1. Introduction
Elevated blood concentration of lipoprotein(a) [Lp(a)] is a defined genetic risk factor for atherosclerotic cardiovascular disease (CVD) and its complications, but the mechanisms underlying the high atherogenicity of Lp(a) are poorly understood. It has been shown that Lp(a) through oxidized phospholipids bound to its apolipoprotein(a) contributes to monocyte activation and endothelial dysfunction. In addition, Lp(a) is able to bind pro-inflammatory molecules and shuttle them to the atherosclerotic plaque, thereby maintaining inflammation in the vessel wall [
1].
Monocytes/macrophages are the major immune cell population involved in atherogenesis. In patients with atherosclerosis and other chronic inflammatory diseases, the amount and composition of circulating monocytes is altered. Particularly, the content of CD16+ intermediate monocytes, demonstrating the most pronounced proinflammatory activity, is increased. According to our previous data, the level of circulating intermediate monocytes is associated with the severity of atherosclerosis in younger (<50 years old) but not in older patients with atherosclerotic CVD [
2]. We have also shown an association between Lp(a) levels and elevated amount of circulating CD16+ monocytes (both intermediate and non-classical) in patients with stenotic three-vessel coronary artery disease [
3].
Proprotein convertase subtilisin/kexin type 9 (PCSK9), which is involved in the degradation of low-density lipoprotein (LDL) receptors on hepatocytes, regulates blood levels of LDL cholesterol. Recent studies confirm the association of PCSK9 levels with the occurrence of cardiovascular events [
4], regardless of adequate pharmacological control of LDL cholesterol and correction of traditional risk factors for atherosclerosis [
5]. The use of PCSK9 inhibitors significantly reduces the risk of CVD [
6,
7], with the robust effect in patients with elevated Lp(a), regardless of LDL-C achieved [
6]. These data suggest additional Lp(a)-mediated mechanisms for the proatherogenic (proinflammatory) effects of PCSK9.
Along with hepatocytes, PCSK9 is also synthesized by endothelial cells, smooth muscle cells and macrophages [8-10]. Emerging evidence suggests an immunomodulatory effect of PCSK9 by stimulating the differentiation of lymphocytes with pro-inflammatory properties. An experimental study by Kim et al. [
11] showed higher blood levels of interleukin (IL)-17 and a predominance of T-cell differentiation towards Th17 in atherosclerotic hypolipidemic Ldlr-/-Apobec1-/-(LDb) mice. Additional PCSK9 gene knockout in these mice was associated with significantly lower plasma IL-17 levels, T-helpers (Th) 17 and CD4+ memory T cell levels in the spleen, and RORC mRNA expression compared to LDb mice. Incubation of T cells isolated from carotid plaque samples after endarterectomy or from peripheral blood of healthy donors with oxLDL-treated dendritic cells (for PCSK9 induction) was accompanied by lymphocyte proliferation with preferential differentiation into Th17 and Th1, and production of interferon-γ and IL-17. PCSK9 inhibition prevented the effects of oxLDL on dendritic cells and T-lymphocytes [
12]. According to several studies [
13,
14], PCSK9 binds to CD36 scavenger receptor and regulates its expression by macrophages, enabling this molecule to be considered as a potential damage-associated molecular patterns [
15].
Lp(a) has been shown to form complexes with PCSK9 [
16]. Furthermore, PCSK9 in complex with Lp(a) [
16] is the dominant pool of lipoprotein-associated PCSK9. We hypothesized that Lp(a) may exert its proatherogenic activity via PCSK9. In this study we analyzed the concentration of Lp(a), PCSK9-Lp(a) complexes and the circulating monocyte subsets in males with atherosclerotic CVD and in patients without atherosclerosis.
