Submitted:
01 June 2023
Posted:
02 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
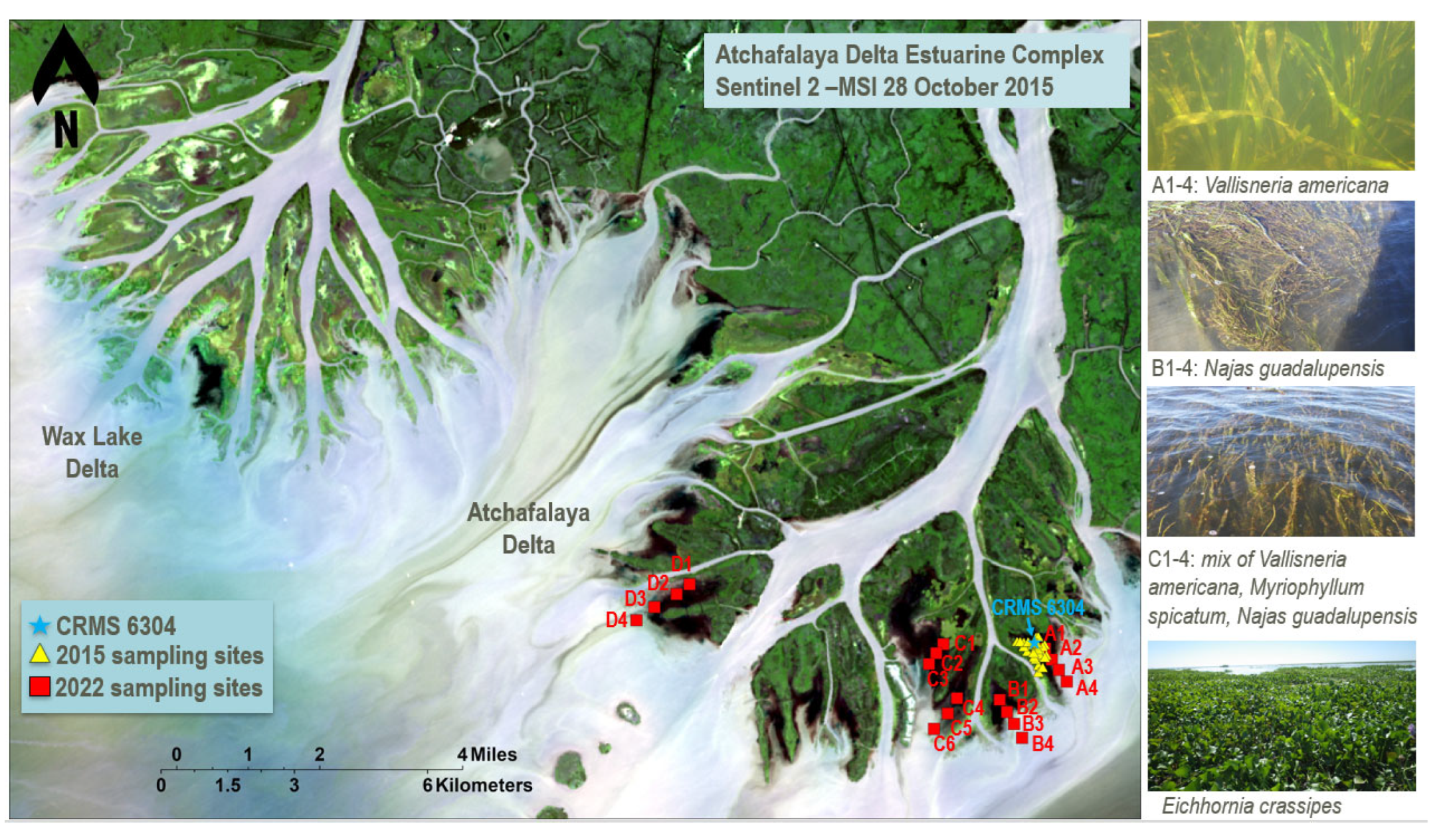
2.1. Study Area
2.2. Field Observations
2.2.1. Historical Data
2.2.2. Field Data Collection for U-Net Model Validation
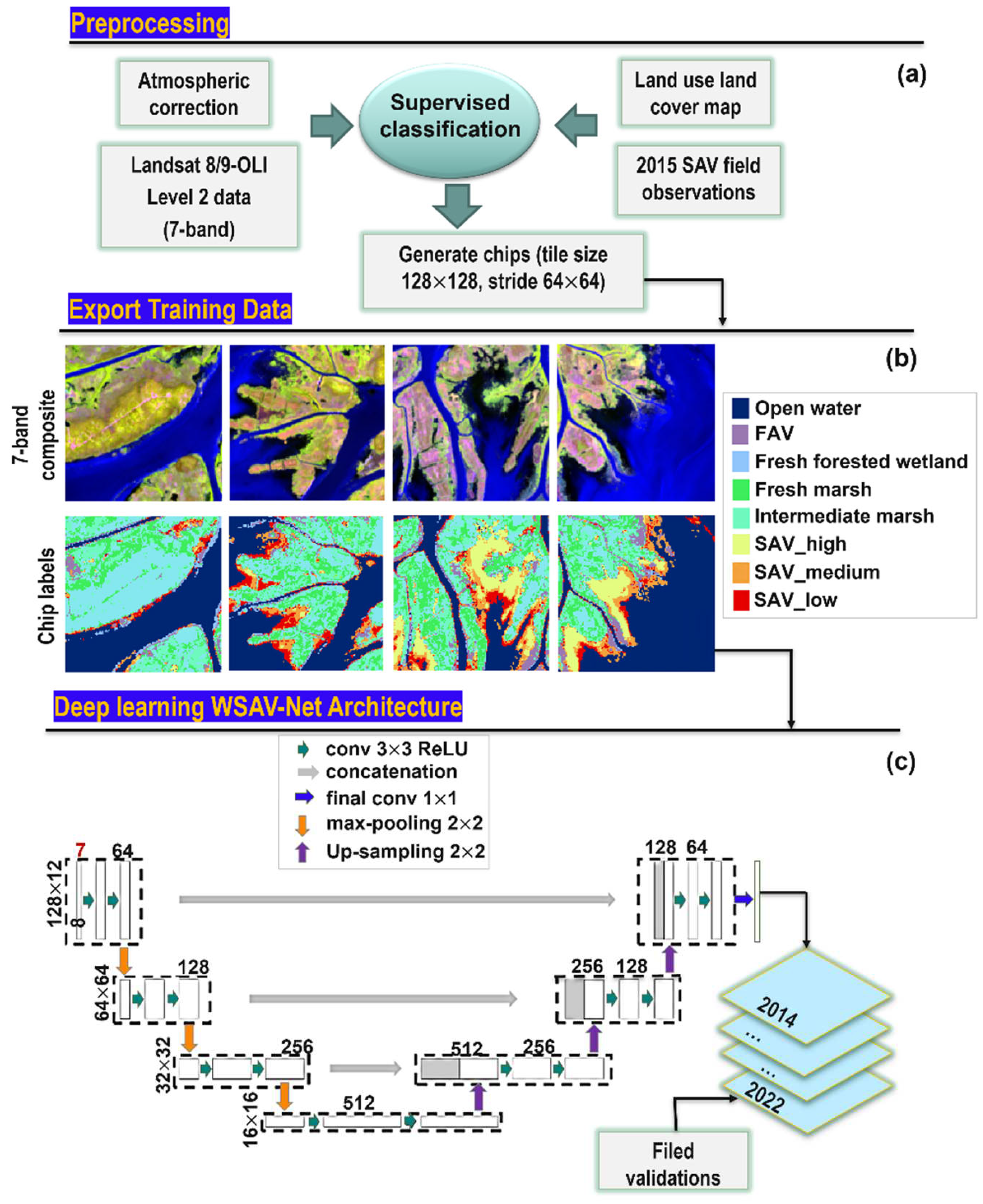
2.3. Deep Learning for Habitat Classification
2.3.1. Remote Sensing Data and Processing
2.3.2. Louisiana Wetland-SAV Network Model (WSAV-Net)
2.3.3. Habitat Percent Cover
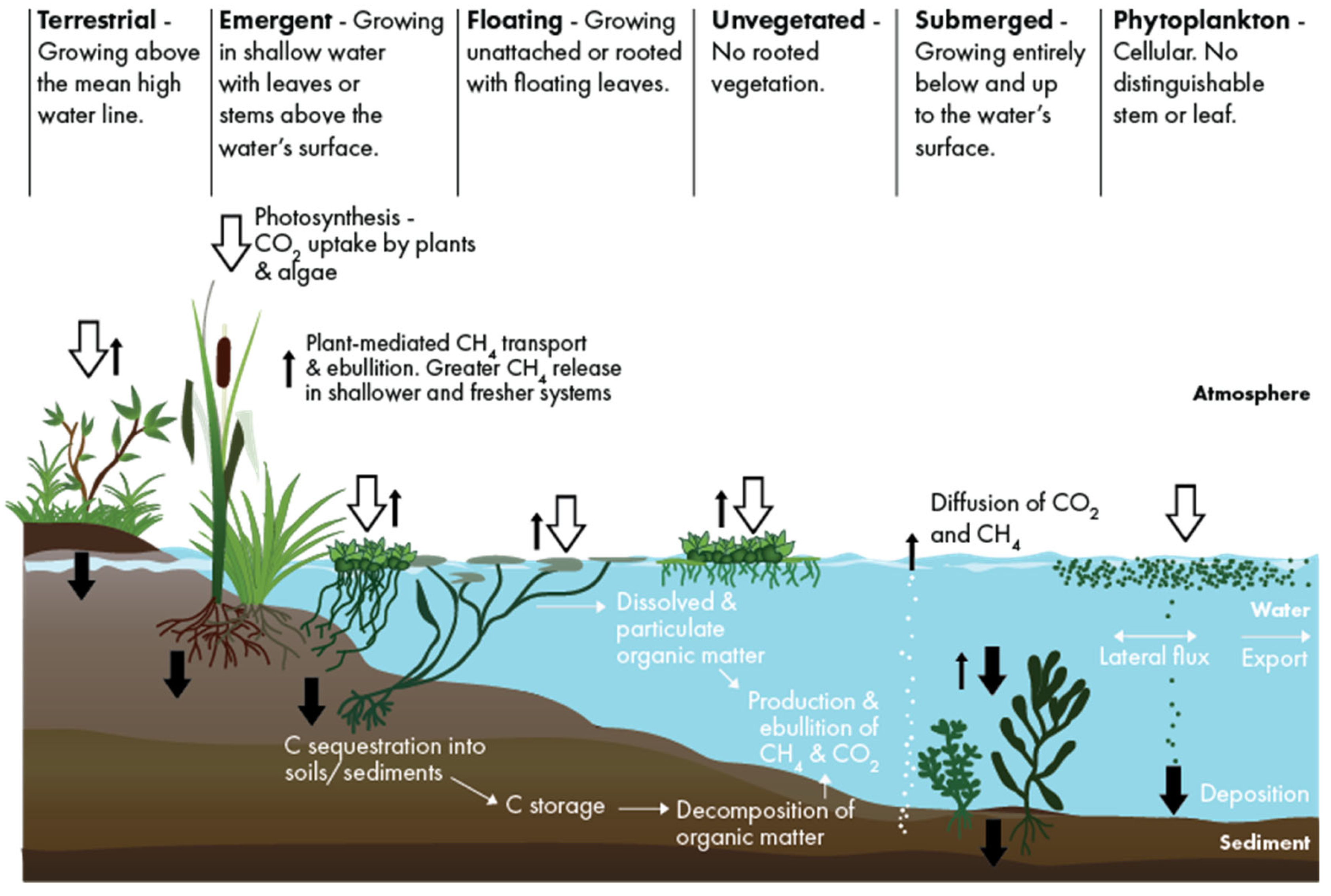
2.4. Carbon Balance Model
- ANPP = Aboveground net primary productivity which represents the live AG biomass produced within one year (tonne CO2e ha-1 yr-1).
