1. Introduction
Recent studies indicated that prolonged exposure to social stress (SS) induces lower urinary tract dysfunction (LUTD) [
1,
2,
3] and causes urinary retention and abnormal urodynamics [
2,
4]. Those results indicated the central parasympathetic neurotransmitters may be decreased by SS and reduced detrusor contraction [
2,
4]. SS also caused copulatory disorder [
5,
6,
7] and depression secondary to neurovascular pathology [
8]. In penile erection, nitric oxide (NO) is an important neurotransmitter as the principal pathway [
9,
10]. Basal NO released from endothelial cells inhibits contractions in mouse corpus cavernosum (CC) [
11]. In unilateral cavernosal denervation rats, intra-cavernous pressure (ICP) and nitric oxide synthase (NOS) fibers were decreased in rat CC [
10]. A decrease in neuronal NOS was accompanied by penile tissue in erectile dysfunction (ED) patients [
12]. However, the role of the peripheral parasympathetic nitrergic nerve in social stress-induced ED is uncertain.
Ketamine is an antagonist of the N-methyl-D-aspartic acid (NMDA) receptor complex [
13,
14]. During the past decade, it is known that a single infusion of a sub-anesthetic dose of ketamine produces a rapid and long-lasting antidepressant effect [
15], and the ketamine may be useful in protecting against stress-induced disorders [
16]. Brain-derived neurotrophic factor in the prefrontal cortex may be related to the long-lasting antidepressant effect in the social defeat stress animal model [
17]. Long term use of ketamine could induce both LUTD and ED in humans with a dose and duration related model [
18,
19]. Recently, our studies have uncovered the crucial role of the interaction between sympathetic and parasympathetic (or axo-axonal) nerve terminals in the relaxation of the CC. This interaction is mediated by both nicotinic acetylcholine receptors and NMDA receptors. Additionally, we have observed that ketamine directly targets the major pelvic ganglia (MPG) in vivo, leading to an inhibition of cavernosal nerve neurotransmission. Furthermore, our in vitro experiments have demonstrated that ketamine impairs the ability of nicotine to induce relaxation in the CC [
20]. Notably, our investigations involving chronic ketamine use did not yield positive results in terms of reversing lower urinary tract dysfunction (LUTD) in mice subjected to SS [
21]. Therefore, the objective of this study is two-fold: firstly, to delve into the mechanism of axo-axonal interaction in SS-related ED; and secondly, to investigate the effects of a single sub-anesthetic dose of ketamine on SS-related ED.
2. Results
2.1. Effects of social stress on voiding frequency
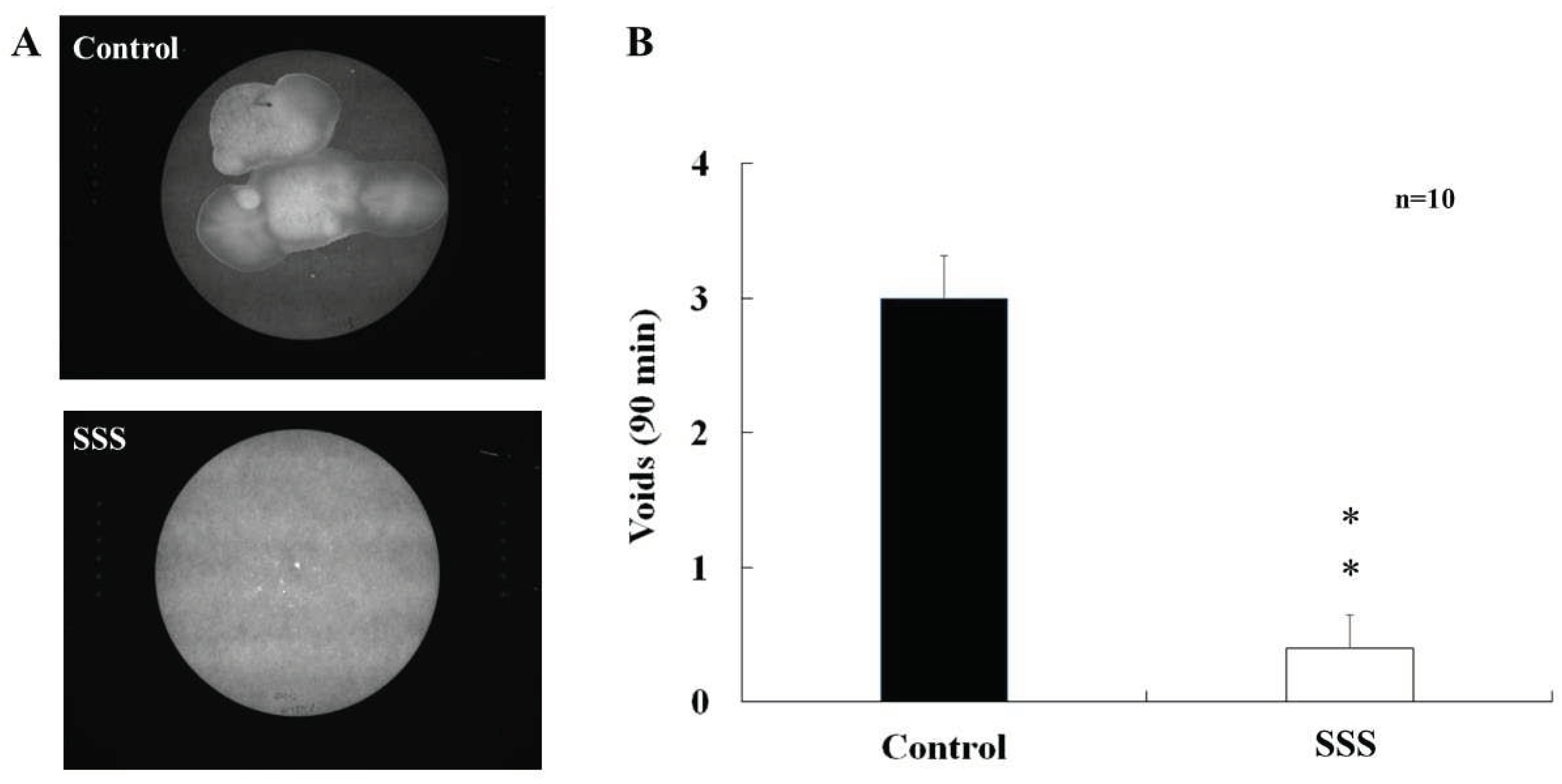
Figure 1 depicts voiding spots over filters of the control and SSS mice. Most SSS mice were scared to void (
Figure 1A). Voiding frequencies of SSS was significantly lower than that of control (0.4±0.24 vs. 3±0.31,
p<0.05,
Figure 1B).
