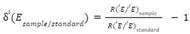1. Background
In the recent years there has been a growing demand for organic products as consumers consider them as safer and healthier than conventional products. Several studies have been carried out over the years to investigate the authenticity of products obtained using organic cultivation methods. They have focused on the identification of new quality "markers" that allow to differentiate, from field to fork, the organic product compared to the conventional one. In particular, the monitoring of some chemical components, deriving from primary and/or secondary metabolism of organic and conventional products, has highlighted the diversity induced by the two production techniques. Indeed, based on biochemical and transcriptional approaches, crucial evidences have been provided about the physiological changes which can occur in organic and conventional cultivated crops. Moreover, it is well known that the difference in fertilization practices of the two cultivation methods influences the isotopic distribution of some elements present in fruits and vegetables, with particular reference to nitrogen (N). Starting from this shared knowledge, this paper proposes an innovative approach for the N fertilization traceability of organic farming products.
2. Up-to Date Literature
Following the sustainable development goals introduced by the United Nations [
1] and the targets of the European green deal [
2], it is known that one of the main worldwide shared goal is to increase the percentage of organically managed farms within 2030. The focused target is to have the 25% of the whole European cultivated area devoted to organic agriculture. In this context, it has been widely discussed how organic agriculture is more environmental sustainable in respect to conventional farming for different reasons, including lower nutrients losses, reduced fossil energy consumption and the ban of synthetic pesticides and fertilizers. [
3]. One of the most important aspects of organic agriculture is to manage N fertilization since the synthetic fertilizers are not allowed. In fact, the N is a crucial element for plant growth and it has been estimated that about half amount of the N provided to the soil is lost [
4] and therefore there is a need to synchronize the amount of N demand and the N supply especially when different fertilization strategies are adopted [
5]. Furthermore, the consumers are increasingly interested in organic food and they strongly demand for reliability of the traceability of the organic products they usually buy and eat. The actual European regulation (EU Reg. 2018/848) establishes that official controls are to be performed in accordance with Regulation (EU) 2017/625 (art. 14) [
6]. Specifically, this regulation establishes that official control methods and techniques shall include examination of documents and traceability records which may be relevant to the assessment of compliance with the rules of the organic management. As a matter of the fact, in most of the organic farms, the only traceability system actually in place is based on the documentary evidence that agricultural management complies with European regulation. Also, multiresidue analysis are carried out only upon specific request of the certification bodies or the distribution buyers and relative samplings are randomly performed [
7]. Indeed, this could not be enough to certify the authenticity of organic products as farmers could fraudulently use fertigation to supply mineral fertilizers to the soil or apply pesticides observing large shortage intervals and all these behaviors would not be easily detected [
8]. So, it comes the need of an objective traceability of the organic management, based on scientifically collected data [
9].
The possibility of differentiating two samples on the basis of isotopic analysis is linked to isotopic fractionation that occurs in raw materials. Isotopic ratios of natural elements such as nitrogen, carbon, oxygen, hydrogen, and sulfur have been conveniently used for food authenticity purposes as their fractionation occur in response to very different biogeochemical phenomena [
10]. It has been demonstrated that isotopic analysis can be reliably used to check for food authenticity [
11,
12,
13]. Specifically for organic products, a deviation from the natural isotopic distribution of N happens during the production and maturation of organic fertilizers. Indeed, an enrichment of the lighter isotope (
14N) happens, due to its preferential volatilization. As a consequence, the N isotopic ratio [
δ(
15N) =
15N/
14N]
is increased in organic fertilizers and amendments of both vegetable and animal origin [
14]. On the other hand, air has more or less the same N isotopic composition throughout the world (
δ(
15N) = 0‰ by definition and internationally recognized) and industrially produced mineral fertilizers typically have
δ(
15N) close to 0 ‰, because their N directly derives from atmospheric nitrogen and there is basically no isotope fractionation during their industrial synthesis [
14]. Conversely, organic fertilizers allowed in organic farming have positive values of
δ(
15N) (ranging from +1‰ to +37‰), therefore being easily distinguishable from synthetic fertilizers. By the way, it has been demonstrated that these differences are reflected in the final vegetable product, thereby confirming the great potential of N isotopic analysis to check the authenticity of organic products [
14,
15,
16,
17,
18,
19,
20,
21,
22,
23]. At the same time, it must be stressed that it has been widely argued that
δ(
15N) may not be discriminating for all the crops and in every management condition, e.g., when organic fertilizers are contemporarily employed also in conventional practice and vice versa, or for crops which are characterized by low N requirements [
23]. In addition, it is also worth noting that, few studies on the effect of the employment of agricultural service crops (such as N-fixing plants (legumes) or not N-fixing plants) for green manure in organic management on the isotopic signature of the final product have been reported [
24]. As a matter of fact, N-fixing plants fix the nitrogen from the air (having
δ(
15N) values close to 0 ‰) in root nodules, thus N isotopic signature of the final products could reflect this root uptake, putatively leading to misclassification of authentic organic products.
