1. Introduction
Methadone (MTD) is the most commonly used drug in the world to treat opioid addiction due to its safety, efficacy, and low cost. The benefits of using MTD as a treatment for opioid-addicted patients include craving control, the disappearance of the effect-abstinence cycle, the complete remission of withdrawal symptoms and the tolerance to MTD's euphoria, sedation, and narcotic effects. MTD treatment reduces mortality, the risk of overdose and infection and the need to commit crimes, allowing patients to gradually return to their normal lives [
1].
MTD is a chiral compound and its two enantiomers (R and S) have different pharmacokinetic and pharmacodynamic properties; it is typically administered orally, with the plasmatic peak occurring after 2-4 hours. It has a half-life of 24 to 36 hours, allowing for daily administration. MTD and its active and inactive metabolites are excreted by the kidney after being metabolized in the liver by cytochrome P450: specifically, cytochrome CYP3A4, CYP2B6, and CYP2D6 [
2]. MTD is an agonist of the µ-opioid receptors, with the R-enantiomer (Levomethadone) being a stronger agonist than the S-enantiomer. Levomethadone (LevoMTD), indeed, has a tenfold higher affinity for the µ1 and µ2 receptors and an analgesic potential that is 8 to 50 times greater [
3]. Constipation, nausea, sleepiness, vomiting, headache, and erectile dysfunction are the most common side effects that can occur during MTD treatment; these effects usually appear during the induction phase and generally disappear after a few weeks of treatment, the only exception being stypsis. Dyspnoea, thoracic oppression, respiratory depression, and mental confusion are more severe symptoms that indicate an excessive accumulation of the drug [
1]. Another significant side effect of MTD use is the prolongation of the ECG's QT interval, which increases the risk of torsade de pointes (TdP) and sudden cardiac death [
4,
5].
In the general population, the elongation of the ECG's QT interval can be caused by genetic variants or by acquired long QT syndrome, the main risk factor for which is drug use. The QT interval reflects the duration of the ventricular depolarization and repolarization caused by inward depolarizing currents (involving sodium and calcium channels) and outward repolarizing currents (involving potassium channels). A malfunctioning in these channels can lead to an excess of intracellular positive ions, prolonging the ventricular repolarization and the QT interval [
6]. Prolonged QT interval can result in Torsade De Pointes (TdP), a polymorphic ventricular tachycardia associated with a high risk of sudden cardiac death [
7]. A variety of factors can cause the QT interval to be prolonged; those associated with a higher level of evidence include the use of drugs with a known risk of QT interval prolongation and hypokalemia. Female sex, old age, cardiovascular and endocrine comorbidities, and the use of drugs with a possible or conditional risk of prolonging the QT interval are other important risk factors with a lower level of evidence. (
Table 1) [
8].
The list of drugs that can prolong the QT interval and cause TdP is constantly updated and can be found online (
www.crediblemeds.org), where drugs are classified as having a known, possible, or conditional risk of TdP (
Table 2) [
9].
MTD use can result in cardiologic side effects, most notably a prolongation of the ECG's QT interval: this can happen with a wide range of dosages, including those recommended for maintenance therapy [
4,
10,
11]. MTD's action on the cardiac hERG (human ether-a-gogo related gene) channels, which are voltage-dependent potassium channels responsible for the cardiac repolarizing current, is the mechanism by which it can prolong the QT interval. As MTD binds to the channel, it inhibits the delayed rectifier potassium current, slowing down the cardiac repolarization (caused by the outward flux of potassium ions): the resulting excess of positive ions inside the cells prolongs the cardiac ventricular repolarization, resulting in an increase in the QT interval seen on the ECG [
12]. The two MTD enantiomers, R and S, inhibit hERG channels in different ways: R-MTD inhibits 40% of the channels while S-MTD inhibits 65% of them. Furthermore, R-MTD inhibits the channels much less potently than the S enantiomer [
13,
14]. Because R-MTD, or LevoMTD, is the main responsible for the drug's therapeutic activity and has a 3,5-fold lower affinity for hERG channels than S MTD, using LevoMTD alone could be beneficial in order to reduce the risk of TdP without sacrificing the racemic mixture's therapeutic efficiency [
2].
The purpose of this study was to determine the prevalence of QT prolongation in the studied population and to assess the other factors that influence the QT interval. Another goal was to compare manual and automatic QT interval readings and to assess the benefits of using LevoMTD in patients with prolonged QT intervals.
2. Materials and Methods
This study was carried out in three Addiction Services (Servizi Dipendenze (Ser.D)) of the Social Health Unit of Treviso, North-Eastern Italy: data were collected in the Ser.Ds of Treviso, Oderzo, and Castelfranco Veneto for a total of 165 patients (31 women and 134 men) on MTD maintenance therapy at various dosages. Gender, age, length of MTD treatment, daily MTD dosage, and other drugs taken by the patients at the time of the ECG were the variables considered for each patient. Furthermore, the control ECG was evaluated in patients who had a prolonged QT interval during MTD treatment and were then switched to LevoMTD.
The QT interval was measured manually, since many studies have shown that manual measurements are more accurate than automatic measurements and that the latter underestimates the QT interval [
15,
16,
17]. The QT interval begins with the onset of the QRS complex, the first manifestation of ventricular depolarization, and ends with the termination of the T wave, the final manifestation of ventricular repolarization [
18]. The correct evaluation of the QT interval requires the selection of the appropriate cardiac lead: in general, lead II or V5 are preferable, even though the American Heart Association recommends evaluating the lead with the best visibility of the T wave [
19]. When suitable, the II and V5 leads were evaluated in this study; otherwise, the better defined one was used. After selecting the best lead, the QT interval was measured using the tangent technique to determine the end of the T wave [
20]. Because the QT interval varies with heart rate, it is necessary to use a correction formula after measuring the QT interval to obtain the QTc value (corrected QT interval), which is related to the risk of TdP. Bazett’s formula is the most commonly used correction formula, and it was also used in this study:
RR indicates the RR interval, measured in seconds, preceding the QT under consideration, measured in milliseconds [
21]. Although the Bazett’s formula appears to be inaccurate for extreme values of heart rate [
22] and many alternative formulas are available (such as Bogossian’s formula for values of QRS greater than 120ms or Fridericia’s formula for bradycardiac patients), it has never been proven that these formulas are superior to Bazett's one.
Because there is no gold standard, the concordance between manually and automatically measured QTc was assessed using the Bland-Altman method. The Wilcoxon-Mann-Whitney test was used to determine whether there is a significant QTc difference between the sexes. The Spearman coefficient was used to assess the relationship between QTc on one hand and age, duration, and dosage of MTD treatment on the other. Finally, a linear regression model was used to evaluate the determinants of the QTc interval.
