Submitted:
31 May 2023
Posted:
01 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methodology
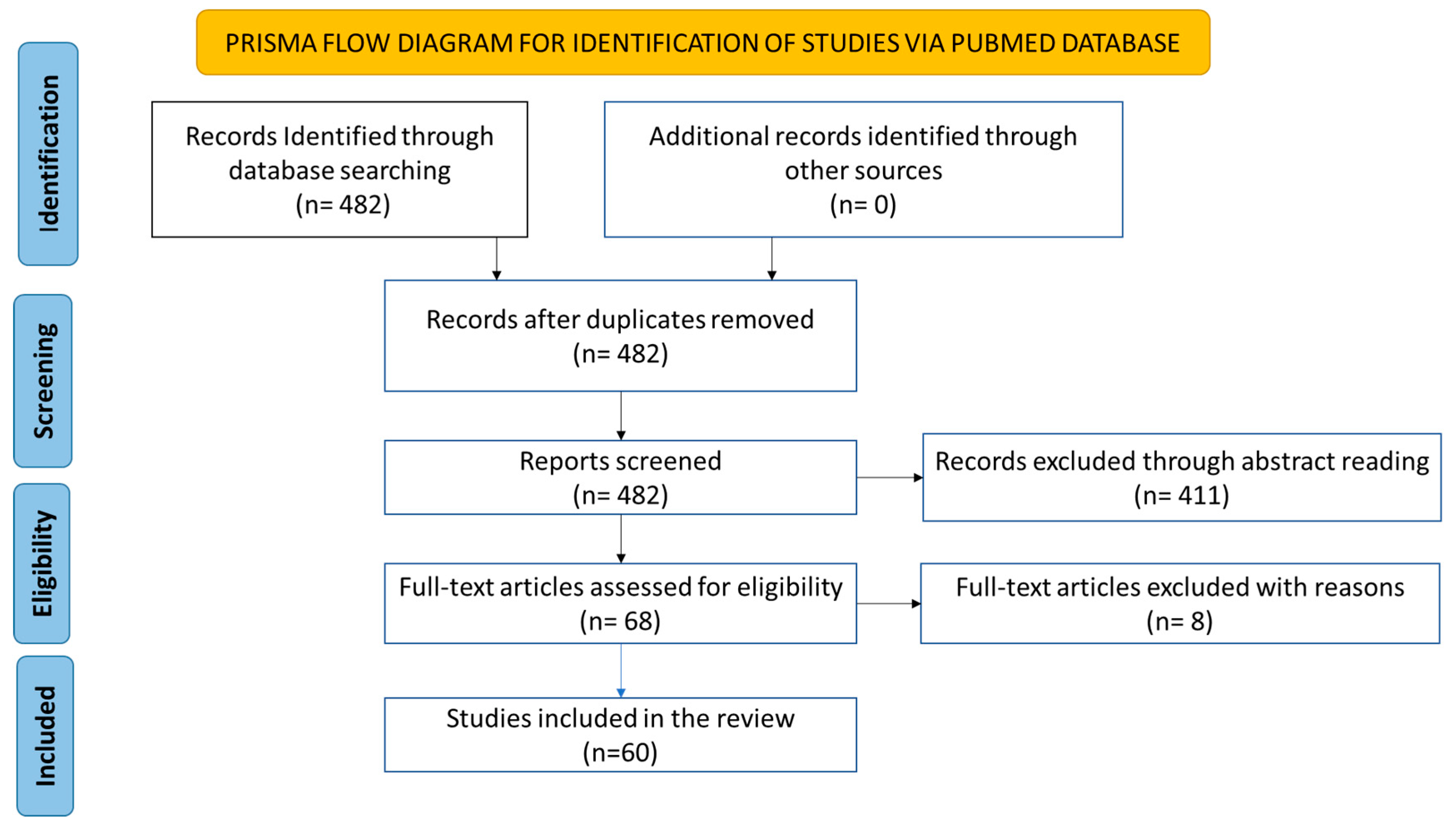
2.1. Literature Search
2.2. Paper Selection
2.3. Quality Criteria using Newcastle-Ottawa Quality Assessment Scale
2.4. Relevant Data Extraction
3. Result and Discussion
3.1. Analogy
3.2. Biological Plausibility and Coherence
3.3. Identification of Human Papillomaviruses in Prostate Tissues
4. Strength of Association
4.1. Serology
4.2. Polymerase Chain Reaction
4.3. Specificity
4.4. Transmission of Virus
4.5. Temporality
4.6. Oncogenic Mechanism
5. Consistency
5.1. Consistency in Tissue Collection
5.1.1. Consistency in Tissue Storage
6. Biological Gradient
7. Experimental Evidence
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abumsimir, B. et al. (2022) ‘Molecular characterization of human papillomavirus and mouse mammary tumor virus-like infections in prostate cancer tissue and relevance with tumor characteristics.’, Molecular and clinical oncology, 16(5), p. 97. [CrossRef]
- Adami, H.-O. et al. (2003) ‘Prostate cancer risk and serologic evidence of human papilloma virus infection: a population-based case-control study.’, Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 12(9), pp. 872–875.
- Aghakhani, A. et al. (2010) ‘The role of human papillomavirus infection in prostate carcinoma’, Scandinavian Journal of Infectious Diseases, 43(1), pp. 64–69. [CrossRef]
- Ahmed, M.Y. et al. (2023) ‘Detection of high - risk Human Papillomavirus in prostate cancer from a UK based population’, Scientific Reports, pp. 1–9. [CrossRef]
- Anderson, M. et al. (1997) ‘Analysis of prostate tissue DNA for the presence of human papillomavirus by polymerase chain reaction, cloning, and automated sequencing.’, Journal of medical virology, 52(1), pp. 8–13. [CrossRef]
- Anwar, K. et al. (1992) ‘Presence of ras oncogene mutations and human papillomavirus DNA in human prostate carcinomas.’, Cancer research, 52(21), pp. 5991–5996.
- Araldi, R.P. et al. (2018) ‘Biomedicine & Pharmacotherapy The human papillomavirus ( HPV ) -related cancer biology : An overview’, Biomedicine & Pharmacotherapy, 106(June), pp. 1537–1556. [CrossRef]
- Atashafrooz, F. and Rokhbakhsh-Zamin, F. (2016) ‘Frequency and type distribution of human papilloma virus in patients with prostate cancer, Kerman, southeast of Iran’, Asian Pacific Journal of Cancer Prevention, 17(8), pp. 3951–3956.