2.2. Effects of social stress on urinary bladder pressure and intra-cavernosal pressure (ICP)
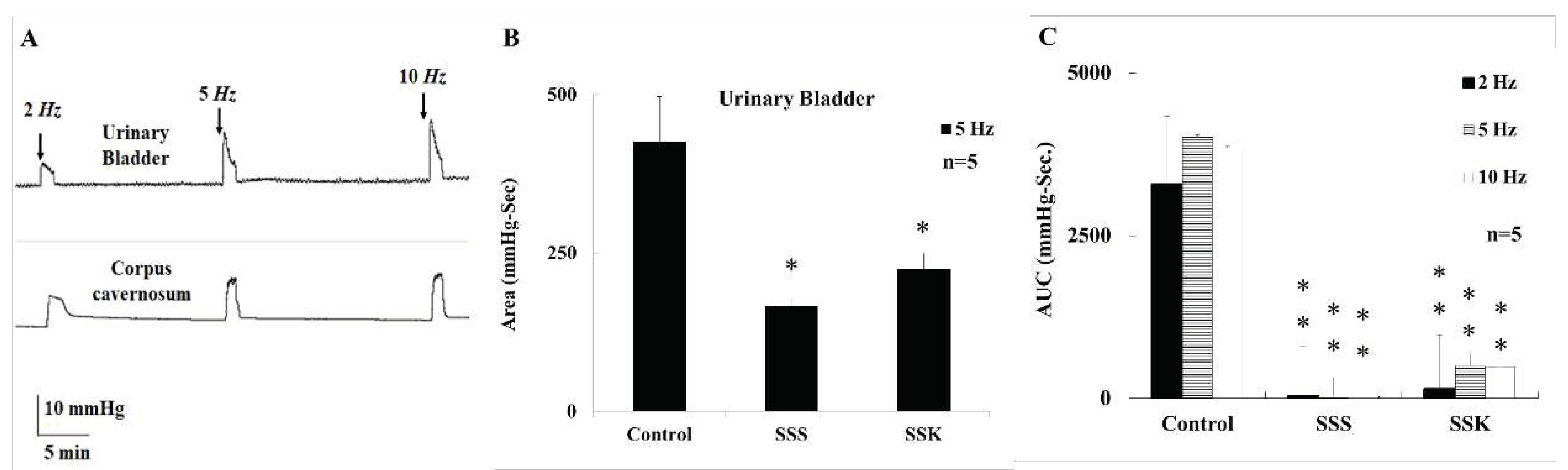
The area under the curve (AUC) of urinary bladder pressure of control mice was induced by electrical stimulation (2, 5, and 10 Hz,
Figure 2A). Both SSS and SSK mice demonstrated a lower AUC than that of control mice at 5 Hz (
Figure 2B), while no statistical difference between SSS and SSK mice (
Figure 2B).
Both SSS and SSK mice demonstrated a lower AUC of ICP and urinary bladder pressure than those of control mice (
Figure 2C), while no statistical difference was noted between SSS and SSK mice (
Figure 2C).
2.3. Effects of social stress and social stress plus ketamine on relaxation of corpus cavernosum strips
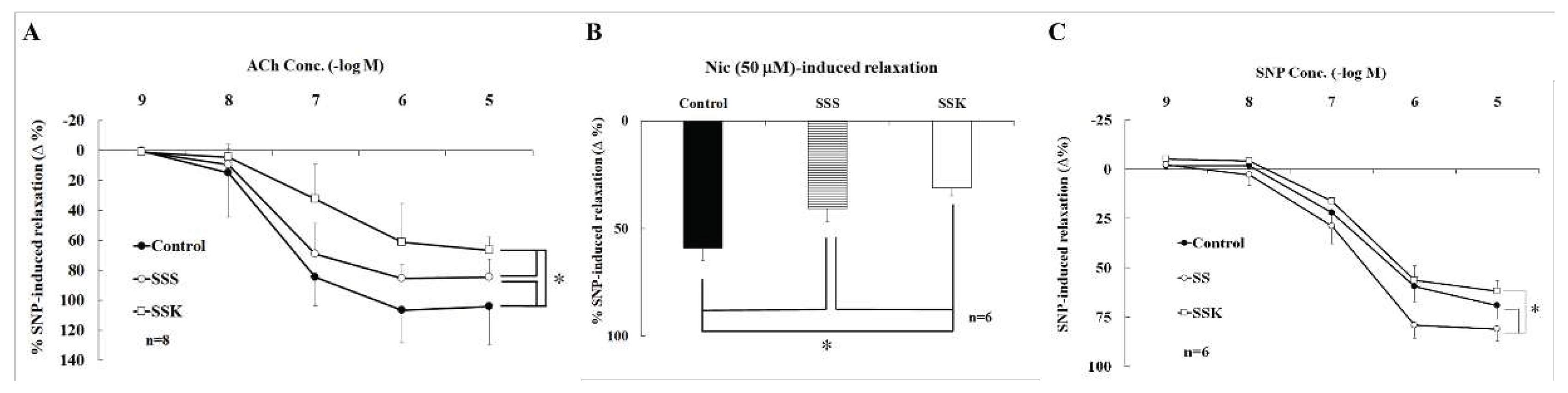
In the presence of active muscle tone induced by phenylephrine (10 µM), the CC strips relaxed upon applications of acetylcholine in concentration-dependent manner (10
-9~10
-5 M). The degree of relaxation of CC strip of SSS and SSK were significantly lower than that of control mice (
Figure 3A), while statistically different between SSS and SSK mice (
Figure 3A). Similarly, the CC strips relaxed upon applications of nicotine (50 µM). This relaxation was significantly lower in SSS and SSK than the control mice (
Figure 3B). In addition, the relaxation of CC strips was induced by sodium nitroprusside (SNP) in a concentration (10
-9~10
-5 M) manner. The relaxation was significantly enhanced in SSS than the control mice (
Figure 3C), while no statistical difference was observed between SSK and control mice (
Figure 3C).
