1. Introduction
Barnyardgrass (
Echinochloa crus-galli L.) refers to a highly prevalent and damaging weed species that infests rice cultivation globally[
1,
2]. The worldwide rice yield losses due to barnyardgrass competition were estimated at approximately 35%[
3]. As herbicides are the primary control method for managing weed infestations, their prolonged and excessive use has led to the evolution of herbicide-resistant weeds[
4]. The emergence and growing global incidence of herbicide-resistant weeds pose a significant threat to rice production[
5,
6].
Integrated weed management proposed crop allelopathy as a non-herbicidal alternative[
7,
8]. The interference mechanism of allelopathy was characterized by the chemical suppression of neighbor plants through the release of allelochemicals from focal plants[
9]. Allelopathic crop cultivars had been found to release their own 'herbicides' (i.e. allelochemicals) to inhibit the growth of associated neighbor weeds, thereby reducing the need for herbicides in paddy fields[
10]. Root-secreted signaling chemicals drove plant neighbor detection and allelochemical response[
11]. The chemical relationship between allelopathic rice and weed species mainly occurred between the underground root systems. Allelopathic rice could interfere with the growth of herbicide-resistant barnyardgrass through allelochemical-mediated root interactions[
12,
13,
14]. However, the differential responses of root traits in allelopathic rice and non-allelopathic rice to herbicide-resistant and -susceptible barnyardgrass remain unclear.
The critical roles that plant hormones playing in plant growth, development, and physiological responses to biotic and abiotic stresses have been extensively studied[
15,
16]. Biotic stress typically triggered complex changes in physiological processes in plants, such as growth changes and the synthesis of various protective compounds. In the case of systemic acquired resistance (SAR), the resulting protection was orchestrated through salicylic acid (SA) signaling, which mainly leaded to protection against biotrophic pathogens[
17,
18]. To regulate stress-induced adaptive responses, a complex network of hormonal signals in plants interacted with each other. Abscisic acid (ABA) was a major phytohormone that regulates stress responses and interacted with jasmonic acid (JA) and salicylic acid (SA) signaling pathways to allocate resources towards mitigating the effects of stresses[
19]. In response to brown planthopper infection, the levels of SA and JA in rice increased simultaneously[
20]. Therefore, it is crucial to study changes in various plant hormones in the reaction of rice to barnyardgrass stress.
In this study, quinclorac-resistant (R) and -susceptible (S) E. crus-galli lines were isolated from the progeny of a single plant. These two lines had a similar genetic background, and were utilized to investigate the above- and below-ground biomass as well as differences in root traits between allelopathic and non-allelopathic rice with barnyardgrass co-culture after 7 and 14 days. In addition, we measured the contents of four representative phytohormones in rice in response to barnyardgrass stress, which provided a theoretical basis for the hormone regulation level of weed control.
2. Materials and Methods
2.1. Plant Growth and Sampling
In this study, two rice cultivars, namely 'PI312777' (PI) and 'Lemont' (LE), were utilized as experimental materials to investigate allelopathic effects. PI is a well-known allelopathic cultivar, while LE is non-allelopathic. The stress treatment included quinclorac-resistant (R) and -susceptible (S) E. crus-galli lines, which were derived from a single plant. Each treatment was replicated four times in a completely randomized design. To initiate pre-germination, sterilized rice and barnyardgrass seeds were sown separately onto moistened filter paper in Petri dishes (9 cm diameter) and kept in a chamber maintained at 28 ℃.
The experiment was designed to include four treatments: PI (R), LE (R), PI (S), and LE (S), where PI and LE were co-cultured with R/S barnyardgrass, respectively. Additionally, PI and LE monocultures without any barnyardgrass stress were considered as the controls. Seven uniform rice seedlings were transplanted into each 96-well hydroponic box (12.7cm ×8.4cm ×11.4 cm), which contained 1L of Kimura B nutrient solution. Daily addition of distilled water was done to maintain a consistent volume, and replacement of Kimura B nutrient solution was carried out every 7 days. At the 2-leaf stage of rice, one pre-germinated barnyardgrass seedling was transplanted into the center of the hydroponic box (the co-culture ratio of rice and barnyardgrass was 7:1). After 7 and 14 days of co-culturing rice and barnyardgrass, the fresh weight of shoot and root of rice were measured after harvesting the rice seedlings. The roots were further collected to study morphological and proliferation traits.
2.2. Root Traits Calculation
The analysis of plant root traits was conducted according to the protocol described by Lupini et al(2016)[
21]. For root analysis, four randomly selected plants from each rice cultivar were used. The clean and undamaged roots were scanned using a Microtek ScanWizard EZ scanner (WSeen, China) to produce a grayscale image. The LA-S image analysis software (Hangzhou Wanshen Detection Technology Co., Ltd, China) was employed to process the image and determine the root length, root surface area, root volume in finer diameter cutoffs (0.5, 2.0, and 3.0mm) of fine-roots, and root tips number. These morphological traits were analyzed using the LA-S software.
2.3. Extraction, Purification and Determination of Plant Hormones in rice
The analysis of phytohormones in plant tissues was carried out in accordance with a modified protocol based on Zhou et al(2017)[
22]. A total of 200 mg of fresh plant tissues were subjected to cryogenic grinding using liquid N2, and subsequently extracted with 1 mL of 80% HPLC grade methanol (Merck, Germany) at 4℃ overnight. The extracted sample was centrifuged at 15000 g for 10 min, and the residue was subjected to re-extraction using 0.5 mL of 80% methanol. The supernatants obtained were vacuum freeze-dried at -60℃ and then reconstituted in 200 mL of 0.1 M sodium phosphate buffer (pH 7.8). The aqueous phase was purified using a Waters Sep-Pak C18 cartridge (Waters, USA) which was washed with 800 μL of ultrapure water, and then eluted with 1.4 mL of 80% methanol. The eluate with 80% methanol was vacuum freeze-dried, and the resulting dried extract was dissolved in 40 μL of 10% methanol. This solution was then utilized for LC-MS/MS assay in a Shimadzu LC-20AD-8030 Plus MS system (Shimadzu, Japan).