- Aydin, M. et al. (2017) ‘Lack of evidence of HPV etiology of prostate cancer following radical surgery and higher frequency of the Arg/Pro genotype in Turkish men with prostate cancer.’, International braz j urol : official journal of the Brazilian Society of Urology, 43(1), pp. 36–46. [CrossRef]
- Bergh, J. et al. (2007) ‘No link between viral findings in the prostate and subsequent cancer development.’, British journal of cancer, 96(1), pp. 137–139. [CrossRef]
- Bostwick, D.G. et al. (2004) ‘Human prostate cancer risk factors’, Cancer, 101(10 SUPPL.), pp. 2371–2490. [CrossRef]
- Burns, M.B., Temiz, N.A. and Harris, R.S. (2013) ‘Evidence for APOBEC3B mutagenesis in multiple human cancers.’, Nature genetics, 45(9), pp. 977–983. [CrossRef]
- De Carolis, S. et al. (2019) ‘HPV DNA Associates With Breast Cancer Malignancy and It Is Transferred to Breast Cancer Stromal Cells by Extracellular Vesicles’, Frontiers in Oncology, 9(September), pp. 1–12. [CrossRef]
- Carozzi, F. et al. (2004) ‘Association of human papillomavirus with prostate cancer: analysis of a consecutive series of prostate biopsies.’, The International journal of biological markers, 19(4), pp. 257–261. [CrossRef]
- Caval, V. et al. (2014) ‘A prevalent cancer susceptibility APOBEC3A hybrid allele bearing APOBEC3B 3’UTR enhances chromosomal DNA damage.’, Nature communications, 5, p. 5129. [CrossRef]
- Chang, H.-J. et al. (2023) ‘A matched case-control study in Taiwan to evaluate potential risk factors for prostate cancer’, Scientific Reports, 13(1), pp. 1–10. [CrossRef]
- Chang, R.T.M., Kirby, R. and Challacombe, B.J. (2012) ‘Is there a link between BPH and prostate cancer?’, The Practitioner, 256(1750), pp. 2,13-16.
- Chen, A.C.H. et al. (2011) ‘Human papillomavirus in benign prostatic hyperplasia and prostatic adenocarcinoma patients’, Pathology and Oncology Research, 17(3), pp. 613–617. [CrossRef]
- Chen, Y. and Wei, J. (2015) ‘Identification of pathogen signatures in prostate cancer using RNA-seq’, PLoS ONE, 10(6), pp. 1–13. [CrossRef]
- Cheng, A.Z. et al. (2019) ‘Epstein-Barr virus BORF2 inhibits cellular APOBEC3B to preserve viral genome integrity.’, Nature microbiology. England, pp. 78–88. [CrossRef]
- Choo, C.K. et al. (1999) ‘Immortalization of human prostate epithelial cells by HPV 16 E6/E7 open reading frames.’, The Prostate, 40(3), pp. 150–158. [CrossRef]
- Chughtai, B. et al. (2016) ‘Benign prostatic hyperplasia.’, Nature reviews. Disease primers, 2, p. 16031. [CrossRef]
- Cirakoglu, A., Benli, E. and Yuce, A. (2018) ‘Polygamy, sexual behavior in a population under risk for prostate cancer diagnostic: an observational study from the Black Sea Region in Turkey.’, International braz j urol : official journal of the Brazilian Society of Urology, 44(4), pp. 704–708. [CrossRef]
- Combes, J.D. et al. (2014) ‘Antibodies against high-risk human papillomavirus proteins as markers for invasive cervical cancer’, International Journal of Cancer, 135(10), pp. 2453–2461. [CrossRef]
- Dagnelie, P.C. et al. (2004) ‘Diet, anthropometric measures and prostate cancer risk: a review of prospective cohort and intervention studies.’, BJU international, 93(8), pp. 1139–1150.
- Díaz-Cano, S.J. and Brady, S.P. (1997) ‘DNA extraction from formalin-fixed, paraffin-embedded tissues: protein digestion as a limiting step for retrieval of high-quality DNA.’, Diagnostic molecular pathology : the American journal of surgical pathology, part B, 6(6), pp. 342–346. [CrossRef]
- Dillner, J. et al. (1998) ‘Sero-epidemiologal association between human-papillomavirus infection and risk of prostate cancer’, International Journal of Cancer, 75(4), pp. 564–567. [CrossRef]
- Dodd, J.G., Paraskevas, M. and McNicol, P.J. (1993) ‘Detection of human papillomavirus 16 transcription in human prostate tissue.’, The Journal of urology, 149(2), pp. 400–402. [CrossRef]
- Eghbali, S.S. et al. (2012) ‘Oncogenic human papillomavirus genital infection in southern Iranian women : population-based study versus clinic-based data’, pp. 1–6.
- Fabbri, M. et al. (2012) ‘MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response’, Proceedings of the National Academy of Sciences of the United States of America, 109(31). [CrossRef]
- Falcaro, M. et al. (2021) ‘The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study’, The Lancet, 398(10316), pp. 2084–2092. [CrossRef]
- Faraoni, I. et al. (2009) ‘miR-155 gene: a typical multifunctional microRNA.’, Biochimica et biophysica acta, 1792(6), pp. 497–505. [CrossRef]
- Fatemipour, M. et al. (2021) ‘Human papillomavirus and prostate cancer: The role of viral expressed proteins in the inhibition of anoikis and induction of metastasis’, Microbial Pathogenesis, 152, p. 104576. [CrossRef]
- Fedak, K.M. et al. (2015) ‘Applying the Bradford Hill criteria in the 21st century: How data integration has changed causal inference in molecular epidemiology’, Emerging Themes in Epidemiology, 12(1), pp. 1–9. [CrossRef]
- Gandaglia, G. et al. (2021) ‘Epidemiology and Prevention of Prostate Cancer’, European urology oncology, 4(6), pp. 877–892. [CrossRef]
- Gansmo, L.B. et al. (2018) ‘APOBEC3A/B deletion polymorphism and cancer risk.’, Carcinogenesis, 39(2), pp. 118–124. [CrossRef]
- Gazzaz, F.S. and Mosli, H.A. (2009) ‘Lack of detection of human papillomavirus infection by hybridization test in prostatic biopsies.’, Saudi medical journal, 30(5), pp. 633–637.
- Ghasemian, E. et al. (2013) ‘Evaluation of human papillomavirus infections in prostatic disease: A cross-sectional study in Iran’, Asian Pacific Journal of Cancer Prevention, 14(5), pp. 3305–3308. [CrossRef]
- Gironella, M. et al. (2007) ‘Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development.’, Proceedings of the National Academy of Sciences of the United States of America, 104(41), pp. 16170–16175. [CrossRef]
- Glenn, W.K. et al. (2017) ‘High risk human papilloma viruses (HPVs) are present in benign prostate tissues before development of HPV associated prostate cancer’, Infectious Agents and Cancer, 12(1), pp. 1–10. [CrossRef]
- Graham, S. V (2017) ‘The human papillomavirus replication cycle, and its links to cancer progression : a comprehensive review’, 0(July), pp. 2201–2221.
