Submitted:
01 June 2023
Posted:
02 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Development in the FREECAD Software of the Virtual 3D Object
2.2. Obtaining the G-CODE Format using the CURA 3D Slicer
2.3. Printing the Drug Using a Cartesian 3D Printer
2.4. Obtaining a Predicted Drug Mass Value by Adapting the Object Sequencing Mode and Interpreting the Experimental Data using the Design Expert Statistical Software
3. Results
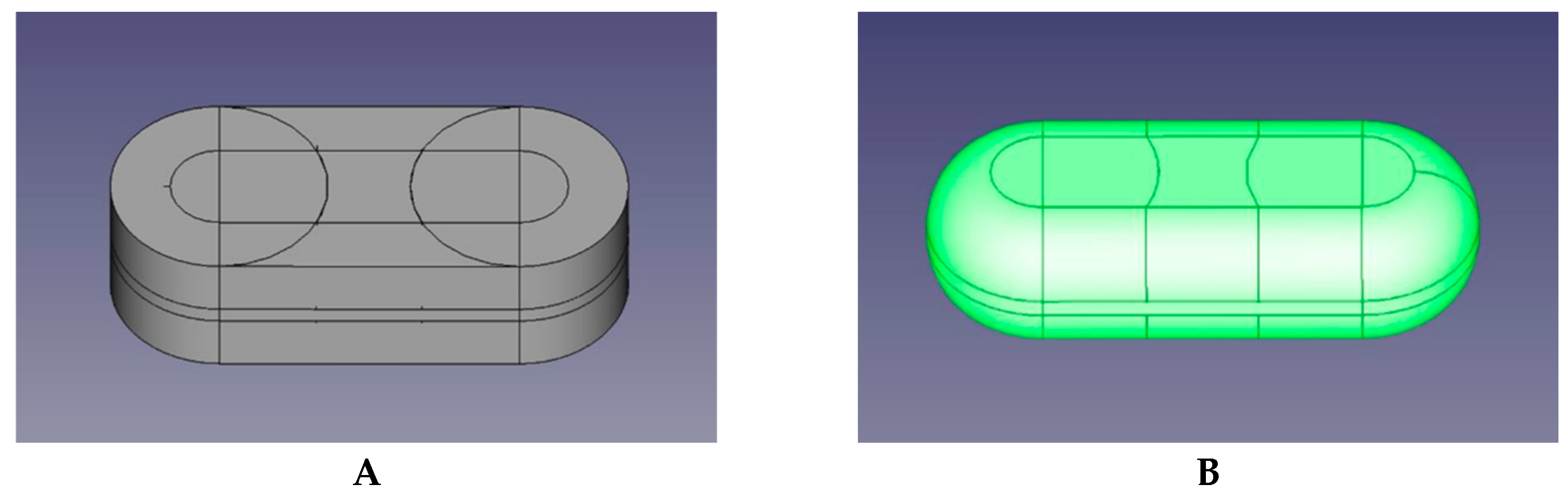
3.1. Development in the FREECAD Software of the Virtual 3D Object
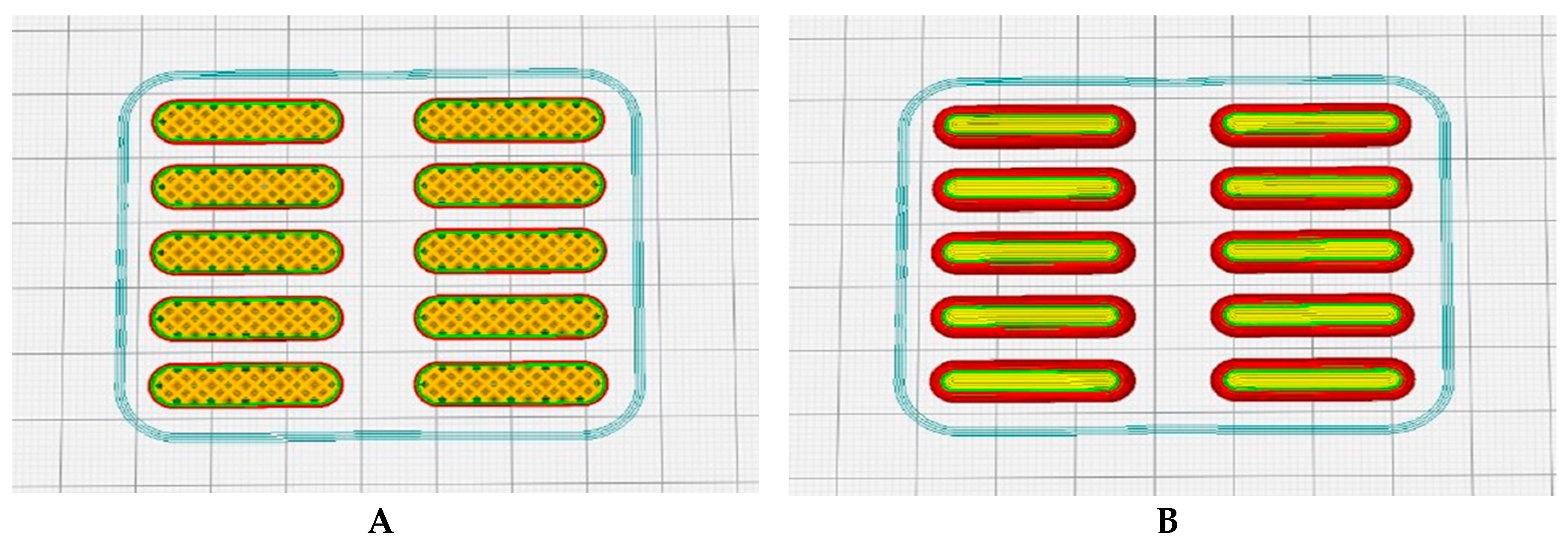
3.2. Obtaining the G-CODE Format using the CURA 3D Slicer
