1. Introduction
Chemical warfare agents (CWAs) are extremely toxic chemicals that are relatively easy to produce and that can be used against military personnel as a part of asymmetric warfare or against civilians by terrorist groups. Chemical warfare agents have been classified based on different properties [
1]. Generally, the CW comprise of six categories, lung insurance (choking agents), blood agents (cyanogens), vasicants (blister agents), nerve agents (anticholinesterase), incapacitants and riot control agents (lacrimators) [
2,
3,
4]. CWAs cause adverse health effects upon contact or by inhalation. Vesicants cause severe blistering of tissue, nerve agents inhibit the enzyme acetylcholinesterase, and blood agents cause tissue hypoxia [
5,
6,
7].
Decontamination, along with contamination avoidance and protection, can reduce or remove adverse health effects of CWAs if applied in time. The environmental degradation process of CWAs based on hydrolysis, oxidation, photolysis and microbial degradation has been studied and reviewed [
8,
9,
10,
11,
12,
13].
Whereas most of the work in the past focused on hydrolysis and oxidation-based degradation of CWAs, additional decontamination strategies such as the use of non-photochemical or photochemical advanced oxidation process [
2,
14,
15,
16], heterogenous processes such as metal-organic frameworks [
3,
17,
18], metal oxides [
19] polymers [
20], direct energy application [
21] or biotechnological [
22] degradation may also be a viable option.
In recent years, alternative decontamination strategies have been explored, such as non-photochemical or photochemical advanced oxidation processes, heterogeneous processes, including metal-organic frameworks, metal oxides, and polymers, as well as direct energy application or biotechnological degradation. Ferrate(VI) and nanoscale zero-valent iron are effective and environmentally friendly decontamination reagents for the destruction of CWAs [
4,
23,
24].
The ferrate(VI) was tested in our work for the degradation of the chemical warfare agent HN3 (tris(2-chloroethyl)amine) under different conditions to optimize the effectiveness of the decontamination system. This research contributes to the development of more efficient and environmentally friendly decontamination strategies for CWAs.
