1. Introduction
Schistosomiasis is a parasitic disease caused by blood flukes (trematode) of the genus
Schistosoma. Schistosomiasis one of the neglected tropical diseases (NTDs) targeted for elimination by World Health Organization [
1]. Today, at least 251.4 million people worldwide required preventive chemotherapy (or mass drug administration: MDA) in 2021 [
2].
Schistosoma mekongi is endemic along the Mekong River basin in Champasak Province, southern part of the Lao People’s Democratic Republic (Lao PDR) and Kratié Province, northern part of Cambodia. In the Lao PDR, two Districts: Khong (152 villages: 86,095 people) and Mounlapamok (50 villages: 37,063 people), are the endemic areas of
S. mekongi [
3,
4]. Significant progress has been made in the past decades to reduce the prevalence of schistosomiasis mekongi through preventive chemotherapy using praziquantel and community awareness programs or health educations [
5]. According to the results of the recent reports on schistosomiasis mekongi, the prevalence of human schistosomiasis ranged from 0.0% to 5.0% without heavy infection in sentinel site villages and endemic communities using Kato-Katz thick smear method for parasite egg detection in stool samples [
5,
6]. Although the prevalence of human schistosomiasis has been relatively well monitored and gradually declining over the past decades in the Lao PDR, the prevalence among potential domestic reservoir animals in the endemic areas has not been well studied. A few studies with limited sample size showed that 14.7% (10/68) of dogs in Don Khone and Don Som in 2011-2012 [
7] and 12.2% (12/98) of pigs in Had Xay Khoun village in 1999 were infected with
S. mekongi [
8]. Previous studies report that cats, buffaloes, and cattle were not infected with
S. mekongi in Don Khone and Don Som. However, it is important to note that these studies used solely the traditional diagnostic method, e.g., microscopy. Therefore, it is possible that light intensity infections could be missed due to the relatively low sensitivity of the tests.
In 2017, WHO adopted a new strategy that accelerates the elimination of Asian schistosomiasis in the Western Pacific Region, i.e., transmission interruption by 2025 and verifying elimination by 2030 [
1]. One of the criteria of transmission interruption is “no new case of animal infection.” Toward this goal, we conducted a cross-sectional study to determine the infection status among the potential domestic reservoir animals with adequate sample size and high sensitivity diagnostic methods in the endemic villages in Khong and Mounlapamok Districts, Champasak Province, southern Lao PDR.
2. Methods
2.1. Study area and domestic animals
This study was conducted along the Mekong River basin in the Khong and Mounlapamok Districts in Champasak Province, southern part of the Lao PDR in 2018. These two Districts are S. mekongi endemic areas. Five villages in Khong District and two villages in Mounlapamok District were selected for the study sites. These villages were selected by the Lao Ministry of Health as sentinel sites for monitoring the prevalence of schistosomiasis in 2017. Five domestic animal species: cattle, buffalo, dog, pig, and goat, were investigated in this study.
2.2. Sample size
Assuming 10% of prevalence of schistosomiasis in animal [
7] using 5% precision with 95% confidence interval, the sample size is 139 for each animal species. Thus, 140 animals per species were enrolled in this study (20 animals per village × 7 villages = 140 animals per species). This assumption of the 10% of prevalence was taken from the highest prevalence of human schistosomiasis in this endemic area in 2016.