2. Materials and Methods
The study was approved by the Institutional Ethics Committee and carried out in accordance with the principles of the Declaration of Helsinki. Written consent was obtained from each patient. 257 males with coronary atherosclerosis verified by coronary angiography were screened. The exclusion criteria were as follows: acute coronary syndrome or interventions in the previous 6 months, history of stroke, neoplasms, liver or renal failure, infectious/inflammatory diseases, decompensated diabetes mellitus, current use of immunosuppressive drugs, statin therapy for less than 6 months prior to enrolment in the trial.
68 men without coronary artery disease or stenotic atherosclerosis in the coronary, carotid and lower extremity arteries comprised the control group. Patients in the control group were not on statins. Other therapies (beta-blockers, ACE inhibitors/sartans, aspirin, calcium channel blockers) were comparable between the groups.
Coronary angiography was performed via a transradial approach using a standard technique. The presence of coronary atherosclerosis was assessed with the degree of the stenosis of the main coronary arteries. The severity of coronary atherosclerosis was assessed in the projection with the greatest degree of stenosis by one experienced independent observer. Stenotic atherosclerosis was identified as the narrowing of the artery lumen by more than 50%. All patients underwent ultrasound duplex scanning of carotid and femoral arteries. The degree of stenosis of the carotid and femoral arteries was assessed as the total percentage of stenosis.
Concentrations of total cholesterol (TC), triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C) were measured using the enzymatic colorimetric method on an Architect C-8000 analyser (Abbott, Chicago, IL, USA). Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald formula with modifications [
17]: LDL-Ccorr (mM/L) = TC – HDL-C – TG/2.2 – 0.3 × Lp(a) mass (md/dL)/38.7 where LDL-Ccorr is the level of LDL-C corrected to the level of Lp(a) cholesterol. Lp(a) concentration was measured by enzyme-linked immunosorbent assay (ELISA) using monospecific polyclonal sheep antibodies against human Lp(a) [
18]. The method was validated with two kits, TintElize Lp(a) (Biopool AB, Umea, Sweden) and Immunozym Lp(a) (Progen Biotechnik GmbH, Heidelberg, Germany). The control serum (Technoclone, Vienna, Austria) was approved by the International Federation of Clinical Chemistry and was used to standardize the ELISA. AutoAbs against Lp(a) and LDL were detected by ELISA according to a previously developed method [
19]. The concentration of PSCK9 was measured by ELISA using commercial kits (R&D Systems, USA).The concentration of PSCK9-Lp(a) complexes were detected by ELISA with laboratory monoclonal antibodies against PCSK9 and polyclonal antibodies against Lp(a) [
20].
Monocyte immunophenotyping was performed in the blood samples by direct immunofluorescence using fluorescently labeled antibodies against CD45, CD14, CD16 antigens (Beckman Coulter, Brea, CA, USA) and a lysis solution (BD Immunocytometry Systems) according to the manufacturer’s instructions. Samples were analysed on FACS Calibur and FACS Canto flow cytometers (BD Immunocytometry Systems). Monocytes were gated according to light scatter parameters and CD45 expression pattern. Monocyte subsets were identified as classical (CD14++CD16-), intermediate (CD14++CD16+) and non-classical (CD14+CD16++) according to the routinely used protocol [
21].
Statistics. Statistical analysis was performed using the MedCalc package. The data are presented as a median (25–75th percentile). Kruskal–Wallis ANOVA and Mann–Whitney U tests were used in multiple or paired comparisons, respectively. Chi-square or Fisher’s exact two-tailed test was used in multiple or paired comparisons of binary features, respectively. Spearman’s test was used for correlation analysis. A linear and multivariate linear regression were performed to identify the association between the studied parameters. The differences were considered statistically significant at p < 0.05.
3. Results
The clinical and immunological characteristics of the patients with coronary atherosclerosis and the control group are shown in
Table 1.
50% of patients in coronary atherosclerosis group had three- or multivessel coronary artery disease, 75% of patients had a history of myocardial infarction, 82% of patients underwent coronary stenting and 21% of patients underwent coronary artery bypass surgery.