3. Results
3.1. Sample Characteristics
The total number of evaluated patients was 165, with 31 women (18.8%) and 134 men (81.2%). The sample's mean (standard deviation) age was 42 (11.1) years, with a minimum of 18 years and a maximum of 68 years (
Table 3).
The median of the duration of the MTD treatment in the population is 10 years (p25-p75=3-22 years) (
Table 4); the median of the daily dosage of MTD is 80mg (p25-p75=40-120 mg), with a minimum of 5mg and a maximum of 440mg (
Table 5). Both distributions show significant positive asymmetry.
In addition to MTD, 35% of the population (57 patients) used benzodiazepines, 18.8% (31 patients) used antidepressants, 15.2% (25 patients) used atypical antipsychotics, 6% used typical antipsychotics, and 4.8% used mood stabilizers.
3.2. ECGs Evaluation
As the ECGs were being performed, all patients had sinusal rhythm. The sample's mean heart rate (HR) was 71 (12) bpm, and the mean QRS length was 95 (11) ms. (
Table 6).
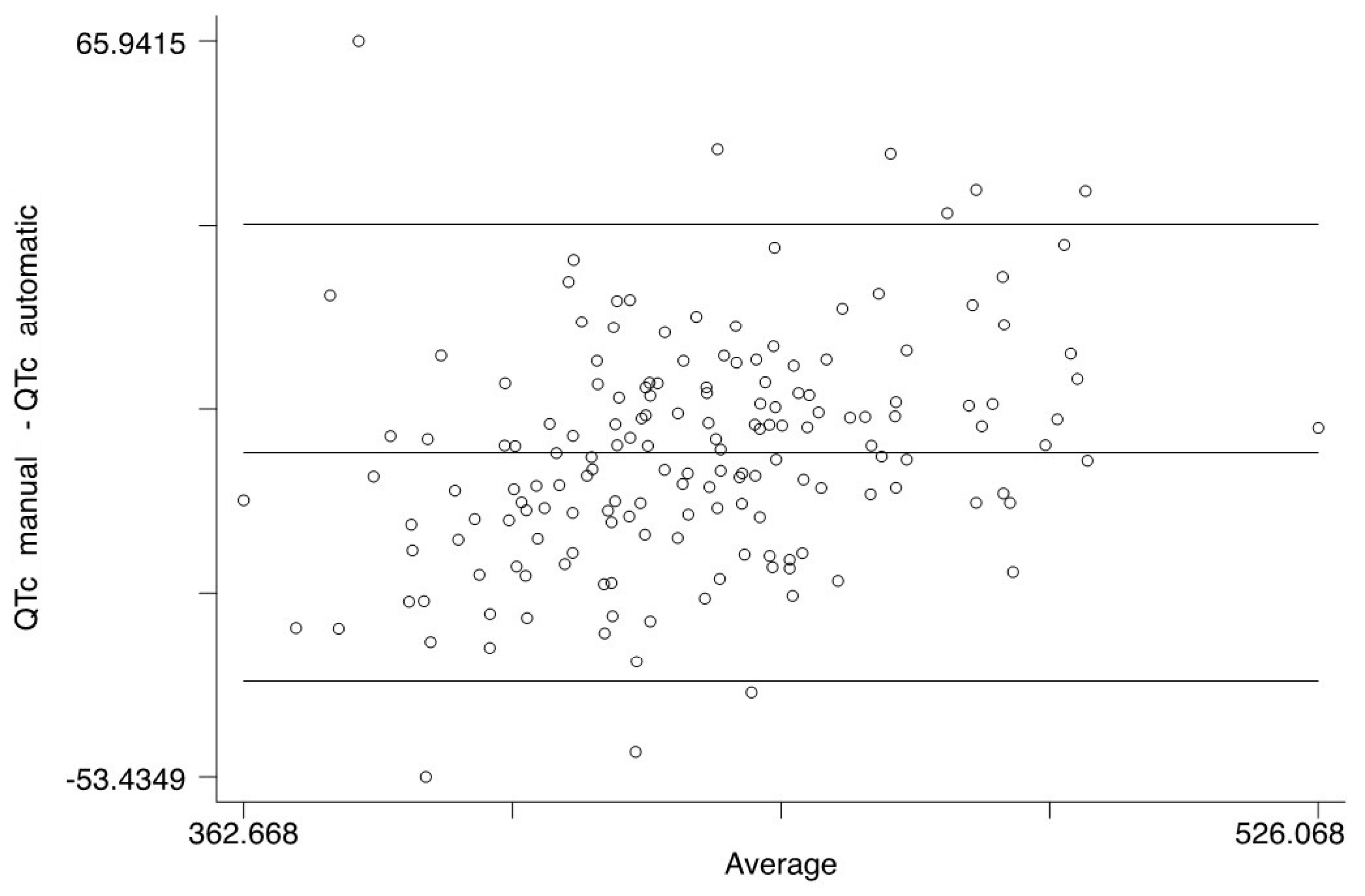
When the manually measured QTc values were compared to the automatic ones, no significant difference was found: on the contrary, the two measurements had a nearly perfect correspondence. (
Figure 1 and
Figure 2). Anyway, because many articles claim that manual measurement is superior, we used manually measured QTc values for the statistical analysis [
15,
16].
The mean QTc value (manually measured) in the sample was 431.7 (±32) ms with a minimum of 356ms and a maximum of 527ms (
Table 7).
