1. Introduction
The oral microbiome naturally serves to maintain an equilibrated ecosystem, facilitating the protection against disease development. This community of oral bacteria is enclosed in a self-produced extracellular polymeric matrix, forming oral biofilms known as plaque. The composition and delicate balance within this community influences the function of the oral microbiome. Oral diseases remain a major health problem worldwide, with the World Health Organisation (WHO) 2022 reporting that oral health neglect affects almost half of the world’s population (1). Furthermore, oral health has been associated with other systemic health conditions. In an effort to improve overall healthcare and well-being, a greater understanding of the environment within the oral cavity is essential in oral and dental research(2).
Quorum sensing (QS) is a form of bacterial communication, regulating gene expression in response to cell density-dependent signalling molecules. For an oral biofilm to exist, QS is essential in coordinating bacterial behavior to configure complex functional communities (3). Quorum sensing molecules (QSMs) have been found to influence oral biofilms in various ways, including growth, iron and hemin uptake, stress-related gene expression and virulence factor production (4). Furthermore, QSMs have been discovered to indirectly influence host cells by shifting characteristics of an oral biofilm from a mutualistic to a pathology-associated state via changes in species population ratios and/or virulence factor expressions such as proteolytic activity, well-known indicators of the shift away from commensal biofilms (4,5).
The understanding of microbiology has vastly advanced over time. From the idea that bacteria exist as single cellular organisms to the understanding that biofilms hold actively coordinated multispecies communities, comes the current paradigm that cross-kingdom communication exists between bacteria and eukaryotic host cells (6). From this, the suggestion that QSMs influence oral disease progression has emerged (7). However, knowledge of direct oral QSM-host interaction is limited, and such studies could provide more insight into the cross-kingdom communication occurring during oral disease development. This review aims to explore the literature on oral QSM-host interaction and highlight areas of advancement. Developments in this field can open new avenues for targeting QS as a therapeutic method to treat oral diseases.
2. The role of host cells in the progression of oral diseases
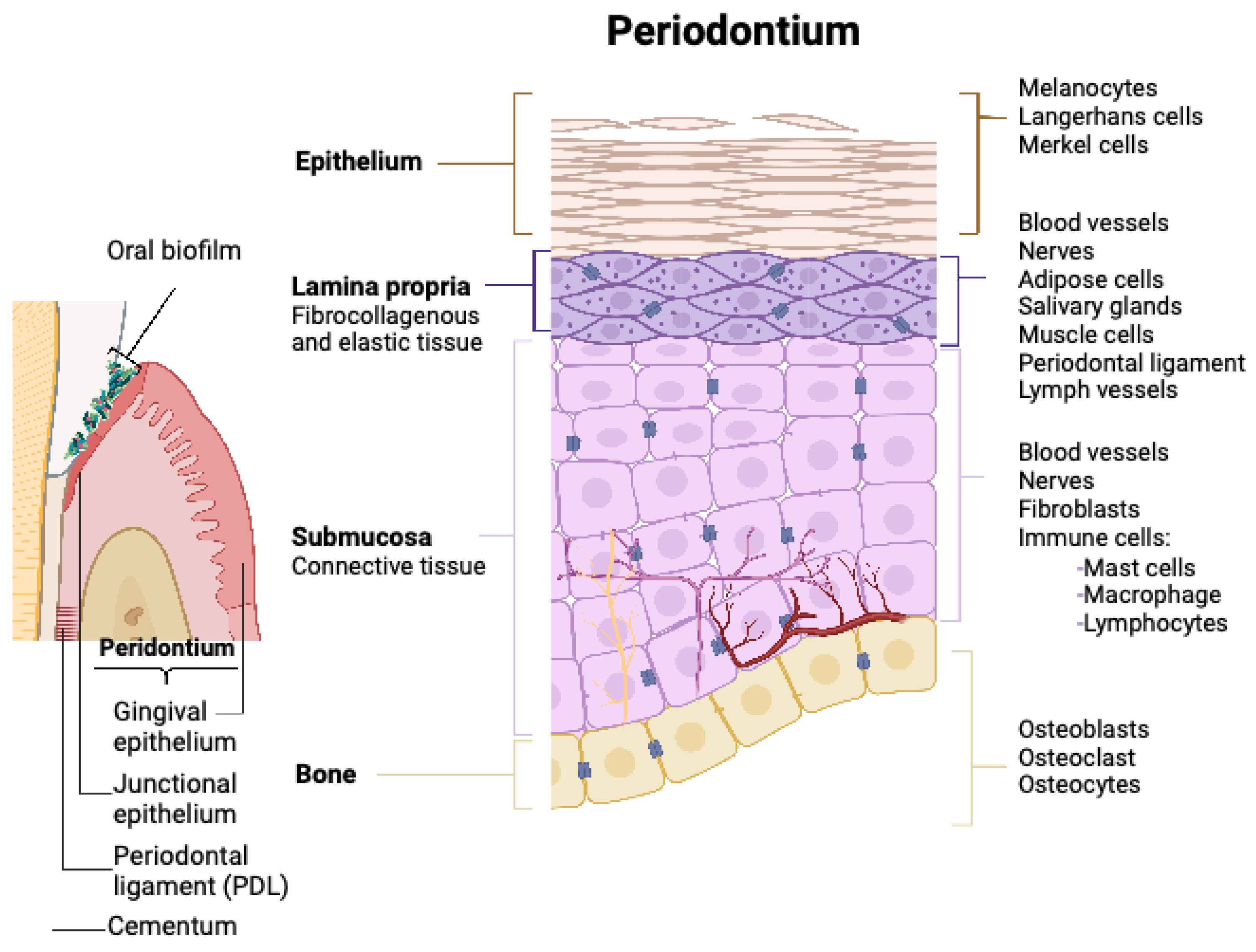
To understand the possible interactions between oral QSMs and the host response, it is beneficial first to recognise the cell configuration within the oral cavity and their role in oral disease development. The tissue and cell arrangement in the oral cavity is illustrated in
Figure 1. According to the WHO, the following are classified as the most common oral diseases, estimated to affect 3.5 billion people (1).
