1. Introduction
With the availability of advanced imaging techniques like computed tomography (CT) and magnetic resonance imaging (MRI), it has become easier to identify abnormalities in the spine (1). However, some spinal lesions may have nonspecific clinical and radiological features, and a confirmatory diagnosis of the pathological tissue type is necessary for initiating the appropriate treatment (2). A precise biopsy is crucial in accurately diagnosing neoplastic lesions in such cases. Open spine procedure was executed first by Duncan and Ferguson (3); it has traditionally been considered the most reliable method for spinal lesions, despite its invasiveness and higher morbidity rates (4). In addition, it typically provides sufficient tissue for histological and immunohistochemical analysis, resulting in a highly informative procedure (5–7). Given the challenges associated with open biopsy complications, there arose a need for improved biopsy techniques.
As a result, closed biopsy techniques emerged as a promising alternative, offering advantages such as lower cost and reduced invasiveness compared to the open biopsy (8). Subsequent advancements in closed biopsy included the utilization of local anesthesia and incorporating of nerve root monitoring, further enhancing the procedure (9). However, ensuring improved accuracy remains a crucial challenge to establishing closed biopsy as a superior technique to open biopsy (10). Fine-needle aspiration and, most importantly, image-guided percutaneous spine biopsy have emerged as viable alternatives to the open biopsy (11).
In contrast to previous studies, Fidler and Niers (12) highlighted the advantages of an open transpedicular approach over a percutaneous procedure. They emphasize that the open approach allows the surgeon to perform block resection and avoid potential damage to the pedicle wall, reducing the risk of contamination in the epidural space or paravertebral structures. Their findings suggest that the open transpedicular approach offers certain benefits that differ from those reported in the existing literature.
The efficacy of imaging-guided percutaneous spine biopsy has been thoroughly examined in various studies and has gained popularity as a management tool for spinal lesions (13–16). Robertson and Ball first described the Percutaneous spinal biopsy (17) in 1935 and later by Valls, Ottolenghi, and Schajowicz in 1948 (18). Fluoroscopy, ultrasound, CT, and MRI have been the most commonly used imaging modalities for guiding percutaneous interventions, but each has limitations that can hinder the accuracy of the biopsy (6,11,13,14,19).
Fluoroscopy, a commonly used technique in percutaneous spine biopsy, offers certain advantages and considerations compared to computed tomography (CT) guidance (10). While fluoroscopy may have limitations in accurately visualizing vital structures surrounding the lesion and paravertebral lesions, CT provides a more precise image of these structures (20). However, it is essential to note that CT-guided procedures may result in longer procedure times and an increased risk of needle slippage into adjacent structures, as real-time imaging is not feasible with CT (21). In cases where fluoroscopy fails to identify the lesion or when dealing with paraspinal soft-tissue tumors, CT becomes necessary (22). Moreover, CT is considered safer for evaluating lesions in challenging areas such as the upper thoracic and cervical spine (10).
Some authors argued that despite these differences, it is worth noting that the outcomes between fluoroscopy and CT in terms of accuracy and overall effectiveness do not show a significant difference (20). Therefore, the choice between the two techniques should be based on the specific requirements of the case, considering the need for precise visualization, real-time imaging, and safety considerations in different anatomical areas.
Even though some authors have reported a lower accuracy in CT-guided needle biopsies in dorsal levels (14), CT-guided biopsies remain the preferred method for evaluating suspected spinal lesions in many countries (14). This preference can be attributed to several factors: A) the ability to perform the procedure without general anesthesia, making it quicker; B) cost-effectiveness; and C) high success rates in some spinal lesions. However, it has been reported that in some cases, the biopsy procedure had to be repeated due to the indecisiveness of the sample obtained, either due to the insufficient amount of the sample tissue or a necrotic part of the lesion in other cases (14). Hence, researchers must prioritize the development of a more accurate diagnostic method.
This study aims to enhance spinal biopsy procedures’ accuracy, efficiency, and adequacy by integrating CT and MRI modalities using an O-arm CT navigation system. In addition, we aim to develop a comprehensive and reliable approach for spinal biopsies that addresses the limitations of individual imaging techniques, ensuring the procurement of viable, non-necrotic samples of the tumor for accurate diagnosis and subsequent treatment planning.
In conclusion, our study advocates for integrating CT and MRI imaging modalities in spinal biopsy procedures, emphasizing the potential for remarkable diagnostic accuracy and efficiency enhancements. While we anticipate this approach becoming a valuable tool for clinicians, it is crucial to recognize the need for larger-scale studies to validate these findings.
2. Materials and Methods
The study population consisted of 18 patients with different spinal tumors who were scheduled to undergo surgery. The Stealth Station navigation system Spine 8 (SSS8) (Medtronic, Medtronic Sofamor Danek, Minneapolis, MN, USA) protocol was followed, and a gadolinium contrast MRI scan of the spinal segment was performed using the SIGNA™ Voyager 1.5T MRI machine.
After informed obtained consent, all biopsies were performed under general anesthesia, and the patients were positioned in the prone position on a Maquet carbon operating table. The O-arm device was sterilely isolated using the Sterile Tube Drape O-arm System.
