Submitted:
31 May 2023
Posted:
02 June 2023
You are already at the latest version
Abstract
Keywords:
Introduction
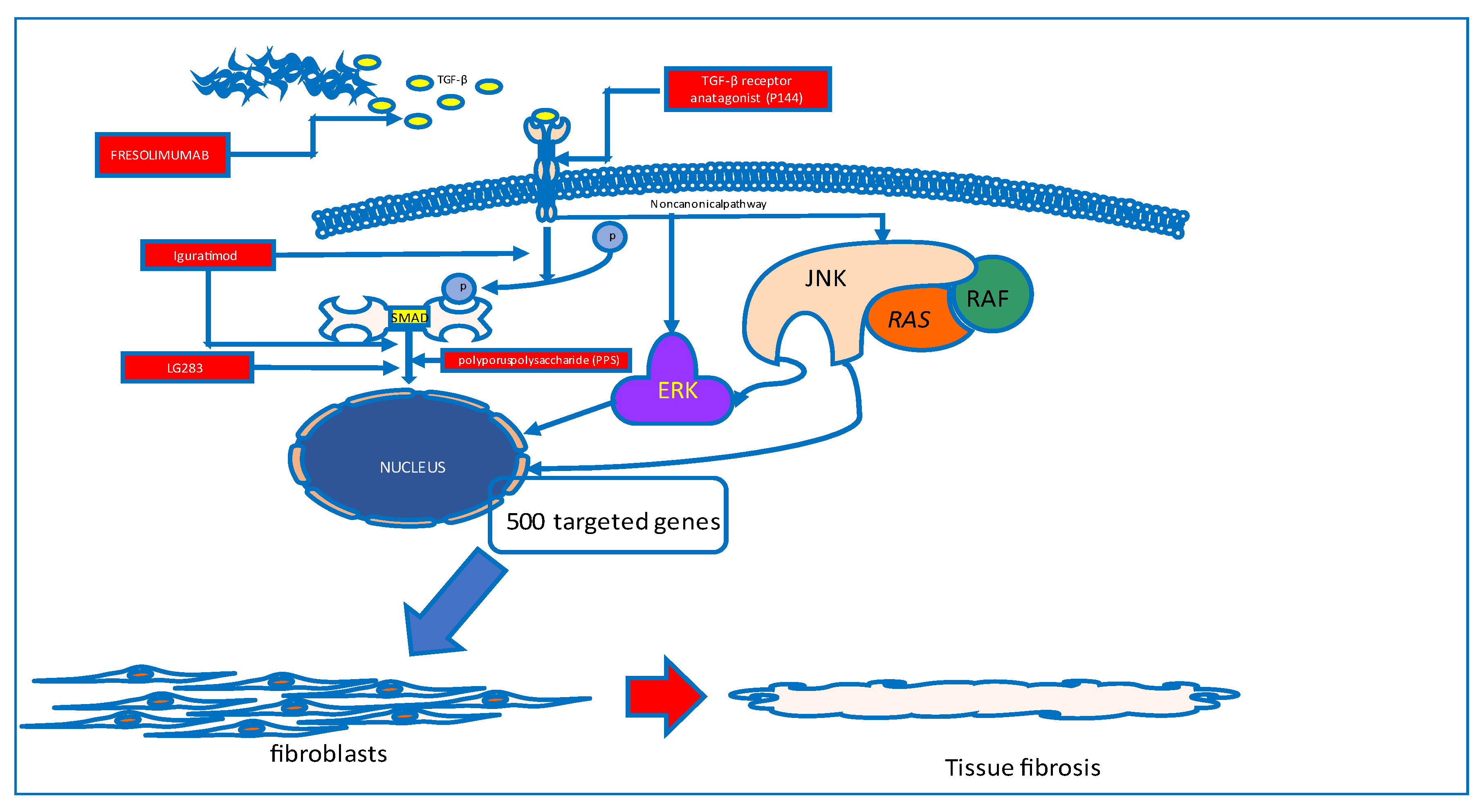
Skin and visceral organ fibrosis
Immunocompetent cells in systemic sclerosis
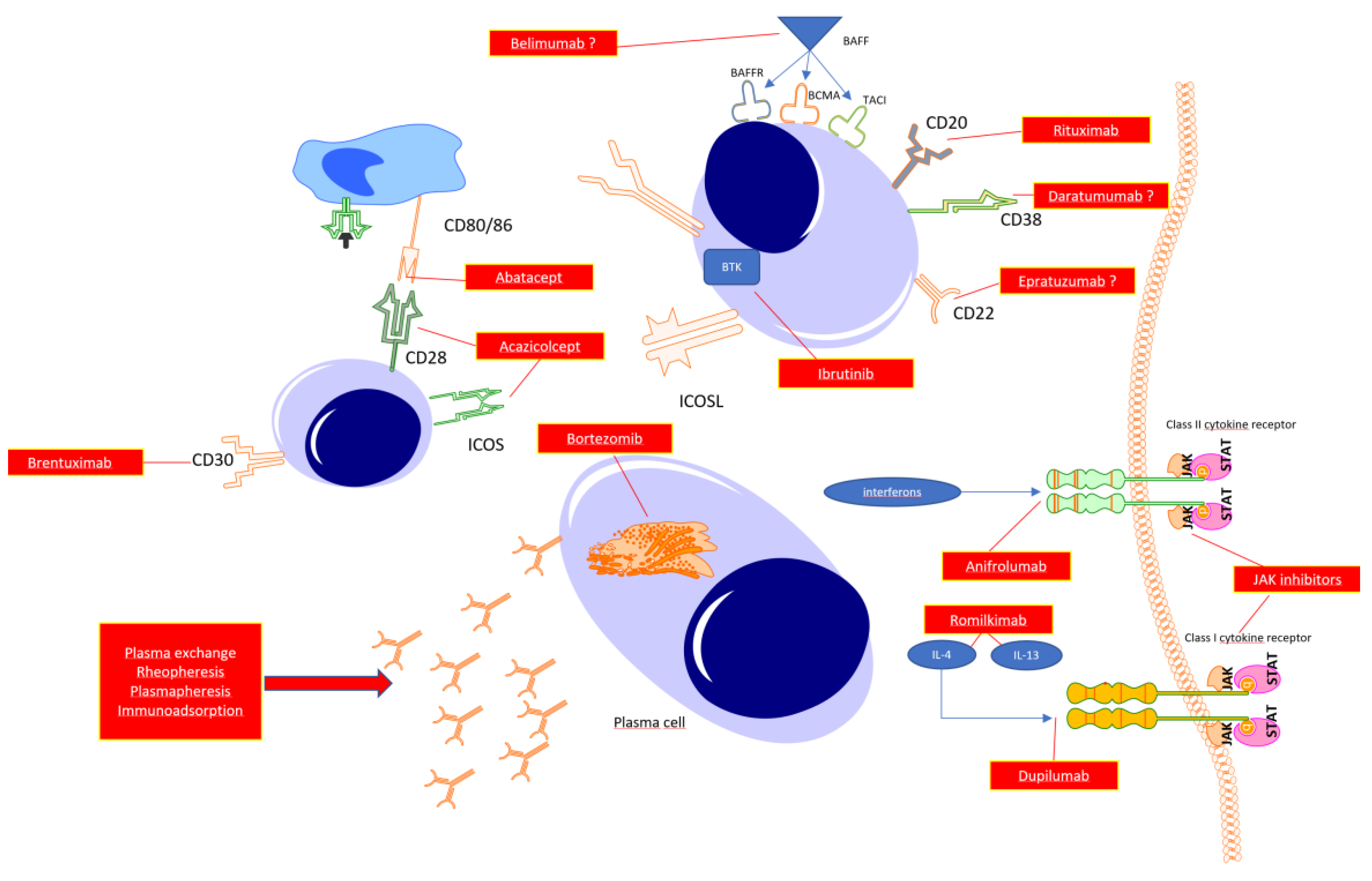
B cells in systemic sclerosis
T cells
Targeting the specific cytokines
Autoantibody -targeted therapy
Bruton’s kinase inhibitors
Targeting CD38 molecule
Mechanical removal of autoantibodies
Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosendahl, A.-H.; Schönborn, K.; Krieg, T. Pathophysiology of systemic sclerosis (scleroderma). The Kaohsiung journal of medical sciences 2022, 38, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Bergamasco, A.; Hartmann, N.; Wallace, L.; Verpillat, P. Epidemiology of systemic sclerosis and systemic sclerosis-associated interstitial lung disease. Clinical epidemiology 2019, 11, 257–273. [Google Scholar] [CrossRef] [PubMed]
- LeRoy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A., Jr.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. The Journal of rheumatology 1988, 15, 202–205. [Google Scholar] [PubMed]
- Mehra, S.; Walker, J.; Patterson, K.; Fritzler, M.J. Autoantibodies in systemic sclerosis. Autoimmunity reviews 2013, 12, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.G.; Fritzler, M.J. Update on autoantibodies in systemic sclerosis. Current opinion in rheumatology 2007, 19, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Bellando-Randone, S.; Matucci-Cerinic, M. Very early systemic sclerosis. Best Practice & Research Clinical Rheumatology 2019, 33, 101428. [Google Scholar]
- Kowal-Bielecka, O.; Fransen, J.; Avouac, J.; Becker, M.; Kulak, A.; Allanore, Y.; Distler, O.; Clements, P.; Cutolo, M.; Czirjak, L.; et al. Update of eular recommendations for the treatment of systemic sclerosis. Annals of the rheumatic diseases 2017, 76, 1327–1339. [Google Scholar] [CrossRef]
- Prud'homme, G.J. Pathobiology of transforming growth factor β in cancer, fibrosis and immunologic disease, and therapeutic considerations. Laboratory Investigation 2007, 87, 1077–1091. [Google Scholar] [CrossRef]
- Lomelí-Nieto, J.A.; Muñoz-Valle, J.F.; Baños-Hernández, C.J.; Navarro-Zarza, J.E.; Godínez-Rubí, J.M.; García-Arellano, S.; Ramírez-Dueñas, M.G.; Parra-Rojas, I.; Villanueva-Pérez, A.; Hernández-Bello, J. Transforming growth factor beta isoforms and tgf-βr1 and tgf-βr2 expression in systemic sclerosis patients. Clinical and experimental medicine 2022. [Google Scholar] [CrossRef]
- Grainger, D.J.; Mosedale, D.E.; Metcalfe, J.C. Tgf-β in blood: A complex problem. Cytokine & Growth Factor Reviews 2000, 11, 133–145. [Google Scholar]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. Tgf-β signaling in fibrosis. Growth factors 2011, 29, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Zavadil, J.; Böttinger, E.P. Tgf-β and epithelial-to-mesenchymal transitions. Oncogene 2005, 24, 5764–5774. [Google Scholar] [CrossRef] [PubMed]
- Santiago, B.; Gutierrez-Cañas, I.; Dotor, J.; Palao, G.; Lasarte, J.J.; Ruiz, J.; Prieto, J.; Borrás-Cuesta, F.; Pablos, J.L. Topical application of a peptide inhibitor of transforming growth factor-β1 ameliorates bleomycin-induced skin fibrosis. Journal of Investigative Dermatology 2005, 125, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, A.; Chino, T.; Kasamatsu, H.; Hasegawa, T.; Utsunomiya, N.; Luong, V.H.; Matsushita, T.; Sasaki, Y.; Ogura, D.; Niwa, S.-i.; et al. The compound lg283 inhibits bleomycin-induced skin fibrosis via antagonizing tgf-β signaling. Arthritis Res Ther 2022, 24, 94. [Google Scholar] [CrossRef]
- Mu, R.; Li, C.; Li, X.; Ke, Y.; Zhao, L.; Chen, L.; Wu, R.; Wu, Z.; Zuo, X.; Xie, Y.; et al. Effectiveness and safety of iguratimod treatment in patients with active rheumatoid arthritis in chinese: A nationwide, prospective real-world study. The Lancet regional health. Western Pacific 2021, 10, 100128. [Google Scholar] [CrossRef]
- Nozaki, Y. Iguratimod: Novel molecular insights and a new csdmard for rheumatoid arthritis, from japan to the world. Life (Basel, Switzerland) 2021, 11. [Google Scholar] [CrossRef]