2.4. Effects of lidocaine on nicotine-induced relaxation
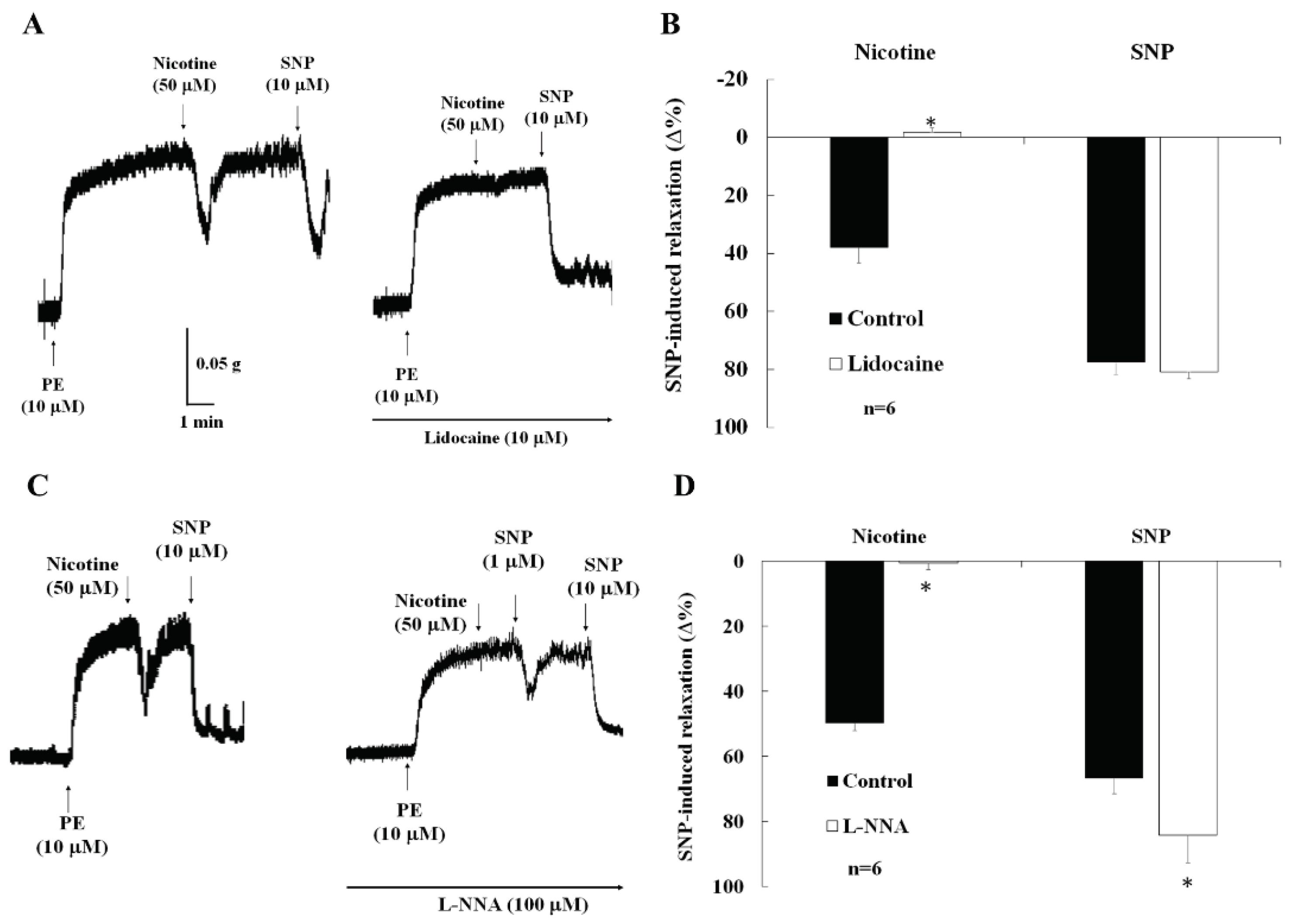
In the present of active muscle tone induced by phenylephrine (10 µM), the relaxation of CC strips were induced by nicotine (50 µM). This relaxation was significantly abolished by lidocaine (10 µM,
Figure 4A,B, 39.8±5.0% vs 0.6±2.7%,
p<0.05). In contrast, the relaxation of CC strips was induced by SNP (0.1 µM), which was not inhibited by lidocaine (10 µM, 75.5±4.1% vs 80.9±1.8%,
p>0.05,
Figure 4A,B).
2.5. Effects of nitric oxide synthase inhibitor (L-NNA) on nicotine-induced relaxation and intra-cavernosal pressure (ICP)
In the presence of active muscle tone induced by phenylephrine (10 µM), the relaxation of CC strips were induced by nicotine (50 µM). This relaxation was significantly abolished by L-NNA (
Figure 4C,D, 49.8±2.3 vs 0.6±1.9,
p<0.05). In contrast, the relaxation of CC strips were induced by SNP (0.1 µM), which was significantly enhanced by L-NNA (
Figure 4D, 66.7±4.7 vs 84.3±8.3,
p<0.05).
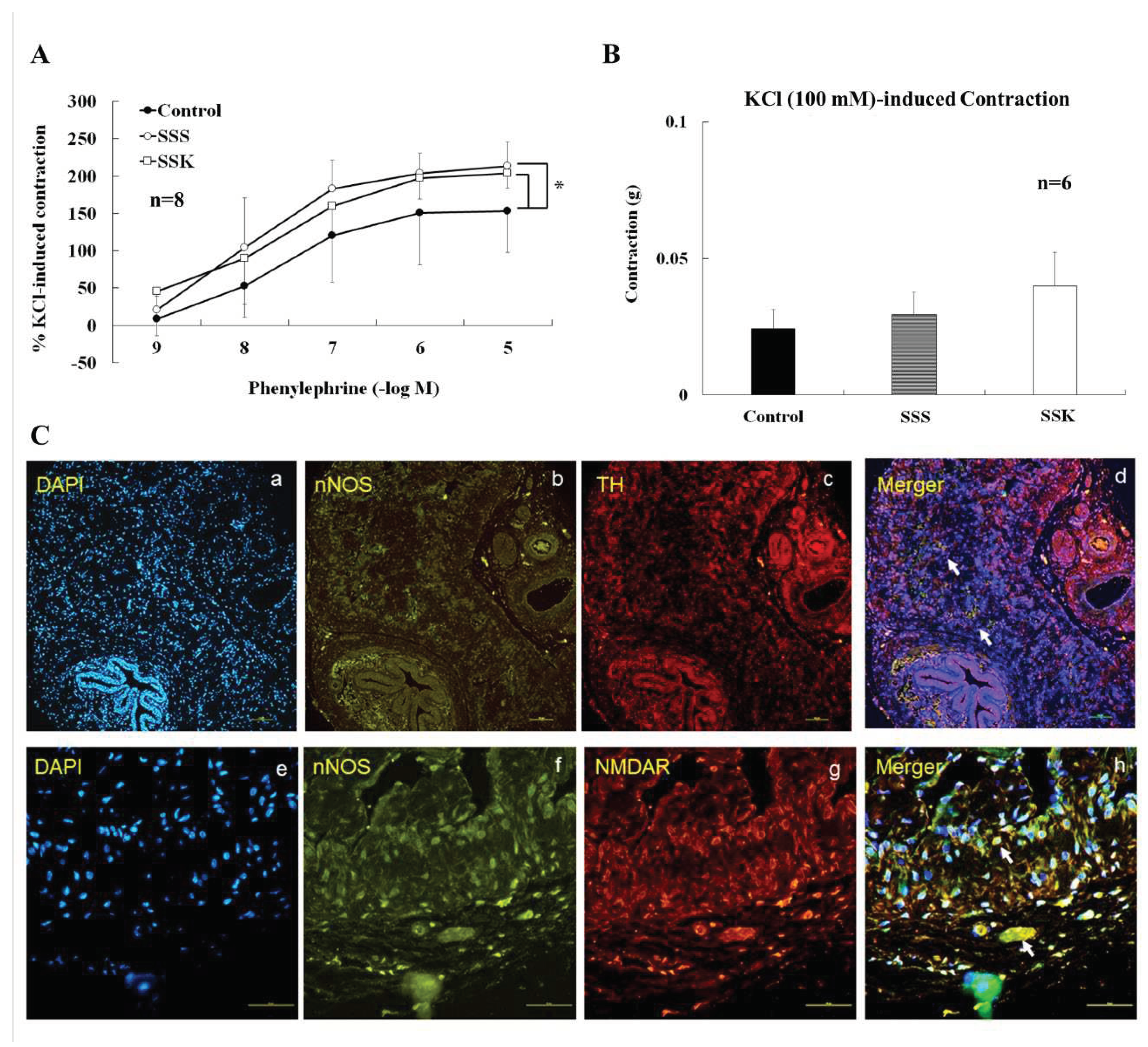
2.6. Effects of social stress and social stress plus ketamine on contraction of corpus cavernosum strips
In the absence of active muscle tone, the contraction of CC strips was induced by phenylephrine in a concentration-dependent manner (10
-9~10
-5 M). The contractility of CC strip of SSS and SSK was significantly higher than that of the control mice (
Figure 5A,
p<0.05). There was no statistical difference of KCl (100 mM)-induced maximal contraction between the 3 groups of mice (
Figure 5B,
p>0.05). Freshly dissected CC preparations were fixed using a formalin solution. Subsequently, we conducted observations of the association between tyrosine hydroxylase (TH)- and neuronal nitric oxide synthase (nNOS)-immunoreactive fibers, as well as the presence of double-labeled TH/nNOS fibers (a-d), in the CC of FVB mice. Additionally, we observed nNOS-immunoreactive fibers and their association with NMDA receptors (NMDAR), along with the presence of double-labeled nNOS/NMDAR fibers (e-h), in the CC of FVB mice. Notably, these TH/nNOS-immunoreactive fibers and NMDAR/nNOS-immunoreactive fibers exhibited co-localization within the same nerve fibers (
Figure 5C).