2. Materials and Methods
2.1. Preparation of Ferrate Fe(VI)
Ferrate(VI) was prepared electrochemically in molten hydroxide according to the process described in detail elsewhere [
31].
2.2. Preparation of Nitrogen Mustard HN3
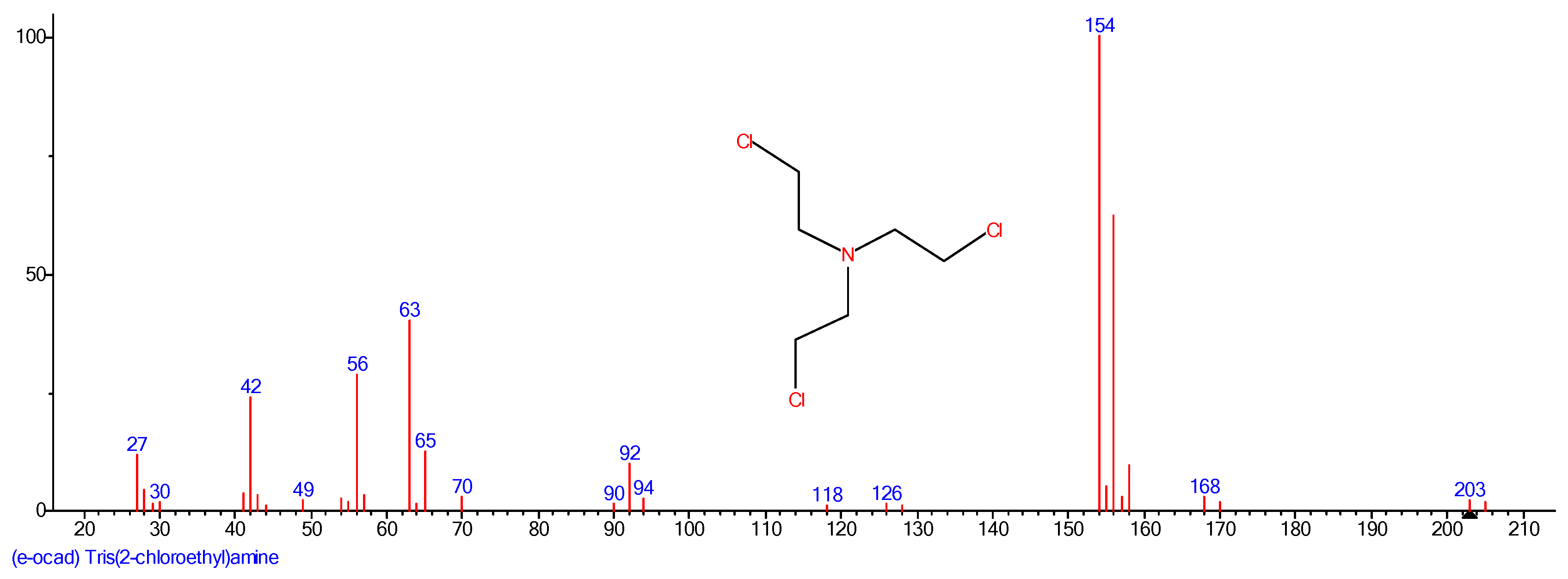
The nitrogen mustard HN3 was prepared by a reaction of triethanolamine with thionyl chloride in chloroform. The prepared by-product HN3 hydrochloride was treated with a solution of sodium carbonate in water. The resulting brownish oily product was separated by a separatory funnel and distilled. The product was then confirmed by GC-MS analysis as shown in
Figure 1.
2.3. Reaction of Ferrate Fe(VI) with Nitrogen Mustard HN3
To study the degradation rate of degradation of chemical warfare agent HN3 (tris(2-chloroethyl)amine), 0.7 mL of phosphate buffer solutions (sodium dihydrogen phosphate) with pH 3, 4, 5 and 6 were prepared in 4 mL glass vials. Then 0.1 mL of a solution of HN3 in n-Hexane (200 ppm, 1.09 µM) mL was added to the buffer containing vials. Immediately, the addition of 0.2 mL freshly prepared ferrate(VI) solution in distilled water (1.09 mM) followed. After a selected time (2, 4, 8, 16 min), 250 µL of the reaction solution was transferred to 1 mL vials containing 500 µL of n-Hexane. The vials were shaken at 1000 RPM for 1 min [
24]. After the organic layer separation, 200 µL of n-Hexane layer was collected, dried with sodium sulphate, and analysed using GC-FID to determine the concentration of remaining HN3.
3. Results and Discussion
3.1. Degradation of Nitrogen Mustard
Since, HN3 undergoes spontaneous hydrolysis in water [
25], at the beginning of our experiments the hydrolysis the hydrolysis rate of prepared HN3 was studied. The GC-FID analysis confirmed that hydrolysis of HN3 fulfil the first order kinetics equation [
24]. Therefore, for analysing the kinetics data of HN3 hydrolysis, an Equation (1)
was used, where c
t denotes the residual concentration of HN3 in time t and c
0 stands for the initial concentration of HN3. The rate constant of the spontaneous hydrolysis of HN3 was at pH 6 determined to be 0.029±0.008 min
−1 which is in line with previously reported results [
25].
