Submitted:
31 May 2023
Posted:
01 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
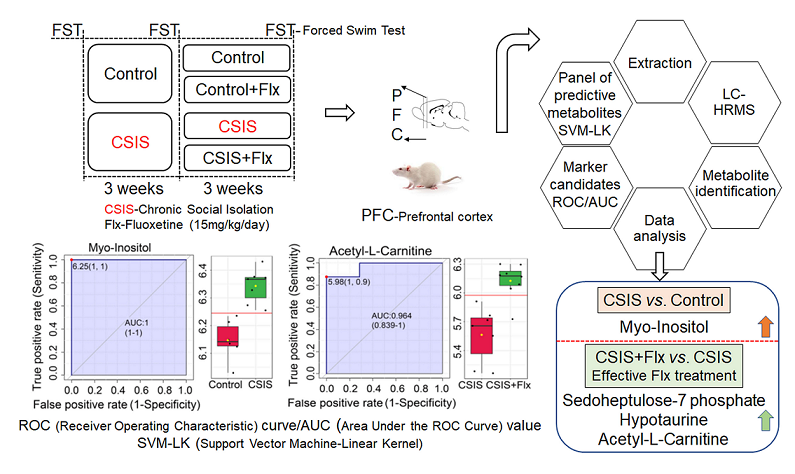
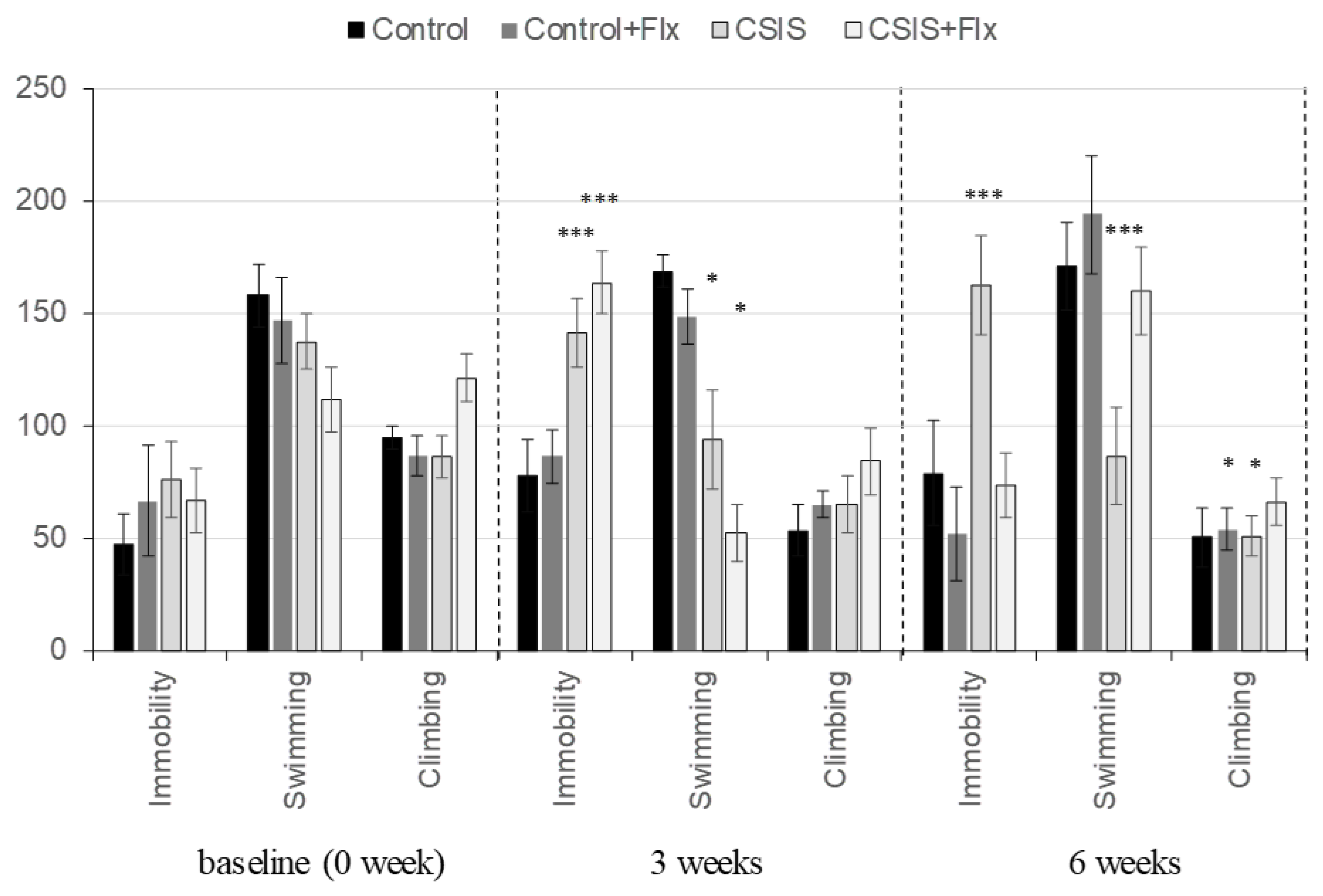
2.1. Behavioral testing
2.2. PFC metabolic fingerprints following CSIS and/or effective Flx treatment and controls
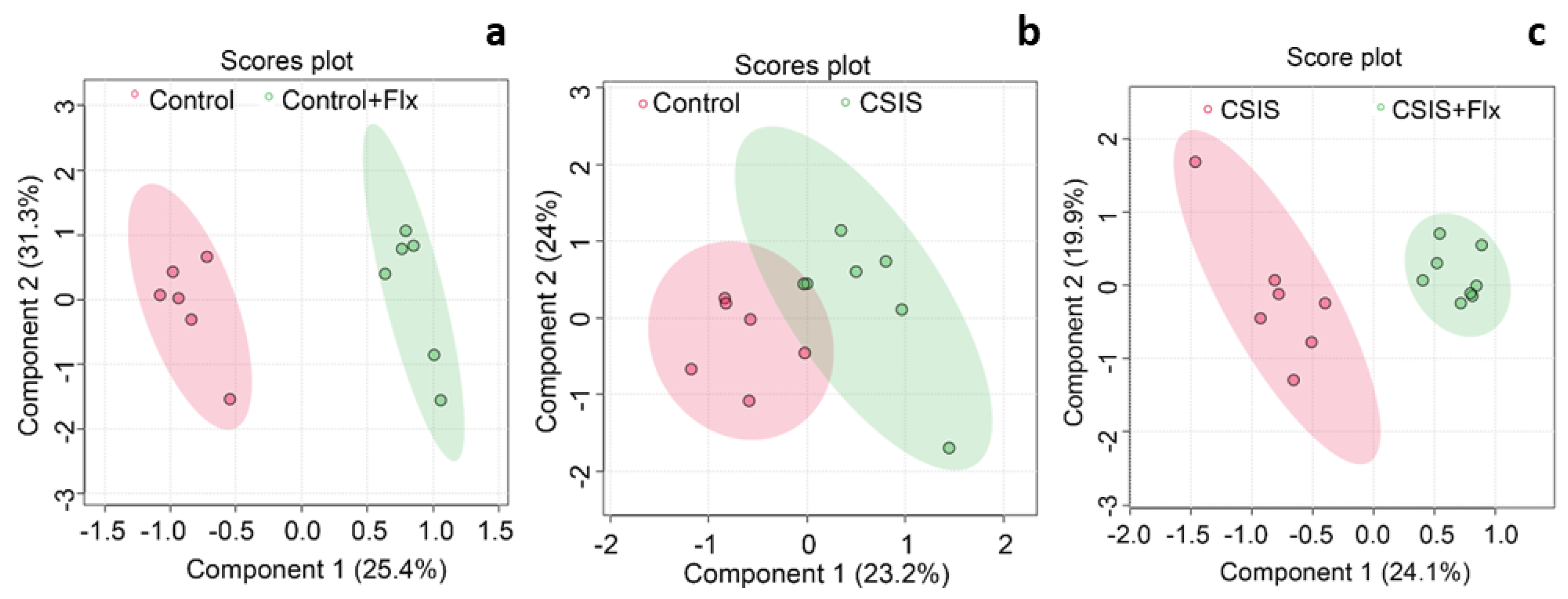
2.3. Multivariate data analysis
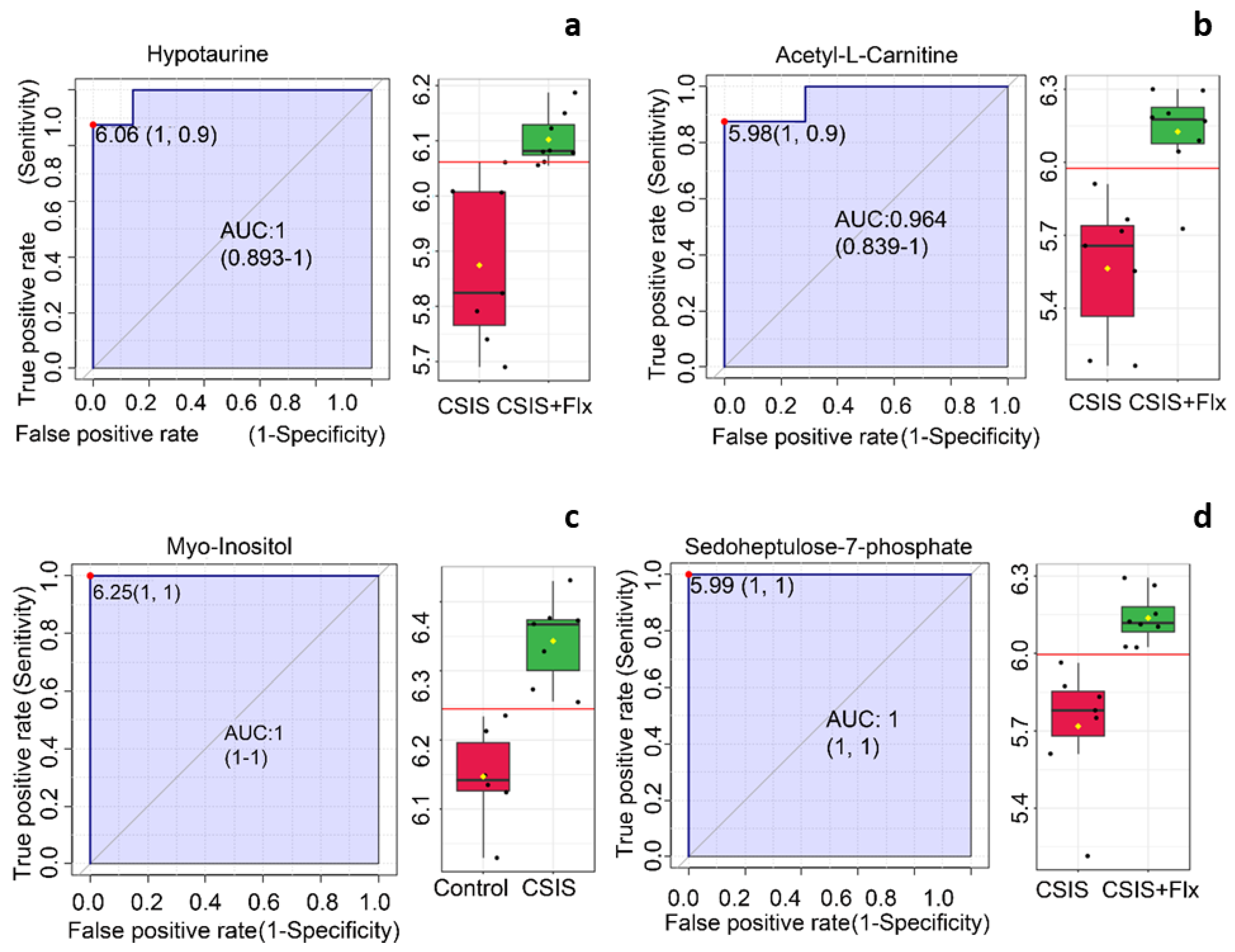
2.4. Marker candidates identification
2.5. SVM classification
2.6. Correlation of behavioral phenotype with the metabolomic data
3. Discussion
4. Material and methods
4.1. Animals
4.2. Fluoxetine-hydrochloride administration
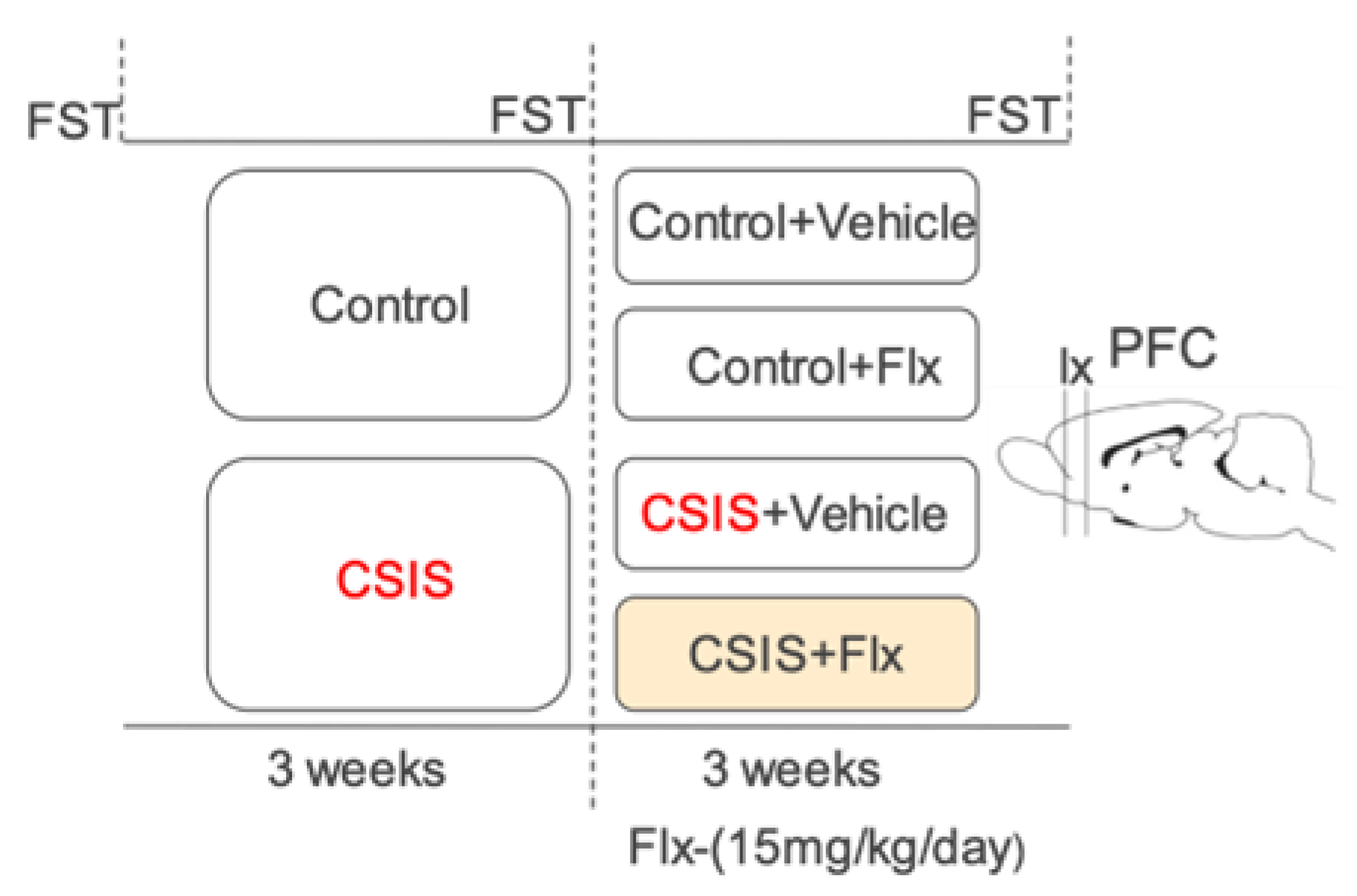
4.3. Experimental design
4.4. Forced swim test
4.5. Metabolomics analysis by LC-HRMS
4.5.1. Optimization of sample preparation for LC-HRMS analysis
4.5.2. Metabolic profiles analyzed by LC-HRMS
4.5.3. Metabolite data statistic and analysis
4.6. Identification of marker candidates
4.7. SVM-LK-based binary classification
4.8. Statistical analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albert, P.R.; Benkelfat, C.; Descarries, L. The Neurobiology of Depression—Revisiting the Serotonin Hypothesis. I. Cellular and Molecular Mechanisms. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2378. [Google Scholar] [CrossRef] [PubMed]
- Lang, U.E.; Borgwardt, S. Molecular Mechanisms of Depression: Perspectives on New Treatment Strategies. Cell. Physiol. Biochem. 2013, 31, 761–777. [Google Scholar] [CrossRef]
- Stahl, S.M. Basic Psychopharmacology of Antidepressants, Part 1: Antidepressants Have Seven Distinct Mechanisms of Action. J. Clin. Psychiatry 1998, 59, 5–14. [Google Scholar] [PubMed]
- Cacioppo, J.T.; Cacioppo, S.; Capitanio, J.P.; Cole, S.W. The Neuroendocrinology of Social Isolation. Annu. Rev. Psychol. 2015, 66, 733–767. [Google Scholar] [CrossRef] [PubMed]
- Filipović, D.; Todorović, N.; Bernardi, R.E.; Gass, P. Oxidative and Nitrosative Stress Pathways in the Brain of Socially Isolated Adult Male Rats Demonstrating Depressive- and Anxiety-like Symptoms. Brain Struct. Funct. 2017, 222. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, F.; Khan, M.I.; Zubair, M.; Dehpour, A.R. Neurobiology and Consequences of Social Isolation Stress in Animal Model-A Comprehensive Review. Biomed. Pharmacother. 2018, 105, 1205–1222. [Google Scholar] [CrossRef] [PubMed]
- Filipović, D.; Zlatković, J.; Inta, D.; Bjelobaba, I.; Stojiljkovic, M.; Gass, P. Chronic Isolation Stress Predisposes the Frontal Cortex but Not the Hippocampus to the Potentially Detrimental Release of Cytochrome c from Mitochondria and the Activation of Caspase-3. J. Neurosci. Res. 2011, 89, 1461–1470. [Google Scholar] [CrossRef]
- Hare, B.D.; Duman, R.S. Prefrontal Cortex Circuits in Depression and Anxiety: Contribution of Discrete Neuronal Populations and Target Regions. Mol. Psychiatry 2020, 25, 2742. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Roberts, A.C. Prefrontal Cortex and Depression. Neuropsychopharmacology 2022, 47, 225–246. [Google Scholar] [CrossRef]
- T Arnsten, A.F. Stress Signalling Pathways That Impair Prefrontal Cortex Structure and Function. Nat. Publ. Gr. 2009. [Google Scholar] [CrossRef]