- Guenat, D. et al. (2017) ‘Exosomes and other extracellular vesicles in HPV transmission and carcinogenesis’, Viruses, 9(8), pp. 1–17. [CrossRef]
- Hanahan, D. and Weinberg, R.A. (2011) ‘Hallmarks of cancer: the next generation.’, Cell, 144(5), pp. 646–674. [CrossRef]
- Hill, A. (1965) ‘President’ s Address The Environment and Disease: Association or causation?’, Proc R Soc Med, (58), pp. 295–300.
- Hodgson, A. et al. (2019) ‘International Endocervical Adenocarcinoma Criteria and Classification (IECC): correlation with adverse clinicopathological features and patient outcome.’, Journal of clinical pathology, 72(5), pp. 347–353. [CrossRef]
- Hrbacek, J. et al. (2011) ‘Serum antibodies against genitourinary infectious agents in prostate cancer and benign prostate hyperplasia patients: a case-control study.’, BMC cancer, 11, p. 53. [CrossRef]
- Ibrahim, G.K. et al. (1992) ‘Detection of human papillomavirus in the prostate by polymerase chain reaction and in situ hybridization’, Journal of Urology, pp. 1822–1826. [CrossRef]
- Itoh, Y. and Nagase, H. (2002) ‘Matrix metalloproteinases in cancer.’, Essays in biochemistry, 38, pp. 21–36. [CrossRef]
- Jeon, S., Allen-hoffmann, B.L. and Lambert, P.F. (1995) ‘Integration of Human Papillomavirus Type 16 into the Human Genome Correlates with a Selective Growth Advantage of Cells †’, 69(5), pp. 2989–2997.
- Jian, Z. et al. (2018) ‘Sexual Activity and Risk of Prostate Cancer: A Dose-Response Meta-Analysis.’, The journal of sexual medicine, 15(9), pp. 1300–1309. [CrossRef]
- Johnson, A.M. et al. (2012) ‘Epidemiology of, and behavioural risk factors for, sexually transmitted human papillomavirus infection in men and women in Britain’, Sexually Transmitted Infections, 88(3), pp. 212–217. [CrossRef]
- Khatami, A. et al. (2022) ‘Human papilloma virus (HPV) and prostate cancer (PCa): The potential role of HPV gene expression and selected cellular MiRNAs in PCa development’, Microbial Pathogenesis, 166(March), p. 105503. [CrossRef]
- Korodi, Z. et al. (2005) ‘Human papillomavirus 16, 18, and 33 infections and risk of prostate cancer: A Nordic nested case-control study’, Cancer Epidemiology Biomarkers and Prevention, 14(12), pp. 2952–2955. [CrossRef]
- Lawson, J.S. and Glenn, W.K. (2020) ‘Evidence for a causal role by human papillomaviruses in prostate cancer-A systematic review’, Infectious Agents and Cancer, 15(1), pp. 1–11. [CrossRef]
- Leechanachai, P. et al. (1992) ‘The E5 gene from human papillomavirus type 16 is an oncogene which enhances growth factor-mediated signal transduction to the nucleus.’, Oncogene, 7(1), pp. 19–25.
- Leiros, G.J. et al. (2005) ‘Detection of human papillomavirus DNA and p53 codon 72 polymorphism in prostate carcinomas of patients from Argentina.’, BMC urology, 5, p. 15. [CrossRef]
- Leitzmann, M.F. and Rohrmann, S. (2012) ‘Risk factors for the onset of prostatic cancer: age, location, and behavioral correlates.’, Clinical epidemiology, 4, pp. 1–11. [CrossRef]
- Lenze, D., Müller, H.H. and Hummel, M. (2012) ‘Considerations for the use of formalin-fixed and paraffin-embedded tissue specimens for clonality analysis’, Journal of Hematopathology, 5(1–2), pp. 27–34. [CrossRef]
- Madani, T.A. (2006) ‘Sexually transmitted infections in Saudi Arabia.’, BMC infectious diseases, 6, p. 3. [CrossRef]
- Madden, S.K. et al. (2021) ‘Taking the Myc out of cancer: toward therapeutic strategies to directly inhibit c-Myc.’, Molecular cancer, 20(1), p. 3. [CrossRef]
- Mahesh, G. and Biswas, R. (2019) ‘MicroRNA-155: A Master Regulator of Inflammation.’, Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research, 39(6), pp. 321–330. [CrossRef]
- Martinez-Fierro, M.L. et al. (2010) ‘Identification of viral infections in the prostate and evaluation of their association with cancer.’, BMC cancer, 10, p. 326. [CrossRef]
- Masood, S. et al. (1991) ‘Human papillomavirus in prostatic cancer: no evidence found by in situ DNA hybridization.’, Southern medical journal, 84(2), pp. 235–236. [CrossRef]
- Mcbride, A.A. and Warburton, A. (2017) ‘The role of integration in oncogenic progression of HPV-associated cancers’, pp. 1–7.
- McLaughlin-Drubin, M.E. and Münger, K. (2009) ‘Oncogenic activities of human papillomaviruses’, Virus Research, 143(2), pp. 195–208. [CrossRef]
- McNicol, P.J. and Dodd, J.G. (1990) ‘Detection of human papillomavirus DNA in prostate gland tissue by using the polymerase chain reaction amplification assay’, Journal of Clinical Microbiology, 28(3), pp. 409–412. [CrossRef]
- McNicol, P.J. and Dodd, J.G. (1991) ‘High prevalence of human papillomavirus in prostate tissues’, Journal of Urology, 145(4), pp. 850–853. [CrossRef]
- Medel-Flores, O. et al. (2018) ‘Association between HPV infection and prostate cancer in a Mexican population.’, Genetics and molecular biology, 41(4), pp. 781–789. [CrossRef]
- Michopoulou, V. et al. (2014) ‘Detection of human papillomavirus (HPV) DNA prevalence and p53 codon 72 (Arg72Pro) polymorphism in prostate cancer in a Greek group of patients’, Tumor Biology, 35(12), pp. 12765–12773. [CrossRef]
- Miller, D.M. et al. (2012) ‘c-Myc and cancer metabolism.’, Clinical cancer research : an official journal of the American Association for Cancer Research, 18(20), pp. 5546–5553. [CrossRef]
- Moghoofei, M. et al. (2019) ‘Association between human papillomavirus infection and prostate cancer: A global systematic review and meta-analysis’, Asia-Pacific Journal of Clinical Oncology, 15(5), pp. e59–e67. [CrossRef]
- Mokhtari, M., Taghizadeh, F. and Hani, M. (2013) ‘Is prostatic adenocarcinoma in a relationship with Human Papilloma Virus in Isfahan- Iran’, Journal of Research in Medical Sciences, 18(8), pp. 707–710.