- Sed./Soilaccum. = Net carbon accumulation in sediment for SAV and open water or in soils for wetlands (tonne CO2e ha-1 yr-1). Incorporates the live or net primary productivity of belowground biomass, the accumulation of dead belowground biomass of roots and rhizomes, aboveground litter, and allochthonous carbon [76].
- GHG = GHG emissions (tonne CO2e ha-1 yr-1) including CH4 and N2O. CO2 is excluded because ANPP and Sed./Soilaccum. represents net value of CO2 balance.
3. Results
3.1 Field Observations of SAV Percent Cover
3.2. Carbon Flux Look-Up Table
| Area of interest (Location) | Salinity (ppt) | Carbon fluxes (tonne CO2e ha-1 yr-1) | |||
|---|---|---|---|---|---|
| ANPP | Sed.accum. | GHG fluxes | Citation | ||
| Wuliangsu Lake, China | Fresh | N/A | N/A | 4.73 | [89] |
| Luanhaizi wetland, China | Fresh | N/A | N/A | 2.84 | [90] |
| Lake Taihu, China | Fresh | N/A | N/A | 2.04 | [91] |
| Everglades Stormwater Treatment Areas, FL | 0–10 | N/A | -17.8 | N/A | [92] |
| Indoor mesocosm of Myriophyllum spicatum | Fresh | N/A | -5.60 | N/A | [93] |
| Mississippi River Delta Plain, LA | 0–0.2 | -2.34 | N/A | N/A | [18] |
| Mississippi River Delta Plain, LA | 0.2–7.2 | -2.50 | N/A | N/A | [18] |
| Fresh and intermediate marsh SAV, LA | 0.5–5 | -2.50 | N/A | N/A | [94] |
| Gulf coast sites | 0–0.5 | -1.32 | N/A | N/A | [17] |
| Atchafalaya delta, LA | 0.5–5 | -0.27 | N/A | N/A | [95] |
| Birds foot delta, LA | 0.5–5 | -1.70 | N/A | N/A | [96] |
| Chenier Plain, LA | 0.5–6.5 | -0.34 | N/A | N/A | [97] |
| Barataria bay, LA | 0.5–5 | -0.47 | N/A | N/A | [98] |
| Rockefeller State Wildlife Refuge, LA |
0–6 | -0.78 | N/A | N/A | [99] |
| Overall mean ± SE | - | -1.40 ± 0.31 | -11.7 ± 6.1 | 3.20 ± 0.79 | - |
3.3. Remote Sensing of Habitats
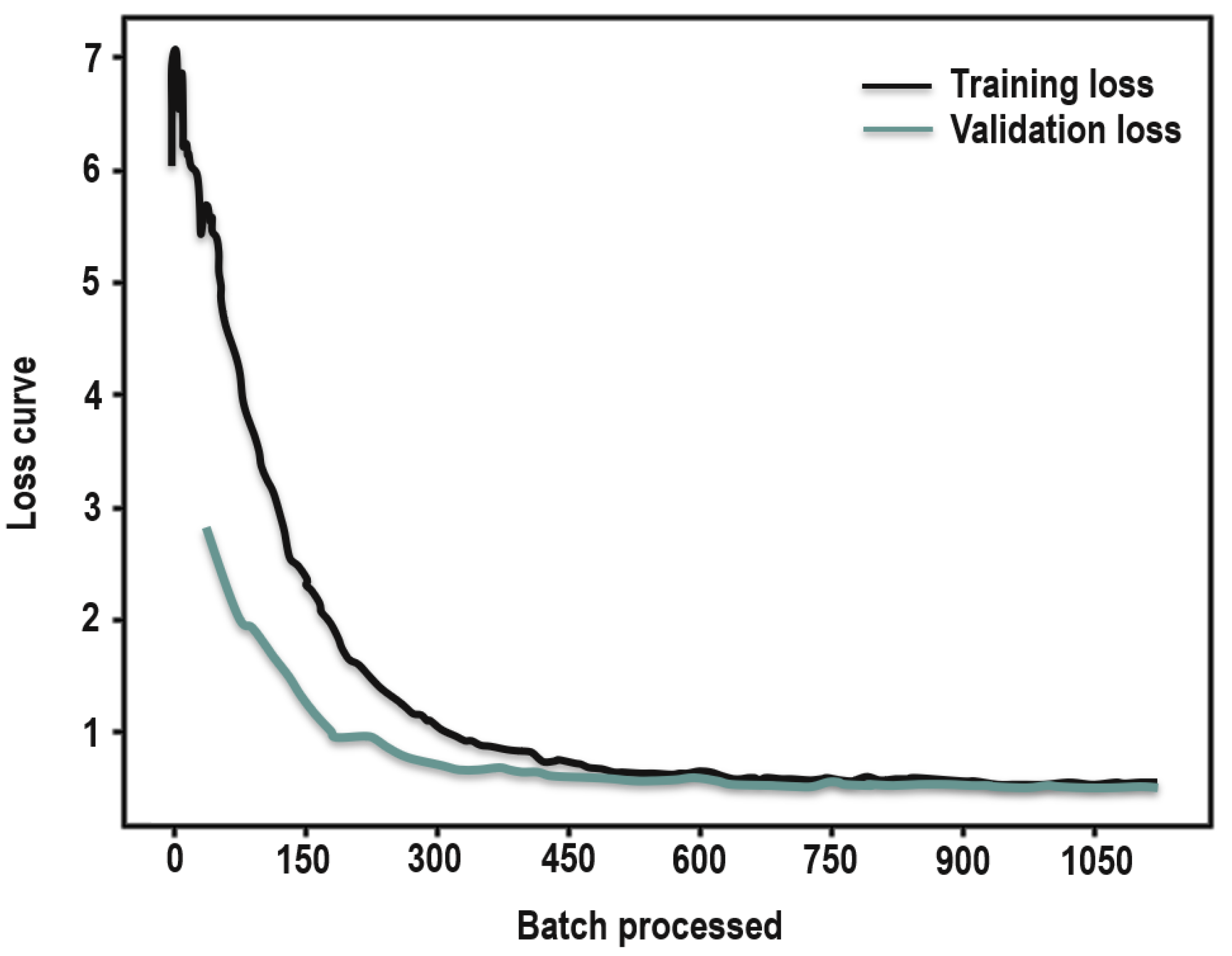
3.3.1. Deep Learning Model Performance
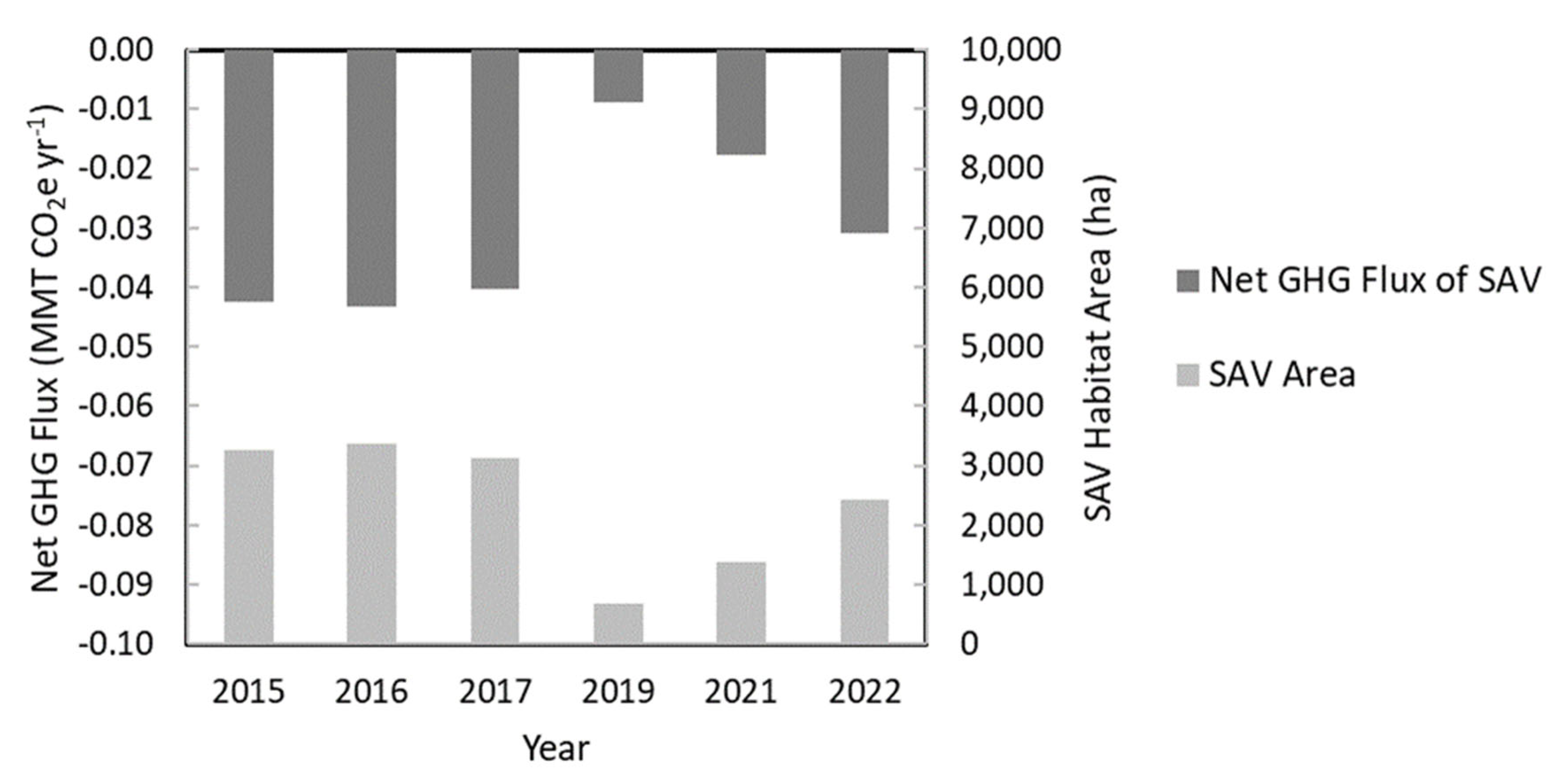
3.3.2. Habitat Changes From 2015 to 2022
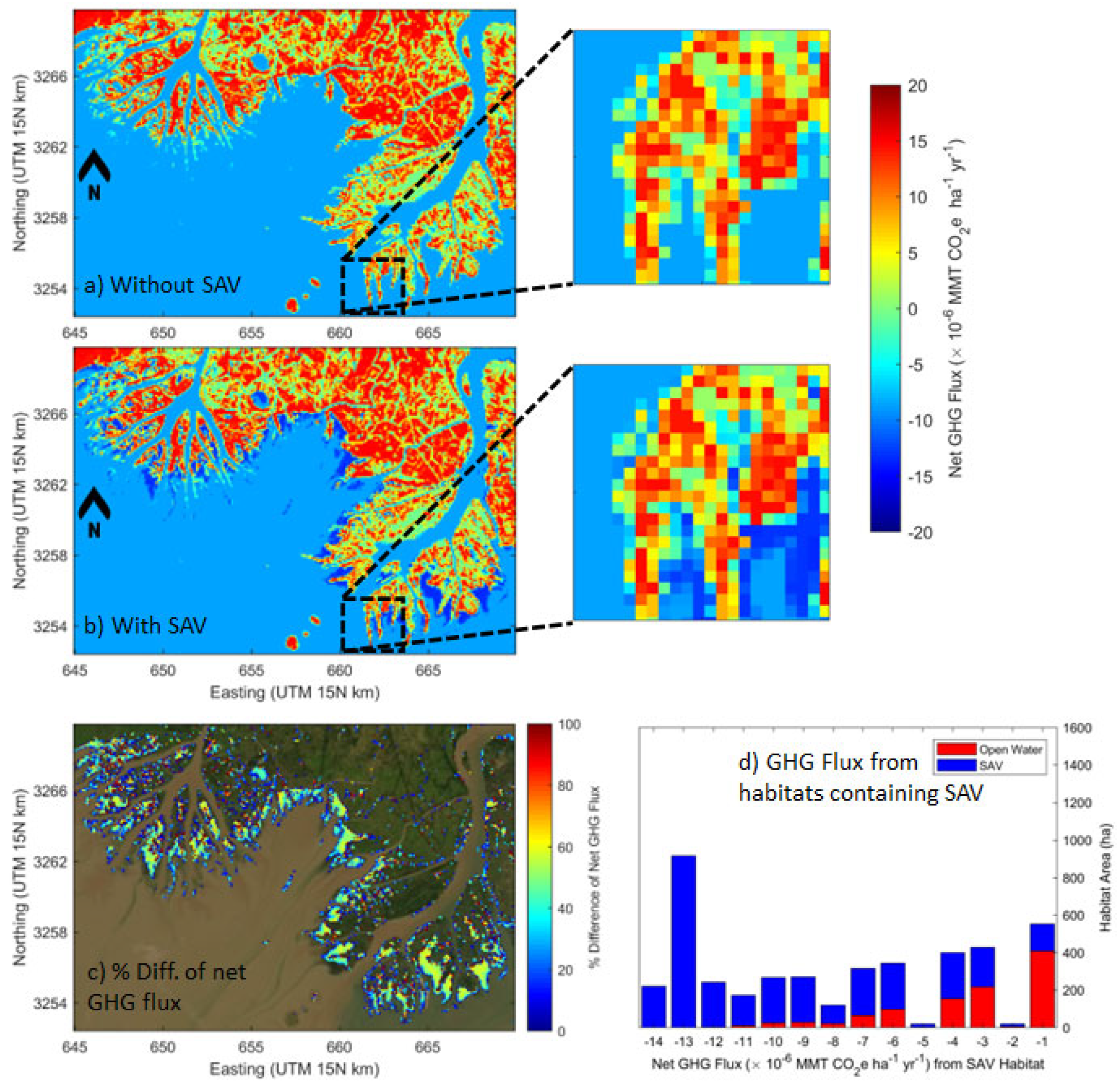
3.4. The Effect of SAV on Net GHG Flux
3.4.1. Net GHG Fluxes
3.4.2. Spatial Distribution of Net GHG Fluxes
3.4.3. Dynamics of Net GHG Fluxes pre- and Post-Hurricanes
4. Discussion
4.1. SAV Habitat Dynamics and Carbon Fluxes
4.2. SAV Contributions to Net GHG Flux
4.3. Future Methane Quantification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castellanos, D.L.; Rozas, L.P. Nekton Use of Submerged Aquatic Vegetation, Marsh, and Shallow Unvegetated Bottom in the Atchafalaya River Delta, a Louisiana Tidal Freshwater Ecosystem. Estuaries 2001, 24, 184. [Google Scholar] [CrossRef]
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A Global Crisis for Seagrass Ecosystems. BioScience 2006, 56, 987. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating Loss of Seagrasses across the Globe Threatens Coastal Ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The Value of Estuarine and Coastal Ecosystem Services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Costanza, R.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Raskin, R.G.; Sutton, P. The Value of the World’s Ecosystem Services and Natural Capital. Nature 1997, 387, 253–260. [Google Scholar]
- Heck, K.; Hays, G.; Orth, R. Critical Evaluation of the Nursery Role Hypothesis for Seagrass Meadows. Mar. Ecol. Prog. Ser. 2003, 253, 123–136. [Google Scholar] [CrossRef]
- Carr, J.; D’Odorico, P.; McGlathery, K.; Wiberg, P. Stability and Bistability of Seagrass Ecosystems in Shallow Coastal Lagoons: Role of Feedbacks with Sediment Resuspension and Light Attenuation. J. Geophys. Res. 2010, 115, G03011. [Google Scholar] [CrossRef]
- Gurbisz, C.; Kemp, W.M.; Sanford, L.P.; Orth, R.J. Mechanisms of Storm-Related Loss and Resilience in a Large Submersed Plant Bed. Estuar Coasts 2016, 39, 951–966. [Google Scholar] [CrossRef]
- McGlathery, K.; Sundbäck, K.; Anderson, I. Eutrophication in Shallow Coastal Bays and Lagoons: The Role of Plants in the Coastal Filter. Mar. Ecol. Prog. Ser. 2007, 348, 1–18. [Google Scholar] [CrossRef]
- McLeod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A Blueprint for Blue Carbon: Toward an Improved Understanding of the Role of Vegetated Coastal Habitats in Sequestering CO2. Front Ecol Environ 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo, M.A.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J.; et al. Seagrass Ecosystems as a Globally Significant Carbon Stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Tokoro, T.; Hosokawa, S.; Miyoshi, E.; Tada, K.; Watanabe, K.; Montani, S.; Kayanne, H.; Kuwae, T. Net Uptake of Atmospheric CO 2 by Coastal Submerged Aquatic Vegetation. Global. Chang. Biol. 2014, 20, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Chmura, G.L.; Anisfeld, S.C.; Cahoon, D.R.; Lynch, J.C. Global Carbon Sequestration in Tidal, Saline Wetland Soils. Global Biogeochem Cycles 2003, 17, 1–11. [Google Scholar]
- Hopkinson, C.S.; Cai, W.-J.; Hu, X. Carbon Sequestration in Wetland Dominated Coastal Systems—A Global Sink of Rapidly Diminishing Magnitude. Curr. Opin. Environ. Sustain. 2012, 4, 186–194. [Google Scholar] [CrossRef]