3.3. Correlations
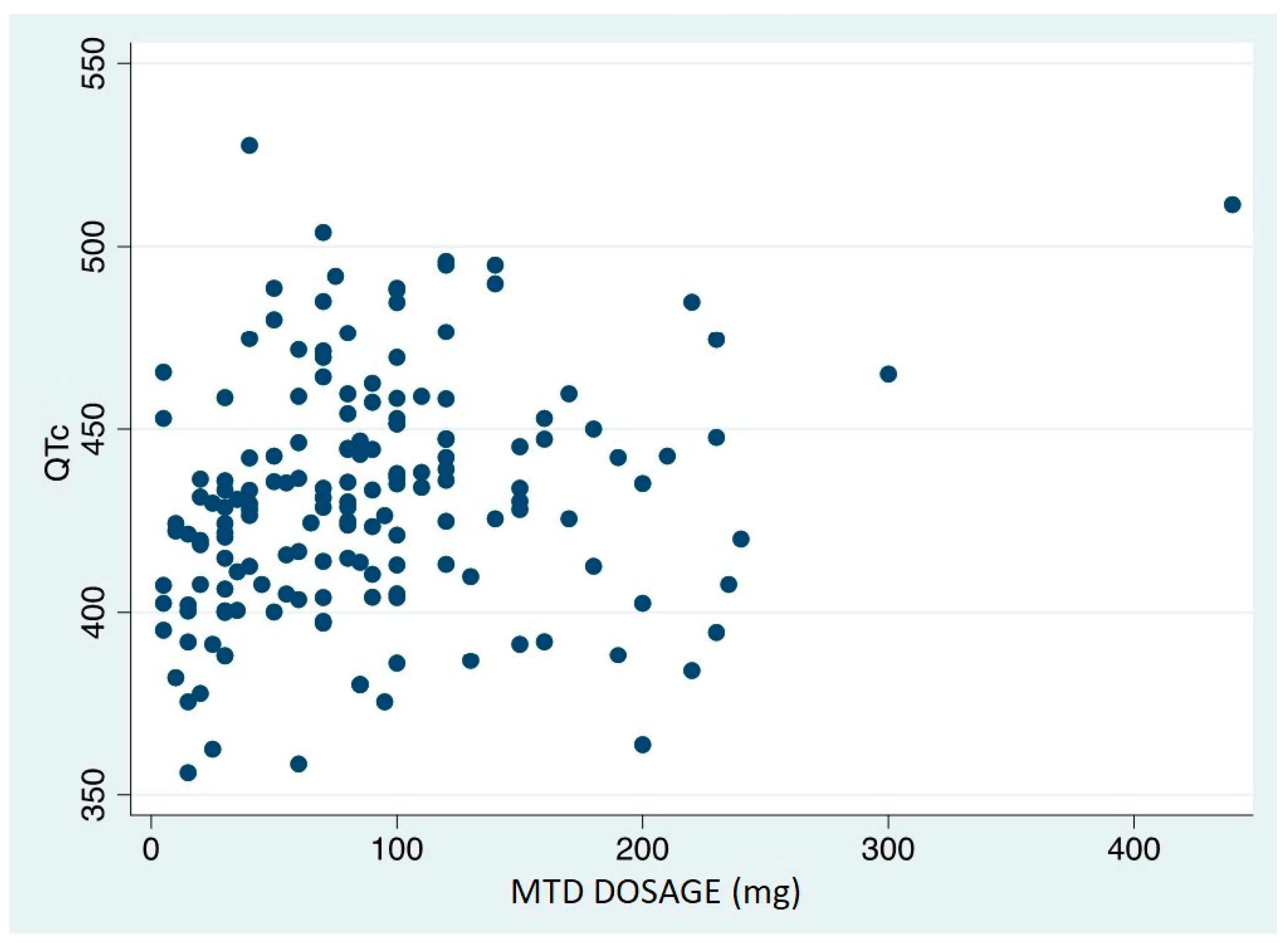
The data analysis revealed a significant correlation between QTc duration and daily MTD dosage: Spearman's Rho is 0.27 (p=0.0005) (
Figure 3). The discovered relationship is a linear correlation in which the QTc values increase as the daily dosage increases.
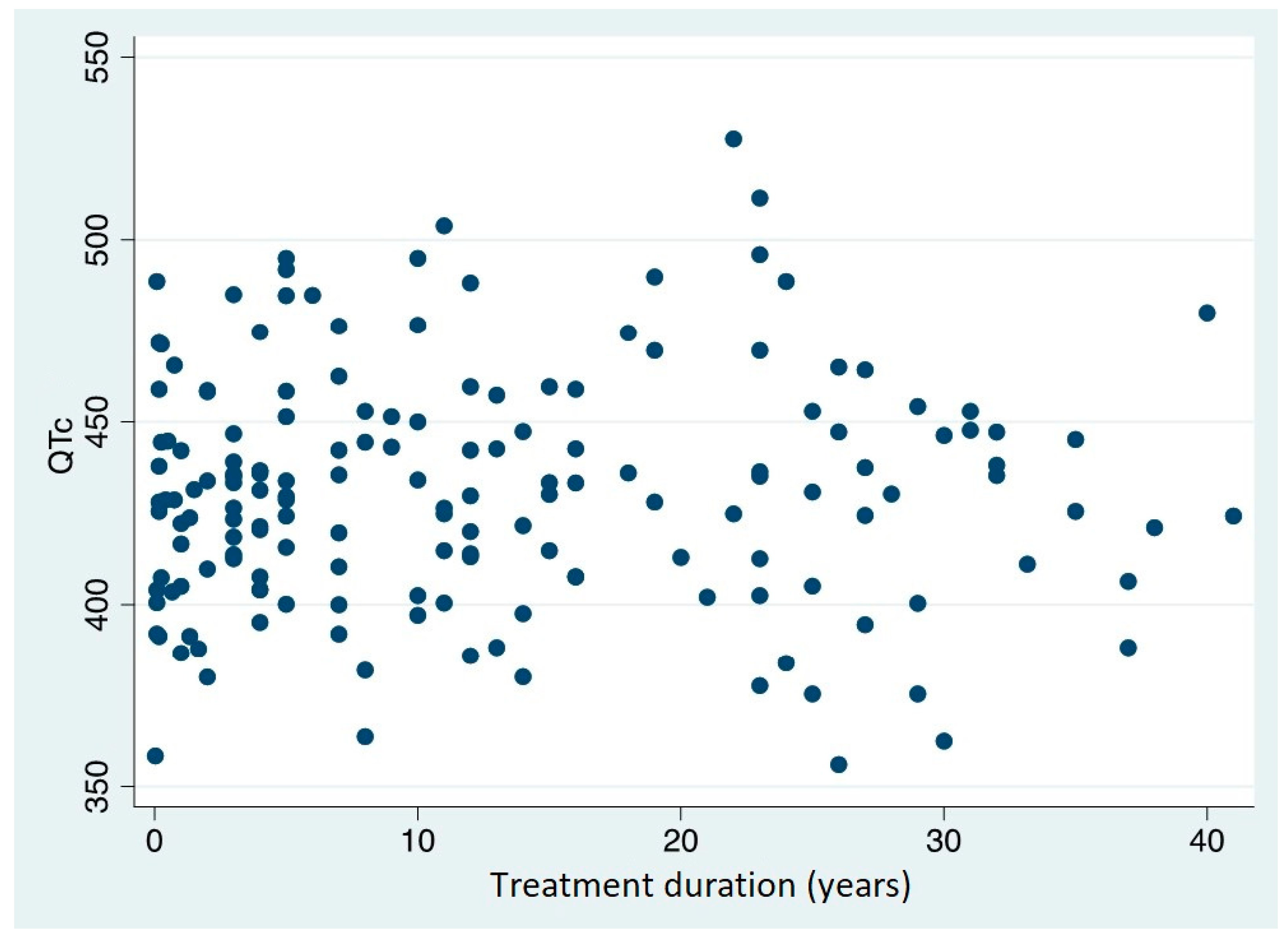
The data analysis, on the contrary, revealed no significant correlation between QTc and treatment duration: the Spearman's Rho is 0.052 (p=0.510) (
Figure 4).
