Submitted:
02 June 2023
Posted:
05 June 2023
You are already at the latest version
Abstract
Keywords:
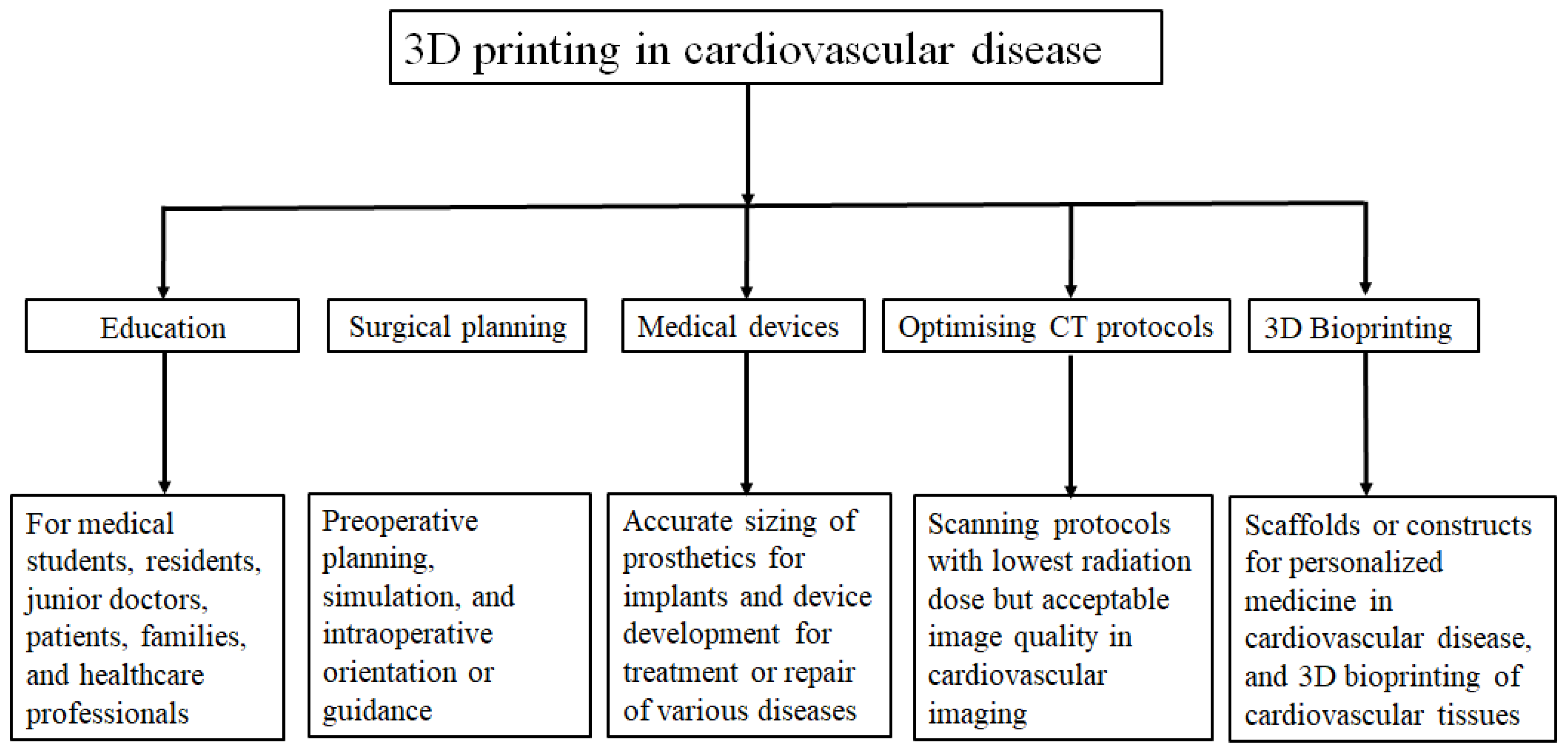
1. Introduction
2. 3D Bioprinting: Where Are We Now?
3. 3D Bioprinting-Bioinks
4. 3D Bioprinting Technologies
4.1. Inkjet-Based Bioprinting
4.2. Extrusion-Based Bioprinting
4.3. Light-Based Bioprinting
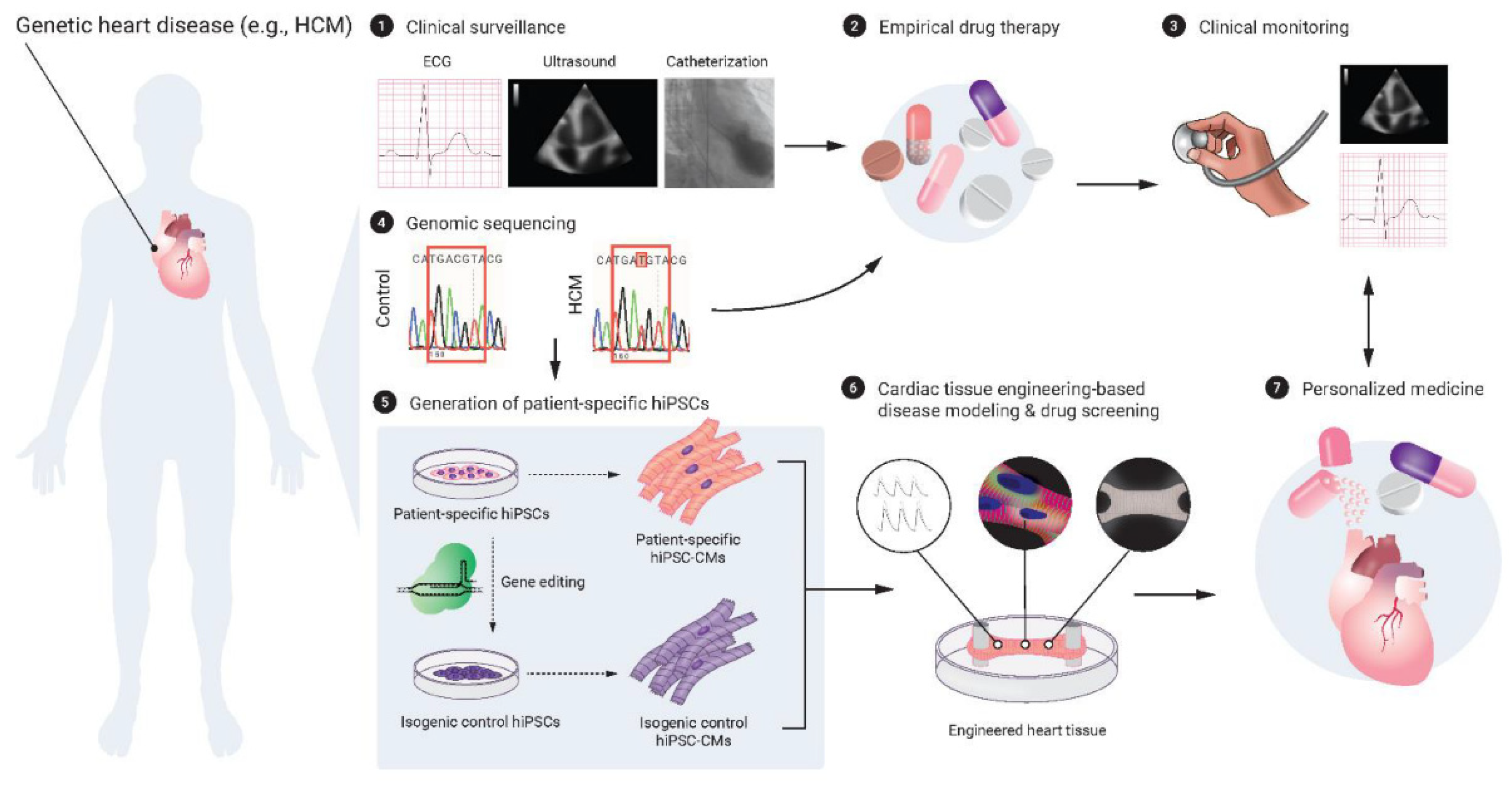
5. 3D Bioprinting Cardiac Tissues
5.1. Human Pluripotent Stem Cells and Cardiac Tissue Engineering
5.2. Cellular Maturity
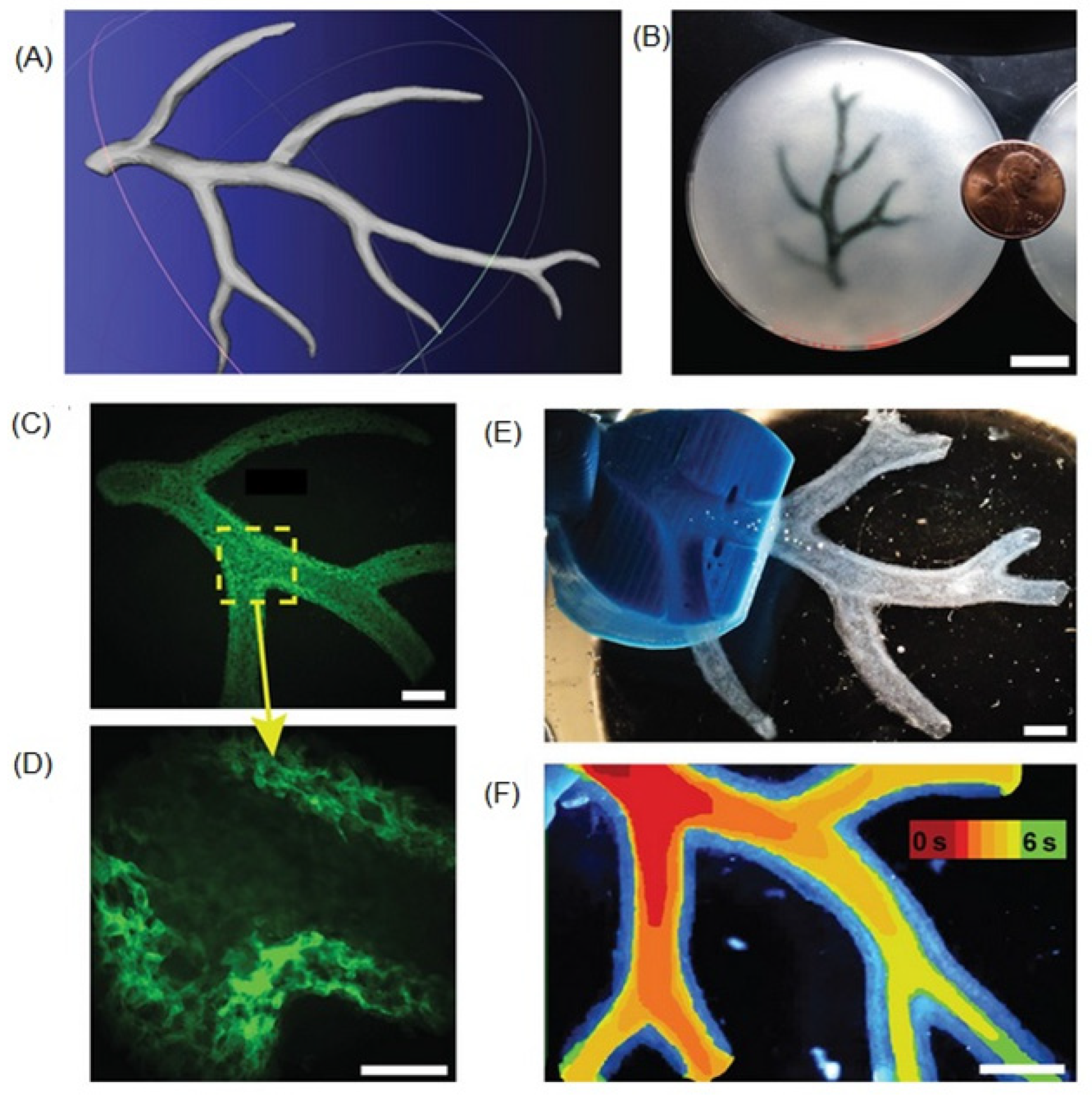
5.3. Microvasculature Constructs
5.4. Other Issues
6. 3D Bioprinting of Vascular Constructs and Grafts
6.1. Requirements of a TEVG
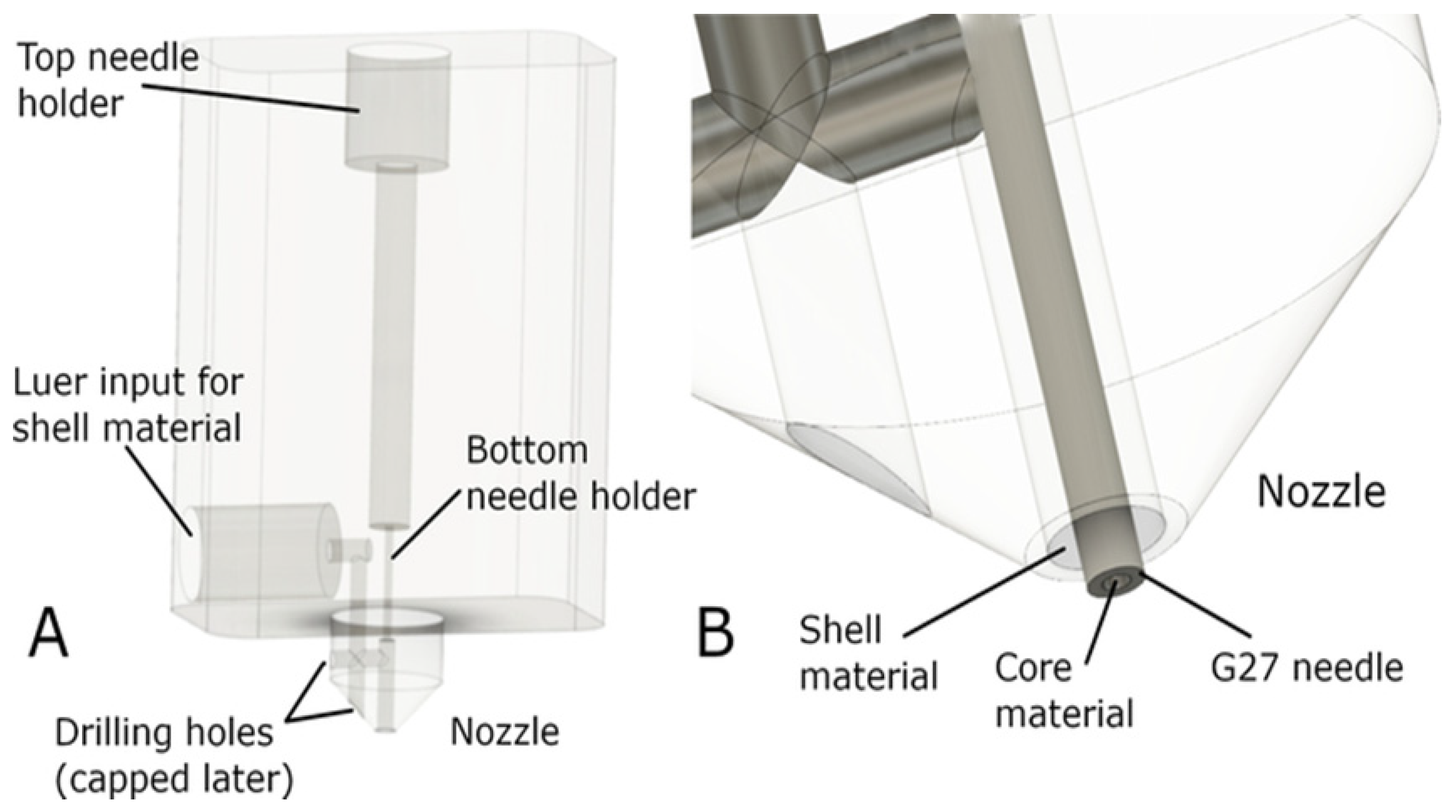
6.2. Methods of 3D Bioprinting of TEVGs
6.3. Summary
7. 3D Bioprinting of Heart Valves
7.1. Extrusion Based TEHVs
7.2. Light-Based TEHVs
7.3. Bioplotted TEHVs
8. 3D Bioprinting of Myocardium and Heart
9. Summary and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Virani, S.S.; Alvonso, A.; Aparicio, J.E.; Benjamin, J.E.; Bittencourt, M.; Callaway, C.; Clifton, W.; et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Al Moudi, M.; Cao, Y. CT angiography in the diagnosis of cardiovascular disease: a transformation in cardiovascular CT practice. Quant. Imaging. Med. Surg. 2014, 4, 376–396. [Google Scholar] [PubMed]
- Russo, V.; Lovato, L.; Ligabue, G. Cardiac MRI: technical basis. Radiol. Med. 2020, 125, 1040–1055. [Google Scholar] [CrossRef]
- Giannopoulos, A.A.; Steigner, M.L.; George, E.; Barile, M.; Hunsaker, A.R.; Rybicki, F.J.; Mitsouras, D. Cardiothoracic applications of 3-dimensional printing. J. Thorac. Imaging 2016, 31, 253–272. [Google Scholar] [CrossRef] [PubMed]
- Lau, I.; Sun, Z. Dimensional accuracy and clinical value of 3D printed models in congenital heart disease: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 1483. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Squelch, A.; Sun, Z. Quantitative assessment of 3D printed model accuracy in delineating congenital heart disease. Biomolecules 2021, 11, 270. [Google Scholar] [CrossRef]
- Valverde, I.; Gomez-Ciriza, G.; Hussain, T.; Suarez-Mejias, C.; Velasco-Forte, M.N.; Byrne, N.; Ordonex, A.; Gonzalez-Calle, A.; Anderson, D.; Hazekamp, M.G.; et al. Three dimensional printed models for surgical planning of complex congenital heart defects: An international multicenter study. Eur. J. Cardiothorac. Surg. 2017, 52, 1139–1148. [Google Scholar] [CrossRef]
- Sun, Z.; Wee, C. 3D printed models in cardiovascular disease: An exciting future to deliver personalized medicine. Micromachines 2022, 13, 1575. [Google Scholar] [CrossRef]
- Sun, Z. Clinical applications of patient-specific 3D printed models in cardiovascular disease: Current status and future directions. Biomolecules 2020, 10, 1577. [Google Scholar] [CrossRef]
- Sun, Z.; Wong, Y.H.; Yeong, C.H. Patient-specific 3D printed low-cost models in medical education and clinical practice. Micromachines 2023, 14, 464. [Google Scholar] [CrossRef]
- Anwar, S.; Singh, G.K.; Miller, J.; Sharma, M. .; Manning, P.; Billadello, J.J.; Eghtesady, P.; Woodard, P.K. 3D printing is a transformative technology in congenital heart disease. JACC. Basic. Transi. Sci. 2018, 3, 294–312. [Google Scholar] [CrossRef]
- Gallo, M.; D’Onofrio, A.; Tarantini, G.; Nocerino, E.; Remondino, F.; Gerosa, G. 3D-printing model for complex aortic transcatheter valve treatment. Int. J. Cardiol. 2016, 210, 139–140. [Google Scholar] [CrossRef]
- Ripley, B.; Kelil, T.; Cheezum, M.K.; Goncalves, A.; Di Carli, M.F.; Rybicki, F.J.; Steigner, M.; Mitsouras, D.; Blankstein, R. 3D printing based on cardiac CT assists anatomic visualization prior to transcatheter aortic valve replacement. J. Cardiovasc. Comput. Tomor. 2016, 10, 28–36. [Google Scholar] [CrossRef]
- Kiraly, L.; Shah, N.C.; Abdullah, O.; Al-Ketan, O.; Rowshan, R. Three-dimensional virtual and printed prototypes in complex congenital and pediatric cardiac surgery-A multidisciplinary team-learning experience. Biomolecules. 2021, 11, 1703. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Szary, J.; Luis. M.S.; Mikulski, S.; Patel, A.; Schulz, F.; Tretiakow, D.; Fercho, J.; Jaguszewska, K.; Frankiewicz. M.; Pawłowska, E.; Targoński, R.; Szarpak, Ł.; Dądela, K.; Sabiniewicz, R.; Kwiatkowska, J. The role of 3D printing in planning complex medical procedures and training of medical professionals-cross-sectional multispecialty review. Int. J. Environ. Res. Public. Health. 2022, 19, 3331. [Google Scholar] [PubMed]