- Jobson, D.D.; Hase, Y.; Clarkson, A.N.; Kalaria, R.N. The Role of the Medial Prefrontal Cortex in Cognition, Ageing and Dementia. Brain Commun. 2021, 3. [Google Scholar] [CrossRef]
- Filipović, D.; Gavrilović, L.; Dronjak, S.; Radojčić, M.B. Brain Glucocorticoid Receptor and Heat Shock Protein 70 Levels in Rats Exposed to Acute, Chronic or Combined Stress. Neuropsychobiology 2005, 51, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Maripuu, M.; Wikgren, M.; Karling, P.; Adolfsson, R.; Norrback, K.F. Relative Hypo- and Hypercortisolism Are Both Associated with Depression and Lower Quality of Life in Bipolar Disorder: A Cross-Sectional Study. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M.; Lightman, S.L. The HPA Axis in Major Depression: Classical Theories and New Developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef]
- Filipović, D.; Novak, B.; Xiao, J.; Yan, Y.; Yeoh, K.; Turck, C.W. Chronic Fluoxetine Treatment of Socially Isolated Rats Modulates Prefrontal Cortex Proteome. Neuroscience 2022, 501, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, P.; Cipriani, A.; Hotopf, M.; Barbui, C. Side-Effect Profile of Fluoxetine in Comparison with Other SSRIs, Tricyclic and Newer Antidepressants: A Meta-Analysis of Clinical Trial Data. Pharmacopsychiatry 2005, 38, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Filipović, D.; Costina, V.; Perić, I.; Stanisavljević, A.; Findeisen, P. Chronic Fluoxetine Treatment Directs Energy Metabolism towards the Citric Acid Cycle and Oxidative Phosphorylation in Rat Hippocampal Nonsynaptic Mitochondria. Brain Res. 2017, 1659, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Filipović, D.; Novak, B.; Xiao, J.; Yan, Y.; Bernardi, R.E.; Turck, C.W. Chronic Fluoxetine Treatment in Socially-Isolated Rats Modulates the Prefrontal Cortex Synaptoproteome. J. Proteomics 2023, 282, 104925. [Google Scholar] [CrossRef]
- Casarotto, P.C.; Girych, M.; Fred, S.M.; Kovaleva, V.; Moliner, R.; Enkavi, G.; Biojone, C.; Cannarozzo, C.; Sahu, M.P.; Kaurinkoski, K.; et al. Antidepressant Drugs Act by Directly Binding to TRKB Neurotrophin Receptors. Cell 2021, 184, 1299–1313.e19. [Google Scholar] [CrossRef]
- Ohira, K.; Takeuchi, R.; Shoji, H.; Miyakawa, T. Fluoxetine-Induced Cortical Adult Neurogenesis. Neuropsychopharmacology 2013, 38, 909–920. [Google Scholar] [CrossRef]
- Popova, D.; Castrén, E.; Taira, T. Chronic Fluoxetine Administration Enhances Synaptic Plasticity and Increases Functional Dynamics in Hippocampal CA3-CA1 Synapses. Neuropharmacology 2017, 126, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yang, S.; Li, W.; Liu, Y.; Li, Z.; Zhang, Y.; Li, L.; Liu, S. The Potential Biomarker Panels for Identification of Major Depressive Disorder (MDD) Patients with and without Early Life Stress (ELS) by Metabonomic Analysis. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Bot, M.; Milaneschi, Y.; Al-Shehri, T.; Amin, N.; Garmaeva, S.; Onderwater, G.L.J.; Pool, R.; Thesing, C.S.; Vijfhuizen, L.S.; Vogelzangs, N.; et al. Metabolomics Profile in Depression: A Pooled Analysis of 230 Metabolic Markers in 5283 Cases With Depression and 10,145 Controls. Biol. Psychiatry 2020, 87, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Brietzke, E. Omics-Based Biomarkers: Application of Metabolomics in Neuropsychiatric Disorders. Int. J. Neuropsychopharmacol. 2016, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, X.; Zhang, Y.; Liu, Y.; Yang, L.; Pu, J.; Zhu, D.; Zhou, C.; Xie, P. The Identification of Metabolic Disturbances in the Prefrontal Cortex of the Chronic Restraint Stress Rat Model of Depression. Behav. Brain Res. 2016, 305, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Zhang, M. Di; Tao, X.; Zhou, Y.F.; Liu, X.M.; Pan, R. Le; Liao, Y.H.; Chang, Q. LC-MS/MS-Based Quantification of Tryptophan Metabolites and Neurotransmitters in the Serum and Brain of Mice. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2019, 1112, 24–32. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Feng, J.; Zhang, C.; Duan, L.; Fan, R.; Li, T.; Yang, Z.; Hu, E.; Yu, Z.; Tian, J.; et al. Metabolomics Analysis of the Prefrontal Cortex in a Rat Chronic Unpredictable Mild Stress Model of Depression. Front. Psychiatry | www.frontiersin.org 2022, 1, 815211. [Google Scholar] [CrossRef]
- Caspani, G.; Turecki, G.; Lam, R.W.; Milev, R. V.; Frey, B.N.; MacQueen, G.M.; Müller, D.J.; Rotzinger, S.; Kennedy, S.H.; Foster, J.A.; et al. Metabolomic Signatures Associated with Depression and Predictors of Antidepressant Response in Humans: A CAN-BIND-1 Report. Commun. Biol. 2021 41 2021, 4, 1–11. [Google Scholar] [CrossRef]
- Kaddurah-Daouk, R.; Weinshilboum, R. Metabolomic Signatures for Drug Response Phenotypes: Pharmacometabolomics Enables Precision Medicine. Clin. Pharmacol. Ther. 2015, 98, 71–75. [Google Scholar] [CrossRef]
- Duan, L.; Fan, R.; Li, T.; Yang, Z.; Hu, E.; Yu, Z.; Tian, J.; Luo, W.; Zhang, C. Metabolomics Analysis of the Prefrontal Cortex in a Rat Chronic Unpredictable Mild Stress Model of Depression. Front. Psychiatry 2022, 13, 815211. [Google Scholar] [CrossRef]
- Barberis, E.; Khoso, S.; Sica, A.; Falasca, M.; Gennari, A.; Dondero, F.; Afantitis, A.; Manfredi, M. Precision Medicine Approaches with Metabolomics and Artificial Intelligence. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Mendez, K.M.; Reinke, S.N.; Broadhurst, D.I. A Comparative Evaluation of the Generalised Predictive Ability of Eight Machine Learning Algorithms across Ten Clinical Metabolomics Data Sets for Binary Classification. Metabolomics 2019, 15. [Google Scholar] [CrossRef]
- Djordjevic, A.; Djordjevic, J.; Elaković, I.; Adzic, M.; Matić, G.; Radojcic, M.B. Effects of Fluoxetine on Plasticity and Apoptosis Evoked by Chronic Stress in Rat Prefrontal Cortex. Eur. J. Pharmacol. 2012, 693, 37–44. [Google Scholar] [CrossRef]
- Perić, I.; Stanisavljević, A.; Gass, P.; Filipović, D. Fluoxetine Reverses Behavior Changes in Socially Isolated Rats: Role of the Hippocampal GSH-Dependent Defense System and Proinflammatory Cytokines. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 737–749. [Google Scholar] [CrossRef]
