1. Introduction
Several governments worldwide are interested in reducing their country’s dependence on oil. Among the alternatives for replacing oil, the growing use of renewable energy sources and Electric Vehicles (EVs) are the most promising. EVs are desirable because they can reduce high emissions of greenhouse gases locally. The carbon dioxide (CO
2) emissions make the land transport sector one of the main ones responsible for climate change [
1].
Although EVs are an alternative to decarbonizing the transport sector, several challenges are still to be overcome. Among them, it is worth highlighting the price of battery modules. Although battery module prices have fallen in recent years, it is estimated that battery modules cost approximately 2/3 of the total EV cost. The price of battery modules must be reduced by half its current value to make these vehicles economically viable and competitive with combustion engine vehicles [
2,
3,
4,
5].
Lithium-Ion Batteries (LIBs) are used in the majority of EVs because of their high specific energy, low self-discharge, extended lifespan, safety, and cost [
6,
7,
8,
9]. During the operation of EVs, the batteries undergo various degradation processes that depend on numerous factors (e.g., road conditions, driver behavior, ambient temperature, and cabin temperature) [
1]. These degradation mechanisms reduce battery capacity and power. The battery capacity and power reduction result in a shorter EV range, causing
range anxiety in customers. The accelerated battery degradation also reduces charging and discharging efficiency, increasing their internal resistance and shortening their lifetime [
10].
Automakers recommend replacing batteries when it remains 70 to 80% of the initial charge range [
11]. This limit is still uncertain, and many studies have reported that batteries will be used below this limit. On the other hand, early battery replacement will be recommended if the battery's degradation mechanisms are not adequately mitigated [
12].
Battery deterioration processes are critical to understanding the battery for technical, economic, and scientific purposes. Understanding the degradation process of batteries will allow companies to determine the best time to replace EV batteries, optimize their design (i.e., maximize their efficiency), accelerate the product development cycle, and ensure that the battery is safe and has the adequate performance to operate on EVs and a second application.
In terms of economic aspects, it is possible to estimate and reduce the return on the investment, identify new ways to capture the value and maximize the value captured during the operation of the batteries based on the degradation mechanisms. Understanding battery degradation mechanisms is essential for optimizing battery models that will be used in embedded systems responsible for battery control and monitoring. These systems can extend battery life and, consequently, enable the market for second-life batteries [
13]. From the research perspective, it is possible to identify the factors that accelerate the batteries' degradation, predict the moment that the battery will fail, identify new viable applications for the batteries, and identify possible battery defects. Thus, it is possible to design new models and solutions to overcome the existing issues [
12].
Predictive maintenance is also essential to ensure battery safety. Most battery manufacturers provide predictive maintenance services based on vehicle distance traveled and lifetime. However, this process has high costs, low efficiency, and is time-consuming. Therefore, understanding battery degradation is essential for the battery manufacturer to provide the maintenance service at the ideal moment, avoid unscheduled maintenance, and reduce costs and maintenance time. This is relevant for the tooling and EV markets. This process can be optimized using machine learning algorithms defining the optimal time to provide service intelligently based on previous maintenance histories and battery operation data.
Battery degradation processes are complex, and their understanding is not a trivial task due to the numerous factors that influence each other. However, identifying the optimal operating range of current, voltage, and temperature for this Energy Storage System (ESS) operation is crucial for diagnosing and prognosis battery failures and predicting and extending battery life. A systematic review of the literature is needed to understand battery behavior in critical situations, predict failures and lifespan, and implement safety functions in the Battery Management System (BMS).
The non-linear characteristics of the Solid-Electrolyte Interphase (SEI), lithium coating, and loss of active material make it challenging to comprehend, model, and manage battery degradation mechanisms. The fact that numerous of these events coexist and have an impact on one another makes it difficult to simulate each degradation mechanism. Therefore, more research is necessary to comprehend the battery deterioration mechanism and to develop dependable novel monitoring and diagnostic technologies.
Usually, the degradation mechanisms are investigated in analyses carried out after the batteries reach their useful lifetime, called post-mortem analyses. In these analyses, the components of the aged cells are separated and individually analyzed. The cell must be disassembled to perform this type of analysis. This technique's advantage is that it makes it feasible to pinpoint each cell's component's unique contribution. Otherwise, one of the main disadvantages is the need to carry out stress tests on the cell to evaluate the degradation mechanisms, making this analysis time-consuming and costly [
14].
Recent research carried out shown a great advance in the development of models to predict the mechanisms of battery degradation. These models can be classified as electrochemical, empirical, semi-empirical, and based on data. Different classifications of models can be developed to predict battery parameters.
Electrochemical models are used to simulate the behavior of cells. They are accurate because they are based on mathematical equations describing the chemical characteristics of the cell's materials and the design variables. The main disadvantage of these models is the difficulty of describing mathematical equations. The chemical behavior of cells and their degradation mechanisms are complex once many of these phenomena co-occur. They depend on numerous external factors and have non-linear characteristics. From a computational perspective, these complex mathematical equations require a high computational cost to be solved, which can take a long time to model and predict. From a practical point of view, this kind of model usually requires cell disassembly and the exposition of its operator to high voltages, making the procedure slow and challenging to be scalable [
12,
15].
Empirical models are built from direct measurements of battery observables. Although empirical models do not require battery disassembly, their major drawback is performing cycle tests to measure the model's variables. These tests could be expensive and take a long time. This model is built for a specific scenario, e.g., batteries employed in EVs, and consequently, cannot be used to forecast the battery deterioration processes in a different scenario, such as the battery's use in a second application [
17]. As shown in
Figure 1, semi-empirical models combine the physical and chemical characteristics of batteries with measured [
12,
18]. Electrochemical Impedance Spectroscopy (EIS) is often used to identify equivalent circuit model parameters that are used to estimate battery states such as internal impedance, Li-ion diffusion dynamics, electrode contact impedance, SOC, and State of Health (SOH). The EIS are signals rich in information about the aging of batteries and are often used as input parameters to estimate the useful life of batteries. However, most commercial BMSs still do not collect EIS on board the vehicle due to the high cost of the equipment and the results are subject to variations in temperature, SOC and the test is time consuming [
19].
Due to the need to avoid collecting EIS in real-time, in [
19], the authors proposed a method for predicting EIS based on battery charge curves. The method can map the battery charge voltage curve with the electrochemical spectrum using machine learning algorithms. The method presented reliable results with errors below 4 mΩ.
Data-based Models (DbMs) have become attractive due to the greater processing power of computers [
19,
20,
21]. DbMs are increasingly being applied in the industry because they can reduce the design time, make predictions about premature battery failures, and do not require the batteries to disassemble to build these models. Data from EIS [
19,
22], and Open-Circuit Voltage (OCV) [
23,
24], among others, are employed to build models using pattern recognition and machine learning techniques [
9,
12,
22,
25,
26,
27]. DbM can be classified as empirical when parametric, i.e., built based on the battery's prior knowledge. On the other hand, DbM can also be classified as non-empirical when built from real-time measurements, i.e., the model emits an output for each new sample measured [
28].
Figure 2 shows a flowchart of data-driven models applied to batteries.
Battery degradation is a complex phenomenon that needs to be modeled and controlled by systems capable of keeping battery operation within operating limits, increasing battery life in EVs and other applications. Several works have been proposed in the literature. In [
29], the authors investigated the current collector's aging mechanism, which is responsible for the power reduction and the increase of the battery impedance. In [
30], the authors showed that the loss of capacity occurs due to changes in the cyclable lithium and active material loss. In [
31], an excellent review of the battery degradation mechanisms is presented. The collected information in [
31] motivated new works focusing on diagnosing battery degradation, offering prognoses, understanding the effects of cycling conditions on degradation, and understanding how the degradation mechanisms are interrelated.
Despite many scientific and technical papers in the literature that aim to clarify the battery degradation process, there are still issues that need to be clarified. In other words, it is possible to say that a review of the new knowledge created since the last review studies carried out to explain the degradation mechanisms of the batteries is still lacking. Therefore, this work seeks to clarify the mechanisms of battery degradation with a focus on comprehensively explaining how cycle and calendar effects affect battery degradation and diagnosing and predicting these mechanisms and their impact on battery safety. Different from most studies in the literature (see
Table 1), the contribution of this work is to provide a systematic review of battery degradation mechanisms, the main causes of battery failure, and ways to mitigate these effects. In addition, this systematic review presents several ways of diagnosing and proposing the different battery degradation mechanisms. The cathode has a fundamental role and the battery degradation mechanisms, and few works describe the degradation mechanisms that happen in the Cathode-Electrolyte Interface (CEI).