- Yamasaki, T.; Toba, S.; Sanders, S.P.; Carreon, C.K. Perfusion-distension fixation of heart specimens: a key step in immortalizing heart specimens for wax infiltration and generating 3D imaging data sets for reconstruction and printed 3D models. Cardiovasc. Pathol. 2022, 58, 107404. [Google Scholar] [CrossRef]
- Ghosh, R.M.; Jolley, M.A.; Mascio, C.E.; Chen, J.M.; Fuller, S.; Rome, J.J.; Silvestro, E.; Whitehead, K.K. Clinical 3D modeling to guide pediatric cardiothoracic surgery and intervention using 3D printed anatomic models, computer aided design and virtual reality. 3D. Pring. Med. 2022, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.J.; Spray, T.; Austin, E.H.; Yun, T.J.; van Arsdell, G.S. Hands-on surgical training of congenital heart surgery suing 3-dimensional print models. J. Thorac. Cardiovasc. Surg. 2017, 153, 15301–15540. [Google Scholar] [CrossRef]
- Gomez-Ciriza, G.; Gomez-Cia, T.; Rivas-Gonzalez, J.A.; Velasco Forte, M.N.; Valverde, I. Affordable three-dimensional printed heart models. Front. Cardiovasc. Med. 2021, 8, 642011. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Dai, J.; Ren, C.; Li, J.; Lai, Y. Application of 3D printing in the surgical planning of hypertrophic obstructive cardiomyopathy and physician-patient communication: A preliminary study. J. Thorac. Dis. 2018, 10, 867–873. [Google Scholar] [CrossRef]
- Ryan, J.; Plasencia, J.; Richardson, R.; Velez, D.; Nigro, J.J.; Pophal, S.; Frakes, D. 3D printing for congenital heart disease: A single site’s initial three-year experience. 3D. Print. Med. 2018, 4, 10. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, S.; Fan, T.; Li, B.; Liang, W.; Dong, H. Three-dimensional printing enhances preparation for repair of double outlet right ventricular surgery. J. Card. Surg. 2018, 33, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yang, F.; Cheung, S.H.; Chan, A.K.Y.; Wang, D.D.; Lam, Y.Y.; Chow, M.C.K.; Leong, M.C.W.; Kam, K.K.; So, K.C.Y.; et al. Device sizing guided by echocardiography-based three-dimensional printing is associated with superior outcome after percutaneous left atrial appendage occlusion. J. Am. Soc. Echocardiogr. 2019, 32, 708–719. [Google Scholar] [CrossRef]
- Hell, M.H.; Achenbach, S.; Yoo, I.S.; Franke, J.; Blackutzik, F.; Roether, J.; Graf, V.; Raaz-Schrauder, D.; Marvann, M.; Schlundt, C. 3D printing for sizing left atrial appendage closure device: Head-to head comparison with computed tomography and transoesophageal echocardiography. EuroIntervention 2017, 13, 1234–1241. [Google Scholar] [CrossRef]
- Li, H.; Yao, Q.; Shen, B.; Shu, M.; Zhong, L.; Wang, X.; Song, Z. Application of 3D printing technology to left atrial appendage occlusion. Int. J. Cardiol. 2017, 231, 258–263. [Google Scholar] [CrossRef]
- Conti, M.; Marconi, S.; Muscogiuri, G.; Guglielmo, M.; Baggiano, A.; Italiano, G.; Mancini, M.E.; Auricchio, F.; Andreini, D.; Rabbat, M.G.; et al. Left atrial appendage closure guided by 3D computed tomography printing technology: A case control study. J. Cardiovasc. Comput. Tomogr. 2019, 13, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Goitein, O.; Fink, N.; Guettar, V.; Beinart, R.; Brodov, Y.; Konen, E.; Goitein, D.; Segni, E.D.; Grupper, A.; Glikson, M. Printed MDCT 3D models for prediction of left atrial appendage (LAA) occlude device size: A feasibility study. EuroIntervention 2017, 13, e1076–e1079. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.O.; De Luccia, N. A simulator for training in endovascular aneurysm repair: The use of three dimensional printers. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 247–253. [Google Scholar] [CrossRef]
- Karkkainen, J.M.; Sandri, G.; Tenorio, E.R.; Alexander, A.; Bjellum, K.; Matsumoto, J.; Morris, J.; Mendes, B.C.; DeMartino, R.R.; Oderich, G.S. Simulation of endovascular aortic repair using 3D printed abdominal aortic aneurysm model and fluid pump. Cardiovasc. Intervent. Radiol. 2019, 42, 1627–1634. [Google Scholar] [CrossRef]
- Kaufmann, R.; Zech, C.J.; Takes, M.; Brantner, P.; Thieringer, F.; Dentschmann, M.; Hergan, K.; Scharinger, B.; Hecht, S.; Rezar, R.; et al. Vascular 3D printing with a novel biological tissue mimicking resin for patient-specific procedure simulations in interventional radiology: A feasibility study. J. Digit. Imaging 2022, 35, 9–20. [Google Scholar] [CrossRef]