- Kim, H.; McGrath, B.M.; Silverstone, P.H. A Review of the Possible Relevance of Inositol and the Phosphatidylinositol Second Messenger System (PI-Cycle) to Psychiatric Disorders--Focus on Magnetic Resonance Spectroscopy (MRS) Studies. Hum. Psychopharmacol. 2005, 20, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Hipps, P.P.; Eveland, M.R.; Laird, M.H.; Sherman, W.R. The Identification of Myo-Inositol:NAD(P)+ Oxidoreductase in Mammalian Brain. Biochem. Biophys. Res. Commun. 1976, 68, 1133–1138. [Google Scholar] [CrossRef]
- Novak, J.E.; Turner, R.S.; Agranoff, B.W.; Fisher, S.K. Differentiated Human NT2-N Neurons Possess a High Intracellular Content of Myo-Inositol. J. Neurochem. 1999, 72, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.; Richter-Landsberg, C.; Leibfritz, D. Multinuclear NMR Studies on the Energy Metabolism of Glial and Neuronal Cells. Dev. Neurosci. 1993, 15, 289–298. [Google Scholar] [CrossRef]
- Chang, L.; Munsaka, S.M.; Kraft-Terry, S.; Ernst, T. Magnetic Resonance Spectroscopy to Assess Neuroinflammation and Neuropathic Pain. J. Neuroimmune Pharmacol. 2013, 8, 576–593. [Google Scholar] [CrossRef]
- Chen, S.; Lu, D.; Wang, W.; Chen, W.; Zhang, S.; Wei, S. Plasma Metabolomic Profiling of Repeated Restraint Stress in Rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1160, 122294. [Google Scholar] [CrossRef]
- Pekny, M.; Pekna, M.; Messing, A.; Steinhäuser, C.; Lee, J.M.; Parpura, V.; Hol, E.M.; Sofroniew, M. V.; Verkhratsky, A. Astrocytes: A Central Element in Neurological Diseases. Acta Neuropathol. 2016, 131, 323–345. [Google Scholar] [CrossRef] [PubMed]
- von Leden, R.E.; Khayrullina, G.; Moritz, K.E.; Byrnes, K.R. Age Exacerbates Microglial Activation, Oxidative Stress, Inflammatory and NOX2 Gene Expression, and Delays Functional Recovery in a Middle-Aged Rodent Model of Spinal Cord Injury. J. Neuroinflammation 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Todorović, N.; Filipović, D. The Antidepressant- and Anxiolytic-like Effects of Fluoxetine and Clozapine in Chronically Isolated Rats Involve Inhibition of Hippocampal TNF-α. Pharmacol. Biochem. Behav. 2017, 163, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Egorova, P.A.; Bezprozvanny, I.B. Inositol 1,4,5-Trisphosphate Receptors and Neurodegenerative Disorders. FEBS J. 2018, 285, 3547–3565. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Lipp, P. Calcium - a Life and Death Signal. Nat. 1998 3956703 1998, 395, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, C.; Miller, R.A.; Smith, I.; Bui, T.; Molgó, J.; Müller, M.; Vais, H.; Cheung, K.H.; Yang, J.; Parker, I.; et al. Essential Regulation of Cell Bioenergetics by Constitutive InsP3 Receptor Ca2+ Transfer to Mitochondria. Cell 2010, 142, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Zlatković, J.; Filipović, D. Bax and B-Cell-Lymphoma 2 Mediate Proapoptotic Signaling Following Chronic Isolation Stress in Rat Brain. Neuroscience 2012, 223, 238–245. [Google Scholar] [CrossRef]