- Mitra, S.; Wassmann, R.; Vlek, P.L.G. An Appraisal of Global Wetland Area and Its Organic Carbon Stock. Curr. Sci. 2005, 88, 25–35. [Google Scholar]
- Watanabe, K.; Kuwae, T. How Organic Carbon Derived from Multiple Sources Contributes to Carbon Sequestration Processes in a Shallow Coastal System? Global Chang. Biol 2015, 21, 2612–2623. [Google Scholar] [CrossRef]
- Hillmann, E.R.; DeMarco, K.E.; Peyre, M.L. Establishing a Baseline of Estuarine Submerged Aquatic Vegetation Resources Across Salinity Zones Within Coastal Areas of the Northern Gulf of Mexico. J. Southeast. Assoc. Fish Wildl. Agencies 2016, 8, 25–32. [Google Scholar]
- Hillmann, E.R.; Rivera-Monroy, V.H.; Nyman, J.A.; La Peyre, M.K. Estuarine Submerged Aquatic Vegetation Habitat Provides Organic Carbon Storage across a Shifting Landscape. Sci Total Environ 2020, 717, 137217. [Google Scholar] [CrossRef]
- DeMarco, K.; Couvillion, B.; Brown, S.; La Peyre, M. Submerged Aquatic Vegetation Mapping in Coastal Louisiana through Development of a Spatial Likelihood Occurrence (SLOO) Model. Aquatic Botany 2018, 151, 87–97. [Google Scholar] [CrossRef]
- Massicotte, P.; Bertolo, A.; Brodeur, P.; Hudon, C.; Mingelbier, M.; Magnan, P. Influence of the Aquatic Vegetation Landscape on Larval Fish Abundance. J. Great Lakes Res. 2015, 41, 873–880. [Google Scholar] [CrossRef]
- Zhang, Y.; Jeppesen, E.; Liu, X.; Qin, B.; Shi, K.; Zhou, Y.; Thomaz, S.M.; Deng, J. Global Loss of Aquatic Vegetation in Lakes. Earth-Sci. Rev. 2017, 173, 259–265. [Google Scholar] [CrossRef]
- Day, J.W.; Britsch, L.D.; Hawes, S.R.; Shaffer, G.P.; Reed, D.J.; Cahoon, D. Pattern and Process of Land Loss in the Mississippi Delta: A Spatial and Temporal Analysis of Wetland Habitat Change. Estuaries 2000, 23, 425. [Google Scholar] [CrossRef]
- Yuill, B.; Lavoie, D.; Reed, D.J. Understanding Subsidence Processes in Coastal Louisiana. J Coast Res 2009, 54, 23–36. [Google Scholar] [CrossRef]
- Couvillion, B.R.; Beck, H. Marsh Collapse Thresholds for Coastal Louisiana Estimated Using Elevation and Vegetation Index Data. J Coast Res 2013, 63, 58–67. [Google Scholar] [CrossRef]
- Hamberg, J.; Findlay, S.E.G.; Limburg, K.E.; Diemont, S.A.W. Post-storm Sediment Burial and Herbivory of Vallisneria Americana in the Hudson River Estuary: Mechanisms of Loss and Implications for Restoration. Restor Ecol 2017, 25, 629–639. [Google Scholar] [CrossRef]
- Kennish, M.J. Coastal Salt Marsh Systems in the U.S.: A Review of Anthropogenic Impacts. J Coast Res 2001, 17, 731–748. [Google Scholar]
- Kuwae, T.; Watanabe, A.; Yoshihara, S.; Suehiro, F.; Sugimura, Y. Implementation of Blue Carbon Offset Crediting for Seagrass Meadows, Macroalgal Beds, and Macroalgae Farming in Japan. Mar Pol 2022, 138, 104996. [Google Scholar] [CrossRef]
- Carruthers, T.J.B.; Kiskaddon, E.P.; Baustian, M.M.; Darnell, K.M.; Moss, L.C.; Perry, C.L.; Stagg, C. Tradeoffs in Habitat Value to Maximize Natural Resource Benefits from Coastal Restoration in a Rapidly Eroding Wetland: Is Monitoring Land Area Sufficient? Restor. Ecol. 2021, e13564. [Google Scholar]
- Asner, G.P. Automated Mapping of Tropical Deforestation and Forest Degradation: CLASlite. J. Appl. Remote Sens 2009, 3, 033543. [Google Scholar] [CrossRef]
- Johansen, K.; Duan, Q.; Tu, Y.-H.; Searle, C.; Wu, D.; Phinn, S.; Robson, A.; McCabe, M.F. Mapping the Condition of Macadamia Tree Crops Using Multi-Spectral UAV and WorldView-3 Imagery. ISPRS J. Photogramm. 2020, 165, 28–40. [Google Scholar] [CrossRef]
- Phinn, S.; Roelfsema, C.; Dekker, A.; Brando, V.; Anstee, J. Mapping Seagrass Species, Cover and Biomass in Shallow Waters: An Assessment of Satellite Multi-Spectral and Airborne Hyper-Spectral Imaging Systems in Moreton Bay (Australia). Remote Sens. Environ. 2008, 112, 3413–3425. [Google Scholar] [CrossRef]
- Sanders, J.T.; Jones, E.A.L.; Minter, A.; Austin, R.; Roberson, G.T.; Richardson, R.J.; Everman, W.J. Remote Sensing for Italian Ryegrass [Lolium Perenne L. Ssp. Multiflorum (Lam.) Husnot] Detection in Winter Wheat (Triticum Aestivum L.). Front. Agron. 2021, 3, 687112. [Google Scholar] [CrossRef]
- Tiner, R.W.; Lang, M.W.; Klemas, V.V. Remote Sensing of Wetlands: Applications and Advances; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-1-4822-3738-2. [Google Scholar]
- Lyons, M.; Phinn, S.; Roelfsema, C. Integrating Quickbird Multi-Spectral Satellite and Field Data: Mapping Bathymetry, Seagrass Cover, Seagrass Species and Change in Moreton Bay, Australia in 2004 and 2007. Remote Sens. 2011, 3, 42–64. [Google Scholar] [CrossRef]
- Hedley, J.; Roelfsema, C.; Chollett, I.; Harborne, A.; Heron, S.; Weeks, S.; Skirving, W.; Strong, A.; Eakin, C.; Christensen, T.; et al. Remote Sensing of Coral Reefs for Monitoring and Management: A Review. Remote Sens. 2016, 8, 118. [Google Scholar]