This work aims to present the working principle of LIBs and their main degradation mechanisms simply and directly. The purpose of
Section 2 is to briefly present the motivation for this work. The goal of
Section 3 is to answer the following questions: "how do LIBs work?", "which are the main components of LIBs?", "which LIB chemicals are most used in EVs?" and "which cell types are most used in EVs?".
Section 3 aims to investigate the LIBs degradation in EVs.
Section 4 presents the degradation mechanisms of LIBs and
Section 5 presents a discussion of the results. Finally, a conclusion is presented.
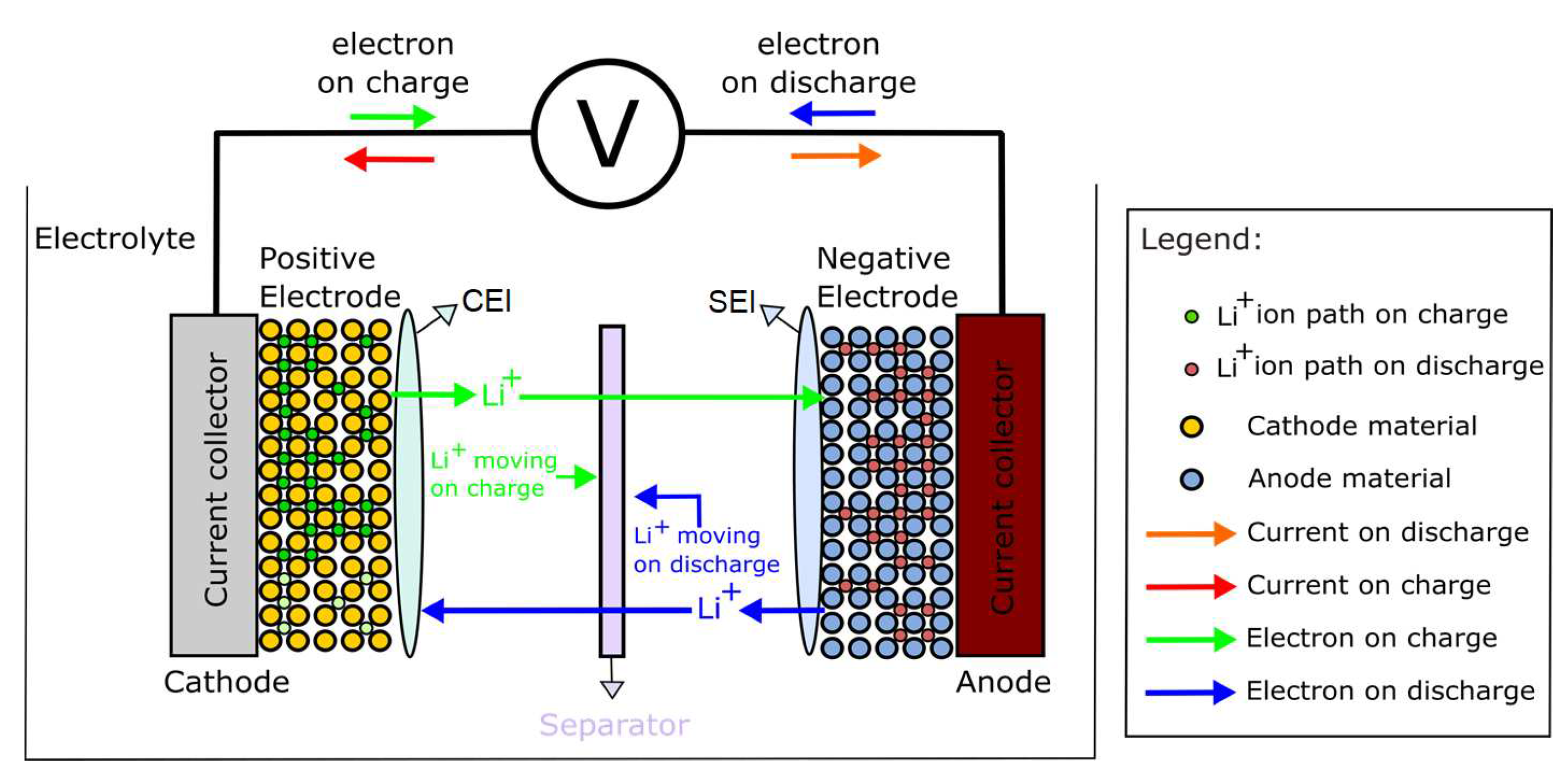
3. Basic Structure of LIBs
The major components of the LIBs comprise the positive electrode, negative electrode, electrolyte, current collector, separating membrane, and casing.
Figure 3 details the atomic structure of a LIB.
The battery has a positive electrode and a negative electrode. The positive electrode presents a crystalline structure, e.g., generally made of lithium manganese oxide (LMO); lithium cobalt oxide (LCO); lithium nickel oxide, cobalt, and aluminum (NCA); and lithium oxide, nickel manganese, and cobalt (NMC). They can also comprise olivine-type materials, such as lithium iron phosphate (LFP) [
41]. Positive electrodes generally consist of materials whose specific capacity is less when compared to negative electrode materials. However, when high-capacity materials are inserted into the positive electrode, the battery's degradation process is accelerated, reducing its useful lifespan [
42,
43,
44,
45]. For this reason, several studies are being performed to develop new materials to increase the positive electrode's specific capacity.
Initially, the LIBs that dominated the battery market contained LCO as positive electrodes. Batteries with the LCO cathode have high working voltage, excellent performance rate, and good cycling performance even at high temperatures. The main disadvantage of this technology is that cobalt has a low specific capacity, and high cost and can cause serious environmental impacts due to its toxicity [
46,
47,
48,
49,
50]. Furthermore, charging LCO-based batteries at high voltages causes instability [
51]. Recent research has shown some strategies can be adopted to mitigate this limitation, such as (i) the use of additives in electrolytes [
52,
53], (ii) lattice element doping [
54,
55], and, (iii) surface coating/modification with other active/inactive materials [
56,
57,
58,
59,
60].
According to [
51], deep discharge in batteries constructed with LCO cathode causes mechanical damage and a large change in the dimension
c of the shaft. The degradation of batteries starts in the first cycles with a structural change of the structure that causes the increase of the grain size, reduction of the surface potential, and loss of the contact rigidity, concomitant with the irreversible fading of the capacity [
51].
However, with the development of new technologies and the need to increase battery life and safety, new technologies have been developed, such as NMC (LiNi
xMn
yCo
zO
2) with the following limits 0≤x, y, z≤1 [
37]. The major advantages of LIBs comprising NMC-positive electrodes are that they present reversible capacity, lower cost, and are environmentally friendly. However, they may show cycle and chemical instability when exposed to air, restricting their use from an industrial point of view [
38]. The increase in nickel content in the electrodes of battery cells allows greater extraction of lithium ions at the same cut-off voltage. Therefore, it is possible to affirm that the increase in the proportion of nickel in the battery cells allows an increase in the capacity of the battery cells [
61]. Manganese improves the Depth of Discharge (DOD) of the battery [
32]. The exposition of NMC-based batteries with air components (e.g., CO
2 and H
2O) produces a reaction that forms Li
2CO
3 and LiOH, considered impurities on the NCM surface. This phenomenon produces a large amount of highly reactive Li, which causes serious safety issues and reduces the electrochemical performance of the battery [
46,
61,
62,
63,
64,
65,
66,
67].
The main degradation mechanisms of NMC-based batteries are particle breakdown, gasification, phase transformations, and cation mixing. These factors are mainly caused by the formation of highly reactive nickel in batteries. Among the main strategies adopted to reduce the instability caused by the high reactivity of nickel are a surface coating on the electrode of the active material, doping of the active material of the electrode, and conversion of the morphology of the NMC particles from a polycrystalline structure to a monocrystalline structure.
Still, other materials such as titanate or silicone can also be employed. Specific characteristics of lithium titanate (LTO) have attracted the attention of academia in recent years, e.g., long lifespan, without significant structural changes during each cycle [
50,
68], safety, thermal stability, and high potential. Also, these advantages prevent the formation of dendrites [
51] at the cost of a reasonably lower voltage [
32,
51].