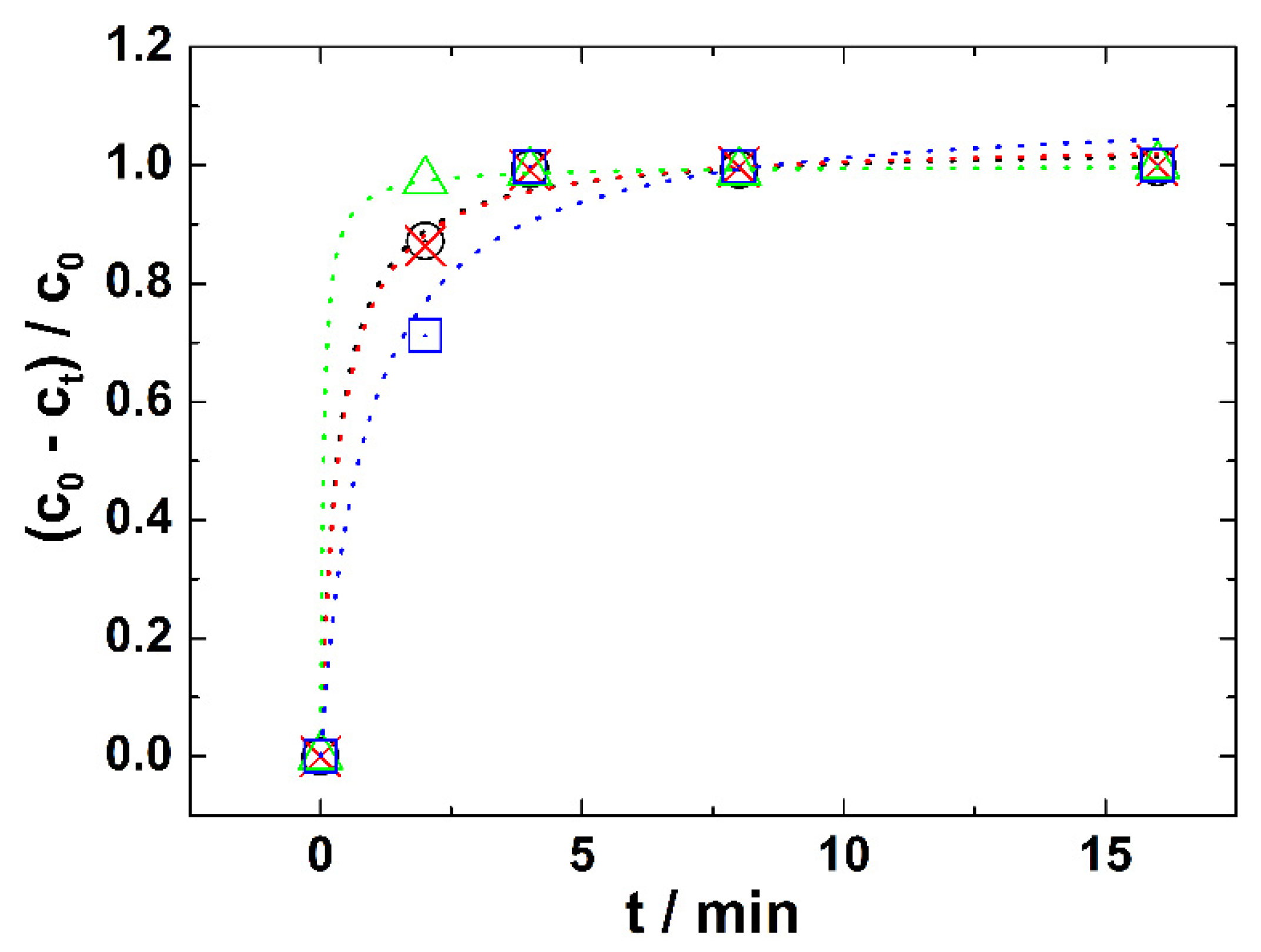
After these initial experiments the oxidation power of ferrate(VI) towards HN3 was tested. The degradation of the nitrogen mustard HN3 was studied as a function of the concentration of remaining nitrogen mustard HN3 in a solution and time at various pH (
Figure 2).
As expected, after the addition of purple ferrate Fe(VI) solution to a reaction mixture, the colour changed and a brown precipitate containing iron(III)/iron(II) products as the final species was formed [
26]. It can be supposed that oxidation of HN3 by ferrate(VI) is 2e
- transfer step (Fe
VI→Fe
IV→Fe
II) rather than 1e
- transfer step, as suggested by previous reports concerning reactions of iron(VI) with amines [
5,
27,
28].
