1. Introduction
Mineral TL refers to the condition where crystalline solids can generate free electrons and holes in the crystal when exposed in ionizing radiation. A part of the free electrons and holes are captured by the trap in the crystal. When the crystal is heated the trapped electrons and holes regain energy and escape from the trap, return to the ground status, and release excess energy in the form of TL. TL of marine sediment minerals is widely applied in dating(Huntley and Johnson,1976;Wintle and Huntley,1979,1980), natural dose measurement (Yassin,2016), stratigraphic division, environmental changes, and other fields (Chen et al.,2013;Joseph et al.,2016; Noriyuki et al.,2017; Qiu et al.,2019). Marine sediments are composed of calcareous and siliceous biological components and nonbiological components, such as terrestrial elements, autogenous elements, and volcanic and cosmic dust. In accordance with the relative abundance and formation mechanism of the components, they are divided into five main types, namely, calcareous biological ooze, siliceous ooze, oceanic clay, terrestrial detrital, and pyroclastic. The calcareous biological ooze from eupelagic deposit is rich in carbonate minerals and is the most widely distributed in the world’s seas. It also contains abundant information on the Earth’s climate changes.
At the 2013 annual meeting of paleoceanography in Barcelona, Spain, an important theme was to develop new alternative indicators of paleoceanography.Carbonate has been proved an excellent TL carrier mineral(Johnson,1965;Vaz and Zeller,1966; Medlin,1967; Bothner and Johnson,1969; Christodoulides and Fremlin,1971; Nambi,1978; Sunta,1984; Calderon et al., 1984; Carmichael et al.,1994 ). The research on TL through the marine carbonate has achieved results in the research of dating (Ninagawa et al., 1988; Duller et al., 2009; Stirling et al., 2012) and paleoclimate changes (Liu et al., 2008). In this study, calcareous biological ooze TL of two cores collected from the Ninetyeast Ridge of the equatorial Northeast Indian Ocean and in the high latitude of North Atlantic were analyzed. The TL peak intensities in calcareous biological ooze to paleoclimatic changes since the middle and late Pleistocene are explored by comparing the results of the δ18O , and are compared with the carbonate contents in sediments and the Milankovitch spectrum period extracted from the TL- time series.
2. Geographical setting
2.1. The ninetyeast ridge of the equatorial northeast Indian ocean
MD81349 piston core was collected near the equatorial ninetyeast ridge, which was acquired by the French scientific vessel Marion Dufresne. The ninetyeast ridge in the northeast Indian Ocean was formed in the South Indian Ocean during the Cretaceous. Since the Cenozoic era, the collision between the Indian plate and the Eurasian plate led to the uplift of the Himalayas and the Tibetan Plateau, thereby affecting global climate and environmental changes.With the Indian Plate moving northward along the Ninetyeast, it was extended from 31°S to 10°N, lasting for approximately 4000 km long and 2000–3000 m high. The ninetyeast ridge is far from the continental margin. Land-based debris accounts for a small proportion of sediments.Unaffected by the input of terrestrial materials, its sedimentary environment is stable. It belongs to the area where the equatorial east wind and the south Asian monsoon meet and is an important geographical tectonic unit in the northeast Indian Ocean. It echoes with the Himalayas and the Qinghai–Tibet Plateau in the north. The Ninetyeast Ridge is a typical studying area for paleoceanography and paleoclimatology (Chen and Farrell,1990; Farrell and Janecek,1990).
Abyssal sediments are regarded as suitable carriers for high-resolution paleoclimatology research and pose extremely important significance for global climate change research. The marine sediments in the northeast Indian Ocean region have preserved information on paleoclimate and environmental evolution, making it an ideal area for studying the response of sedimentary records to the changes in paleoclimate and paleoenvironment.
The calcareous biological ooze in the core MD81349 represented by the oceanic origin of gray-white ooze constitute the main sedimentary components and are excellent carriers of climate signals. The carbonate content is as high as 80%–90%. The sediments are primarily nannofossils, foraminifers, with an average deposition rate of 1.45 cm/ka. The foraminifer assemblages are dominated by planktonic foraminifers, and benthic foraminifers are few and small.