LFP-based batteries are highly thermally stable and have high cycle life and power. The use of this battery technology is also beneficial in high-power applications where high discharge rates are required. During charge and discharge cycles, lithium intercalation and deintercalation can cause phase transitions in the active material, leading to volume changes and mechanical stress. Certain crystalline structures can more effectively accommodate these volume variations, thereby reducing mechanical stress and deterioration. For instance, anisotropic structures like those found in LiFePO4 (olivine) can ameliorate mechanical stress during phase transitions, thereby contributing to a longer shelf life. The primary mechanisms associated with this type of battery are the decomposition of electrolytes, the loss of active material, the formation of lithium dendrites, the structural degradation of the electrodes, and the modification of the separator's morphology.
The negative electrode usually consists of graphite-like material. Graphite dominates commercial batteries because its main advantages are high cycle stability, small voltage hysteresis, and high tap density. However, batteries with graphite anodes still have limited power and energy density in EV scenarios and large-scale power supplies. New anode materials that have low redox potential for high output voltage, excellent Li
+ reversibility, intercalation/deintercalation (or plating/stripping for lithium metal anodes), structural stability during cycling, high ionic/electronic conductivity, low cost, and should be friendly to the environment [
69].
As mentioned above, LIBs have a high energy density and low memory effect, and they are lightweight, whose benefits have targeted them as the best option for EV application. The high energy density is critical because it enables the cell to reach a high capacity to store energy in the same cell volume. The low memory effect allows the cell to be recharged at any current charge level without significantly losing the maximum energy capacity [
11].
LIBs' electrodes are prepared by mixing binders (i.e., polymeric-based to "glue" particles) to connect materials among themselves and to the current collector [
32]. The electrodes are assembled face-to-face and separated by a mesoporous membrane (i.e., separator), and electrodes are soaked in electrolytes. Under polarization, the ions move out from the positive electrode and into the negative electrode. Electrolytes transport lithium ions and also directly influence battery electrochemistry [
70]. The electrolyte's ions move in the same direction, i.e., from the positive electrode to the negative electrode, neutralizing each piece of the system locally. Most of the electrolytes employed comprise carbonate solvent blends. The most widely used electrolytes are mixtures of various carbonates (e.g., ethylene, dimethyl, and propylene carbonate) and dissolved salt (e.g., LITFSI and LiPF
6) [
32].
During the charging phase, Lithium ions (Li
+) migrate towards the negatively charged electrode, a process scientifically referred to as intercalation. Concurrently, electrons are compelled to traverse from the cathode to the anode, facilitated by an external Direct Current (DC) source, thus maintaining the overall equilibrium of the electrochemical cell. In the discharge cycle, the ions return from the electrolyte to the positive electrode, and electrons are extracted from the positive electrode to the external circuit spontaneously. In these processes, the materials are oxidized or reduced. The positive electrode consumes electrons as the electrode is reduced; the oxidation occurs at the negative electrode electrons from the external circuit [
60].
Regarding the battery design, according to battery manufacturers, the positive electrode should be as close as possible to the negative electrode, which will reduce the ions impedance in the electrolyte. Therefore, it is essential to have a separator to prevent contact between the electrodes and avoid a short circuit. The separator is a permeable membrane that serves as an ionic conductor and an electrical insulator. This indicates that it allows the passage of lithium ions but not electrons. Thus, it means that the separator is ionically conducting and electronically insulating.
EV batteries can have three structural shapes: pouch, prismatic cells, and cylindrical cells. Cylindrical cells have a lower manufacturing cost (
$/kWh) because they have been mass-manufactured for a long time, providing fast production compared to other types of cells. The assembly of cylindrical cells consists of wrapping the electrodes in a cylindrical shape encapsulated with a metal. This type of encapsulation reduces the delamination of the active material of the current collector, increasing the resistance of this type of cell to mechanical shocks, thermal charging, discharging cycles, and current collectors' mechanical expansion. They have a high energy density, and if one cell fails on a battery package, it will culminate in a low impact. Also, the temperature control of these cells is more accessible than prismatic cells [
54]. Cylindrical cells are combined into packages and modules and the circular cross-section of the cell does not optimally utilize the available space, which is a significant disadvantage when compared to other types of shapes [
71].
Prismatic cells are lighter and mechanically robust. However, this cell type has a high manufacturing cost, and lower energy density and mechanical stability than cylindrical batteries. Prismatic cells may experience swelling due to their operation outside of safe conditions. This swelling is due to increased pressure in the cell when the safety vent opening is obstructed. This cell shape presents a lower energy density than cylindrical cells [
54].
Soft pouch cells have the advantages of being lightweight, their manufacturing cost is not very high, and they have a greater density when compared to the other two models mentioned above. On the other hand, they need a robust mechanical structure for their protection. They are more likely to suffer an increase in volume because they may not have a designated ventilation mechanism. The swelling effect observed in pouch cells is due to battery degradation, which is caused by the physical expansion of the battery as a consequence of gas accumulation or other factors. This expansion can result in an increase in internal pressure, which may eventually cause the battery to rupture. The expansion effect is influenced by several technical factors, including the increased thickness and flexibility of electrodes and separators, the cell design, and the battery shape. To prevent and mitigate battery enlargement in pouch cells, it is essential to carefully consider and optimize these technical parameters.
Gas generation is an additional form of battery deterioration that can be influenced by battery shape. Gases can be produced inside the battery as a result of the chemical reactions that take place during the charging and discharging process, leading to swelling, pressure build-up, and even battery rupture. The main types of gases produced by these chemical reactions that take place inside the battery are CO, HF, SO
2, NO
2, NO, and HCl. There are several ways in which the design of the battery can affect gas production. For example, flat pouch batteries have a higher ratio of surface area to volume than cylindrical batteries, which can increase gas production due to increased electrochemical reactions on the battery's surface. Due to the design of the electrodes and separators, certain regions of a pouch battery may be more susceptible to gas accumulation [
71].
Lithium plating is another form of battery deterioration that can occur, particularly during rapid charging or at low temperatures. This occurs when metallic lithium accumulates on the anode's surface, decreasing the quantity of available lithium ions and causing a capacity loss. The geometry of the battery can influence lithium plating in several ways. For example, cylindrical batteries have a lower ratio of surface area to volume than flat pouch batteries, thereby reducing the likelihood of lithium plating. In addition, the geometry of the anode and cathode can influence the distribution of lithium ions and the likelihood of lithium plating.
The formation of an SEI occurs when a layer of solid electrolyte forms on the anode's surface, reducing the availability of lithium ions and resulting in capacity loss. By affecting the ratio of surface area to volume and the geometry of the electrodes and separators, the battery's configuration can influence the distribution of lithium ions and the likelihood of solid electrolyte formation.
Dendrite proliferation is an additional form of battery degradation, especially in LIBs. This occurs when lithium deposits form on the surface of the anode and grow into sharp, needle-like structures that can breach the separator and cause a short circuit. The configuration of the battery can impact dendrite growth by influencing the geometry of the anode and cathode and the distribution of lithium ions.
Separator cracking is an additional form of battery degradation that can occur, especially in LIBs. This occurs when the separator, which is typically made of polymer, dissolves due to excessive heat or flame exposure. The geometry of the battery can affect this process by altering the thickness and thermal conductivity of the separator, as well as the cell's design.
Decomposition of the positive active material can also contribute to battery degradation. Positive active material, which is typically composed of lead dioxide, degrades over time due to electrochemical reactions. The configuration of the battery can influence this process by influencing the geometry of the anode and cathode and the lead distribution.
Generally, these EV batteries can reach an energy density of nearly 300 Wh/kg [
51,
52] and the predominant average cost of current battery generation is approximately 100 to 200
$/kWh [
56,
72]. During the phase design of LIBs, the goal is to maximize the potential difference between the positive and negative electrodes, minimize the active material mass and volume, and prevent the electrolyte from undergoing the oxidation/reduction process [
73].