2.3. Field and sample collection
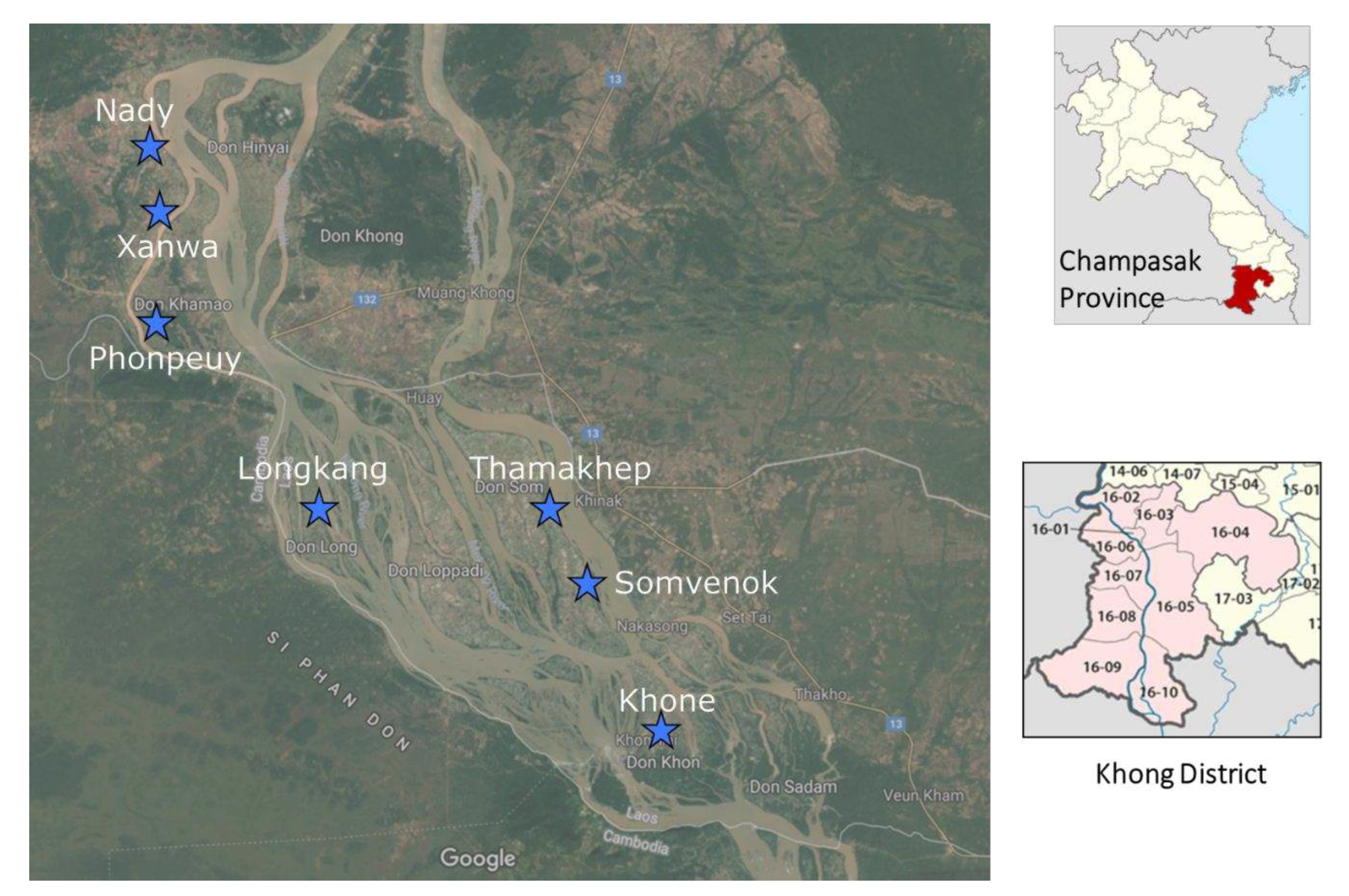
Three field surveys were conducted in the seven sentinel site villages (Khone, Longkang, Thamakhep, Somvenok, and Phonpheuy) in Khong District and two sentinel site villages (Xanwa and Nady) in Mounlapamok District, Champasak Province, Lao PDR (
Figure 1). The first survey was conducted in Longkang, Thamakhep, and Somvenok villages between May 6 and 23, 2018. The second survey was conducted in Khone and Phonpheuy villages between July 1 and 15, 2018. The third survey was conducted in Xanwa and Nady villages between July 22 and August 2, 2018. In each field study, a meeting with villagers and village authorities was conducted to explain the aim and field activities. Prior to sample collection, a list of domestic animals in the study villages was created with the assistance of the villagers and village chief. For each animal species, the number of animals per household was counted. Based on the list, 20 individual animals per species were randomly selected per study village. In a village where the number of animals was 20 or less per species, all animals were enrolled in the study. A total of 2.5g of stool samples from each study animal were collected. Approximately 2g of collected samples were fixed in a 10% formalin solution and transported to the Lao Tropical and Public Health Institute, Vientiane Capital, Lao PDR, for parasitological analysis to detect the eggs of helminth parasites. Another 0.5g of samples were preserved in 70% ethanol for loop-mediated isothermal amplification (LAMP) analysis to detect