2.2. The North Atlantic ice-rafted detritus belt
Core IODP306-U1312B was continuously cored with the variable-length hydraulic piston coring (VLHPC) system at U1312 station from March 2 to April 25, 2005 of Integrated Ocean
Drilling Program (IODP306). The north is directly related to Arctic glacier activity and is the starting point of deep water in the global warm-salt circulation. Low-temperature, high-salinity seawater invades the Labrador and Greenland Seas, driving the current global ocean cycle and heat exchange and affecting the global climate. Therefore, this area is regarded as the driver of global climate change (Gerard and Rusty,1995). Different from the stable sedimentary environment of the Ninetyeast Ridge longitude of the Indian Ocean, The north Atlantic ice-rafted detritus belt ( IRD belt) was affected by the alternation of cold and warm climates throughout history. The interconversion of material composition and sedimentation process under the influence of glacial and interglacial periods was recorded. Therefore, this area is regarded as the sensitive area to experience the climate change in the Earth. The IRD belt (Dansgaard et al., 1993;Stein et al.,2006 ) is another hotspot for research. It refers to the floating ice carrying debris from the land to the sea area away from the land margin. With the gradual melting of ice floes, debris is released into the water body and sinks through the water column to the bed of the sea, which is a sign of highly sensitive climate change. This belt is an abundant carbonate deposit area, with the average deposition rate of 2.0 cm/ka, making it conducive to the study on the climate change and air–sea interaction on the short time scale at the millennium level and the long time scale of millions of years since the Pliocene.
Sediments at Site U1312B are composed of varying mixtures of biogenic and detrital components, primarily nannofossils, foraminifers, and clay minerals. Lithologies include nannofossil ooze, foraminifer nannofossil ooze, foraminifer nannofossil ooze with clay, nannofossil ooze with foraminifers, nannofossil ooze with clay, nannofossil ooze with clay and foraminifers, silty clay nannofossil ooze with foraminifers, silty clay nannofossil ooze, nannofossil silty clay, and silty clay calcareous ooze. Most contacts between these lithologies are bioturbated or gradational. Common smear slide estimates of biogenic components include nannofossils (30%–90%), foraminifers (5%–30%), diatoms (<5%), radiolarians (trace), silicoflagellates (trace), and sponge spicules (trace).Total carbonate contents range from 59 to 98 wt% in the core. (Channell et al.,2006).
The geographic location is shown in
Figure 1. The core parameters are shown in
Table 1.
3. Analysis methods
3.1. Sample preparation
The sampling spacing of TL analysis of the core MD81349 is 3–5 cm, and is
2–4 cm of the core U1312B. Each sample of calcareous biological ooze is weighed with a fixed volume sample splitter. The quality of the sample was tested with an electronic balance with an accuracy of 0.1mg, and the mass was about 4.0 mg.
The planktonic foraminifer (Globigerinoides ruber,G.ruber) were picked out of the samples under a microscope and measured after ultrasonic cleaning. It is gray-white calcareous biological ooze sample and sample splitter seen in
Figure 2.
3.2. Measurements
TL of calcareous biological ooze, and CaCO3 measurements of the cores MD81349 and IODP306-U1312B were carried out at the Radiation and Environmental Laboratory and School of Oceanography, China University of Geosciences (Beijing). TL measurements were taken using an RGD-3 model. The samples of the core MD81349 were evenly measured on a stainless steel heating plate at a heating rate of 6°C/s in 2001 under an air atmosphere, and with 5°C/s in 2011 for the core U1312B under an air atmosphere. The optical signal is converted into an electrical signal output with a highly sensitive photomultiplier tube. The measurement result is the total TL peak intensity(a.u./4mg) including chemiluminescence (CL) derived from high temperature oxidation of organic matter (Carmichael et al.,1994;Roque et al., 2001;Wang et al.,2015) and spurious TL(Pagonis et al.,1997).
Oxygen isotopes in the G.ruber of the core MD81349 were determined at the French National Research Center for Climate and Environmental Sciences. After sieving and washing the samples, 15–20 planktonic foraminifers larger than 150 μm G.ruber were collected. The shell is reacted with 100% phosphoric acid at 25 °C. After vacuuming and dehydration, the generated CO2 gas is collected and used for the isotope test with a British OPTIMA mass spectrometer.
Due to rare G. ruber for oxygen isotope test (Liu et al.,2018). The global ocean standard LR04 (Lisiecki and Raymo, 2005)is cited to determine the stages of oxygen isotopes and establish an age model of the core U1312B.
To study the fine structure of G.ruber, the samples were analyzed with a JEM-2010F field emission transmission electron microscopy (FE-TEM) in Tsinghua University(Beijing). Point resolution: 0.23 nm; crystalline lattice resolution: 0.102 nm; minimum beam spot size: 0.5 nm; maximum magnification factor: 1.5 M; maximum tilt angle of the sample table: X=±35°, Y=±30°.