- Granados-Domínguez, L.M.; Flores-Gutiérrez, E.O.; Alcauter, S.; Cervantes, J.J.; Torres-Álvarez, M.; Corsi-Cabrera, M.; Granados-Domínguez, L.M.; Flores-Gutiérrez, E.O.; Alcauter, S.; Cervantes, J.J.; et al. Increased Myo-Inositol in the Posterior Cingulate Cortex in First-Episode Major Depressive Patients. Adv. Biosci. Biotechnol. 2013, 4, 45–52. [Google Scholar] [CrossRef]
- Pu, J.; Liu, Y.; Gui, S.; Tian, L.; Yu, Y.; Song, X.; Zhong, X.; Chen, X.; Chen, W.; Zheng, P.; et al. Metabolomic Changes in Animal Models of Depression: A Systematic Analysis. Mol. Psychiatry 2021, 26. [Google Scholar] [CrossRef]
- Shirayama, Y.; Takahashi, M.; Osone, F.; Hara, A.; Okubo, T. Myo-Inositol, Glutamate, and Glutamine in the Prefrontal Cortex, Hippocampus, and Amygdala in Major Depression. Biol. psychiatry. Cogn. Neurosci. neuroimaging 2017, 2, 196–204. [Google Scholar] [CrossRef]
- Taylor, M.J.; Selvaraj, S.; Norbury, R.; Jezzard, P.; Cowen, P.J. Normal Glutamate but Elevated Myo-Inositol in Anterior Cingulate Cortex in Recovered Depressed Patients. J. Affect. Disord. 2009, 119, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Thomas, A.; Lavretsky, H.; Yue, K.; Huda, A.; Curran, J.; Venkatraman, T.; Estanol, L.; Mintz, J.; Mega, M.; et al. Frontal White Matter Biochemical Abnormalities in Late-Life Major Depression Detected with Proton Magnetic Resonance Spectroscopy. Am. J. Psychiatry 2002, 159, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Lind, A.; Boraxbekk, C.J.; Petersen, E.T.; Paulson, O.B.; Siebner, H.R.; Marsman, A. Regional Myo-Inositol, Creatine, and Choline Levels Are Higher at Older Age and Scale Negatively with Visuospatial Working Memory: A Cross-Sectional Proton Mr Spectroscopy Study at 7 Tesla on Normal Cognitive Ageing. J. Neurosci. 2020, 40, 8149–8159. [Google Scholar] [CrossRef] [PubMed]
- Urrila, A.S.; Hakkarainen, A.; Castaneda, A.; Paunio, T.; Marttunen, M.; Lundbom, N. Frontal Cortex Myo-Inositol Is Associated with Sleep and Depression in Adolescents: A Proton Magnetic Resonance Spectroscopy Study. Neuropsychobiology 2017, 75, 21–31. [Google Scholar] [CrossRef]
- Chiappelli, J.; Rowland, L.M.; Wijtenburg, S.A.; Muellerklein, F.; Tagamets, M.; McMahon, R.P.; Gaston, F.; Kochunov, P.; Hong, L.E. Evaluation of Myo-Inositol as a Potential Biomarker for Depression in Schizophrenia. Neuropsychopharmacology 2015, 40, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Yamashiro, D.; Ogawa, S.; Kobayashi, M.; Cho, D.; Iizuka, A.; Tsukamoto-Yasui, M.; Takada, M.; Isokawa, M.; Nagao, K.; et al. Intake of Seven Essential Amino Acids Improves Cognitive Function and Psychological and Social Function in Middle-Aged and Older Adults: A Double-Blind, Randomized, Placebo-Controlled Trial. Front. Nutr. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.C.; McKenna, M.C. L-Carnitine and Acetyl-L-Carnitine Roles and Neuroprotection indeveloping Brain. Neurochem. Res. 2017, 42, 1661. [Google Scholar] [CrossRef]
- Scafidi, S.; Fiskum, G.; Lindauer, S.L.; Bamford, P.; Shi, D.; Hopkins, I.; McKenna, M.C. Metabolism of Acetyl-L-Carnitine for Energy and Neurotransmitter Synthesis in the Immature Rat Brain. J. Neurochem. 2010, 114, 820–831. [Google Scholar] [CrossRef]