- Abdullah, K.A.; McEntee, M.F.; Reed, W.; Kench, P.L. Development of an organ-specific insert phantom generated using a 3D printer for investigations of cardiac computed tomographic protocols. J. Med. Radiat. Sci. 2018, 65, 175–183. [Google Scholar] [CrossRef]
- Morup, S.D.; Stowe, J.; Precht, H.; Gervig, M.H.; Foley, S. Design of a 3D printed coronary artery model for CT optimization. Radiography 2022, 28, 426–432. [Google Scholar] [CrossRef]
- Sun, Z.; Ng, C.K.C.; Wong, Y.H.; Yeong, C.H. 3D-printed coronary plaques to simulate high calcification in the coronary arteries for investigation of blooming artifacts. Biomolecules 2021, 11, 1307. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ng, C.K.; Squelch, A. Synchrotron radiation computed tomography assessment of calcified plaques and coronary stenosis with different slice thicknesses and beam energies on 3D printed coronary models. Quant. Imaging Med. Surg. 2019, 9, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z. 3D printed coronary models offer new opportunities for developing optimal coronary CT angiography protocols in imaging coronary stents. Quant. Imaging. Med. Surg. 2019, 9, 1350–1355. [Google Scholar] [CrossRef]
- Sun, Z.; Jansen, S. Personalized 3D printed coronary models in coronary stenting. Quant. Imaging Med. Surg. 2019, 9, 1356–1367. [Google Scholar] [CrossRef]
- Sommer, K.N.; Lyer, V.; Kumamaru, K.K.; Rava, R.A.; Ionita, C.N. Method to simulate distal flow resistance in coronary arteries in 3D printed patient specific coronary models. 3D. Print. Med. 2020, 6, 19. [Google Scholar] [CrossRef]
- Wu, C.; Squelch, A.; Sun, Z. Assessment of optimization of computed tomography angiography protocols for follow-up type B aortic dissection patients by using a 3D-printed model. J. 3D. Print. Med. 2022, 6, 117–127. [Google Scholar] [CrossRef]
- Aldosari, S.; Jansen, S.; Sun, Z. Optimization of computed tomography pulmonary angiography protocols using 3D printed model with simulation of pulmonary embolism. Quant. Imaging Med. Surg. 2019, 9, 53–62. [Google Scholar] [CrossRef]
- Aldosari, S.; Jansen, S.; Sun, Z. Patient-specific 3D printed pulmonary artery model with simulation of peripheral pulmonary embolism for developing optimal computed tomography pulmonary angiography protocols. Quant. Imaging Med. Surg. 2019, 9, 75–85. [Google Scholar] [CrossRef]
- Cui, H.; Miao, S.; Esworthy, T.; Zhou, X.; Lee, S.J.; Liu, C.; Yu, Z.; Fisher, J.P.; Mohiuddin, M.; Zhang, L.G. 3D bioprinting for cardiovascular regeneration and pharmacology. Adv. Drug. Deliv. Rev. 2018, 132, 252–269. [Google Scholar] [CrossRef]
- Alonzo, M.; Anilkumar, S.; Roman, B.; Tasnim, N.; Joddar, B. 3D bioprinting of cardiac tissue and cardiac stem cell therapy. Transl. Res. 2019, 211, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wehrle, E.; Rubert, M.; Muller, R. 3D bioprinting of human tissues: Biofabrication, bioinks and bioreactors. Int. J. Mol. Sci. 2021, 22, 3971. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, S.J.; Cheng, H.J.; Yoo, J.J.; Atala, A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta. Biomater. 2018, 70, 48–56. [Google Scholar] [CrossRef]
- Xia, Z.; Jin, S.; Ye, K. Tissue and organ 3D bioprinting. SLAS. Technol. 2018, 23, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Kato, B.; Wisser, G.; Agrawal, D.K.; Wood, T.; Thankam, F.G. 3D bioprinting of cardiac tissue: Current challenges and perspectives. J. Mater. Sci. Mater. Med. 2021, 32, 54. [Google Scholar] [CrossRef] [PubMed]
- Chessa, M.; Van De Bruaene, A.; Farooqi, K.; Valverde, I.; Jung, C.; Votta, E.; Sturla, F.; Paul Diller, G.; Brida, M.; Sun, Z.; et al. Three-dimensional printing, holograms, computational modelling, and artificial intelligence for adult congenital heart disease care: An exciting future. Eur. Heart J. 2022, 43, 2672–2684. [Google Scholar] [PubMed]
- Jain, P.; Kathuria, H.; Dubey, N. Advances in 3D printing of tissues/organs for regenerative medicine and in-vitro models. Biomaterials. 2022, 287, 121639. [Google Scholar] [CrossRef]
- Hoy, S.; Frisbee, J. Common postoperative heart transplant complications. Crit. Care. Nurs. Q. 2018, 41, 383–388. [Google Scholar] [CrossRef]
- Award, M.A.; Shah, A.; Griffith, B.P. Current status and outcomes in heart transplantation: a narrative review. Rev. Cardiovasc. Med. 2022, 23, 11. [Google Scholar]
- Shi, W.Y.; Smith, J.A. Role of coronary artery bypass surgery in acute myocardial infarction, in: Prim. Angioplasty, Springer Singapore, 2018, pp. 211–221.