4. Results
4.1. TL measurement results
The TL of 89 calcareous biological ooze samples and 92 planktonic foraminifers samples (G.ruber)in the core MD81349 was determined. Among them, 73 samples are consistent with the sampling depth of oxygen isotopes. The maximum TL of the samples of the core MD81349 is 174.7 a.u. (TL peak intensity of the sample at a.u./4 mg, arbitrary unit, same below). The minimum value is 27.3 a.u. the average value is 94.1 a.u.. The TL value of planktonic foraminifers are higher than that of the TL of biological ooze sample, where the maximum value is 300.10 a.u., the minimum value is 114 a.u., the average value is 186.84 a.u..
The maximum value of the TL of calcareous biological ooze of the core U1312B is 199.23 a.u., the minimum value is 28.49 a.u., the average value is 84.7 a.u. at a total of 139 samples.
4.2. FE-TEM of planktonic foraminifer shell
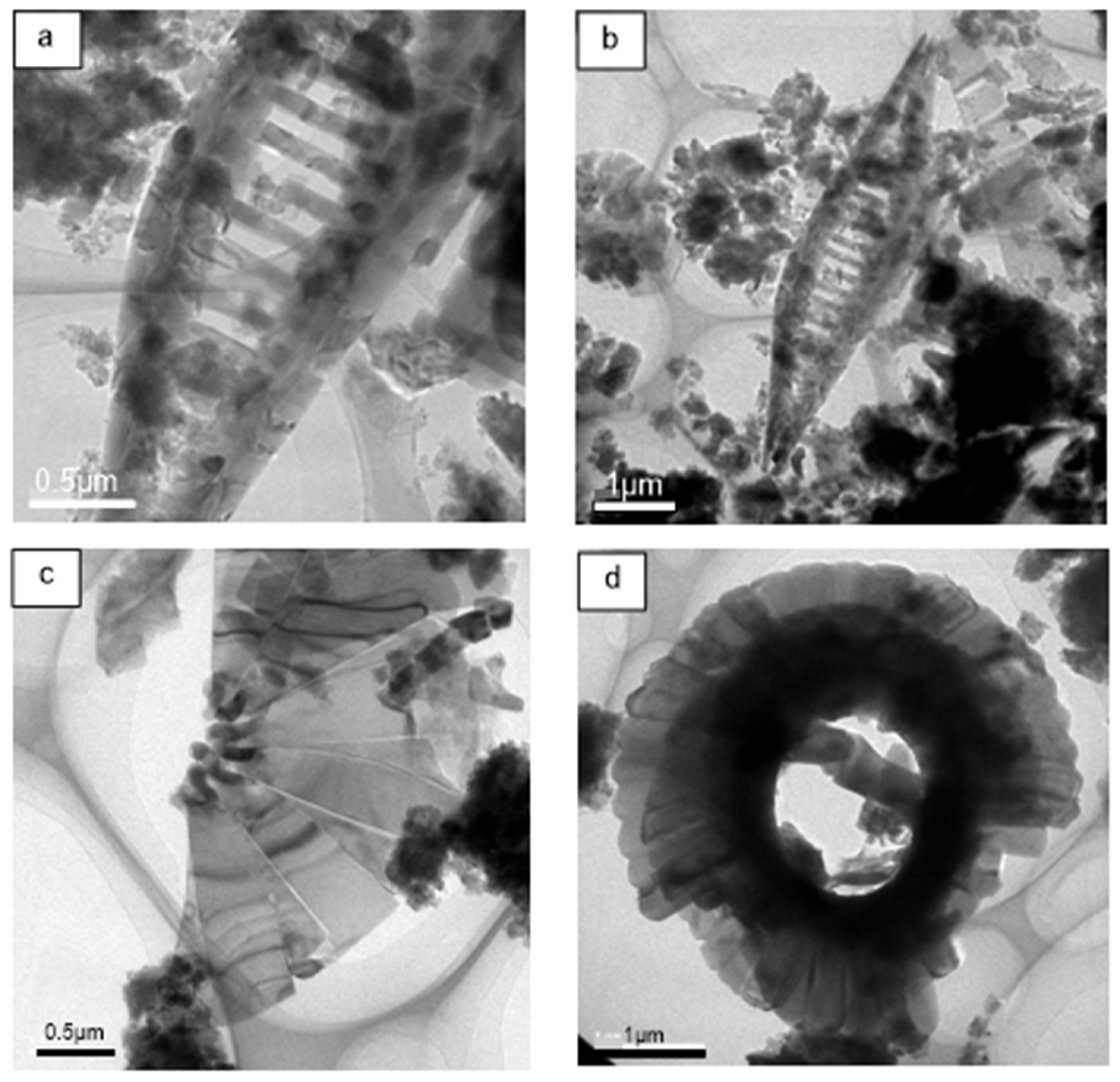
The FE-TEM microscopic imaging of planktonic foraminifer is shown in
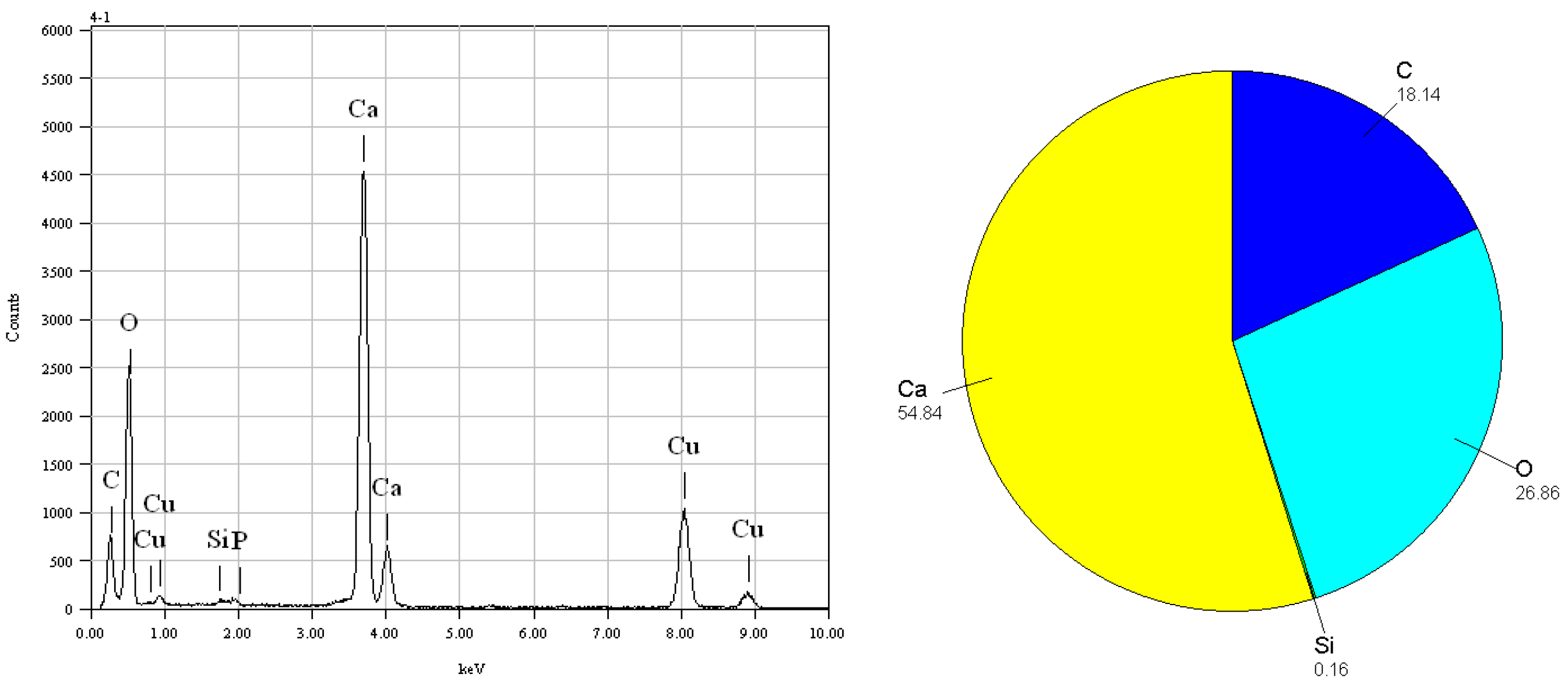
Figure 3, indicating the evident shape of foraminifer nannofossil ooze. The elemental composition of calcareous organisms is mainly Ca, O, C, and trace P,Si. The main minerals constituting is carbonate, as shown in
Figure 4.