3. Discussion
Social stress induced both lower urinary tract dysfunction and erectile dysfunction in mice. Single dose administration of ketamine could not reverse both LUTD and ED in mice. Social stressed mice had statistically lower voiding frequency and smaller voided volume than those of control group (
Figure 1). These findings concurred with previous reports that social stress resulted in LUTD in experimental animals [
1,
2,
3,
21]. In addition, SS mice had detrusor overactivity [
21] and impaired detrusor contractility which were not reversed by short-term ketamine (
Figure 2B). Under electrical stimulation, SS mice had lower ICP than control and this cannot be reversed single-dose sub-anesthetic ketamine administration (
Figure 2C). These results suggested that SS may impair peripheral parasympathetic transmission, detrusor contractility, and erectile function.
Social stress related ED may be associated with reduction of NO inside CC, and exogenous NO enhanced CC relaxation of SSS mice (
Figure 3C), possibly as a compensatory mechanism. SNP induced CC relaxation in a concentration-dependent manner, and this phenomenon was enhanced by social stress, but that compensatory mechanism was abolished by a sub-anesthesia dose of ketamine administration (
Figure 3A). It is well known that norepinephrine can induce CC contractions, while acetylcholine can induce endothelial dependent relaxation [
22,
23,
24]. In this study, acetylcholine induced a concentration-dependent and endothelial NO dependent relaxation, which was decreased in both SSS mice, and single dose ketamine administration further decreased acetylcholine-induced relaxation in CC (
Figure 3B). It has reported that ketamine can decrease endothelial NO synthase activity in blood vessels due to a reduction in intracellular calcium levels [
25], and a reduction of intracellular Ca
2+ concentration in vascular smooth muscle cells [
26]. Therefore, ketamine may be decreased intracellular calcium levels of endothelial cell in CC.
In control mice, nicotine-induced CC relaxation was inhibited by lidocaine (
Figure 4A,B) and L-NNA (
Figure 4C,D), which suggests nicotine-induced a neurogenic NO dependent relaxation in CC. In neurophysiology, voltage-dependent ion channels play a fundamental role in the generation and propagation of the nerve impulse [
27]. That Blockade of Na
+ channels by ketamine, an important feature of local anesthetic action [
27]. That Blockade of Na
+ channels by ketamine, an important feature of local anesthetic action [
28]. In the central neuron system (CNS), ketamine inhibits Na
+ but not K
+ channel causing a decrease in brainstem parasympathetic cardiac neurons activity [
29]. In addition to CNS, it was reported that nicotine acted on the nicotinic acetylcholine receptors (nAChRs) located on the peripheral parasympathetic nitrergic nerves, thereby evoked the release of NO from these nerve terminals and induced a relaxation response in rabbit CC [
30]. The α7-nAChR was expressed in rat CC and modulated the neurogenic relaxation response to nicotine [
31]. SS decreased nicotine-induced CC relaxation due to damage of parasympathetic nitrergic nerves, while ketamine may not reverse this change. Taken together current results indicated that the neurogenic- and endothelial-dependent relaxation was reduced by SS and further decreased by ketamine in CC.
KCl induced maximal CC contraction was not different in the 3 groups, but phenylephrine-induced CC contraction was enhanced in SSS and SSK mice (
Figure 5A,B). These results suggested that contractility of smooth muscle of CC were not different in the 3 groups. Current findings in the control mice concurred with previous observation that in the endothelium and neuronal nitric oxide synthase knockout mice, the maximum contractions to phenylephrine and relaxation responses to nitroprusside were increased in CC [
32]. Meanwhile, SNP-induced relaxation was enhanced by pretreated L-NNA in the present study. This result further provides evidence that NO-dependent relaxation was impaired by social stress and ketamine in CC.
Our recent results indicated that NMDAR plays an essential role in this sympathetic-parasympathetic (or axo-axonal) nerve interaction transmitting pathway [
20]. Present results demonstrated that the TH-/nNOS-immunoreactive fibers and NMDAR/nNOS-immunoreactive fibers appeared co-localized in the same nerve fibers (
Figure 5C). These unique features of the close apposition between the sympathetic and parasympathetic nerve terminals suggest possible functional interactions between these nerves in regulating of CC muscle tone. In our current study, nicotine can induce relaxation of CC. Nicotine acts on adrenergic nerve terminals, causing norepinephrine to be released, which then acts on beta-adrenergic receptors on cholinergic nerve terminals. Excitation of beta-adrenergic receptors facilitates NMDAR action, which enhances the transformation of L-arginine to NO via nNOS. Furthermore, this axo-axonal interaction mechanism was dependent on intact sympathetic nerves. Nicotine-induced corpus cavernosum relaxation was inhibited by MK-801 and ketamine, for the impact on NMDAR.