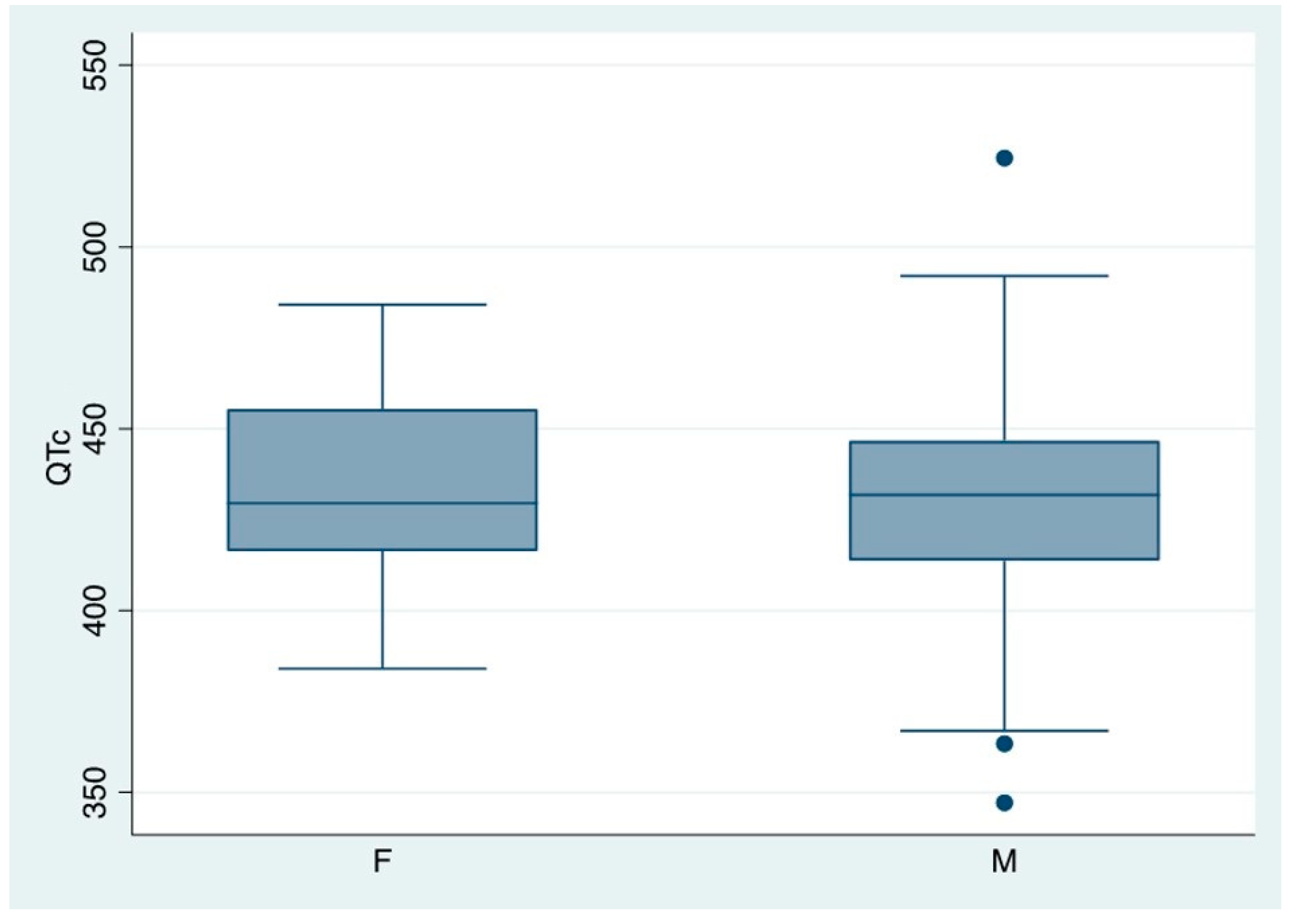
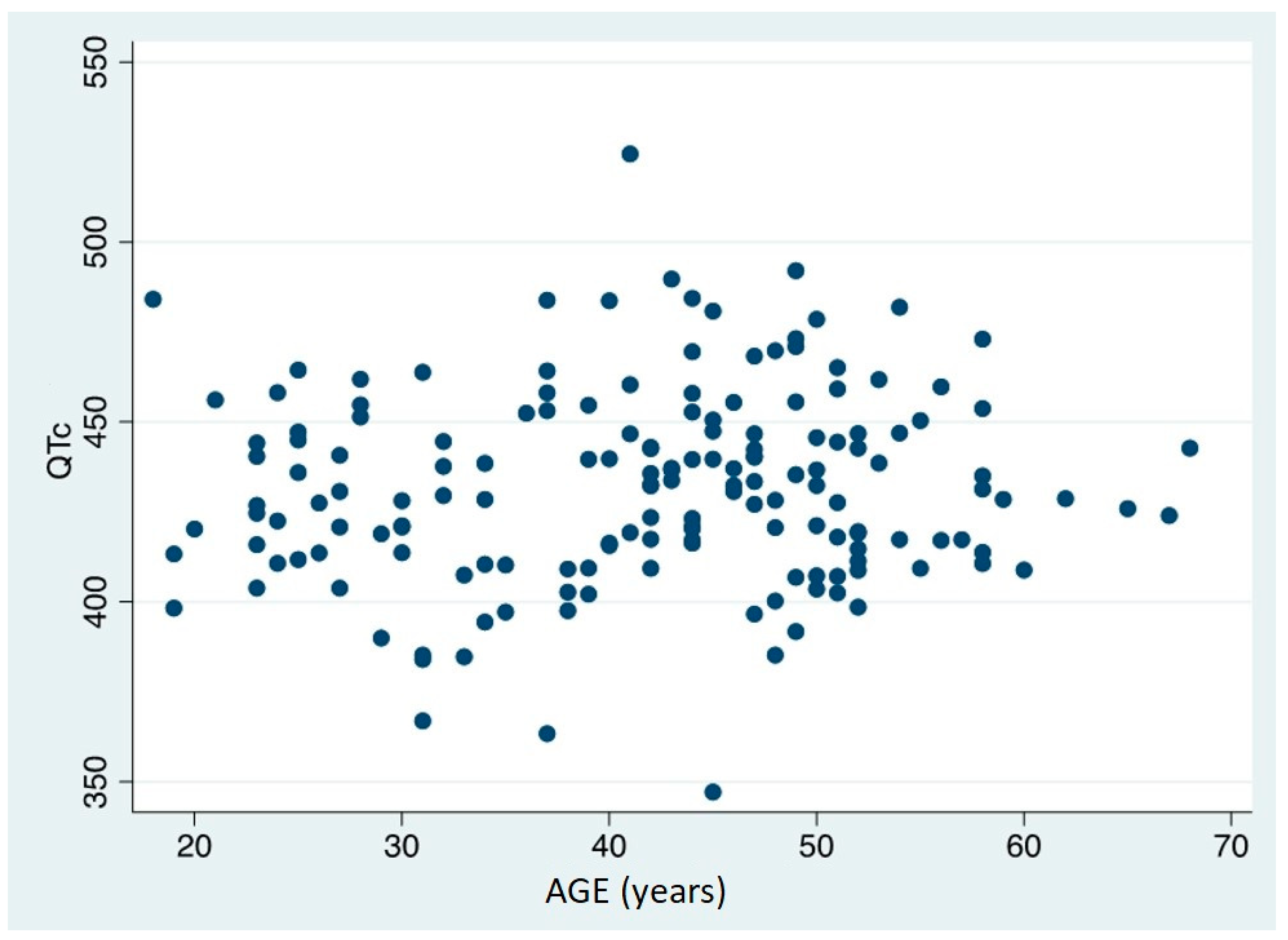
The value of QTc was discovered to be both gender and age independent (Spearman’s Rho =-0,1945, p=0,590) (
Figure 5 and
Figure 6).