The obtained data were studied using several kinetics models. Based on our measured kinetic data of the decomposition of HN3 using Fe(VI) it follows that the best fit was found a pseudo-second order kinetics model. This observation is consistent with previous results, where the kinetics of amine oxidation by ferrate(VI) was monitored [
5,
27,
28]. Therefore, the following equation was applied to calculate the second-order rate constant k
2 for all degradation processes at all pH used:
where c
e is an equilibrium concentration of HN3. The rate of HN3 degradation is represented by kinetic curves in
Figure 3.
As can be seen from
Figure 3 the complete degradation of HN3 by ferrate(VI) was reached after ca 4 min in all solutions of different pH. All the curves that were calculated according to Equation (2) fit the experimental points well, while the individual statistical parameters are summarized in
Table 1. This confirms the correctness of our reasoning that it is a reaction with pseudo-second order kinetics.
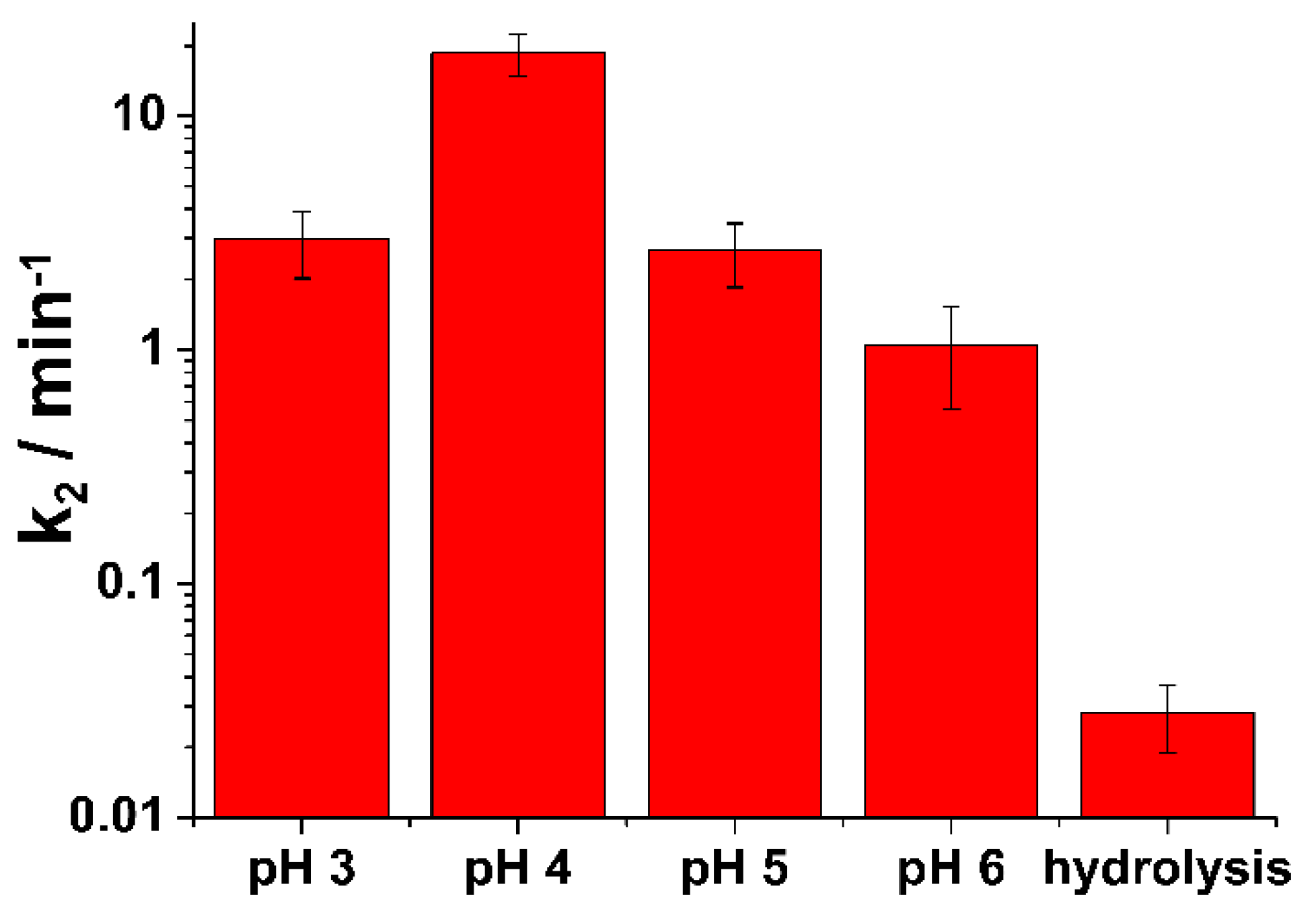
In the following
Figure 4, the bar diagrams of calculated rate constants k
2 according to the Equation (2) at different pH are plotted.