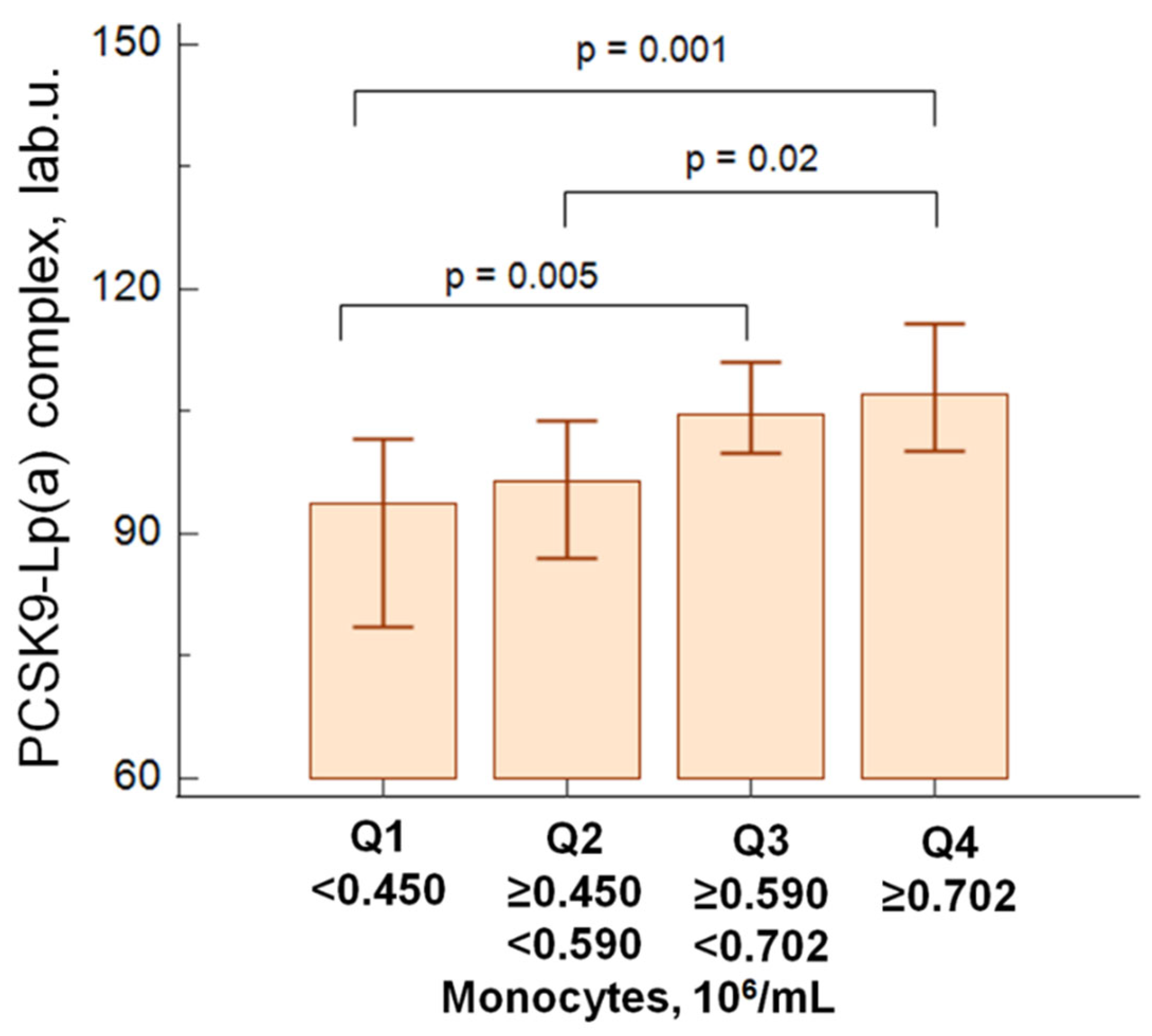
In patients with atherosclerosis, PCSK9 - Lp(a) complex levels were associated to higher quartiles of absolute monocyte values (
Figure 1).