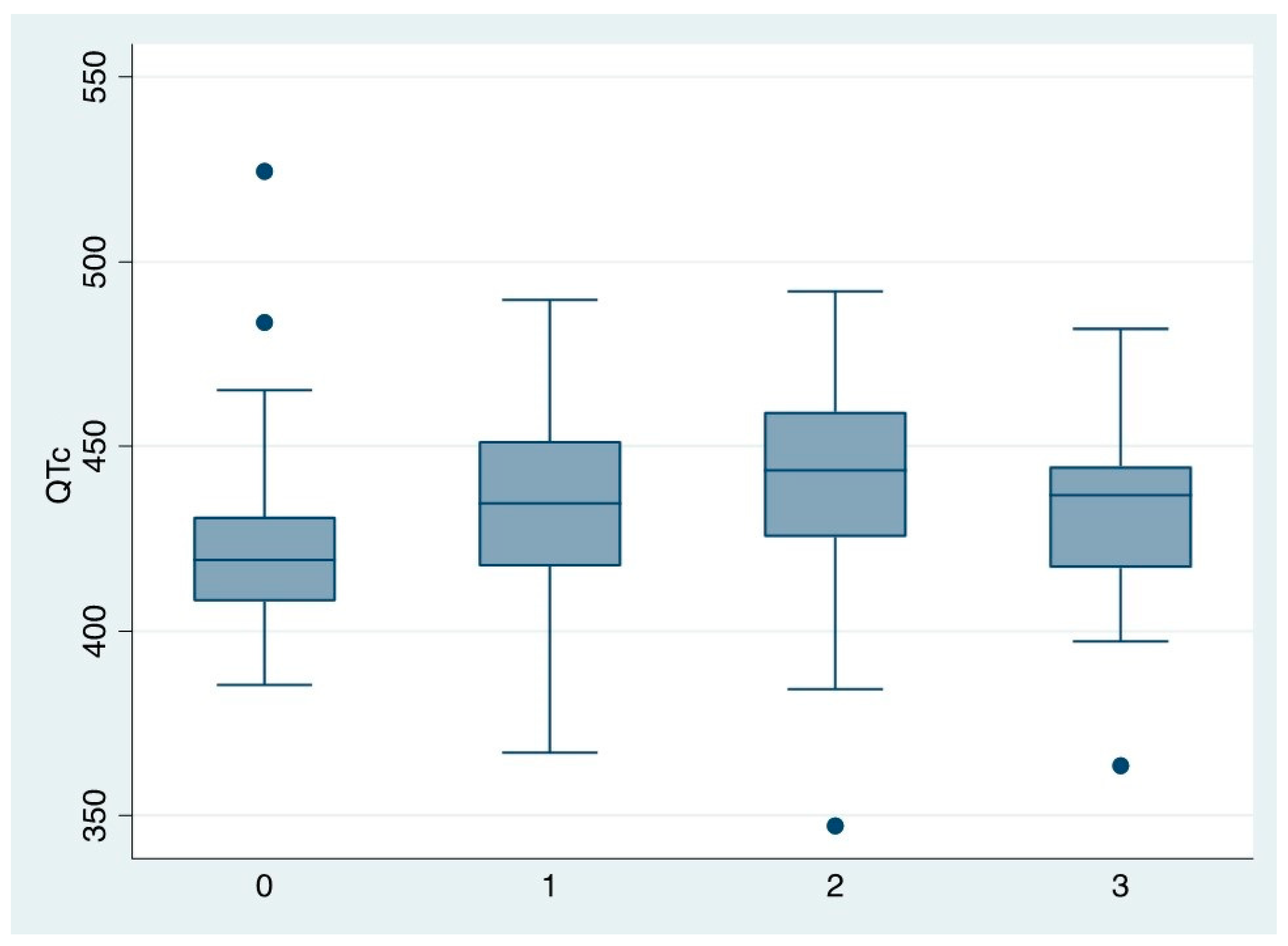
The dose of MTD emerged as the only independent risk factor for QTc prolongation in the multivariate analyses: the QTc increased by 28 msec when the dose of MTD increased from 50 to >=150 mg/day.
Gender, age, length of MTD therapy, use of antidepressants, and typical and atypical antipsychotic drugs were not associated with QTc prolongation (
Table 8,
Figure 7)
3.4. Patients with QTc Prolongation
Using 470ms for men and 480ms for women as cut-offs [
23], 23 patients (14%) with QTc elongation were identified: there were three women and twenty men among these patients. The mean age of those patients was 43 years, the mean treatment duration was 12 years, and the mean MTD dosage was 113 mg/die (
Table 9).