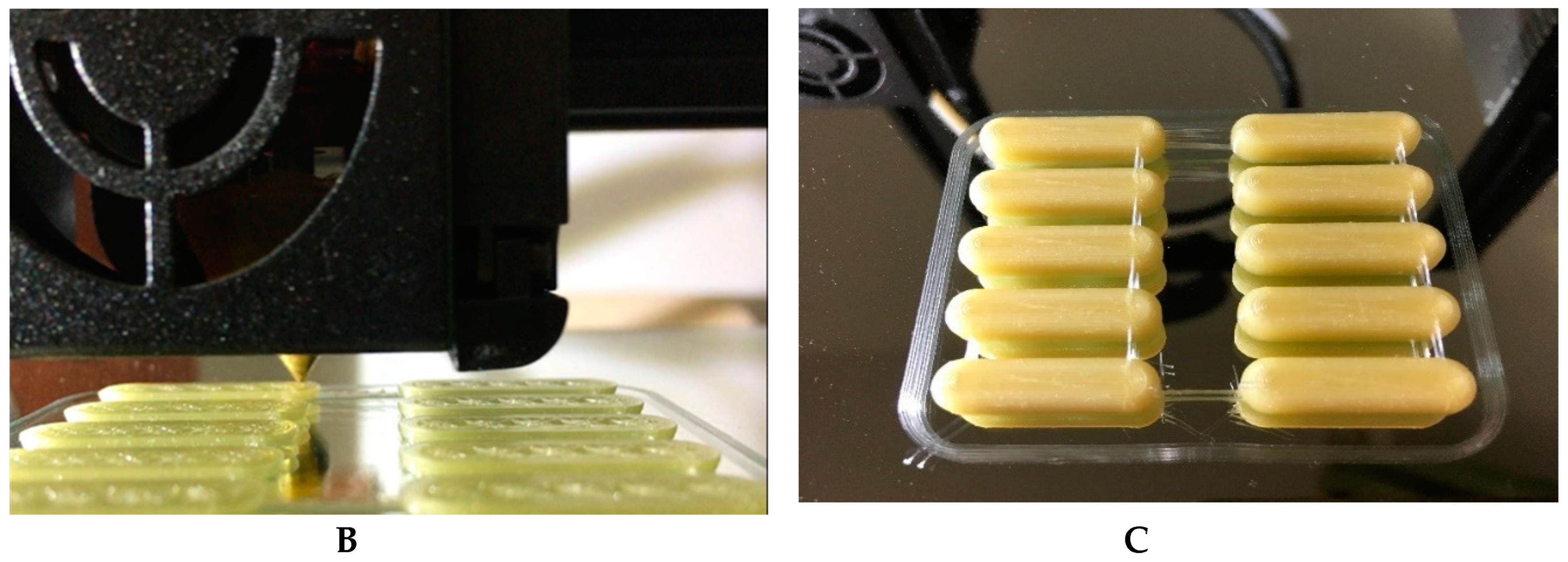
3.3. Printing the Drug Using a Cartesian 3D Printer
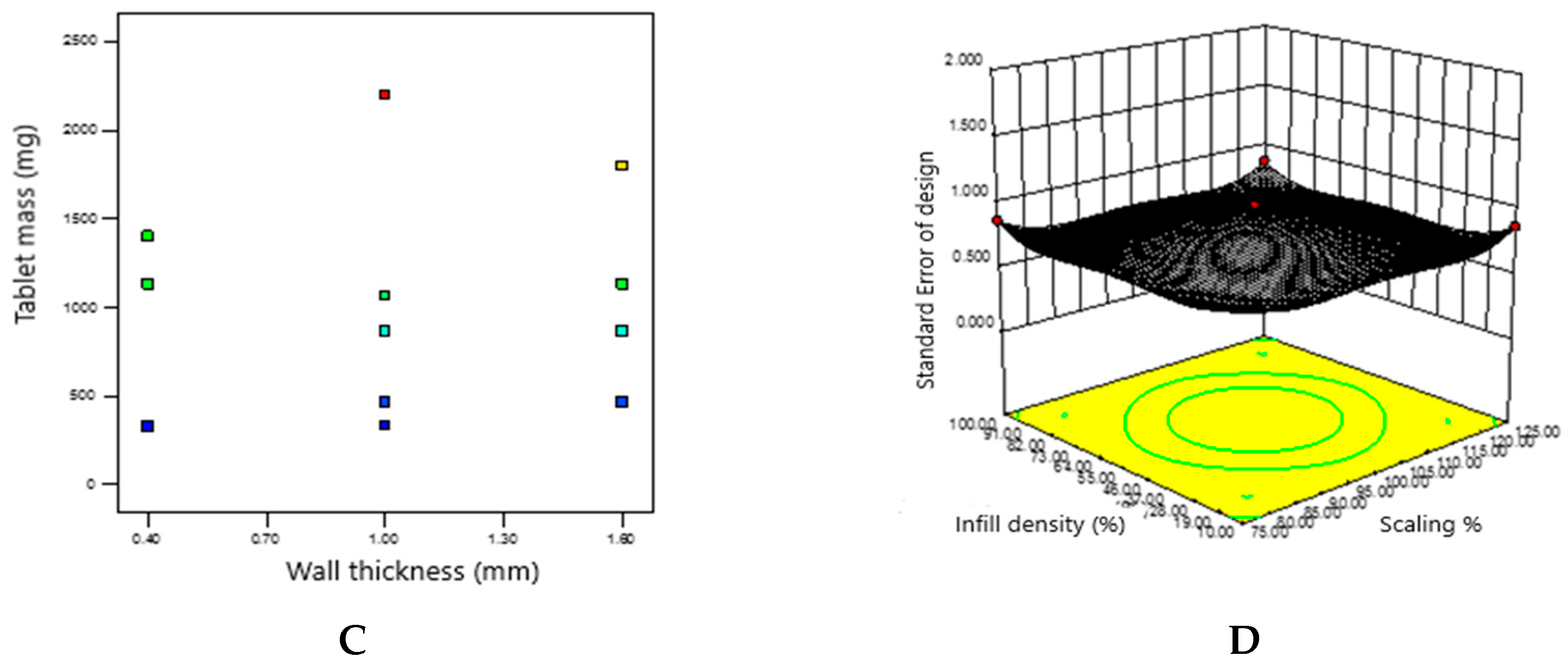
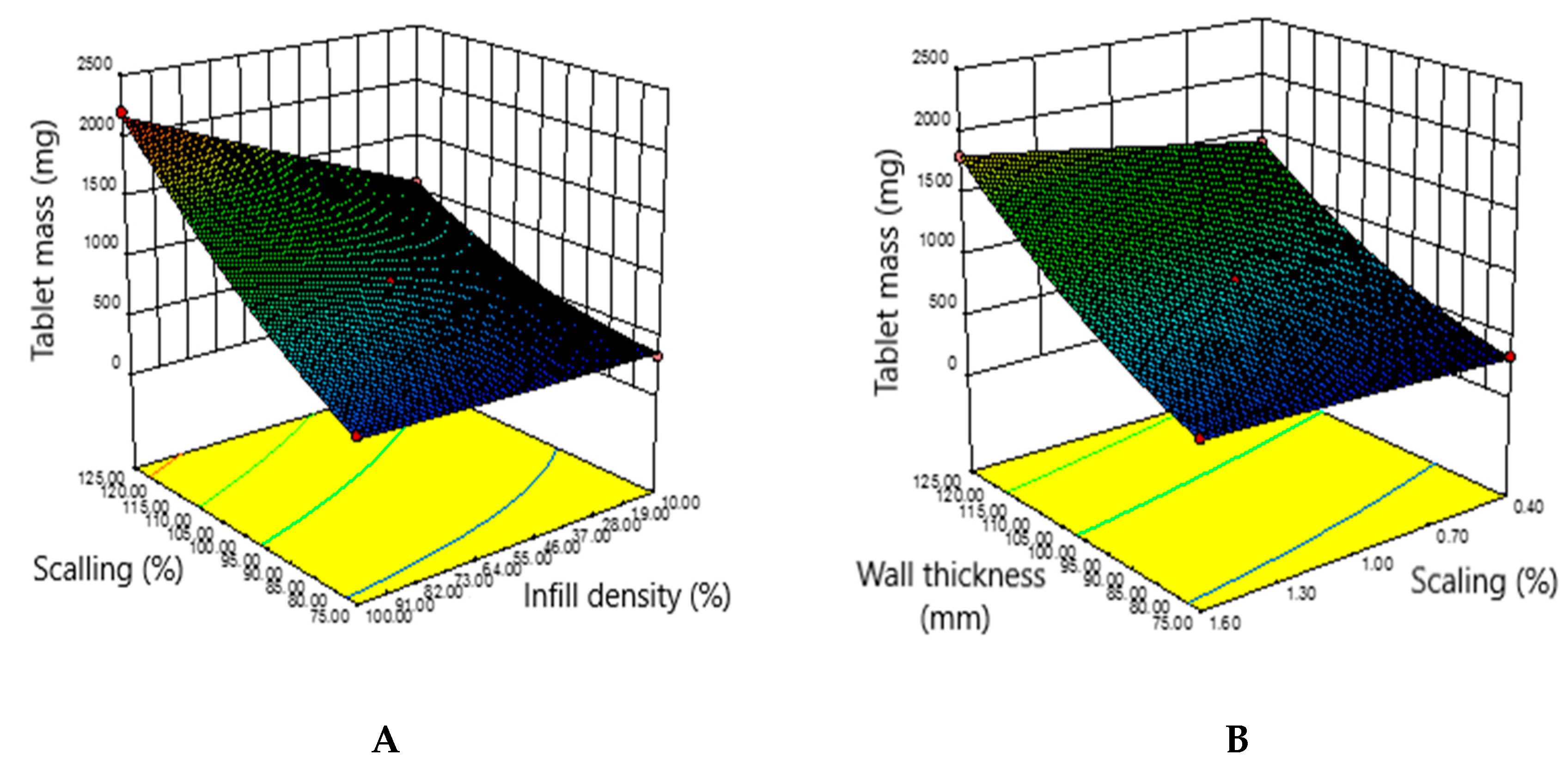
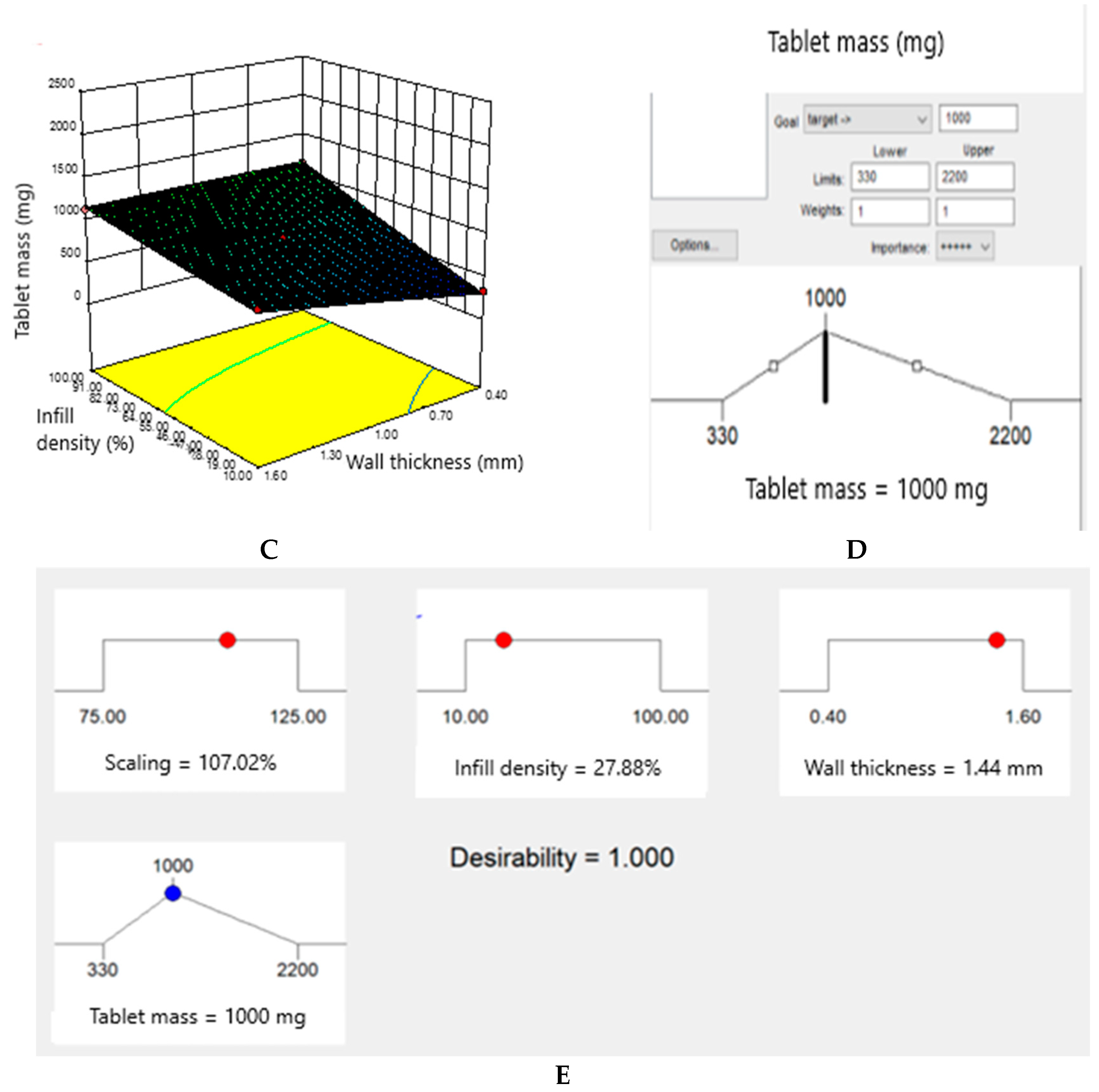
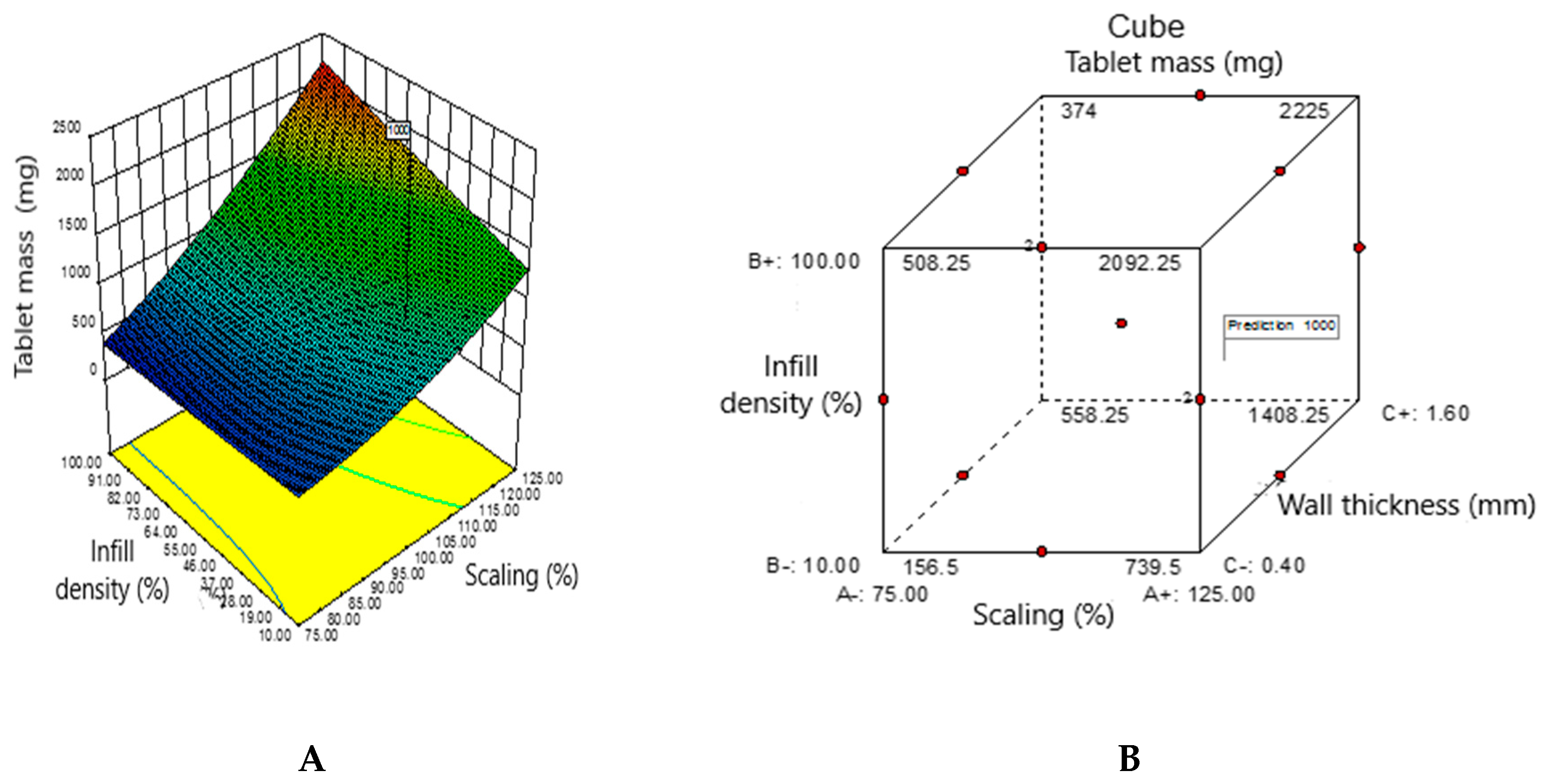
3.4. Obtaining a Predicted Drug Mass Value by Adapting the Object Sequencing Mode and Interpreting the Experimental Data using the Design Expert Statistical Software
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hemanth, K.G.; Hemamanjushree, S.; Abhinaya, N.; Pai, R.; Girish Pai, K. 3d Printing: A Review on Technology, Role in Novel Dosage Forms and Regulatory Perspective. Res. J. Pharm. Technol. 2021, 14, 562–572. [Google Scholar] [CrossRef]
- Singh, P.; Yadav, S.; Parvej, N.; Singh, G. 3D Printing Technology in Pharmaceutical Industry. Int. J. Sci. Res. Sci. Technol. 2022, 9, 592–604. [Google Scholar]
- Lakkala, P.; Munnangi, S.R.; Bandari, S.; Repka, M. Additive Manufacturing Technologies with Emphasis on Stereolithography 3D Printing in Pharmaceutical and Medical Applications: A Review. Int J Pharm X 2023, 5, 100159. [Google Scholar] [CrossRef] [PubMed]
- Aprecia Pharmaceuticals FDA Approves the First 3D Printed Drug Product. Aprecia Pharm. Co. 2015, 44.