From
Figure 4 it is easily visible that the decomposition rate of HN3 by ferrate(VI) strongly depends on the pH of the solution. The highest rate was observed for pH=4, followed by pH=5 and pH=3. The lowest rate was observed for the pH=6. The lower decomposition rate of nitrogen mustard at pH=3 compare to pH=4 is probably caused by the strong self-decomposition reaction of ferrate(VI) to Fe(III)/Fe(II) by water via the intermediary of Fe(IV) and Fe(V) species, which could reduce the efficiency of the degradation process itself. At pH=3 H
2FeO
4 is mainly present due to the pKa = 3.5 ± 0.2 [
6,
29,
30]. At this acidic pH the formation of a diferrate(VI) with fast intramolecular oxo-coupling producing O
2 and diferryl(IV) species was proposed. However, the authors of this study point out that in the acidic solutions, the kinetics of ferrate(VI)-mediated water oxidation (ferrate decomposition) becomes a complex problem. Similarly to our study with HN3 a second-order decay process can be resolved. However, the self-decomposition of ferrate(VI) is faster than oxidation of nitrogen mustard with condensation and dimerization of monomeric ferrate as the rate-determining step [
29]. In the following
Table 1, the calculated values of all rate constants are summarised.
It can be easily seen that the rate of the decomposition of HN3 by ferrate(VI) is at the same pH levels approximately two orders of magnitude higher than that of spontaneous hydrolysis of HN3. This means that the impact of hydrolysis on the decomposition of HN3 is very low compare to ferrate(VI) and can be during calculations neglected. In addition, this means that Fe(VI) is a strong enough oxidizing agent against nitrogen mustard even at an almost neutral pH, which in field conditions means simplification of handling during eventual decontamination of surfaces, people, vehicles, etc. ferrate(VI) solution.
4. Conclusions
This study aimed to test a potentially fast-acting and environmentally friendly decontamination agent based on ferrate Fe(VI) for its ability to degrade the persistent chemical warfare agent HN3. Our research focused on identifying the optimal conditions for degradation, with particular attention to reaction rates under different pH levels to achieve the fastest and most effective degradation.
Our experiments revealed that the reaction rate was fastest at a lower pH of 3. However, the degradation of the nitrogen mustard HN3 was nearly 100% within just four minutes across the full range of pH levels tested. This remarkable result demonstrates the efficiency and effectiveness of ferrate Fe(VI) as a promising candidate for the decontamination of persistent chemical warfare agents.
Furthermore, the formation of Fe(III) and Fe(II) species as ferrate reduction products is acceptable from an environmental perspective, as these products pose no significant harm to the environment.
Our future work will focus on the degradation of other persistent chemical warfare agents and optimizing the degradation conditions for practical applications of ferrate Fe(VI) based decontamination agents. These efforts hold great promise for the development of safer, more efficient, and environmentally friendly solutions for the decontamination of hazardous materials.
Author Contributions
Conceptualization, T.M. and M.L.; methodology, M.L.; validation, M.L.; formal analysis, M.G.; investigation, M.L.; data curation, M.L. and M.G.; writing—original draft preparation, M.L..; writing—review and editing, M.G.; visualization, M.L.; supervision, T.M.; funding acquisition, T.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by Norway through the Norway Grants; project: “Innovative carbon-based sorbents as an effective method of wastewater treatment”, grant number 3213200008.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prentiss, A.M. Classification of Chemical Agents, A Treatise on Chemical Warfare. New York : McGraw Hill, 1937.
