. Region-by-region analysis of PET, MRI, and histology in en bloc-resected oligodendrogliomas reveals intra-tumoral heterogeneity. Eur. J. Nucl. Med. 2019, 46, 569–579, . [CrossRef]
- Verburg, N.; Koopman, T.; Yaqub, M.M.; Hoekstra, O.S.; A Lammertsma, A.; Barkhof, F.; Pouwels, P.J.W.; Reijneveld, J.C.; Heimans, J.J.; Rozemuller, A.J.M.; et al. Improved detection of diffuse glioma infiltration with imaging combinations: a diagnostic accuracy study. Neuro-Oncology 2020, 22, 412–422, . [CrossRef]
- Schön, S.; Cabello, J.; Liesche-Starnecker, F.; Molina-Romero, M.; Eichinger, P.; Metz, M.; Karimov, I.; Preibisch, C.; Keupp, J.; Hock, A.; et al. Imaging glioma biology: spatial comparison of amino acid PET, amide proton transfer, and perfusion-weighted MRI in newly diagnosed gliomas. Eur. J. Nucl. Med. 2020, 47, 1468–1475, . [CrossRef]
- Filss CP, Cicone F, Shah NJ, Galldiks N, Langen KJ. Amino acid PET and MR perfusion imaging in brain tumours. Clin Transl Imaging. 2017;5:209-23.
- Cicone, F.; Filss, C.P.; Minniti, G.; Rossi-Espagnet, C.; Papa, A.; Scaringi, C.; Galldiks, N.; Bozzao, A.; Shah, N.J.; Scopinaro, F.; et al. Volumetric assessment of recurrent or progressive gliomas: comparison between F-DOPA PET and perfusion-weighted MRI. Eur. J. Nucl. Med. 2015, 42, 905–915, . [CrossRef]
- Göttler, J.; Lukas, M.; Kluge, A.; Kaczmarz, S.; Gempt, J.; Ringel, F.; Mustafa, M.; Meyer, B.; Zimmer, C.; Schwaiger, M.; et al. Intra-lesional spatial correlation of static and dynamic FET-PET parameters with MRI-based cerebral blood volume in patients with untreated glioma. Eur. J. Nucl. Med. 2017, 44, 392–397, . [CrossRef]
- Henriksen OM, Larsen VA, Muhic A, Hansen AE, Larsson HB, Poulsen HS, et al. Simultaneous evaluation of brain tumour metabolism, structure and blood volume using [(18)F]-fluoroethyltyrosine (FET) PET/MRI: feasibility, agreement and initial experience. Eur J Nucl Med Mol Imaging. 2016;43:103-12.
- Yuan, Y.; Yu, Y.; Guo, Y.; Chu, Y.; Chang, J.; Hsu, Y.; Liebig, P.A.; Xiong, J.; Yu, W.; Feng, D.; et al. Noninvasive Delineation of Glioma Infiltration with Combined 7T Chemical Exchange Saturation Transfer Imaging and MR Spectroscopy: A Diagnostic Accuracy Study. Metabolites 2022, 12, 901, . [CrossRef]
- D’souza, M.M.; Sharma, R.; Jaimini, A.; Panwar, P.; Saw, S.; Kaur, P.; Mondal, A.; Mishra, A.; Tripathi, R.P. 11C-MET PET/CT and Advanced MRI in the Evaluation of Tumor Recurrence in High-Grade Gliomas. Clin. Nucl. Med. 2014, 39, 791–798, . [CrossRef]
- Dandois, V.; Rommel, D.; Renard, L.; Jamart, J.; Cosnard, G. Substitution of 11C-methionine PET by perfusion MRI during the follow-up of treated high-grade gliomas: Preliminary results in clinical practice. J. Neuroradiol. 2010, 37, 89–97, . [CrossRef]