The Spine Stealth Air reference frame (RF)was attached to the spinous process of the above the target vertebral body. The O-arm registration menu was prepared in the Cranial software on the SSS8. The navigation camera was used to ensure that the O-arm trackers and the RF were visible, and the O-arm device was connected to the SSS8 via an Ethernet cable.
2.1. Imaging Procedure
The first step of the imaging procedure involved taking O-arm scans of axial and sagittal planes to verify the target area, followed by a three-dimensional (3D) scan of the selected spinal region. For the lumbar spine, 3-4 levels were scanned; for the thoracic spine, 4-5 levels were scanned. After verification, the 3D scan was performed with the O-arm device. Later, 3D scans were registered and automatically transmitted to the SSS8. Then, in the Images menu of the SSS8 Cranial software, the patient’s O-arm and MR scans performed according to the SSS8 protocol were selected.
2.2. Images Processing and Analysis:
In the "Merge Images" menu, the O-arm and MR scans were merged manually using the "Manual Merge" function. Then the "Verify Merge" option was selected to confirm the merging process of the O-arm and MRI scans. The memory function of the O-arm was used to determine the scan and parking positions of the O-arm device. The navigating instruments were registered on Patient Reference, including the Passive Planar Blunt Probe, SureTrak II Large Clamp, and SureTrak II Small Passive Tracker Orange. The SureTrak II Large Clamp was attached to the Johnson and Johnson 8G Vertebroplasty Needle. The SureTrak II Small Passive Tracker Orange was linked to the SureTrack II Large Clamp.
2.3. Biopsy Procedure
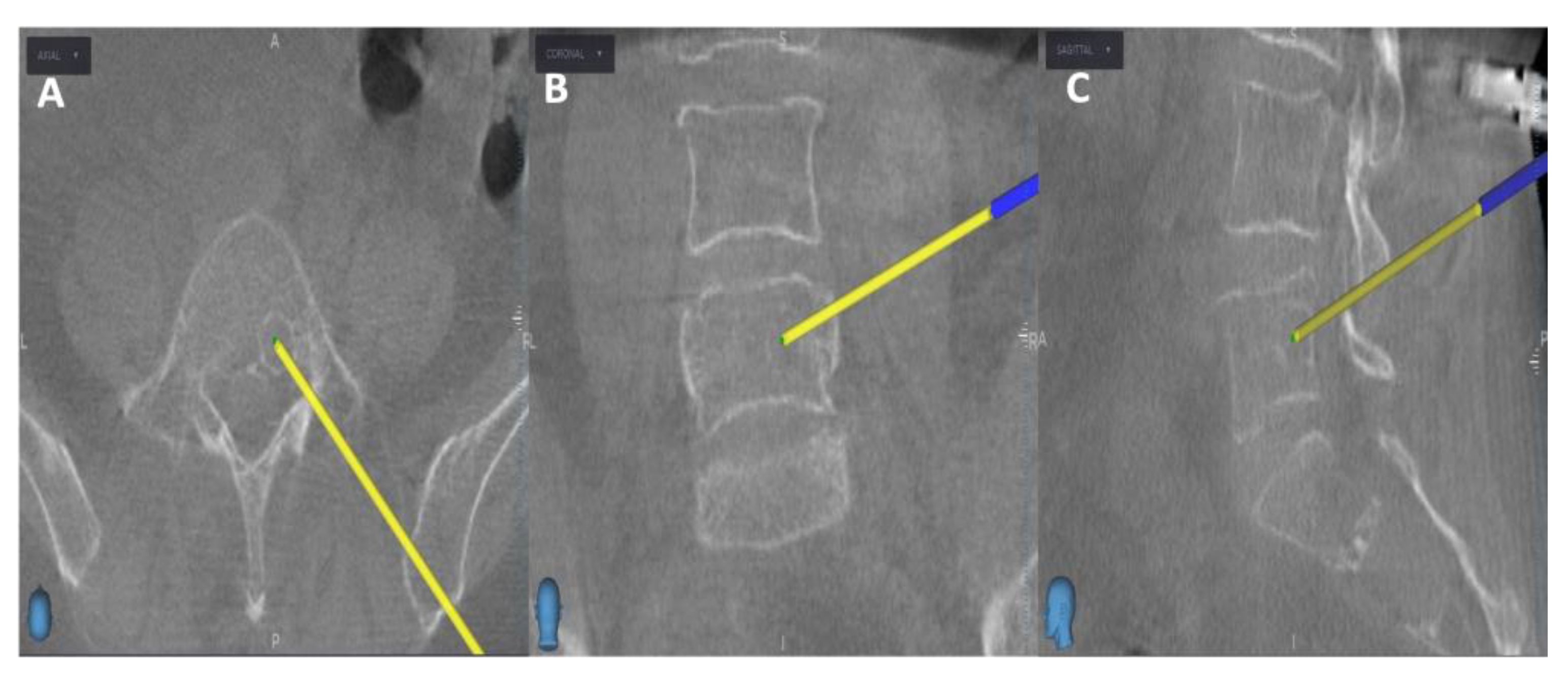
Initially, the entry point is determined through the O-arm scan (
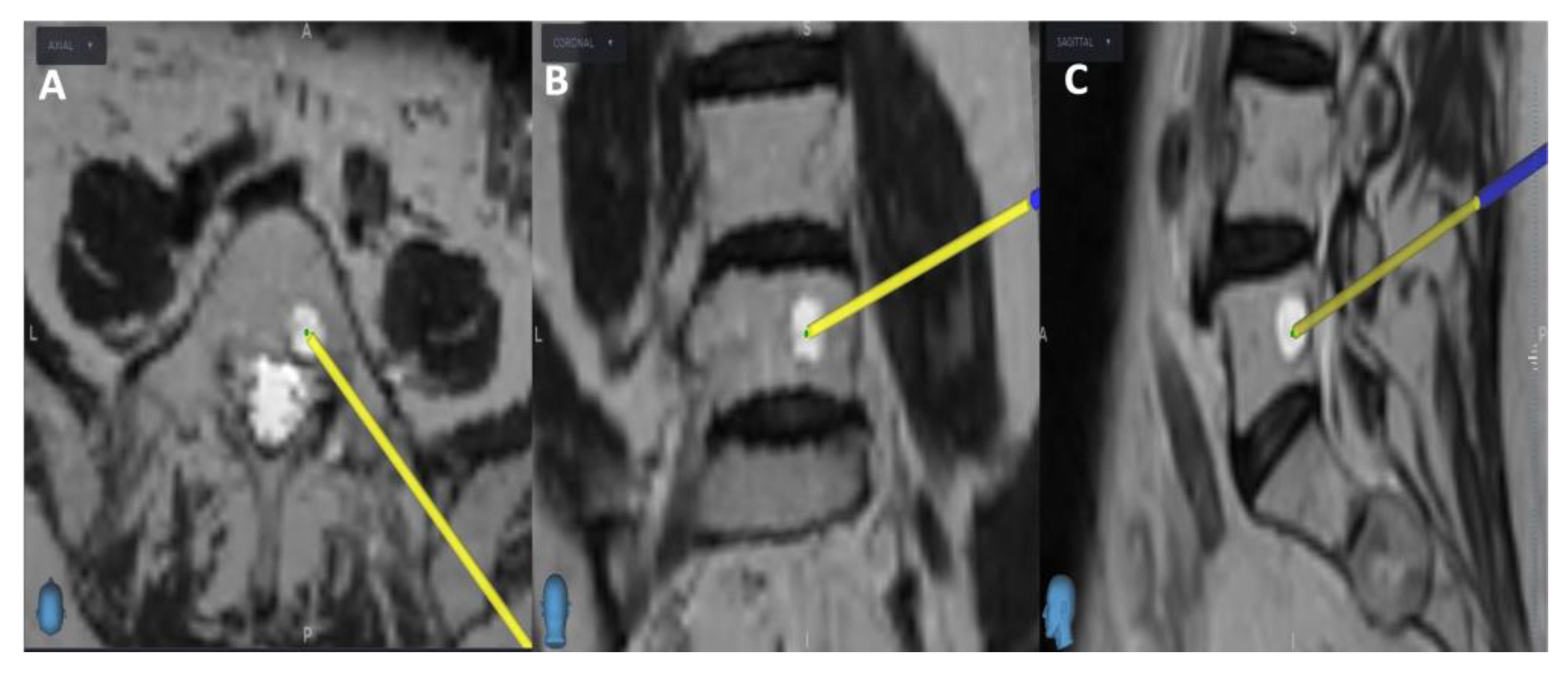
Figure 1). Next, the target point for sampling is set using an MRI scan (