- Kwon, Y.W.; Yang, H.M.; Cho, H.J. Cell therapy on myocardial infarction. Int. J. Stem. Cells. 2010, 3, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Nam, H.; Jang, J. 3D bioprinting of stem cell-laden cardiac patch: a promising alternative for myocardial repair. APL. Bioeng. 2021, 5, 031508. [Google Scholar] [CrossRef] [PubMed]
- Vukicevic, M.; Mosadegh, B.; Min, J.K.; Little, S.H. 3D printing and its future directions. JACC. Cardiovasc. Imaging. 2017, 10, 171–134. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Ayna, B.; Undieh, A.A.; Yang, Y.P.; Huang, N.F. Advances in three-dimensional bioprinted stem cell-based tissue engineering for cardiovascular regeneration. J. Mol. Cell. Cardiol. 2022, 169, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Ye, X.; Yao, B.; Zhao, M.; Wu, P.; Liu, G.; Zhuang, D.; Jiang, H.; Chen, X.; He, Y.; et al. Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration. Bioact. Mater. 2020, 6, 1388–1401. [Google Scholar] [CrossRef]
- Liu, J.; He, J.; Liu, J.; Ma, X.; Chen, Q.; Lawrence, N.; Zhu, W.; Xu, Y.; Chen, S. rapid 3D bioprinting of in vitro cardiac tissue models using human embryonic stem cell-derived cardiomyocytes. Bioprinting. 2019, 13, 1–6. [Google Scholar] [CrossRef]
- Muffarih, S.H.; Mahmood, F.; Qureshi, B.Q.; Yunus, R.; Quraishi, I.; Baribeau, V.; Sharkey, A.; Matyal, R.; Khabbaz, K. Three-dimensional printing of patient-specific heart valves: separating facts from fiction and myth from reality. J. Cardiovasc. Vasc. Anesth. 2022, 36, 2643–2655. [Google Scholar] [CrossRef]
- Gaetani, R.; Rizzitelli, G.; Chimenti, I, et al. Cardiospheres and tissue engineering for myocardial regeneration: potential for clinical applications. J. Cell. Mol. Med. 2010, 14, 1071–1077. [Google Scholar] [CrossRef]
- Gaetani, R.; Doevendans, R.A.; Metz, C.H.; et al. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials. 2012, 33, 1782–1790. [Google Scholar] [CrossRef]
- Gaetnai, R.; Feyen, D.A.; Verhage, V, et al. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials. 2015, 61, 339–348. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Moncal, K.K.; Gudapati, H. Evaluation of bioprinter technologies. Addit. Manuf. 2017, 3, 179–200. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Pei, B.; Chen, J.; Zhoud, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet bioprinting of biomaterials. Chem Rev. 2020, 122, 10596–10636. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 4, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioeng. 2015, 112, 1047–1055. [Google Scholar] [CrossRef]
- Schneberg, J.; De Lorenzi, F.; Theek, B.; Blaeser, A.; Rommel, D.; Kuehne, A.J.; Kiessling, F.; Fischer, H. Engineering biofunctional in vitro vessel models using a multilayer bioprinting technique. Sci. Rep. 2018, 8, 10430–13. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.E.; Derby, B. Inkjet printing biomaterials for tissue engineering: bioprinting. Int. Mater. Rev. 2014, 59, 430–448. [Google Scholar] [CrossRef]
- Shafiee, A.; Ghadiri, E.; Ramesh, H.; Kengla, C.; Kassis, J.; Calvert, P.; Williams, D.; Khademhosseini, A.; Narayan, R.; Forgacs, G.; Atala, A. Physics of bioprinting. Appl. Phys. Rev. 2019, 6, 21315. [Google Scholar] [CrossRef]
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J. Biomed. Mater. Res. B. Appl. Biomater. 2011, 98B(1), 160–170. [Google Scholar] [CrossRef]
- Rider, P.; Kačarević, Ž.P.; Alkildani, S.; Retnasingh, S.; Barbeck, M. Bioprinting of tissue engineering scaffolds. J. Tissue. Eng. 2018, 9, 2041731418802090. [Google Scholar] [CrossRef]
- Chang, R.; Nam J, Sun W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue. Eng Part. A. 2008, 14, 41–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Kumarwew, P.; Lv, S.; Xiong, D.; Zhao, H.; Cai, Z.; Zhao, X. Recent advances in 3D bioprinting of vascularized tissues. Mater. Des. 2021, 199, 109398. [Google Scholar] [CrossRef]
- Xiong, R.; Zhang, Z.; Chai, W.; Huang, Y.; Chrisey, D.B. Freeform drop-on-demand laser printing of 3D alginate and cellular constructs. Biofabrication. 2015, 7, 045011. [Google Scholar] [CrossRef] [PubMed]
- Häneke, T.; Sahara, M. Progress in bioengineering strategies for heart regenerative medicine. Int. J. Mol. Sci. 2022, 23, 3482. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.J.; Hudson, J.E. Bioengineering adult human heart tissue: How close are we? APL. Bioengineering. 2019, 3, 010901. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Goldfracht, I.; Protze, S.; Shiti, A.; Setter, N.; Gruber, A.; Shaheen, N.; Nartiss, Y.; Keller, G.; Gepstein, L. Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes. Nat. Comun. 2020, 11, 75. [Google Scholar] [CrossRef]
- Bremner, S.B.; Gaffney, K.S.; Sniadecki, N.J.; Mack, D.L. A Change of Heart: Human cardiac tissue engineering as a platform for drug development. Curr. Cardiol. Rep. 2022, 24, 473–486. [Google Scholar] [CrossRef]
- Quadri, F.; Soman, S.S.; Vijayavenkataraman, S. Progress in cardiovascular bioprinting. Artif. Organs. 2021, 45, 652–664. [Google Scholar] [CrossRef]
- Marchianò, S.; Bertero, A.; Murry, C.E. Learn from your elders: developmental biology lessons to guide maturation of stem cell-derived cardiomyocytes. Pediatr. Cardiol. 2019, 40, 1367–1387. [Google Scholar] [CrossRef]
- Sedlakova, V.; McTiernan, C.; Cortes, D.; Suuronen, E.J.; Alarcon, E.I. 3D bioprinted cardiac tissues and devices for tissue maturation. Cells. Tissues. Organs. 2022, 211, 406–419. [Google Scholar] [CrossRef]
- Tomasina, C.; Bodet, T.; Mota, C.; Moroni, L.; Camarero-Espinosa, S. Bioprinting vasculature: materials, cells and emergent techniques. Materials. 2019, 12, 2701. [Google Scholar] [CrossRef] [PubMed]
- Seymour, A.J.; Westerfield, A.D.; Cornelius, V.C.; Skylar-Scott, M.A.; Heilshorn, S. Bioprinted microvasculature: progressing from structure to function. Biofabrication. 2022, 14, 022002. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, L.; Li, T.; Liu, S.; Guo, B.; Huang, W.; Wu, Y. 3D bioprinting in cardiac tissue engineering. Theranostics. 2021, 11, 7948–7969. [Google Scholar] [CrossRef] [PubMed]