- Moody, C.A. and Laimins, L.A. (2010) ‘Human papillomavirus oncoproteins : pathways to transformation’, Nature Publishing Group, 10(8), pp. 550–560. [CrossRef]
- Moyret-Lalle, C. et al. (1995) ‘ras, p53 and HPV status in benign and malignant prostate tumors.’, International journal of cancer, 64(2), pp. 124–129. [CrossRef]
- Murphy, W.M. et al. (1983) ‘Papillomavirus structural antigens in condyloma acuminatum of the male urethra.’, The Journal of urology, 130(1), pp. 84–85. [CrossRef]
- Nik-Zainal, S. et al. (2014) ‘Association of a germline copy number polymorphism of APOBEC3A and APOBEC3B with burden of putative APOBEC-dependent mutations in breast cancer.’, Nature genetics, 46(5), pp. 487–491. [CrossRef]
- Ohba, K. et al. (2014) ‘In vivo and in vitro studies suggest a possible involvement of HPV infection in the early stage of breast carcinogenesis via APOBEC3B induction.’, PloS one, 9(5), p. e97787. [CrossRef]
- Pascale, M. et al. (2013) ‘Is human papillomavirus associated with prostate cancer survival?’, Disease Markers, 35(6), pp. 607–613. [CrossRef]
- Peng, Y. and Croce, C.M. (2016) ‘The role of MicroRNAs in human cancer’, Signal Transduction and Targeted Therapy, 1(1), p. 15004. [CrossRef]
- Pereira, N.M. et al. (2023) ‘Presence of HPV in prostate tissue from patients submitted to prostate biopsy’, Acta Cirurgica Brasileira, 37(12), pp. 1–8. [CrossRef]
- Pernar, C.H. et al. (2018) ‘The Epidemiology of Prostate Cancer.’, Cold Spring Harbor perspectives in medicine, 8(12). [CrossRef]
- Pim, D. and Banks, L. (2010) ‘Interaction of viral oncoproteins with cellular target molecules: Infection with high-risk vs low-risk human papillomaviruses’, Apmis, 118(6–7), pp. 471–493. [CrossRef]
- Vande Pol, S.B. and Klingelhutz, A.J. (2013) ‘Papillomavirus E6 oncoproteins’, Virology, 445(1–2), pp. 115–137. [CrossRef]
- Rawla, P. (2019) ‘Epidemiology of Prostate Cancer.’, World journal of oncology, 10(2), pp. 63–89. [CrossRef]
- Rodriguez, M.I.D. et al. (2016) ‘Human papilloma virus detection by INNOLiPA HPV in prostate tissue from men of Northeast Mexico’, Asian Pacific Journal of Cancer Prevention, 17(11), pp. 4863–4865. [CrossRef]
- Roman, A. and Munger, K. (2013) ‘The papillomavirus E7 proteins’, Virology, 445(1–2), pp. 138–168. [CrossRef]
- Rosenblatt, K.A. et al. (2003) ‘Serologic evidence of human papillomavirus 16 and 18 infections and risk of prostate cancer.’, Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 12(8), pp. 763–768.
- Rotola, A. et al. (1992) ‘Presence and physical state of HPV DNA in prostate and urinary-tract tissues’, International Journal of Cancer, 52(3), pp. 359–365. [CrossRef]
- Sarkar, F.H. et al. (1993) ‘Detection of human papillomavirus (HPV) DNA in human prostatic tissues by polymerase chain reaction (PCR).’, The Prostate, 22(2), pp. 171–180. [CrossRef]
- Serth, J. et al. (1999) ‘Increased levels of human papillomavirus type 16 DNA in a subset of prostate cancers.’, Cancer research, 59(4), pp. 823–825.
- Sfanos, K.S. et al. (2008) ‘A molecular analysis of prokaryotic and viral DNA sequences in prostate tissue from patients with prostate cancer indicates the presence of multiple and diverse microorganisms.’, The Prostate, 68(3), pp. 306–320. [CrossRef]
- Singh, N. et al. (2015) ‘Implication of high risk Human papillomavirus HR-HPV infection in prostate cancer in Indian population-A pioneering case-control analysis’, Scientific Reports, 5, pp. 7–10. [CrossRef]
- Stoler, M.H. et al. (1991) ‘Small-cell neuroendocrine carcinoma of the cervix. A human papillomavirus type 18-associated cancer.’, The American journal of surgical pathology, 15(1), pp. 28–32. [CrossRef]
- Stolnicu, S. et al. (2018) ‘International Endocervical Adenocarcinoma Criteria and Classification (IECC): A New Pathogenetic Classification for Invasive Adenocarcinomas of the Endocervix.’, The American journal of surgical pathology, 42(2), pp. 214–226. [CrossRef]
- Strickler, H.D. et al. (1998) ‘A multifaceted study of human papillomavirus and prostate carcinoma.’, Cancer, 82(6), pp. 1118–1125.
- Sung, H. et al. (2021) ‘Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries’, CA: A Cancer Journal for Clinicians, 71(3), pp. 209–249. [CrossRef]
- Sutcliffe, S. et al. (2007) ‘Plasma antibodies against Chlamydia trachomatis, human papillomavirus, and human herpesvirus type 8 in relation to prostate cancer: a prospective study.’, Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 16(8), pp. 1573–1580. [CrossRef]
- Sutcliffe, S. et al. (2010) ‘Human papillomavirus types 16, 18, and 31 serostatus and prostate cancer risk in the Prostate Cancer Prevention Trial.’, Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 19(2), pp. 614–618. [CrossRef]
- Tachezy, R. et al. (2012) ‘HPV persistence and its oncogenic role in prostate tumors.’, Journal of medical virology, 84(10), pp. 1636–1645. [CrossRef]
- Terris, M.K. and Peehl, D.M. (1997) ‘Human papillomavirus detection by polymerase chain reaction in benign and malignant prostate tissue is dependent on the primer set utilized.’, Urology, 50(1), pp. 150–156. [CrossRef]
- Torjesen, I. (2021) ‘HPV vaccine cut cervical cancer rates in England by 87\%’, BMJ, 375. [CrossRef]
- Tu, H. et al. (1994) ‘Rare incidence of human papillomavirus types 16 and 18 in primary and metastatic human prostate cancer.’, Urology, 44(5), pp. 726–731. [CrossRef]
- Tung, C.-H. et al. (2020) ‘MicroRNA-150-5p promotes cell motility by inhibiting c-Myb-mediated Slug suppression and is a prognostic biomarker for recurrent ovarian cancer.’, Oncogene, 39(4), pp. 862–876. [CrossRef]
- Vieira, V.C. et al. (2014) ‘Human papillomavirus E6 triggers upregulation of the antiviral and cancer genomic DNA deaminase APOBEC3B.’, mBio, 5(6). [CrossRef]
- Whitaker, N.J. et al. (2013) ‘Human papillomavirus and Epstein Barr virus in prostate cancer: Koilocytes indicate potential oncogenic influences of human papillomavirus in prostate cancer’, Prostate, 73(3), pp. 236–241. [CrossRef]
- Williams, S.M.G. et al. (2005) ‘Requirement of Epidermal Growth Factor Receptor for Hyperplasia Induced by E5, a High-Risk Human Papillomavirus Oncogene’, (15), pp. 6534–6542.