When we examined if these patients were taking medications associated with QTc prolongation, we discovered that none of them were taking drugs with a known risk of TdP. However, three of the 23 patients were taking medications with a possible risk of TdP (atomoxetine, clotiapine, paliperidone, and ritonavir), and two were taking medications with a conditional risk of TdP (trazodone). The other 18 patients were not taking any medication (
Table 10).
3.5. Change to levoMTD therapy
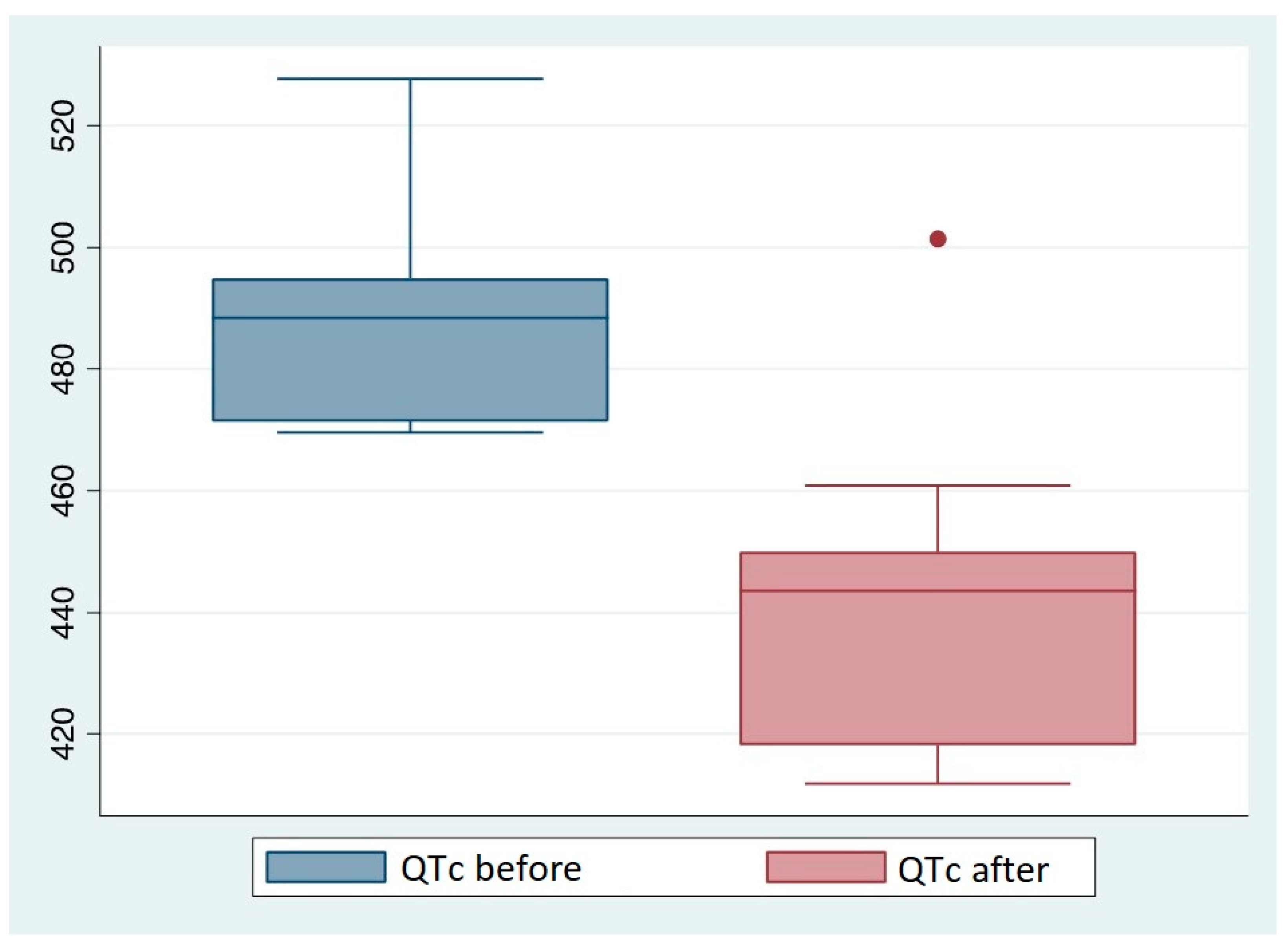
Among the 23 patients with prolonged QTc intervals, 10 (nine men and one woman) switched from MTD to LevoMTD maintenance therapy. Following the therapy change, the QTc values were reduced in 9 of these patients (
Table 11).
At the time of the control ECG, the mean duration of Levometadone treatment was 19,8 months, with a minimum of 1,6 months and a maximum of 48 months. The mean LevoMTD dosage was 70,5 mg/die. It is worth noting that the only patient who did not have a normalization of the QT interval following the switch in therapy was the patient who had been on levoMTD for the shortest amount of time: the control ECG was performed only 1 month and 20 days after the start of the levoMTD therapy.
To evaluate the difference in QTc after switching to levoMTD we used the Wilcoxon signed rank test with a significant p value of 0.007. We discovered a mean decrease of 47ms in QTc before and after switching. Only one subject had an increase of QTc after switching therapy, and this may be due to inter-subject variability in drug response.
Figure 8.
Comparison between QTc value before and after switching to LevoMTD.
Figure 8.
Comparison between QTc value before and after switching to LevoMTD.
4. Discussion
MTD is a powerful opioid agonist that is used as a first-line treatment for heroin and prescription-opioid addiction. Furthermore, due to its distinctive pharmacokinetic and pharmacodynamic properties, such as its high oral bioavailability, its longer half-life when compared to other opioids, its non-competitive antagonist action on NMDA receptors, and its inhibitory action on serotonin and noradrenaline reuptake, MTD is a drug that can be used to treat both chronic and acute pain [
24]. MTD is now used to treat both acute post-operatory pain and chronic pain, both cancer and non-cancer related. [
25,
26,
27]. It is estimated that over 300.000 patients in Europe are receiving MTD maintenance therapy for opioid addiction (750.000 patients worldwide). MTD was added to the WHO's list of essential drugs in 2005 due to the reduction in mortality, relapses, and illicit activities it ensures [
2].
Despite its widespread use and relative safety and handling, MTD use, particularly when long-term, is not without risk: the risks include intoxication and overdose, respiratory depression, and sudden cardiac death due to TdP. These risks are primarily related to the drug's long half-life, high bioavailability, and tendency to accumulate in the organism due to slow excretion [
5]. TdP is a polymorphic ventricular tachycardia that occurs in patients with an elongated QT interval: this event occurs in 5% of antiarrhythmic drug patients and less frequently in patients on other drugs, such as MTD [
7]. MTD, in fact, like the majority of other QT-prolonging drugs, acts by blocking the hERG potassium channels involved in the ventricular repolarizing currents, increasing the repolarization time and prolonging the QT interval [
28].