Several studies have shown that organically grown fruit are richer in antioxidants compared with conventionally grown fruit [
25,
26,
27,
28]. Since many years, the theory according to which N deficiency may induce the production of carbon-rich phenylpropanoids has been postulated as an explanation of the higher healthy value of organic products. Recent research [
29] showed that long-term organic fields have been associated with no consistent difference in N and carbon (C) content, nor in C:N ratio, between the organic and conventional produces. At the same time, pest attacks were preferentially settled on conventional plants respect to organic ones. Organic management reduced insect population but no evidence about plant nutrient unbalance was directly linked with. The study demonstrated that organic soil management promoted salicylic acid build-up, which resulted in discouraging plant–insect interactions. Indeed, salicylic acid accumulation was not associated with lower nitrogen content of organic plants, but it depended on alterations in soil microbial communities associated with long-term organic management. Based on recent experimental evidence reported on how soil microbiota influences plant resistance to pests, it may also be hypothesized that polyphenolics may accumulate in organic plant due to functional shifts in soil microflora. Furthermore, based on a biochemical and molecular approach, it has been recently demonstrated [
30] that the different fertilization practice (organic or conventional) induced the different accumulation of free amino acids, with higher concentrations of proline and contemporarily lower concentrations of glutamate, in addition to the upregulation of glutamate dehydrogenase gene expression, in fruits from organically managed orange trees, thus identifying a possible adaptive response of common orange plants to organic fertilizers based on a metabolism switch aimed at ensuring the turnover of the tricarboxylic acid cycle to continuous supply carbon skeletons for biosynthetic demands, as a regulatory phenomenon.
3. Methodology
In order to achieve the goal of implementing a reliable system for tracing the authenticity of organic products, different treatments should be adopted and compared. One of the possibilities is to include in the experimental design conventional, organic and mixed N fertilization strategies, comparing them at different percentages of fertilizers. It is also relevant to include the application of agronomic methods for the management of soil fertility, such as the introduction of agroecological service crops [
31]. The variations of
δ(
15N) induced by the combination of the different fertilizations together with the use of agroecological service crops in the organic production system should be quantified, thus evaluating how the soil management based on the agroecological approach affects the
δ(
15N) in the final product, in comparison with the organic management based on the full substitution and/or conventional agriculture approaches. At the same time, the variations in physicochemical and nutraceutical attributes induced by the different treatments should be evaluated. Once collected, the obtained data should be treated by a chemometric multivariate approach (such as Linear Discriminant Analysis) able to discriminate, at a high confidence level, between organic and conventional products. Finally, the use of the implemented database should be validated on a multiyear basis.
4. Perspectives
The main aim of the present communication is to provide insights and general suggestions for the application of a feasible methodology for the implementation of a chemical and chemometric investigation system. In particular, the main perspectives of the application of this kind of approach are linked to the possibility to: i) allow, through the acquisition of isotopic data and other chemical and biochemical parameters, to discern between productions obtained with synthetic fertilizers, typically employed in conventional agriculture and not allowed in organic agriculture, and productions obtained with the organic agriculture method; ii) improve the general understanding of the factors influencing the quality of organic products and their differentiation from conventional ones; iii) allow the traceability of organic products from field to fork which, in turn, would contribute to protect and reward virtuous farmers; iv) valorize the environmental externalities linked to the application of organic farming practices and v) support policy makers in identifying methodologies for contrasting food fraud.
Author Contributions
“Conceptualization, S.F., L.B., G.C., S.C. and F.M.; methodology, S.F., L.B., G.C., S.C. and F.M.; resources, S.F., L.B., G.C., S.C. and F.M.; writing—original draft preparation, S.F., L.B. and F.M.; writing—review and editing, S.F., L.B., G.C., S.C. and F.M.; visualization, S.F., L.B., G.C., S.C. and F.M.; supervision, S.F., L.B. and F.M.; project administration, S.F.; funding acquisition, S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Italian Ministry of Agriculture, Food Sovereignty and Forestry, grant number 93173/2017, 'Application of innovative methods for the traceability of organic farming products' – INNOVABIO project and by European Commission Research Executive Agency, Grant Agreement (GA) Number 101084527, ‘Supporting UPtake Integrated Pest Management and lOw-Risk pesTicide Use’ – SUPPORT project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United Nations. Available online: https://www.un.org/sustainabledevelopment/ (accessed on May, 15 2023).