2.4. Statistical Analysis
The data were presented as means ± standard error (SE) derived from four replicates for each experiment or determination. A one-way analysis of variance (ANOVA) was employed to determine any significant differences among the treatments, followed by Tukey's honestly significant differences (HSD) tests at a significance level of P<0.05. When necessary, transformations were conducted to meet the assumptions of the ANOVA. Pearson’s correlation coefficients (r) between the root morphological traits and phyto-hormones contents were calculated for two rice cultivars with barnyardgrass at two periods. All data analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA).
3. Results
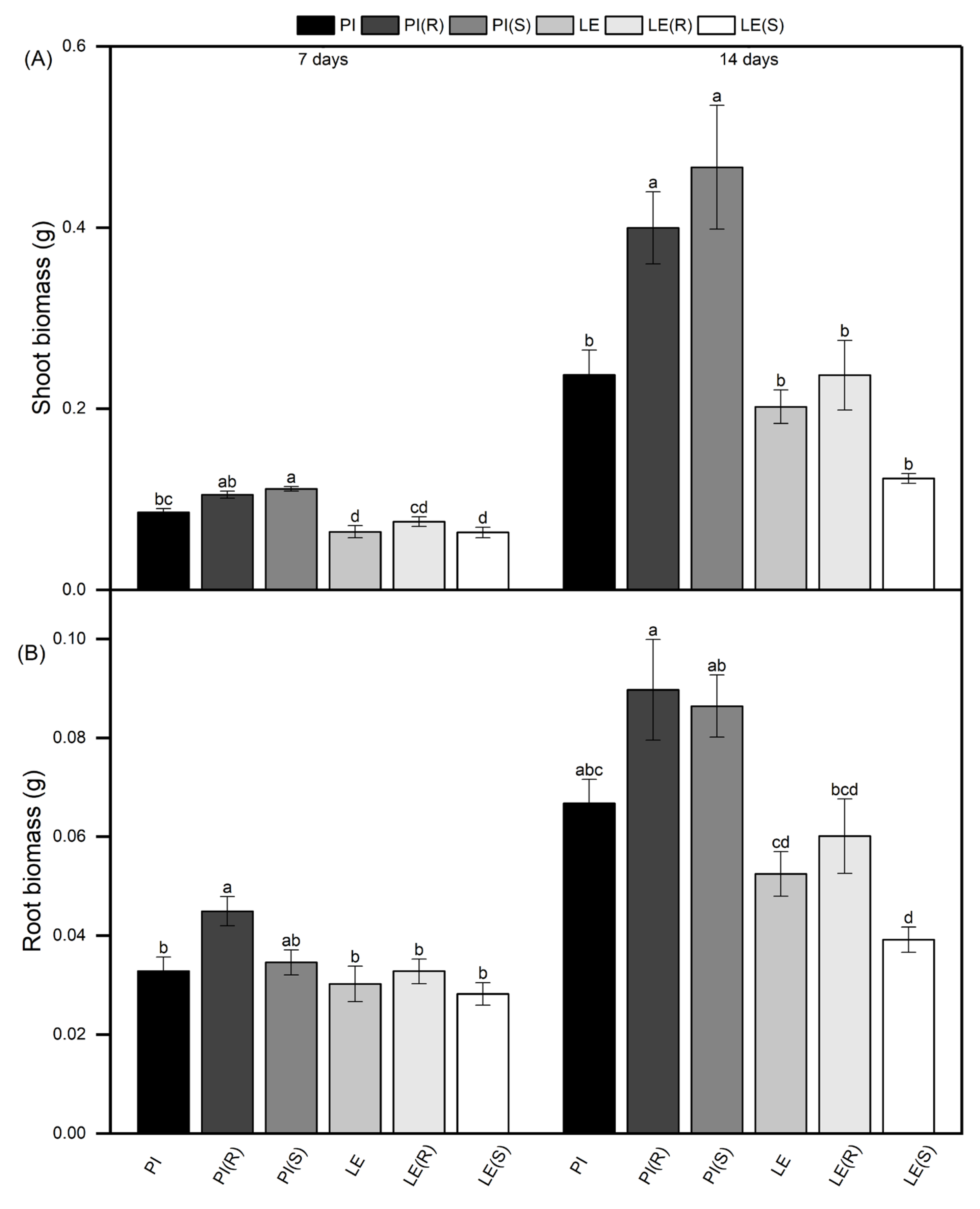
3.1. Different Reaction of Allelopathic Rice and Non-allelopathic Rice on Shoot and Root Biomass under Barnyardgrass Stress
Due to the competition between rice and barnyardgrass, differences were observed in the shoot and root biomass among allelopathic rice and non-allelopathic rice (
Figure 1). Specifically, PI displayed higher shoot and root biomass than LE in all treatments, indicating a stronger growth advantage. Notably, barnyardgrass stress resulted in a significant increase in the shoot and root biomass of PI in both time points. In contrast, LE showed increased shoot and root biomass only under resistant barnyardgrass stress, while susceptible barnyardgrass stress resulted in a decrease in the biomass. However, changes in shoot and root biomass of LE were not significant. Interestingly, PI (S) exhibited greater shoot biomass compared to PI (R), while the opposite trend was observed for root biomass. Additionally, under susceptible barnyardgrass stress, LE experienced a significant decrease of nearly 40% in shoot biomass and 25% in root biomass after 14 days of co-culture.