We did not observe correlations between the level of Lp(a) and the concentration of PCSK9-Lp(a) complexes, nor between the level of Lp(a) or PCSK9 and the total number of monocytes in both groups. A slight positive correlation between the concentration of PCSK9-Lp(a) complexes and the absolute level of monocytes was obtained (r=0.20, p=0.002) in patients with atherosclerosis predominantly due to intermediate monocyte subsets (r=0.33, p=0.04). There were no correlations between the level of Lp(a), the concentration of PCSK9-Lp(a) and the classical and non-classical monocyte subsets.
In patients with atherosclerosis, a positive correlation was found between PCSK9-Lp(a) complex levels and blood monocyte counts, predominantly in the hyperLp(a) group (>30 mg/dL) (r=0.24, p=0.01).
According to regression analysis both PCSK9-Lp(a) complex concentration and BMI were related to the absolute number of blood monocytes (
Table 2) in patients with atherosclerosis. When age, hypertension, diabetes mellitus, Lp(a) and LDL-C levels were entered into a multivariate regression model, the above relationships remained independent and statistically significant (
Table 2).
4. Discussion
Elevated lipoprotein(a) concentrations are now recognized as a genetic risk factor for atherosclerotic cardiovascular disease and as a major residual lipid risk factor. Inflammation is another major contributor to atherogenesis, with a confirmed role in the development of cardiovascular complications in patients achieving target LDL cholesterol levels. Despite the obvious role of both dyslipidaemia and inflammation as key determinants of atherogenesis, the diversity and sequencing of inflammatory processes and the contribution of atherogenic lipoproteins to chronic inflammation in atherosclerosis is an extremely important area for investigation.
The results of our previous study showed a redistribution of monocyte subpopulations towards an increased number of non-classical monocytes in hyperLp(a) patients. The combination of elevated Lp(a) and intermediate monocytes was associated with a significant increase in the odds of severe stenotic lesions in all three vascular beds [
3]. According to our observational study, monocyte levels above the median accompanied by Lp(a) concentrations above 30 mg/dL, were associated with a 2.7-fold increased risk of cardiovascular events in patients with early-onset coronary artery disease [
22].
There is evidence that hyperLp(a) is associated with monocyte activation. Monocytes isolated from the blood of patients with hyperLp(a) showed an increased capacity for transendothelial migration [
23]. Transcriptome analysis of CD14+ monocytes from patients with elevated Lp(a) levels before and after treatment with AKCEA-APO(a)-LRx antisense oligonucleotides, inhibiting apo(a) synthesis, showed a significant decrease in monocyte proinflammatory gene expression, leading to a reduction in monocyte transendothelial migration and decreased expression of TLR2 and the chemokine receptors CCR2 and CX3CR1 [
24]. These observations suggest an additive contribution of Lp(a) and immune cells to the progression of atherosclerosis and the development of cardiovascular complications. However, a clear understanding of the mechanism of this phenomenon is lacking.
PCSK9 is a key regulator of apoB-containing lipoprotein metabolism through its ability to induce lysosomal degradation of internalized LDL receptors. According to a 15-year prospective cohort study, PCSK9 blood concentrations were associated with the development of cardiovascular complications, even after adjustment for traditional risk factors, including LDL cholesterol levels [
4].
There is evidence that PCSK9 has pro-inflammatory properties independent of LDL metabolism. In addition to the predominant expression of PCSK9 by hepatocytes, it is also expressed by cells of the vascular wall (including smooth muscle cells (SMC), endothelium, macrophages) [8-10]. PCSK9 suppresses the expression of ABCA1 transporter by macrophages, thereby inhibiting reverse cholesterol transport [
25]. PCSK9 increases macrophage expression of scavenger receptors, particularly CD36 and LOX-1 [
26], which may contribute to foam cell differentiation and growth of atherosclerotic lesions. Cultivation of macrophages derived from donor blood monocytes and THP-1 monocyte line cells in the presence of recombinant human PCSK9 was associated with induction of pro-inflammatory cytokine IL-1beta, IL-6, tumor necrosis factor, CXCL2 and monocyte chemotactic protein (MCP)-1 mRNA synthesis. Clinical data from the ATHEROREMO IVUS study [
27] on the association of PCSK9 levels with the extent of necrotic core in atherosclerotic plaques indirectly support the in vitro data.