- Xie, X.; Gan, H.; Tian, J.; Li, F.; Chen, J.; Wang, J.; Liao, J.; Li, S. Iguratimod inhibits skin fibrosis by regulating tgf-β1/smad signalling pathway in systemic sclerosis. European journal of clinical investigation 2022, 52, e13791. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, F.; Luo, A.; Lin, S.; Feng, X.; Yan, W.; Shi, Y.; Zhang, Q.; Gu, X.; Cui, G.; et al. Polyporus polysaccharide ameliorates bleomycin-induced pulmonary fibrosis by suppressing myofibroblast differentiation via tgf-β/smad2/3 pathway. Frontiers in pharmacology 2020, 11, 767. [Google Scholar] [CrossRef]
- Denton, C.P.; Merkel, P.A.; Furst, D.E.; Khanna, D.; Emery, P.; Hsu, V.M.; Silliman, N.; Streisand, J.; Powell, J.; Akesson, A.; et al. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: A multicenter, randomized, placebo-controlled phase i/ii trial of cat-192. Arthritis and rheumatism 2007, 56, 323–333. [Google Scholar] [CrossRef]
- Rice, L.M.; Padilla, C.M.; McLaughlin, S.R.; Mathes, A.; Ziemek, J.; Goummih, S.; Nakerakanti, S.; York, M.; Farina, G.; Whitfield, M.L.; et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest 2015, 125, 2795–2807. [Google Scholar] [CrossRef]
- Sanges, S.; Guerrier, T.; Launay, D.; Lefèvre, G.; Labalette, M.; Forestier, A.; Sobanski, V.; Corli, J.; Hauspie, C.; Jendoubi, M. Role of b cells in the pathogenesis of systemic sclerosis. La Revue de médecine interne 2017, 38, 113–124. [Google Scholar] [CrossRef]
- Steen, V.D. In Autoantibodies in systemic sclerosis, Seminars in arthritis and rheumatism, 2005; Elsevier: pp 35-42.
- Liem, S.I.E.; Neppelenbroek, S.; Fehres, C.M.; Wortel, C.; Toes, R.E.M.; Huizinga, T.W.J.; Scherer, H.U.; de Vries-Bouwstra, J.K. Autoreactive b cell responses targeting nuclear antigens in systemic sclerosis: Implications for disease pathogenesis. Seminars in arthritis and rheumatism 2023, 58, 152136. [Google Scholar] [CrossRef] [PubMed]
- Merino-Vico, A.; Frazzei, G.; van Hamburg, J.P.; Tas, S.W. Targeting b cells and plasma cells in autoimmune diseases: From established treatments to novel therapeutic approaches. European journal of immunology n/a.
- Boonstra, M.; Meijs, J.; Dorjée, A.L.; Marsan, N.A.; Schouffoer, A.; Ninaber, M.K.; Quint, K.D.; Bonte-Mineur, F.; Huizinga, T.W.; Scherer, H.U. Rituximab in early systemic sclerosis. RMD open 2017, 3, e000384. [Google Scholar] [CrossRef] [PubMed]
- Sircar, G.; Goswami, R.P.; Sircar, D.; Ghosh, A.; Ghosh, P. Intravenous cyclophosphamide vs rituximab for the treatment of early diffuse scleroderma lung disease: Open label, randomized, controlled trial. Rheumatology 2018, 57, 2106–2113. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.P.; Ray, A.; Chatterjee, M.; Mukherjee, A.; Sircar, G.; Ghosh, P. Rituximab in the treatment of systemic sclerosis–related interstitial lung disease: A systematic review and meta-analysis. Rheumatology 2021, 60, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Gumkowska-Sroka, O.; Jagoda, K.; Owczarek, A.; Helbig, G.; Giemza-Stokłosa, J.; Kotyla, P.J. Cytometric characterization of main immunocompetent cells in patients with systemic sclerosis: Relationship with disease activity and type of immunosuppressive treatment. Journal of clinical medicine 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Stochmal, A.; Czuwara, J.; Trojanowska, M.; Rudnicka, L. Antinuclear antibodies in systemic sclerosis: An update. Clinical reviews in allergy & immunology 2020, 58, 40–51. [Google Scholar]
- Wynn, T.A. Fibrotic disease and the th1/th2 paradigm. Nature Reviews Immunology 2004, 4, 583–594. [Google Scholar] [CrossRef]
- Baraut, J.; Farge, D.; Jean-Louis, F.; Kesmandt, H.; Durant, C.; Verrecchia, F.; Michel, L. Cytokines in systemic sclerosis. Pathologie-biologie 2010, 60, 127–139. [Google Scholar] [CrossRef]
- Guo, R.H.; Cheng, H.; Zhang, X.Y.; Yu, Z.; Wang, G.H.; Hao, S.Y.; Gao, X.P.; Wen, H.Y. Changes in peripheral t-lymphocyte subsets and serum cytokines in patients with systemic sclerosis. Frontiers in pharmacology 2022, 13, 986199. [Google Scholar] [CrossRef]