- Barrs, R.W.; Jia, J.; Silver, S.E.; Yost, M.; Mei, Y. Biomaterials for bioprinting microvasculature. Chem. Rev. 2020, 120, 10887–10949. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, A.; Stevens, M.M.; Sattler, S.; Rosenthal, N. Toward regeneration of the heart: bioengineering strategies for immunomodulation. Front. Cardiovasc. Med. 2019, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Roche, C.D.; Brereton, R.J.; Ashton, A.W.; Jackson, C.; Gentile, C. Current challenges in three-dimensional bioprinting heart tissues for cardiac surgery. Eur. J. Cardiothorac. Surg. 2020, 58, 500–510. [Google Scholar] [CrossRef]
- Tillman, B.; Hardin-Young, J.; Shannon, W.; Russell, A.J.; Parenteau, N.L. Meeting the need for regenerative therapies: translation-focused analysis of U.S. regenerative medicine opportunities in cardiovascular and peripheral vascular medicine using detailed incidence data. Tissue. Eng. Part. B. Rev. 2021, 19, 99–115. [Google Scholar] [CrossRef]
- Fazal, F.; Raghav, S.; Callanan, A.; Koutsos, V.; Radacsi, N. Recent advancements in the bioprinting of vascular grafts. Biofabrication. 2021, 13, 32003. [Google Scholar] [CrossRef]
- Wenger, R.; Giraud, M. 3D printing applied to tissue engineered vascular grafts. Appl. Sci (Basel). 2018, 8, 2631. [Google Scholar] [CrossRef]
- Klinkert, P.; Post, P.N.; Breslau, P.J.; Bockel, J.H. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 357–362. [Google Scholar] [CrossRef]
- Catto, V.; Fare, S.; Freddi, G.; Tani, M.C. Vascular tissue engineering: recent advances in small diameter blood vessel regeneration. ISRN. Vasc. Med. 2014, 2014, 1–27. [Google Scholar] [CrossRef]
- Syedain, Z.H.; Prunty, A.; Li, J.; Tranquillo, R.T. Evaluation of the probe burst test as a measure of strength for a biologically-engineered vascular graft. J. Mech. Behav. Biomed. Mater. 2021, 119, 104527. [Google Scholar] [CrossRef] [PubMed]
- Pensalfini, M.; Meneghello, S.; Lintas, V.; Bircher, K.; Ehret, A.E.; Mazzza, E. The suture retention test, revisited. J. Mech. Behav. Biomed. Mater. 2018, 77, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Deo, K.A.; Singh, K.A.; Peak, C.W.; Algae, D.L.; Gaharwar, A.K. Bioprinting 101: design, fabrication, and evaluation of cell-laden 3D bioprinted scaffolds. Tissue. Eng. Part A. 2020, 26, 318–338. [Google Scholar] [CrossRef]
- Xu, C.; Christensen, K.; Zhang, Z.; Huang, Y.; Fu, J.; Markwald, R.R. Predictive compensation-enabled horizontal inkjet printing of alginate tubular constructs. Manuf. Lett. 2013, 1, 28–32. [Google Scholar] [CrossRef]
- Xu, C.; Chai, W.; Huang, Y.; Markwald, R.R. Scaffold-free inkjet printing of three-dimensional zigzag cellular tubes. Biotechno Bioeng. 2012, 109, 3152–3160. [Google Scholar] [CrossRef] [PubMed]
- Gudapati, H.; Dey, M.; Ozbolat, I. A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials. 2016, 102, 20–42. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H, Ramadan MH, Hudson AR, Feinberg AW. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef]
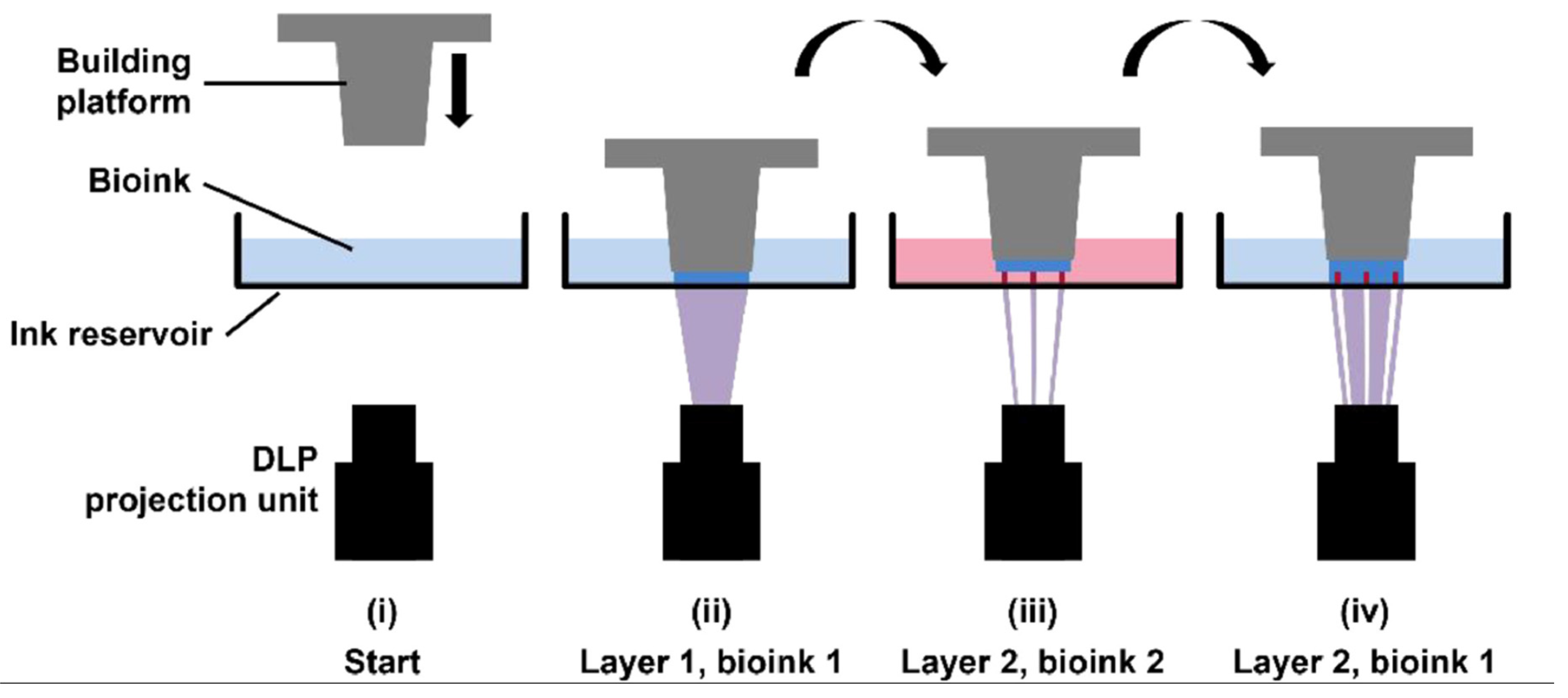
- Dikyol, C.; Altunbek, M.; Bartolo, P.; Koc, B. Multimaterial bioprinting approaches and their implementations for vascular and vascularized tissues. Bioprinting. 2021, 24, e00159. [Google Scholar] [CrossRef]
- Hong, S.; Kim, J.S.; Jung, B.; Won, C.; Hwang, C. Coaxial bioprinting of cell-laden vascular constructs using a gelatin-tyramine bioink. Biomater. Sci. 2019, 7, 4578–4587. [Google Scholar] [CrossRef]
- Mohan, T.S.; Datta, P.; Nesaei, S.; Ozbolat, V.; Ozbolat, I.T. 3D coaxial bioprinting: process mechanisms, bioinks and applications. Prog. Biomed. Eng (Bristol). 2022, 4, 22003. [Google Scholar]
- Milojević, M.; Vihar, B.; Banović, L.; Miško, M.; Gradišnik, L.; Zidarič, T.; Maver, U. Core/shell printing scaffolds for tissue engineering of tubular structures. J. Vis. Exp. 2019, 151, e59951. [Google Scholar]
- Wang, Y.; Kankala, R.K.; Zhu, K.; Wang, S.; Zhang, Y.S.; Chen, A.Z. Coaxial extrusion of tubular tissue constructs using a gelatin/GelMA blend bioink. ACS. Biomater. Sci. Eng. 2019, 5, 5514–5524. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhong, Z.; Hu, N.; Zhou, Y.; Maggio, L.; Miri, A.K.; Fragasso, A.; Jin, Z.; Khademhosseine, A.; Zhang, Y.S. Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favourable gelatin methacryloyl microenvironments. Biofabrication. 2018, 10, 024102. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yey, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; Khademhosseini, A. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. 2016, 106, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, Z.; Lin, Z.; Qiu, J.; Liu, Y.; Liu, A.; Wang, Y.; Xiang, M.; Chen, B.; Fu, J.; He, Y. 3D bioprinting of vessel-like structures with multilevel fluidic channels. ACS. Biomater. Sci. Eng. 2017, 3, 399–408. [Google Scholar] [CrossRef]
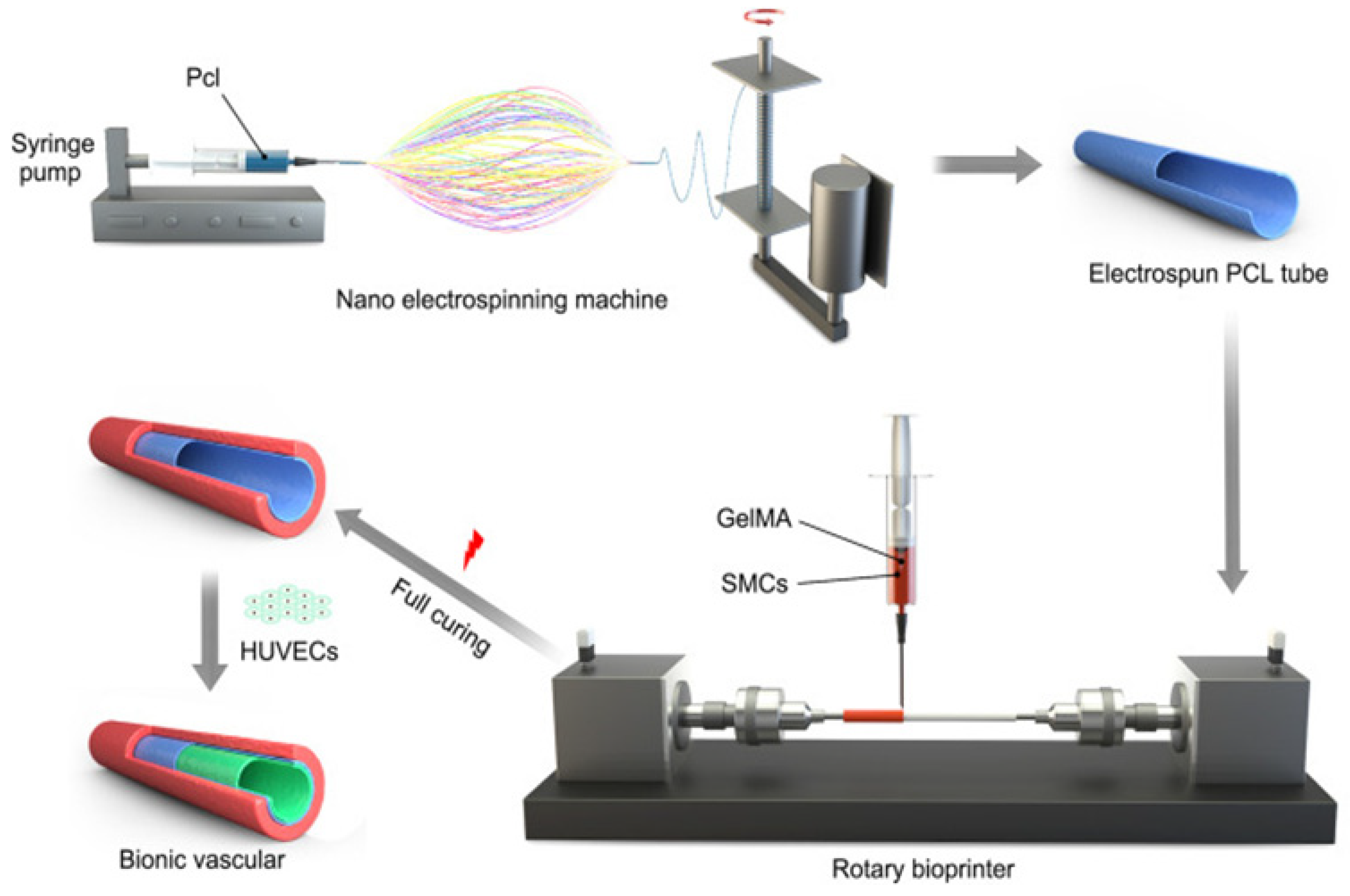
- Jin, Q.; Zhang, G.; Xu, L.; Jin, G.; Tang, L.; Ju, J.; Zhao, W.; Hou, R. Nanofiber electrospinning combined with rotary bioprinting for fabricating small-diameter vessels with endothelium and smooth muscle. Compos. B. Eng. 2022, 234, 109691. [Google Scholar] [CrossRef]
- Xu, L.; Varkey, M.; Jorgensen, A.; Ju, J.; Jin, Q.; Park, J.H.; Zhang, G.; Ke, D.; Zhao, W.; Hou, R.; Atala, A. Bioprinting small diameter blood vessel constructs with an endothelial and smooth muscle cell bilayer in a single step. Biofabrication. 2020, 12, 045012. [Google Scholar] [CrossRef]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Gold, K.A.; Saha, B.; Pandian, N.K.; Walther, B.K.; Palma, J.A.; Jo. J.; Cooke, J.P.; Jain, A.; Gahawar, A.K. 3D bioprinted multicellular vascular models. Adv. Health Mater. 2021, 10, e2101141. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Vascular tissue engineering: the role of 3D bioprinting. In: Walpoth BH, Bergmeister H, Bowlin GL, Kong D, Rotmans JI, Zilla P, editors. Tissue-engineered vascular grafts. Springer Cham; 2020, p. 321-38.