Figure 2). Afterward, the surgical plan for the needle biopsy is progressed by utilizing the instrument projection function.
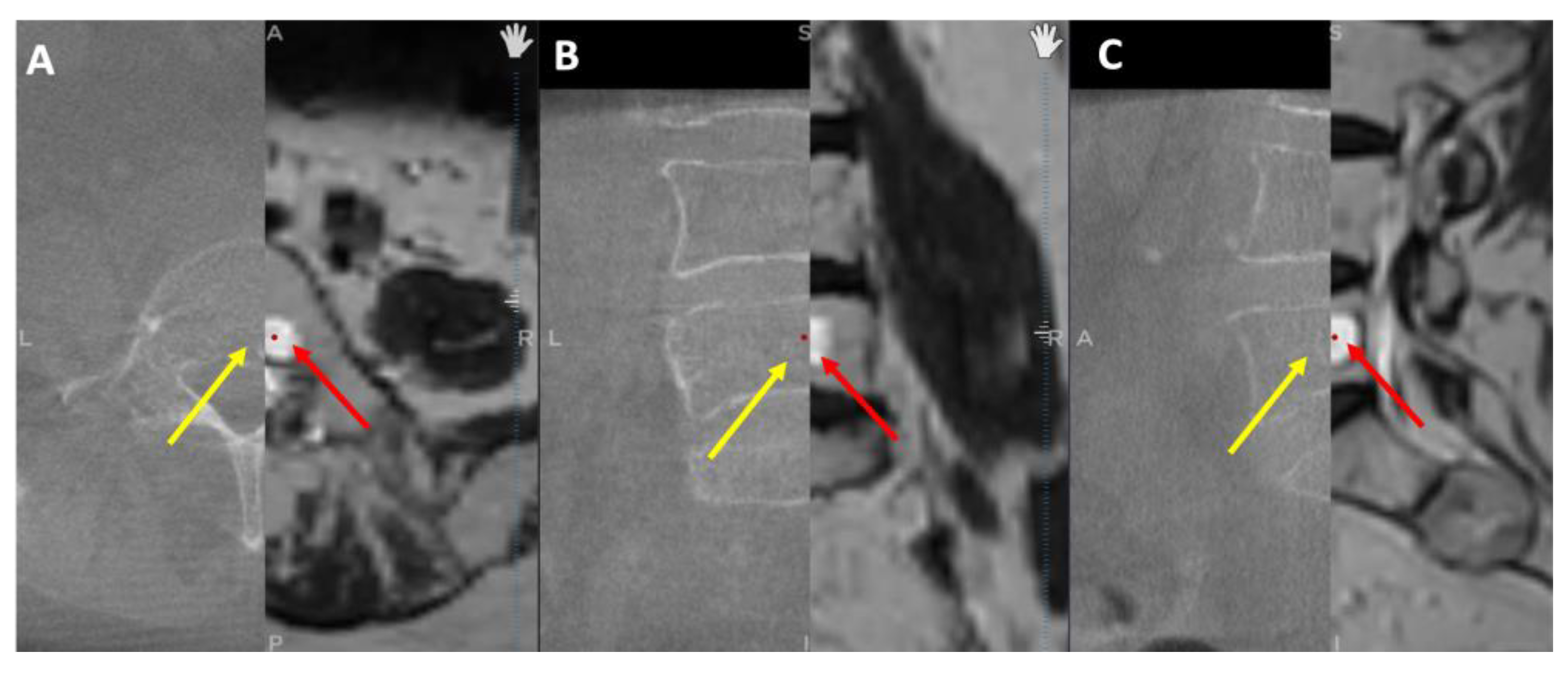
After a 5 mm skin incision, the surgical technique projected on the screen entered the vertebral body and performed a needle biopsy sampling using the Johnson and Johnson 8G vertebroplasty needle. During vertebroplasty sampling, the position of the needle to be inserted was also checked on the O-arm scan, not only on the MRI scan. After the needle biopsy sampling, the O-arm device was returned to the previous O-arm scanning position. Next, an O-arm scan of the same spinal segment was performed, and then the O-arm scan was merged with the needle biopsy plan previously selected in the MRI scan using the "Merge Image" function. If the position of the needle biopsy channel in the second O-arm scan matched the needle biopsy plan selected in the MRI scan, the needle biopsy sampling was considered successful (