4. Degradation of LIBs
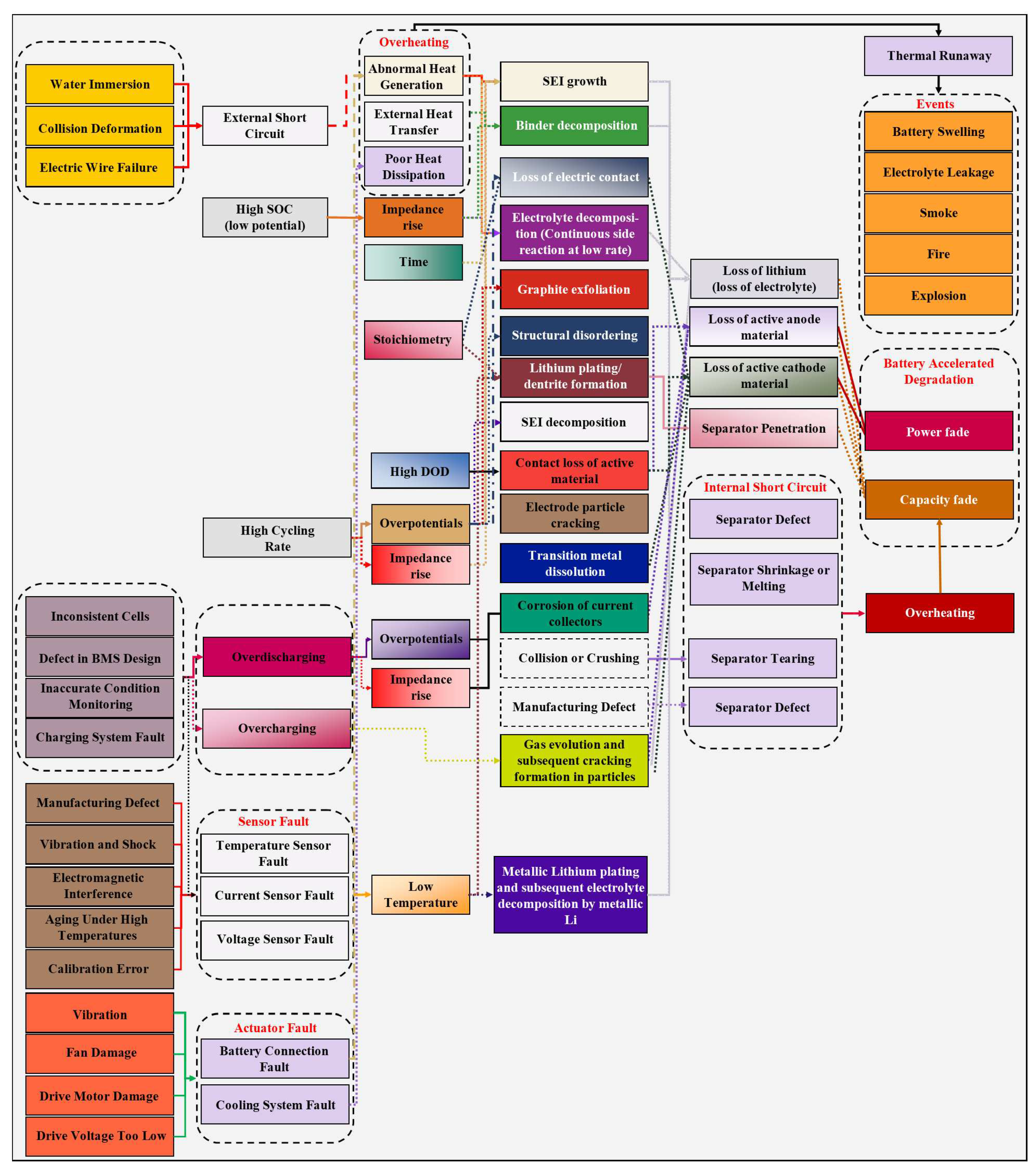
LIBs are subject to the phenomenon known as “thermal runaway” when subjected to abuse conditions, such as vehicle collision, overvoltage, overcurrent, and deep discharge [
74,
75]. Thermal runaway is an exothermic phenomenon in which reactions inside the cell cause an increase in temperature [
76,
77,
78,
79]. These reactions within the cell can lead to electrolyte decomposition, gas formation, voltage drop, and internal pressure increase. It can culminate in the cell's rupture and swell, causing electrolyte leakage, which may cause fire/flame and explosion in contact with air.
The gases inside the cell can also be produced by the electrolyte reduction, resulting from the decomposition reaction of electrolyte solvent and by the structural release of cathodic materials [
36,
80]. These released gases, such as hydrogen, organic products, and ethylene, can be toxic and flammable, which can cause severe harm to the individual's health [
36,
58]. These gases can also cause uncontrolled thermal runaway in the cell and compromise the vehicle's safety [
32,
59]. The gas evolution inside the cell is also associated with electrolytic displacement, increased internal resistance that reduces the battery's efficiency, the number of cycles, and its lifespan [
36,
58,
81].
The aging of LIBs is still the subject of research to understand and minimize electrolyte decomposition and gas evolution. To understand the degradation mechanisms, it is essential to understand the phenomena that occur at the electrode/electrolyte interface, considering that changes in this interface are responsible for the aging of the electrodes. The electrode's combination with lithium ions and electrons occurs in the electrode/electrolyte interface. The ion is stored in the electrode, intercalated, as an alloy, or simply as Li metal. The Li
+ intercalation implies electron absorption for the sake of electrode neutrality [
19,
58,
73,
82]. The charge transfer in and out electrode also affects the current collector, which suffers from corrosion as an effect of cycling. This corrosion effect takes place at the negative electrode (the degradation mechanisms that occur at this electrode are discussed in more detail in Section 3.2) and causes irreversible capacity loss in the cell [
59].
In aging, the main problems are the effects of the cycle and the calendar, as these effects influence both the energy (capacity) and the power (impedance) of the battery due to loss of lithium inventory, loss of active material, negative electrode capacity, and positive electrode capacity [
19,
46,
55,
60,
61,
83]. Loss of cell power happens due to loss of local contact, reduction of electrode reaction surface, structural changes in host materials, changes in electrolyte properties, structural changes in the separator, and the current collector's corrosion [
51]. The decrease in battery capacity refers to reducing the amount of charge that a battery can store per unit of time, usually expressed as a percentage. The energy decrease minimizes the ability to supply energy because of the internal resistance increase [
28]. In practice, the cycling and calendar effects are interrelated, especially when batteries have low cycle depth and low current rates [
56,
57,
62,
63,
64,
73]. The two effects coexist simultaneously, undergoing a mutual action with each other. Thus, every degradation mechanism has its behavior influenced by each other [
46].
The cycling effect is directly related to the battery charging and discharging and refers to the degradation mechanisms and capacity loss caused by the electrode and electrolyte decomposition [
46,
64]. In cycling, it is possible to detect battery degradation due to impedance increases. That is caused by the formation of passivation layers in the electrode-electrolyte interfaces, resulting from the mechanical stress in the electrode's active materials or lithium coating [
20,
55,
62,
64,
66]. Therefore, the cycling effect mainly impairs the reversibility of materials, and it is directly related to battery parameters, such as SOC, high/low temperature, time, charge and discharge currents, deep-of-discharge, and charge efficiency [
64,
66,
67,
70].
The calendar effect is irreversible and refers to all battery degradation processes that occur over time regardless of the battery's charge/discharge cycle. When batteries are stored in open circuit conditions, no current flows inside the battery [
20,
55,
70]. This effect has no linear behavior on the SOH and can be accelerated at high temperatures that increase the dissolution of the metal and produce a reduction in the cell capacity [
20,
65]. Calendar aging is a result of electrolyte reduction and oxidation and surface film growth on active materials [
20,
56,
57,
65]. It is accelerated at higher SOC, longer time intervals, and/or high temperatures [
56,
70].
In general, some factors have a great influence on the battery degradation process, they are (i) temperature, (ii) SOC variation, (iii) DOD, and (iv) load/voltage limit. discharge. The temperature affects cell aging because high temperatures increase the agitation of the molecules and consequently accelerate the processes of insertion and/or removal of lithium in the host network. At low temperatures, lithium metal grows due to the slow transport of lithium into and within the negative electrode host network. This increases the local lithium-ion concentration and makes the lithium metal stable. The high temperatures accelerate the side reactions, alter the composition of the SEI layer and its thickness, increase the battery's internal resistance, and certainly enhance the degradation process. This makes the cell more prone to the thermal leakage process, leading to batteries' fire and explosion. It is also important to mention that a very thick SEI layer is the main factor for the power loss in the cell. Low temperatures only matter if the cell is operating. Low temperatures can reduce the electrolyte viscosity, decrease the lithium-ion conductivity process, and cause the slow diffusion of the lithium ions within the electrode, reducing the battery discharge capacity.