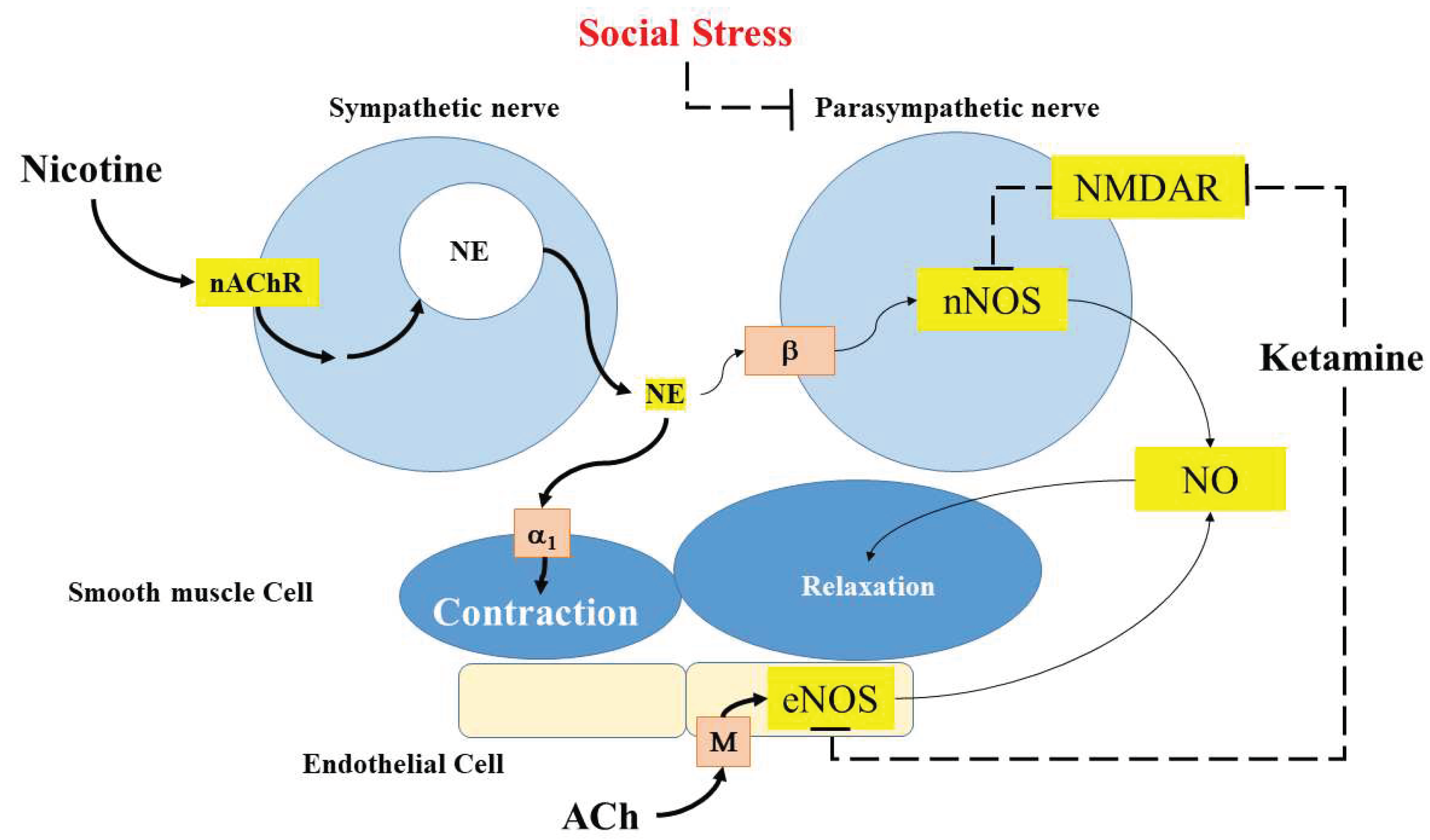
Figure 6 summarizes the proposed mechanism of SS related ED based on results of current research. CC of all species, including humans, is densely innervated by autonomic nerves and sympathetic nervous system is important for penile erection and may contribute to the maintenance of a non-erect state [
2]. Parasympathetic nitrergic/cholinergic transmission may be impaired by SS. In addition to reducing parasympathetic nitrergic/cholinergic transmission, SS may decrease the function of the endothelial cell of the corpus cavernosum and decrease basal level NO release caused erectile dysfunction. In addition, SS related ED was not reversed by single-dose sub-anesthetic ketamine treatment.
4. Materials and Methods
4.1. Social stress model
This experiment was approved by the Laboratory Animal Care and Use Committee of our institute (109-IACUC-006). Male 5-week FVB mice were maintained under controlled light (12-hour light/dark cycles from 7:00 AM to 7:00 PM) and temperature (21°C to 23°C) conditions. The FVB mice were randomly assigned to either SS exposure for 60 minutes on seven consecutive days for 4 weeks or control without SS. During each SS exposure, an intruder FVB mouse was placed into the home cage territory of an unfamiliar C57BL/6 resident previously screened for high aggression. A typical SS exposure resulted in intruder subordination, termed defeat, and is operationally defined as the FVB mice intruder assuming a supine posture. Following defeat, a wire mesh partition was placed in the cage to prevent physical contact between the resident and intruder. Auditory, olfactory, and visual contact were allowed to continue between FVB and C57BL/6 resident mice for 60 minutes. Control mice were placed in a novel cage behind a wire partition for 60 minutes daily. Mice were returned to their home cage after each session [
21].
4.2. Ketamine administration procedures
The FVB mice were maintained in a SS protocol for three weeks, then the mice were under stress for another week and randomly divided into two groups, namely the SSS group receiving single intraperitoneal injection of saline, SSK group receiving single dose intraperitoneal ketamine injection. The dose of ketamine was 25 mg/kg (United Biomedical, Inc., Asia).
4.3. Urinary bladder pressure
In vivo urinary bladder pressure was performed at the end of SS protocol for 4 weeks. Upon completing the SS exposure, subjects were anesthetized with urethane (500 mg/kg) and chloralose (50 mg/kg). A saline-filled polyethylene (PE) 10 catheter with a blunted end was sutured in place at the bladder dome after a lower abdominal wall incision and identification of urinary bladder. Upon determining the optimal length, the PE10 catheter was affixed with 6-0 sutures. The exteriorized cannula was connected to one port of a pressure transducer; the other port was connected to a syringe pump (1.8 ml/h, 37℃). Muscle and skin layers were closed separately using nonabsorbable sutures. The bladder pressure was recorded with a MP36 polygraph (Biopac Systems Inc., Santa Barbara, CA, USA) and Biopic Student Lab (BSL) 3.7.3 software (Biopac Systems Inc., Santa Barbara, CA, USA).
4.4. Intra-cavernosal pressure (ICP) measure
After 4-weeks of SS, mice were subjected to in vivo ICP measurement. Mice were anesthetized with urethane (500 mg/kg) and chloralose (50 mg/kg). After anesthesia, incised the abdominal wall along the white line of the lower abdomen to the bladder, carefully removed the connective tissue to avoid bleeding, exposed the major pelvic ganglia (MPG), carefully passed the electrode wire through and hook the cavernosal nerve and MPG, the other end was connected to an electrical stimulator (Grass, SD9J) [
21]. Filled the polyethylene (PE) 10 catheter with normal saline containing heparin (100 IU) with one end connected to a 30-gauge needle, carefully pierced the needle into the CC of the penis, and then connected the other end of the PE10 catheter to a port of the pressure sensor. The ICP was recorded on an MP36 polygraph (Biopac Systems Inc., Santa Barbara, CA, USA) and Biopac Student Lab (BSL) 3.7.3 software (Biopac Systems Inc., Santa Barbara, CA, USA). The cavernous nerve and major pelvic ganglia (MPG) were then electrical stimulated (ES, 2-10 Hz, 50 msec, and 2.5 V) in 60-second while measuring ICP, and total ICP was monitored by calculating the area under erectile curve (AUC) from the beginning of cavernous nerve stimulation to return of the ICP to the baseline.