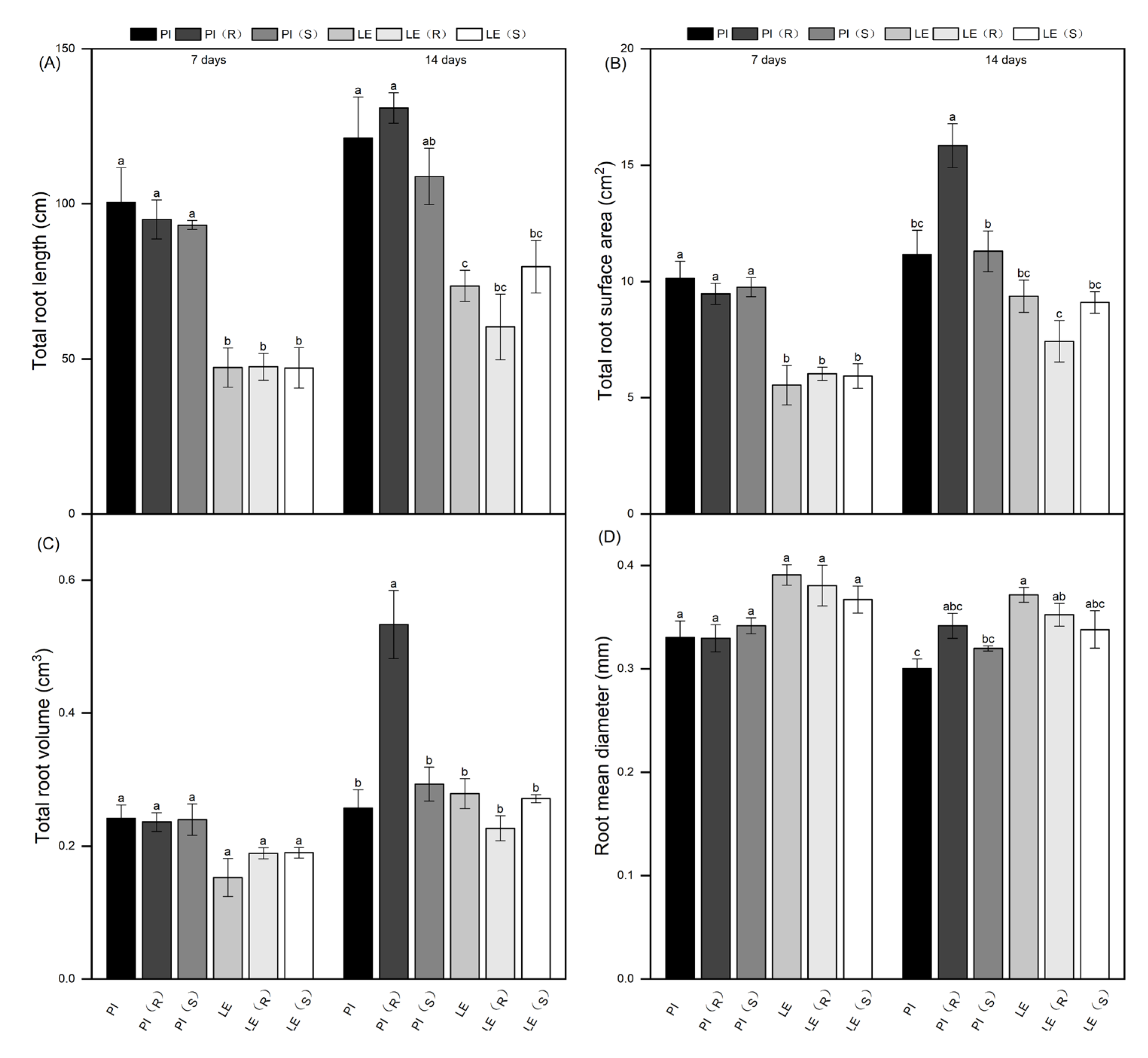
3.2. Analysis of Root Length, Root Surface Area, Root Volume and Root Diameter of Allelopathic and Non-allelopathic Rice under Barnyardgrass Stress
The root development differences between two rice cultivars were illustrated through root length, root surface area, root volume, and root diameter (
Figure 2). The findings indicated that PI had notably higher total root length and root surface area compared to LE. After 7 days of treatment, both rice cultivars displayed only marginal changes in total root length relative to their respective untreated controls. Following 14 days of co-cultivation with resistant barnyardgrass, PI showed a 10% increase in total root length, while LE exhibited a 20% decrease. Conversely, after 14 days of co-cultivation with susceptible barnyardgrass, total root length decreased slightly in PI, but increased in LE. A similar trend was observed in total root surface area for both rice cultivars at each treatment period. Resistant barnyardgrass co-culture stress significantly induced the total root volume of PI. Interestingly, the root mean diameter of LE was higher than that of PI. However, there was no significant difference in root mean diameter between allelopathic rice and non-allelopathic rice at 7 days of treatment, with a slight change in the two rice cultivars observed after 14 days.