The thermal stability of battery cells, as well as the kinetics of internal battery reactions, can be evaluated using the Accelerated Rate Calorimetry (ARC) technique. ARC involves heating the battery at a high rate while monitoring its temperature and heat output using a sensitive calorimeter. This enables the observation and analysis of the battery's thermal behavior, including the onset temperature and peak temperature of thermal events, the heat generated during thermal events, and the rate of heat generation. This is feasible because ARC is an adiabatic system. It facilitates the quantification of the system's inherent heat generation and the development of gaseous products via pressure evaluation. Battery safety can be investigating lithium content, particle size, material density, lithium salt, solvent, additive, binder, and initial heating temperature using ARC [
79]. By analyzing the data obtained from ARC experiments, researchers can gain a detailed understanding of the thermal behavior of LIBs under different conditions, such as changes in SOC, c-rate of discharge, and temperature. This information can be used to develop more accurate models of LIBs, which can be used to design safer and more reliable batteries.
The SOC refers to the amount of lithium stored in the electrode, which means that a higher SOC implies an increase in the amount of cycling active material and, consequently, a reduction in battery capacity. In [
19,
22,
74,
84] it is shown that the variation of SOC (ΔSOC) has a strong influence on battery degradation. The scientists demonstrated that greater battery degradation is achieved at high ΔSOC rates caused by changes in the material structure of the positive electrode associated with phase changes. Another study published in [
26] shows that the imbalance between the cathode and anode SOC reduces capacitance and complicates the relationship between cell voltage and internal resistance. The results were obtained by investigating the EIS for a given SOC of the electrode and suggesting that the electrodes should be designed in a way to minimize this imbalance. Another parameter widely discussed and investigated in the literature is the DOD, which is complementary to the SOC. The study shown in [
85], shows that high DOD rates cause a reduction in cell capacity and energy. Finally, the battery charge/discharge voltage threshold can also accelerate or reduce the degrading effects of the battery. This means that high and low charge and discharge voltages increase impedance and accelerate cell degradation mechanisms [
1,
9,
33,
86].
The SOC should be estimated mainly to equalize the cells and reduce the range anxiety of the customers. However, collecting labeled samples for training data-driven models is expensive and time-consuming. In [
21], the authors investigated this problem by developing a deep neural network applied to estimate the SOC for a limited number of available labeled samples and considering a large number of unlabeled samples. The results were promising, and the SOC estimation error was less than 0.6%.
The SEI layer process can explain these effects associated with battery degradation. In the SEI, there is a thin layer of electrolyte decomposition products (i.e. carbonates) known as the SEI layer. It is formed at the electrolyte/electrode interface when the electrode's redox potential is not within the Electrochemical Stability Window (ESW) of the electrolyte [
87,
88,
89]. The composition of the SEI layer is not yet known in detail, but it is known that this layer is formed by the products of the decomposition reactions between the electrolyte, electrode, and lithium [
90]. The main products reported in the literature are lithium fluoride (LiF), lithium carbonate (Li
2CO
3), lithium methyl carbonate (LiOCO
2CH
3), lithium ethylene dicarbonate (LiOCO
2CH
2)
2, and lithium oxide (Li
2O) [
86,
91]. The reactions must take place within the electrode's ESW. Thus, reversibility is guaranteed, and the batteries are rechargeable [
92]. This passivation layer usually is (but not exclusively) formed in the first load cycles of the LIBs, mainly at the negative electrode because this electrode operates at voltages outside the electrolyte's ESW.
The SEI layer is also formed at the electrode/electrolyte interface on the surface of the positive electrode and in such cases is called the CEI. It is crucial to note that one of the major shortcomings of the literature concerns the lack of an adequate description of CEI. The CEI plays a key role in improving coulombic efficiency and overall battery capacity retention [
93]. The detection, measurement, and characterization of this layer are not trivial due to the high potentials in this electrode that are close to the stability window of commercial carbonate electrolytes [
33,
94,
95,
96]. In addition, the complexity of determining and understanding the formation phenomena of CEI is increased due to the numerous chemical reactions that occur near the positive electrode, among them, nucleophilic reactions, induced polymerizations, and dissolution of transition metals stand out.
On the other hand, understanding SEI is also of great importance. The formation of SEI consumes cyclable lithium and electrolytic materials due to the irreversible electrochemical process of electrolyte decomposition. Therefore, there is a reduction at the negative electrode interface and oxidation at the positive electrode interface [
31,
89,
95].
It is essential to mention that SEI prevents Li ions from bringing their solvation layer during intercalation. Without this protection function, any graphite electrode would break into pieces after a few cycles. The most significant development in LIB technology was the discovery of an electrolyte that could produce stable SEI layers. These layers can perform this filtering operation while limiting their sustained growth.
The SEI layer is essential to ensure the chemical and electrochemical stability of the battery because it allows the transportation of Li
+ while blocking electrons, ensuring to continue of the electrochemical reactions, but avoiding the additional electrolyte decomposition, because it is almost impenetrable by the electrolyte molecules [
88,
95,
97,
98]. In addition to these factors, SEI contributes to stabilizing the electrode, allowing a greater number of battery charge and discharge cycles [
99]. However, the SEI layer is formed by four main factors: (i) breakdown of solvents and electrolytic salts, (ii) chemical breakdown of electrode materials, (iii) consumption of lithium, and (iv) co-insertion of organic solvents in the electrodes. All of these effects cause battery capacity loss, reducing the energy density and increasing the cell's internal resistance and temperature. Consequently, it is going to accelerate the battery degradation mechanisms [
90,
100,
101].
A parameter that significantly impacts the battery degradation process is the temperature. The temperature affects cell aging because high temperatures increase the agitation of the molecules and consequently accelerate the processes of insertion and/or removal of lithium in the host network. At low temperatures, lithium metal grows due to the slow transport of lithium into and within the negative electrode host network. This increases the local lithium-ion concentration and makes the lithium metal stable. The high temperatures accelerate the side reactions, alter the composition of the SEI layer and its thickness, increase the battery's internal resistance, and certainly enhance the degradation process. This makes the cell more prone to the thermal leakage process, leading to batteries' fire and explosion. It is also important to mention that a very thick SEI layer is the main factor for the power loss in the cell. Low temperatures only matter if the cell is operating. Low temperatures can reduce the electrolyte viscosity, decrease the lithium-ion conductivity process, and cause the slow diffusion of the lithium ions within the electrode, reducing the battery discharge capacity. Consequently, there is an increase in the internal resistance of the batteries and the parasitic reactions during the battery charging process, such as the metallic lithium coating and the growth of lithium dendrite. Therefore, low temperatures also accelerate the degradation of batteries, reducing their safety [
31,
102,
103,
104].
Battery degradation can happen in two ways: decreased capacity and decreased power due to loss of inventory lithium, loss of active material, negative electrode capacity, and positive electrode capacity. Loss of cell power happens due to loss of local contact, reduction of electrode reaction surface, structural changes in host materials, changes in electrolyte properties, structural changes in the separator, and the current collector's corrosion [
105]. The decrease in battery capacity refers to the decrease in the amount of charge that a battery can store per unit of time, usually expressed as a percentage. The energy decrease refers to reducing the ability to supply energy because of the internal resistance increase [
40].
The negative and positive electrodes are related to several battery aging mechanisms. Therefore, the next sections aim to explain in detail the degradation processes that occur in the negative electrode, positive electrode, separator, and electrolyte, and, finally, a discussion will be presented.