- Chauhan S, Chauhan S, D’Cruz R, Faruqi S, Singh KK, Varma S, Singh, M., Karthik, V. Chemical warfare agents. Environ Toxicol Pharmacol. 2008, Vol. 26, 2.
- Ganesan, K., Raza, S.K., & Vijayaraghavan, R. Chemical warfare agents. Journal of pharmacy & bioallied sciences. 2010, Vol. 2, 3.
- Szinicz, L. History of chemical and biological warfare agents. Toxicology. 2005, Vol. 214, 3. [CrossRef]
- GUPTA, Ramesh, C. Handbook of toxicology of chemical warfare agents. s.l. : Academic Press, 2015. ISBN: 978–0-12–800159–2.
- Marrs, Timothy, T., Robert, L. Maynard, and Frederick Sidell, eds. Chemical warfare agents: Toxicology and treatment. s.l. : John Wiley & Sons, 2007. ISBN 978–0-470–01359–5.
- Romano, J.A. Jr., Lukey, B.J., Salem, H.,. Chemical Warfare Agents: Chemistry, Pharmacology, Toxicology, and Therapeutics. s.l. : CRC Taylor & Francis, 2007. ISBN13: 978–1-4200–4661–8.
- Small, M.J. Compounds Formed from the Chemical Decontamination of HD, GB, and VX and Their Environmental Fate. Fort Detrick, Frederick MD; : U.S. Army Medical Bioengineering Research and Development Laboratory, 1984. Technical Report 8304, AD A149515.
- Trapp, R. The Detoxification and Natural Degradation of Chemical Warfare Agents. . Stockholm, Sweden : Stockholm International Peace Institute (SIPRI),, 1985. [CrossRef]
- Clark, D.N. Review of Reactions of Chemical Agents in Water. Battelle, Columbus, OH. : Defense Technical Information Center, Ft. Belvoir, VA, 1989. AD-A213 287.
- MacNaughton, M.G. and Brewer, J.H.,. Environmental Chemistry and Fate of Chemical Warfare Agents. Final report.,. San Antonio, TX : Southwest Research Institute, 1994. SwRI Project 01–5864.
- Munro, N.B.; et al. The Sources, Fate, and Toxicity of Chemical Warfare Agent Degradation Products. Environ. Health Persp.,. 1999, Vol. 107, 933. [CrossRef]
- Talmage, S.S., Watson, A.P., Hauschild, V., Munro, N.B., King, J.,. Chemical Warfare Agent Degradation and Decontamination. Current Organic Chemistry,. 2007, Vol. 11. [CrossRef]
- Snelson, A., Taylor, K., O’Neill, H.J.,. Reaction of CW agents simulants on surfaces in the presence of O3, UV and O3+UV. J. Environ. Sci. Health. 1984, Vol. A19, 7. [CrossRef]
- Popiel, S., Nalepa, T., Dzierziak, D., Stankiewicz, R., Witkiewicz, Z.,. Rate of dibutylsulfide decomposition by ozonation and the O3/H2O2 advanced oxidation process. Journal of Hazardous Materials. 2009, Vol. 164. [CrossRef]
- Popiel, S., Witkiewicz, Z., Chrzanowski, M.,. Sulfur mustard destruction using ozone, UV, hydrogen peroxide and their combination. Journal of Hazardous Materials. 2007, Vol. 153. [CrossRef]
- Liu. Y., Howarth, A.J., Vermeulen, N.A., Moon, S.-Y.,. Catalytic degradation of chemical warfare agents and their simulants by metal-organic frameworks. Coordination Chemistry Reviews. 2017, Vol. 346. [CrossRef]
- Tang, J.,Li, P.,Islamoglu, T., Li, S., Zhang, X.,Son, F.A., Chen, Z.,. Micropore environment regulation of zirconium MOFs for instantaneous hydrolysis of an organophosphorus chemical. Cell Reports Physical Science. 2, 2021. [CrossRef]