- Orvain, C.; Cauvet, A.; Prudent, A.; Guignabert, C.; Thuillet, R.; Ottaviani, M.; Tu, L.; Duhalde, F.; Nicco, C.; Batteux, F.; et al. Acazicolcept (alpn-101), a dual icos/cd28 antagonist, demonstrates efficacy in systemic sclerosis preclinical mouse models. Arthritis Res Ther 2022, 24, 13. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.; Simms, P.; Slivnick, D.; Jäck, H.; Fisher, R. Cd30 is a signal-transducing molecule that defines a subset of human activated cd45ro+ t cells. The Journal of Immunology 1993, 151, 2380–2389. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, B.; Reddish, M.; Longenecker, B.M. Cd30 expression on human cd8+ t cells isolated from peripheral blood lymphocytes of normal donors. Journal of immunology (Baltimore, Md. : 1950) 1996, 157, 3229–3234. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, G.; De Carli, M.; D'Elios, M.M.; Daniel, K.C.; Almerigogna, F.; Alderson, M.; Smith, C.A.; Thomas, E.; Romagnani, S. Cd30-mediated signaling promotes the development of human t helper type 2-like t cells. J Exp Med 1995, 182, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Ihn, H.; Yazawa, N.; Kubo, M.; Yamane, K.; Sato, S.; Fujimoto, M.; Kikuchi, K.; Soma, Y.; Tamaki, K. Circulating levels of soluble cd30 are increased in patients with localized scleroderma and correlated with serological and clinical features of the disease. The Journal of rheumatology 2000, 27, 698–702. [Google Scholar] [PubMed]
- Nagy, Z.; Czirják, L. Increased levels of amino terminal propeptide of type iii procollagen are an unfavourable predictor of survival in systemic sclerosis. Clinical and experimental rheumatology 2005, 23, 165–172. [Google Scholar] [PubMed]
- Amedei, A.; Pimpinelli, N.; Grassi, A.; Bella, C.D.; Niccolai, E.; Brancati, S.; Benagiano, M.; D'Elios, S.; Bosi, A.; D'Elios, M.M. Skin cd30(+) t cells and circulating levels of soluble cd30 are increased in patients with graft versus host disease. Auto- immunity highlights 2014, 5, 21–26. [Google Scholar] [CrossRef]
- Giacomelli, R.; Cipriani, P.; Lattanzio, R.; Di Franco, M.; Locanto, M.; Parzanese, I.; Passacantando, A.; Ciocci, A.; Tonietti, G. Circulating levels of soluble cd30 are increased in patients with systemic sclerosis (ssc) and correlate with serological and clinical features of the disease. Clinical and experimental immunology 1997, 108, 42–46. [Google Scholar] [CrossRef]
- Higashioka, K.; Kikushige, Y.; Ayano, M.; Kimoto, Y.; Mitoma, H.; Kikukawa, M.; Akahoshi, M.; Arinobu, Y.; Horiuchi, T.; Akashi, K.; et al. Generation of a novel cd30(+) b cell subset producing gm-csf and its possible link to the pathogenesis of systemic sclerosis. Clinical and experimental immunology 2020, 201, 233–243. [Google Scholar] [CrossRef]
- Mavalia, C.; Scaletti, C.; Romagnani, P.; Carossino, A.M.; Pignone, A.; Emmi, L.; Pupilli, C.; Pizzolo, G.; Maggi, E.; Romagnani, S. Type 2 helper t-cell predominance and high cd30 expression in systemic sclerosis. The American journal of pathology 1997, 151, 1751–1758. [Google Scholar]
- Walker, L.S.K.; Sansom, D.M. The emerging role of ctla4 as a cell-extrinsic regulator of t cell responses. Nature Reviews Immunology 2011, 11, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Linsley, P.S.; Greene, J.L.; Brady, W.; Bajorath, J.; Ledbetter, J.A.; Peach, R. Human b7-1 (cd80) and b7-2 (cd86) bind with similar avidities but distinct kinetics to cd28 and ctla-4 receptors. Immunity 1994, 1, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Lingel, H.; Brunner-Weinzierl, M.C. Ctla-4 (cd152): A versatile receptor for immune-based therapy. Seminars in Immunology 2019, 42, 101298. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.C.; Becker, J.-C.; Schiff, M.; Luggen, M.; Sherrer, Y.; Kremer, J.; Birbara, C.; Box, J.; Natarajan, K.; Nuamah, I. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. New England Journal of Medicine 2005, 353, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Connolly, S.E.; Jabado, O.; Ye, J.; Kelly, S.; Maldonado, M.A.; Westhovens, R.; Nash, P.; Merrill, J.T.; Townsend, R.M. Identification of biomarkers of response to abatacept in patients with sle using deconvolution of whole blood transcriptomic data from a phase iib clinical trial. Lupus Sci Med 2017, 4, e000206. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, T.; Hashimoto, S.; Sato, R.; Fujita, M.; Aizawa, T.; Tsugawa, K.; Tanaka, H. Abatacept as an alternative therapy for the treatment of drug-intolerant lupus nephritis: A case of underlying monosomy 1p36 deletion syndrome. Clinical nephrology 2022, 97, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Zhu, M.; Lin, Y.; Zhong, H.; Cai, L.; He, L.; Chou, K.C. The preliminary efficacy evaluation of the ctla-4-ig treatment against lupus nephritis through in-silico analyses. Journal of theoretical biology 2019, 471, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, M.; Bird, P.; Pachai, C.; Du, S.; Wu, C.; Landis, J.; Fuerst, T.; Ahmad, H.A.; Connolly, S.E.; Conaghan, P.G. Implementation of the omeract psoriatic arthritis magnetic resonance imaging scoring system in a randomized phase iib study of abatacept in psoriatic arthritis. Rheumatology (Oxford, England) 2022, 61, 4305–4313. [Google Scholar] [CrossRef]
- Tang, Q.; Ramsköld, D.; Krystufkova, O.; Mann, H.F.; Wick, C.; Dastmalchi, M.; Lakshmikanth, T.; Chen, Y.; Mikes, J.; Alexanderson, H.; et al. Effect of ctla4-ig (abatacept) treatment on t cells and b cells in peripheral blood of patients with polymyositis and dermatomyositis. Scandinavian journal of immunology 2019, 89, e12732. [Google Scholar] [CrossRef]
- Tjärnlund, A.; Tang, Q.; Wick, C.; Dastmalchi, M.; Mann, H.; Tomasová Studýnková, J.; Chura, R.; Gullick, N.J.; Salerno, R.; Rönnelid, J.; et al. Abatacept in the treatment of adult dermatomyositis and polymyositis: A randomised, phase iib treatment delayed-start trial. Annals of the rheumatic diseases 2018, 77, 55–62. [Google Scholar] [CrossRef]
- Toussirot, E.; Michaud, M.; Wendling, D.; Devauchelle, V. Abatacept as adjunctive therapy in refractory polymyalgia rheumatica. The Journal of rheumatology 2021, 48, 1888–1889. [Google Scholar] [CrossRef]
- Jin, W.; Zheng, Y.; Zhu, P. T cell abnormalities in systemic sclerosis. Autoimmunity reviews 2022, 21, 103185. [Google Scholar] [CrossRef] [PubMed]
- Elhai, M.; Meunier, M.; Matucci-Cerinic, M.; Maurer, B.; Riemekasten, G.; Leturcq, T.; Pellerito, R.; Von Mühlen, C.A.; Vacca, A.; Airo, P. Outcomes of patients with systemic sclerosis-associated polyarthritis and myopathy treated with tocilizumab or abatacept: A eustar observational study. Annals of the rheumatic diseases 2013, 72, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Spino, C.; Johnson, S.; Chung, L.; Whitfield, M.L.; Denton, C.P.; Berrocal, V.; Franks, J.; Mehta, B.; Molitor, J. Abatacept in early diffuse cutaneous systemic sclerosis: Results of a phase ii investigator-initiated, multicenter, double-blind, randomized, placebo-controlled trial. Arthritis & rheumatology 2020, 72, 125–136. [Google Scholar]
- Chung, L.; Spino, C.; McLain, R.; Johnson, S.R.; Denton, C.P.; Molitor, J.A.; Steen, V.D.; Lafyatis, R.; Simms, R.W.; Kafaja, S.; et al. Safety and efficacy of abatacept in early diffuse cutaneous systemic sclerosis (asset): Open-label extension of a phase 2, double-blind randomised trial. The Lancet. Rheumatology 2020, 2, e743–e753. [Google Scholar] [CrossRef] [PubMed]
- Katsumoto, T.R.; Whitfield, M.L.; Connolly, M.K. The pathogenesis of systemic sclerosis. Annual review of pathology 2011, 6, 509–537. [Google Scholar] [CrossRef] [PubMed]
- Kurzinski, K.; Torok, K.S. Cytokine profiles in localized scleroderma and relationship to clinical features. Cytokine 2011, 55, 157–164. [Google Scholar] [CrossRef]
- Boin, F.; De Fanis, U.; Bartlett, S.J.; Wigley, F.M.; Rosen, A.; Casolaro, V. T cell polarization identifies distinct clinical phenotypes in scleroderma lung disease. Arthritis & Rheumatism 2008, 58, 1165–1174. [Google Scholar]
- Skaug, B.; Assassi, S. Type i interferon dysregulation in systemic sclerosis. Cytokine 2020, 132, 154635. [Google Scholar] [CrossRef]
- Brkic, Z.; van Bon, L.; Cossu, M.; van Helden-Meeuwsen, C.G.; Vonk, M.C.; Knaapen, H.; van den Berg, W.; Dalm, V.A.; Van Daele, P.L.; Severino, A. The interferon type i signature is present in systemic sclerosis before overt fibrosis and might contribute to its pathogenesis through high baff gene expression and high collagen synthesis. Annals of the rheumatic diseases 2016, 75, 1567–1573. [Google Scholar] [CrossRef]