- Kim, Y.H.; Oh, S.W.; Lim, Y.J.; Park, C.-K.; Lee, S.-H.; Kang, K.W.; Jung, H.-W.; Chang, K.H. Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: Assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI. Clin. Neurol. Neurosurg. 2010, 112, 758–765, . [CrossRef]
- Deuschl, C.; Kirchner, J.; Poeppel, T.D.; Schaarschmidt, B.; Kebir, S.; El Hindy, N.; Hense, J.; Quick, H.H.; Glas, M.; Herrmann, K.; et al. 11C–MET PET/MRI for detection of recurrent glioma. Eur. J. Nucl. Med. 2018, 45, 593–601, . [CrossRef]
- Verger, A.; Filss, C.P.; Lohmann, P.; Stoffels, G.; Sabel, M.; Wittsack, H.-J.; Kops, E.R.; Galldiks, N.; Fink, G.R.; Shah, N.J.; et al. Comparison of O-(2- 18 F-Fluoroethyl)-L-Tyrosine Positron Emission Tomography and Perfusion-Weighted Magnetic Resonance Imaging in the Diagnosis of Patients with Progressive and Recurrent Glioma: A Hybrid Positron Emission Tomography/Magnetic Resonance Study. World Neurosurg. 2018, 113, e727–e737, . [CrossRef]
- Henriksen, O.M.; Hansen, A.E.; Muhic, A.; Marner, L.; Madsen, K.; Møller, S.; Hasselbalch, B.; Lundemann, M.J.; Scheie, D.; Skjøth-Rasmussen, J.; et al. Diagnostic yield of simultaneous dynamic contrast-enhanced magnetic resonance perfusion measurements and [18F]FET PET in patients with suspected recurrent anaplastic astrocytoma and glioblastoma. Eur. J. Nucl. Med. 2022, 49, 4677–4691, . [CrossRef]
- Steidl, E.; Langen, K.-J.; Abu Hmeidan, S.; Polomac, N.; Filss, C.P.; Galldiks, N.; Lohmann, P.; Keil, F.; Filipski, K.; Mottaghy, F.M.; et al. Sequential implementation of DSC-MR perfusion and dynamic [18F]FET PET allows efficient differentiation of glioma progression from treatment-related changes. Eur. J. Nucl. Med. 2020, 48, 1956–1965, . [CrossRef]
- Qiao, Z.; Zhao, X.; Wang, K.; Zhang, Y.; Fan, D.; Yu, T.; Shen, H.; Chen, Q.; Ai, L. Utility of Dynamic Susceptibility Contrast Perfusion-Weighted MR Imaging and 11C-Methionine PET/CT for Differentiation of Tumor Recurrence from Radiation Injury in Patients with High-Grade Gliomas. Am. J. Neuroradiol. 2019, 40, 253–259, . [CrossRef]
- Jena A, Taneja S, Gambhir A, Mishra AK, D'Souza M M, Verma SM, et al. Glioma Recurrence Versus Radiation Necrosis: Single-Session Multiparametric Approach Using Simultaneous O-(2-18F-Fluoroethyl)-L-Tyrosine PET/MRI. Clin Nucl Med. 2016;41:e228-36.
- Gambhir, A.; Sogani, S.K.; Jena, A.; Taneja, S.; Mishra, A.K.; D’souza, M.M.; Verma, S.M.; Hazari, P.P.; Negi, P.; Jadhav, G.K.R. Potential for differentiation of glioma recurrence from radionecrosis using integrated18F-fluoroethyl-L-tyrosine (FET) positron emission tomography/magnetic resonance imaging: A prospective evaluation. Neurol. India 2017, 65, 293–301, . [CrossRef]
- Jena A, Taneja S, Khan AA, Sogani SK. Recurrent Glioma: Does Qualitative Simultaneous 18F-DOPA PET/mp-MRI Improve Diagnostic Workup? An Initial Experience. Clin Nucl Med. 2021;46:703-9.