4.1. Degradation process at the negative electrode
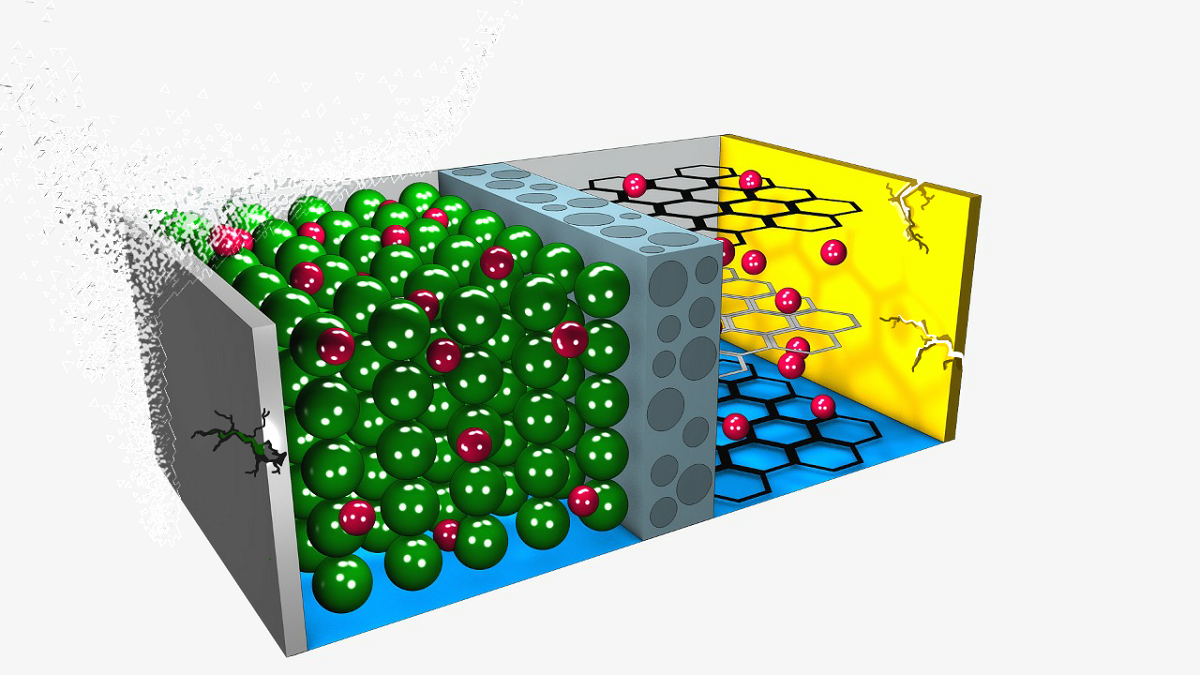
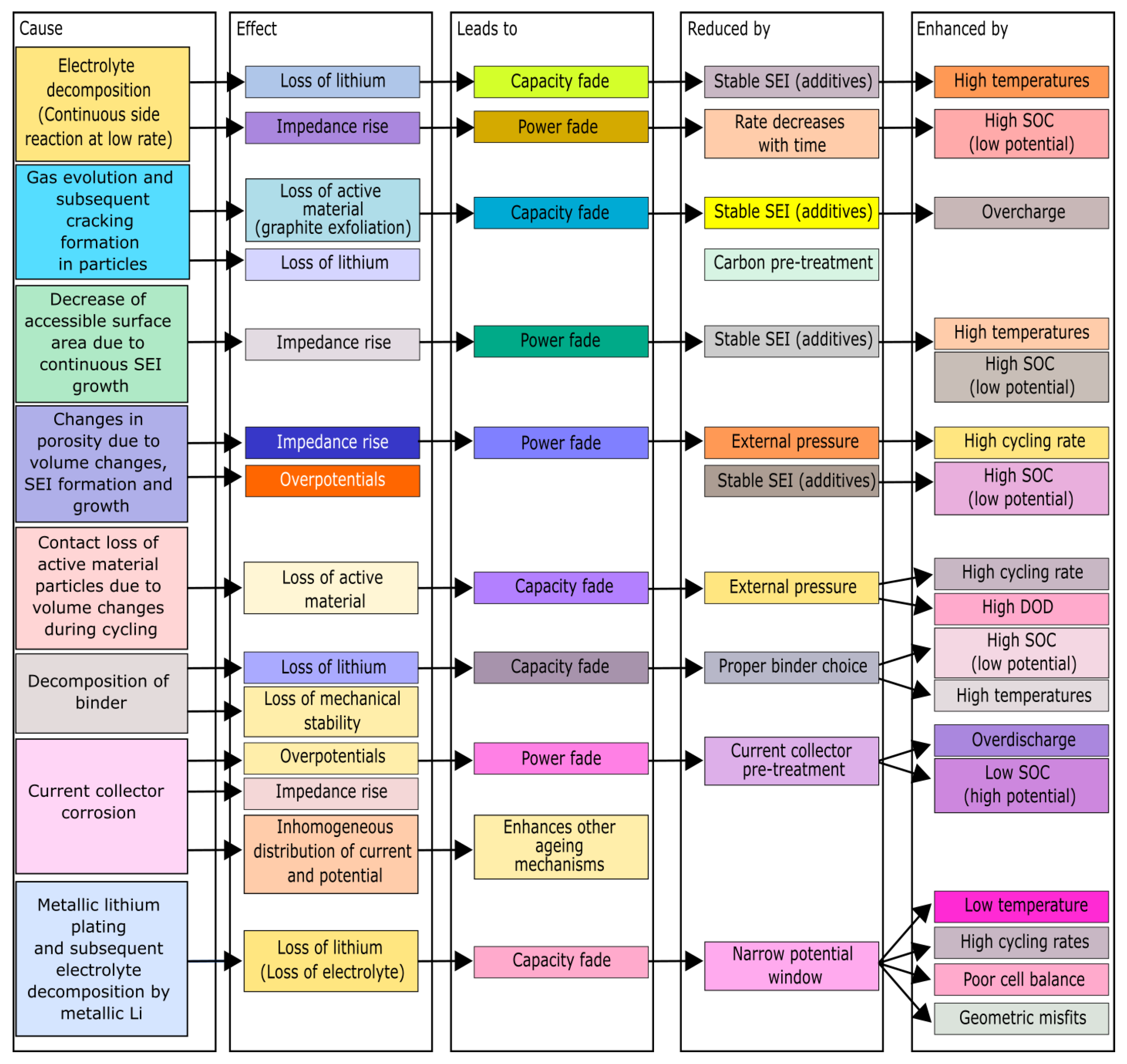
Negative electrode aging is mainly caused by lithium coating, electrolyte decomposition, solvent co-intercalation, gas evolution, decreased accessible surface area (due to the SEI layer formation), changes in porosity, loss of particle contact (due to changes in volume due to cycling), decomposition of the binder and electrolyte, and corrosion of the current collector, as detailed in
Figure 4 [
31].
After assembly, the batteries are initially discharged because the carbon lithium is unstable in the air. Therefore, lithium-ions may exist only in the electrolyte or interspersed at the cathode. As mentioned above, there is the SEI layer formation in the battery's first cycle, which is a thin film formed at the anode due to the reaction of the lithium ions of the cathode and the organic compounds of the electrolyte solvent [
40].
The formation of the SEI layer can consume 10 to 15% of the battery's initial capacity, but during the operation, this capacity loss is lower than when the SEI layer is formed, and this passivation layer is stable most of the time the batteries operate within their stability window [
106,
107,
108,
109]. This SEI layer formation process requires controlled conditions and technical knowledge about the batteries. The battery manufacturers must carry out the first charge before placing them on the market to prevent them from losing much of their capacity on the first charge [
90]. Despite this capacity loss, batteries can be used in EVs for many years [
95]. The SEI depends on the specific surface area (SSA) of graphite and the conditions of the passivation layer. The specific surface area is related to the type and morphology of the graphite. On the other hand, the formation conditions of SEI depend on the electrolyte's concentration, electrochemical conditions, and cell temperature. After long periods, corrosion of the SEI occurs and the formation of an additional SEI produces a new capacity loss [
40].
The interaction between the solvent and the graphite induces the exfoliation of the graphite and produces a gas capable of breaking the SEI. Therefore, it expands, increasing the internal cell's pressure and causing mechanical stress [
110]. The cycling effect increases and reduces the graphite particle diameter, which implies a variation in the cell volume, and the graphite structure is lithiated and de-lithiated in this process. This volume variation causes an increase in the cell's mechanical tension, which results in graphite exfoliation by breaking the particles. The reduction in the amount of active material available allows the growth of the SEI layer in a more significant number of locations that become available at the electrode/electrolyte interface. The simultaneous insertion of the electrolyte components with the insertion of lithium in the spaces available by the cracks caused by the negative electrode expansion in the SEI layer increases the thickness, and the cell's resistance, consuming the cyclable lithium and reducing the system's capacity [
40,
90].
Delamination can occur in two ways: the first occurs when the increase in the volume of the electrode breaks the connection between the electrode and the collector. The second possible way of delamination is caused when the current collector is not able to insert lithium. In that case, the electrode does not increase in volume but increases the surface tension of the interface between the negative electrode and current collector, causing the connection between the electrode and current collector and resulting in delamination. Delamination implicates higher internal resistance and current congestion at the interface, which can cause short circuits [
40,
90]. Current congestion at the interface can induce local lithium metal growth, which can eventually lead to dendrites and short circuits.