- Gillery, P.; Fertin, C.; Nicolas, J.F.; Chastang, F.; Kalis, B.; Banchereau, J.; Maquart, F.X. Interleukin-4 stimulates collagen gene expression in human fibroblast monolayer cultures. Potential role in fibrosis. FEBS letters 1992, 302, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Postlethwaite, A.E.; Holness, M.A.; Katai, H.; Raghow, R. Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J Clin Invest 1992, 90, 1479–1485. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujimoto, M.; Kikuchi, K.; Takehara, K. Elevated serum levels of interleukin 4 (il-4), il-10, and il-13 in patients with systemic sclerosis. The Journal of rheumatology 1997, 24, 328–332. [Google Scholar] [PubMed]
- Makhluf, H.A.; Stepniakowska, J.; Hoffman, S.; Smith, E.; LeRoy, E.C.; Trojanowska, M. Il-4 upregulates tenascin synthesis in scleroderma and healthy skin fibroblasts. Journal of investigative dermatology 1996, 107, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Chomarat, P.; Banchereau, J. Interleukin-4 and lnterleukin-13: Their similarities and discrepancies. International reviews of immunology 1998, 17, 1–52. [Google Scholar] [CrossRef]
- McCormick, S.M.; Heller, N.M. Commentary: Il-4 and il-13 receptors and signaling. Cytokine 2015, 75, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Chiaramonte, M.G.; Mentink-Kane, M.; Jacobson, B.A.; Cheever, A.W.; Whitters, M.J.; Goad, M.E.; Wong, A.; Collins, M.; Donaldson, D.D.; Grusby, M.J. Regulation and function of the interleukin 13 receptor α 2 during a t helper cell type 2–dominant immune response. The Journal of experimental medicine 2003, 197, 687–701. [Google Scholar] [CrossRef]
- Nguyen, J.K.; Austin, E.; Huang, A.; Mamalis, A.; Jagdeo, J. The il-4/il-13 axis in skin fibrosis and scarring: Mechanistic concepts and therapeutic targets. Archives of dermatological research 2020, 312, 81–92. [Google Scholar] [CrossRef]
- O'Reilly, S. Role of interleukin-13 in fibrosis, particularly systemic sclerosis. Biofactors 2013, 39, 593–596. [Google Scholar] [CrossRef]
- Wohlfahrt, T.; Usherenko, S.; Englbrecht, M.; Dees, C.; Weber, S.; Beyer, C.; Gelse, K.; Distler, O.; Schett, G.; Distler, J.H.; et al. Type 2 innate lymphoid cell counts are increased in patients with systemic sclerosis and correlate with the extent of fibrosis. Annals of the rheumatic diseases 2016, 75, 623–626. [Google Scholar] [CrossRef]
- Fuschiotti, P.; Medsger Jr, T.A.; Morel, P.A. Effector cd8+ t cells in systemic sclerosis patients produce abnormally high levels of interleukin-13 associated with increased skin fibrosis. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology 2009, 60, 1119–1128. [Google Scholar]
- Kotyla, P.; Gumkowska-Sroka, O.; Wnuk, B.; Kotyla, K. Jak inhibitors for treatment of autoimmune diseases: Lessons from systemic sclerosis and systemic lupus erythematosus. Pharmaceuticals (Basel, Switzerland) 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; Wang, Y.J.; Yan, J.W.; Wan, Y.N.; Chen, B.; Li, B.Z.; Yang, G.J.; Wang, J. Role of anti-inflammatory cytokines il-4 and il-13 in systemic sclerosis. Inflammation research : official journal of the European Histamine Research Society... [et al.] 2015, 64, 151–159. [Google Scholar] [CrossRef]
- Gasparini, G.; Cozzani, E.; Parodi, A. Interleukin-4 and interleukin-13 as possible therapeutic targets in systemic sclerosis. Cytokine 2020, 125, 154799. [Google Scholar] [CrossRef] [PubMed]
- Allanore, Y.; Wung, P.; Soubrane, C.; Esperet, C.; Marrache, F.; Bejuit, R.; Lahmar, A.; Khanna, D.; Denton, C.P. A randomised, double-blind, placebo-controlled, 24-week, phase ii, proof-of-concept study of romilkimab (sar156597) in early diffuse cutaneous systemic sclerosis. Annals of the rheumatic diseases 2020, 79, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Brusselle, G.; Quirce, S.; Papi, A.; Kuna, P.; Chipps, B.E.; Hanania, N.A.; Blaiss, M.; Msihid, J.; Jacob-Nara, J.A.; Deniz, Y.; et al. Dupilumab efficacy in patients with uncontrolled or oral corticosteroid-dependent allergic and non-allergic asthma. The journal of allergy and clinical immunology. In practice, 2022. [Google Scholar]
- Kamphuis, E.; Boesjes, C.M.; Loman, L.; Bakker, D.S.; Poelhekken, M.; Zuithoff, N.P.A.; Kamsteeg, M.; Romeijn, G.L.E.; van Wijk, F.; de Bruin-Weller, M.S.; et al. Dupilumab in daily practice for the treatment of pediatric atopic dermatitis: 28-week clinical and biomarker results from the bioday registry. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology 2022, 33, e13887. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The jak/stat signaling pathway: From bench to clinic. Signal transduction and targeted therapy 2021, 6, 402. [Google Scholar] [CrossRef]