This study found that more than one-eighth of MTD-treated patients had a QTc prolongation. This value is not significantly different from those found in other prevalence studies [
29,
30]. No correlation was found between prolonged QTc and the gender or age of the patients. Furthermore, despite MTD's capacity for storage in the body [
5], no correlation was found between MTD treatment duration and QTc value. However, a linear relationship was discovered between the patients' MTD dose at the time the ECG was performed and the QTc value, implying that MTD dosage plays a role in the prolongation of the QT interval.
No one in the sample of patients with prolonged QTc was taking any drugs that had a known risk of QT prolongation, and only a minority of them were taking drugs that had a possible or conditional risk of QT prolongation and TdP. It can be deduced that in the vast majority of patients, the prolongation of the QT interval was caused by MTD alone, and that in the remaining patients, MTD may have played a synergistic role with the other drugs.
Another point to note is that benzodiazepines were used by more than a third of the patients in the study: even though they play no role in QT interval prolongation, benzodiazepines should be used with caution in opioid-addicted patients being treated with MTD because they, too, can be abused and can cause respiratory depression in conjunction with MTD [
31,
32].
MTD is a chiral molecule that has R and S isomers. The R-enantiomer (LevoMTD) is primarily responsible for MTD's therapeutic effects, as it has a greater ability to bind opioid receptors; on the other hand, the S-enantiomer is primarily responsible for MTD's cardiotoxic effects, as it blocks the hERG potassium channels more strongly [
13,
33]. Given the lower cardiotoxic ability of LevoMTD compared to the racemic mixture (which contains equal proportions of both enantiomers) usually administered to patients [
34], we investigated the role of LevoMTD in the normalization of QTc values in patients who had a prolongation of the QT value following MTD treatment.
The QT value was completely normalized in 9 of the ten patients who underwent the therapy change, confirming the potential benefits of LevoMTD maintenance therapy. It is also worth noting that the only patient who did not have a QT value normalization was the one who had more recently switched to LevoMTD therapy. Despite the small sample size, this study confirms the benefits of using LevoMTD in patients who have risk factors for QT prolongation. Despite its higher cost [
35], LevoMTD should be recommended as a first-line treatment for patients with pre-existing structural cardiac diseases, patients who take QT prolonging drugs, patients with other risk factors, or patients who already have a prolonged QT interval prior to MTD treatment. In clinical practice, it is therefore necessary to thoroughly evaluate each patient in order to determine an individualized therapy [
33]. Furthermore, if other studies confirm the correspondence between manual and automatic QT measurement, the evaluation of patients will become easier for clinicians with fewer cardiologic skills, as in low access threshold services and/or risk-reduction programs. Even though there is plenty of research on MTD's propensity to lengthen the QT interval, very few studies investigated the impact of methadone in correcting this risky side-effect.
One of the study's limitations is the absence of a baseline ECG prior to the start of MTD treatment. Another limitation is the lack of information about the patients' plasmatic electrolyte levels; in particular, hypokalemia can be associated with QT elongation [
36] and should thus be monitored. Other limitations that should be mentioned are the lack of a thorough history of the patients, the possibility of the patients abusing other illicit drugs, and the variability of their adherence to the therapy.
5. Conclusions
This study analysed a sample of patients undergoing MTD treatment in the addiction centres in Italy's northeast: it showed a linear relationship between the daily dose of MTD and the measured QTc value; however, there appeared to be no relationship between the QTc value and the length of the therapy.
It has been observed that one-eighth of the patients taken into consideration had a QTc prolongation over the normal ranges, validating the inclusion of MTD among the lists of QTc prolonging drugs due to its ability to block the hERG potassium channels, which are responsible for the repolarizing cardiac currents.
LevoMTD's weaker ability to interact with hERG channels and, as a result, its weaker association with QTc prolongation and TdP, have been confirmed by the observation that 90% of patients receiving MTD treatment with a QTc prolongation underwent a normalization of the QT value (<470ms in men and <480ms in women) after switching from MTD to levoMTD.
In order to reduce the risk of QTc prolongation and the mortality connected with it, this study demonstrated the recommendation of an accurate familiar and pathological anamnesis in patients needing MTD, the execution of a basal ECG prior to the start of the treatment and a thorough evaluation of the other risk factors (such as the serum levels of electrolytes and the concomitant therapy). As LevoMTD is a safer drug, it should be available in all addiction services.
Author Contributions
Conceptualization, F.L; validation, F.L, G.V; formal analysis, G.V., A.T.; investigation, L.S., R.M., G.Z.; resources, R.M., G.Z.; data curation, L.S., A.T.; writing—original draft preparation, L.S.; writing—review and editing, L.S., F.L., F.F., G.V.; supervision, F.L.; project administration, F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of AOUI Verona (protocol code 875CESC, date of approval: 14-04-2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting reported results can be found by contacting medicina.dipendenze@aovr.veneto.it.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Giancane, S. Il metadone: un trattamento riabilitativo. In In sostanza: manuale sulle dipendenze patologiche; Lugoboni, F., Zamboni, L., Eds.; Edizioni CLAD onlus: Verona, Italy, 2018, pp. 337–347.
- Masini, E.; Lanzi, C.; Mannaioni, G. Le terapie sostitutive della tossicodipendenza da oppioidi: la terapia metadonica. In Analgesici oppiodi - uso abuso e addiction; Lugoboni, F., Faccin, M., Zamboni, L., Eds.; Edizioni CLAD Onlus: Verona, Italy, 2018, pp. 110–120.
- De Vos, J.W. et al. L-methadone and D,L-methadone in methadone maintenance treatment: A comparison of therapeutic effectiveness and plasma concentrations. Eur Addict Res 1998, 4, 134-141. [CrossRef]
- Pani, P.P.; Trogu, E.; Maremmani, I.; Pacini, M. QTc interval screening for cardiac risk in methadone treatment of opioid dependence. Cochrane Database of Systematic Reviews 2013, 2013. https://doi.org/10.1002/14651858.CD008939.pub2.
- Lugoboni, F.; Fantozzi, F.; Nava, F. Curare l’abuso degli analgesici oppioidi. In Analgesici oppioidi: uso, abuso e addiction; Lugoboni, F., Faccin, M., Zamboni, L., Eds.; Edizioni Clad-Onlus: Verona, Italy, 2018, pp. 357–368.