- Reuter, S.E.; Evans, A.M. Carnitine and Acylcarnitines: Pharmacokinetic, Pharmacological and Clinical Aspects. Clin. Pharmacokinet. 2012, 51, 553–572. [Google Scholar] [CrossRef]
- Nasca, C.; Xenos, D.; Barone, Y.; Caruso, A.; Scaccianoce, S.; Matrisciano, F.; Battaglia, G.; Mathé, A.A.; Pittaluga, A.; Lionetto, L.; et al. L-Acetylcarnitine Causes Rapid Antidepressant Effects through the Epigenetic Induction of MGlu2 Receptors. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 4804–4809. [Google Scholar] [CrossRef]
- Russo, S.J.; Charney, D.S. Next Generation Antidepressants. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 4441. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, Y.; Xue, Z.; Li, C.; Wang, C.; Zhao, X.; Zhang, J.; Wei, X.; Chen, X.; Cui, W.; et al. Rapid-Acting Antidepressant-like Effects of Acetyl-l-Carnitine Mediated by PI3K/AKT/BDNF/VGF Signaling Pathway in Mice. Neuroscience 2015, 285, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Chiechio, S.; Canonico, P.L.; Grilli, M. L-Acetylcarnitine: A Mechanistically Distinctive and Potentially Rapid-Acting Antidepressant Drug. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.J.; Liang, J.; Shan, F.; Wang, B.S.; Mu, Y.Y.; Zhou, X.H.; Xia, Q.R. L-Carnitine and Acetyl-L-Carnitine: Potential Novel Biomarkers for Major Depressive Disorder. Front. Psychiatry 2021, 12, 671151. [Google Scholar] [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.C.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.M.; Krüger, A.; Tauqeer Alam, M.; et al. The Return of Metabolism: Biochemistry and Physiology of the Pentose Phosphate Pathway. Biol. Rev. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- Aruoma, O.; Halliwell, B.; Hoey, B.; Journal, J.B.-B. ; 1988, undefined The Antioxidant Action of Taurine, Hypotaurine and Their Metabolic Precursors. portlandpress.com 1988, 256, 251–255. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Yao, J.K.; Dougherty, G.G.; Reddy, R.D.; Keshavan, M.S.; Montrose, D.M.; Matson, W.R.; McEvoy, J.; Kaddurah-Daouk, R. Homeostatic Imbalance of Purine Catabolism in First-Episode Neuroleptic-Naïve Patients with Schizophrenia. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Qiu, W.; Duarte-Guterman, P.; Eid, R.S.; Go, K.A.; Lamers, Y.; Galea, L.A.M. Postpartum Fluoxetine Increased Maternal Inflammatory Signalling and Decreased Tryptophan Metabolism: Clues for Efficacy. Neuropharmacology 2020, 175, 108174. [Google Scholar] [CrossRef]
- Kovacevic, I.; Pokrajac, M.; Miljkovic, B.; Jovanovic, D.; Prostran, M. Comparison of Liquid Chromatography with Fluorescence Detection to Liquid Chromatography-Mass Spectrometry for the Determination of Fluoxetine and Norfluoxetine in Human Plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 830, 372–376. [Google Scholar] [CrossRef]
- Dulawa, S.C.; Holick, K.A.; Gundersen, B.; Hen, R. Effects of Chronic Fluoxetine in Animal Models of Anxiety and Depression. Neuropsychopharmacology 2004, 29, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Naito, R.; Leong, D.P.; Bangdiwala, S.I.; McKee, M.; Subramanian, S. V.; Rangarajan, S.; Islam, S.; Avezum, A.; Yeates, K.E.; Lear, S.A.; et al. Impact of Social Isolation on Mortality and Morbidity in 20 High-Income, Middle-Income and Low-Income Countries in Five Continents. BMJ Glob. Heal. 2021, 6, e004124. [Google Scholar] [CrossRef] [PubMed]