Figure 3). Finally, the Spine Stealth Air reference frame was removed.
2.4. Measurement and Statistical Analysis:
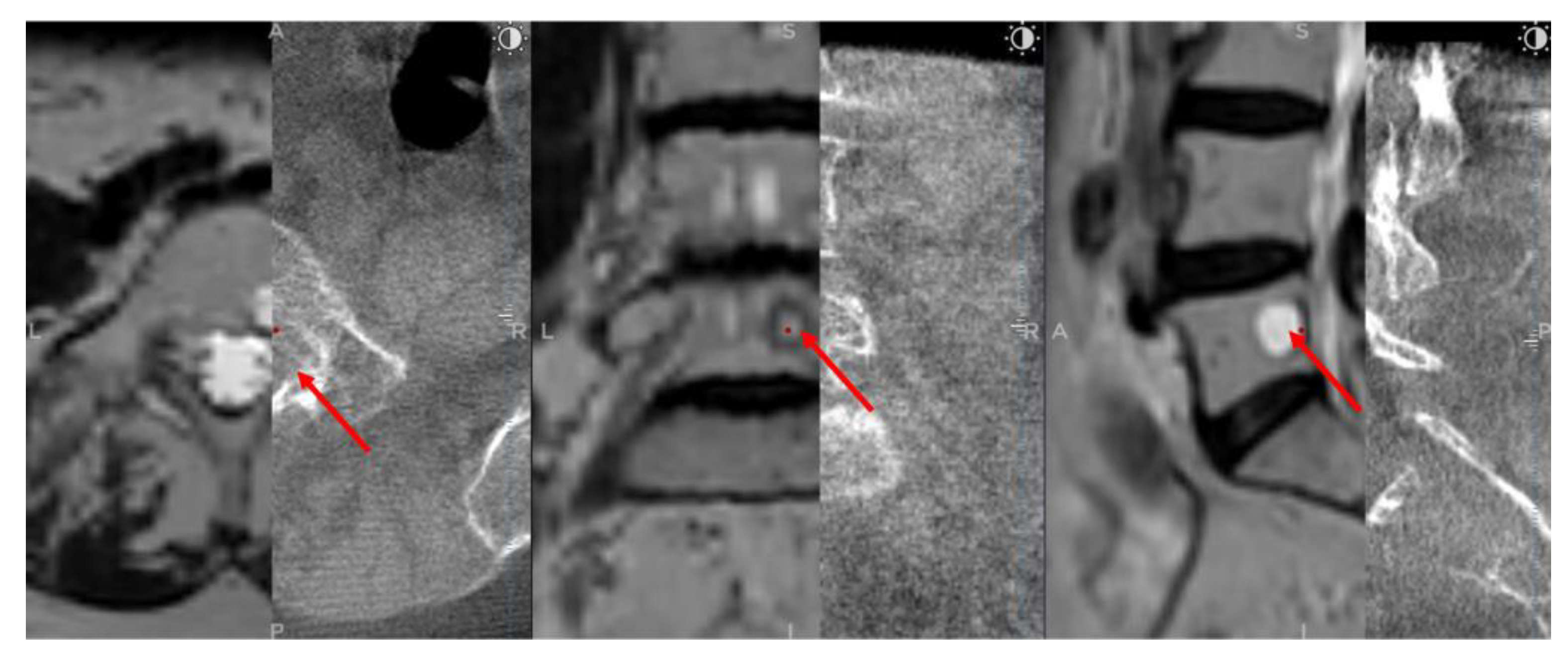
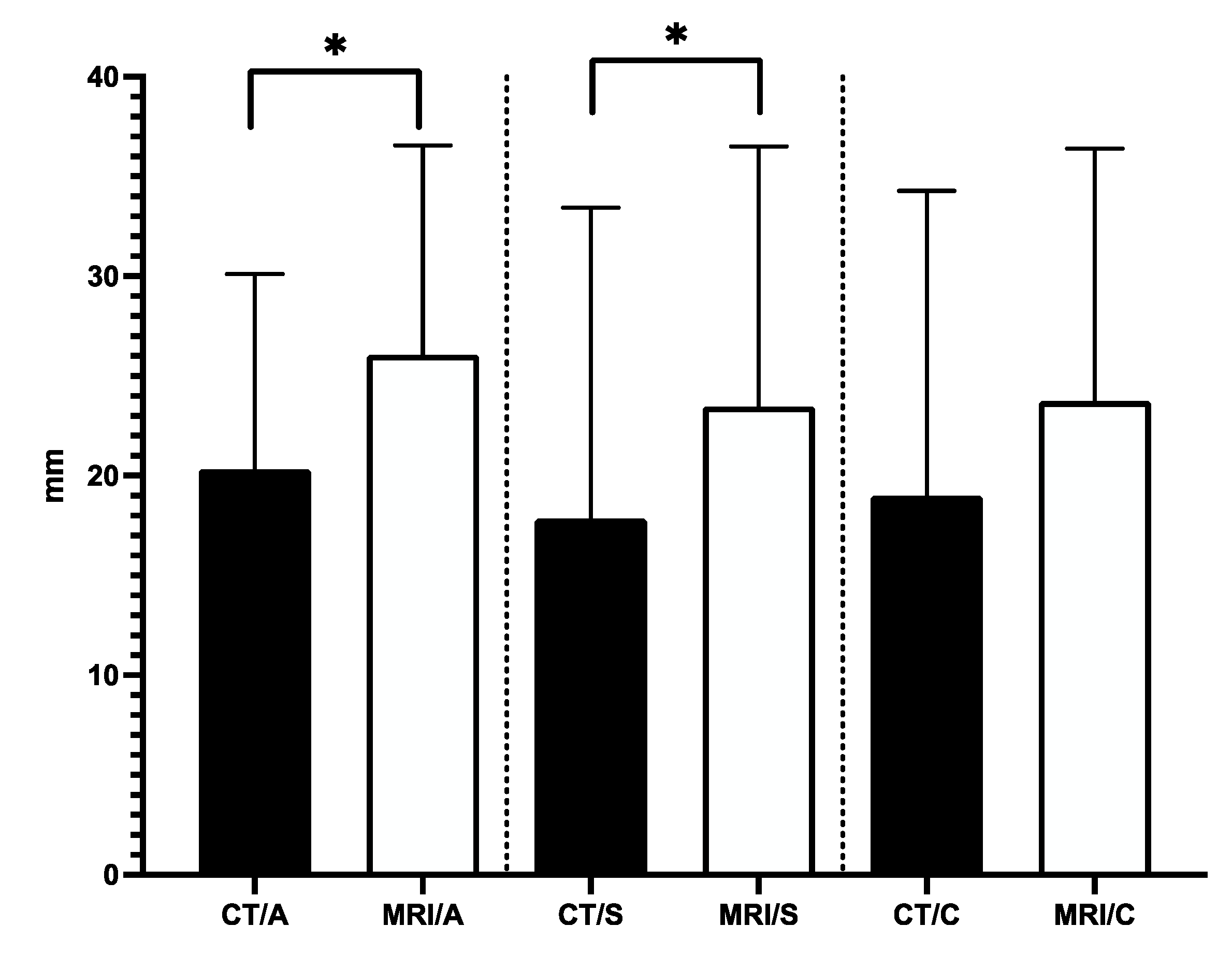
To validate that CT images were improved through merging with MRI images, the tumor dimensions were measured separately on axial, sagittal, and coronal planes in cases where the tumor was found in both modalities. While MRI scans revealed tumors in all 17 patients, CT scans revealed tumors in only 8 cases, implying that some tumors were not visible on CT (
Figure 4). As a result, we could only compare tumor dimensions in these 8 cases. GraphPad Prism 9 software was used to perform statistical analysis with a significance level set at p ≤ 0.05. Normality was checked for all data distributions, and a student t-test was employed depending on the results. The Pearson correlation coefficient was also calculated to examine the correlation between data sets.