4.5. Tissue preparation
The FVB mice were sacrificed by cervical dislocation after anesthesia with urethane (500 mg/kg, ip) and chloralose (50 mg/kg, ip). The CC was dissected and placed in oxygenated (95% O2 and 5% CO2) Krebs’ solution at 4℃. The composition of the Krebs’ bicarbonate solution (in mM) was NaCl 117, NaHCO3 25, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, glucose 11.1, and calcium disodium ethylenediamine tetraacetate (EDTA) 0.023.
4.6. Tissue Bath Wire Myography
The CC were dissected and cleaned of surrounding tissue under a dissecting microscope and were mounted on a stainless-steel rod and a platinum wire in a tissue bath containing 20 ml Krebs' solution which was equilibrated with 95% O2 and 5% CO2 and maintained at 37 °C. Tension changes were measured by an isometric transducer (FT03C; Grass). CC strips were equilibrated in the Krebs' solution for 60 minutes and mechanically stretched to a resting tension of 0.2 gm. Step 1: After equilibration, the resting muscle tone of CC strips was changed by cumulative applications of phenylephrine (10-9~10-5 M). Step 2: The CC strips were pre-contracted with phenylephrine (10 µM), and then relaxation effects were induced by acetylcholine (10-9~10-5 M), and nicotine (50 µM) on an MP36 polygraph (Biopac Systems Inc., Santa Barbara, CA, USA) and Biopac Student Lab (BSL) 3.7.3 software (Biopac Systems Inc., Santa Barbara, CA, USA), respectively. Between step 1 and 2, there were 45 minutes washes with Kreb’s solution. Step 3: The maximal contraction of CC strip was induced by KCl (100 mM). Only one isolated CC strip per animal was used in the myography study. The phenylephrine-induced CC smooth muscle contraction was estimated as a percentage of KCl (100 mM)-induced maximum contraction. The ACh- and nicotine-induced CC smooth muscle relaxation were estimated as a percentage of sodium nitroprusside (SNP, 0.1 mM)-induced maximum relaxation.
4.7. Double-labeled immunohistochemistry
Fresh CC was dissected and placed into formalin solution (neutral buffered, 10%) at room temperature. After five washes in PBS (pH 7.4), the CC were permeabilized, and nonspecific sites were blocked with 2% bovine serum albumin in 0.25% Triton X-100-PBS for 30 minutes at room temperature. The CC was incubated with the following primary antibodies at 4°C for 24 hr: rabbit anti-nNOS (1:500), combined mouse anti-NMDAR (1:500) or rabbit anti-nNOS (1:500), combined mouse anti-tyrosine hydroxylase (TH, 1:500). After being rinsed with PBS (pH 8.2) three times, the CC was incubated with the secondary antibodies for 1 h at room temperature. The secondary antibodies were: FITC-labeled rabbit anti-rabbit IgG (1:500) and rhodamine-labeled mouse anti-mouse IgG (1:1000). After washing out the secondary antibodies, sections were incubated with 4’, 6-diamidino-2-pheny-lindole (DAPI) for nuclear staining. After being rinsed with PBS (pH 8.2), each CC was whole-mounted with Vectashield mounting medium on Vectabondcoated slides (Vector Laboratories, Burlingame, CA, USA). The labeled specimens were then observed and photographed under a fluorescence microscope fitted (Nikon E800 microscope).
4.8. Drugs used and statistical analysis
The following chemicals were used: NaCl, NaHCO3, KCl, CaCl2, MgCl2, Glucose, NaH2PO4, EDTA, ACh, SNP, Nicotine, Phenylephrine, rabbit anti-nNOS, mouse anti-NMDAR, mouse anti-tyrosine hydroxylase, FITC-labeled rabbit anti-rabbit IgG (1:500), rhodamine-labeled mouse anti-mouse, and DAPI (all from Sigma-Aldrich, ST Louis, MO, USA). A paired t-test was used to compare difference in the same strip. An ANOVA of variance followed by post-hoc tests (Bonferroni) was used to compare difference between different strips. All values are presented as mean ± SEM. A value of p < 0.05 was considered statistically significant.
5. Conclusions
In conclusion, our findings suggest that the development of SS-related erectile dysfunction (ED) may be associated with a decrease in neuronal nitric oxide synthase (nNOS) and compromised nicotine-induced neurogenic relaxation, which is mediated through the axo-axonal interaction mechanism. Additionally, our study demonstrates that a single-dose treatment of ketamine does not possess the ability to reverse SS-related ED. These results emphasize the intricate nature of SS-related ED and the limited effectiveness of ketamine as a potential therapeutic intervention in this condition. Further research is warranted to explore alternative strategies for managing SS-related ED.
Author Contributions
Conceptualization, S.S.-D.Y.; methodology, H.-H.C. and S.S.-D.Y.; formal analysis, S.-Y.W. and C.-K.H.; investigation, S.-Y.W.; T.-C.C.; C.-K.H. and H.-H.C.; resources, S.-Y.W.; T.-C.C.; C.-K.H. and S.S.-D.Y.; writing—original draft preparation, S.-Y.W.; writing—review and editing, H.-H.C. and S.S.-D.Y.; funding acquisition, S.-Y.W. ; T.-C.C.; C.-K.H. and S.S.-D.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Buddhist Tzu Chi Medical Foundation (TCMF-JCT-112-11) and Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (TCRD-TPE-109-RT-3, TCRD-TPE-110-05, TCRD-TPE-110-12, TCRD-TPE-111-49, and TCRD-TPE-112-12).
Institutional Review Board Statement
This study was approved by the Laboratory Animal Care and Use Committee of Taipei Tzu Chi Hospital (Approval Number: 109-IAUCU-006).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available on request to the corresponding author.