- Pyka, T.; Hiob, D.; Preibisch, C.; Gempt, J.; Wiestler, B.; Schlegel, J.; Straube, C.; Zimmer, C. Diagnosis of glioma recurrence using multiparametric dynamic 18F-fluoroethyl-tyrosine PET-MRI. Eur. J. Radiol. 2018, 103, 32–37, . [CrossRef]
- Lohmeier, J.; Bohner, G.; Siebert, E.; Brenner, W.; Hamm, B.; Makowski, M.R. Quantitative biparametric analysis of hybrid 18F-FET PET/MR-neuroimaging for differentiation between treatment response and recurrent glioma. Sci. Rep. 2019, 9, 1–9, . [CrossRef]
- Werner, J.-M.; Stoffels, G.; Lichtenstein, T.; Borggrefe, J.; Lohmann, P.; Ceccon, G.; Shah, N.J.; Fink, G.R.; Langen, K.-J.; Kabbasch, C.; et al. Differentiation of treatment-related changes from tumour progression: a direct comparison between dynamic FET PET and ADC values obtained from DWI MRI. Eur. J. Nucl. Med. 2019, 46, 1889–1901, . [CrossRef]
- Paprottka, K.J.; Kleiner, S.; Preibisch, C.; Kofler, F.; Schmidt-Graf, F.; Delbridge, C.; Bernhardt, D.; Combs, S.E.; Gempt, J.; Meyer, B.; et al. Fully automated analysis combining [18F]-FET-PET and multiparametric MRI including DSC perfusion and APTw imaging: a promising tool for objective evaluation of glioma progression. Eur. J. Nucl. Med. 2021, 48, 4445–4455, . [CrossRef]
- D’amore, F.; Grinberg, F.; Mauler, J.; Galldiks, N.; Blazhenets, G.; Farrher, E.; Filss, C.; Stoffels, G.; Mottaghy, F.M.; Lohmann, P.; et al. Combined 18F-FET PET and diffusion kurtosis MRI in posttreatment glioblastoma: differentiation of true progression from treatment-related changes. Neuro-Oncology Adv. 2021, 3, vdab044, . [CrossRef]
- Dang H, Zhang J, Wang R, Liu J, Fu H, Lin M, et al. Glioblastoma Recurrence Versus Radiotherapy Injury: Combined Model of Diffusion Kurtosis Imaging and 11C-MET Using PET/MRI May Increase Accuracy of Differentiation. Clin Nucl Med. 2022;47:e428-e36.
- Lombardi, G.; Spimpolo, A.; Berti, S.; Campi, C.; Anglani, M.G.; Simeone, R.; Evangelista, L.; Causin, F.; Zorzi, G.; Gorgoni, G.; et al. PET/MR in recurrent glioblastoma patients treated with regorafenib: [18F]FET and DWI-ADC for response assessment and survival prediction. Br. J. Radiol. 2022, 95, 20211018, . [CrossRef]
- Marner, L.; Nysom, K.; Sehested, A.; Borgwardt, L.; Mathiasen, R.; Henriksen, O.M.; Lundemann, M.; Rosenschöld, P.M.A.; Thomsen, C.; Bøgeskov, L.; et al. Early Postoperative 18F-FET PET/MRI for Pediatric Brain and Spinal Cord Tumors. J. Nucl. Med. 2019, 60, 1053–1058, . [CrossRef]
- Marner, L.; Lundemann, M.; Sehested, A.; Nysom, K.; Borgwardt, L.; Mathiasen, R.; Wehner, P.S.; Henriksen, O.M.; Thomsen, C.; Skjøth-Rasmussen, J.; et al. Diagnostic accuracy and clinical impact of [18F]FET PET in childhood CNS tumors. Neuro-Oncology 2021, 23, 2107–2116, . [CrossRef]
- Bezrukov, I.; Schmidt, H.; Gatidis, S.; Mantlik, F.; Schäfer, J.F.; Schwenzer, N.F.; Pichler, B.J. Quantitative Evaluation of Segmentation- and Atlas-Based Attenuation Correction for PET/MR on Pediatric Patients. J. Nucl. Med. 2015, 56, 1067–1074, . [CrossRef]
- Ladefoged, C.N.; Law, I.; Anazodo, U.; Lawrence, K.S.; Izquierdo-Garcia, D.; Catana, C.; Burgos, N.; Cardoso, M.J.; Ourselin, S.; Hutton, B.; et al. A multi-centre evaluation of eleven clinically feasible brain PET/MRI attenuation correction techniques using a large cohort of patients. NeuroImage 2017, 147, 346–359, . [CrossRef]
- Dunkl, V.; Cleff, C.; Stoffels, G.; Judov, N.; Sarikaya-Seiwert, S.; Law, I.; Bøgeskov, L.; Nysom, K.; Andersen, S.B.; Steiger, H.-J.; et al. The Usefulness of Dynamic O-(2-18F-Fluoroethyl)-l-Tyrosine PET in the Clinical Evaluation of Brain Tumors in Children and Adolescents. J. Nucl. Med. 2015, 56, 88–92, . [CrossRef]
- Morana, G.; Piccardo, A.; Puntoni, M.; Nozza, P.; Cama, A.; Raso, A.; Mascelli, S.; Massollo, M.; Milanaccio, C.; Garrè, M.L.; et al. Diagnostic and prognostic value of18F-DOPA PET and1H-MR spectroscopy in pediatric supratentorial infiltrative gliomas: a comparative study. Neuro-Oncology 2015, 17, 1637–1647, . [CrossRef]
- Pirotte, B.J.M.; Lubansu, A.; Massager, N.; Wikler, D.; Van Bogaert, P.; Levivier, M.; Brotchi, J.; Goldman, S. Clinical impact of integrating positron emission tomography during surgery in 85 children with brain tumors. J. Neurosurgery: Pediatr. 2010, 5, 486–499, . [CrossRef]
- Kertels O, Krauss J, Monoranu CM, Samnick S, Dierks A, Kircher M, et al. [(18)F]FET-PET in children and adolescents with central nervous system tumors: does it support difficult clinical decision-making? Eur J Nucl Med Mol Imaging. 2023.