3. Results
In this study, we conducted 18 biopsy procedures on 18 patients to obtain viable portions of suspected lesions for cytological examination (
Table 1). The procedures included 10 lumbar, 1 sacral, and 8 dorsal biopsies. For 16 out of the 18 patients, PET-CT FDG imaging was initially performed to identify the lesions; however, for all patients, we followed up with MRI investigations due to low resolution and sensitivity. The remaining two patients underwent MRI imaging directly. Contrasting with the remaining ten biopsies that had no previous attempts, seven (4 lumbar and three dorsal) had prior negative cytology results from C-arm free-hand guided biopsies.
Of the 18 patients, 13 had confirmed primary malignancies. Among them, 7 cases were diagnosed with breast cancer, with 3 of these patients having an additional primary tumor, including 2 cases of adenocarcinoma and 1 case of renal cell carcinoma. Four cases were identified as non-Hodgkin lymphoma, including 2 follicular lymphomas and 2 diffuse large B-cell lymphomas. The remaining 2 cases involved ovarian squamous cell carcinoma and prostate cancer. We aimed to identify metastatic spinal tumors in these cases, while 5 patients had no confirmed primary tumor.
All 18 biopsies yielded positive outcomes. Our cytology examination exposed non-Hodgkin lymphoma in 5 cases, with 4 confirming the previously known tumor and 1 detecting another follicular non-Hodgkin lymphoma with no known primary tumor, for the 7 patients with a history of breast cancer, metastatic tumors in the spine were confirmed through cultures. Among the patients with multiple primary tumors, 2 had metastatic breast cancer, while 1 had lung adenocarcinoma in the vertebrae. In addition, the biopsies for the patients with ovarian squamous cell carcinoma and prostate cancer supported a definitive diagnosis. The remaining 5 lesions were identified as 2 cases of monoclonal gammopathy of undetermined significance, 1 hemangioma, and 1 benign synovial cyst.
Our analysis showed that the average tumor dimensions in CT axial, sagittal, and coronal planes were 20.3 mm (±9.7), 17.8 mm (±15.6), and 18.9 mm (±15.2), respectively. On the other hand, the mean tumor dimensions in MRI axial, sagittal, and coronal planes were 26mm (±10.4), 23.4 mm (±13), and 23.7 mm (±12.6), respectively. The variations of the measured tumor dimensions between CT and MRI in axial and sagittal planes were statistically significant (
Figure 5).
4. Discussion
The examination of spinal lesions, whether infectious, tumor-related, or of other origins, has been dramatically enhanced by CT and MRI imaging. This advancement primarily provides a detailed visualization of the anatomical extent and identification of the active regions of the lesion following medium contrast injection. Nevertheless, the limited specificity of these imaging techniques often precludes a definitive etiological diagnosis based solely on radiographic findings. Consequently, histological analysis is typically necessary to accurately diagnose most spinal tumors and pseudo-tumorous lesions.
Open biopsy has long been regarded as the gold standard technique for obtaining tissue samples for histological analysis due to its perceived accuracy and adequacy. However, the significant complications associated with open biopsy have led to the exploration of alternative approaches, particularly closed biopsy techniques. In recent years, notable advancements have been made in closed biopsy, with a key focus on incorporating imaging modalities to ensure precise and sufficient sampling from the target tissue. The outcomes of numerous studies have been remarkable, demonstrating the potential of closed biopsy to overcome the challenges associated with open biopsy while retaining its advantages. Nevertheless, these compelling results warrant further investigation and the continuous search for a technique that strikes the balance between accuracy, adequacy, and minimized complications, such a technique holds immense promise for the future of tissue sampling in diagnostic procedures. Fluoroscopy, ultrasound, CT, and MRI have been the most commonly used imaging modalities for guiding percutaneous interventions, but each has limitations that can hinder the accuracy of the biopsy (6,11,13,14,19).
Percutaneous spine biopsy performed under fluoroscopy guidance has been extensively studied regarding its accuracy and adequacy. The reported accuracy of this procedure ranges between 16% and 92%, while the complication rates range from 0% to 10% (21). Notably, Kamei et al. conducted a series of 128 cases and reported a high accuracy rate of 93.8% for percutaneous spine biopsy performed under fluoroscopy guidance (23). Similarly, Dave et al. reported an adequacy rate of 88.7% for percutaneous spine biopsy conducted under fluoroscopy guidance (24). These findings highlight the promising outcomes and reliability of fluoroscopy-guided percutaneous spine biopsy as a valuable diagnostic tool for obtaining accurate and adequate tissue samples.
Renfrew et al. (25) proposed that CT-guided percutaneous transpedicular spine biopsy is recommended in cases where the location of a vertebral body lesion makes it challenging to access using the posterolateral approach. This recommendation was due to the proximity of neural elements to the pedicle. However, with the availability of high-resolution image intensifiers, it is possible to visualize the vertebral aspects in sufficient detail, allowing for the protection of the medial and inferior walls of the pedicle during biopsy and thereby avoiding injury to neural elements. On the contrary, Dave et al. (24) did not observe any distinct advantages of CT guidance over image intensification. Furthermore, image intensification offers the benefits of maintaining an aseptic environment in the operating room and being more cost-effective than CT scans.