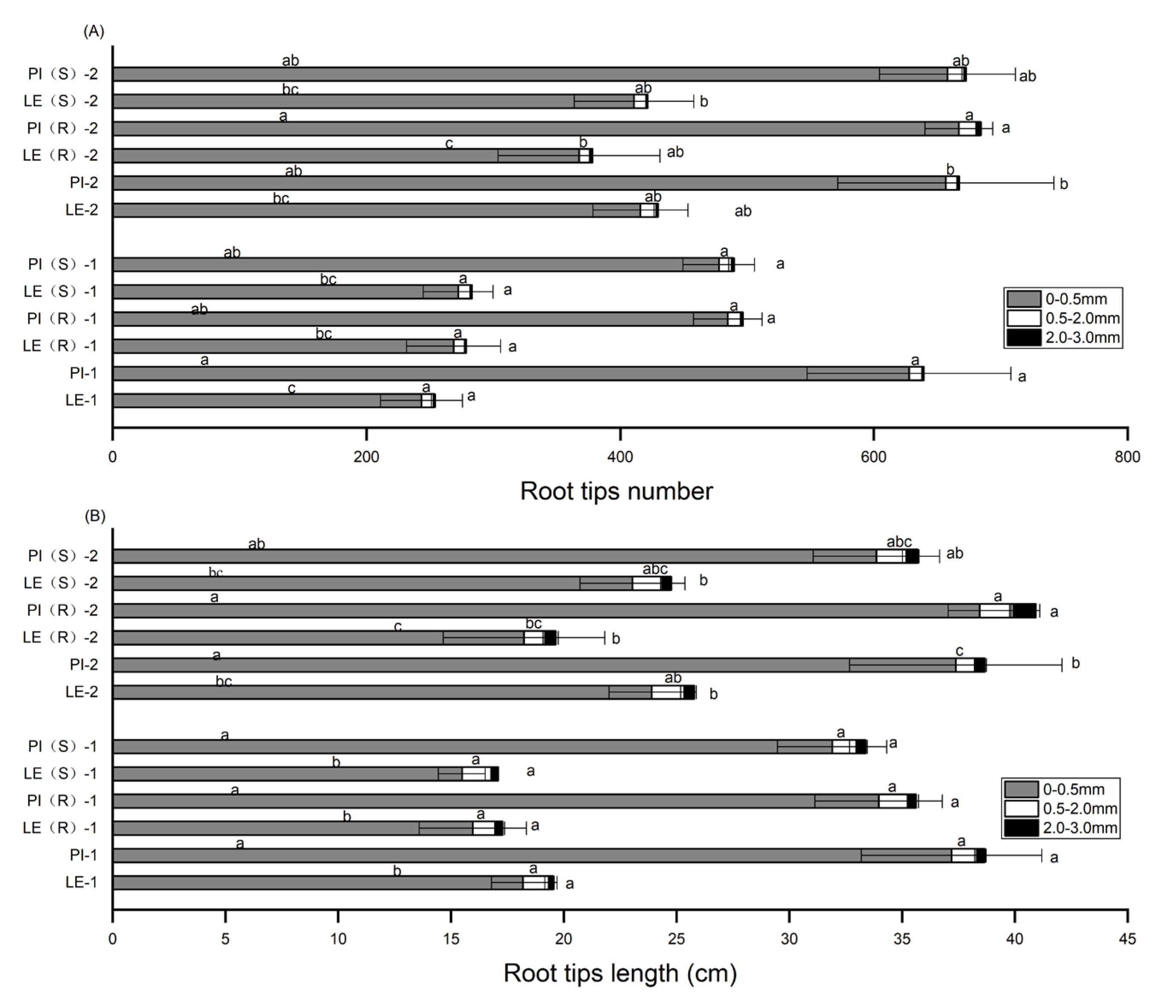
3.3. Changes of Root Tips Number and Root Tips Length of Allelopathic and Non-allelopathic Rice under Barnyardgrass Stress
The analysis of root tips number and root tips length in three diameter ranges: 0-0.5mm, 0.5-2.0mm, and 2.0-3.0mm were presented in
Figure 3. The findings revealed that 95-98% of the roots in both PI and LE belonged to the fine root category, with diameters <0.5mm. Notably, the total root tips number and root tips length of PI in the diameter <0.5mm category were significantly higher than those of LE under the different treatments.
At 7 days, a decrease in root tips number was observed in PI, while an increase was observed in LE, relative to their respective untreated controls. This trend was reversed at 14 days. Under the stress of resistant barnyardgrass co-culture, root tips number in PI increased and reached a peak at 14 days. Conversely, resistant and susceptible barnyardgrass co-culture resulted in a 23% and 24% decrease, respectively, in root tips number of PI at 7 days. Subsequently, a slight increase of 2% and 0.2% in root tips number was observed in PI in response to resistant and susceptible barnyardgrass individually at 14 days.
Following 7 days of treatment, a reduction in root tips length was observed in both PI and LE varieties under barnyardgrass-induced stress, with the former exhibiting a 9% and 14% decrease in root tips length in response to resistant and susceptible barnyardgrass, respectively, compared to the controls. Additionally, the root tips length of LE also decreased under barnyardgrass-induced stress. Notably, the peak root tips length in PI occurred at day 14 in the presence of resistant barnyardgrass co-culture.
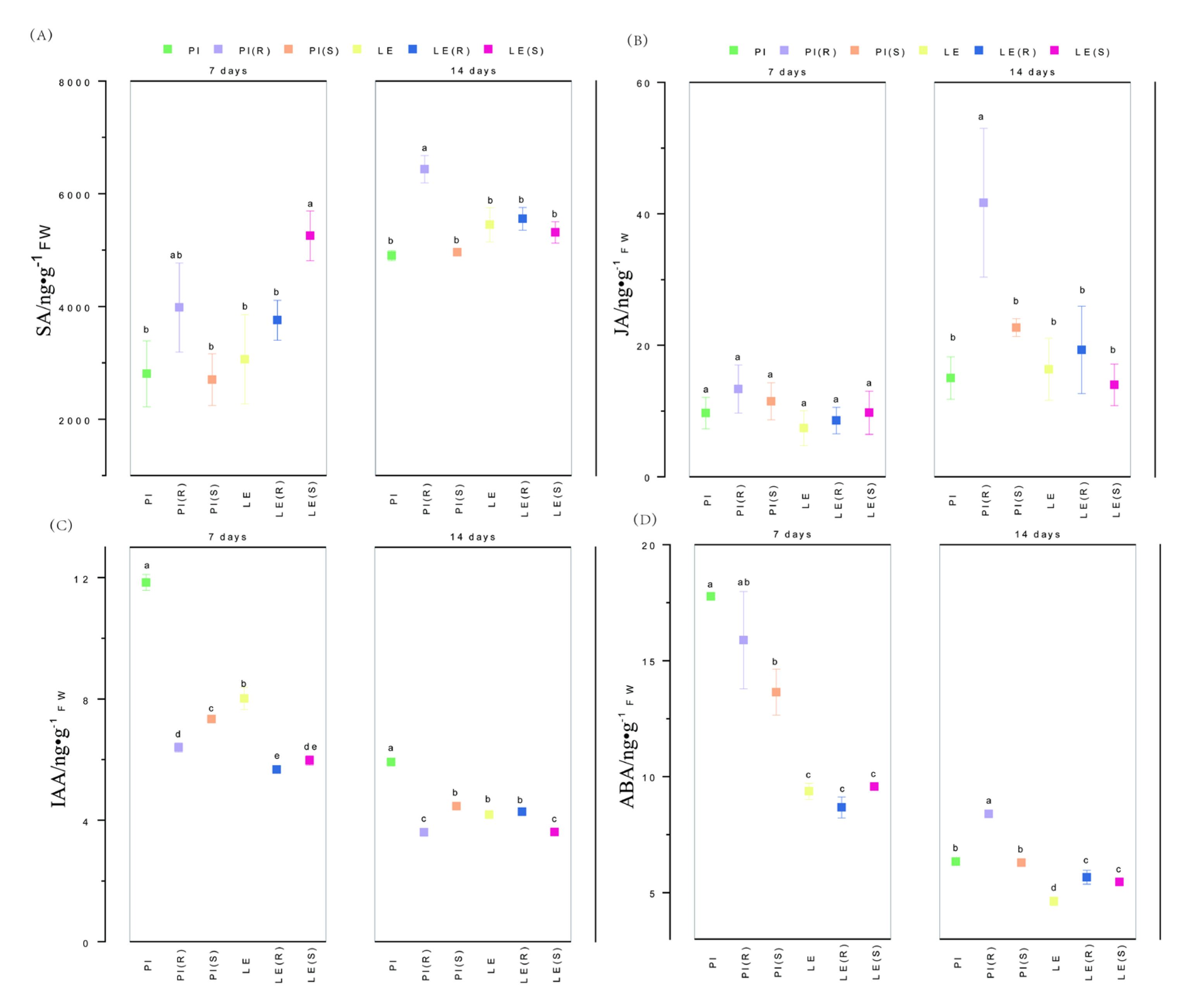
3.4. Impact of Barnyardgrass Stress on Phytohormones Level
This experiment aimed to estimate the levels of four distinct plant hormones in allelopathic and non-allelopathic rice varieties under barnyardgrass-induced stress (
Figure 4). The analysis revealed a significant decrease in IAA and ABA levels over time, whereas SA and JA levels increased. Notably, SA levels were consistently higher in PI (R) than in PI and PI (S) at both time points, with the highest concentration observed in PI (R) at 14 days. Moreover, SA content was relatively higher in LE(S) than in the other treatments at 7 days. At 14 days, there was no significant difference observed in SA levels in LE under different stress conditions. Similar to SA, the peak level of JA was detected in PI (R) at 14 days, with a similar pattern of changes observed in both rice samples. Compared to the control, a reduction in IAA content was noted in PI and LE under both R and S barnyardgrass stress. Additionally, IAA content was notably lower in PI (R) and LE (S) at 14 days. Moreover, ABA content was consistently higher in PI than in LE under barnyardgrass-induced stress and was reduced by barnyardgrass co-culture at 7 days. However, at 14 days, ABA content was significantly higher in PI (R), and its levels were induced by R and S stress in LE.