- Shen, Z., Zhong, J.-Y., Han, X.-Y., Wanga, L.-Y., Cui, Y., Chen, L.-K.,. Decontamination of Chemical Warfare Agents on sensitive equipment materials using Zr4+ and Ge4+ co-doped TiO2 and hydrofluoroether suspension. Chemical Engineering Journal. 2016, Vol. 302. [CrossRef]
- Bromberg, L., Schreuder-Gibson, H., Creasy, W.R., McGarvey, D.J., Fry, R.A., Hatton, T.A.,. Degradation of Chemical Warfare Agents by Reactive Polymers. Ind. Eng. Chem. Res. 2009, Vol. 48. [CrossRef]
- Iwai, T., Inoue, H., Kakegawa, K., Ohrui, Y., Nagoya, T., Nagashima, H., Miyahara, H., Chiba, K., Seto, Y., Okino, A.,. Development of a High-Efficiency Decomposition Technology for Volatile Chemical Warfare Agent Sarin Using Dielectric Barrier Discharge. Plasma Chemistry and Plasma Processing. 2020, Vol. 40. [CrossRef]
- Ohmori, T., Kawahara, K., Nakayama, K., Shioda, A., Ishikawa, S., Kanamori-Kataoka, M., Kishi, S., Komano, A., Seto, Y.,. Decontamination of nerve agents by immobilized organophosphorus hydrolase. Forensic Toxicology. 2013, Vol. 31. [CrossRef]
- Organization, Science and Technology Organization North Atlantic Treaty. Long-Term Scientific Study on CBRN Defence. Neuilly-sur-Seine Cedex, France: NATO Science and Technology organization, TR-HFM-273, 2021. ISBN 978–92–837–2327–1.
- Zboril, R., Andrle, M., Oplustil, F., Machala, L., Tucek, J., Filip, J., Marusak, Z., Sharma, V.K. Treatment of chemical warfare agents by zero-valent iron nanoparticles and ferrate(VI)/(III) composite. Journal of Hazardous Materials. 2012, Vols. 211–212. [CrossRef]
- Bartlett, P.D., Ross, S.D., Swain, C.G.,. Kinetics and Mechanism of the Reactions of Tertiary β-Chloroethylamines in Solution. III. β-Chloroethyldiethylamine and tris-β-Chloroethylamine. J. Am. Chem. Soc. 1947, Vol. 71, 4. [CrossRef]
- Czölderova, M., Behúl, M., Filip, J., Zajíček, P., Grabic, R., Vojs-Staňová, A.,Gál, M., Kerekeš, K., Híveš, J., Ryba, J., Rybanská, M., Brandeburová, P., Mackuľak, T.,. 3D printed polyvinyl alcohol ferrate(VI) capsules: Effective means for the removal of pharmaceuticals and illicit drugs from wastewater,. Chemical Engineering Journal,. 2018, Vol. 349. [CrossRef]
- Sharma, V.K. Oxidation of nitrogen-containing pollutants by novel ferrate (VI) technology: A review. Journal of Environmental Science and Health Part, A. 2010, Vol. 45, 6, pp. 645–667. [CrossRef]
- Ferrate (VI) and ferrate (V) oxidation of organic compounds: Kinetics and mechanism. Coordination Chemistry Reviews. 2013, Vol. 257, 2, pp. 495–510. [CrossRef]
- Sarma, R.; et al. Studies of the di-iron (VI) intermediate in ferrate-dependent oxygen evolution from water. Journal of the American Chemical Society. 2012, Vol. 134, 37, pp. 15371–15386. [CrossRef]
- Luo, C., Feng, M., Sharma, V.K., Huang, Ch-H. Revelation of ferrate(VI) unimolecular decay under alkaline conditions: Investigation of involvement of Fe(IV) and Fe(V) species. Chemical Engineering Journal. 2020, Vol. 338, pp. 124–134. [CrossRef]
- J.; Híves, J., Benova, M., Bouzek, K., Sitek, J., Sharma, V.K. The cyclic voltammetric study of ferrate(VI) formation in a molten Na/K hydroxide mixture. Electrochimica Acta. 2008, Vol. 54. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).