- Chen, R.X.; Wang, Z.M.; Han, X.L.; Liu, Z.C.; Zheng, A.P. The Principle of Drop-on-Powder 3D Printing and Its Application and Challenge in Solid Preparation. Acta Pharm. Sin. 2020, 55, 2862–2868. [Google Scholar]
- In Seok, C.; Yong-Ha, K. A Study on GMP Application to 3D Printing for Medical Service Enhancement: Focusing on Related Cases and Regulations. J. Korea Serv. Manag. Soc. 2015, 16, 71–87. [Google Scholar] [CrossRef]
- Kulinowski, P.; Malczewski, P.; Łaszcz, M.; Baran, E.; Milanowski, B.; Kuprianowicz, M.; Dorożyński, P. Development of Composite, Reinforced, Highly Drug-Loaded Pharmaceutical Printlets Manufactured by Selective Laser Sintering—In Search of Relevant Excipients for Pharmaceutical 3D Printing. Materials 2022, 15, 2142. [Google Scholar] [CrossRef]
- Moroni, S.; Khorshid, S.; Aluigi, A.; Tiboni, M.; Casettari, L. Poly(3-Hydroxybutyrate): A Potential Biodegradable Excipient for Direct 3D Printing of Pharmaceuticals. Int J Pharm 2022, 623, 121960. [Google Scholar] [CrossRef]
- van den Heuvel, K.A.; de Wit, M.T.W.; Dickhoff, B.H.J. Evaluation of Lactose Based 3D Powder Bed Printed Pharmaceutical Drug Product Tablets. Powder Technol 2021, 390, 97–102. [Google Scholar] [CrossRef]
- Sadia, M.; Sośnicka, A.; Arafat, B.; Isreb, A.; Ahmed, W.; Kelarakis, A.; Alhnan, M.A. Adaptation of Pharmaceutical Excipients to FDM 3D Printing for the Fabrication of Patient-Tailored Immediate Release Tablets. Int J Pharm 2016, 513, 659–668. [Google Scholar] [CrossRef]
- Shuaib, M.; Haleem, A.; Kumar, S.; Javaid, M. Impact of 3D Printing on the Environment: A Literature-Based Study. Sustain. Oper. Comput. 2021, 2, 57–63. [Google Scholar] [CrossRef]
- Trenfield, S.; Basit, A.W. Personalising Drug Products Using 3D Printing. ONdrugDelivery 2019, 2019, https://www.ondrugdelivery.com/wp–content/uploads/2019/08/FabRxpdf. [Google Scholar]
- Basit, A. Recent Innovations in 3D-Printed Personalized Medicines: An Interview with Abdul Basit. J 3d Print Med 2020, 4, 5–7. [Google Scholar] [CrossRef]
- Available online:. Available online: https://www.labiotech.eu/best-biotech/five-companies-personalizing-treatments-with-3d-printed-drugs/ (accessed on 25 May 2023).
- Chamberlain, R.; Windolf, H.; Geissler, S.; Quodbach, J.; Breitkreutz, J. Precise Dosing of Pramipexole for Low-Dosed Filament Production by Hot Melt Extrusion Applying Various Feeding Methods. Pharmaceutics 2022, 14, 216. [Google Scholar] [CrossRef]
- Varghese, R.; Salvi, S.; Sood, P.; Karsiya, J.; Kumar, D. Recent Advancements in Additive Manufacturing Techniques Employed in the Pharmaceutical Industry: A Bird's Eye View. Annals of 3D Printed Medicine 2022, 8, 100081. [Google Scholar] [CrossRef]
- Mukai, S.; Mukai, E.; Santos-Junior, J.A.; Shibli, J.A.; Faveri, M.; Giro, G. Assessment of the Reproducibility and Precision of Milling and 3D Printing Surgical Guides. BMC Oral Health 2021, 21, s12903. [Google Scholar] [CrossRef]
- Sahai, N.; Gogoi, M. Techniques and Software Used in 3D Printing for Nanomedicine Applications. In 3D Printing Technology in Nanomedicine; Ahmad, N., Gopinath, P., Dutta, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–41. [Google Scholar]
- Ultimaker Cura: Powerful, Easy-to-Use 3D Printing Software. Available online: https://ultimaker.com/software/ultimaker-cura/.