- Yang, L. et al. (2015) ‘Worldwide Prevalence of Human Papillomavirus and Relative Risk of Prostate Cancer: A Meta-analysis’, Scientific Reports, 5, pp. 1–10. [CrossRef]
- Yow, M.A. et al. (2014) ‘Detection of infectious organisms in archival prostate cancer tissues’, BMC Cancer, 14(1), pp. 1–5. [CrossRef]
- Zambrano, A. et al. (2002) ‘Detection of human polyomaviruses and papillomaviruses in prostatictissue reveals the prostate as a habitat for multipleviral infections’, Prostate, 53(4), pp. 263–276. [CrossRef]
- Zhang, Z. et al. (2018) ‘MicroRNA-150 promotes cell proliferation, migration, and invasion of cervical cancer through targeting PDCD4.’, Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 97, pp. 511–517. [CrossRef]
- Zhao, H. et al. (2021) ‘Inflammation and tumor progression: signaling pathways and targeted intervention’, Signal Transduction and Targeted Therapy, 6(1), p. 263. [CrossRef]
- Zhao, X. et al. (2017) ‘Role of antibodies to human papillomavirus 16 in prostate cancer: A seroscreening by peptide microarray.’, Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine, 39(6), p. 1010428317698371. [CrossRef]
- Zhou, Y. et al. (2017) ‘Diagnosis of cancer as an emergency: A critical review of current evidence’, Nature Reviews Clinical Oncology, 14(1), pp. 45–56. [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Primary studies | Secondary data (e.g., Meta-Analysis and Systematic reviews) |
| Tissues only infected with HPV | Coinfections |
| Male participants over the age of 18 | Male participants with age < 18 |
| Paper in English | Studies published languages other than English |
| Duplicated papers |
| S/N | Study | Selection | Comparability | Exposure | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1` | 2 | 3 | 4 | 5 | 6 | 7 | |||
| 1 | Medel Flores et al. (2018) | * | * | - | * | * | * | * | 6 |
| 2 | Chen et al. (2011) | * | * | - | * | * | * | * | 6 |
| 3 | Aghakhani et al. (2011) | * | * | - | * | * | * | * | 6 |
| 4 | Zhao et al. (2017) | * | * | - | * | - | * | * | 5 |
| 5 | Tachezy et al. (2012) | * | * | - | * | * | * | * | 6 |
| 6 | Ghasemian et al. (2013) | * | * | - | * | * | * | * | 6 |
| 7 | Rodriguez et al. (2016) | * | * | - | * | * | * | * | 6 |
| 8 | Khatami et al. (2022) | * | * | - | * | * | * | * | 6 |
| 9 | Rotola et al. (1992) | * | * | - | * | * | * | * | 6 |
| 10 | Moyret-Lalle et al. (1995) | * | * | - | * | * | * | * | 6 |
| 11 | Atashafrooz et al. (2016) | * | * | - | * | * | * | * | 6 |
| 12 | Singh et al. (2015) | * | * | - | * | * | * | * | 6 |
| 13 | Sarkar et al. (1993) | * | * | - | - | * | * | * | 5 |
| 14 | Noda et al. (1998) | * | * | - | * | * | * | * | 6 |
| 15 | Korodi et al. (2005) | * | * | * | * | - | * | * | 6 |
| 16 | Carozzi et al. (2004) | * | * | - | * | * | * | * | 6 |
| 17 | Adami et al. (2003) | * | * | * | * | - | * | * | 6 |
| 18 | Leiros et al. (2005) | * | * | - | * | * | * | * | 6 |
| 19 | Wideroff et al. (1996) | * | * | - | * | * | * | * | 6 |
| 20 | Martinez-Fierro et al. (2010) | * | * | * | * | * | * | * | 7 |
| 21 | Sutcliffe et al. (2010) | * | * | * | * | - | * | * | 6 |
| 22 | Silvestre et al. (2009) | * | * | - | * | * | * | * | 6 |
| 23 | Michopoulo et al. (2014) | * | * | - | * | * | * | * | 6 |
| 24 | McNicol and Dodd (1990) | * | * | - | * | * | * | * | 6 |
| 25 | Rosenblatt et al. (2003) | * | * | * | * | - | * | * | 6 |
| 26 | Aydin et al. (2017) | * | * | - | * | * | * | * | 6 |
| 27 | McNicol and Dodd (1991) | * | * | - | * | * | * | * | 6 |
| 28 | Masood et al. (1991) | * | * | - | * | - | * | * | 5 |
| 29 | Anwar et al. (1992) | * | * | - | * | - | * | * | 5 |
| 30 | Terris and Peehl (1997) | * | * | - | - | * | * | * | 5 |
| 31 | Suzuki et al. (1996) | * | * | - | * | - | * | * | 5 |
| 32 | Ibrahim et al. (1992) | * | * | - | * | * | * | * | 6 |
| 33 | Serth et al. (1999) | * | * | - | * | * | * | * | 6 |
| 34 | Dodd et al. (1993) | * | * | - | * | * | * | * | 6 |
| 35 | Salehi and Hadavi (2012) | * | * | - | * | * | * | * | 6 |
| 36 | Strickler et al. (1998) | * | * | - | * | * | * | * | 6 |
| 37 | Hrbacek et al. (2011) | * | * | - | * | - | * | * | 5 |
| 38 | Sutcliffe et al. (2007) | * | * | * | * | - | * | * | 6 |
| 39 | Bergh et al. (2006) | * | * | - | * | * | * | * | 6 |
| 40 | Groom et al. 2012) | * | * | - | * | * | * | * | 6 |
| 41 | Tu et al. (1994) | * | * | - | * | * | * | * | 6 |
| 42 | Sitas et al. (2007) | * | * | - | * | - | * | * | 6 |
| 43 | Anderson et al., (1997) | * | * | - | * | * | * | * | 6 |
| 44 | Effert et al., (1992) | * | * | - | - | - | * | 3 | |
| 45 | Noda et al., (1998) | * | * | - | - | * | * | * | 5 |
| 46 | Araujo-Neto et al., (2016) | * | * | - | - | - | * | * | 4 |