The relationship between PCSK9 and the phenotype and functional activity of peripheral blood monocytes in patients with familial hypercholesterolaemia and healthy individuals has been previously studied. Monocyte expression of the MCP-1 receptor CCR2 was 3-fold higher in these patients. Monocytes from patients showed increased ex vivo migration capacity. Anti-PCSK9 monoclonal antibody therapy was associated with suppression of inflammation by reducing CCR2-mediated monocyte migration [
28]. SMC-secreted PCSK9 has been shown to reduce LDL receptor exposure on monocytes and increase LDL-mediated CCR2 expression by monocytes [
29]. Krychtiuk K.A. [
30] demonstrated a positive correlation between the percentage of classical monocytes and a negative correlation between the relative content of non-classical monocytes and PCSK9 blood levels in patients receiving statins. However, patients with PCSK9 levels above the median showed higher relative values of classical monocytes and lower values of non-classical monocytes.
Previously, Tavori et al [
16] demonstrated that the major pool of lipoprotein-associated PCSK9 resides in a complex with Lp(a). Accumulating data on the immunomodulatory effects of PCSK9 provided the rationale to investigate the relationship between Lp(a)-associated PCSK9 levels [PCSK9-Lp(a) complex] and the number and composition of circulating monocytes. In this study, we analyzed the levels of Lp(a), PCSK9 and circulating PCSK9-Lp(a) complexes as well as the frequency of circulating monocytes in patients with stenotic coronary atherosclerosis. Age-matched patients without stenotic atherosclerosis of the coronary and peripheral arteries were included as a control group. In order to exclude the possible influence of gender differences on the indices analyzed, only male patients were included.
The proportion of patients with hyperLp(a) was two times higher in the group with stenotic coronary atherosclerosis than in the control group (45.5% vs. 20.6%), but no differences in monocyte levels were observed. No apparent correlation was found between total monocyte content and Lp(a) concentration, which is consistent with previous studies [
3]. A positive correlation between blood Lp(a) and PCSK9 concentrations was observed in both atherosclerotic and control patients, confirming our previous findings in patients with hypercholesterolaemia [
31]. However, no correlation was detected between Lp(a) and circulating PCSK9-Lp(a) complex.
5. Study Limitation
Our study has some limitations. The retrospective study design does not allow assessing the causal relationship between the studied parameters and the development of atherosclerosis. Unlike patients in the main group, patients in the control group did not take statins. Due to the possible effect of statins on the studied parameters, statistical analysis and relationship assessment were performed separately within each group. It was not possible to include a group of patients without AC of coronary and peripheral arteries taking statins.
6. Conclusions
Here we demonstrate that the level of the PCSK9-Lp(a) complex, but not Lp(a), directly correlates with the level of circulating monocytes. The described correlation was only observed in the group of patients with atherosclerosis, but not in the control group. The level of PCSK9-Lp(a) complexes was independently associated with the absolute number of peripheral blood monocytes in patients with atherosclerosis according to single-factorial and multivariate linear regression including other factors such as age, body mass index, hypertension, diabetes, Lp(a) and LDL-C levels. The independent direct relationship between the level of circulating PCSK9-Lp(a) complex and absolute content of blood monocytes suggests additional molecular and cellular mechanisms of high atherogenicity of Lp(a) as a transporter of immunologically active molecules, in particular PCSK9. Further studies are required to determine the pathogenetic contribution of PCSK9-Lp(a) complexes to the development of atherosclerosis.