S. mekongi DNA.
Laboratory procedures
2.5.1. LAMP technique for detecting S. mekongi DNA
The LAMP test for detecting S. mekongi DNA was performed in a laboratory at the Institut Pasteur du Laos. Primers for the LAMP test were designed based on the internal transcribed spacer 1 (ITS1) region in the ribosomal RNA gene of S. mekongi [9]. Positive control DNA for the S. mekongi LAMP was extracted from S. mekongi adult worms collected by a previous study. S. mekongi DNA was extracted by alkali-boil method from the stool samples. The extracted DNA was applied to LAMP reaction tube containing S. mekongi specific primers with an enzyme (LoopampTM DNA Amplification Kit, Eiken Chemical, Co., Ltd., Japan) and Fluorescent Detection Reagent (Eiken Chemical, Co., Ltd., Japan). The reaction tubes were incubated at 65oC for 60 minutes, and then incubated at 80oC for 5 minutes for inactivation of DNA amplification enzyme by LoopampTM LF-160 Incubator (Eiken Chemical, Co., Ltd., Japan). Result of the LAMP test was evaluated using a detector unit (UV light) of the LF-160 Incubator. When S. mekongi DNA is present in the stool samples, the DNA is amplified by the LAMP reaction and can then be detected by a color change (transparent to green) under UV light as well as by change in turbidity (transparent to white) by the naked eye.
2.5.2. FECT analysis for helminth infections
Upon the arrival of preserved stool samples at the Tropical and Public Health Institute, the samples were proceeded and analyzed by experienced laboratory technicians using formalin-ethyl acetate concentration technique (FECT) [10,11]. In brief, a 15-mL tube containing the preserved sample was well checked before covering an open top with a two-layer gauze for sample filtering into a funnel jar. A filtered sample solution was poured back into the tube, and 3 mL of 0.9% sodium chloride was added to balance the tube before centrifuging at 1,500 rpm for 5 minutes. After centrifuging, the supernatant was removed, and the sediment was re-suspended with 10 mL of a 10% formalin solution and 3 mL of ethyl acetate and well checked for 1 minute before centrifuging the tube again at 1,500 rpm for 5 minutes. The fatty plug (debris) was loosened with an applicator stick and poured away by quickly inverting the tube. The sediment was re-suspended with 1 mL of a 10% formalin solution and well mixed. Up to four slides (eight drops of sediment) were prepared and read for each sample. For each slide, two drops of sediment were prepared. On the first drop of sediment, a drop of 0.9 sodium chloride was added, and a drop of 1% iodine solution was added to the second drop of sediment. The prepared slides were placed under a light microscope and read by experienced microscopists. All detected helminth parasite eggs were identified and recorded separately by species. About 10% of reading slides were re-examined by a senior laboratory technician. Any discrepancy of findings was discussed among microscopist to conclude the consensus findings.
3. Results
3.1. Study animals
A total of 699 domestic animals in seven sentinel site villages in two endemic districts (Khong and Mounlapamok), Champasack province, southern Lao PDR, were enrolled in the study. From these 699 animals, 691 (98.9%) had enough stool samples for both LAMP and FECT analyses, which included 160 cattle, 154 pigs, 149 buffaloes, 143 dogs, and 85 goats. Nady (124 animals) was the village with the highest number of study animals, followed by Phonpeuy (123 animals), Khone (107 animals), Xanwa (101 animals), Thamakheb (88 animals), Longkang (77 animals), and Somven-Ork (71 animals), respectively (
Table 1).
3.2. LAMP test
From 691 study animals, a stool sample of a dog (0.7%, 1/143) and a stool sample of a pig (0.6%, 1/154) were
S. mekongi DNA positive during the screening phase using the LAMP method in Phonpeuy village. The validation of the positivity was performed in three independent tests including DNA extraction. Only dog sample was positive by the three independent LAMP tests and it was counted as confirmed positive (
Table 2). For the pig sample, the positive result was obtained only one test alongside two negative results. Thus, the pig sample was considered a suspected positive, rather than a confirmed positive. The age of the