Dental caries results from the complex interaction of fermentable sugars and acid-producing microorganisms such as Streptococcus mutans. Four factors determine the progression of dental caries: the oral biofilm dynamics, the oral environment, the presence of a tooth surface and time (8). From this, it could be considered that the synchronised increase in QSMs with increased time may facilitate the progression of dental caries, however, no studies have explored this to date.
Periodontitis is the dysregulated, excessive inflammatory response of periodontal tissues towards a dysbiotic biofilm. Bacteria in built-up plaque release virulence factors, leading to the recruitment of inflammatory cells (9). This often leads to the loss of marginal gingival connective tissue and tight junctions between epithelial cells (10). As QS and periodontal disease are both being bacterial density influenced, it could be hypothesised that there may be a correlation between the two. Research exploring this has become an area of recent interest and is reviewed within this paper.
Periodontitis has been found to induce increased epithelial migration, cell proliferation, and release of growth factors and inflammatory cytokines, all of which are closely associated with the development of oral cancers (8). Currently, there is no direct correlation between QSMs and oral cancer development, however QSMs present in the oral cavity have been discovered to upregulate some factors associated with cancer development, as described in this review (11–13).
This review will explore if any of the discovered oral QSMs have an influence on the host response or the integrity of oral tissues, highlighting how QS may directly or indirectly contribute to the onset and progression of oral diseases.
3. Quorum Sensing in the oral cavity
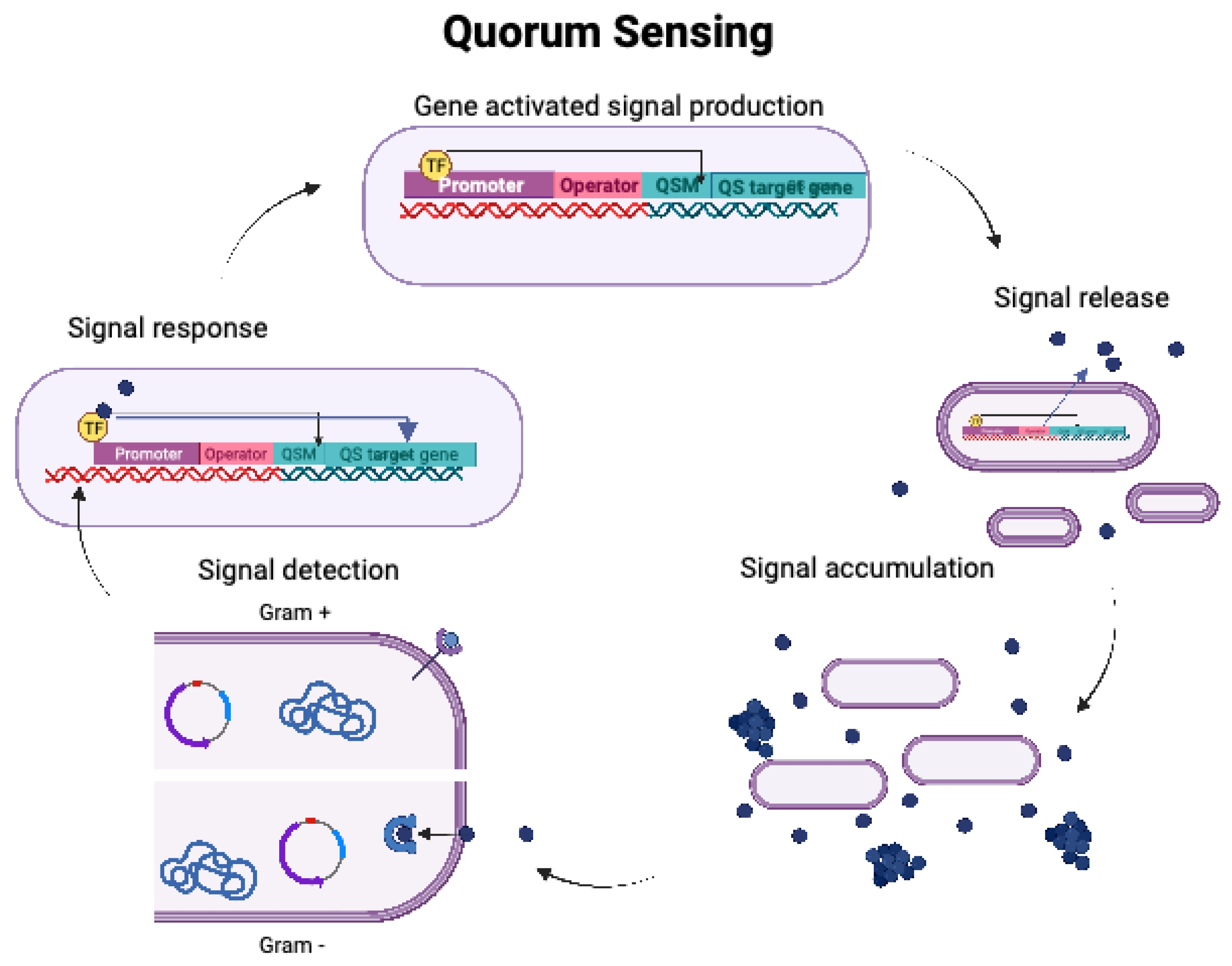
QS follows the general mechanism of gene-activated signal production, signal release, signal accumulation, signal detection and response, as illustrated in
Figure 2. Initially, communication was thought to occur exclusively between Gram-positive and Gram-negative bacterial strains, respectively. However, Autoinducer-2 (AI-2) has demonstrated to mediate cross-species communication between both domains (3).