Author Contributions
Conceptualization and design of the study A.Y.F., O.I.A, T.I.A. and A.V.P.; methodology O.I.A., A.Y.F., T.I.A., E.A.K., O.A.R.; validation O.I.A., A.Y.F., T.I.A., M.V.E. and S.N.P.; investigation A.Y.F., A.V.T., E.A.K., O.A.R. and T.I.A.; resources T.I.A. and O.I.A.; writing— original draft preparation, A.Y.F.; writing—review and editing, T.I.A., O.I.A. and A.V.P.; supervision M.V.E. and S.N.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by RSF, grant number 22-25-00051.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of National Medical Research Center of Cardiology named after ac. E.I. Chazov of Ministry of Healthcare of Russian Federation (protocol No. 251 from 25 November 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to acknowledge the cooperation of the Department of Endovascular Diagnostics and Treatment and the Ultrasound Department of NMRCC named after ac. E.I. Chazov for the clinical examination of the study patients.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Afanasieva, O.I. Arefieva, T.I., Ezhov, M.V., Pokrovsky, S.N. Lipoprotein(a) and immunity. In Lipoprotein(a); Kostner, K., Kostner, G.M., Toth, P.P., Eds.; Publisher: Humana press, Springer Nature; 2023; pp. 2761–2274. [Google Scholar]
- Filatova, A.Y. , Potekhina, A.V., Radyukhina, N.V., Ruleva, N.Yu., Provatorov, S.I., Arefieva, T.I. Circulating monocyte populations in patients with coronary atherosclerosis. Future Cardiology 2022, 18, 455–460. [Google Scholar] [CrossRef]
- Afanasieva, O.I.; Filatova AYu Arefieva, T.I.; Klesareva, E.A.; Tyurina, A.V.; Radyukhina, N.V.; Ezhov, M.V.; Pokrovsky, S.N. The association of lipoprotein(a)and circulating monocyte subsets with severe coronary atherosclerosis. J Cardiovasc Dev Dis 2021, 8, 63. [Google Scholar] [CrossRef]
- Leander, K. , Mälarstig, A., Van’t Hooft, F.M., Hyde, C., Hellénius, M.L., Troutt, J.S., Konrad, R.J., Öhrvik, J., Hamsten, A., de Faire, U. Circulating proprotein convertase subtilisin/kexin type 9 (PCSK9) predicts future risk of cardiovascular events independently of established risk factors. Circulation 2016, 133, 1230–1239. [Google Scholar] [PubMed]
- Werner, C. , Hoffmann, M.M., Winkler, K., Böhm, M., Laufs, U. Risk prediction with proprotein convertase subtilisin/kexin type 9 (PCSK9) in patients with stable coronary disease on statin treatment. Vascul Pharmacol 2014, 62, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S. , Giugliano, R.P., Keech, A.C., Honarpour, N., Wiviott, S.D., Murphy, S.A., Kuder, J.F., Wang, H., Liu, T., Wasserman, S.M., Sever, P.S., Pedersen, T.R., FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017, 376, 1713–1722. [Google Scholar] [PubMed]
- Robinson, J.G. , Farnier, M., Krempf, M., Bergeron, J., Luc, G., Averna, M., Stroes, E.S., Langslet, G., Raal, F.J., El Shahawy, M., Koren, M.J., Lepor, N.E., Lorenzato, C., Pordy, R., Chaudhari, U., Kastelein, J.J., ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015, 372, 1489–1499. [Google Scholar] [PubMed]
- Ferri, N. , Tibolla, G., Pirillo, A., Cipollone, F., Mezzetti, A., Pacia, S., Corsini, A., Catapano, A.L. Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Secreted by Cultured Smooth Muscle Cells Reduces Macrophages LDLR Levels. Atherosclerosis 2012, 220, 381–386. [Google Scholar] [CrossRef]