S. mekongi positive dog was 2 years old, while the pig was only 3 months old. All the other stool samples were
S. mekongi DNA negative by the LAMP test.
3.2. Microscopy by FECT
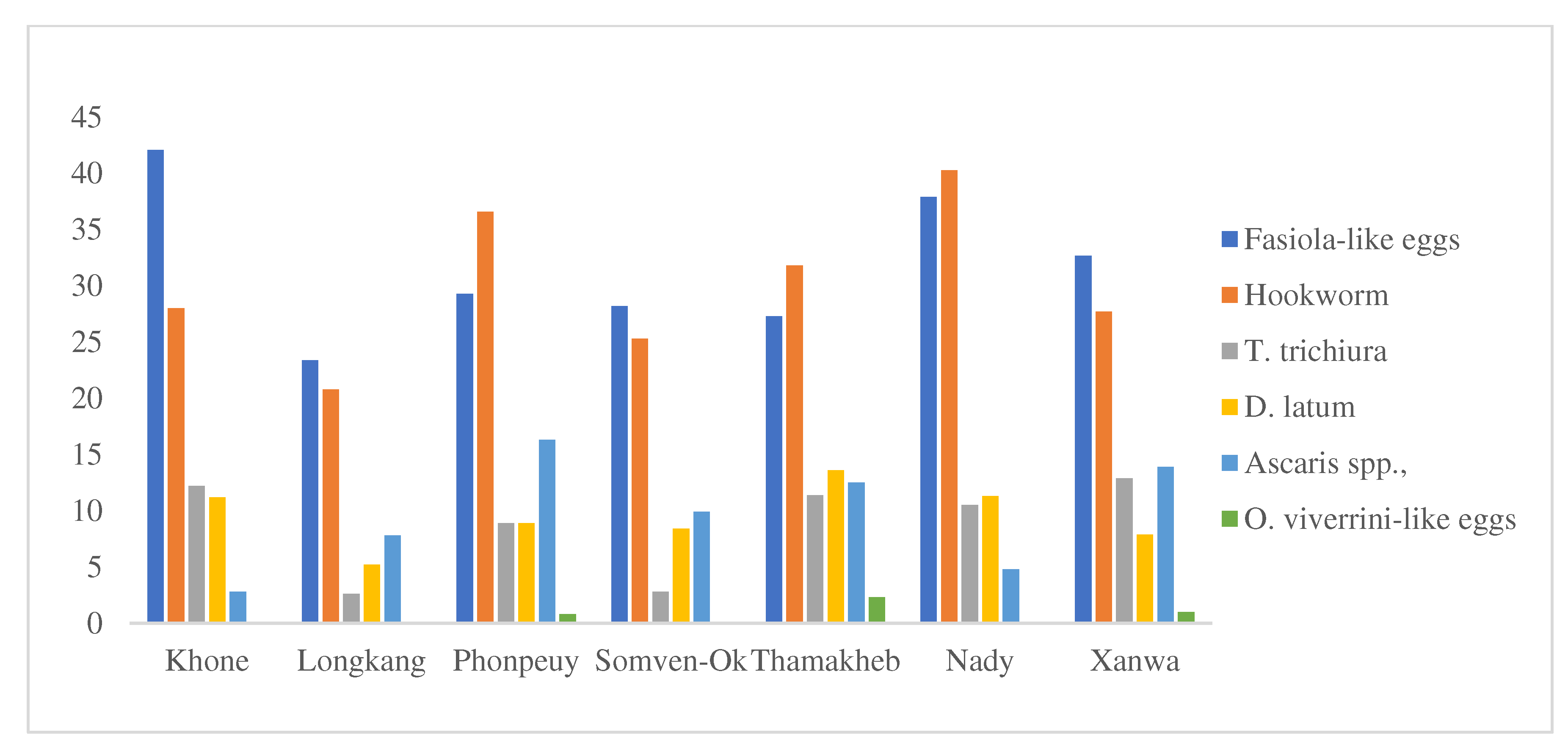
From the 691 study animals, the FECT analysis detected helminth infections in 72.4% of the study animals (500/691). Domestic animals in Nady village had the highest rate of helminth infections (79.8%), followed by animals in Phonpeuy village (79.7%), in Thamakhep village (73.9%), in Khone village (73.8%), in Xanwa village (70.3%), in Somven-ork village (62.0%), and in Longkang village (57.1%), respectively.
Table 3 displays the helminth infections detected in the FECT analysis by animal species. Stool analysis of the study animals did not detect any
S. mekongi eggs in all preserved samples.
Fasciola spp. infection in cattle and buffalo was detected in 65.0% and 60.4%, respectively. Hookworm and
Diphylobothrium latum infections in dogs were 62.5% and 46.5%, respectively.
Trichuris suis,
Ancylostoma spp., and
Ascaris spp. infections in pigs were 19.5%, 40.9%, and 39.6%, respectively.