- McKenzie, L.J.; Langlois, L.A.; Roelfsema, C.M. Improving Approaches to Mapping Seagrass within the Great Barrier Reef: From Field to Spaceborne Earth Observation. Remote Sens. 2022, 14, 2604. [Google Scholar]
- Giardino, C.; Bresciani, M.; Valentini, E.; Gasperini, L.; Bolpagni, R.; Brando, V.E. Airborne Hyperspectral Data to Assess Suspended Particulate Matter and Aquatic Vegetation in a Shallow and Turbid Lake. Remote Sens. Environ. 2015, 157, 48–57. [Google Scholar] [CrossRef]
- Santos, M.J.; Khanna, S.; Hestir, E.L.; Greenberg, J.A.; Ustin, S.L. Measuring Landscape-Scale Spread and Persistence of an Invaded Submerged Plant Community from Airborne Remote Sensing. Ecol Appl 2016, 26, 1733–1744. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, R.; Hao, Y.; Wu, L.; Zhang, W.; Zhang, Q.; Bu, X. A New Method for Mapping Aquatic Vegetation Especially Underwater Vegetation in Lake Ulansuhai Using GF-1 Satellite Data. Remote Sens. 2018, 10, 1279. [Google Scholar] [CrossRef]
- Rowan, G.S.L.; Kalacska, M. A Review of Remote Sensing of Submerged Aquatic Vegetation for Non-Specialists. Remote Sens. 2021, 13, 623. [Google Scholar] [CrossRef]
- Kovacs, E.M.; Roelfsema, C.; Udy, J.; Baltais, S.; Lyons, M.; Phinn, S. Cloud Processing for Simultaneous Mapping of Seagrass Meadows in Optically Complex and Varied Water. Remote Sens. 2022, 14, 609. [Google Scholar] [CrossRef]
- Dierssen, H.M.; Ackleson, S.G.; Joyce, K.E.; Hestir, E.L.; Castagna, A.; Lavender, S.; McManus, M.A. Living up to the Hype of Hyperspectral Aquatic Remote Sensing: Science, Resources and Outlook. Front. Environ. Sci. 2021, 9, 649528. [Google Scholar] [CrossRef]
- Maasri, A.; Jähnig, S.C.; Adamescu, M.C.; Adrian, R.; Baigun, C.; Baird, D.J.; Batista-Morales, A.; Bonada, N.; Brown, L.E.; Cai, Q.; et al. A Global Agenda for Advancing Freshwater Biodiversity Research. Ecol Lett 2022, 25, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Orth, R.J.; Dennison, W.C.; Gurbisz, C.; Hannam, M.; Keisman, J.; Landry, J.B.; Lefcheck, J.S.; Moore, K.A.; Murphy, R.R.; Patrick, C.J.; et al. Long-Term Annual Aerial Surveys of Submersed Aquatic Vegetation (SAV) Support Science, Management, and Restoration. Estuar Coasts 2022, 45, 1012–1027. [Google Scholar] [CrossRef]
- Huber, S.; Hansen, L.B.; Nielsen, L.T.; Rasmussen, M.L.; Sølvsteen, J.; Berglund, J.; Paz von Friesen, C.; Danbolt, M.; Envall, M.; Infantes, E.; et al. Novel Approach to Large-Scale Monitoring of Submerged Aquatic Vegetation: A Nationwide Example from Sweden. Integr Environ Assess Manag. 2022, 18, 909–920. [Google Scholar] [CrossRef]
- Liu, Z.Y.-C.; Chamberlin, A.J.; Tallam, K.; Jones, I.J.; Lamore, L.L.; Bauer, J.; Bresciani, M.; Wolfe, C.M.; Casagrandi, R.; Mari, L.; et al. Deep Learning Segmentation of Satellite Imagery Identifies Aquatic Vegetation Associated with Snail Intermediate Hosts of Schistosomiasis in Senegal, Africa. Remote Sens. 2022, 14, 1345. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.; Han, L.; Zhu, L. How Well Do Deep Learning-Based Methods for Land Cover Classification and Object Detection Perform on High Resolution Remote Sensing Imagery? Remote Sens. 2020, 12, 417. [Google Scholar] [CrossRef]
- Mahdianpari, M.; Rezaee, M.; Zhang, Y.; Salehi, B. Wetland Classification Using Deep Convolutional Neural Network. In Proceedings of the IGARSS 2018 - 2018 IEEE International Geoscience and Remote Sensing Symposium; July 2018; pp. 9249–9252. [Google Scholar]
- Rezaee, M.; Mahdianpari, M.; Zhang, Y.; Salehi, B. Deep Convolutional Neural Network for Complex Wetland Classification Using Optical Remote Sensing Imagery. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2018, 1–10. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention – MICCAI 2015; Navab, N., Hornegger, J., Wells, W.M., Frangi, A.F., Eds.; Springer International Publishing: Cham, 2015; pp. 234–241. [Google Scholar]
- Guo, M.; Yu, Z.; Xu, Y.; Huang, Y.; Li, C. ME-Net: A Deep Convolutional Neural Network for Extracting Mangrove Using Sentinel-2A Data. Remote Sens. 2021, 13, 1292. [Google Scholar] [CrossRef]
- Guo, Y.; Liao, J.; Shen, G. Mapping Large-Scale Mangroves along the Maritime Silk Road from 1990 to 2015 Using a Novel Deep Learning Model and Landsat Data. Remote Sens. 2021, 13, 245. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Q.; Zhang, Y.; Yan, H. A Deep Convolution Neural Network Method for Land Cover Mapping: A Case Study of Qinhuangdao, China. Remote Sens. 2018, 10, 2053. [Google Scholar] [CrossRef]
- Cisneros, A.; Fiorio, P.; Menezes, P.; Pasqualotto, N.; Van Wittenberghe, S.; Bayma, G.; Furlan Nogueira, S. Mapping Productivity and Essential Biophysical Parameters of Cultivated Tropical Grasslands from Sentinel-2 Imagery. Agronomy 2020, 10, 711. [Google Scholar] [CrossRef]
- Singh, M.; Tyagi, K.D. Pixel Based Classification for Landsat 8 OLI Multispectral Satellite Images Using Deep Learning Neural Network. Remote Sens. Appl. Soc. Environ. 2021, 24, 100645. [Google Scholar]