- Ding, Z. , Liu, S., Wang, X., Deng, X., Fan, Y., Shahanawaz, J., Shmookler Reis, R.J., Varughese, K.I., Sawamura, T., Mehta, J.L. Cross-Talk between LOX-1 and PCSK9 in Vascular Tissues. Cardiovasc Res 2015, 107, 556–567. [Google Scholar] [CrossRef]
- Ding, Z. , Liu, S., Wang, X., Mathur, P., Dai, Y., Theus, S., Deng, X., Fan, Y., Mehta, J.L. Cross-Talk Between PCSK9 and Damaged MtDNA in Vascular Smooth Muscle Cells: Role in Apoptosis. Antioxid Redox Signal 2016, 25, 997–1008. [Google Scholar]
- Kim, Y.U. , Kee, P., Danila, D., Teng, B.B. A critical role of PCSK9 in mediating IL-17-producing T cell responses in hyperlipidemia. Immune Netw 2019, 19, e41. [Google Scholar] [CrossRef]
- Liu, A.; Frostegard, J. PCSK9 plays a novel immunological role in oxidized LDL-induced dendritic cell maturation and activation of T cells from human blood and atherosclerotic plaque. J Intern Med 2018, 284, 193–210. [Google Scholar] [CrossRef]
- Ding, Z. , Liu, S., Wang, X., Theus, S., Deng, X., Fan, Y., Zhou, S., Mehta, J.L. PCSK9 regulates expression of scavenger receptors and ox-LDL uptake in macrophages. Cardiovasc Res 2018, 114, 1145–53. [Google Scholar] [CrossRef]
- Qi, Z. , Hu, L., Zhang, J., Yang, W., Liu, X., Jia, D., Yao, Z., Chang, L., Pan, G., Zhong, H., Luo, X., Yao, K., Sun, A., Qian, J., Ding, Z., Ge, J. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Enhances Platelet Activation, Thrombosis, and Myocardial Infarct Expansion by Binding to Platelet CD36. Circulation 2021, 143, 45–61. [Google Scholar]
- Silverstein, R.L. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Goes "DAMP". Circulation 2021, 143, 62–64. [Google Scholar] [CrossRef]
- Tavori, H. , Christian, D., Minnier, J., Plubell, D., Shapiro, M.D., Yeang, C., Giunzioni, I., Croyal, M., Duell, P.B., Lambert, G., Tsimikas, S., Fazio, S. PCSK9 Association with lipoprotein(a). Circ Res 2016, 119, 29–35. [Google Scholar] [CrossRef]
- Dahlen, G.H. Incidence of Lp(a) among populations. In Lipoprotein(a); Scanu, A.M., Ed.; ISBN 0-12-620990-1; Academic Press: New York, NY, USA, 1990; pp. 151–173. ISBN 0-12-620990-1. [Google Scholar]
- Afanas’eva, O.I.; Adamova, I.Y. .; Benevolenskaya, G.F.; Pokrovskii, S.N. Enzyme immunoassay of lipoprotein(a). Bull Exp Biol Med 1995, 120, 1030–1033. [Google Scholar]
- Tmoyan, N.A. ,Afanasieva, O.I., Ezhov, M.V., Klesareva, E.A., Balakhonova, T.V., Pokrovsky, S.N. Lipoprotein(a), Immunity, and Inflammation in Polyvascular Atherosclerotic Disease. J Cardiovasc Dev Dis 2021, 8, 11. [Google Scholar]
- Razova, O.A. , Afanasieva, O.I., Egiazaryan, M., Sherstyuk, E., Klesareva, E.A., Pokrovsky, S.N. Circulating complex of lipoprotein(a) and proprotein convertase subtilisin kexin type 9 in the serum measured by ELISA. Bull Exp Biol Med 2020, 169, 639–643. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L. Blood monocytes and their subsets: established features and open questions. Front Immunol 2015, 6, 423. [Google Scholar]
- Afanasieva, O.I.; Tyurina, A.V.; Klesareva, E.A.; Arefieva, T.I.; Ezhov, M.V.; Pokrovsky, S.N. Lipoprotein(a), immune cells and cardiovascular outcomes in patients with premature coronary heart disease. J Pers Med 2022, 12, 269.