- Agashe, R.P.; Lippman, S.M.; Kurzrock, R. Jak: Not just another kinase. Mol Cancer Ther 2022, 21, 1757–1764. [Google Scholar] [CrossRef]
- Waickman, A.T.; Park, J.-Y.; Park, J.-H. The common γ-chain cytokine receptor: Tricks-and-treats for t cells. Cellular and molecular life sciences 2016, 73, 253–269. [Google Scholar] [CrossRef]
- Pant, H.; Hercus, T.R.; Tumes, D.J.; Yip, K.H.; Parker, M.W.; Owczarek, C.M.; Lopez, A.F.; Huston, D.P. Translating the biology of β common receptor-engaging cytokines into clinical medicine. The Journal of allergy and clinical immunology 2022. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 family cytokines. Cold Spring Harbor perspectives in biology 2018, 10, a028415. [Google Scholar] [CrossRef] [PubMed]
- Vignali, D.A.; Kuchroo, V.K. Il-12 family cytokines: Immunological playmakers. Nat Immunol 2012, 13, 722–728. [Google Scholar] [CrossRef]
- Ni, S.; Shan, F.; Geng, J. Interleukin-10 family members: Biology and role in the bone and joint diseases. International immunopharmacology 2022, 108, 108881. [Google Scholar] [CrossRef] [PubMed]
- Kotyla, P.J. Are janus kinase inhibitors superior over classic biologic agents in ra patients? BioMed research international 2018, 2018, 7492904. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, E.; Bonomi, F.; Peretti, S.; Orlandi, M.; Lepri, G.; Matucci Cerinic, M.; Bellando Randone, S.; Guiducci, S. Potential role of jak inhibitors in the treatment of systemic sclerosis-associated interstitial lung disease: A narrative review from pathogenesis to real-life data. Life (Basel, Switzerland) 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.H.; Chung, W.H.; Wu, P.C.; Chen, C.B. Jak-stat signaling pathway in the pathogenesis of atopic dermatitis: An updated review. Frontiers in immunology 2022, 13, 1068260. [Google Scholar] [CrossRef]
- Hutton, J.; Mease, P.; Jadon, D. Horizon scan: State-of-the-art therapeutics for psoriatic arthritis. Best practice & research. Clinical rheumatology, 2022; 101809. [Google Scholar]
- Nash, P. Jak inhibitors: New indication and emerging safety data in 2022. Nature reviews. Rheumatology 2022. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, S.; Jinnin, M.; Inoue, K.; Yamane, K.; Honda, N.; Kajihara, I.; Makino, T.; Masuguchi, S.; Fukushima, S.; Ihn, H. Regulatory mechanisms of collagen expression by interleukin-22 signaling in scleroderma fibroblasts. Journal of dermatological science 2018, 90, 52–59. [Google Scholar] [CrossRef]
- Matsushita, T.; Hamaguchi, Y.; Hasegawa, M.; Takehara, K.; Fujimoto, M. Decreased levels of regulatory b cells in patients with systemic sclerosis: Association with autoantibody production and disease activity. Rheumatology 2016, 55, 263–267. [Google Scholar] [CrossRef]
- Mavropoulos, A.; Simopoulou, T.; Varna, A.; Liaskos, C.; Katsiari, C.G.; Bogdanos, D.P.; Sakkas, L.I. Breg cells are numerically decreased and functionally impaired in patients with systemic sclerosis. Arthritis & rheumatology 2016, 68, 494–504. [Google Scholar]
- Aydoğdu, E.; Pamuk, Ö.N.; Dönmez, S.; Pamuk, G.E. Decreased interleukin-20 level in patients with systemic sclerosis: Are they related with angiogenesis? Clinical rheumatology 2013, 32, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, V.; Assassi, S.; Allanore, Y.; Kuwana, M.; Denton, C.P.; Khanna, D.; Del Galdo, F. Type 1 interferon activation in systemic sclerosis: A biomarker, a target or the culprit. Current opinion in rheumatology 2022, 34, 357–364. [Google Scholar] [CrossRef]
- Wu, M.; Assassi, S. Dysregulation of type i interferon signaling in systemic sclerosis: A promising therapeutic target? Current Treatment Options in Rheumatology 2021, 7, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.; Geppert, T.; Schiopu, E.; Frech, T.; Hsu, V.; Simms, R.W.; Peng, S.L.; Yao, Y.; Elgeioushi, N.; Chang, L.; et al. Dose-escalation of human anti-interferon-α receptor monoclonal antibody medi-546 in subjects with systemic sclerosis: A phase 1, multicenter, open label study. Arthritis Res Ther 2014, 16, R57. [Google Scholar] [CrossRef]