- Krishnamoorthy, S.; Wadnap, S.; Noorani, B.; Xu, H.; Xu, C. Investigation of gelatin methacrylate working curves in dynamic optical projection stereolithography of vascular-like constructs. Eur. Poly J. 2020, 124, 109487. [Google Scholar] [CrossRef]
- Thomas, A.; Orellano, I.; Lam, T.; Noichl, B.; Geiger, M.; Amler, A.; Kreuder, A.; Palmer, C.; Duda, G.; Lauster, R.; Kloke, L. Vascular bioprinting with enzymatically degradable bioinks via multi-material projection-based stereolithography. Acta. Biomater. 2020, 117, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; et al. Heart disease and stroke statistics—2021 Update: A report from the American Heart Association. Circulation. 2021, 143. [Google Scholar] [CrossRef]
- Sanz-Garcia, A.; Oliver-de-la-Cruz, J.; Mirabet, V.; Gandía, C.; Villagrasa, A.; Sodupe, E.; et al. Heart valve tissue engineering: how far is the bedside from the bench? Expert. Rev. Mol. Med. 2015, 17, e16. [Google Scholar] [CrossRef] [PubMed]
- Butcher, J.T.; Mahler, G.J.; Hockaday, L.A. Aortic valve disease and treatment: The need for naturally engineered solutions. Adv. Drug. Deliv. Rev. 2011, 63, 242–268. [Google Scholar] [CrossRef] [PubMed]
- Goldbarg, S.H.; Elmariah, S.; Miller, M.A.; Fuster, V.; York. N.; York, N. Insights into degenerative aortic valve disease. J. Am. Coll. Cardiol. 2007, 50, 1205–1213. [Google Scholar] [CrossRef]
- Hammermeister, K.; Sethi, G.K.; Henderson, W.G.; Grover, F.L.; Oprian, C.; Rahimtoola, S.H. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J. Am. Coll. Cardiol. 2000, 36, 1152–1158. [Google Scholar] [CrossRef]
- Head, S.J.; Çelik, M.; Kappetein, A.P. Mechanical versus bioprosthetic aortic valve replacement. Eur. Heart. J. 2017, 38, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Kostyunin, A.E.; Yuzhalin, A.E.; Rezvova, M.A.; Ovcharenko, E.A.; Glushkova, T.V.; Kutikhin, A.G. Degeneration of bioprosthetic heart valves: update 2020. J. Am. Heart. Assoc. 2020, 9, e018506. [Google Scholar] [CrossRef]
- Dvir, D.; Bourguignon, T.; Otto, C.M.; Hahn, R.T.; Rosenhek, R.; Webb, J.G.; et al. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation. 2018, 137, 388–399. [Google Scholar] [CrossRef]
- Capodanno, D.; Petronio, A.S.; Prendergast, B.; Eltchaninoff, H.; Vahanian, A.; Modine, T.; et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart. J. 2017, 38, 3382–3390. [Google Scholar] [PubMed]
- Sathananthan, J.; Lauck, S.; Polderman, J.; Yu, M.; Stephenson, A.; Sathananthan, G.; et al. Ten year follow-up of high-risk patients treated during the early experience with transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2021, 97, E431–7. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Tefft, B.J.; Jana, S.; Tefft, B.J.; Spoon, D.B.; Simari, R.D. Scaffolds for tissue engineering of cardiac valves. Acta. Biomaterialia. 2014, 17, 2877–2893. [Google Scholar] [CrossRef] [PubMed]
- Mela, P. Subject- and leaflet-specific remodeling of polymeric heart valves for in situ tissue engineering: Challenges towards Clinical translation. JACC. Basic. Transl. Sci. 2020, 5, 32–34. [Google Scholar] [CrossRef]
- Vesely, I. Heart Valve Tissue Engineering. Circ. Res. 2005, 97, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Wissing, T.B.; Bonito, V.; Bouten, C.V.C.; Smits, A.I.P.M. Biomaterial-driven in situ cardiovascular tissue engineering—a multi-disciplinary perspective. NPJ. Regen. Med. 2017, 2, 1–19. [Google Scholar] [CrossRef]
- Butcher, J.T. The root problem of heart valve engineering. Sci. Transl. Med. 2018, 10, eaat5850. [Google Scholar] [CrossRef]
- Zhuang, R.Z.; Lock, R.; Liu, B.; Vunjak-Novakovic, G. Opportunities and challenges in cardiac tissue engineering from an analysis of two decades of advances. Nat. Biomed. Eng. 2022, 6, 327–338. [Google Scholar] [CrossRef]
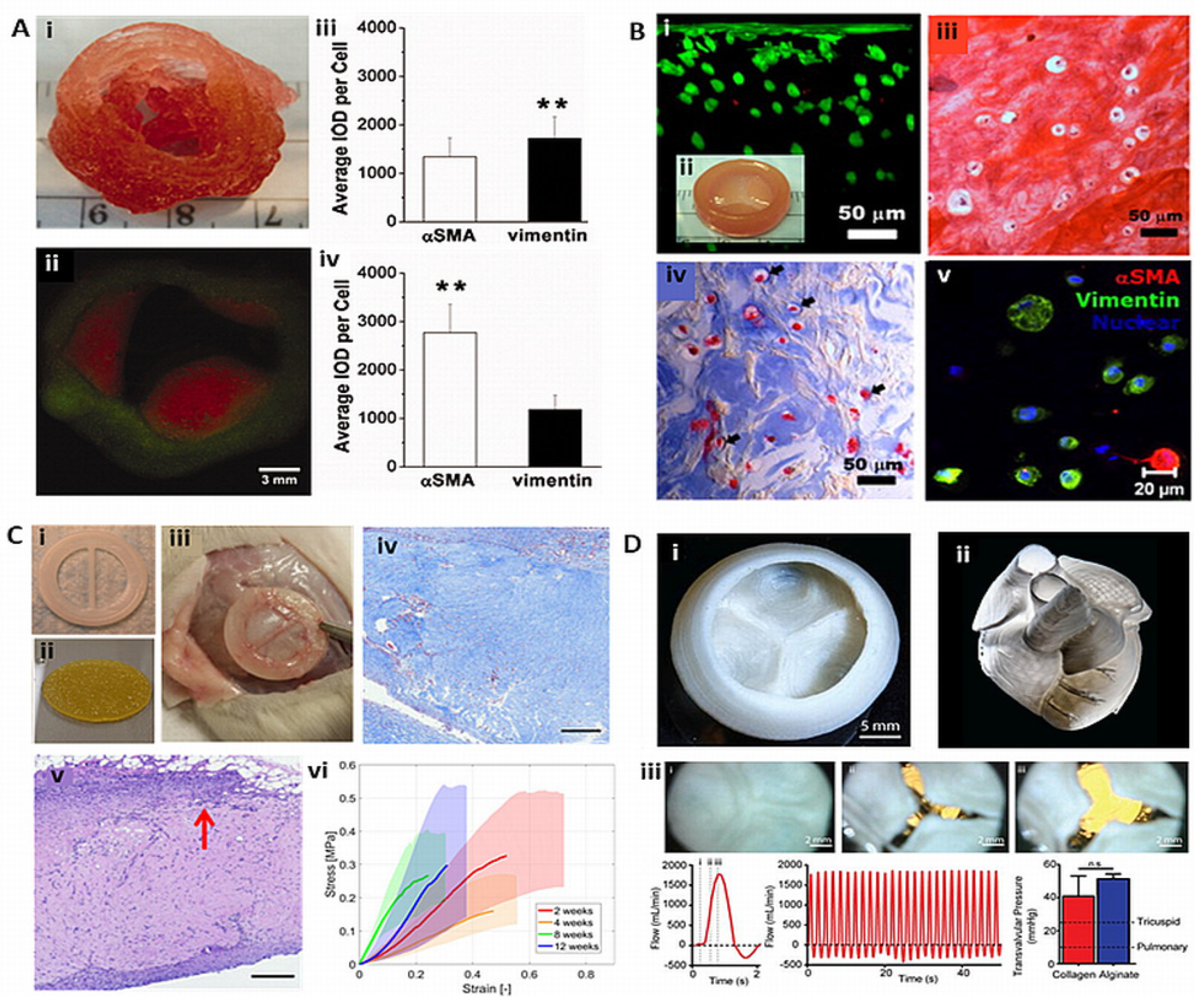
- Hockaday, L.A.; Kang, K.H.; Colangelo, N.W.; Cheung, P.Y.C.; Duan, B.; Malone, E.; et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication. 2012, 4, 035005. [Google Scholar] [CrossRef]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. 2013, 101A(5), 1255–1264. [Google Scholar] [CrossRef]