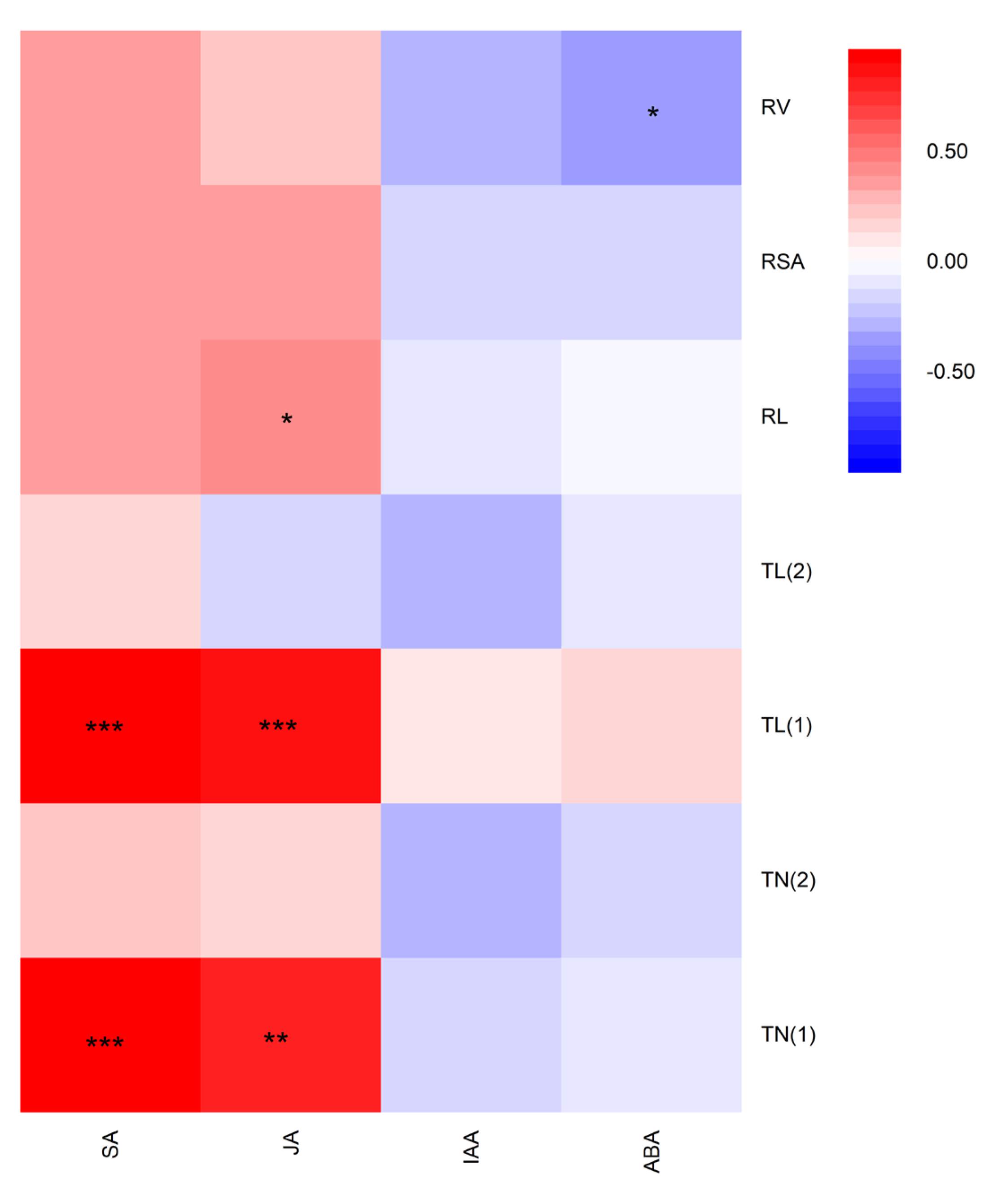
3.5. Correlation Between Root Traits and Phytohormones Contents
Highly significant and positive correlations were found between SA content and root tips number (r = 0.962, P < 0.001) and root tips length (r=0.919, P < 0.001) at 0-0.5mm. However, SA content was not significantly correlated with the total root length, root surface area, root volume, root tips number and root tips length at 0.5–2.0 mm (
Figure 5). Similarly, JA was highly-significantly correlated with the root tips number (r = 0.807, P <0.01) and root tips length at 0-0.5mm (r =0.847, P <0.001), but not significantly correlated with root tips number and root tips length at 0.5-2.0mm, root surface area and root volume. In contrast, the contents of IAA and ABA had the slightly negative correlation with fine-root traits. In addition, ABA was negatively correlated with total root volume (r=-0.359, P <0.05). In summary, there were significant correlations among SA and JA contents of rice with root tips number and root tips length at 0-0.5mm.
4. Discussion
Barnyardgrass, a C4 plant, exhibits higher photosynthetic rate and utilization efficiency compared to rice[
23]. The presence of barnyardgrass co-culture significantly reduces the leaf photosynthetic rate, root biomass, root oxidation activity, and dry matter accumulation of rice[
24]. Despite the efficacy of chemical herbicides in controlling weeds, their frequent use results in the selection of herbicide-resistant barnyardgrass strains, leading to environmental and health issues. Therefore, non-herbicide control tactics are crucial in reducing selection pressure for weed resistance evolution. The importance of allelopathic interactions between rice and barnyardgrass, and the mechanisms that underlie them, have been a significant area of research in non-herbicidal alternative barnyardgrass management for an extended period[
25,
26].
Plants with greater phenotypic plasticity have a better chance of survival in response to environmental changes[
27]. Previous studies found that the root biomass was relatively larger than non-allelopathic rice cultivar[
28,
29]. In this study, it was found that the allelopathic rice cultivar “PI312777” (PI) showed greater competitiveness with higher shoot and root biomass than the non-allelopathic rice cultivar “Lemont” (LE) (