- Morana, G.; Piccardo, A.; Tortora, D.; Puntoni, M.; Severino, M.; Nozza, P.; Ravegnani, M.; Consales, A.; Mascelli, S.; Raso, A.; et al. Grading and outcome prediction of pediatric diffuse astrocytic tumors with diffusion and arterial spin labeling perfusion MRI in comparison with 18F–DOPA PET. Eur. J. Nucl. Med. 2017, 44, 2084–2093, . [CrossRef]
| Reference | Year | PET Tracer | MR-methods | Tumor type | No of subjects | Remarks | Main Result |
|---|---|---|---|---|---|---|---|
| Verburg et al. [86] | 2020 | 18F-FET | PWI, DWI, MRS | Newly diagnosed gliomas | 20 | Tumor infiltration, Verification of tumor extent by biopsies | Best result for combined 18F-FET + ADC in depicting enhancing gliomas |
| Haubold et al. [78] | 2020 | 18F-FET | DWI, ADC, SWI | Phenotyping of newly diagnosed gliomas | 42 | Radiomics, multiparametric MRI and 18F-FET PET parameters | Best differentiation of high-grade and low-grade glioma by combination of 18F-FET PET, T1ce and SWI |
| Song et al.[77] | 2021 | 18F-FET | PWI | Phenotyping of newly diagnosed gliomas | 52 | Retrospective evaluation after surgery | Improved differentiation of IDH status by combination of 18F-FET PET and PWI |
| Pyka et al. [76] | 2022 | 18F-FET | MRSI | Newly diagnosed gliomas | 67 | Characterization of intracranial gliomas | Improved differentiation of high-grade from low-grade glioma and of glioblastoma from non-glioblastoma |