- European Commission. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on May, 15 2023).
- Reganold, J.P. , Wachter, J.M. Organic agriculture in the twenty-first century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef] [PubMed]
- Diacono, M. , Montemurro, F. Towards a Better Understanding of Agronomic Efficiency of Nitrogen: Assessment and Improvement Strategies. Agronomy 2016, 6, 31. [Google Scholar] [CrossRef]
- Persiani, A. , Diacono, M., Monteforte, A., Montemurro, F. Agronomic performance, energy analysis and carbon balance comparing different fertilization strategies in horticulture under Mediterranean conditions. Environ. Sci. Pollut. Res. 2019, 26, 19250–19260. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on organic production and labelling of organic products and repealing Council Regulation (EC) No 834/2007.
- Ambrus, A. Reliability of measurements of pesticide residues in food. Accred. Qual. Assur. 2004, 9, 288–304. [Google Scholar] [CrossRef]
- Rapisarda, P. , Calabretta, M.L., Romano, G., Intrigliolo, F. Nitrogen Metabolism Components as a Tool to Discriminate between Organic and Conventional Citrus Fruits. J. Agric. Food Chem. 2005, 53, 2664–2669. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission, CAC/GL 60-Principles for Traceability / Product Tracing as a Tool Within a Food Inspection and Certification System, 2006. Available online: www.codexalimentarius.net/input/download/standards/.../CXG_060e.pdf (accessed on May, 15 2023).
- Picó, Y., Barceló, D. Isotopic Mass Spectrometry in Food and Environmental Chemistry. In: Picó, Y., Campo, J. (eds) Mass Spectrometry in Food and Environmental Chemistry. The Handbook of Environmental Chemistry, 2022, vol 119. Springer, Cham. [CrossRef]
- Zhi, L., Yuan, W., Yudi, H., Wei, L., Bin, L., Guiyuan, M. Multi-stable isotope and multi-element origin traceability of rice from the main producing regions in Asia: A long-term investigation during 2017–2020, Food Chem. 2023, 412, 135417. [CrossRef]
- Bontempo, L.; Perini, M.; Pianezze, S.; Horacek, M.; Roßmann, A.; Kelly, S.D.; Thomas, F.; Heinrich, K.; Schlicht, C.; Schellenberg, A.; Hoogewerff, J.; Heiss, G.; Wimmer, B.; Camin, F. Characterization of Beef Coming from Different European Countries through Stable Isotope (H, C, N, and S) Ratio Analysis. Molecules 2023, 28, 2856. [Google Scholar] [CrossRef] [PubMed]
- Popîrdă, A.; Luchian, C.E.; Colibaba, L.C.; Focea, E.C.; Nicolas, S.; Noret, L.; Cioroiu, I.B.; Gougeon, R.; Cotea, V.V. Carbon-Isotope Ratio (δ13C) and Phenolic-Compounds Analysis in Authenticity Studies of Wines from Dealu Mare and Cotnari Regions (Romania). Agronomy 2022, 12, 2286. [Google Scholar] [CrossRef]
- Bateman, A.S., Kelly. Nitrogen Isotope Relationships between Crops and Fertilizer: Implications for Using Nitrogen Isotope Analysis as an Indicator of Agricultural Regime. J. Agric. Food Chem. 2005, 53, 5760–5765. [Google Scholar] [CrossRef]
- Flores, P. , Fenoll, J., Hellin, P. The Feasibility of Using δ15N and δ13C Values for Discriminating between Conventionally and Organically Fertilized Pepper (Capsicum annuum L.). J. Agric. Food Chem. 2007, 55, 5740–5745. [Google Scholar] [CrossRef]
- Bateman, A.S. , Kelly, S.D., Woolfe, M. Nitrogen Isotope Composition of Organically and Conventionally Grown Crops. J. Agric. Food Chem. 2007, 55, 2664–2670. [Google Scholar] [CrossRef]
- Rapisarda, P. , Camin, F., Fabroni, S., Perini, M., Torrisi, B., Intrigliolo, F. Influence of Different Organic Fertilizers on Quality Parameters and the δ15N, δ13C, δ2H, δ34S, and δ18O Values of Orange Fruit (Citrus sinensis L. Osbeck). J. Agric. Food Chem. 2010, 58, 3502–3506. [Google Scholar] [CrossRef]