4. Influence of Autoinducer peptides (AIP) on host cells
QS in Gram-positive bacteria utilises AIP signalling molecules. Receptors are usually transmembrane proteins that channel this signal intracellularly, and sensor histidine kinases transduce this signal to QS-regulated target genes (14)In streptococci, 124 competence-stimulating peptides (CSP) -genes have been identified, affecting multiple pathways, including biofilm formation, acid tolerance, and virulence activity (15–17). QS systems that have been described in Gram-positive oral bacteria include the CSP/ComABCDE, and comX- inducing peptide (XIP)-ComRS signalling systems. A further PdrA/WGK system was recently identified in S. mutans, along with the fatty acid signalling molecule trans-2-decanoic acid (18,19).
Host cell membrane proteins have recently been discovered to recognise bacterial CSP molecules. Bitter taste receptors (taste family 2 bitter receptor proteins; T2R) are G protein-coupled receptors (GPCRs) that signal through Gα-mediated, C-di-adenosine monophosphate (cAMP) decrease and Gβγ activation of phospholipase C (Gβγ/PLC) leading to calcium release. The majority of T2Rs reside in the respiratory pathway, with the oral cavity containing 25 variations of T2R. A number of studies have detected genes for T2R receptors in non-respiratory tissues, including the gastrointestinal tract, kidneys and lymphocytes, suggesting that T2R may have a chemosensory function in other organs too. In oral tissues, T2Rs have been discovered to serve a diverse array of chemosensory functions, including innate immunity within the oral epithelium. Furthermore, T2Rs have been shown to act as essential sensors towards bacterial QSMs (11,20,21).
It has been discovered that out of CSP-1, 2 and 3 produced by S. mutans, only CSP-1 induced a robust intracellular calcium mobilisation via the receptor complexes T2R14- Gβγ/PLC-β pathway within gingival epithelial cells (TIGK-hTERT). The immune response of this interaction was further examined by Enzyme-Linked Immunosorbent Assay (ELISA), monitoring the expression of inflammatory cytokines. An increase in interleukin-6 (IL-6), IL-8, and tumour necrosis factor- α (TNF-α) was observed upon 18-hour treatment of the gingival epithelial cells with 50 μM CSP-1, whilst IL-2, IL-4, and IL-10 were unaffected. CSP-1 was also observed to mediate NF-kB signalling. The regulation pathways leading to the observed characteristics remain an area for further research(21) (Medapati et al., 2021). It is possible that the activation of IL-8 and TNF-α by CSP-1 is followed by an infiltration of immune cells such as neutrophils and macrophages, however only chemotactic immune cell testing can confirm this (22). Distinguishing the mechanism as to why the structure and chemical characteristics of CSP-2 and CSP-3 may not elicit a host response, may hold the key towards a more comprehensive understanding of oral QSM-host interactions.
Medapati et al. (2021a) further examined the interaction between T2R14 and Staphylococcus aureus and S. mutans. It was found that oral epithelial gene knockout cells depleted of T2R14 significantly decreased the internalisation of S. aureus whilst S. mutans internalization remained unaffected. T2R14 affected the cytoskeleton of oral epithelial cells, thereby inhibiting S. aureus internalization. Interestingly, when oral epithelial cells were primed with S. mutans CSP-1, the growth of S. aureus was inhibited, but S. mutans growth was unaffected (21). Recent work reports that bioactive molecules, isolated from S. mutans biofilms, demonstrate bactericidal effects on other commensal bacteria (23). The results from the T2R14 study, in conjunction with S. mutans biofilms’ bactericidal effects, indicate that the underlying mechanism for this inhibitory effect may be attributable to QSMs (24).
Further to the two studies described, no other work has specifically evaluated the effects of CSP present in the oral cavity on oral host cells. Nonetheless, other studies add value to this field. The influence of CSP from Streptococcus mitis on breast cancer cell invasion was investigated. Microscopic, transcriptomic and chick chorioallantoic membrane (CAM) analysis demonstrated that CSP from S. mitis promoted tumour cell invasion and angiogenesis(12). The new insight into oral QSMs’ influence on breast cancer progression may be translatable to oral epithelial carcinoma. However, to the best of our knowledge, no published research exists to investigate this hypothesis. If QSMs can be confirmed to have tumorigenic effects by multiple independent studies, antagonists to the identified QSMs may present opportunities in cancer prevention and therapy.
5. Influence of Oral Acetyl homoserine lactones (AHLs) on host cells
5.1. QS molecules produced by Gram-negative oral bacteria
AHLs are the membrane permeable QSMs of Gram-negative bacteria. Receptors are often cytoplasmic, and when activated act as transcription factors for QS-regulated target genes (3).