| Reference | Year | PET Tracer | MR-methods | Tumor type | No of subjects | Remarks | Main Result |
|---|---|---|---|---|---|---|---|
| Jena et al. [101] | 2016 | 18F-FET | PWI, DWI, MRSI | Tumor recurrence in pretreated gliomas | 26 | Verification by surgery (9) and clinical follow-up (17) | Best AUC by combination of 18F-FET PET, rCBV and MRS (0.94) versus 18F-FET PET (0.89), ADC (0.74), PWI (0.85), MRS (0.89) |
| Sogani et al. [102] | 2017 | 18F-FET | PWI, DWI, MRSI | Tumor recurrence in pretreated gliomas | 32 | Verification by surgery (12) and clinical follow-up (20) | Best accuracy by combination of 18F-FET PET, ADC, rCBV and MRS (97%) |
| Pyka et al. [104] | 2018 | 18F-FET | PWI, DWI | Tumor recurrence in pretreated gliomas | 47(63 lesions) | Verification by surgery (23) and clinical follow-up (40) | Improved accuracy by combination of 18F-FET PET, ADC and rCBV (AUC 0.89) |
| Lohmeier et al. [105] | 2019 | 18F-FET | DWI-ADC | Recurrent high and low grade gliomas | 42 | Verification by surgery (36) and clinical follow-up (6) | Best AUC by combination of static 18F-FET PET and ADC (90%) versus 18F-FET PET (0.81) or ADC alone (0.82) |
| Qiao et al. [100] | 2019 | 11C-MET | PWI-DSC | Recurrent high and low grade gliomas | 42 | Verification by surgery (32) and clinical follow-up (10) | Best AUC by combination of 11C-MET PET and rCBV (0.95) versus 11C-MET PET (0.85) or rCBV alone (0.85) |