- Van Noord, C.; Eijgelsheim, M.; Stricker, B.H.C. Drug- and non-drug-associated QT interval prolongation. British Journal of Clinical Pharmacology, 2010, 70, 16-23. [CrossRef]
- Roden, D.M. Cellular basis of drug-induced torsades de pointes. British Journal of Pharmacology 2008, 154, 1502-1507. [CrossRef]
- Vandael, E.; Vandenberk, B.; Vandenberghe, J.; Willems, R.; Foulon, V. Risk factors for QTc-prolongation: systematic review of the evidence. Int J Clin Pharm 2017, 39, 16-25. [CrossRef]
- Crediblemeds Home Page. Available online www.crediblemeds.org. (accessed on 10 March 2023).
- Alinejad, S.; Kazemi, T.; Zamani, N.; Hoffman, R. S.; Mehrpour, O. A systematic review of the cardiotoxicity of methadone. EXCLI Journal 2015, 14, 577-600. [CrossRef]
- Zerdazi, E.H.; et al. QT length during methadone maintenance treatment: gene × dose interaction. Fundam Clin Pharmacol 2019, 33, 96–106. [CrossRef]
- Sanguinetti, M.C.; Tristani-Firouzi, M. hERG potassium channels and cardiac arrhythmia. Nature 2006, 440, 463–469. [CrossRef]
- Eap, C.B. et al. Stereoselective block of hERG channel by (S)-methadone and QT interval prolongation in CYP2B6 slow metabolizers. Clin Pharmacol Ther 2007, 81, 719-728. [CrossRef]
- Titus-Lay E.N. et al. Methadone-associated QT interval prolongation in patients undergoing maintenance therapy in an urban opioid treatment program. Pharmacotherapy 2021, 41, 238-246. [CrossRef]
- Talebi, S. et al. Underestimated and unreported prolonged QTc by automated ECG analysis in patients on methadone: Can we rely on computer reading?. Acta Cardiol 2015, 70, 211-216. [CrossRef]
- Garg, A.; Lehmann, M.H. Prolonged QT interval diagnosis suppression by a widely used computerized ECG analysis system. Circ Arrhythm Electrophysiol 2013, 6, 76-83. [CrossRef]
- Viskin, S. et al. Inaccurate electrocardiographic interpretation of long QT: The majority of physicians cannot recognize a long QT when they see one. Heart Rhythm 2005, 2, 569-574. [CrossRef]
- Rautaharju, P.M.; Surawicz, B.; Gettes, L.S. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. Part IV: The ST Segment, T and U Waves, and the QT Interval A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Journal of the American College of Cardiology 2009, 53, 982-991. [CrossRef]
- Indraratna, P.; Tardo, D.; Delves, M.; Szirt, R.; Ng, B. Measurement and Management of QT Interval Prolongation for General Physicians. Journal of General Internal Medicine 2020, 35, 865-873. [CrossRef]
- Postema, P.G.; De Jong, J.S.S.G.; Van der Bilt, I.A.C.; M. Wilde, A.A. Accurate electrocardiographic assessment of the QT interval: Teach the tangent. Heart Rhythm 2008, 5, 1015-1018. [CrossRef]
- BAZETT, H.C. AN ANALYSIS OF THE TIME-RELATIONS OF ELECTROCARDIOGRAMS. Annals of Noninvasive Electrocardiology 1997, 2. [CrossRef]
- Sagie, A.; Larson, M.G.; Goldberg, R.J.; Bengtson, J.R.; Levy, D. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiol 1992, 70, 797-801. [CrossRef]
- Priori, S.G. et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Russian Journal of Cardiology 2016, 135, 2793-2867. [CrossRef]
- Lai, G.; Aroke, E.N.; Zhang, S. J. Rediscovery of Methadone to Improve Outcomes in Pain Management. Journal of PeriAnesthesia Nursing 2022, 37, 425–434. [CrossRef]
- Hanna, V.; Senderovich, H. Methadone in Pain Management: A Systematic Review. Journal of Pain 2021, 22, 233-245. [CrossRef]
- Treillet, E.; Giet, O.; Picard, S.; Laurent, S.; Seresse, L. Methadone Switching for Cancer Pain: A New Classification of Initiation Protocols, Based on a Critical Literature Review. Journal of Palliative Medicine 2021, 24, 1884-1894. [CrossRef]
- Kreutzwiser, D.; Tawfic, Q.A. Methadone for Pain Management: A Pharmacotherapeutic Review. CNS Drugs 2020, 34, 827-839. [CrossRef]
- Abriel, H. et al. Molecular and clinical determinants of drug-induced long QT syndrome: An iatrogenic channelopathy. Swiss Medical Weekly 2004, 134, 685-694. [CrossRef]
- Mayet, S.; Gossop, M.; Lintzeris, N.; Markides, V.; Strang, J. Methadone maintenance, QTc and torsade de pointes: Who needs an electrocardiogram and what is the prevalence of QTc prolongation?. Drug Alcohol Rev 2011, 30, 388-396. [CrossRef]
- Vallecillo, G.; et al. Risk of QTc prolongation in a cohort of opioid-dependent HIV-infected patients on methadone maintenance therapy. Clin Infect Dis 2013, 57, 1189-1194. [CrossRef]
- Chevillard, L.; Declèves, X.; Baud, F.J.; Risède, P.; Mégarbane, B. Respiratory effects of diazepam/methadone combination in rats: A study based on concentration/effect relationships. Drug Alcohol Depend 2013, 131, 298-307. [CrossRef]
- Boon, M.; Van Dorp, E.; Broens, S.; Overdyk, F. Combining opioids and benzodiazepines: Effects on mortality and severe adverse respiratory events. Annals of Cardiothoracic Surgery 2020, 9, 542-557. [CrossRef]
- Mccance-Katz, E.F. (R)-methadone versus racemic methadone: What is best for patient care? Addiction 2011, 106, 687-688. [CrossRef]
- Ansermot, N. Substitution of (R,S)-Methadone by (R)-Methadone. Arch Intern Med 2010, 170, 529, 529-536. [CrossRef]
- Reimer, J.; Vogelmann, T.; Trümper, D.; Scherbaum, N. Opioid use disorder in Germany: Healthcare costs of patients in opioid maintenance treatment. Subst Abuse Treat Prev Policy 2019, 14, 57. [CrossRef]
- TeBay, C.; Hill, A.P.; Windley, M. J. Metabolic and electrolyte abnormalities as risk factors in drug-induced long QT syndrome. Biophysical Reviews 2022, 14, 353-367. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).