- Camin, F.; Perini, M.; Bontempo, L.; Fabroni, S.; Faedi, W.; Magnani, S.; Baruzzi, G.; Bonoli, M.; Tabilio, M. R.; Musmeci, S.; Rossmann, A.; Kelly, S. D.; Rapisarda, P. Potential isotopic and chemical markers for characterizing organic fruits. Food Chem. 2011, 125, 1072–1082. [Google Scholar] [CrossRef]
- Hayashi, N. , Ujihara, T., Tanaka, E., Kishi, Y., Ogwa, H., Matsuo, H. Annual Variation of Natural 15N Abundance in Tea Leaves and Its Practicality as an Organic Tea Indicator. J. Agric. Food Chem. 2011, 59, 10317–10321. [Google Scholar] [CrossRef] [PubMed]
- Gatzert, X., Chun, K. P., Boner, M., Hermanowski, R., Mäder, R., Breuer, L., Gattinger, A., Orlowski, N. Assessment of multiple stable isotopes for tracking regional and organic authenticity of plant products in Hesse, Germany. Isotopes in Environmental and Health Studies, 2021, 57, 281–300. [CrossRef]
- Bontempo, L. , van Leeuwen, K. A., Paolini, M., Holst Laursen, K., Micheloni, C., Prenzler, P.D., Ryan, D., Camin, F. Bulk and compound-specific stable isotope ratio analysis for authenticity testing of organically grown tomatoes. Food Chem. 2020, 318, 126426. [Google Scholar] [CrossRef]
- Wassenaar, LI, Kelly, SD, Douence, C, et al. Assessment of rapid low-cost isotope (δ15N, δ18O) analyses of nitrate in fruit extracts by Ti(III) reduction to differentiate organic from conventional production. Rapid Commun. Mass Spectrom. 2022; 36, e9259. [CrossRef]
- Inácio, C. T. , Chalk, P. M., & Magalhães, A. M. Principles and limitations of stable isotopes in differentiating organic and conventional foodstuffs: 1. Plant products. Crit. Rev. Food Sci. Nutr. 2015, 55, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Yun, S-I. , Lim, S-S., Lee, G-S., Lee, S-M., Kim, H-Y., Ro, H-M. and Choi, W-J. Natural 15N abundance of paddy rice (Oryza sativa L.) grown with synthetic fertilizer, livestock manure compost, and hairy vetch. Biol. Fertil. Soils. 2011, 47, 607–617. [Google Scholar] [CrossRef]
- Lester G.E., Manthey J.A., Buslig B.S. Organic vs conventionally grown Rio Red whole grapefruit and juice: comparison of production inputs, market quality, consumer acceptance, and human health-bioactive compounds. J. Agric. Food Chem. 2007 55, 4474-80. [CrossRef]
- Navarro P., A. J. Pérez-López, M.T. Mercader, A.A. Carbonell-Barrachina, and J.A. Gabaldon. Antioxidant activity, color, carotenoids composition, minerals, vitamin C and sensory quality of organic and conventional mandarin juice, cv. Orogrande. Food Sci. Technol. Int. 2011, 17, 241–248. [Google Scholar] [CrossRef]
- Esch, JR. , Friend, J.R., Kariuki, J.K. Determination of the vitamin c content of conventionally and organically grown fruits by cyclic voltammetry. Int. J. Electrochem. Sci. 2010, 5, 1464–1474. [Google Scholar] [CrossRef]
- Chebrolu KK, Jayaprakasha GK, Jifon J, Patil BS. Production system and storage temperature influence grapefruit vitamin C, limonoids, and carotenoids. J. Agric. Food Chem. 2012, 60, 7096–103. [CrossRef]
- Blundell, R. , Schmidt, J.E., Igwe, A. et al. Organic management promotes natural pest control through altered plant resistance to insects. Nat. Plants 2020, 6, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Fabroni, S. , Amenta, M., Rapisarda, S., Torrisi, B., & Licciardello, C. Amino acid metabolism and expression of genes involved in nitrogen assimilation in common oranges cv. Valencia Late. Biol. Plant. 2022, 66, 155–162. [Google Scholar] [CrossRef]
- Canali, S. , Diacono, M., Campanelli, G., & Montemurro, F. Organic no-till with roller crimpers: Agro-ecosystem services and applications in organic Mediterranean vegetable productions. Sustain. Agric. Res. 2015, 4, 70. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).