- Roberts, H.H. Dynamic Changes of the Holocene Mississippi River Delta Plain: The Delta Cycle. J. Coastal Res 1997, 23, 605–627. [Google Scholar]
- Thomas, N.; Simard, M.; Castañeda-Moya, E.; Byrd, K.; Windham-Myers, L.; Bevington, A.; Twilley, R.R. High-Resolution Mapping of Biomass and Distribution of Marsh and Forested Wetlands in Southeastern Coastal Louisiana. Int. J. Appl. Earth Obs. Geoinf 2019, 80, 257–267. [Google Scholar]
- Carle, M. Spatial Structure and Dynamics of the Plant Communities in a Pro-Grading River Delta : Wax Lake Delta, Atchafalaya Bay, Louisiana. Doctor of Philosophy, Louisiana State University and Agricultural and Mechanical College, 2013.
- Chabreck, R.H.; Condrey, R.E. Common Vascular Plants of the Louisiana Marsh; Louisiana State University Center for Wetland Resources, 1979; p. 117;
- Holm, G.O., Jr.; Sasser, C.E. Differential Salinity Response between Two Mississippi River Subdeltas: Implications for Changes in Plant Composition. Estuaries 2001, 24, 78–89. [Google Scholar] [CrossRef]
- Lane, R.R.; Day, J.W.; Marx, B.; Reves, E.; Kemp, G.P. Seasonal and Spatial Water Quality Changes in the Outflow Plume of the Atchafalaya River, Louisiana, USA. Estuaries 2002, 25, 30–42. [Google Scholar] [CrossRef]
- Pasch, R.J.; Roberts, D.P.; Blake, E.S. The 2019 Atlantic Hurricane Season: An Active and Destructive Year. Weatherwise 2020, 73, 32–39. [Google Scholar] [CrossRef]
- Yao, Q.; Cohen, M.C.L.; Liu, K.; de Souza, A.V.; Rodrigues, E. Nature versus Humans in Coastal Environmental Change: Assessing the Impacts of Hurricanes Zeta and Ida in the Context of Beach Nourishment Projects in the Mississippi River Delta. Remote Sens. 2022, 14, 2598. [Google Scholar] [CrossRef]
- Clarito, Q.Y.; Suerte, N.O.; Bontia, E.C.; Clarito, I.M. Determining Seagrassess Community Structure Using the Braun – Blanquet Technique in the Intertidal Zones of Islas de Gigantes, Philippines. SJES 2020, 4. [Google Scholar] [CrossRef]
- Roy, D.P.; Wulder, M.A.; Loveland, T.R.; C. E., W.; Allen, R.G.; Anderson, M.C.; Helder, D.; Irons, J.R.; Johnson, D.M.; Kennedy, R.; et al. Landsat-8: Science and Product Vision for Terrestrial Global Change Research. Remote Sens. Environ. 2014, 145, 154–172. [Google Scholar] [CrossRef]
- Wulder, M.A.; Hilker, T.; White, J.C.; Coops, N.C.; Masek, J.G.; Pflugmacher, D.; Crevier, Y. Virtual Constellations for Global Terrestrial Monitoring. Remote Sens. Environ. 2015, 170, 62–76. [Google Scholar] [CrossRef]
- Li, J.; Roy, D. A Global Analysis of Sentinel-2A, Sentinel-2B and Landsat-8 Data Revisit Intervals and Implications for Terrestrial Monitoring. Remote Sens. 2017, 9, 902. [Google Scholar] [CrossRef]
- Lin, C.; Gong, Z.; Zhao, W. The Extraction of Wetland Hydrophytes Types Based on Medium Resolution TM Data. Sheng Tai Xue Bao 2010, 30, 6460–6469. [Google Scholar]
- Zhao, D.; Lv, M.; Jiang, H.; Cai, Y.; Xu, D.; An, S. Spatio-Temporal Variability of Aquatic Vegetation in Taihu Lake over the Past 30 Years. PLoS ONE 2013, 8, e66365. [Google Scholar] [CrossRef] [PubMed]
- Couvillion, B. 2017 Coastal Master Plan Modeling: Attachment C3-27: Landscape Data. Version Final; Baton Rouge, LA, 2017; p. 84.
- Visser, J.M.; Duke-Sylvester, S. LaVegMod v2: Modeling Coastal Vegetation Dynamics in Response to Proposed Coastal Restoration and Protection Projects in Louisiana, USA. Sustainability 2017, 9, 1625. [Google Scholar] [CrossRef]
- Chapin, F.S.; Woodwell, G.M.; Randerson, J.T.; Rastetter, E.B.; Lovett, G.M.; Baldocchi, D.D.; Clark, D.A.; Harmon, M.E.; Schimel, D.S.; Valentini, R.; et al. Reconciling Carbon-Cycle Concepts, Terminology, and Methods. Ecosystems 2006, 9, 1041–1050. [Google Scholar] [CrossRef]
- Hopkinson, C.S. Net Ecosystem Carbon Balance of Coastal Wetland-Dominated Estuaries: Where’s the Blue Carbon? In A Blue Carbon Primer; CRC Press: Boca Raton, FL, USA, 2018 ISBN 978-0-429-43536-2.
- Poungparn, S.; Komiyama, A.; Sangteian, T.; Maknual, C.; Patanaponpaiboon, P.; Suchewaboripont, V. High Primary Productivity under Submerged Soil Raises the Net Ecosystem Productivity of a Secondary Mangrove Forest in Eastern Thailand. J. Trop. Ecology 2012, 28. [Google Scholar] [CrossRef]
- Taillardat, P.; Thompson, B.S.; Garneau, M.; Trottier, K.; Friess, D.A. Climate Change Mitigation Potential of Wetlands and the Cost-Effectiveness of Their Restoration. Interface Focus. 2020, 10, 20190129. [Google Scholar] [CrossRef]
- Troxler, T.G.; Gaiser, E.; Barr, J.; Fuentes, J.D.; Jaffé, R.; Childers, D.L.; Collado-Vides, L.; Rivera-Monroy, V.H.; Castañeda-Moya, E.; Anderson, W.; et al. Integrated Carbon Budget Models for the Everglades Terrestrial-Coastal-Oceanic Gradient: Current Status and Needs for Inter-Site Comparisons. Oceanography 2013, 26, 98–107. [Google Scholar] [CrossRef]
- Twilley, R.; Castañeda-Moya, E.; Rivera-Monroy, V.H.; Rovai, A.S. Chapter 5: Productivity and Carbon Dynamics in Mangrove Wetlands. In Mangrove Ecosystems: A Global Biogeographic Perspective: Structure, Function, Services; 2017; pp. 113–162.