Trichuris suis, hookworm, and
Fasciola spp. infection rates in goats were 28.2%, 67.1%, and 32.9%, respectively.
Fasciola-like eggs had the highest rate of helminth infections among animals in Khone, Longkang, Somven-Ork, and Xanwa villages, while hookworm had the highest rates of helminth infections for animals in Phonpeuy, Thamakheb, and Nady villages (see
Figure 2).
4. Discussions
In the present study, only one dog and one pig stool sample detected S. mekongi DNA by the LAMP test. These dog and pig stool samples were considered confirmed and suspected positive, respectively, due to the varied results for the pig sample LAMP test. Microscopic examination using the FECT method showed negative results with the same stool samples. These different results suggested that the LAMP test is more sensitive than the FECT. All stool samples from other domestic animals (cattle, buffalo, and goat) were negative for S. mekongi by both the LAMP test and the FECT.
Our previous study between October 2011 and August 2012 showed that 14.7% of dogs (10/68) were positive for
S. mekongi eggs using the FECT method in two islands (Donkhone, 15.9%, and Donsom, 12.5%). The prevalence of schistosomiasis among the villagers was as high as 23.6% (112/475) in Donkhon and 21.0% (109/519) in Donsom, with heavy-intensity infections of 1.8% (2/112) and 5.5% (6/109), respectively [
7]. It is important to note that the settings of our present study geographically overlap with a previous study conducted in 2011 and 2012. Khone village is in Donkhone (Khone island), and Thamakhep and Somvenok villages are in Donsom (Som Island). In the previous study, the prevalence of schistosomiasis among the villagers was as high as 23.6% (112/475) in Donkhon and 21.0% (109/519) in Donsom, with heavy-intensity infections of 1.8% (2/112) and 5.5% (6/109), respectively[
7]. The most recent study showed a much lower prevalence of schistosomiasis among villagers in Khone, Thamakhep, and Somven-Ork at 7.3%, 6.0%, and 4.5%, respectively, without a heavy intensity [
5]. The low prevalence of
S. mekongi infection in animals observed in this study may be associated with the significant reduction of schistosomiasis in these endemic communities.
In addition, 88.2% of dogs (127/144) in the present study were less than 3 years old (average 2.2 years old), whereas the S. mekongi positive dog was 4 years old. Although the age of the dogs may not be accurate because there is no official age documentation, this result suggests that S. mekongi transmission among dogs has either not occurred or has been limited since 2015 in the study areas.
Our previous study conducted in 2011–2012, the stool samples of cats (n = 64), pigs (n = 105), and buffaloes (n = 94) were also examined using the FECT method and were all negative for
S. mekongi eggs. However, another study conducted in Hadxaykhoun village, Khong District, in 1999 found a positive rate for
S. mekongi eggs in 12.2% (12/98) of pigs [
8]. Strandgaard et al. suspected that the most likely route of pig infection was ingestion of cercariae-infested drinking water that the owners brought directly from the Mekong River to feed their pigs daily. At that time (1999), most pigs were normally tied up or kept in pens, but occasionally they were allowed to roam freely. During our study, we observed a similar manner of keeping pigs to 1999 in Khong district. However, the extensive intervention conducted over the past decades has reduced the prevalence of
S. mekongi in the endemic areas to a very low level, with no severe cases observed in the community [
5]. In addition, we observed that most of the households in the endemic communities pumped the water from the Mekong River and stored it in a tank or well for hours before using it. Previous studies suggested that
Schistosoma cercariae can remain infective in freshwater for one to three days [
12], depending on the water temperature [
13,
14]. If the owner of the pigs gave the storage water (stored for more than three days) to their pigs, the risk of pig infection with
S. mekongi would be lower compared with previous practices. Moreover, the age of the pigs in this study was relatively young, with an average of 5 months (ranging from 1 month to 2 years old). In fact, the study team did not see any older pigs (>2 years old) in this study. This relatively high turnover of the pig population would decrease the chance of
S. mekongi infection in the study areas.
In the present surveys, we observed that many dogs took baths on the Mekong River side (
Figure 3), while no pigs came to the Mekong River as they were normally kept in pens. Buffaloes like to stay in the water for a long time. Cattle and goats normally come to the Mekong River's riverside only when they want to drink water.