- Ruoppolo, M.; Caterino, M.; Albano, L.; Pecce, R.; Di Girolamo, M.G.; Crisci, D.; Costanzo, M.; Milella, L.; Franconi, F.; Campesi, I. Targeted Metabolomic Profiling in Rat Tissues Reveals Sex Differences. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural Despair in Rats: A New Model Sensitive to Antidepressant Treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Folberth, J.; Begemann, K.; Jöhren, O.; Schwaninger, M.; Othman, A. MS2 and LC Libraries for Untargeted Metabolomics: Enhancing Method Development and Identification Confidence. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1145, 122105. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinforma. 2019, 68, 1–128. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.J.; Chang, Y.C.I.; Hsueh, H.M. Biomarker Selection for Medical Diagnosis Using the Partial Area under the ROC Curve. BMC Res. Notes 2014, 7, 1–15. [Google Scholar] [CrossRef]
- Awad, M.; Khanna, R. Support Vector Machines for Classification. Effic. Learn. Mach. 2015, 39–66. [Google Scholar] [CrossRef]
- Lahmiri, S.; Dawson, D.A.; Shmuel, A. Performance of Machine Learning Methods in Diagnosing Parkinson’s Disease Based on Dysphonia Measures. Biomed. Eng. Lett. 2018, 8, 29–39. [Google Scholar] [CrossRef]
- Lin, S.; Wu, Y.; Fang, Y. A Hybrid Machine Learning Model of Depression Estimation in Home-Based Older Adults: A 7-Year Follow-up Study. BMC Psychiatry 2022, 22, 1–13. [Google Scholar] [CrossRef]
- Yu, J.S.; Xue, A.Y.; Redei, E.E.; Bagheri, N. A Support Vector Machine Model Provides an Accurate Transcript-Level-Based Diagnostic for Major Depressive Disorder. Transl. Psychiatry 2016 610 2016, 6, e931–e931. [Google Scholar] [CrossRef] [PubMed]
| Control+Flx vs. Control | CSIS vs. Control | CSIS+Flx vs. CSIS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Retention Time (min) |
Metabolite | FC | p-value | p-adjusted | FC | p-value | p-adjusted | FC | p-value | p-adjusted | Metabolic pathway |
| 11.28 | N-acetyl-L-arginine | 0.27 | 1.00E-05 | 1.20 E-03 |
Amino acid metabolism | ||||||
| 5.57 | Xanthine | 0.61 | 1.95E-03 | 3.80 E-02 |
Purine metabolism | ||||||
| 9.50 | N1-methyl nicotinamide |
0.65 | 7.26E-04 | 2.12E-02 | Energy metabolism | ||||||
| 14.25 | Sedoheptulose 7-phosphate | 2.24 | 1.09E-04 | 2.56E-02 | 2.4 | 6.89E-04 | 2.02E-02 | Energy metabolism | |||
| 4.16 | 2-hydroxy glutaric acid |
2.28 | 6.84E-04 | 2.12E-02 | Energy metabolism | ||||||
| 4.62 |
Indoxylsulfate |
2.57 |
2.49 E-03 | 4.16 E-02 | Organic acids and derivatives | ||||||
| 10.09 | Stachydrine (proline betain) | 5.08 | 3.10E-05 | 1.80E-03 | Amino acid metabolism | ||||||
| 11.98 | Myo-Inositol | 1.56 | 2.69 E-04 | 3.15 E-02 | Inositol phosphate metabolism | ||||||
| 7.10 | Hexanoyl carnitine |
0.45 | 2.60 E-04 | 2.02 E-02 | Lipid metabolism | ||||||
| 7.98 | Xantosine | 0.62 | 6.83 E-04 |
2.02 E-02 |
Purine metabolism | ||||||