Due to the lack of oral AHLs discovered, it has been suggested that AHLs have a minor influence on oral biofilm development (25,26). However, current literature suggests this assumption may be due to underdeveloped detection technology and techniques, rather than the absence of oral AHL molecules (Whittaker et al., 1996; Fuqua et al., 1994). This is evident by Burgess et al., not detecting any AHLs in Porphyromonas gingivalis W50 solvent-extracted supernatant against a range of AHLs by thin layer chromatography (TLC) with four different bacterial AHL biosensors. However, studies incorporating sophisticated chemical extraction and detection methods such as high-performance liquid chromatography (HPLC) have now established the presence of AHLs in samples extracted from species present in oral biofilms, plaque and saliva samples(27–30)
Additionally, Muras et al. found homologues of AHL-synthase genes hdtS and luxR-type receptor in P. gingivalis W83 and ATCC 33277 strains (5,29). This study identified the presence AHLs of a variety of carbon chain lengths, including C8 AHL in saliva and teeth samples (Muras et al., 2020b) as well as C14, C18, OC8, HC10 AHL in saliva samples (Muras et al., 2020a, 2020b). This was the first in vitro culture to successfully reveal the presence of OC8 AHL by P. gingivalis ATCC 33277, achieved through HPLC/mass spectrometry (29).
5.2. The immune response towards Oral AHLs
In the immunostimulatory analysis of AHLs, human Jurkat T lymphocytes were subjected to seven different AHLs at a concentration of 100 μM, which included the orally detected AHLs C8 and OC8 by Muras et al, (2020). Among the tested AHLs, (3-Oxododecanoyl)-l-homoserine lactone (OdDHL), the well-defined QSM of Pseudomonas aeruginosa, was the only AHL that led to apoptosis via the mitochondrial pathway (31). Despite the orally present AHLs examined in this study not eliciting any stimulation response in immune cells, this work has discovered a possible mechanism whereby newly researched apoptosis activating AHLs may interact with T lymphocytes.
To consolidate oral AHL immunostimulatory influence, oral cell line range, exposure methods, concentrations and analysis techniques such as memory lymphocyte immunostimulation assay and inflammation biomarker detection, remain areas to research.
Other studies have confirmed the possible inert characteristics of AHL molecules found in the oral cavity. C8 and C14, did not stimulate the production of inflammatory cytokines, IL-8, TNF-α or IL-1β in THP-1 cells, a mammalian monocytic cell line. Furthermore, activation of Nuclear factor kappa B (NF-κB), a key signalling molecule involved in inflammatory immune responses, showed no changes when exposed to C8 or C14 AHLs (32).
Further studies have clarified the non-interactive nature of oral AHLs C8 and C14 and a range of cell lines including EL4 T-lymphoblasts, DC2.4 dendritic cells, A20 mouse lymphoma cell line, Raw264.7 mouse monocyte/macrophage-like cells and THP-1 cells (33). This study also investigated the mechanism of OdDHL-host interaction. OdDHL was discovered to incorporate into the mammalian plasma membrane, leading to lipid domain dissolution, resulting in apoptosis, mediated by caspase 3 and caspase 8. This research holds interesting grounds for investigating other oral AHLs’ ability to interact with host cell membranes to induce apoptosis similarly to OdDHL (33).
5.3. Oral AHL molecules influence on bitter taste receptors (T2R)
The interaction between G-coupled T2R receptors and AHLs has also been an area of interest for analysing the influence of AHL QSMs on host cells. The AHL-human interaction was studied through a unique sensory analysis method known as electronic-tongue, an analytical instrument containing chemical sensors capable of sensing and characterising chemical taste (34). Participants scored the bitterness of AHL compounds compared to known bitter standards, with identified T2R receptor activation and in vivo standard bitter score. Individual AHLs and mixtures scored higher bitterness than standards. This data indicated an interaction between T2R and AHLs. This study further analysed the functional characteristics of this observed interaction. Oral C8 AHL was found to activate T2R4, T2R14 and T2R20 receptors on HEK293T human kidney epithelial cells with strong receptor potency, a measure of magnitude of receptor activation. Characterisation of the amino acids in the interaction found that C8 AHL bound to similar sites on all three T2R receptors specifically located on extracellular loop 2 (35). The interaction between C8 AHL and T2R, has provided a steppingstone towards further investigating the receptor activation by oral AHLs. Analysis methods such as Fluorescence resonance energy transfer (FRET) assay, could be incorporated to detect the oligomerisation state of membrane proteins against orally present AHLs.