4.3. TL glow curve and thermal stability of foraminifer nannofossil ooze
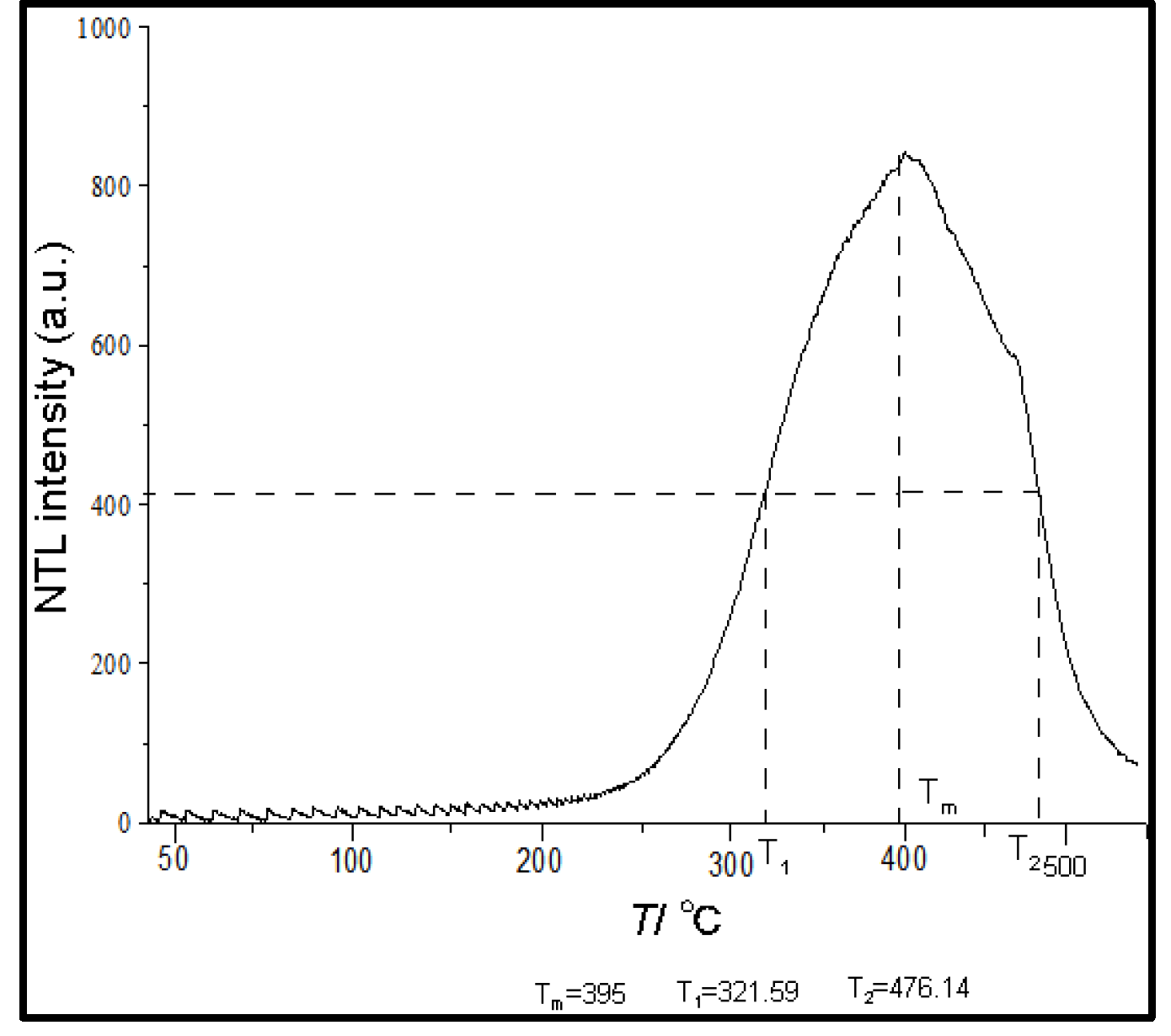
The luminescence peak intensity is related to the heating rate. Medlin (1963) found that the TL of marine foraminifer can show a single peak of 227 ℃(500 K). Ponnusamy et al. (2006) indicated that the TL of the biological shell only shows a TL peak of 330 ℃. Research shows that calcite minerals exhibit three main luminescence peaks, and the corresponding peak temperatures are 120 ℃, 280 ℃, and 350 ℃, respectively, when the heating rate is 5 °C/s. Among them, the TL of 280 ℃ luminescence peak has been demonstrated to have great potential for dating (Zhang and Wang,2020). TL signals by heating to a high temperature (i.e., 400-450 °C), which for calcite could generate spurious TL signals (Huang et al.,2022).The typical TL peak of planktonic foraminifer of the core MD81349 is shown in
Figure 5.
Tm, T1, and
T2 are the maximum peak temperature, the low-end and high-end temperatures corresponding to the TL glow curve at peak temperature and half-peak height. The heating rate is 6 °C/s, and the TL peak is quite brad at about 395 °C.
The thermal stability of the charge in the TL peak at about 395°C has not been measured here. Longer lifetimes are associated with high temperature TL signals, or peaks, and shorter lifetimes are associated with low-temperature TL signals. For examples, high-temperature luminescence peak of feldspar is extremely stable under normal temperature and low-temperature environments (Biswas et al.,2018,2020). The low-temperature (120-130 °C) TL peak of calcite has a lifetime of several hours, while the 280 °C TL peak corresponds to a lifetime of more than 10 Ma (Engin and Güven, 1997;Huang et al.,2022 ).The luminescence peak at 335 °C from calcitic snail opercula has a lifetime of more than 1011 years (Stirling et al., 2012).