- Cheng, Y.; Zha, Y.; Tong, C.; Du, D.; Chen, L.; Wei, G. Estimating the Gaseous Carbon Budget of a Degraded Tidal Wetland. Ecol. Eng. 2021, 160, 106147. [Google Scholar]
- Howard, J.; Hoyt, S.; Isensee, K.; Pidgeon, E.; Telszewski, M. Coastal Blue Carbon: Methods for Assessing Carbon Stocks and Emissions Factors in Mangroves, Tidal Salt Marshes, and Seagrass Meadows; Conservation International, Intergovernmental Oceanographic Commission of UNESCO, International Union for Conservation of Nature: Arlington, VA, 2014. [Google Scholar]
- US EPA Inventory of, U.S. Greenhouse Gas Emissions and Sinks: 1990-2019; 2021.
- Forster, P.; Ramaswamy, V.; Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Raga, G.; et al. Changes in Atmospheric Constituents and in Radiative Forcing. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S.D., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, United Kingdom and New York, NY, USA, 2007; p. 106. [Google Scholar]
- IPCC Climate Change 2007: The Physical Science Basis: Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge; New York, 2007; ISBN 978-0-521-88009-1.
- Lane, R.R.; Mack, S.K.; Day, J.W.; Kempka, R.; Brady, L.J. Carbon Sequestration at a Forested Wetland Receiving Treated Municipal Effluent. Wetlands 2017, 37, 861–873. [Google Scholar]
- Baustian, M.M.; Liu, B.; Moss, L.C.; Dausman, A.; Pahl, J.W. Climate Change Mitigation Potential of Louisiana’s Coastal Area: Current Estimates and Future Projections. Ecol Appl. 2023. [Google Scholar] [CrossRef]
- Sasaki, Y. The Truth of the F-Measure. 2007, 5.
- IPCC 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Prepared by the National Greenhouse Gas Inventories Programme; Institute for Global Environmental Strategies: Japan, 2006.
- IPCC 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands; IPCC: Switzerland, 2014.
- IPCC 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; 2019.
- Duan, X.; Wang, X.; Mu, Y.; Ouyang, Z. Seasonal and Diurnal Variations in Methane Emissions from Wuliangsu Lake in Arid Regions of China. Atmos. Environ. 2005, 39, 4479–4487. [Google Scholar] [CrossRef]
- Hirota, M.; Tang, Y.; Hu, Q.; Hirata, S.; Kato, T.; Mo, W.; Cao, G.; Mariko, S. Methane Emissions from Different Vegetation Zones in a Qinghai-Tibetan Plateau Wetland. Soil Biol. Biochem. 2004, 36, 737–748. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, Q.; Zhang, Z.; Gao, Y.; Zhao, J.; Pu, Y.; Wang, W.; Xiao, W.; Liu, S.; Lee, X. Methane Flux Dynamics in a Submerged Aquatic Vegetation Zone in a Subtropical Lake. Sci Total Environ. 2019, 672, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Hu, J.; Villapando, O.; Bhomia, R.K.; Vardanyan, L.; Osborne, T. Long-Term Accumulation of Macro- and Secondary Elements in Subtropical Treatment Wetlands. Ecosphere 2021, 12, e03787. [Google Scholar] [CrossRef]
- Velthuis, M.; Kosten, S.; Aben, R.; Kazanjian, G.; Hilt, S.; Peeters, E.T.H.M.; van Donk, E.; Bakker, E.S. Warming Enhances Sedimentation and Decomposition of Organic Carbon in Shallow Macrophyte-Dominated Systems with Zero Net Effect on Carbon Burial. Global Chang. Biol. 2018, 24, 5231–5242. [Google Scholar] [CrossRef]
- DeMarco, K.E.; Hillmann, E.R.; Nyman, J.A.; Couvillion, B.; La Peyre, M.K. Defining Aquatic Habitat Zones Across Northern Gulf of Mexico Estuarine Gradients Through Submerged Aquatic Vegetation Species Assemblage and Biomass Data. Estuar Coasts. 2022, 45, 148–167. [Google Scholar] [CrossRef]
- Castellanos, D.L.; Rozas, L.P. Nekton Use of Submerged Aquatic Vegetation, Marsh, and Shallow Unvegetated Bottom in the Atchafalaya River Delta, a Louisiana Tidal Freshwater Ecosystem. Estuaries 2001, 24, 184. [Google Scholar] [CrossRef]
- Taylor, C.; Nyman, J.; La Peyre, M. Nekton Community Dynamics within Active and Inactive Deltas in a Major River Estuary: Potential Implications for Altered Hydrology Regimes. Aquat. Biol. 2022, 31, 1–18. [Google Scholar] [CrossRef]
- O’Connell, J.L.; Nyman, J.A. Marsh Terraces in Coastal Louisiana Increase Marsh Edge and Densities of Waterbirds. Wetlands 2010, 30, 125–135. [Google Scholar] [CrossRef]