After discovering an immune response activation of AHL molecules by several T2Rs receptors expressed in airway epithelial cells Freund et al., (2018) expanded their research to a broader range of spacialised QSMs. Quinolones are naturally occurring plant and bacterial-derived molecules, other than their bactericidal properties, quinolones have been utilised by many species including P. aeruginosa as density-dependent QSMs. 2-heptyl-3-hydroxy-4-quinolone, known as Pseudomonas quinolone signal (PQS), 2,4-dihydroxyquinolone, and 4-hydroxy-2-heptylquinolone (HHQ) T2R activation within HEK293T cells, lung epithelial cell lines (A549, BEAS-2B, and NCI-H292), and primary sinonasal cells was investigated. PQS at 10-100 μM activated T2R4, 16 and 38 receptors in HEK293T cells, HHQ 100 μM activated T2R14 and 2,4-Dihydroxyquinolone had no effect. The activation of T2R was found to lead to an increased calcium induction, indicating activation of G-coupled T2R receptors. This induction led to a decrease in stimulated cAMP levels in cell line cultures and primary airway cells, analysed using a combination of FRET-based protein biosensors and fluorescent indicator dyes. PQS and HHQ in primary sinonasal cells activated nitric oxide (NO) production, to levels that were previously found to be bacteriocidal and increase ciliary beating. Combined, the findings indicate that the presence of QSMs within the airway are utilised by host cells to activate innate immune response via T2R receptor activation, further leading to an increase in NO production promoting clearance of pathogen-trapped mucus. This study explores how broad the examination of QSMs and host interaction can be and that it is not restricted to conventionally known QSMs. This study further indicates that bacterial quinolones, along with AHLs, trigger immune responses mediated by airway T2R. Furthermore, this study’s findings can be translated to the oral cavity where the majority of T2R reside. From this study it is recommended that oral epithelial cell lines such as H400 cells as well as primary oral cells are exposed to PQS and HHQ molecules to understand the immune response induction they may have in the oral cavity. However, this remains an under-researched area and requires research into the AHL detection by T2R. Investigating changes in inflammatory markers/ chemokines will aid in understanding AHL and QS-related molecules in the progression of overactivated immune response of periodontal diseases. (20)
5.4. AHLs Potential carcinogenic influence
The theory that AHLs may have a role in carcinoma induction has been proposed by Sankar et al., hypothesising that AHLs might induce carcinomas via the NF-κB signalling pathway through IκB phosphorylation and peroxisome proliferator-activated receptor (PPAR) inhibition. This theory is based on limited literature, without any conducted tests (36). Hence, in vitro analysis such as oral epithelial cell proliferation investigations in the presence of oral AHLs and AHL inhibitors, are critical tests to evaluate the hypothesis proposed by Sankar et al., (2023). Furthermore, investigating the cascade changes of crucial genes in the NF-κB signalling pathway that may lead to changes in apoptosis would highly advance this area of research (37). Additionally, carcinoma signalling pathways and the expression of carcinoma development genes such as cyclin A, cyclin D1, cyclin-depended kinase 6, PRAD-1/CCND1, OSCCC are advised genes to analyse in aiding the understanding AHL influence on carcinoma development (38)
6. Influence of the universal QS molecule AI-2 on host cells
6.1. AI-2 communication in the oral cavity
The AI-2/LuxS system is considered the central QS system in oral bacteria, holding cross-species communication capability. AI-2 molecules are 4,5-dihydroxy-2,3-pentanedione (DPD) derivates formed from the enzymatic activity of the AI-2 synthases, encoded by the luxS gene. Farias et al., (2001) identified in vitro activity of the AI-2 system in 13 of 33 oral species. It has been shown that S. mutans species communicate with other streptococci utilising the LuxS system, affecting multiple virulence traits including adhesion, cohesion and acidic tolerance of S. mutans biofilms (39,40). Adding AI-2 to S. mutans biofilms upregulates cariogenic, bacterial adhesion, cohesion and biofilm formation genes. AI-2 was also found to regulate iron chelation and iron acquisition in Aggregatibacter actinomycetemcomitans and P. gingivalis (40).
6.2. The immune induction of AI-2
Fusobacterium nucleatum is a specialised oral commensal, playing a crucial role in biofilm development and build up and is associated with a broad spectrum of human diseases. F. nucleatum utilises AI-2 molecules in oral biofilm formation(41). The influence of F. nucleatum’s AI-2 on macrophages has been investigated by Wu et al (2019). A macrophage precursor cell line U937 was exposed to F. nucleatum’s AI-2 molecules (50-400 μM), which were found to significantly increase macrophage migration. This phenomenon ceased in the presence of AI-2 inhibitor D-ribose (50 μM). In addition, this study explored the impact of AI-2 molecules on macrophage immune response, by assessing the markers for the M1-phenotype (pro-inflammatory, mediating the release of inflammatory cytokines) or M2-phenotype (anti-inflammatory and tumour-promoting) induction. ELISA assessment highlighted that M1 markers IL-8, TNF-α and IL-1β were significantly increased at 400 μM AI-2. M2 marker IL-10 significantly decreased at 50μM and 400μM AI-2 (13).
Despite its importance in biofilm formation, no clinical research into the concentrations of AI-2 found in oral disease has been undertaken to date. It could be assumed that disease-associated oral biofilms have a higher abundance of AI-2 molecules with increasing biomass (42–44). Accordingly, macrophages may respond to increased AI-2 levels in these biofilms in an M1 macrophage pro-inflammatory manner. Hence, QSMs may indirectly contribute to increased inflammation, promoting periodontal diseases.
A recent study demonstrated that protein expression in macrophages was altered when co-cultured with purified F. nucleatum AI-2 molecules. Gene expression profiling highlighted 57 genes expressed at > 1.3-fold change, of which 46 were upregulated and 11 downregulated. Further analysis revealed that the proteins overexpressed were associated with inflammatory factors and cytokine production, including TNFSF9, IL-1β and C-C motif chemokine (45). TNFSF9 is highly expressed in pancreatic cancer tissue and is associated with the M1 polarisation of macrophages(46). This highlights the influence that F. nucleatum’s AI-2 may have on progression of inflammatory diseases and carcinoma development.
6.3. The interaction between AI-2 QSMs and periodontium tissue
P. gingivalis holds as a keystone pathogenic bacterium, due to its influence of triggering the immune response in the progression of periodontitis. For this reason, luxS mutants of P. gingivalis have been developed to aid understanding the relationship between AI-2 and periodontitis disease development(47). P. gingivalis luxS mutants showed decreased capabilities to induce an immune response, with reduced expression of inflammatory cytokines IL-6, monocyte chemoattractant protein-1 and IL-1β produced by periodontal ligament (PDL) fibroblasts. This study suggests that AI-2 present in the oral cavity may induce an inflammatory response in PDL-fibroblasts, aggravating periodontal disease development (48).
Additionally, it has been found that a range of mammalian cells can produce AI-2-mimicking molecules. Vibrio harveyi TL26, a reporter strain that is unable to synthesise AI-2 and does not respond to AI-1, was exposed to growth media from Caco-2, Hela, A59, Jurkat E6-1 and U937 cells. Cells were identified to produce AI-2 homologues as luminescence was activated in the reporter strain, which only responds in this manner in the presence of AI-2 molecules. It was identified that AI-2 mimicking molecules are utilised by host cells to communicate with resident microorganisms. Although the investigated cell lines were not oral epithelial cells, it is possible that oral epithelial cells may present similar characteristics. However, studies confirming the described cross-communication assay with oral epithelial cells are still required (49).