- Duan, B.; Kapetanovic, E.; Hockaday, L.A.; Butcher, J.T. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta. Biomater. 2014, 10, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Cheung, D.Y.; Butcher, J.T. Incorporating nanocrystalline cellulose into a multifunctional hydrogel for heart valve tissue engineering applications. J. Biomed. Mater. Res. 2022, 110, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.H.; Armstrong, P.A.; Lee, L.J.; Duan, B.; Kang, K.H.; Butcher, J.T. Optimizing photo-encapsulation viability of heart valve cell types in 3D printable composite hydrogels. Ann. Biomed. Eng. 2017, 45, 360–377. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Duan, B.; Liu, P.; Zhang, C.; Qin, X.; Butcher, J.T. Fabrication of aligned nanofiber polymer yarn networks for anisotropic soft tissue scaffolds. ACS. Appl. Mater. Interfaces. 2016, 8, 16950–16960. [Google Scholar] [CrossRef] [PubMed]
- Sodian, R.; Loebe, M.; Hein, A.; Martin, D.P.; Hoerstrup, S.P.; Potapov, E.V.; et al. Application of stereolithography for scaffold fabrication for tissue engineered heart valves: ASAIO. J. 2002, 48, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Akpek, A. Analysis of biocompatibility characteristics of stereolithography applied three dimensional (3D) bioprinted artificial heart valves. J. Faculty. Eng. Architec. Gazi. Uni. 2018, 3, 929–938. [Google Scholar]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; et al. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Maxson, E.L.; Young, M.D.; Noble, C.; Go, J.L.; Heidari, B.; Khorramirouz, R.; et al. In vivo remodeling of a 3D-Bioprinted tissue engineered heart valve scaffold. Bioprinting. 2019, 16, e00059. [Google Scholar] [CrossRef]
- Noble, C.; Maxson, E.L.; Lerman, A.; Young, M.D. Mechanical and finite element evaluation of a bioprinted scaffold following recellularization in a rat subcutaneous model. J. Mech. Behav. Biomed. Mater. 2020, 102, 103519. [Google Scholar] [CrossRef]
- Zengin, A.; Castro, J.; Habibovic, P.; Van Rijt, S. Injectable, self-healing mesoporous silica nanocomposite hydrogels with improved mechanical properties. Nanoscale. 2021, 13, 1144–1154. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Radinekiyan, F.; Aliabadi, H.A.M.; Sukhtezari, S.; Tahmasebi, B.; Maleki, A.; et al. Chitosan hydrogel/silk fibroin/Mg (OH) 2 nanobiocomposite as a novel scaffold with antimicrobial activity and improved mechanical properties. Sci. Rep. 2021, 11, 650. [Google Scholar] [CrossRef] [PubMed]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today. Bio. 2021, 10, 100098. [Google Scholar] [CrossRef] [PubMed]
- Bas, O.; Lucarotti, S.; Angella, D.D.; Castro, N.J.; Meinert, C.; Wunner, F.M.; et al. Rational design and fabrication of multiphasic soft network composites for tissue engineering articular cartilage: A numerical model-based approach. Chem. Engn. J. 2018, 340, 15–23. [Google Scholar] [CrossRef]
- Visser, J.; Melchels, F.P.W.; Jeon, J.E.; Van Bussel, E.M.; Kimpton, L.S.; Byrne, H.M.; et al. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Ye, X.; Yao, B.; Zhao, M.; Wu, P.; Liu, G.; et al. Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration. Bioact Mater. 2021, 6, 1388–1401. [Google Scholar] [CrossRef]
- Montero, P.; Flandes-Iparraguirre, M.; Musquiz, S.; Pérez Araluce, M.; Plano, D.; Sanmartín, C.; et al. Cells, Materials, and Fabrication Processes for Cardiac Tissue Engineering. Front. Bioeng. Biotechnol. 2020, 8, 955. [Google Scholar] [CrossRef]
- Bejleri, D.; Streeter, B.W.; Nachlas, A.L.Y.; Brown, M.E.; Gaetani, R.; Christman, K.L.; et al. A Bioprinted Cardiac patch composed of cardiac-specific extracellular matrix and progenitor cells for heart repair. Adv. Healthc. Mater. 2018, 7, 1800672. [Google Scholar] [CrossRef]
- Zhu, K.; Shin, S.R.; van Kempen, T.; Li, Y.; Ponraj, V.; Nasajpour, A.; et al. Gold nanocomposite bioink for printing 3D cardiac constructs. Adv. Funct. Mater. 2017, 27, 1605352. [Google Scholar] [CrossRef]
- Erdem, A.; Darabi, M.A.; Nasiri, R.; Sangabathuni, S.; Ertas, Y.N.; Alem, H.; et al. 3D Bioprinting of oxygenated cell-laden gelatin methacryloyl constructs. Adv. Healthc. Mater. 2020, 9, 1901794. [Google Scholar] [CrossRef]
- Ahrens, J.H.; Uzel, S.G.M.; Skylar-Scott, M.; Mata, M.M.; Lu, A.; Kroll, K.T.; et al. Programming cellular alignment in engineered cardiac tissue via bioprinting anisotropic organ building blocks. Adv. Mater. 2022, 34, 2200217. [Google Scholar] [CrossRef]