- Hillmann, E.; DeMarco, K.; La Peyre, M. Salinity and Water Clarity Dictate Seasonal Variability in Coastal Submerged Aquatic Vegetation in Subtropical Estuarine Environments. Aquat. Biol. 2019, 28, 175–186. [Google Scholar] [CrossRef]
- La Peyre, M.K.; Gossman, B.; Nyman, J.A. Assessing Functional Equivalency of Nekton Habitat in Enhanced Habitats: Comparison of Terraced and Unterraced Marsh Ponds. Estuar Coasts. 2007, 30, 526–536. [Google Scholar] [CrossRef]
- Poffenbarger, H.J.; Needelman, B.A.; Megonigal, J.P. Salinity Influence on Methane Emissions from Tidal Marshes. Wetlands 2011, 31, 831–842. [Google Scholar]
- Weston, N.B.; Neubauer, S.C.; Velinsky, D.J.; Vile, M.A. Net Ecosystem Carbon Exchange and the Greenhouse Gas Balance of Tidal Marshes along an Estuarine Salinity Gradient. Biogeochemistry 2014, 120, 163–189. [Google Scholar] [CrossRef]
- Dang, K.B.; Nguyen, M.H.; Nguyen, D.A.; Phan, T.T.H.; Giang, T.L.; Pham, H.H.; Nguyen, T.N.; Tran, T.T.V.; Bui, D.T. Coastal Wetland Classification with Deep U-Net Convolutional Networks and Sentinel-2 Imagery: A Case Study at the Tien Yen Estuary of Vietnam. Remote Sens. 2020, 12, 3270. [Google Scholar]
- Le, Q.T.; Dang, K.B.; Giang, T.L.; Tong, T.H.A.; Nguyen, V.G.; Nguyen, T.D.L.; Yasir, M. Deep Learning Model Development for Detecting Coffee Tree Changes Based on Sentinel-2 Imagery in Vietnam. IEEE Access 2022, 10, 109097–109107. [Google Scholar] [CrossRef]
- Brownlee, J. Better Deep Learning: Train Faster, Reduce Overfitting, and Make Better Predictions; Machine Learning Mastery, 2018.
- Li, H.; Li, J.; Guan, X.; Liang, B.; Lai, Y.; Luo, X. Research on Overfitting of Deep Learning. In Proceedings of the 2019 15th International Conference on Computational Intelligence and Security (CIS); IEEE: Macao, Macao, December, 2019; pp. 78–81. [Google Scholar]
- Huang, W.; Li, C. Contrasting Hydrodynamic Responses to Atmospheric Systems with Different Scales: Impact of Cold Fronts vs. That of a Hurricane. JMSE 2020, 8, 979. [Google Scholar] [CrossRef]
- Orth, R.J. , Carruthers, T.J., Dennison, W.C., Duarte, C.M., Fourqurean, J.W., Heck, K.L., Hughes, A.R., Kendrick, G.A., Kenworthy, W.J., Olyarnik, S.; Short, F.T. A global crisis for seagrass ecosystems. BioScience. 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Cloern, J.E., Abreu; Xu, J. Human activities and climate variability drive fast-paced change across the world's estuarine–coastal ecosystems. Global Chang. Biology 2016, 22, 513–529. [Google Scholar] [CrossRef]
- Beven, J.L.; Hagen, A.; Berg, R. National Hurricane Center Tropical Cyclone Report: Hurricane Ida (AL092021); AL092021 National Hurricane Center (available at: www. nhc. noaa. gov/data …, 2021. [Google Scholar]
- Kinney, E.L.; Quigg, A.; Armitage, A.R. Acute Effects of Drought on Emergent and Aquatic Communities in a Brackish Marsh. Estuar Coasts. 2014, 37, 636–645. [Google Scholar] [CrossRef]
- Lou, S.; Huang, W.; Liu, S.; Zhong, G.; Johnson, E. Hurricane Impacts on Turbidity and Sediment in the Rookery Bay National Estuarine Research Reserve, Florida, USA. Int. J. Sediment Res. 2016, 31, 330–340. [Google Scholar] [CrossRef]
- Morton, R.A.; Barras, J.A. Hurricane Impacts on Coastal Wetlands: A Half-Century Record of Storm-Generated Features from Southern Louisiana. J Coast Res. 2011, 275, 27–43. [Google Scholar] [CrossRef]
- Frazer, T.K.; Notestein, S.K.; Jacoby, C.A.; Littles, C.J.; Keller, S.R.; Swett, R.A. Effects of Storm-Induced Salinity Changes on Submersed Aquatic Vegetation in Kings Bay, Florida. Estuar Coasts. 2006, 29, 943–953. [Google Scholar] [CrossRef]
- Pendleton, L.; Donato, D.C.; Murray, B.C.; Crooks, S.; Jenkins, W.A.; Sifleet, S.; Craft, C.B.; Fourqurean, J.W.; Kauffman, J.B.; Marbà, N.; et al. Estimating Global “Blue Carbon” Emissions from Conversion and Degradation of Vegetated Coastal Ecosystems. PLoS ONE 2012, 7, e43542. [Google Scholar] [CrossRef]
- Sapkota, Y.; White, J.R. Marsh Edge Erosion and Associated Carbon Dynamics in Coastal Louisiana: A Proxy for Future Wetland-Dominated Coastlines World-Wide. Estuar Coast Shelf Sci. 2019, 226, 106289. [Google Scholar] [CrossRef]