| Paprottka et al. [107] | 2021 | 18F-FET | APT-CEST, PWI | Tumor recurrence in pretreated gliomas | 66 (74 lesions) | Verification by surgery (46) and clinical follow-up (31), ADC evaluation guided by 18F-FET PET | Best accuracy by combination of 18F-FET PET, APT-CEST and PWI (0.85) versus 18F-FET PET alone (0.81) |
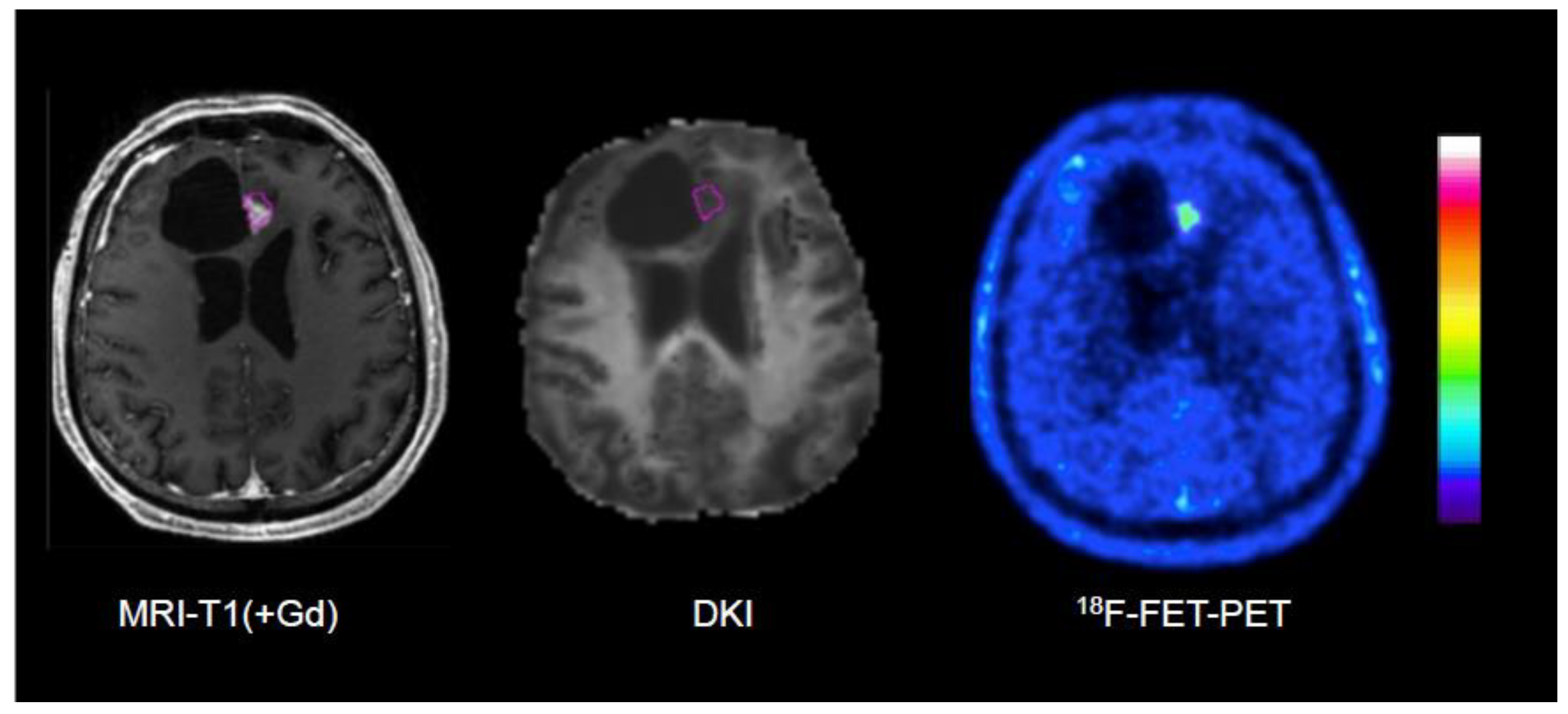
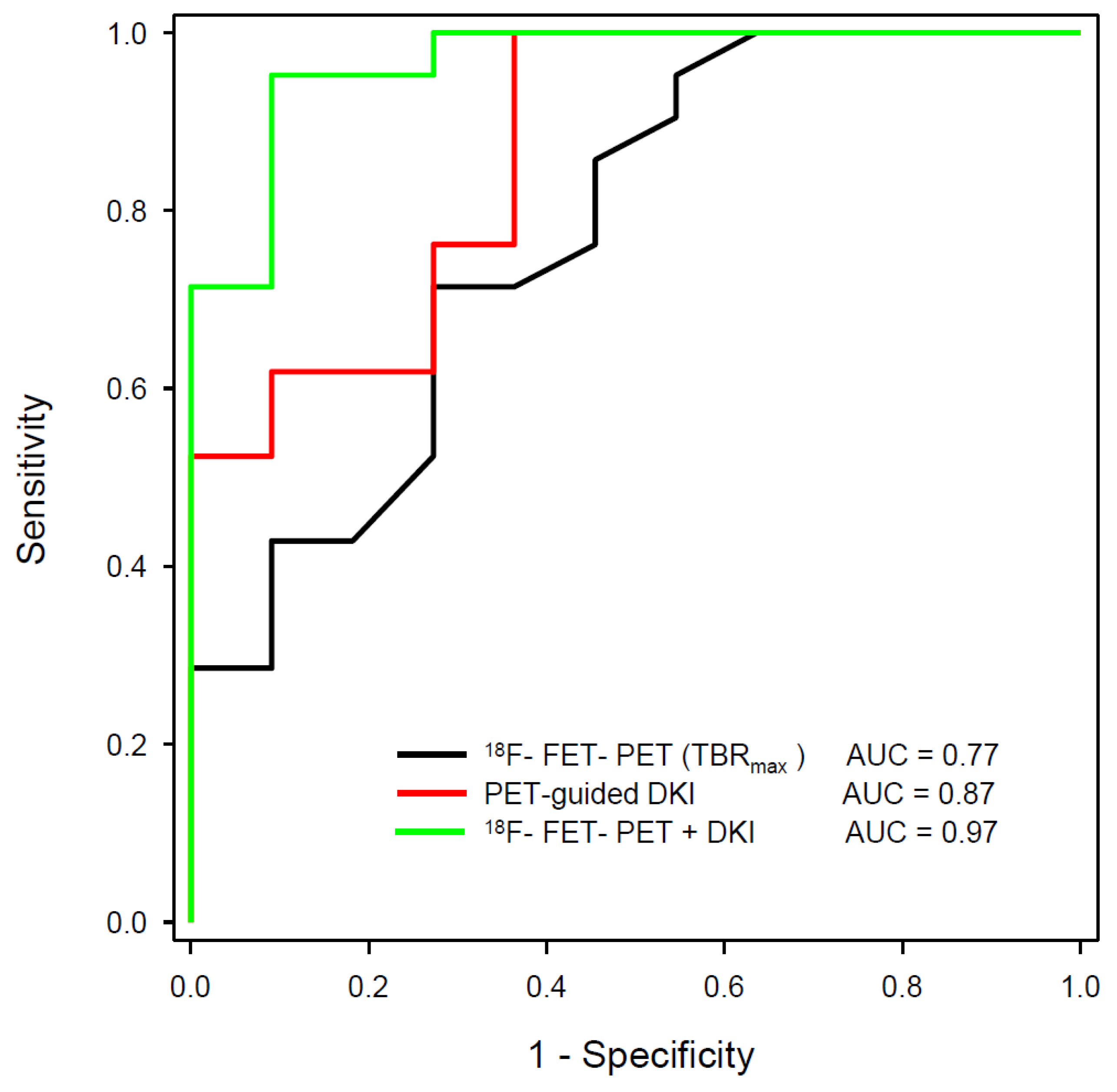
| D’Amore et al. [108] | 2021 | 18F-FET | DWI, DKI | Tumor recurrence in pretreated gliomas | 32 | Verification by surgery (12) and clinical follow-up (20), DKI evaluation guided by 18F-FET PET | Best AUC by combination of static 18F-FET PET and DKI (0.97) versus 18F-FET PET (0.77) or DKI alone (0.87) |
| Jena et al. [103] | 2021 | 18F-FDOPA | PWI, DWI, MRS | Tumor recurrence in pretreated gliomas | 26 | Verification by surgery (4) and clinical follow-up (22) | Best AUC by combination of 18F-FDOPA PET, rCBV, ADC and MRS (0.94) versus 18F-FDOPA-PET (0.81), ADC (0.42), rCBV (0.50) and MRS (0.77) alone |
| Lombardi et al. [110] | 2021 | 18F-FET | DWI | Monitoring of regorafenib therapy in recurrent glioblastoma | 16 | Verification by clinical follow-up, ADC evaluation guided by 18F-FET PET | 18F-FET guided ADC promising for therapy monitoring, better than RANO |
| Dang et al. [109] | 2022 | 11C-MET | DWI, DKI | Tumor recurrence in pretreated gliomas | 86 | Verification by surgery (23) and clinical follow-up (20) | Best AUC by combination of 11C-MET PET and DKI (0.95). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