CT guidance has become the modality of choice due to its higher accuracy and reduced complications. However, the reliability of this technique varies between 70-93%. It is believed that these rates depend significantly on the level (cervical, dorsal, or lumbar) of the biopsy obtained from, the type (primary or secondary) of tumor targeting, and its nature (heterogenous or homogenous) (14,26–29). Hence, a second needle biopsy, or incisional biopsy for the misdiagnosed lesions usually is done.
Since MRI has a superior soft-tissue and bone marrow contrast (11,30) and some lesions like metastatic multiple myeloma and non-Hodgkin’s lymphoma can only be visualized better by MRI (2), MRI guidance is becoming an accurate complementary diagnostic method to other imaging modalities for biopsy guidance spinal tumor biopsies (11). Liu M et al. even argued that MR-guided spinal biopsy alone is enough and has accuracy rates higher than the CT-guided method (2).
To increase diagnostic accuracy and reduce the need for additional invasive procedures, this study examined the effectiveness of combining MRI and CT imaging modalities for directing spinal biopsies in 18 patients with diverse spinal pathologies. Our findings showed that in all 18 biopsies, the combined technique accurately identified the diseased tissue type, offering a solid foundation for other treatment choices.
Our study leveraged the strengths of both MRI and CT imaging by utilizing MRI preoperatively and merging it with the intraoperative CT navigation system. This approach allowed for continuous monitoring of the biopsy process, ensuring accurate sampling of viable, non-necrotic tumor tissue; additionally, comparing pre-and post-operative scans validated the biopsy outcomes.
5. Conclusions
Our study offers a compelling argument for integrating CT and MRI imaging modalities in spinal biopsy procedures, highlighting the potential for significant improvements in the 100% diagnostic accuracy and efficiency rate. Although, as we continue to refine this approach and expand its application, we anticipate that it will become a valuable tool for clinicians, it is essential to acknowledge the need for larger-scale studies to validate these findings and explore the potential benefits of this approach in different clinical scenarios. Moreover, it is time-consuming since the patient must be under general anesthesia, but it is a way to avoid further misdiagnosed lesions. Our study findings reinforce the significance of careful consideration in choosing the appropriate imaging modality for accurate tumor size measurements. Our results indicate a substantial disparity between MRI and CT modalities, particularly in the axial and sagittal planes. This discrepancy highlights the superiority of MRI images in providing more reliable and detailed information, which is crucial for precise diagnosis and effective treatment planning. Moreover, these observations underscore the essential role of utilizing MRI imaging for obtaining biopsy samples with the necessary accuracy and adequacy.
Author Contributions
Conceptualization, A.V., and M.W.A-S.; Methodology, A.V., I.K., and B.B.; Analysis and Investigation, All authors; Data Curation, I.K, and B.B.; Writing – Original Draft Preparation, A.V., M.W.A-S, and S.A.; Writing – Review & Editing, M.W.A-S. and S.A.; Supervision, A.V.
Funding
No external funding was obtained for this research.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Péterfy Sándor Utcai Hospital-Clinic and National Institute of Traumatology, Medical Library (Dr. Manninger Jenő National Traumatology Institution), 1081, Budapest. Approval number is 02/2022 and the date of approval is 02/02/2022.
Informed Consent Statement
All subjects gave their informed consent for inclusion before they participated in the study.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We want to express our sincere gratitude to the staff of the Department of Neurosurgery and Neurotraumatology at Péterfy Hospital - Manninger Jenő National Traumatology Institution in Budapest, Hungary.
Conflicts of Interest
The authors confirm that no financial or commercial relationships could be considered a potential conflict of interest during this research.
References
- Tsukamoto S, Mavrogenis AF, Langevelde K van, Vucht N van, Kido A, Errani C. Imaging of Spinal Bone Tumors: Principles and Practice. Curr Med Imaging. 2022;18(2):142–61.
- Liu M, Sequeiros RB, Xu Y, He X, Zhu T, Li L, et al. MRI-guided percutaneous transpedicular biopsy of thoracic and lumbar spine using a 0.23t scanner with optical instrument tracking. Journal of Magnetic Resonance Imaging. 2015;42(6):1740–6.
- Duncan GA, Ferguson AB. BENIGN GIANT-CELL TUMOR OF THE FOURTH LUMBAR VERTEBRA: A Case Report. JBJS. 1936 Jul;18(3):769.
- Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am. 1996 May;78(5):656–63.
- Errani C, Traina F, Perna F, Calamelli C, Faldini C. Current concepts in the biopsy of musculoskeletal tumors. ScientificWorldJournal. 2013;2013:538152.
- Liu JC, Chiou HJ, Chen WM, Chou YH, Chen TH, Chen W, et al. Sonographically guided core needle biopsy of soft tissue neoplasms. J Clin Ultrasound. 2004;32(6):294–8.
- Rougraff BT, Aboulafia A, Biermann JS, Healey J. Biopsy of soft tissue masses: evidence-based medicine for the musculoskeletal tumor society. Clin Orthop Relat Res. 2009 Nov;467(11):2783–91.
- Stringham DR, Hadjipavlou A, Dzioba RB, Lander P. Percutaneous transpedicular biopsy of the spine. Spine (Phila Pa 1976). 1994 Sep 1;19(17):1985–91.