7. Conclusions
Current literature presents QSM-host interactions as a possible novel pathway to treat oral diseases. However, this can only be achieved with a comprehensive understanding of QSMs within the oral cavity. QSMs from crucial Gram-positive bacteria have been well-defined. QSM CSP-1 produced by S. mutans has been found to interact with T2R receptors, activating NF-kB signalling and to remodel of the cytoskeleton. Gram-positive QSMs’ inflammatory influence may facilitate the progression of periodontal diseases. Nonetheless, this needs to be further evaluated with specific oral cell lines and through clinical sampling.
AI-2 in the oral cavity activates M1 macrophage characteristics known to have a pro-inflammatory role. AI-2 from P. gingivalis has been found to elicit an inflammatory response in PDL fibroblasts. The QSM in this investigation was extracted from a key oral pathogen and the cell line used was highly specific to the oral cavity; hence this study paves the way for understanding oral QSM-host interactions. Research can further build on these findings by analysing a wider range of direct oral QSM extracts against orally specific cell lines, or primary oral cells.
AHL detection and influence within the oral cavity remains an area of limited findings. Further research of AHLs in the oral cavity is critical in understanding oral QSMs’ influence on host cells. The current literature presents chemically inactive in vitro characteristics of two oral AHLs (C8 and C14); however, this has not been explored on oral cells. It is fundamental first to consolidate the findings of QSMs found in the oral cavity. This can be achieved through advancements in sensitive chemical analysis techniques and technology, which may include nuclear magnetic resonance spectroscopy, HPLC-MS and Fourier-transform ion cyclotron resonance (FT-ICR-MS) (50). Ultimately, developments to research in this area would facilitate understanding QS influence on the progression of oral diseases. Through the understanding of QSMs and host cell interactions, oral healthcare and prevention could be further improved through identification of novel molecular targets.
Funding
Please add: This research was funded by is BBSRC ICASE Studentship, GSK Ref BIDS Rec: 100708
Informed Consent Statement
Not applicable.
Data Availability Statement
All data presented within manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO highlights oral health neglect affecting nearly half of the world’s population [Internet]. [cited 2023 Jan 6]. Available from: https://www.who.int/news/item/18-11-2022-who-highlights-oral-health-neglect-affecting-nearly-half-of-the-world-s-population.
- 2. Services I of M (US) B on HC. The Connection Between Oral Health and Overall Health and Well-Being. 2009 [cited 2023 Feb 17]; Available from: https://www.ncbi.nlm.nih.gov/books/NBK219661/.
- Li Z, Nair SK. Quorum sensing: How bacteria can coordinate activity and synchronize their response to external signals? Protein Sci [Internet]. 2012 Oct [cited 2022 May 13];21(10):1403. Available from: /pmc/articles/PMC3526984/.
- Wright, P.P.; Ramachandra, S.S. Quorum Sensing and Quorum Quenching with a Focus on Cariogenic and Periodontopathic Oral Biofilms. Microorganisms 2022, 10, 1783. [Google Scholar] [CrossRef] [PubMed]
- Muras, A.; Mayer, C.; Otero-Casal, P.; Exterkate, R.A.M.; Brandt, B.W.; Crielaard, W.; Otero, A.; Krom, B.P. Short-Chain N -Acylhomoserine Lactone Quorum-Sensing Molecules Promote Periodontal Pathogens in In Vitro Oral Biofilms. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef]
- Hughes, D.T.; Sperandio, V. Inter-kingdom signalling: communication between bacteria and their hosts. Nat. Rev. Microbiol. 2008, 6, 111–120. [Google Scholar] [CrossRef]
- Rajamani S, Lee L, Smith E, Majireck M, Mohan R. Modulation of Bacterial Quorum Sensing by Eukaryotes. Implication of Quorum Sensing and Biofilm Formation in Medicine, Agriculture and Food Industry [Internet]. 2019 [cited 2023 Jan 5];39–56. Available from: https://link.springer.com/chapter/10.1007/978-981-32-9409-7_4.
- Chen, X.; Daliri, E.B.-M.; Kim, N.; Kim, J.-R.; Yoo, D.; Oh, D.-H. Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Carrillo JL, Hernández-Reyes VE, García-Huerta OE, Chávez-Ruvalcaba F, Chávez-Ruvalcaba MI, Chávez-Ruvalcaba KM, et al. Pathogenesis of Periodontal Disease. Periodontal Disease - Diagnostic and Adjunctive Non-surgical Considerations [Internet]. 2019 Jun 6 [cited 2022 ]; Available from: undefined/state.item. 28 May.
- Könönen, E.; Gursoy, M.; Gursoy, U. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef]
- Medapati, M.R.; Singh, N.; Bhagirath, A.Y.; Duan, K.; Triggs-Raine, B.; Batista, E.L.; Chelikani, P. Bitter taste receptor T2R14 detects quorum sensing molecules from cariogenic Streptococcus mutans and mediates innate immune responses in gingival epithelial cells. FASEB J. 2021, 35, e21375. [Google Scholar] [CrossRef]
- De Spiegeleer, B.; Verbeke, F.; D’hondt, M.; Hendrix, A.; Van De Wiele, C.; Burvenich, C.; Peremans, K.; De Wever, O.; Bracke, M.; Wynendaele, E. The Quorum Sensing Peptides PhrG, CSP and EDF Promote Angiogenesis and Invasion of Breast Cancer Cells In Vitro. PLOS ONE 2015, 10, e0119471. [Google Scholar] [CrossRef]
- Wu J, Li K, Peng W, Li H, Li Q, Wang X, et al. Autoinducer-2 of Fusobacterium nucleatum promotes macrophage M1 polarization via TNFSF9/IL-1β signaling. Int Immunopharmacol. 2019 Sep 1;74:105724.