| 5.32 | Riboflavin | 0.64 | 9.14 E-04 |
2.14 E-02 |
Riboflavin metabolism | ||||||
| 11.65 | Hypotaurine | 1.62 |
1.16 E-03 |
2.25 E-02 |
Lipid metabolism | ||||||
| 8.75 | Acetyl-L- carnitine |
3.31 | 5.24 E-04 |
2.02 E-02 |
Lipid metabolism | ||||||
| Group comparison | No of component | R2a | Q2b | Accuracy |
|---|---|---|---|---|
| Control + Flx vs. Control | 5 | 0.99953 | 0.92444 | 1 |
| CSIS vs. Control | 5 | 0.99587 | 0.29245 | 0.84615 |
| CSIS + Flx vs. CSIS | 5 | 0.99636 | 0.86544 | 1 |
| Metabolites | CSIS vs. Control |
||
|---|---|---|---|
| AUC | p-value | Fold Change | |
| Myo-Inositol | 1.000 | 2.69E-04 | 1.56 |
| Methylnicotinamide | 0.95238 | 2.40E-03 | 0.75 |
| cAMP | 0.92857 | 1.13E-02 | 1.66 |
| NAD | 0.90476 | 2.03E-02 | 1.76 |
| Metabolites | CSIS+Flx vs. CSIS |
||
| AUC | p-value | Fold Change | |
| Sedoheptulose-7-phosphate | 1 | 6.89E-04 | 2.40 |
| Hypotaurine | 0.98214 | 1.16E-03 | 1.62 |
| Riboflavin | 0.98214 | 1.29E-03 | 0.64 |
| Acetyl-L-carnitine | 0.96429 | 5.24E-04 | 3.31 |
| Hexanoylcarnitine | 0.96429 | 2.60E-04 | 0.45 |
| Xanthosine | 0.94643 | 6.83E-04 | 0.62 |
| Aconitate | 0.92857 | 4.69E-03 | 0.71 |
| Cytosine | 0.91071 | 2.98E-03 | 1.39 |
| 5-Methylcytosine | 0.91071 | 5.77E-03 | 0.76 |
| Myo-Inositol | 0.91071 | 3.57E-0.3 | 0.76 |
| CSIS vs. Control | CSIS+Flx vs. CSIS | ||
|---|---|---|---|
| Accuracy | 61.50% | Accuracy | 93.30% |
| Sensitivity | 66.70% | Sensitivity | 85.70% |
| Specificity | 57.10% | Specificity | 100.0% |
| Balanced Accuracy | 61.90% | Balanced Accuracy | 92.90% |
| Predictive metabolites | Predictive metabolites | ||
| Name | FC | Name | FC |
| Tyrosine | 0.91 | PLK | 0.82 |
| Methylnicotinamide | 0.75 | Phenylalanine | 0.91 |
| Hypoxantine | 0.78 | Decanoylcarnitine | 0.93 |
| Asparagine | 1.16 | Histidine | 0.90 |
| Succinate | 1.26 | Pantothenic acid | 0.97 |
| Valine | 0.84 | Tyrosine | 0.85 |
| Serine | 1.15 | Inosine monophosphate | 0.78 |
| Alanine | 0.95 | ||
| Phosphatidylcholine | 1.04 | ||
| Glycerophosphocholine | 0.71 | ||
| Fumarate | 0.87 | ||
| Thymine | 0.92 | ||
| Carnitine | 0.88 | ||
| Cytidinemonophosphate | 1.12 | ||
| Creatine | 1.04 | ||
| Cystathionine | 0.75 | ||
| Adenosinediphosphoribose | 0.81 | ||
| N-Acetylaspartylglutamicacid | 0.73 | ||
| C6sugaralcohol | 0.71 | ||
| Succinate | 1.10 | ||
| Indoxylsulfate | 1.65 | ||
| Cytidinediphosphocholine | 1.19 | ||
| Guanosinemonophosphate | 1.19 | ||
| Dihydroxyacetone phosphate | 1.24 | ||
| Xantine | 0.77 | ||
| Metabolites | r | p |
|---|---|---|
| Sedoheptulose-7-phosphate | -0.5698 | 0.0037 |
| Indoxylsulfate | -0.4942 | 0.0141 |
| Cytosine | -0.4642 | 0.0224 |
| C6H13O9P | -0.4622 | 0.0230 |
| Urocanic acid | -0.4517 | 0.0270 |
| Saccharopine | 0.4093 | 0.0471 |
| Adenosinediphosphoribose | 0.4121 | 0.0454 |
| Acetylcholine | 0.4362 | 0.0331 |
| Adenine | 0.4585 | 0.0243 |
| Guanosine | 0.4667 | 0.0215 |
| Acetylarginine | 0.4669 | 0.0215 |
| NAD | 0.5001 | 0.0128 |
| Riboflavin | 0.5359 | 0.0070 |
| cAMP | 0.5521 | 0.0052 |
| Myo-Inositol | 0.5932 | 0.0023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