- Schoolmaster, D.R.; Stagg, C.L.; Creamer, C.; Laurenzano, C.; Ward, E.; Waldrop, M.; Baustian, M.M.; Aw, T.; Merino, S.; Villani, R.; et al. A Model of the Spatiotemporal Dynamics of Soil Carbon Following Coastal Wetland Loss Applied to a Louisiana Salt Marsh in the Mississippi River Deltaic Plain. J Geophys Res Biogeosci. 2022. [Google Scholar] [CrossRef]
- Bos, A.R.; Bouma, T.J.; de Kort, G.L.J.; van Katwijk, M.M. Ecosystem Engineering by Annual Intertidal Seagrass Beds: Sediment Accretion and Modification. Estuar Coast Shelf Sci. 2007, 74, 344–348. [Google Scholar] [CrossRef]
- Gacia, E.; Duarte, C.M.; Middelburg, J.J. Carbon and Nutrient Deposition in a Mediterranean Seagrass (Posidonia Oceanica) Meadow. Limnol. Oceanogr. 2002, 47, 23–32. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon Balance in Salt Marsh and Mangrove Ecosystems: A Global Synthesis. JMSE 2020, 8, 767. [Google Scholar] [CrossRef]
- Al-Haj, A.N.; Fulweiler, R.W. A Synthesis of Methane Emissions from Shallow Vegetated Coastal Ecosystems. Global Chang. Biol. 2020, 26, 2988–3005. [Google Scholar] [CrossRef] [PubMed]
- Myhre, G.; Shindell, D.; Bréon, F.M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.F.; Lee, D.; Mendoza, B. Anthropogenic and Natural Radiative Forcing, Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, 659–740; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Franz, D.; Koebsch, F.; Larmanou, E.; Augustin, J.; Sachs, T. High Net CO2 and CH4 Release at a Eutrophic Shallow Lake on a Formerly Drained Fen. Biogeosciences 2016, 13, 3051–3070. [Google Scholar] [CrossRef]
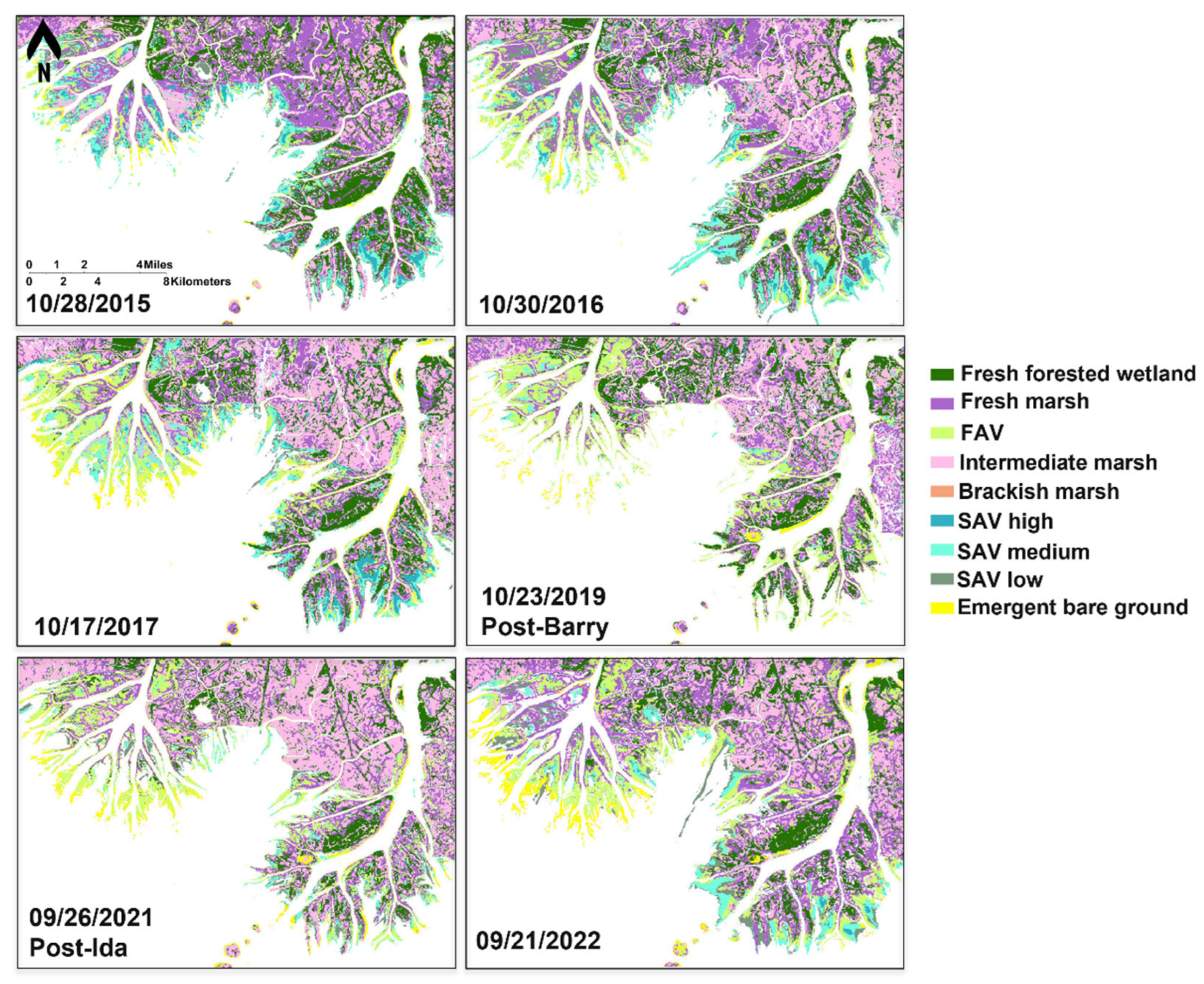
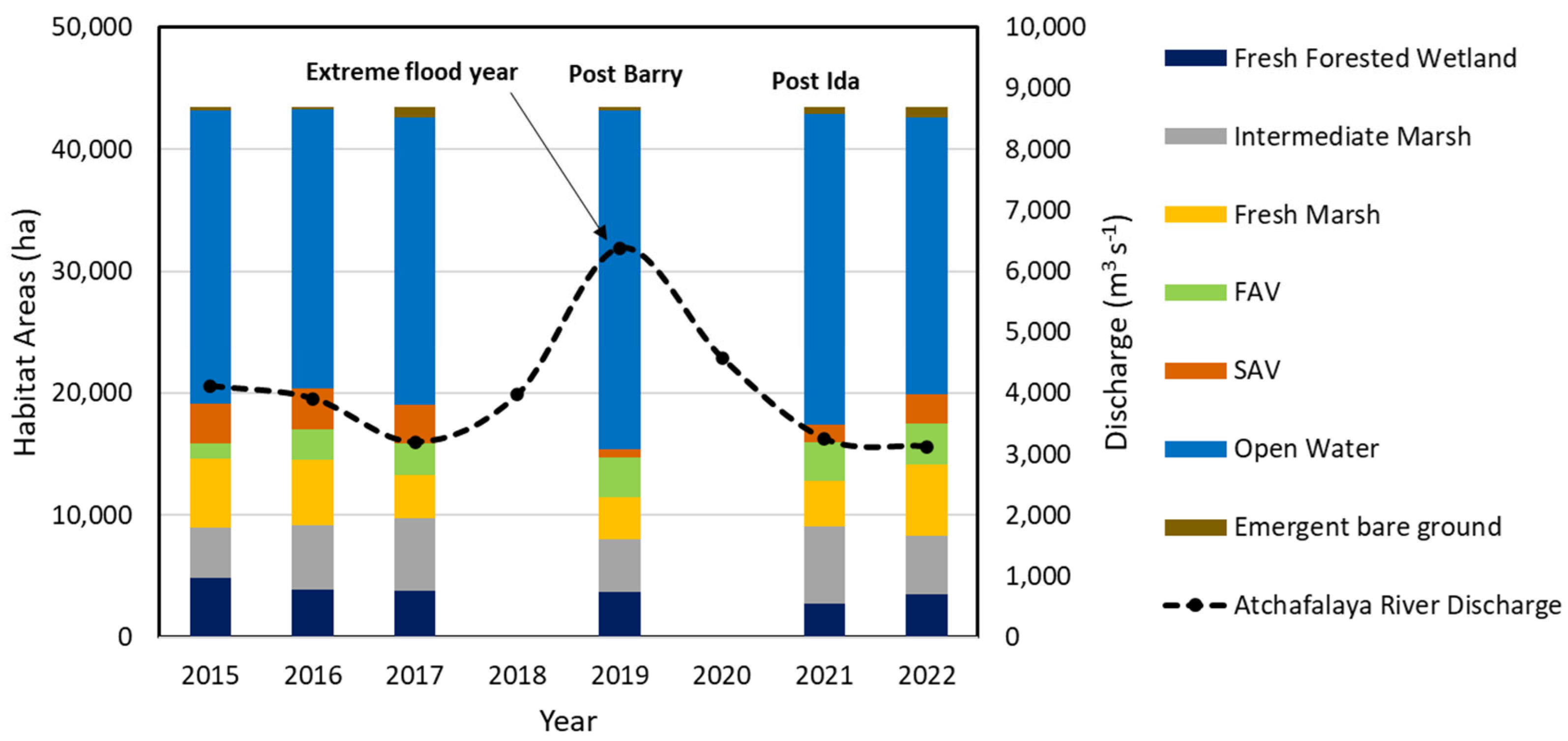
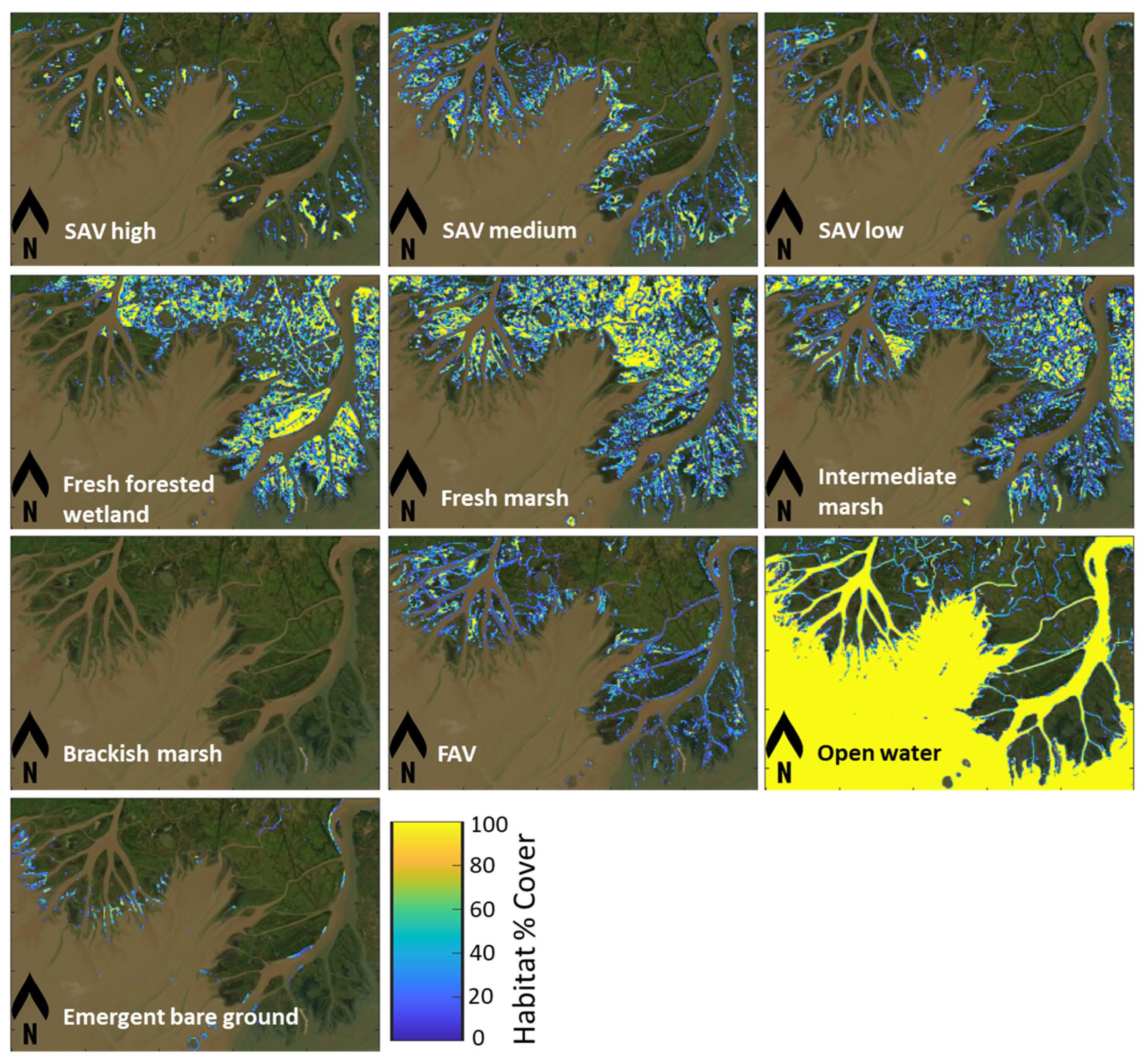
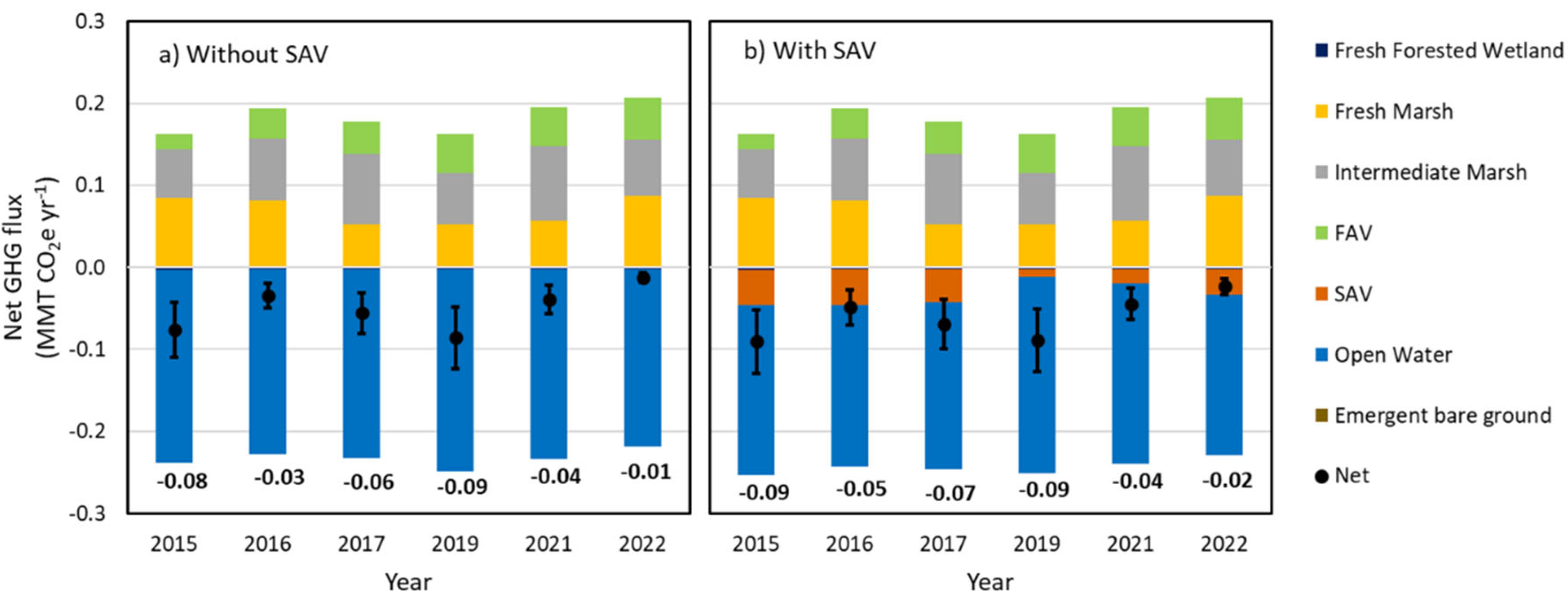
| # | Date | Satellite sensor | File name |
|---|---|---|---|
| 1 | 10/28/2015 | Landsat 8-OLI | LC08_L2SP_023039_20151028_20200908_02_T1 |
| 2 | 10/30/2016 | Landsat 8-OLI | LC08_L1TP_023039_20161030_20200905_02_T1 |
| 3 | 10/17/2017 | Landsat 8-OLI | LC08_L1TP_023039_20171017_20200902_02_T1 |
| 4 | 10/23/2019 | Landsat 8-OLI | LC08_L2SP_023039_20191023_20200825_02_T1 |
| 5 | 09/26/2021 | Landsat 8-OLI | LC08_L2SP_023039_20210926_20211001_02_T1 |
| 6 | 09/21/2022 | Landsat 9-OLI | LC09_L1TP_023039_20220921_20220923_02_T1 |
| 7 | 10/15/2022 | Landsat 9-OLI | LC08_L1TP_023039_20221015_20221021_02_T1 |
| Total SAV % cover from field | SAV % cover categorized from field measurements | SAV % cover classified from remote sensing | Dominant species | % cover of dominant species | Sampling stations |
|---|---|---|---|---|---|
| 61% | High | SAV medium | V. americana | 46% | A3 |
| 64% | High | SAV medium | V. americana | 63% | C4 |
| 64% | High | SAV medium | V. americana | 64% | D3 |
| 72% | High | SAV high | V. americana | 72% | C2 |
| 73% | High | SAV high | N. guadalupensis | 67% | B3 |
| 75% | High | SAV high | V. americana | 73% | A2 |
| 79% | High | SAV high | V. americana | 73% | A1 |
| 85% | High | SAV high | V. americana | 85% | B2 |
| 86% | High | SAV high | N. guadalupensis | 63% | B1 |
| 95% | High | SAV high | V. americana | 93% | D2 |
| 35% | Medium | SAV low | N. guadalupensis | 19% | B4 |
| 44% | Medium | SAV medium | V. americana | 44% | C5 |
| 51% | Medium | SAV medium | V. americana | 51% | C1 |
| 16% | Low | SAV low | N. guadalupensis | 15% | C6 |
| 22% | Low | SAV low | N. guadalupensis | 18% | D4 |
| 23% | Low | SAV low | N. guadalupensis | 11% | C3 |
| 27% | Low | SAV low | V. americana | 27% | A4 |
| Metrics Habitats |
Precision (%) | Recall (%) | F1 score (%) | Overall accuracy (%) |
|---|---|---|---|---|
| Fresh forested wetland | 81.2 | 71.9 | 76.4 | 86.7% |
| Fresh marsh | 86.4 | 73.2 | 79.8 | |
| Intermediate marsh | 74.3 | 83.1 | 78.7 | |
| Brackish marsh | 52.3 | 64.2 | 58.2 | |
| FAV | 79.1 | 79.5 | 79.2 | |
| SAV high | 80.5 | 77.4 | 78.9 | |
| SAV medium | 69.3 | 71.9 | 70.6 | |
| SAV low | 78.5 69.3 | 79.2 | 78.9 | |
| Open water | 97.3 | 99.6 | 98.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