Figure 1). Additionally, under barnyardgrass stress, PI exhibited increased shoot and root bio-mass, LE showed similar trend in response to resistant barnyardgrass stress, whereas the figures were decrease under susceptible barnyardgrass stress. These findings suggested that the allelopathic rice cultivar might pose a stronger neighbor-identity recognition ability in response to barnyardgrass stress, compared to the non-allelopathic rice cultivar. Notably, we observed more pronounced changes in shoot and root biomass of PI when co-cultured with barnyardgrass, indicating the role of chemically mediated signaling interactions between allelopathic crop and competing weeds in influencing crop growth. Plant interactions within mixed-species systems were shaped by the competition for growth resources both above and below ground, with below-ground interactions having a more significant impact on the performance of coexisting plants than aboveground interactions[
30]. This study found that while the shoot biomass of PI (S) was greater than that of PI (R), the opposite trend was observed in root biomass. Thus, we hypothesized that allelopathic rice cultivars may confer a competitive advantage for underground growth under resistant barnyardgrass stress.
Below-ground ecological interactions greatly affected crop-weed allelopathic action[
31]. The development and interaction of roots played crucial roles in the interference of allelopathic rice with barnyardgrass[
14]. Root traits were significantly positive correlated to allelopathic inhibition in rice root-exudates[
30]
. It was found that PI exhibited significantly higher fine-root length, more root tips number and greater root tips length compared to LE (
Figure 2), in agreement with previously reported results by Li
et al(2019)[
30]. After 14 days of co-culture, the total root surface and root volume of PI were significantly induced by resistant barnyardgrass, with the greatest root tips length detected at this time point. Consequently, PI accumulated and released more allelochemicals into the culture solution, resulting in high inhibition of the target plant. Smaller diameter cutoffs of root (e.g., 1.0 or 0.5 mm) were considered to be more functional fine roots[
33]. The study revealed that 95-98% of the roots of both PI and LE had fine roots with a diameter of less than 0.5 mm. Additionally, the total number of root tips and root tips length with a diameter of less than 0.5 mm in PI was higher than that in LE across different treatments (
Figure 3). There could be significant correlations between the allelopathic potential of rice and the length of fine-root tips with a diameter of 0-0.5 mm.