S. mekongi is genetically and morphologically close to
S. japonicum, which has a wide variety of mammal hosts, such as cattle, buffalo, pigs, dogs, rats, and so on [
3,
15]. In contrast,
S. mekongi has only two mammalian hosts as natural reservoirs, namely, dogs and pigs in the endemic areas [
7,
8]. Rats are a mammalian host in a laboratory setting [
16] and buffaloes are suspected as a potential reservoir animal [
3], but this has yet to be proven. The present study and the accumulated data from previous studies suggest that only dogs and pigs are the natural reservoir animals for
S. mekongi. Rats were not examined in this study because they are not domestic animals and it is challenging to collect them. Therefore, it remains unknown whether rats would contribute to the transmission of
S. mekongi in the endemic areas.
5. Conclusions
This study found only one S. mekongi positive case in dogs and one suspicious case in pigs by the LAMP test in the seven sentinel site villages in Lao PDR in 2018. No S. mekongi infection was observed among cattle, buffaloes, or goats. Therefore, we conclude that humans play a key role in the transmission of S. mekongi in the endemic areas of Khong and Mounlapamok districts, Champasack province, southern Lao PDR. Therefore, key interventions focusing on humans, such as community-based chemotherapy coupled with health education and improving access to safe water and sanitation, might significantly reduce the S. mekongi infection in the endemic areas, leading to its elimination.
Author Contributions
Conceptualization, investigation, methodology, formal analysis, validation, writing – original draft, S.S, P.K and M.I; investigation, methodology, S.K, P.P, PS, S.S (Sonesimmaly Sannikone), TK, SP, PI, and BH; writing – review & editing, P.T.B; funding acquisition S.K (Shigeyuki Kano), M.I, Writing – review & editing, S.K (Shigeyuki Kano).
Funding
The study was funded by the World Health Organization Western Pacific Region “to assess the prevalence of S. mekongi in the animal reservoirs in the endemic communities of Khong and Mounlapamok Districts, Champasak Province, southern Lao PDR” (WHO Reference No: 2017/757877-0), by a Japan International Cooperation Agency (JICA) and Japan Agency for Medical Research and Development (AMED) for “the development of innovative research techniques in genetic epidemiology of malaria and other parasitic diseases in the Lao PDR for containing their expanding endemicity” (2013-2019) and by a JICA/AMED SATREPS project for “Project for Malaria and Neglected Parasitic Diseases Control and Elimination using Advanced Research Technique, Communication Tools and Eco-Health Education” (2022-2028).
Institutional Review Board Statement
This study did not require the ethical approval, since the data collection involved only the stool sample collection from domestic animals. No any human data was collected during the study.
Informed Consent Statement
Prior to stool sample collection from study animals, the survey team obtained oral consent from the owners and village authorities. One stool sample was collected from each animal by experienced veterinarians. All study team members who contacted the animals had rabies vaccinations before conducting the field survey.
Data Availability Statement
All data collected from the field and analyzed for this manuscript are available at the Intitut Pasteur du Laos and the Lao Tropical and Public Health Institute. Data will make freely access to interested individuals and institutions upon the official request.
Acknowledgments
The authors sincerely thank the staffs of the Center of Malariology, Parasitology, and Entomology, Ministry of Health, Vientiane Capital, Lao PDR, the Champasak Provincial Health Department, the Khong District Health Department, and the Mounlapamok District Health Department. We also thank the staff of the Animal Health Service Center, the Khong District Agriculture and Forestry Office, the Mounlapamok District Agriculture and Forestry Office, and the Champasack Provincial Department of Agriculture and Forestry. We are also grateful to the chiefs of the study villages, village health volunteers, village animal health volunteers, and all the owners of the animals for their support and contributions to this study. We are grateful for the English correction by Ms. Miley Sinantha-Hu, a native English speaker and research assistant at the Lao Tropical and Public Health Institute.
Declaration of Competing Interest
The authors have declared that no competing interests exist.
References
- World Health Organization. Regional Office for the Western Pacific Expert Consultation to Accelerate Elimination of Asian Schistosomiasis, Shanghai, China, 22-23 May 2017 : Meeting Report; WHO Regional Office for the Western Pacific, 2017.
- World Health Organization Schistosomiasis. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 3 May 2023).