| 47 | Balis et al., (2007) | * | * | - | - | - | * | 3 | |
| 48 | Mokhtari et al., (2013) | - | * | - | * | * | - | * | 4 |
| 49 | Pascale et al., (2013) | * | * | - | - | - | * | - | 3 |
| 50 | Abumsimir et al (2022) | * | * | - | - | - | * | - | 3 |
| 50 | Nahand et al., (2020) | * | * | - | * | * | * | 5 | |
| 51 | Pereira et al., (2023) | * | * | - | - | - | * | - | 3 |
| 52 | Yow et al., (2014) | * | * | - | - | - | * | 3 | |
| 53 | Whitaker et al., (2012) | * | * | - | * | - | * | * | 5 |
| 54 | (Ahmed et al., 2023) | * | * | * | * | * | * | * | 7 |
| 55 | (Chang et al., 2023) | * | * | * | * | * | * | * | 7 |
| Study | Selection | Comparability | Outcome | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Dillner et al. (1998) | * | * | * | - | - | * | * | - | 6 |
| Gazzaz and Mosli (2009) | * | * | * | * | - | * | - | - | 5 |
| Strickler et al (1998) | * | - | * | * | * | * | 5 | ||
| Glenn et al (2017) | * | - | * | - | - | * | * | 4 | |
| Dennis et al., (2009) | * | * | * | * | * | * | - | - | 6 |
| Subjects | HPV types | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author | HPV detection | Collection (storage) | No. | Type | HPV | 6 | 16 | 18 | Other |
| Medel Flores et al. (2018) | L1 PCR consensus primers E6/E7 PCR for HPV 16, 18, 31, 33, 52b, 58 |
RP (FFPE) | 189 | PCa cases | 37 (20%) | 6 | 7 | 8 | 52 (17), 58 (12) |
| RP (FFPE) | 167 | BPH controls | 16 (10%) | 1 | 3 | 52 (16), 58 (5) | |||
|
Pascale et al. (2013) |
E7 IHC staining L1 PCR consensus primer |
Surgery, not fine needle biopsies (FFPE) | 150 | PCa cases | 19 (13%) | 9 | |||
|
Chen et al. (2011) |
L1 PCR consensus primer Type specific PCR primer for HPV 18 ISH for HPV 18 |
Collection not specified (snap frozen) | 51 | PCa cases | 7 (14%) | 7 | |||
| Collection not specified (snap frozen) | 11 | BPH controls | 3 (27%) | 3 | |||||
|
Aghakhani et al. (2011) |
L1 PCR consensus primers | RP, TURP (FFPE) | 104 | PCa cases | 13 (13%) | 1 | 7 | 3 | 11 (2) |
| RP, TURP (FFPE) | 104 | BPH controls | 8 (8%) | 1 | 3 | 2 | 11 (2) | ||
|
Tachezy et al. (2012) |
L1 PCR consensus primers | RRP (FFPE) | 51 | PCa cases | 1 (2%) | 42 (1) | |||
| 11 SP, 84 TURP (FFPE) | 95 | BPH controls | 2 (2%) | 1 | 1 unknown | ||||
|
Mokhtari et al. (2013) |
IHC staining | Collection not specified (PE) | 30 | PCa cases | 3 (10%) | ||||
| Collection not specified (PE) | 90 | BPH controls | 1 (1%) | ||||||
|
Balis et al. (2007) |
L1 PCR consensus primer Type specific PCR primers for HPV 11, 16, 18, 33 |
Collection not specified (22 FFPE, 20 fresh frozen) | 42 | PCa cases | 2 (5%) | Unknown | |||
|
Ghasemian et al. (2013) |
L1 PCR consensus primers | Collection not specified (FFPE) | 29 | PCa cases | 5 (17%) | ||||
| Collection not specified (FFPE) | 167 | BPH controls | 8 (5%) | ||||||
|
Rodriguez et al. (2016) |
INNO-LiPA HPV kit–L1 and 28 HPV genotypes | OP (FFPE) | 62 | PCa cases | 12 (20%) | 11 (46.1%), 51, 52, and 66 (15.4%) | |||
| TURP (FFPE) | 25 | BPH controls | 1 (4%) | 1 | |||||
|
Khatami et al. (2022) |
L1 PCR consensus primer | Collection not specified (snap frozen) | 73 | PCa cases | 21 (29%) | 1 | 9 | 7 | 11 (1), 33 (3) |
| Collection not specified (snap frozen) | 39 | Healthy controls | 7 (8%) | 3 | 3 | 11 (1) | |||
|
Rotola et al. (1992) |
E6 PCR for HPV 6/11, 16 | Collection not specified (snap frozen) | 8 | PCa cases | N/A | 4 | 6 | 11 (4) | |
| Collection not specified (snap frozen) | 17 | BPH controls | N/A | 11 | 14 | 11 (11) | |||
| Moyret-Lalle et al. (1995) | E6 PCR for HPV 16 and 18 | Collection not specified (snap frozen) | 17 | PCa cases | 9 (53%) | 9 | |||
| Collection not specified (snap frozen) | 22 | BPH controls | 7 (32%) | 7 | |||||
|
Atashafrooz et al. (2016) |
Real Time PCR HPV detection/genotyping assay kit–13 genotypes | Collection not specified (PE) | 100 | PCa cases | 20 (20%) | 1 | 16/18 (8), 31/33 (6), 54 (2), 6/11 (3) | ||
| Collection not specified (PE) | 100 | BPH Controls | 8 (8%) | 2 | 16/18 (1), 31/33 (1), 6/11 (4) | ||||
|
Araujo-Neto et al. (2016) |
L1 PCR consensus primers E6/E6 PCR for HPV 16 |
RP (fresh frozen) | 104 | PCa cases | 0 (0%) | ||||
|
Singh et al. (2015) |
L1 PCR consensus primers Type specific PCR primers for HPV 6, 11, 16, 18 |
Collection not specified (storage not specified) | 95 | PCa cases | 39 (41%) | 2 | 30 | 6 | 11 (1) |
| Collection not specified (storage not specified) |
55 | BPH controls | 11 (20%) | 6 | 3 | 1 | 11 (1) | ||
|
Sarkar et al. (1993) |
E6/E7 PCR for HPV 6, 11, 16, and 18 Southern blot hybridisation |
Surgical resection, not TURP (PE) | 23 | PCa cases | 3 (13%) | 3 | |||
| Surgical resection, not TURP (PE) | 23 | PIN controls | 0 (0%) | ||||||
|
Noda et al. (1998) |