The ubiquitous signaling chemicals, SA and JA, have been shown to elicit the production of defensive plant metabolites against microbes, herbivores, or competitors [
34,
35]. It was found that the contents of both SA and JA were significantly increased between 7 to 14 days (
Figure 4 A and B). In addition, the levels of SA and JA were significantly higher in PI co-cultivated with resistant barnyardgrass compared to PI alone or PI co-cultivated with susceptible barnyardgrass. These results indicated that the SA and JA-dependent defense mechanisms were triggered in response to barnyardgrass stress. The most potent allelopathic ability of PI was observed at 14 days, as evidenced by the highest SA and JA contents in PI (R). This finding was consistent with the results of root traits in allelopathic rice in the presence of resistant barnyardgrass. The data generated in this study indicated that the levels of IAA and ABA tended to decrease from 7 to 14 days (
Figure 4 C and D). The authors hypothesized that the allelopathic effects on barnyardgrass were inversely correlated with the contents of IAA and ABA. Auxin not only played a crucial role in plant growth and development but was also closely associated with plant biotic stress[
15,
36]. The barnyardgrass stress resulted in a decrease in IAA content in both rice materials. Additionally, the experiment revealed the response variation rules of rice ABA content under barnyardgrass stress, likely due to the antagonistic role of ABA and SA signaling pathways in rice[
37]. Through correlation analysis, we found that SA and JA in the shoots of rice seedlings were significantly correlated with rice fine-root traits of root tips number and root tips length at 0-0.5mm (
Figure 5), indicating that SA and JA had a potential positive regulation role to suppress barnyadgrass growth in allelopathic rice.
5. Conclusions
This study revealed that the allelopathic rice cultivar “PI” demonstrated greater competitive ability with respect to shoot and root biomass as compared to the non-allelopathic rice cultivar “LE” under conditions of barnyardgrass stress. Meanwhile, allelopathic rice cultivars exhibited significantly greater length of fine roots with a diameter of less than 0.5 mm, increased number of root tips, and longer root tips, as compared to the non-allelopathic rice cultivar co-cultivated with barnyardgrass in a hydroponic system. The results also demonstrated significant correlations between rice allelopathic potential and rice fine-root tips length at 0-0.5 mm in diameter. Additionally, barnyardgrass stress triggered SA and JA-dependent defense responses. The contents of SA and JA in the shoots of rice seedlings were significantly-positviely correlated with root tips number and root tips length at 0-0.5mm. To illustrate the regulating effects of phytohormone-based interactions in improving the ability of rice to resistant barnyardgrass stress, further study is essential to analyse the SA and JA -related gene expression and enzyme activity.
Author Contributions
Conceptualization, Q.Y., J.T., and S.L.; methodology, Q.Y. and Q.P.; software, Q.Y.; validation, J.T., S.L. and Q.P.; formal analysis, Q.P.; investigation, Q.Y., S.L. and J.T.; resources, Q.Y. and Q.P.; data curation, Q.Y.; writing—original draft preparation, Q.Y.; writing—review and editing, Q.Y. and Q.P.; visualization, S.L. and J.T.; supervision, Q.P.; project administration, Q.L. and Q.P.; funding acquisition, Q.P. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: This research was financially supported by the National Natural Science Foundation of China (Grant No.U22A20461, 32261143733).
Institutional Review Board Statement
Not applicable
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aoki, D.; Yamaguchi, H. Genetic relationship between Echinochloa crus-galli and Echinochloa oryzicola accessions inferred from internal transcribed spacer and chloroplast DNA sequences. Weed Biol Manag 2008, 8, 233–242. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Cao, J.J.; Gu, T.; Yang, X.; Peng, Q.; Bai, L.Y.; Li, Y.F. Co-planted barnyardgrass reduces rice yield by inhibiting plant above-and belowground-growth during post-heading stages. Crop J 2021, 9, 1198–1207. [Google Scholar] [CrossRef]
- Oerke, E.C.; Dehne, H.W. Safeguarding production-losses in major crops and the role of crop protection. Crop Prot 2004, 23, 275–285. [Google Scholar] [CrossRef]
- Peterson, M.A.; Collavo, A.; Ovejero, R.; Shivrain, V.; Walsh, M.J. The challenge of herbicide resistance around the world: a current summary. Pest Manag Sci 2018, 74, 2246–2259. [Google Scholar] [CrossRef]
- Heap, I. Global perspective of herbicide-resistant weeds. Pest Manag Sci 2014, 70, 1306–1315. [Google Scholar] [CrossRef]
- Baucom, R.S. Evolutionary and ecological insights from herbicide-resistant weeds: what have we learned about plant adaptation, and what is left to uncover ? New Phytol 2019, 223, 68–82. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Farooq, M.; Jabran, K.; Cheema, Z.A.; Wahid, A.; Siddique, K.H.M. The role of allelopathy in agricultural pest management. Pest Manag Sci 2011, 67, 493–506. [Google Scholar] [CrossRef]
- Meiners, S.J.; Kong, C.H.; Ladwig, L.M.; Pisula, N.L.; Lang, K.A. Developing an ecological context for allelopathy. Plant Ecol 2012, 213, 1221–1227. [Google Scholar] [CrossRef]
- Kong, C.H.; Chen, X.H., Hu, F., Zhang, S.Z. Breeding of commercially acceptable allelopathic rice cultivars in China. Pest Manag Sci 2011, 67, 1100–1106. [CrossRef]
- Kong, C.H.; Zhang, S.Z.; Li, Y.H; Xia, Z.H.; Yang, X.F.; Meiners, S.J.; Wang, P. Plant neighbor detection and allelochemical response are driven by root-secreted signaling chemicals. Nat Commun 2018, 9, 3867. [Google Scholar] [CrossRef]