4.4. Preservation of TL signatures
At least two sources are found for the TL of the deposited calcium carbonate minerals, that is, the source of geological dose and the inherent “defect” sources of mineral crystallization. TL signals of geological dose are that after the crystallization of mineral crystals, they are subjected to the external natural radionuclides uranium (U), radium (Ra), thorium (Th), potassium (K), and their decay daughter nuclides.The TL of geological dose has a positive linear relationship with the deposition time and is frequently used as an important method of dating (Aitken,1985). Furthermore, Castagnoli et al. (1988,1990) indicated that the minerals in the atmospheric dust are energy receivers during periods of strong solar activity and when the solar wind, solar protons, ultrahigh-energy ultraviolet rays, and high-energy gamma rays reach the Earth. They store ray radiant energy, which is also one of the material of sedimentary core and the source of radiant energy, and is consistent with the cause of environmental radiation of TL.Sediments from the Subantarctic Ocean and the Cape Basin (South Atlantic), where oxic conditions currently prevail, show high accumulation rates of authigenic Cd and U during glacial intervals. A third core which located south of the Antarctic Polar Front shows an approximately inverse pattern to the Subantarctic record. The contrasting patterns to the north and south of the Antarctic Polar Front suggest that higher accumulation rates of Cd and U in Subantarctic sediments were driven primarily by increased productivity (Rosenthal et al.,1995). From this point of view, relationship between natural radionuclides and climate change is complex. The U ,Th and their decay daughter nuclides concentration in these biogenic carbonates is not known. We cannot subtract the contribution from geological dose of radioactive elements contained in the samples. The experiment proves that the spurious TL of Marine calcareous biological ooze is dose independent. TL measurements were performed after gamma irradiation using
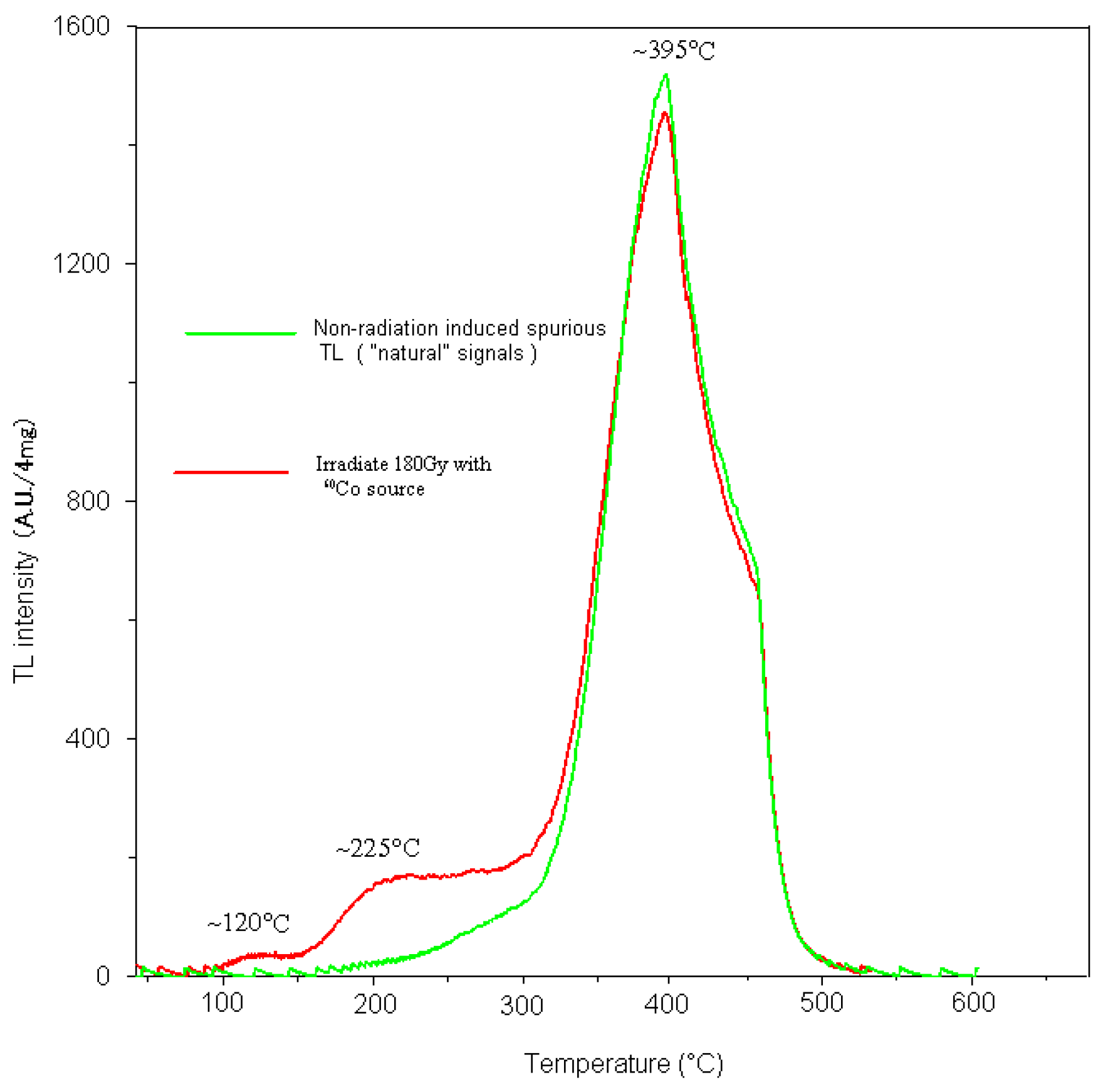
60Co gamma source with increasing doses (irradiation times of 3600s, with irradiation dose rates of 50 mGy/s, irradiation dose 180Gy ). Heating was at 6 °C/s in a nitrogen atmosphere (2 liters of nitrogen per minute ). The main objective of the measurements was to acquire the natural TL intensity of 395 °C peak does not increase with dose of the calcareous biological ooze samples, without effects of additional laboratory treatments. Calcareous biological ooze is TL carrier minerals.The TL glow curves of calcareous biological ooze received a