- Muth, S.; Sayasone, S.; Odermatt-Biays, S.; Phompida, S.; Duong, S.; Odermatt, P. Schistosoma Mekongi in Cambodia and Lao People’s Democratic Republic. Adv Parasitol 2010, 72, 179–203. [Google Scholar] [CrossRef] [PubMed]
- Sayasone, S.; Mak, T.K.; Vanmany, M.; Rasphone, O.; Vounatsou, P.; Utzinger, J.; Akkhavong, K.; Odermatt, P. Helminth and Intestinal Protozoa Infections, Multiparasitism and Risk Factors in Champasack Province, Lao People’s Democratic Republic. PLoS Negl Trop Dis 2011, 5, e1037. [Google Scholar] [CrossRef] [PubMed]
- Khieu, V.; Sayasone, S.; Muth, S.; Kirinoki, M.; Laymanivong, S.; Ohmae, H.; Huy, R.; Chanthapaseuth, T.; Yajima, A.; Phetsouvanh, R.; et al. Elimination of Schistosomiasis mekongi from Endemic Areas in Cambodia and the Lao People’s Democratic Republic: Current Status and Plans. Trop Med Infect Dis 2019, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wang, Y.; Su, Q.; Zhu, J.; Tang, S.; Bergquist, R.; Zhang, Z.; Hu, Y. Mapping Schistosomiasis Risk in Southeast Asia: A Systematic Review and Geospatial Analysis. Int J Epidemiol 2022, dyac227. [Google Scholar] [CrossRef]
- Vonghachack, Y.; Odermatt, P.; Taisayyavong, K.; Phounsavath, S.; Akkhavong, K.; Sayasone, S. Transmission of Opisthorchis viverrini, Schistosoma mekongi and Soil-Transmitted Helminthes on the Mekong Islands, Southern Lao PDR. Infect Dis Poverty 2017, 6, 131. [Google Scholar] [CrossRef] [PubMed]
- Strandgaard, H.; Johansen, M.V.; Pholsena, K.; Teixayavong, K.; Christensen, N.O. The Pig as a Host for Schistosoma Mekongi in Laos. J Parasitol 2001, 87, 708–709. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, T.; Matsumoto-Takahashi, E.L.A.; Ishikawa, H.; Keomalaphet, S.; Khattignavong, P.; Soundala, P.; Hongvanthong, B.; Oyoshi, K.; Sasaki, Y.; Mizukami, Y.; et al. Detection of Schistosoma Mekongi DNA in Human Stool and Intermediate Host Snail Neotricula Aperta via Loop-Mediated Isothermal Amplification Assay in Lao PDR. Pathogens 2022, 11, 1413. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, A.; El-Morshedy, H.; Omer, E.; El-Daly, S.; Barakat, R. Evaluation of the Kato-Katz Thick Smear and Formol Ether Sedimentation Techniques for Quantitative Diagnosis of Schistosoma Mansoni Infection. Am J Trop Med Hyg 1997, 57, 706–708. [Google Scholar] [CrossRef] [PubMed]
- Sayasone, S.; Utzinger, J.; Akkhavong, K.; Odermatt, P. Repeated Stool Sampling and Use of Multiple Techniques Enhance the Sensitivity of Helminth Diagnosis: A Cross-Sectional Survey in Southern Lao People’s Democratic Republic. Acta Trop 2015, 141, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human Schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.R.; Wilson, R.A. The Survival of the Cercariae of Schistosoma Mansoni in Relation to Water Temperature and Glycogen Utilization. Parasitology 1980, 81, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Upatham, E.S.; Kruatrachue, M.; Khunborivan, V. Effects of Physico-Chemical Factors on the Infection of Mice with Schistosoma Japonicum and S. Mekongi Cercariae. Southeast Asian J Trop Med Public Health 1984, 15, 254–260. [Google Scholar] [PubMed]
- Agatsuma, T.; Iwagami, M.; Liu, C.X.; Rajapakse, R.; Mondal, M.M.H.; Kitikoon, V.; Ambu, S.; Agatsuma, Y.; Blair, D.; Higuchi, T. Affinities between Asian Non-Human Schistosoma Species, the s. Indicum Group, and the African Human Schistosomes. 2002, 7–19. [CrossRef]
- Sanpool, O.; Intapan, P.M.; Thanchomnang, T.; Sri-Aroon, P.; Lulitanond, V.; Sadaow, L.; Maleewong, W. Development of a Real-Time PCR Assay with Fluorophore-Labelled Hybridization Probes for Detection of Schistosoma Mekongi in Infected Snails and Rat Feces. Parasitology 2012, 139, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).