- Yang, X.F.; Kong, C.H. Interference of allelopathic rice with paddy weeds at the root level. Plant Biol (Stuttg) 2017, 19, 584–591. [Google Scholar] [CrossRef]
- Yang, X.F.; Kong, C.H.; Yang, X.; Li, Y.F. Interference of allelopathic rice with penoxsulam-resistant barnyardgrass. Pest Manag Sci 2017, 73, 2310–2317. [Google Scholar] [CrossRef]
- Rahaman, F.; Juraimi, A.S.; Rafii, M.Y.; Uddin, M.K.; Hassan, L.; Chowdhury, A.K.; Bashar, K.H.M. Allelopathic effect of selected rice (oryza sativa) varieties against barnyard grass (Echinochloa cruss-gulli). Plants 2021, 10, 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, Y. Current understanding of the interplays between host hormones and plant viral infections. PLoS Pathog 2021, 17, e1009242. [Google Scholar] [CrossRef]
- Aerts, N.; Pereira, M.M.; VanWees, S.C.M. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Peng, Y.J.; Yang, J.F.; Li, X.; Zhang, Y.L. Salicylic acid: biosynthesis and signaling. Annu Rev Plant Biol 2021, 72, 761–791. [Google Scholar] [CrossRef]
- Liang, B.; Wang, H.; Yang, C.; Wang, L.Y.; Qi, L.L.; Guo, Z.J.; Chen, X.J. Salicylic acid is required for broad-spectrum disease resistance in rice. Int J Mol Sci 2022, 23, 1354. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.S.; Sintaha, M.; Cheung, M.Y.; Lam, H.M. Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int J Mol Sci 2018, 19, 3206. [Google Scholar] [CrossRef] [PubMed]
- Harun-Or-Rashid, M.; Kim, H.J.; Yeom, S.I.; Yu, H.A.; Manir, M.M.; Moon, S.S.; Kang, Y.J.; Chung, Y.R. Bacillus velezensis YC7010 enhances plant defenses against brown planthopper through transcriptomic and metabolic changes in rice. Front Plant Sci 2018, 9, 1904. [Google Scholar] [CrossRef]
- Lupini, A.; Sorgonà, A.; Princi, M.P.; Sunseri, F.; Abenavoli, M.R. Morphological and physiological effects of trans-cinnamic acid and its hydroxylated derivatives on maize root types. Plant Growth Regul 2016, 78, 263–273. [Google Scholar] [CrossRef]
- Zhou, L.J.; Xiao, L.T.; Xue, H.W. Dynamic cytology and transcriptional regulation of rice lamina joint development. Plant Physiol 2017, 174, 1728–1746. [Google Scholar] [CrossRef]
- Cui, H.C. Challenges and approaches to crop improvement through C3-to-C4 engineering. Front Plant Sci 2021, 12, 715391. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Gu, T.; Zhao, B.; Yang, X.; Peng, Q.; Li, Y.; Bai, L.Y. Effects of common echinochloa varieties on grain yield and grain quality of rice. Field Crops Res 2017, 203, 163–172. [Google Scholar] [CrossRef]
- Alam, M.A.; Hakim, M.A.; Juraimi, A.S.; Rafii, M.Y.; Hasan, M.M.; Aslani, F. Potential allelopathic effects of rice plant aqueous extracts on germination and seedling growth of some rice field common weeds. Ital J Agron 2018, 13, 134–140. [Google Scholar] [CrossRef]
- Sun, B.; Wang, P.; Kong, C.H. Plant-soil feedback in the interference of allelopathic rice with barnyardgrass. Plant Soil 2014, 377, 309–321. [Google Scholar] [CrossRef]
- Reiss, A.; Fomsgaard, I.S.; Mathiassen, S.K.; Stuart, R.M.; Kudsk, P. Weed suppression by winter cereals: relative contribution of competition for resources and allelopathy. Chemoecology 2018, 28, 109–121. [Google Scholar] [CrossRef]
- Gealy, D.R.; Moldenhauer, K.A.K. Use of 13C isotope discrimination analysis to quantify distribution of barnyardgrass and rice roots in a four-year study of weed suppressive rice. Weed Sci 2012, 60, 133–142. [Google Scholar] [CrossRef]
- Gealy, D.R.; Moldenhauer, K.A.K.; Duke, S. Root distribution and potential interactions between allelopathic rice, sprangletop (Leptochloa spp.), and barnyardgrass (Echinochloa crus-galli) based on 13C isotope discrimination analysis. J Chem Ecol 2013, 39, 186–203. [Google Scholar] [CrossRef] [PubMed]
- Wardle, D.A.; Bardgett, R.R.; Klironomos, J.N.; Setala, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Sci 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Z.; Li, Y.H.; Kong, C.H.; Xu, X.H. Interference of allelopathic wheat with different weeds. Pest Manag Sci 2016, 72, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Lin, S.X.; Zhang, Q.X.; Zhang, Q.; Hu, W.; He, H.B. Fine-root traits of allelopathic rice at the seedling stage and their relationship with allelopathic potential. PeerJ 2019, 7, e7006. [Google Scholar] [CrossRef] [PubMed]
- Iversen, C.M.; McCormack, M.L.; Powell, A.S.; Blackwood, C.B.; Freschet, G.T.; Kattge, J.; Roumet, C.; Stover, D.B.; Soudzilovskaia, N.A.; Valverde-Barrantes, O.J.; Bodegom, P.M.; Violle, C. A global fine-root ecology database to address below-ground challenges in plant ecology. New Phytol 2017, 215, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Medina, A.; Fernandez, I.; Lok, G.B.; Pozo, M.J.; Pieterse, C.M.; Wees, V.S.C. Shifting from priming of salicylic acid- to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol 2017, 213, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Vo, K.T.X.; Rahman, M.M.; Rahman, M.M.; Trinh, K.T.T.; Kim, S.T.; Jeon, J.S. Proteomics and metabolomics studies on the biotic stress responses of rice: an update. Rice 2021, 14, 30. [Google Scholar] [CrossRef]
- Kunkel, B.N.; Johnson, J.M.B. Auxin plays multiple roles during plant-pathogen interactions. CSH Perspect Biol 2021, 13, a040022. [Google Scholar] [CrossRef]
- Xu, J.; Audenaert, K.; Hofte, M.; Vleesschauwer, D.D. Abscisic acid Promotes susceptibility to the rice leaf blight pathogen Xanthomonas oryzae pv oryzae by suppressing salicylic acid-mediated defenses. PloS One 2013, 8, e67413. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).