60Co gamma dose of 180 Gy show prominent peaks at temperatures of 120°C, 225°C, and 395°C.The TL intensity of the dosimetric peak located around 395 °C does not increase with irradiation dose (
Figure 6). We did not conduct saturated absorption dose test.
The lattice defects formed during the crystallization of minerals are called the inherent “defect” sources. TL from the inherent “defect”of mineral crystallization is called spurious TL(Pagonis et al.,1997; Roque et al.,2001). Zeller et al. (1957) found that the fresh calcite formed by simulated seawater evaporation exhibits TL without any radiation and mechanical action.The generation of TL is related to pressure and crystallization temperature (Zeller et al. ,1955), the number and type of mineral lattice defects, the content and type of impurities, such as Mn
2+ substituted Ca
2+ in calcite lattices ( Townsend et al.,1994; Qiu et al.,2019) and the temperature and deposition time of calcite mineral formation (Kononova and Tarashchan,1970). As shown in
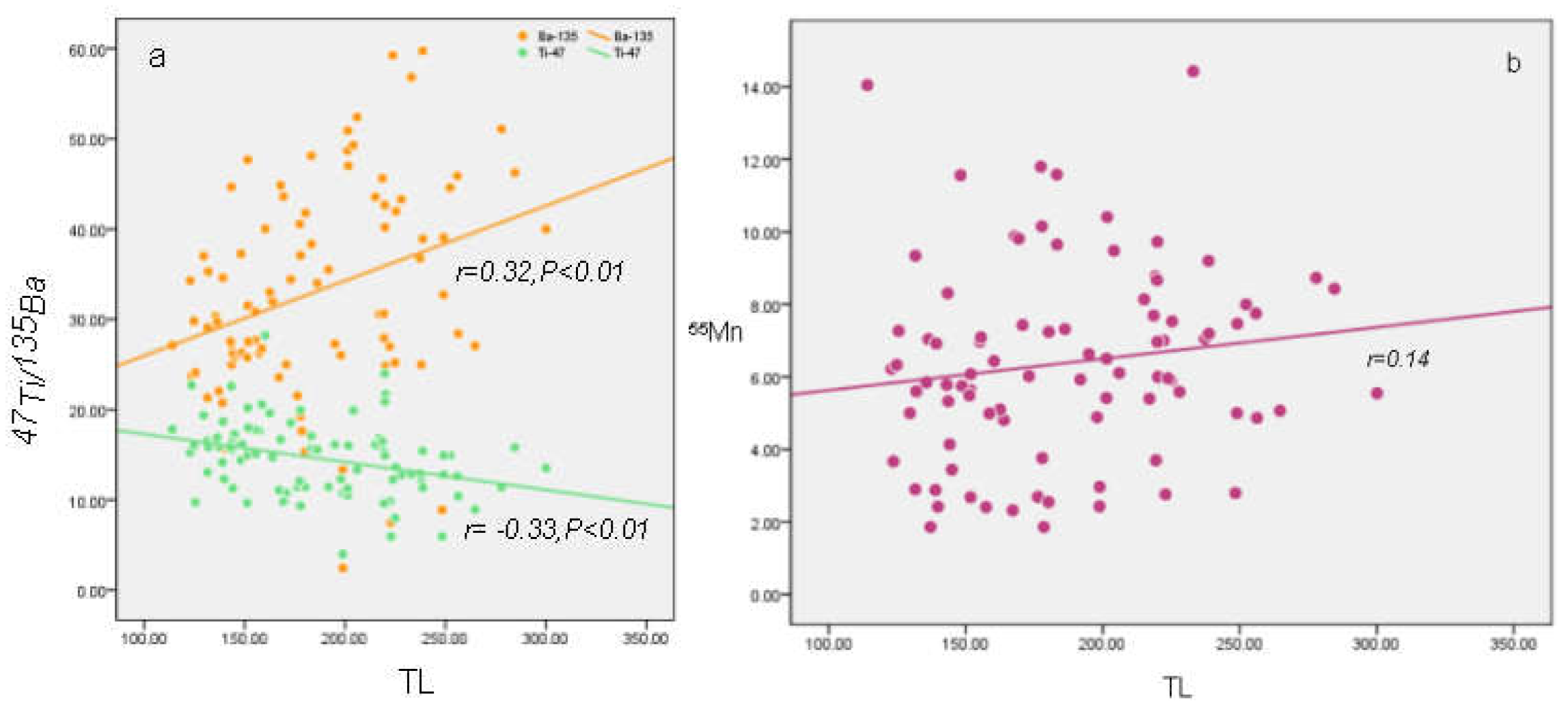
Figure 7(a), TL has a significant positive correlation with trace elements
135Ba (
n=92,
r=0.318,
p<0.01), a significant negative correlation with
47Ti (
n=92,
r=-0.325,
p<0.01).
135Ba acts as an activator in the TL of marine authigenic calcite, while
47Ti may be an impurity for quenching TL.
55Mn content of samples show a positive relationship with TL peak intensities (
n=92,
r=0.144) as shown in
Figure 7(b), Mn
2+ is the key ion, because the TL emission efficiency and the partition coefficient of Mn are greater for calcite than for aragonite (Noriyuki et al.,2017)
.
Thus it can be inferred that this spurious TL of 395 °C peak intensities from the marine calcareous biological ooze is depend on the temperature of formation of minerals. The impurity ions (e.g. Ba2+,Ti2+ and Mn2+) doped in carbonate are acting as activators and suppressants respectively. From the perspective of the formation and crystallization of minerals, the internal defects that form carbonate mineral crystals will produce certain differences under different temperature environments.
5. Discussion