PCR primers for LCR and E7 for HPV 16, 18, 31, 33, 52, 58 | TP (FFPE) | 38 | PCa cases | 0 (0%) | ||||
| 10 SCP, 61 TURP (FFPE) | 71 | BPH controls | 3 (4%) | 3 | |||||
|
Carozzi et al. (2004) |
L1 PCR consensus primer E6/E7 PCR for HPV types 16, 18, 31, 33, 35, 45, 52, 58 Hybridisation |
TPB (formalin) | 26 | PCa cases | 17 (65%) | 1 | 3 | 3 | 58 (4) |
| TPB (formalin) | 25 | BPH controls | 12 (48%) | 1 | 2 | 53 (4) | |||
|
Leiros et al. (2005) |
Type specific PCR primers for HPV 6, 11, 16, 18 L1 PCR consensus primer Southern blot hybridisation |
TRB (FFPE) | 41 | PCa cases | 17 (42%) | 5 | 11 (2) | ||
| TRB (FFPE) | 30 | BPH controls | 0 (0%) | ||||||
| Wideroff et al. (1996) | L1 PCR consensus primers E6 PCR for HPV 6, 11, 16, 18, 31, 33, 45 Hybridisation |
TURP, RP, excision biopsy (FFPE) | 56 | PCa cases | 7 (13%) | ||||
| TURP (FFPE) | 42 | BPH controls | 4 (10%) | ||||||
|
Martinez-Fierro et al. (2010) |
L1 PCR consensus primers Linear Array HPV Genotyping Test |
TRB, TURP (storage not specified) | 55 | PCa cases | 11 (20%) | ||||
| TRB, TURP (storage not specified) | 75 | Non-PCa controls | 4 (5%) | ||||||
|
Silvestre et al. (2009) |
L1 PCR consensus primer Linear Array HPV Genotyping Test |
Collection and storage not specified | 65 | PCa cases | 2 (3%) | 2 | 84 (coinfection) | ||
| Collection and storage not specified | 6 | BPH controls | 0 (0%) | ||||||
|
Michopoulo et al. (2014) |
L1 PCR consensus primer | Collection not specified (FFPE) | 50 | PCa cases | 8 (16%) | 2 | 4 | 31 (1), unknown (1) | |
| Collection not specified (FFPE) | 30 | Healthy controls | 1 (3%) | Unknown (1) | |||||
|
McNiol and Dodd (1990) |
E6 PCR for HPV 16 and 18 | 2 SPP, 17 TURP (fresh frozen) | 4 | PCa cases | 4 (100%) | 4 | |||
| 15 | BPH controls | 14 (93%) | 11 | 16 + 18 (3) | |||||
| Autopsy (fresh frozen) | 5 | Healthy controls | 1 (20%) | 1 | |||||
|
Aydin et al. (2017) |
L1 PCR consensus primer HPV sign® Q24 for genotyping |
RP (FFPE) | 60 | PCa cases | 1 (2%) | 57 | |||
| TVP (FFPE) | 36 | BPH controls | 0 (0%) | ||||||
|
McNiol and Dodd (1991) |
E6 PCR for HPV 16 and 18 Hybridisation |
TURP, SPP (fresh frozen) | 27 | PCa cases | 14 (52%) | 14 | 1 | ||
| TURP, SPP (fresh frozen) | 56 | BPH controls | 34 (63%) | 34 | 3 | ||||
| Autopsy (fresh frozen) | 5 | Healthy controls | 1 (20%) | 1 | |||||
|
Masood et al. (1991) |
In situ hybridisation for HPV 6, 11, 16, 18, 31, 33, 35 | Core needle biopsy, TURP (FFPE) | 20 | PCa cases | 0 (0%) | ||||
| Core needle biopsy, TURP (FFPE) | 20 | BPH controls | 0 (0%) | ||||||
|
Anwar et al. (1992) |
E6 PCR for HPV 16, 18, 33 | TURP, SPP (FFPE) | 68 | PCa cases | 28 (41%) | 11 | 7 | 33 (5) | |
| TURP, SPP (FFPE) | 10 | BPH controls | 0 (0%) | ||||||
| Autopsy (FFPE) | 10 | Healthy controls | 0 (0%) | ||||||
|
Rodriguez et al. (2015) |
L1 PCR consensus primer Type specific PCR for 19 HPV genotypes |
Collection not specified (FFPE) | 69 | PCa cases | 0 (0%) | ||||
| Terris and Peehl (1997) | L1 PCR consensus primer E6 PCR for HPV 16 |
RRP (FFPE) | 53 | PCa cases | 12 (23%) | 12 | |||
| 41 | Peripheral benign tissue | 15 (37%) | 15 | ||||||
| 37 | Healthy controls | 6 (16%) | 6 | ||||||
|
Suzuki et al. (1996) |
L1 PCR consensus primer | 29 TP, 22 autopsy (storage not specified) | 51 | PCa cases | 8 (16%) | 8 | |||
|
Ibrahim et al. (1992) |
L1 PCR consensus primer ISH |
RP, TURP, TRB (FFPE and fresh frozen) | 40 | PCa cases | 6 (15%) | 6 | |||
| RP, TURP, TRB (FFPE) | 12 | BPH controls | 0 (0%) | ||||||
| RP, TURP, TRB (FFPE) | 17 | Healthy controls | 2 (12%) | 2 | |||||
|
Serth et al. (1999) |
E6 PCR for HPV 16 | RP (snap frozen) | 47 | PCa cases | 10 (21%) | 10 | |||
| TVP (snap frozen) | 37 | BPH controls | 1 (3%) | 1 | |||||
|
Dodd et al. (1993) |
Reverse transcription PCR for E6/E7 mRNA of HPV16 | Collection not specified (fresh frozen) |
7 | PCa cases | 3 (43%) | 3 | |||
| Collection not specified (fresh frozen) |
10 | BPH controls | 5 (50%) | 5 | |||||
|
Salehi and Hadavi (2012) |
L1 PCR consensus primer | Collection not specified (snap frozen) |
68 | PCa cases | 3 (4%) | ||||
| Collection not specified (snap frozen) |
85 | BPH controls | 0 (0%) | ||||||
|
Abumsimir et al. (2022) |
L1 PCR consensus primers | Biopsies (fresh) | 50 | PCa cases | 8 (16%) | 8 | |||
|
Strickler et al. (1998) |
L1 PCR consensus primers E6 PCR for HPV 11, 16, 18, 51, 61 Southern blot hybridisation |
Surgery, TURP (fresh frozen) | 63 | PCa cases | 0 (0%) | ||||
| Surgery, TURP (fresh frozen) | 61 | BPH controls | 0 (0%) | ||||||
|
Glenn et al. (2017) |
L1 PCR consensus primer E7 PCR for HPV 16 and 18 IHC for E7 oncoprotein |
Collection not specified (FFPE) | 28 | PCa cases | L1 8 (29%) E7 19 (68%) E7P 8 (29%) |
||||
| 28 | BPH controls–years before | L1 13 (46%) E7 23 (82%) E7P 23 (82%) |