- Guo, X.; Higgs, B.W.; Bay-Jensen, A.C.; Karsdal, M.A.; Yao, Y.; Roskos, L.K.; White, W.I. Suppression of t cell activation and collagen accumulation by an anti-ifnar1 mab, anifrolumab, in adult patients with systemic sclerosis. Journal of Investigative Dermatology 2015, 135, 2402–2409. [Google Scholar] [CrossRef]
- Moriana, C.; Moulinet, T.; Jaussaud, R.; Decker, P. Jak inhibitors and systemic sclerosis: A systematic review of the literature. Autoimmunity reviews 2022, 21, 103168. [Google Scholar] [CrossRef] [PubMed]
- Kranenburg, P.; van den Hombergh, W.M.; Knaapen-Hans, H.K.; van den Hoogen, F.H.; Fransen, J.; Vonk, M.C. Survival and organ involvement in patients with limited cutaneous systemic sclerosis and anti-topoisomerase-i antibodies: Determined by skin subtype or auto-antibody subtype? A long-term follow-up study. Rheumatology 2016, 55, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.I.; Howat, S.; Abraham, D.J.; Pearson, J.D.; Lawson, C. Agonistic anti-icam-1 antibodies in scleroderma: Activation of endothelial pro-inflammatory cascades. Vascular pharmacology 2013, 59, 19–26. [Google Scholar] [CrossRef]
- Tamby, M.C.; Humbert, M.; Guilpain, P.; Servettaz, A.; Dupin, N.; Christner, J.J.; Simonneau, G.; Fermanian, J.; Weill, B.; Guillevin, L. Antibodies to fibroblasts in idiopathic and scleroderma-associated pulmonary hypertension. European Respiratory Journal 2006, 28, 799–807. [Google Scholar] [CrossRef]
- Hysa, E.; Campitiello, R.; Sammorì, S.; Gotelli, E.; Cere, A.; Pesce, G.; Pizzorni, C.; Paolino, S.; Sulli, A.; Smith, V.; et al. Specific autoantibodies and microvascular damage progression assessed by nailfold videocapillaroscopy in systemic sclerosis: Are there peculiar associations? An update. Antibodies (Basel, Switzerland) 2023, 12. [Google Scholar]
- Jerjen, R.; Nikpour, M.; Krieg, T.; Denton, C.P.; Saracino, A.M. Systemic sclerosis in adults. Part i: Clinical features and pathogenesis. J Am Acad Dermatol 2022, 87, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Liem, S.I.E.; Neppelenbroek, S.; Fehres, C.M.; Wortel, C.; Toes, R.E.M.; Huizinga, T.W.J.; Scherer, H.U.; de Vries-Bouwstra, J.K. Autoreactive b cell responses targeting nuclear antigens in systemic sclerosis: Implications for disease pathogenesis. Seminars in arthritis and rheumatism 2023, 58, 152136. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Denton, C.P. Scleroderma autoantibodies in guiding monitoring and treatment decisions. Current opinion in rheumatology 2022, 34, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Nakayamada, S.; Tanaka, Y. Baff- and april-targeted therapy in systemic autoimmune diseases. Inflammation and Regeneration 2016, 36, 6. [Google Scholar] [CrossRef]
- Gordon, J.K.; Martyanov, V.; Franks, J.M.; Bernstein, E.J.; Szymonifka, J.; Magro, C.; Wildman, H.F.; Wood, T.A.; Whitfield, M.L.; Spiera, R.F. Belimumab for the treatment of early diffuse systemic sclerosis. Arthritis & Rheumatology 2018, 70, 308–316. [Google Scholar]
- Ly, S.; Nedosekin, D.; Wong, H.K. Review of an anti-cd20 monoclonal antibody for the treatment of autoimmune diseases of the skin. American journal of clinical dermatology 2023, 1–27. [Google Scholar] [CrossRef]
- Merino-Vico, A.; Frazzei, G.; van Hamburg, J.P.; Tas, S.W. Targeting b cells and plasma cells in autoimmune diseases: From established treatments to novel therapeutic approaches. European journal of immunology 2023, 53, e2149675. [Google Scholar] [CrossRef]
- van den Hoogen, L.L.; Bisoendial, R.J. B-cells and baff in primary antiphospholipid syndrome, targets for therapy? Journal of clinical medicine 2022, 12. [Google Scholar] [CrossRef]
- Zhen, C.; Hou, Y.; Zhao, B.; Ma, X.; Dai, T.; Yan, C. Efficacy and safety of rituximab treatment in patients with idiopathic inflammatory myopathies: A systematic review and meta-analysis. Frontiers in immunology 2022, 13, 1051609. [Google Scholar] [CrossRef]
- Alexander, T.; Cheng, Q.; Klotsche, J.; Khodadadi, L.; Waka, A.; Biesen, R.; Hoyer, B.F.; Burmester, G.R.; Radbruch, A.; Hiepe, F. Proteasome inhibition with bortezomib induces a therapeutically relevant depletion of plasma cells in sle but does not target their precursors. European journal of immunology 2018, 48, 1573–1579. [Google Scholar] [CrossRef]