Acknowledgments
We thank Yu-He Chang of the Department of Research, Taipei Tzu Chi Hospital for taking care of laboratory animals.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mingin, G.C.; Peterson, A.; Erickson, C.S.; Nelson, M.T.; Vizzard, M.A. Social stress induces changes in urinary bladder function, bladder NGF content, and generalized bladder inflammation in mice. Am J Physiol Regul Integr Comp Physiol. 2014, 307, R893–R900. [Google Scholar] [CrossRef]
- Wood, S.K.; Baez, M.A.; Bhatnagar, S.; Valentino, R.J. Social stress-induced bladder dysfunction: Potential role of corticotropin-releasing factor. Am J Physiol Regul Integr Comp Physiol. 2009, 296, R1671–R1678. [Google Scholar] [CrossRef]
- Mingin, G.C.; Heppner, T.J.; Tykocki, N.R.; Erickson, C.S.; Vizzard, M.A.; Nelson, M.T. Social stress in mice induces urinary bladder overactivity and increases TRPV1 channel-dependent afferent nerve activity. Am J Physiol Regul Integr Comp Physiol. 2015, 309, R629–R638. [Google Scholar] [CrossRef]
- Wood, S.K.; McFadden, K.; Griffin, T.; Wolfe, J.H.; Zderic, S.; Valentino, R.J. A corticotropin-releasing factor receptor antagonist improves urodynamic dysfunction produced by social stress or partial bladder outlet obstruction in male rats. Am J Physiol Regul Integr Comp Physiol. 2013, 304, R940–R950. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Kimura, N. Ethopharmacology of copulatory disorder induced by chronic social conflict in male mice. Neurosci Biobehav Rev. 1991, 15, 497–500. [Google Scholar] [CrossRef]
- Sugiura, K.; Yoshimura, H.; Yokoyama, M. An animal model of copulatory disorder induced by social stress in male mice: Effects of apomorphine and L-dopa. Psychopharmacology (Berl). 1997, 133, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Yotsuyanagi, S.; Nagasaka, Y.; Namiki, M. Dehydroepiandrosterone alleviates copulatory disorder induced by social stress in male rats. J Sex Med. 2006, 3, 612–618. [Google Scholar] [CrossRef]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V. , Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci. 2017, 20, 1752–1760. [Google Scholar] [CrossRef]
- Burnett, A.L.; Lowenstein, C.J.; Bredt, D.S.; Chang, T.S.; Snyder, S.H. Nitric oxide: A physiologic mediator of penile erection. Science. 1992, 257, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Ozkara, H.; Alan, C.; Atukeren, P.; Uyaner, I.; Demirci, C.; Gümüştaş, M.K.; Alici, B. Changes of nitric oxide synthase-containing nerve fibers and parameters for oxidative stress after unilateral cavernous nerve resection or manuplation in rat penis. Chin J Physiol. 2006, 49, 160–166. [Google Scholar]
- Priviero, F.B.; Jin, L.M.; Ying, Z.; Teixeira, C.E.; Webb, R.C. Up-regulation of the RhoA/Rho-kinase signaling pathway in corpus cavernosum from endothelial nitric-oxide synthase (NOS), but not neuronal NOS, null mice. J Pharmacol Exp Ther. 2010, 333, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Dashwood, M.R.; Crump, A.; Shi-Wen, X.; Loesch, A. Identification of neuronal nitric oxide synthase (nNOS) in human penis: A potential role of reduced neuronally-derived nitric oxide in erectile dysfunction. Curr Pharm Biotechnol. 2011, 12, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lee, M.H.; Chen, Y.C.; Lin, M.F. Ketamine-snorting associated cystitis. J Formos Med Assoc. 2011, 110, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, S.D.; van den Berg, S.J.; Tytgat, G.N.; Boeckxstaens, G.E. Oral S(+)-ketamine does not change visceral perception in health. Dig Dis Sci. 2004, 49, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Franceschelli, A.; Sens, J.; Herchick, S.; Thelen, C.; Pitychoutis, P.M. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and "depressed" mice exposed to chronic mild stress. Neuroscience. 2015, 290, 49–60. [Google Scholar] [CrossRef]
- Brachman, R.A.; McGowan, J.C.; Perusini, J.N.; Lim, S.C.; Pham, T.H.; Faye, C.; Gardier, A.M.; Mendez-David, I.; David, D.J.; Hen, R.; Denny, C.A. Ketamine as a Prophylactic Against Stress-Induced Depressive-like Behavior. Biol Psychiatry. 2016, 79, 776–786. [Google Scholar] [CrossRef]
- Zhang, J.C.; Yao, W.; Dong, C.; Yang, C.; Ren, Q.; Ma, M.; Han, M.; Hashimoto, K. Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl). 2015, 232, 4325–4335. [Google Scholar] [CrossRef]
- Yang, S.S.; Jang, M.Y.; Lee, K.H.; Hsu, W.T.; Chen, Y.C.; Chen, W.S.; Chang, S.J. Sexual and bladder dysfunction in male ketamine abusers: A large-scale questionnaire study. PLoS ONE. 2018, 13, e0207927. [Google Scholar] [CrossRef]
- Li, C.C.; Wu, S.T.; Cha, T.L.; Sun, G.H.; Yu, D.S.; Meng, E. A survey for ketamine abuse and its relation to the lower urinary tract symptoms in Taiwan. Sci Rep. 2019, 9, 7240. [Google Scholar] [CrossRef] [PubMed]
- Li, M.W.; Chao, T.C.; Lim, L.Y.; Chang, H.H.; Yang, S.S. The Acute Effects and Mechanism of Ketamine on Nicotine-Induced Neurogenic Relaxation of the Corpus Cavernosum in Mice. Int J Mol Sci. 2023, 24, 6976. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.S.; Chang, H.H.; Chang, S.J. Does ketamine ameliorate the social stress-related bladder dysfunction in mice? Neurourol Urodyn. 2020, 39, 935–944. [Google Scholar] [CrossRef]
- Srilatha, B.; Adaikan, P.G. Estrogen and phytoestrogen predispose to erectile dysfunction: Do ER-alpha and ER-beta in the cavernosum play a role? Urology 2004, 63, 382–386. [Google Scholar] [CrossRef]
- Ohmasa, F.; Saito, M.; Tsounapi, P.; Dimitriadis, F.; Inoue, S.; Shomori, K.; Shimizu, S.; Kinoshita, Y.; Satoh, K. Edaravone ameliorates diabetes-induced dysfunction of NO-induced relaxation in corpus cavernosum smooth muscle in the rat. J Sex Med. 2011, 8, 1638–1649. [Google Scholar] [CrossRef]
- Fraga-Silva, R.A.; Costa-Fraga, F.P.; Savergnini, S.Q.; De Sousa, F.B.; Montecucco, F.; da Silva, D.; Sinisterra, R.D.; Mach, F.; Stergiopulos, N.; da Silva, RF.; Santos, R.A. An oral formulation of angiotensin-(1-7) reverses corpus cavernosum damages induced by hypercholesterolemia. J Sex Med. 2013, 10, 2430–2442. [Google Scholar] [CrossRef]
- Chen, R.M.; Chen, T.L.; Lin, Y.L.; Chen, T.G.; Tai, Y.T. Ketamine reduces nitric oxide biosynthesis in human umbilical vein endothelial cells by down-regulating endothelial nitric oxide synthase expression and intracellular calcium levels. Crit Care Med. 2005, 33, 1044–1049. [Google Scholar] [CrossRef]
- Akata, T.; Izumi, K.; Nakashima, M. Mechanisms of direct inhibitory action of ketamine on vascular smooth muscle in mesenteric resistance arteries. Anesthesiology. 2001, 95, 452–462. [Google Scholar] [CrossRef]
- Bezanilla, F. Voltage-gated ion channels. IEEE Trans Nanobioscience. 2005, 4, 34–48. [Google Scholar] [CrossRef]
- Haeseler, G.; Tetzlaff, D.; Bufler, J.; Dengler, R.; Münte, S.; Hecker, H.; Leuwer, M. Blockade of voltage-operated neuronal and skeletal muscle sodium channels by S(+)- and R(-)-ketamine. Anesth Analg. 2003, 96, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Irnaten, M.; Wang, J.; Chang, K.S.; Andresen, M.C.; Mendelowitz, D. Ketamine inhibits sodium currents in identified cardiac parasympathetic neurons in nucleus ambiguus. Anesthesiology. 2002, 96, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, N.B.; Vural, I.M.; Sarioglu, Y.; Pekiner, C. Nicotine potentiates the nitrergic relaxation responses of rabbit corpus cavernosum tissue via nicotinic acetylcholine receptors. Eur J Pharmacol. 2007, 558, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Faghir-Ghanesefat, H.; Rahimi, N.; Yarmohammadi, F.; Mokhtari, T.; Abdollahi, A.R.; Ejtemaei Mehr, S.; Dehpour, A.R. The expression, localization and function of α7 nicotinic acetylcholine receptor in rat corpus cavernosum. J Pharm Pharmacol. 2017, 69, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Nangle, M.R.; Cotter, M.A.; Cameron, N.E. An in vitro investigation of aorta and corpus cavernosum from eNOS and nNOS gene-deficient mice. Pflugers Arch. 2004, 448, 139–145. [Google Scholar] [PubMed]
Figure 1.