|||||||
|
Bergh et al. (2006) |
L1 PCR consensus primer | TURP (FFPE) | 201 | PCa cases | 0 (0%) | ||||
| TURP (FFPE) | 201 | BPH controls | 0 (0%) | ||||||
|
Groom et al. (2012) |
INNO-LiPA HPV Genotyping kit Hybridisation to HPV 16 |
Collection and storage not specified | 100 | PCa cases | 1 (1%) | 1 | |||
| Collection and storage not specified | 62 | Healthy controls | 0 (0%) | ||||||
|
Tu et al. (1994) |
L1 PCR consensus primer Hybridisation |
RP (FFPE) | 43 | PCa cases | 1 (2%) | 1 | |||
| Collection not specified (snap frozen) | 17 | Metastatic pelvic lymph nodes | 1 (6%) | 1 | |||||
| RRP (not specified) | 1 | Normal prostate | 0 (0%) | ||||||
| Effert et al. (1992) | E6 PCR for HPV 16 and 18 | RP (FFPE) | 30 | PCa cases | 0 (0%) | ||||
|
Gazzaz and Mosli (2009) |
Hybridisation using Hybrid Capture 2 kit | TURP, TRB (fresh) | 6 | PCa cases | 0 (0%) | ||||
| TURP, TRB (fresh) | 50 | 21 BPH, 29 nodular hyperplasia | 0 (0%) | ||||||
|
Anderson et al. (1997) |
L1 PCR consensus primer E6 and E2 ORF PCR for HPV 16 |
TURP (fresh frozen) | 14 | PCa cases | 0 (0%) | ||||
| TURP (fresh frozen) | 10 | Benign controls | 0 (0%) | ||||||
| Pereira et al., (2023) | L1 PCR consensus primer for HPV 16, 18, 56, 59 and 66. | TRUS Biopsies | 162 | PCa cases, | 10(6.2%) | ||||
| Nahand et al., (2020) | L1 and E7 PCR consensus primer and INNO-LiPA HPV Genotyping Kit | Surgery | 58 | PCa cases | 19(32.7%) | 9 | 6 | 33(3) | |
| Yow et al., (2014) | PapType High-Risk (HR) HPV Detection and Genotyping kit | TRUP (FFPE) RP (FFPE) |
221 | PCa cases | 0 (0%) | ||||
| Whitaker et al (2012) | L1 PCR consensus primer, and In Situ PCR | Collection not specified (FFPE; Fresh frozen) | 10 | PCa cases | 7 (70%) | 1 | |||
| Ahmed et al (2023) | HPV-HCR Genotype-Eph kit | Biopses (Fresh) | 49 | PCa cases, Benign controls | 16 (32.7%) | 4 | 33, 35, 45, 52, 56, 58 | ||
| Chang et al (2023) | Cobas 4800 HPV Test and DR HPV Genotyping IVD Kit | FFPE | 178 | PCa cases, Benign controls | 12 (6.7%) | 2 | 52 (1), 53(3), 62(1), others (5) | ||
| Author | Year | Pathogen studied | PCa present | % Seropositive | No. of controls without PCa | % Seropositive | RR/OR | 95% CI | Evidence of association? | Method |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al. | 2011 | HPV 16, 18,31,33, 52, 58 | 26/53 | 49 | 35/104 | 33.7 | OR = 0.71-4.16 | 0.18–48.0 | No | Fluorescent assay |
| Zhao et al. | 2017 | HPV 16 | 48/75 | 64 | 14/80 | 17.5 | N/A | N/A | Yes (p < 0.001) | Peptide microarray |
| Tachezy et al. | 2012 | HPV 16,18,31,33 | 14/51 | 27 | 172 | 21.5 | 1.44 | 0.69–2.97 | P =0.329 | ELISA |
| Dillner et al. | 1998 | HPV 16,18,33 | 31/165 | 18.8 | 35/290 | 12.1 | RR = 2.59, 2.38, 0.66 | 1.17–5.75 0.75–7.58 0.26–1.66 | Yes for HPV 16 and 18 (P< 0.001) | ELISA |
| Korodi et al. | 2005 | HPV 16,18,33 | 107/799 | 13.4 | 363/2596 | 14.0 | OR 0.94 | 0.74-1.19 | No | ELISA |
| Adami et al. | 2003 | HPV 16, 18, 33 | 129/238 | 54 | 105/210 | 50 | OR= 0.7, 0.9, 1.6 | 0.4-1.3, 0.5-1.9, 1.0-2.7 | Yes for HPV 33 | ELISA |
| Sutcliffe et al. | 2010 | HPV 16, 18,33 | 180/616 | 29.2 | 179/616 | 29 | OR 1.07, 0.87, 1.15 | 0.77-1.48 0.47-1.63 | No | ELISA |
| Rosenblatt et al. | 2003 | HPV 16, 18 | 81/642 | 12.6 | 64/570 | 11.3 | OR = 1.06, 1.36 | 0.71-1.57 0.69-2.69 | No | ELISA |
| Strickler et al. | 1998 | HPV 16 | 1/63 | 1.6 | 7/144 | 4.9 | N/A | N/A | No (p = 0.44) | ELISA |
| Hrbacek et al. | 2011 | HPV 16, 18, 31, 33 | 50/329 | 15.2 | 33/105 | 31 | OR = 0.48, 023, 073, 0.43 | 0.21-1.13 0.09-0.61 0.32-1.83 0.13-1.48 |
No | ELISA |
| Sutcliffe et al. | 2007 | HPV 16 | 144/ 691 | 20.8 | 145/691 | 21 | OR = 0.83,1.04,1.14 | 0.57-1.23 0.66-1.64 0.76-1.72 | No | ELISA |
| Sitas et al. | 2007 | HPV 16 | 59/205 | 28.78 | 173/673 | 25.71 | OR 1.33 | 0.86–2.07 | No | ELISA |
| Dennis et al | 2009 | HPV 16 and 18 | 50/267 | 19 | 45/267 | 17 | OR 1.33 | 0.73-1.75 | No | ELISA |
| S/N | Author | OR (95% Cl) | P value |
|---|---|---|---|
| 1 | Chen et al (2011) | P>0.05 | |
| 2 | Aghakhani et al., (2011) |
- | P>0.05 |
| 3 | Khatami et al., (2022) | 2.01 (0.8-5.68) | P= 0.102 |
| 4 | Atashafrooz et al., (2016) |
- | P= 0.413 |
| 5 | Noda et al., (1998) | - | P=0.19 |
| 6 | Wideroff et al., (1996) | 1.36(0.37, 4.98) | p>0.05 |
| 7 | Michopoulou et al., (2014) | 5.52(0.66-45.6) | P=0.086 |
| 8 | Rotola et al., (1992) | - | P>0.05 |
| 9 | McNicol and Dodd (1991) | P>0.05 | |
| 10 | Ibrahim et al., (1992) | - | P= 0.343 |
| 11 | Salehi and M. Hadavi (2012) | P=0.71 | |
| 12 | Nahand et al (2020) | P=0.078 | |
| 13 | Periera et al (2023) | P=0.487 | |
| 14 | Ahmed et al (2023) | P>0.05 | |
| 15 | Chang et al (2023) | P>0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