- Van der Valk, F.M. , Bekkering, S., Kroon, J., Yeang, C., Van den Bossche, J., Van Buul, J.D., Ravandi, A., Nederveen, A.J., Verberne, H.J., Scipione, C., Nieuwdorp, M., Joosten, L.A., Netea, M.G., Koschinsky, M.L., Witztum, J.L., Tsimikas, S., Riksen, N.P., Stroes, E.S. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation 2016, 134, 611–624. [Google Scholar] [PubMed]
- Stiekema, L.C. , Prange, K.H., Hoogeveen, R.M., Verweij, S.L., Kroon, J., Schnitzler, J.G., Dzobo, K.E., Cupido, A.J., Tsimikas, S., Stroes, E.S., de Winther, M.P., Bahjat, M. Potent lipoprotein(a) lowering following apolipoprotein(a) antisense treatment reduces the pro-inflammatory activation of circulating monocytes in patients with elevated lipoprotein(a). Eur Heart J 2020, 41, 2262–2271. [Google Scholar] [PubMed]
- Adorni, M.P. , Cipollari, E., Favari, E., Zanotti, I., Zimetti, F., Corsini, A., Ricci, C., Bernini, F., Ferri, N. Inhibitory effect of PCSK9 on Abca1 protein expression and cholesterol efflux in macrophages. Atherosclerosis 2017, 256, 1–6. [Google Scholar] [CrossRef]
- Ding, Z. , Wang, X., Liu, S., Shahanawaz, J., Theus, S., Fan, Y., Deng, X., Zhou, S., Mehta, J.L. PCSK9 expression in the ischaemic heart and its relationship to infarct size, cardiac function, and development of autophagy. Cardiovasc Res 2018, 114, 1738–1751. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.M. , Oemrawsingh, R.M., Garcia-Garcia, H.M., Boersma, E., van Geuns, R.J., Serruys, P.W., Kardys, I., Akkerhuis, K.M. PCSK9 in relation to coronary plaque inflammation: results of the ATHEROREMO-IVUS study. Atherosclerosis 2016, 248, 117–22. [Google Scholar]
- Bernelot Moens, S.J. , Neele, A.E., Kroon, J., van der Valk, F.M., van den Bossche, J., Hoeksema, M.A., Hoogeveen, R.M., Schnitzler, J.G., Baccara-Dinet, M.T., Manvelian, G., de Winther, M., Stroes, E. PCSK9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia. Eur Heart J 2017, 38, 1584–1593. [Google Scholar]
- Grune, J. , Meyborg, H., Bezhaeva, T., Kappert, K., Hillmeister, P., Kintscher, U., Pieske, B., Stawowy, P. PCSK9 regulates the chemokine receptor CCR2 on monocytes. Biochem Biophys Res Commun 2017, 485, 312–318. [Google Scholar] [CrossRef]
- Krychtiuk, K.A. , Lenz, M., Hohensinner, P., Distelmaier, K., Schrutka, L., Kastl, S.P., Huber, K., Dostal, E., Oravec, S., Hengstenberg, C., Wojta, J., Speidl, W.S. Circulating levels of proprotein convertase subtilisin/kexin type 9 (PCSK9) are associated with monocyte subsets in patients with stable coronary artery disease. J Clin Lipidol 2021, 15, 512–521. [Google Scholar]
- Afanasieva, O.I. , Ezhov, M.V., Razova, O.A., Afanasieva, M.I., Utkina, E.A., Pokrovsky, S.N. Apolipoprotein(a) phenotype determined the correlations of lipoprotein(a) and proprotein convertase subtilisin/kexin type 9 levels in patients with potential familial hypercholesterolemia. Atherosclerosis 2018, 277, 477–482. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).