- Hong, Q.; Lin, L.; Li, Q.; Jiang, Z.; Fang, J.; Wang, B.; Liu, K.; Wu, Q.; Huang, C. A Direct Slicing Technique for the 3D Printing of Implicitly Represented Medical Models. Comput Biol Med 2021, 135, 104534. [Google Scholar] [CrossRef]
- Guan, Y.; Sun, X.; Jin, L.; Guo, X. li; Zhang, Z. min; Shui, G. yan; Ma, L. bo Development of 3D Printing Entity Slicing Software. China Foundry 2021, 18, s41230. [Google Scholar] [CrossRef]
- Spinelli, G.; Kotsilkova, R.; Ivanov, E.; Petrova-Doycheva, I.; Menseidov, D.; Georgiev, V.; Di Maio, R.; Silvestre, C. Effects of Filament Extrusion, 3D Printing and Hot-Pressing on Electrical and Tensile Properties of Poly(Lactic) Acid Composites Filled with Carbon Nanotubes and Graphene. Nanomaterials 2020, 10, 35. [Google Scholar] [CrossRef]
- Sarbu, I.; Fița, A.C.; Popovici, V.; Lupuliasa, D.; Mitu, M.A.; Birman, V.S.; Ozon, E.A. Innovative methods for the characterisation of a novel pharmaceutical adhesive for 3D printing drugs. Farmacia 2022, 70, 1140. [Google Scholar] [CrossRef]
- Kechagias, J.D.; Vidakis, N. Parametric Optimization of Material Extrusion 3D Printing Process: An Assessment of Box-Behnken vs. Full-Factorial Experimental Approach. Int. J. Adv. Manuf. Technol. 2022, 121, s00170. [Google Scholar] [CrossRef]
- European Pharmacopoeia (Ph. Eur.) 10th ed. Available online: https://www.Edqm.Eu/En/European-Pharmacopoeia-Ph-Eur-10th-Edition (accessed on 20 May 2023).
- Hoffmann, L.; Breitkreutz, J.; Quodbach, J. Fused Deposition Modeling (FDM) 3D Printing of the Thermo-Sensitive Peptidomimetic Drug Enalapril Maleate. Pharmaceutics 2022, 14, 2411. [Google Scholar] [CrossRef] [PubMed]
- Siemiński, P. Introduction to Fused Deposition Modeling. In Additive Manufacturing; Pou, J., Riveiro, A., Davim, J.P., Eds.; Elsevier Inc: Amsterdam, The Netherlands, 2021; pp. 217–275. [Google Scholar]
- Bruère, V.M.; Lion, A.; Holtmannspötter, J.; Johlitz, M. Under-Extrusion Challenges for Elastic Filaments: The Influence of Moisture on Additive Manufacturing. Prog. Addit. Manuf. 2022, 7, s40964. [Google Scholar] [CrossRef]
- Hoque, M.; Kabir, H.; Jony, M.H. Design and Construction of a Bowden Extruder for a FDM 3D Printer Uses 1.75 mm Filament. Int. J. Tech. Res. Sci. 2018, 3, 282–288. [Google Scholar] [CrossRef]
- Silva, L.B.; de Oliveira, R.O.; Barbosa, G.F.; Shiki, S.B.; Fu, K. Influence of the Single-Screw Extruder Nozzle Diameter on Pellet-Based Filaments for Additive Manufacturing. J. Braz. Soc. Mech. Sci. Eng. 2022, 44, s40430. [Google Scholar] [CrossRef]
- Do, Q.D.; Tran, H.Q.; Le, T.B.; Phung, L.X.; Nguyen, T.K. Thermal Analysis by Finite Element Model for Powder Screw Extruder for 3D Printing Method. In The AUN/SEED-Net Joint Regional Conference in Transportation, Energy, and Mechanical Manufacturing Engineering, Lecture Notes in Mechanical Engineering; Le, A.T., Pham, V.S., Le, M.Q., Pham, H.L., Eds.; Springer Nature Pte Ltd: Singapore, 2022. [Google Scholar]
- Darling, C.; Smith, D.A. Syringe Pump Extruder and Curing System for 3D Printing of Photopolymers. HardwareX 2021, 9, e00175. [Google Scholar] [CrossRef]
- Goyanes, A.; Kobayashi, M.; Martínez-Pacheco, R.; Gaisford, S.; Basit, A.W. Fused-Filament 3D Printing of Drug Products: Microstructure Analysis and Drug Release Characteristics of PVA-Based Caplets. Int J Pharm 2016, 514, 290–295. [Google Scholar] [CrossRef]
- Goyanes, A.; Wang, J.; Buanz, A.; Martínez-Pacheco, R.; Telford, R.; Gaisford, S.; Basit, A.W. 3D Printing of Medicines: Engineering Novel Oral Devices with Unique Design and Drug Release Characteristics. Mol Pharm 2015, 12, 4077–84. [Google Scholar] [CrossRef]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Influence of Geometry on the Drug Release Profiles of Stereolithographic (SLA) 3D-Printed Tablets. AAPS PharmSciTech 2018, 19, s12249. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