A high SOC reduces the negative electrode's potential, but the negative electrode is highly lithiated. If the anode potential is lower than 0 V, a thermodynamic process can occur. In this process, lithium is deposited on the negative electrode. These low potentials lithium can be deposited on the negative electrode instead of being intercalated during charging. To avoid this problem and to prevent the negative electrode from being fully lithiated, battery manufacturers design this electrode with 10% of the positive electrode capacity [
40,
111]. In addition to the SOC, the temperature also influences the batteries' degradation; once the high temperature increases the SEI solubility can create lithium crystals less permeable to lithium ions, which increases the negative electrode impedance [
95].
The lithium coating and the SEI formation are some of the main degradation mechanisms and compromise the safety of batteries. The lithium coating consists of the coating of negative electrodes with lithium. This is caused because the lithium ions (Li+) move from the positive electrode to the negative electrode while the battery is charging. Then, they intercalate in the active material of the negative electrode, which in most cases is graphite.
Two factors cause lithium coating. The first factor is charging batteries at low temperatures with a high current rate and high SOC. This factor is caused because charging batteries at low temperatures, with high current rates, and high SOC limits lithium diffusion and the transfer of charge at the interface formed by the particle and the SEI. This makes the graphite particle surface saturated with lithium ions polarising the negative electrode and forcing the graphite potential to reduce below the lithium potential limit (0 V). Consequently, the negative electrode is coated by lithium [
112,
113]. In this context, the potential difference between Li intercalation in graphite and the formation of metallic lithium must be less than 90 mV if the cell is nearly fully charged [
114]. Other negative electrode materials (e.g., LTO) are safer than graphite-like electrodes, but at the cost of lower cell voltage and reduced energy density.
The second factor is an imbalance in the capacity of a cell, specifically a capacity loss of the negative electrode, which drives it below one of the positive electrodes. This can create (local) litigation and lithium plating even at higher temperatures. The lithium coating can be responsible for serious safety failures because the deposition of lithium on the negative electrode forms dents or mosses, which can cause a short circuit in the cell and capacity loss [
112,
113]. It also increases the cell resistance due to the formation of thin films in the coated lithium metal and leads to the electrolyte ionic conductivity reduction [
112].
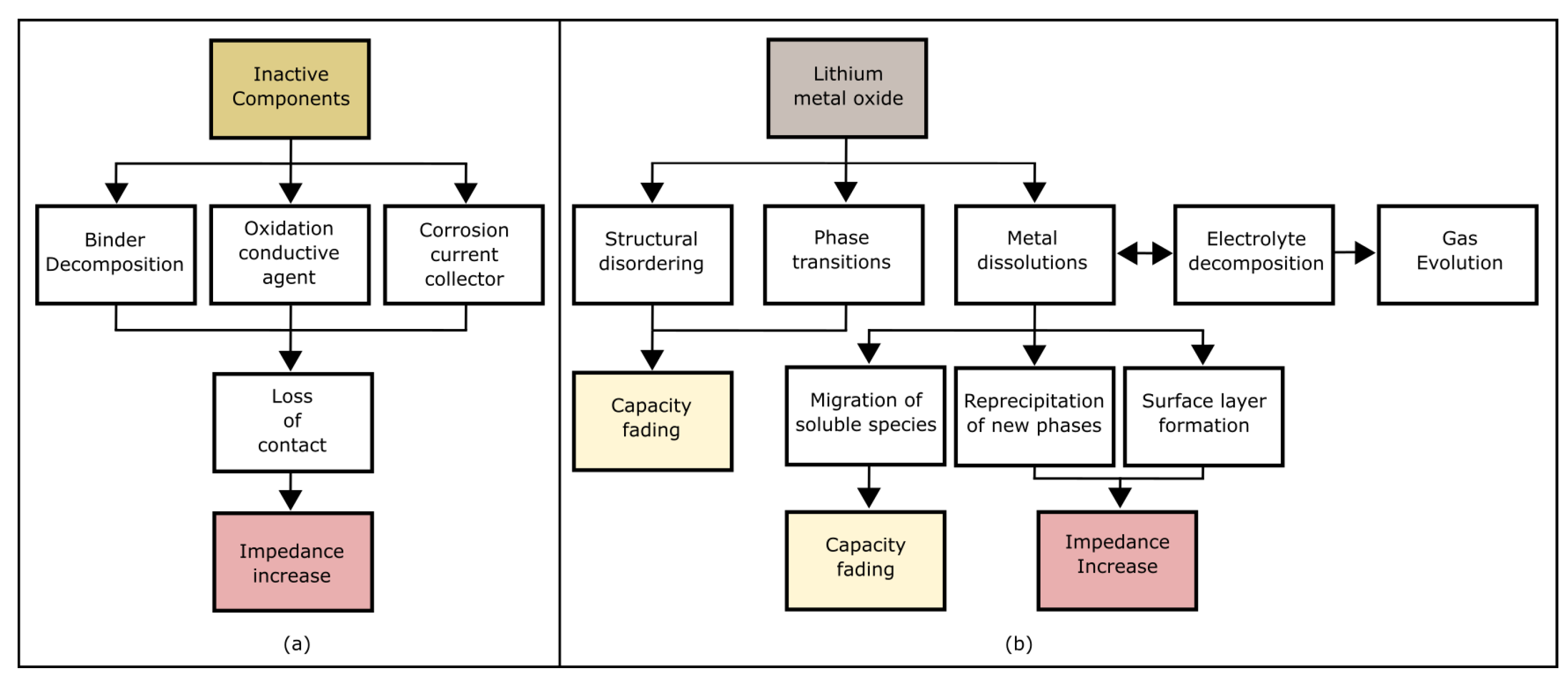
4.2. Degradation process at the positive electrode
The positive electrode degradation is mainly because of material loss and SEI layer growth. The active material loss occurs due to the dissolution of the transition metals in the positive electrode and reacts with the electrolyte. These effects can occur, and they are accelerated at high temperatures. The presence of water in the batteries can cause hydrolysis with the LiPF
6 salt to form hydrofluoric acid, causing the dissolution of the transition metals. Positive electrodes containing manganese usually dissolve the transition metals when the electrode is fully discharged. The cathode transition metals that have been dissolved in the electrolyte can react with the SEI layer formed on the negative electrode's surface, increasing the conductivity, forming additional SEI and dendrites, and reducing the amount of active material available in the electrode [
40].
When the cathode is exposed to the electrolyte there is a reaction between them that causes the loss of inventory lithium. The SOC also influences the positive electrode degradation, considering that a low SOC can reduce the amount of lithium that can intercalate in the positive electrode, promoting structural changes in this electrode and reducing the amount of active material in that electrode. At the positive electrode, high temperatures can culminate in the loss of oxygen from the metal oxide, and, together with the electrolytic decomposition promoted by high voltages, it can generate the cracking of particles and produce gases inside the batteries [
40].
Therefore, based on the factors that affect the battery's degradation mechanisms, it is possible to say that the batteries are degraded more quickly when the cell operates outside its ESW. The ESW consists of a voltage range and temperature that the cell can safely operate with minimized degradation mechanisms. When the cell operates outside its ESW, effects can occur that accelerate cell degeneration, and other more serious effects that degrade the cell quickly can also compromise its safety. That is why a BMS is required to control the parameters (e.g., voltage, current, temperature, etc.) to keep the cell operating within its stability window.
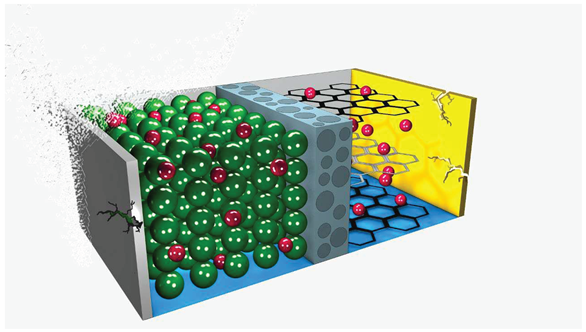
To summarize the degradation mechanisms, it is possible to highlight that the positive electrode degrades due to a combination of factors. These include active mass attrition, electrolyte degradation, gas generation, binder corrosion, and the formation of an SEI (please, see
Figure 5). Concerning the positive electrode, wear is strongly related to SOC and temperature [
39,
95,
101].