- Monnet, V.; Gardan, R. Quorum-sensing regulators in Gram-positive bacteria: ‘cherchez le peptide ’. Mol. Microbiol. 2015, 97, 181–184. [Google Scholar] [CrossRef]
- Suntharalingam, P.; Cvitkovitch, D.G. Quorum sensing in streptococcal biofilm formation. Trends Microbiol. 2005, 13, 3–6. [Google Scholar] [CrossRef]
- Peterson, S.N.; Sung, C.K.; Cline, R.; Desai, B.V.; Snesrud, E.C.; Luo, P.; Walling, J.; Li, H.; Mintz, M.; Tsegaye, G.; et al. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol. Microbiol. 2003, 51, 1051–1070. [Google Scholar] [CrossRef]
- Cvitkovitch DG, Li YH, Ellen RP. Quorum sensing and biofilm formation in Streptococcal infections. Journal of Clinical Investigation. 2003 Dec;112(11):1626–32.
- Steinberg, D. Dental Chatter: Bacterial Cross-Talk in the Biofilm of the Oral Cavity. Isr. J. Chem. 2015, 56, 273–281. [Google Scholar] [CrossRef]
- Underhill SAM, Shields RC, Kaspar JR, Haider M, Burne RA, Hagen SJ. Intracellular Signaling by the comRS System in Streptococcus mutans Genetic Competence. mSphere [Internet]. 2018 Oct 31 [cited 2023 Feb 20];3(5). Available from: https://pubmed.ncbi.nlm.nih. 3038.
- Freund JR, Mansfield CJ, Doghramji LJ, Adappa ND, Palmer JN, Kennedy DW, et al. Activation of airway epithelial bitter taste receptors by Pseudomonas aeruginosa quinolones modulates calcium, cyclic-AMP, and nitric oxide signaling. J Biol Chem [Internet]. 2018 Jun 22 [cited 2023 Jan 13];293(25):9824–40. Available from: https://pubmed.ncbi.nlm.nih.gov/29748385/.
- Medapati, M.R.; Bhagirath, A.Y.; Singh, N.; Schroth, R.J.; Bhullar, R.P.; Duan, K.; Chelikani, P. Bitter Taste Receptor T2R14 Modulates Gram-Positive Bacterial Internalization and Survival in Gingival Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 9920. [Google Scholar] [CrossRef] [PubMed]
- Meyle, J.; Dommisch, H.; Groeger, S.; Giacaman, R.A.; Costalonga, M.; Herzberg, M. The innate host response in caries and periodontitis. J. Clin. Periodontol. 2017, 44, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Kudo, Y.; Baker, J.L.; LaBonte, S.; Jordan, P.A.; McKinnie, S.M.K.; Guo, J.; Huan, T.; Moore, B.S.; Edlund, A. Cariogenic Streptococcus mutans Produces Tetramic Acid Strain-Specific Antibiotics That Impair Commensal Colonization. ACS Infect. Dis. 2020, 6, 563–571. [Google Scholar] [CrossRef]
- Alves, L.A.; Nomura, R.; Mariano, F.S.; Harth-Chu, E.N.; Stipp, R.N.; Nakano, K.; Mattos-Graner, R.O. CovR Regulates Streptococcus mutans Susceptibility To Complement Immunity and Survival in Blood. Infect. Immun. 2016, 84, 3206–3219. [Google Scholar] [CrossRef]
- Guo, L.; He, X.; Shi, W. Intercellular communications in multispecies oral microbial communities. Front. Microbiol. 2014, 5, 328. [Google Scholar] [CrossRef]
- Whittaker, C.J.; Klier, C.M.; Kolenbrander, P.E. MECHANISMS OF ADHESION BY ORAL BACTERIA. Annu. Rev. Microbiol. 1996, 50, 513–552. [Google Scholar] [CrossRef]
- A Burgess, N.; Kirke, D.F.; Williams, P.; Winzer, K.; Hardie, K.R.; Meyers, N.L.; Aduse-Opoku, J.; A Curtis, M.; Cámara, M. LuxS-dependent quorum sensing in Porphyromonas gingivalis modulates protease and haemagglutinin activities but is not essential for virulence. Microbiology 2002, 148, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Yin WF, Purmal K, Chin S, Chan XY, Koh CL, Sam CK, et al. N-Acyl Homoserine Lactone Production by Klebsiella pneumoniae Isolated from Human Tongue Surface. Sensors 2012, Vol 12, Pages 3472-3483 [Internet]. 2012 Mar 12 [cited 2023 Jan 5];12(3):3472–83. Available from: https://www.mdpi. 1424.
- Muras, A.; Otero-Casal, P.; Blanc, V.; Otero, A. Acyl homoserine lactone-mediated quorum sensing in the oral cavity: a paradigm revisited. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Muras, A.; Otero-Casal, P.; Blanc, V.; Otero, A. Acyl homoserine lactone-mediated quorum sensing in the oral cavity: a paradigm revisited. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Jacobi CA, Schiffner F, Henkel M, Waibel M, Stork B, Daubrawa M, et al. Effects of bacterial N-acyl homoserine lactones on human Jurkat T lymphocytes-OdDHL induces apoptosis via the mitochondrial pathway. International Journal of Medical Microbiology. 2009 Nov 1;299(7):509–19.