The mice exposed to the 4-week social stress demonstrated a diminished number of urine spots on the filter paper (A) and a lower voiding frequency.
Figure 1.
The mice exposed to the 4-week social stress demonstrated a diminished number of urine spots on the filter paper (A) and a lower voiding frequency.
Figure 2.
A representative tracing depicts activation of MPG by electrical stimulation (2, 5, and 10 Hz), which caused urinary bladder contract and ICP increase in male mice (A). The electrical stimulation-induced contraction of urinary bladder was significantly greater in control mice than in the SSS and SSK mice (*p<0.05 vs control) (B). The electrical stimulation-induced elevation of ICP was significantly greater in control mice than in the SSS and SSK mice (*p<0.05 vs control) (C).
Figure 2.
A representative tracing depicts activation of MPG by electrical stimulation (2, 5, and 10 Hz), which caused urinary bladder contract and ICP increase in male mice (A). The electrical stimulation-induced contraction of urinary bladder was significantly greater in control mice than in the SSS and SSK mice (*p<0.05 vs control) (B). The electrical stimulation-induced elevation of ICP was significantly greater in control mice than in the SSS and SSK mice (*p<0.05 vs control) (C).
Figure 3.
In the present of active muscle tone induced by PE (10 μM), the relaxation of corpus cavernosum was induced by acetylcholine (ACh, 10-9~10-5 M). The ACh-induced relaxation was lower in SSS and SSK than that of control mice (n=6, *p<0.05, A), and that was lower in SSK than that of SSS mice (n=6, *p<0.05, A). Inactive CC muscle tone induced by phenylephrine (PE, 10 µM), the nicotine (50 μM)-induced relaxation were significantly decreased between SSS and SSK (n=6, *p<0.05), and SSK was significantly decreased than that of SSS mice (n=6, *p<0.05, B). The sodium nitroprusside (SNP, 10-9~10-5 M, C)-induced relaxation was enhanced in SSK mice (n=6, *p<0.05, C) than the control mice, and the relaxation in SSK was lower than the SSS mice. This SNP-induced relaxation did not reach statistical difference between SSK and control mice.
Figure 3.
In the present of active muscle tone induced by PE (10 μM), the relaxation of corpus cavernosum was induced by acetylcholine (ACh, 10-9~10-5 M). The ACh-induced relaxation was lower in SSS and SSK than that of control mice (n=6, *p<0.05, A), and that was lower in SSK than that of SSS mice (n=6, *p<0.05, A). Inactive CC muscle tone induced by phenylephrine (PE, 10 µM), the nicotine (50 μM)-induced relaxation were significantly decreased between SSS and SSK (n=6, *p<0.05), and SSK was significantly decreased than that of SSS mice (n=6, *p<0.05, B). The sodium nitroprusside (SNP, 10-9~10-5 M, C)-induced relaxation was enhanced in SSK mice (n=6, *p<0.05, C) than the control mice, and the relaxation in SSK was lower than the SSS mice. This SNP-induced relaxation did not reach statistical difference between SSK and control mice.
Figure 4.
A representative tracing in A showing that in CC, in active muscle tone induced by phenylephrine (PE, 10 µM), the nicotine (50 µM)-induced relaxation was abolished by lidocaine (10 µM). This result was summary in B. The sodium nitroprusside (SNP)-induced relaxation was not inhibited by lidocaine (n=6, *p<0.05 vs control, A and B). A representative tracing in C showing that in CC, in active muscle tone induced by phenylephrine (PE, 10 µM), the nicotine-induced relaxation was abolished by L-NNA (100 µM). This result was summary in D. The sodium nitroprusside (SNP)-induced relaxation was enhanced by L-NNA (n=6, *p<0.05 vs control, D).
Figure 4.
A representative tracing in A showing that in CC, in active muscle tone induced by phenylephrine (PE, 10 µM), the nicotine (50 µM)-induced relaxation was abolished by lidocaine (10 µM). This result was summary in B. The sodium nitroprusside (SNP)-induced relaxation was not inhibited by lidocaine (n=6, *p<0.05 vs control, A and B). A representative tracing in C showing that in CC, in active muscle tone induced by phenylephrine (PE, 10 µM), the nicotine-induced relaxation was abolished by L-NNA (100 µM). This result was summary in D. The sodium nitroprusside (SNP)-induced relaxation was enhanced by L-NNA (n=6, *p<0.05 vs control, D).
Figure 5.
In the absence of active muscle tone, corpus cavernosum contracted upon application of phenylephrine (PE, 10-9~10-5 M) in a concentration-response manner. The PE-induced contraction was significantly greater in SSS and SSK than those of control mice (n=6, *p<0.05, A). KCl (100 mM)-induced maximal CC contraction did not reach statistical difference between the 3 groups (n=6, *p>0.05 vs control, B). Neuronal NOS-containing fibers (f), NMDA receptor (g), and DAPI (e) merger (h) in the corpus cavernosum of FVB mice. The tyrosine hydroxylase (TH)-/nNOS-immunoreactive fibers, and double-labeled TH/nNOS (a-d) fibers were observed in the CC of FVB mice. In addition, nNOS-containing fibers (f), NMDA receptor (g), and DAPI (e) merger (h) in the corpus cavernosum of FVB mice. The scale represents as 100 μm.
Figure 5.
In the absence of active muscle tone, corpus cavernosum contracted upon application of phenylephrine (PE, 10-9~10-5 M) in a concentration-response manner. The PE-induced contraction was significantly greater in SSS and SSK than those of control mice (n=6, *p<0.05, A). KCl (100 mM)-induced maximal CC contraction did not reach statistical difference between the 3 groups (n=6, *p>0.05 vs control, B). Neuronal NOS-containing fibers (f), NMDA receptor (g), and DAPI (e) merger (h) in the corpus cavernosum of FVB mice. The tyrosine hydroxylase (TH)-/nNOS-immunoreactive fibers, and double-labeled TH/nNOS (a-d) fibers were observed in the CC of FVB mice. In addition, nNOS-containing fibers (f), NMDA receptor (g), and DAPI (e) merger (h) in the corpus cavernosum of FVB mice. The scale represents as 100 μm.
Figure 6.
Summarizes the proposed mechanism of social stress related ED based on results of current research.
Figure 6.
Summarizes the proposed mechanism of social stress related ED based on results of current research.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).