4.3. Degradation process in the electrolyte
Electrolytes undergo a degradation process caused by the decomposition of salts and solvents and by the formation of electrolyte interphases during cycling [
115]. In the first few cycles of the cell, the electrolyte comes into contact with the negative electrode which generally operates at voltages below the window of electrochemical stability of the electrolyte. This contact between the electrolyte and the negative electrode on the surface of the electrode accelerates the redox processes causing the decomposition of the electrolyte and reducing the performance of the battery. In a nutshell, it is possible to say that the decomposition of electrolyte solvents is the main degradation mechanism that occurs in electrolytes.
The products formed by the reactions that result from electrolyte decomposition can be used as a marker of the health status of electrolytes in batteries [
70]. In [
116], the authors noted that organophosphate molecules can be a type of marker to assess the health status of LiPF
6-based electrolytes. However, the products of the reaction can have a variety of molecules such as ether, organocarbonate, and organophosphate species [
70]. Therefore, further studies should be performed to identify more markers that can be used to assess electrolyte health status. This will make it possible to assess the need for predictive battery maintenance for the second-life battery market, develop a unique identifier and estimate the battery safety level.
The decomposition of solvents also causes the formation of CEI and SEI. To prevent these electrolyte decompositions, multifunctional additives can be used to form protective films on the cathode and anode surfaces. In [
117], researchers evaluated the degradation mechanisms in an electrolyte composed of lithium hexafluorophosphate dissolved in a binary mixture of cyclic and linear organic carbonates. The results showed that the electrolytes undergo thermally and electrochemically induced degradation. And, therefore, high temperatures can accelerate the degradation mechanisms in certain types of batteries because it causes the formation of ethylene glycols via EC polymerization and subsequent decarboxylation. Ways to suppress electrolyte decomposition still need to be explored as a way to increase the thermal and electrochemical stability of batteries.
The results discussed in [
118], show that the performance of batteries built with nickel-rich cathodes, for example, NMC811 (LiNi
0,8Mn
0,1Co
0,1O
2), can be limited by the main component of conventional electrolytes, known as Ethylene Carbonate (EC). The main reason for this limitation is that in scenarios where batteries are charged at high potentials (above 4.4 V vs Li/Li
+), EC can increase oxygen release causing oxidation/breakdown of electrolytes and degradation of the cathode surface. However, this increase in oxygen release was not observed in NMC111-based batteries independent of the electrolyte. Therefore, it is possible to observe that the development of electrodes with different chemistries has made it possible to increase the useful life and improve the performance of the batteries. On the other hand, electrolytes compatible with these electrodes need to be developed to ensure battery safety.
Degradation of NMC811-based batteries can also occur below the cutoff potential. In this case, the electrolyte over-decomposition processes are mainly caused by electrolytic oxidation of electrolytic solvents [
118]. This electrolytic oxidation is caused by the release of oxygen as discussed earlier.
4.4. Degradation process in the separator
The separator is of fundamental importance to avoid short circuits and, consequently, to ensure the safety and reliability of the batteries. The separators are a fundamental component for battery safety and must be disconnected in the event of an abnormal increase in temperature or the event of a thermal runaway [
119]. Short circuits are responsible for serious safety failures in batteries. The short circuit can cause fires and explosions. Short circuits can be classified as external or internal. The short circuit in the batteries is caused by the penetration of an electrical conductor in the separator. As a result, an increase in temperature is observed and, consequently, the melting of the separator [
74,
120].
In [
119], the authors highlighted four main phenomena that cause separator degradation: (i) growth of lithium dendrites caused by separator pores, (ii) blocking passes in the separator during cycling, and (iii) structural degradation due to high temperature or a high number of cycles. Internal Short Circuit (ISC) was also investigated in [
120]. The authors conducted electrochemical impedance spectroscopy tests of cells without LiPF
6 to assess the short-circuit resistance. Additionally, accelerated calorimetry tests and separator oven tests to evaluate thermoelectric behaviors and short-circuit failure modes. The findings suggest that voltage failure occurs as a result of self-discharge brought on by ISC, such as those of the Al-Cu type and the Al-An type, at low SOC. In contrast, voltage failure occurs as a result of separator collapse, and at high levels of SOC, a distinct extension of the ISC region, such as the Al-An type of ISC, indicates a bigger potential hazard. The expansion behavior of ISC, which affects the safety characteristics of the battery, is significantly influenced by the separator's thermal stability.
The aging of the separators causes a reduction in the mechanical strength of the separator as the number of cycles increases. This reduces the battery's ability to withstand mechanical impact, reducing battery safety.
However, high temperatures can overheat the cell, causing thermal shrinkage or even melting the separator and resulting in short-circuit. Thermal shrinkage is typified by a decrement in the pore size of the polymer separator, an effect that is induced by the separator's swelling. This, in turn, curtails the comprehensive coverage of the separator, leading to a consequential decrease in pore dimensions. This alteration precipitates a decline in the velocity at which lithium ions traverse the separator, thereby impairing the battery's capacity to supply elevated current rates. Existing works in the literature also showed that increasing the number of cycles causes a reduction in pore size. The reduction of separator pores is accentuated at high temperatures. Pore reduction causes an increase in battery impedance and a reduction in ionic conductivity.
The separator is influenced by the electrode cycling process. During the battery charging process, the electrode undergoes expansion and compresses the separator. This understanding causes the reduction of the useful life of the separator. The electrolyte can also influence the elasticity of the separator and, consequently, the performance of the separator. Therefore, elasticity is an important indicator of the degradation level of the separator. The penetration of the electrolyte liquid into the separator causes a reduction in the elasticity of this separator.
In [
121], the authors evaluated the performance of polyolefin separators in puncture, expansion, and softening tests in electrolytic solvents. The exposure of the separators to cyclic understanding caused a reduction in the ionic conductivity, a reduction of the C-rate capacity, and a worsening of the electrochemical performance of the separator, in the scenarios of cyclic understanding. Consequently, the battery has reduced its useful life.
6. Conclusions
LIBs can be used in all-EVs, hybrid vehicles, and in stationary applications such as microgrids, power tools, short-range vehicles, ships, and in grid-connected applications. The degradation mechanisms that occur in batteries can be accelerated depending on the application. The accelerated degradation of batteries can lead to severe safety failures, cause accidents, and increase the safety risk of people and equipment. Therefore, understanding battery degradation mechanisms is critical to increasing safety, reliability and extending battery life.
However, mitigating the effects of battery degradation is challenging. Despite the relevance of this subject to the scientific community and industry, a review of recent discoveries in this field was warranted. Therefore, this paper aims to present a comprehensive and didactic review of battery degradation mechanisms. The systematic review also presents some recommendations on how the EV owner can operate his vehicle and the factors that affect the equipment's health to minimize battery degradation mechanisms and reduce damage. In addition, companies will be able to improve the existing BMS as well as the user manuals. Updating user manuals is important to avoid contradictory information, and not compatible with battery behavior, preserving your customer's life.
The results of the work show that the understanding of battery degradation mechanisms influences each other and occurs on a microscopic scale. Some phenomena that occur mainly in the CEI layer are still poorly understood and need further studies. The results show that battery degradation can be accelerated by several factors, including operating conditions, temperature, SOC, DOD, voltage, and current. All battery components are affected by calendar aging and cyclic aging.
Knowledge of battery degradation mechanisms helps to understand the behaviour of batteries when they are operating on EVs and a second application. From this, it is possible to control the conditions of use and the parameters of the batteries to minimize the mechanisms of battery degradation, maximizing and predicting their helpful life in both the first life (in EVs) and in the second life (in an application secondary).
Extending the life of EV batteries enables company revenue because the longer the batteries operate on EVs, the greater the product is added value, and the lower the recycling costs for these batteries. It is also possible to generate value for the environment by reducing the number of batteries that will reach their end of life and will be discarded in the environment or recycled.
This systematic review aims to stimulate future studies that investigate the degradation mechanisms, in particular, the SEI and CEI layers, describing each phenomenon more reliably. The results show the need to understand battery degradation mechanisms for the development of new BMS that are battery agnostic and easily adaptable to second-life batteries.
There are still many gaps to be filled, and more studies are needed to clarify to companies and users the mechanisms of battery degradation and how to manage them. In this way, it will be possible for companies to improve the existing manuals and devise new materials to instruct the EV owner and the user of an ESS manufactured with second-life batteries on how to operate the vehicle or system to maximize its use and avoid accidents.