- Gomi K, Kikuchi T, Tokue Y, Fujimura S, Uehara A, Takada H, et al. Mouse and Human Cell Activation by N-Dodecanoyl-dl-Homoserine Lactone, a Chromobacterium violaceum Autoinducer. Infect Immun [Internet]. 2006 Dec [cited 2023 Jan 17];74(12):7029. Available from: /pmc/articles/PMC1698062/.
- Song, D.; Meng, J.; Cheng, J.; Fan, Z.; Chen, P.; Ruan, H.; Tu, Z.; Kang, N.; Li, N.; Xu, Y.; et al. Pseudomonas aeruginosa quorum-sensing metabolite induces host immune cell death through cell surface lipid domain dissolution. Nat. Microbiol. 2018, 4, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Podrazka M, Báczyńska E, Kundys M, Jeleń PS, Nery EW. Electronic Tongue—A Tool for All Tastes? Biosensors (Basel) [Internet]. 2018 Dec 31 [cited 2023 Feb 21];8(1). Available from: /pmc/articles/PMC5872051/.
- Jaggupilli, A.; Singh, N.; De Jesus, V.C.; Duan, K.; Chelikani, P. Characterization of the Binding Sites for Bacterial Acyl Homoserine Lactones (AHLs) on Human Bitter Taste Receptors (T2Rs). ACS Infect. Dis. 2018, 4, 1146–1156. [Google Scholar] [CrossRef]
- Sankar, S.; Yuwanati, M.; Ganesh, P.S. Acyl homoserine lactone inhibitors for oral squamous cell carcinoma – Novel insights and therapeutic perspective. Med Hypotheses 2023, 170. [Google Scholar] [CrossRef]
- Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-κB Function in Growth Control: Regulation of Cyclin D1 Expression and G0/G1-to-S-Phase Transition. Mol Cell Biol [Internet]. 1999 Apr [cited 2023 Jan 17];19(4):2690. Available from: /pmc/articles/PMC84062/.
- Duckett CS, Perkins ND, Leung K, Agranoff AB, Nabel GJ. Cytokine induction of nuclear factor kappa B in cycling and growth-arrested cells. Evidence for cell cycle-independent activation. J Biol Chem [Internet]. 1995 [cited 2023 ];270(32):18836–40. Available from: https://pubmed.ncbi.nlm.nih. 22 May 7642.
- Rocha, F.R.; Regis, W.F.; Duarte, S.; Muniz, F.W.; Rodrigues, L.K. Effect of bioactive compounds on the regulation of quorum sensing network-associated genes and virulence in Streptococcus mutans—A systematic review. Arch. Oral Biol. 2020, 119, 104893. [Google Scholar] [CrossRef]
- Niazy, A.A. LuxS quorum sensing system and biofilm formation of oral microflora: A short review article. Saudi Dent. J. 2020, 33, 116–123. [Google Scholar] [CrossRef]
- Frias, J.; Olle, E.; Alsina, M. Periodontal Pathogens Produce Quorum Sensing Signal Molecules. Infect. Immun. 2001, 69, 3431–3434. [Google Scholar] [CrossRef] [PubMed]
- Velsko, I.M.; Yates, J.A.F.; Aron, F.; Hagan, R.W.; Frantz, L.A.F.; Loe, L.; Martinez, J.B.R.; Chaves, E.; Gosden, C.; Larson, G.; et al. Microbial differences between dental plaque and historic dental calculus are related to oral biofilm maturation stage. Microbiome 2019, 7, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gedif Meseret, A. Oral Biofilm and Its Impact on Oral Health, Psychological and Social Interaction. International Journal of Oral and Dental Health. 2021 Mar 12;7(1).
- Ahmed NA, Petersen FC, Scheie AA. AI-2/LuxS is involved in increased biofilm formation by Streptococcus intermedius in the presence of antibiotics. Antimicrob Agents Chemother [Internet]. 2009 Oct [cited 2023 Feb 21];53(10):4258–63. Available from: https://pubmed.ncbi.nlm.nih. 1959.
- Wu, J.; Wang, Y.; Jiang, Z. Immune induction identified by TMT proteomics analysis in Fusobacterium nucleatum autoinducer-2 treated macrophages. Expert Rev. Proteom. 2020, 17, 175–185. [Google Scholar] [CrossRef]
- Sun Y, Zhao Y, Wang X, Zhao L, Li W, Ding Y, et al. Wogonoside prevents colitis-associated colorectal carcinogenesis and colon cancer progression in inflammation-related microenvironment via inhibiting NF-κB activation through PI3K/Akt pathway. Oncotarget [Internet]. 2016 Apr 18 [cited 2023 Jan 17];7(23):34300–15. Available from: https://www.oncotarget.com/article/8815/text/.
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Scheres, N.; Lamont, R.J.; Crielaard, W.; Krom, B.P. LuxS signaling in Porphyromonas gingivalis-host interactions. Anaerobe 2015, 35, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.S.; Valastyan, J.S.; Bassler, B.L. A Host-Produced Autoinducer-2 Mimic Activates Bacterial Quorum Sensing. Cell Host Microbe 2016, 19, 470–480. [Google Scholar] [CrossRef]
- Hiwrale I, Shukla V, Pal S, Dhodapkar R. Chapter 1 Advanced Chemical Extraction and Detection Methods Applied in the Photochemical Treatment Methods for Removal of CECs from Different Water Matrices. 2022 [cited 2023 Jan 17];1–33. Available from: https://pubs.rsc.org/en/content/chapterhtml/2022/bk9781839167355-00001.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).