- Adams, J. The proteasome: A suitable antineoplastic target. Nature reviews. Cancer 2004, 4, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Richardson, P.G.; Anderson, K.C. Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma. Molecular Cancer Therapeutics 2011, 10, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Fineschi, S.; Bongiovanni, M.; Donati, Y.; Djaafar, S.; Naso, F.; Goffin, L.; Barazzone Argiroffo, C.; Pache, J.-C.; Dayer, J.-M.; Ferrari-Lacraz, S. In vivo investigations on anti-fibrotic potential of proteasome inhibition in lung and skin fibrosis. American journal of respiratory cell and molecular biology 2008, 39, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Duan, W.; Cu, X.; Liang, C.; Xin, M. Bruton’s tyrosine kinase (btk) inhibitors in treating cancer: A patent review (2010-2018). Expert Opinion on Therapeutic Patents 2019, 29, 217–241. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.N. Regulation of b lymphocyte development and activation by bruton's tyrosine kinase. Immunologic research 2001, 23, 147–156. [Google Scholar] [CrossRef]
- Du, Y.; Lei, L.; Ding, H.; Chen, Y.; Pathak, S.; Hicks, J.; Tran, P.T.; Wu, M.; Chang, B.; Wirtz, U.; et al. Targeting multiple end organs in lupus and other systemic rheumatic diseases by inhibiting bruton's tyrosine kinase. Frontiers in immunology 2022, 13, 893899. [Google Scholar] [CrossRef] [PubMed]
- Einhaus, J.; Pecher, A.-C.; Asteriti, E.; Schmid, H.; Secker, K.-A.; Duerr-Stoerzer, S.; Keppeler, H.; Klein, R.; Schneidawind, C.; Henes, J.; et al. Inhibition of effector b cells by ibrutinib in systemic sclerosis. Arthritis Res Ther 2020, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Ringheim, G.E.; Wampole, M.; Oberoi, K. Bruton's tyrosine kinase (btk) inhibitors and autoimmune diseases: Making sense of btk inhibitor specificity profiles and recent clinical trial successes and failures. Frontiers in immunology 2021, 12, 662223. [Google Scholar] [CrossRef]
- Agarbati, S.; Benfaremo, D.; Viola, N.; Paolini, C.; Svegliati Baroni, S.; Funaro, A.; Moroncini, G.; Malavasi, F.; Gabrielli, A. Increased expression of the ectoenzyme cd38 in peripheral blood plasmablasts and plasma cells of patients with systemic sclerosis. Frontiers in immunology 2022, 13, 1072462. [Google Scholar] [CrossRef]
- Peclat, T.R.; Shi, B.; Varga, J.; Chini, E.N. The nadase enzyme cd38: An emerging pharmacological target for systemic sclerosis, systemic lupus erythematosus and rheumatoid arthritis. Current opinion in rheumatology 2020, 32, 488–496. [Google Scholar] [CrossRef]
- Benfaremo, D.; Gabrielli, A. Is there a future for anti-cd38 antibody therapy in systemic autoimmune diseases? Cells 2019, 9. [Google Scholar] [CrossRef]
- Cole, S.; Walsh, A.; Yin, X.; Wechalekar, M.D.; Smith, M.D.; Proudman, S.M.; Veale, D.J.; Fearon, U.; Pitzalis, C.; Humby, F.; et al. Integrative analysis reveals cd38 as a therapeutic target for plasma cell-rich pre-disease and established rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther 2018, 20, 85. [Google Scholar] [CrossRef] [PubMed]
- De Flora, A.; Guida, L.; Franco, L.; Zocchi, E. The cd38/cyclic adp-ribose system: A topological paradox. The international journal of biochemistry & cell biology 1997, 29, 1149–1166. [Google Scholar]
- Shi, B.; Wang, W.; Korman, B.; Kai, L.; Wang, Q.; Wei, J.; Bale, S.; Marangoni, R.G.; Bhattacharyya, S.; Miller, S.; et al. Targeting cd38-dependent nad(+) metabolism to mitigate multiple organ fibrosis. iScience 2021, 24, 101902. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.A.; Boslett, J.; Varadharaj, S.; De Pascali, F.; Hemann, C.; Druhan, L.J.; Ambrosio, G.; El-Mahdy, M.; Zweier, J.L. Depletion of nadp (h) due to cd38 activation triggers endothelial dysfunction in the postischemic heart. Proceedings of the National Academy of Sciences 2015, 112, 11648–11653. [Google Scholar] [CrossRef]
- Graßhoff, H.; Fourlakis, K.; Comdühr, S.; Riemekasten, G. Autoantibodies as biomarker and therapeutic target in systemic sclerosis. Biomedicines 2022, 10, 2150. [Google Scholar] [CrossRef]
- Tsou, P.-S.; Varga, J.; O’Reilly, S. Advances in epigenetics in systemic sclerosis: Molecular mechanisms and therapeutic potential. Nature Reviews Rheumatology 2021, 17, 596–607. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