- Ong, C.S; Fukunishi, T.; Zhang, H.; Huang, C.Y.; Nashed, A.; Blazeski, A.; et al. Biomaterial-free three-dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes. Sci. Rep. 2017, 7, 4566. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.; Fukunishi, T.; Bai, Y.; Bedja, D.; Pitaktong, I.; Mattson, G.; et al. Cardiac regeneration using human-induced pluripotent stem cell-derived biomaterial-free 3D-bioprinted cardiac patch in vivo. J. Tissue. Eng. Regen. Med. 2019, 13, 2031–2039. [Google Scholar] [CrossRef]
- Asulin, M. ; Michael, I,; Shapira, A.; Dvir, T. One-step 3D printing of heart patches with built-in electronics for performance regulation. Adv. Sci 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Edri, R.; Gal, I.; Noor, N.; Harel, T.; Fleischer, S.; Adadi, N.; et al. Personalized hydrogels for engineering diverse fully autologous tissue implants. Adv. Mater. 2019, 31, 1803895. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of personalized thick and perfusable cardiac patches and hearts. Adv. Sci (Weinh). 2019, 6, 1900344. [Google Scholar] [CrossRef]
- Kupfer, M.E.; Lin, W.H.; Ravikumar, V.; Qiu, K.; Wang, L.; Gao, L.; et al. In Situ expansion, differentiation, and electromechanical coupling of human cardiac muscle in a 3D bioprinted, chambered organoid. Circ. Res. 2020, 127, 207–224. [Google Scholar] [CrossRef]
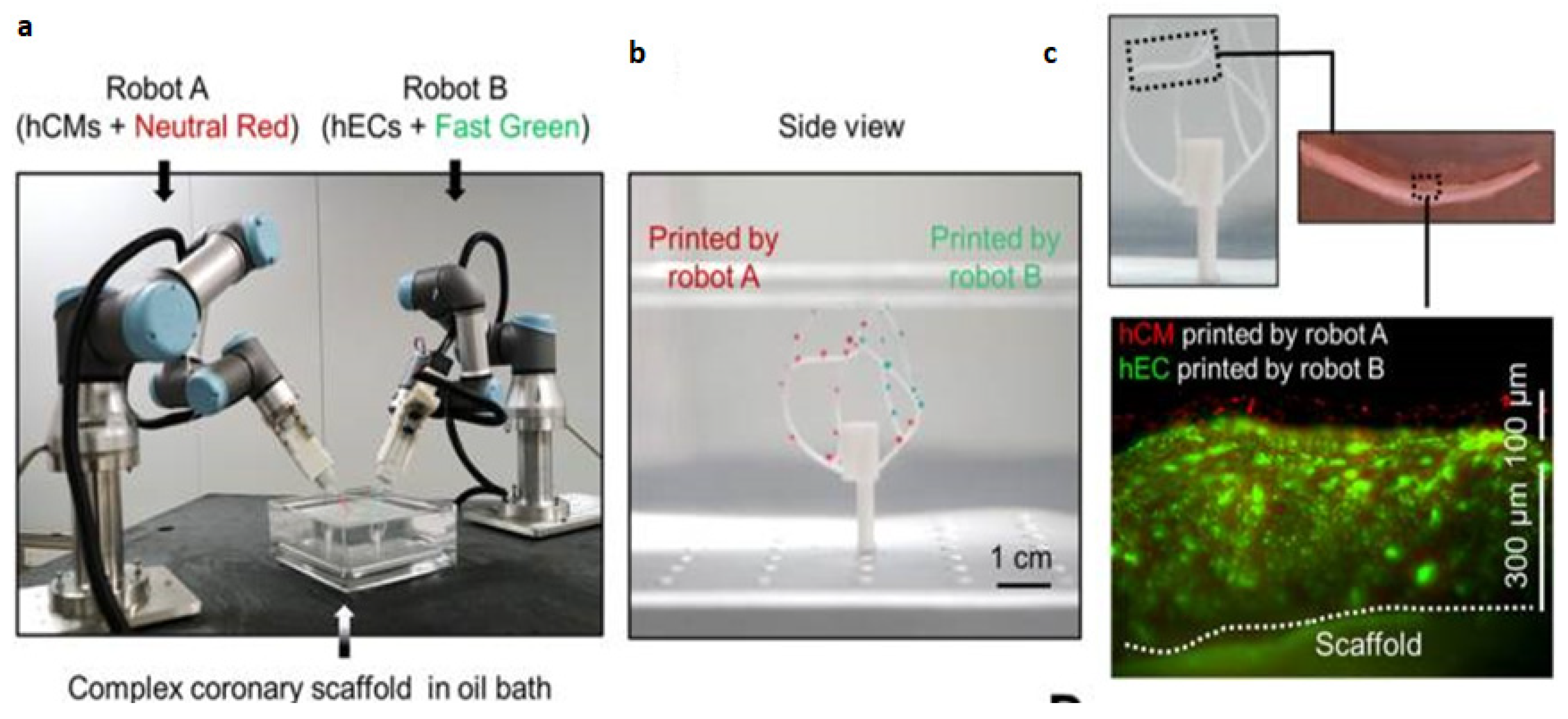
- Zhang, Z.; Wu, C.; Dai, C.; Shi, Q.; Fang, G.; Xie, D.; et al. A multi-axis robot-based bioprinting system supporting natural cell function preservation and cardiac tissue fabrication. Bioact. Mater. 2022, 18, 138–150. [Google Scholar] [CrossRef]

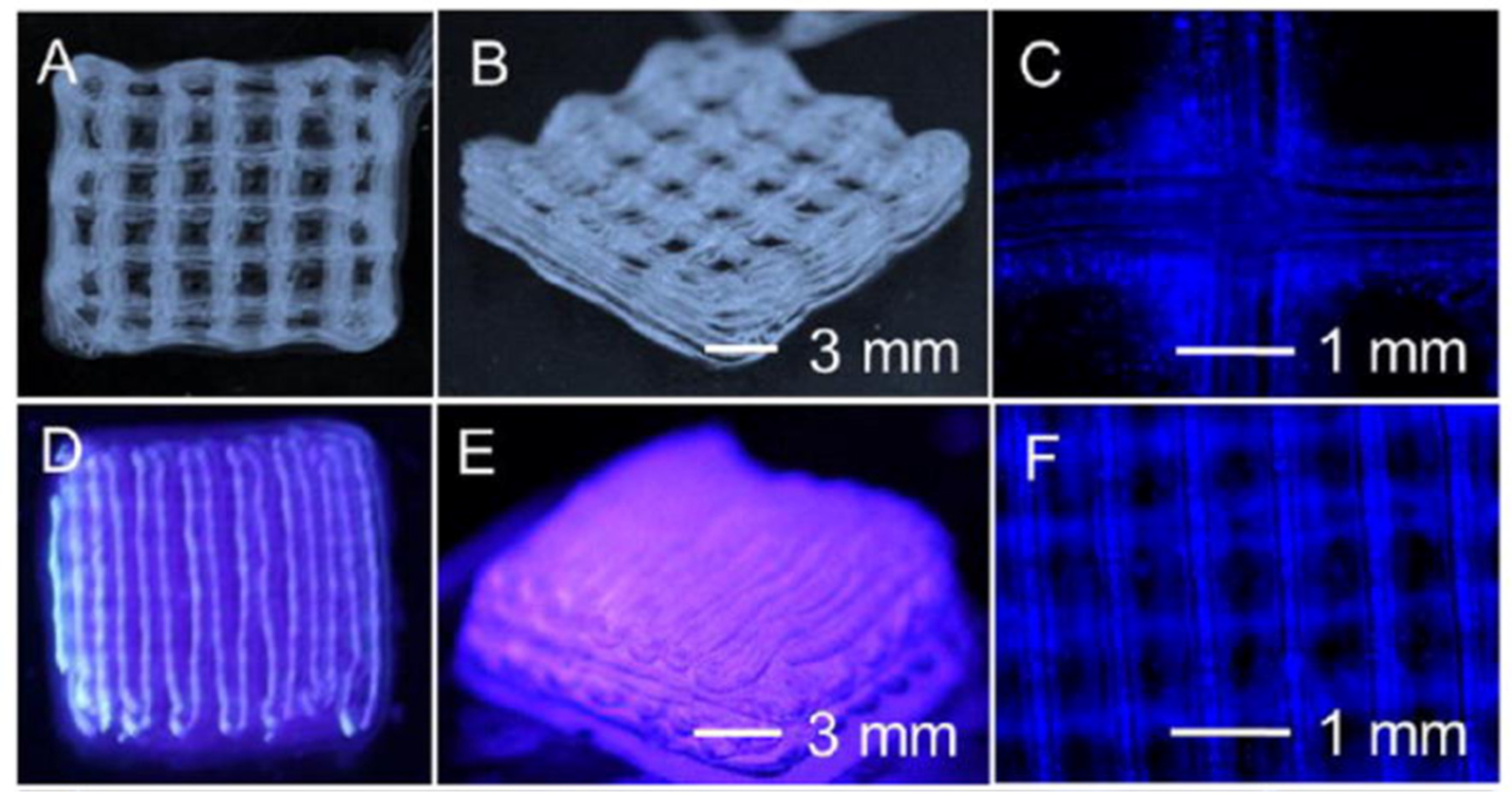
| References | Construct shape | Bioinks | Key aspect of study | Examined benefits | |
|---|---|---|---|---|---|
| Cells | Hydrogel | ||||
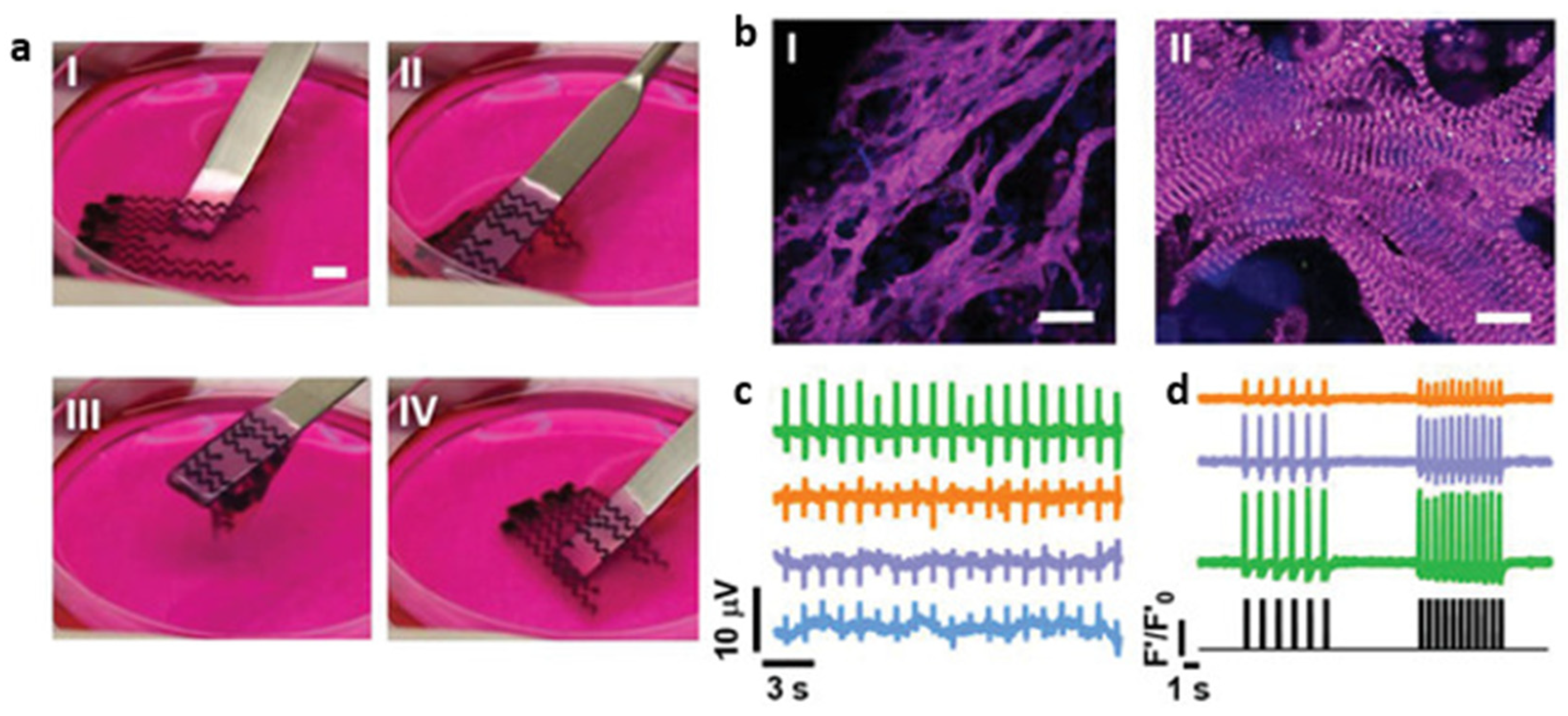
| [153] | Patch | Spheroids of hiPSC-derived CM, CB and EC | - | Biomaterial-free | Spontaneous contraction, ability to pace constructs, rudimentary vascularization, in vivo engraftment |
| [150] | Grid | Neonatal rat CM + CF | GelMA + alginate + GNR | GNR to improve electrical conduction | Higher Cx43 expression, higher synchronous contractile frequency than constructs without GNR |
| [149] | Grid, patch | hCPCs | ECM + GelMA | Cardiac-ECM specific bioink | Higher cardiac and endothelial-specific gene expression than GelMA-only constructs, retention and vascularization after in vivo implantation |
| [44] | Patch | Neonatal rat CM | Fibrinogen + gelatin | PCL frame to impart auxotonic mechanical stress | Cell alignment, physiologic response to drugs altering force and frequency of contraction |
| [139] | Ellipsoid | hESC-CM | Collagen | Ventricle-like shape | Spontaneous, synchronous contraction, pacing at 1 and 2 Hz. |
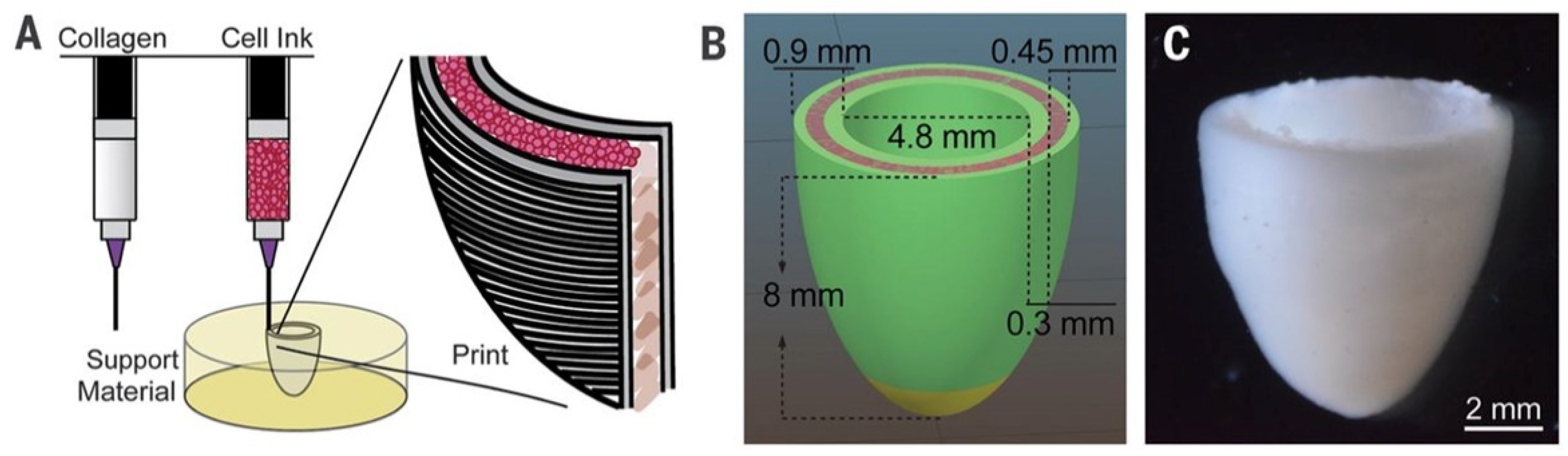
| [157] | Patch, two-chambered ellipsoid | hiPSC-derived CM and EC | ECM | Patient specificity, vascularization, shape | Cardiac patch with perfusable, vascular-like channels. Spontaneous and synchronous contraction |
| [154] | Patch | Spheroids of hiPSC-derived CM, CF and EC | - | In vivo study of patch described in (7) | Smaller scar, greater vascularization than control (omentum patch). Greater ejection fraction and cardiac output, although not significant |
| [151] | Grid | Neonatal rat CM + mouse fibroblasts | GelMA + CPO | Oxygen-releasing bioink | Enhanced viability and function under hypoxic conditions |
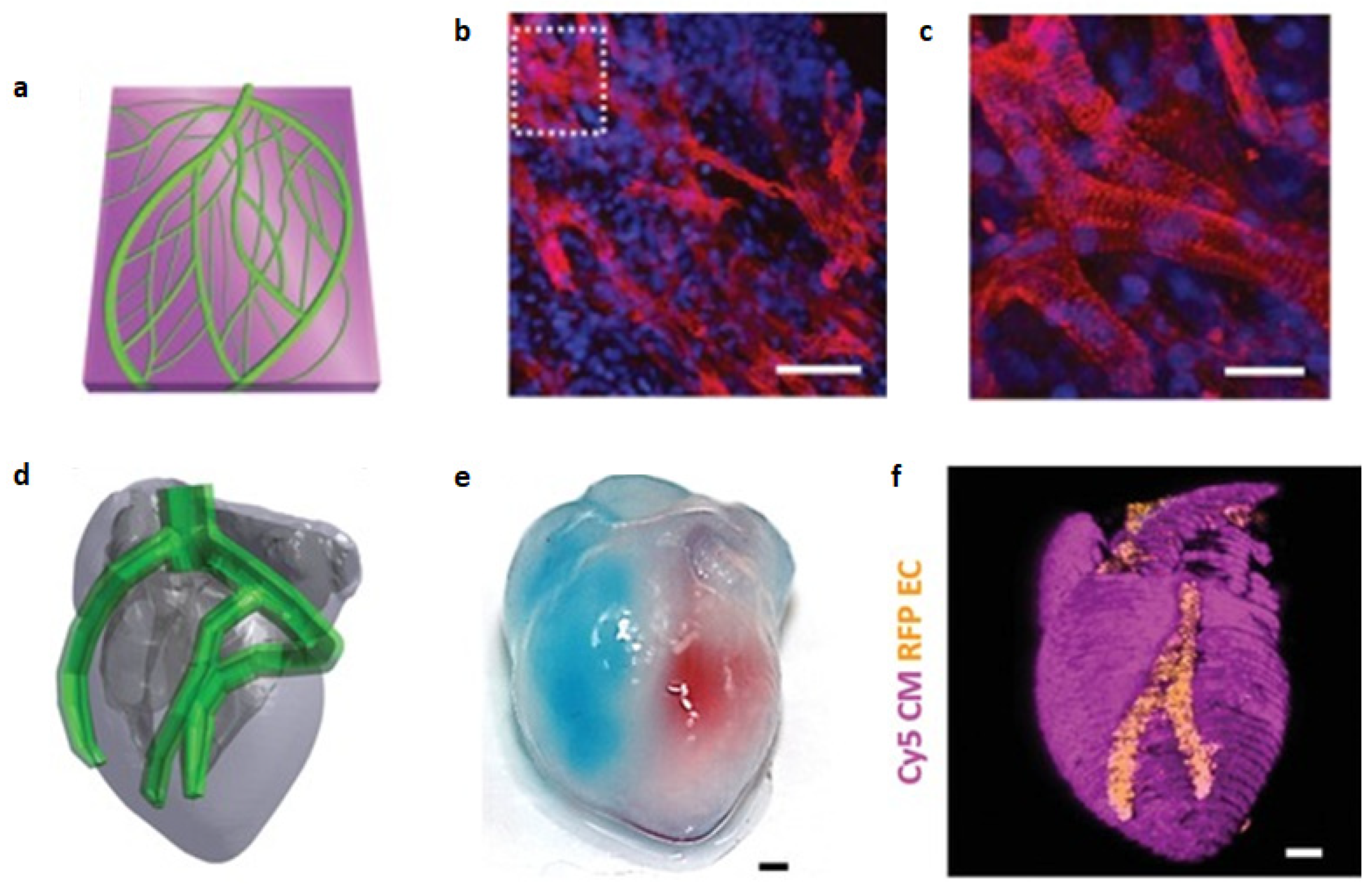
| [158] | Chambered ellipsoid | hiPSCs | GelMA + ColMA | Ventricular-like shape, pump-like function, differentiation after printing | Differentiation to CM, SMC and EC. Spontaneous and synchronous contraction, physiologic response to isoproterenol, for up to 6 weeks in culture |
| [155] | Patch | Neonatal rat CM/hiPSC-CM | ECM, PDMS + graphite, PDMS + surfactant | Integrated electrodes for sensing and pacing | Good cell viability, spontaneous contraction and actinin expression. Sensing and pacing at 1 and 2 Hz |
| [152] | Struts, patch | hiPSC-CM microtissues | Fibrinogen + gelatin | High cellular density, alignment | Higher directionality, conduction velocity and force generation than spheroid-based constructs |
| [159] | Lining of vascular model | hESC-CM + EC | - | Ability to print in any direction | No damage in viability or activity after printing, evidence of vasculogenesis, synchronous and spontaneous contraction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