- Hadjipavlou AG, Kontakis GM, Gaitanis JN, Katonis PG, Lander P, Crow WN. Effectiveness and pitfalls of percutaneous transpedicle biopsy of the spine. Clin Orthop Relat Res. 2003 Jun;(411):54–60.
- Nourbakhsh A, Grady JJ, Garges KJ. Percutaneous spine biopsy: a meta-analysis. J Bone Joint Surg Am. 2008 Aug;90(8):1722–5.
- Carrino JA, Khurana B, Ready JE, Silverman SG, Winalski CS. Magnetic resonance imaging-guided percutaneous biopsy of musculoskeletal lesions. J Bone Joint Surg Am. 2007 Oct;89(10):2179–87.
- Fidler MW, Niers BB. Open transpedicular biopsy of the vertebral body. J Bone Joint Surg Br. 1990 Sep;72(5):884–5.
- Möller S, Kothe R, Wiesner L, Werner M, Rüther W, Delling G. Fluoroscopy-guided transpedicular trocar biopsy of the spine--results, review, and technical notes. Acta Orthop Belg. 2001 Dec;67(5):488–99.
- Rimondi E, Staals EL, Errani C, Bianchi G, Casadei R, Alberghini M, et al. Percutaneous CT-guided biopsy of the spine: results of 430 biopsies. Eur Spine J. 2008 Jul;17(7):975–81.
- Skrzynski MC, Biermann JS, Montag A, Simon MA. Diagnostic accuracy and charge-savings of outpatient core needle biopsy compared with open biopsy of musculoskeletal tumors. J Bone Joint Surg Am. 1996 May;78(5):644–9.
- Sucu HK, Ciçek C, Rezanko T, Bezircioğlu H, Erşahin Y, Tunakan M, et al. Percutaneous computed-tomography-guided biopsy of the spine: 229 procedures. Joint Bone Spine. 2006 Oct;73(5):532–7.
- Robertson RC, Ball RP. DESTRUCTIVE SPINE LESIONS: Diagnosis by Needle Biopsy. JBJS. 1935 Jul;17(3):749.
- Valls J, Ottolenghi CE, Schajowicz F. Aspiration biopsy in diagnosis of lesions of vertebral bodies. J Am Med Assoc. 1948 Feb 7;136(6):376–82.
- López JI, Del Cura JL, Zabala R, Bilbao FJ. Usefulness and limitations of ultrasound-guided core biopsy in the diagnosis of musculoskeletal tumours. APMIS. 2005 May;113(5):353–60.
- Ghelman B, Lospinuso MF, Levine DB, O’Leary PF, Burke SW. Percutaneous computed-tomography-guided biopsy of the thoracic and lumbar spine. Spine (Phila Pa 1976). 1991 Jul;16(7):736–9.
- Metzger CS, Johnson DW, Donaldson WF. Percutaneous biopsy in the anterior thoracic spine. Spine (Phila Pa 1976). 1993 Mar 1;18(3):374–8.
- Babu NV, Titus VT, Chittaranjan S, Abraham G, Prem H, Korula RJ. Computed tomographically guided biopsy of the spine. Spine (Phila Pa 1976). 1994 Nov 1;19(21):2436–42.
- Kamei Y, Nishida J, Mimata Y, Shiraishi H, Ehara S, Satoh T, et al. Core Needle Percutaneous Transpedicular Vertebral Body Biopsy: A Study of 128 Cases. J Spinal Disord Tech. 2015 Aug;28(7):E394-399.
- Dave BR, Nanda A, Anandjiwala JV. Transpedicular percutaneous biopsy of vertebral body lesions: a series of 71 cases. Spinal Cord. 2009 May;47(5):384–9.
- Renfrew DL, Whitten CG, Wiese JA, el-Khoury GY, Harris KG. CT-guided percutaneous transpedicular biopsy of the spine. Radiology. 1991 Aug;180(2):574–6.
- Hau A, Kim I, Kattapuram S, Hornicek FJ, Rosenberg AE, Gebhardt MC, et al. Accuracy of CT-guided biopsies in 359 patients with musculoskeletal lesions. Skeletal Radiol. 2002 Jun;31(6):349–53.
- Issakov J, Flusser G, Kollender Y, Merimsky O, Lifschitz-Mercer B, Meller I. Computed tomography-guided core needle biopsy for bone and soft tissue tumors. Isr Med Assoc J. 2003 Jan;5(1):28–30.
- Monti C, Rimondi E, Rollo G, Bettini N, Picci P, Marchi M. [Percutaneous computed tomography-guided biopsy in spinal diseases]. Radiol Med. 1994 Mar;87(3):299–304.
- Puri A, Shingade VU, Agarwal MG, Anchan C, Juvekar S, Desai S, et al. CT-guided percutaneous core needle biopsy in deep seated musculoskeletal lesions: a prospective study of 128 cases. Skeletal Radiol. 2006 Mar;35(3):138–43.
- Kerimaa P, Marttila A, Hyvönen P, Ojala R, Lappi-Blanco E, Tervonen O, et al. MRI-guided biopsy and fine needle aspiration biopsy (FNAB) in the diagnosis of musculoskeletal lesions. Eur J Radiol. 2013 Dec;82(12):2328–33.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).