5.1. Relevance between TL and Oxygen stable isotope formation
The seawater oxygen isotope composition is an indicator of sea level rise and fall, reflecting the global changes in climateand the global ice volume information. The oxygen isotope of planktonic foraminifera of marine organisms not only contains the information of global ice volume, but also is affected by the surface temperature and salinity of seawater, which can reflect regional climate change. Globigerinoides ruber(G.ruber) is a widely distributed species of planktonic foraminifer. In tropical and temperate zones, the Coccidia population frequently accounts for a large proportion. The suitable temperature for the survival of this species is about 12 °C–30 °C, which is used to reflect the warm water temperature environment. Another important ecological feature is that it only lives at a sea water depth of 50m in its entire life cycle. The oxygen isotopes of benthic foraminifer can reflect the nature of the bottom water, and the δ18O values in the foraminifer shells of various oceanic regions show a synchronized history of change (Lisiecki and Raymo, 2005).
Therefore, the paleoclimate cycle reflected by the abysmal oxygen isotope value is an important criterion for high-precision and large-scale stratigraphic division and comparison (Martinson et al.,1987; Shemesh et al.,1992). From the new to old, the stages corresponding to oxygen isotopes are sorted by numbers, and the stages corresponding to odd numbers 1, 5, and 7 are defined as interglacial stages that represent climate warming. The stage corresponding to even numbers 2, 4, 6, and 8 is defined as the ice age, indicating that the climate is getting colder (Berger, et al.,2016). Since 300 ka to now, the Earth has experienced eight cold and warm fluctuations with long duration.
Bioclastic carbonate minerals are sensitive carriers for TL (Johnson,1960; Noriyuki et al., 2017). Carbonate minerals grow on networks formed by organic polymers (Carmichael et al., 1994).Many biomineralized crystals are made up of inorganic minerals, but they can also contain trace elements of organic compounds that can control the process of biomineralization. The deposition of calcium carbonate from a supersaturated solution caused by the biochemical activities of microbial cells is called microbially induced calcite precipitation (MICP). Organisms may excrete one or more metabolic products (CO32- ) that react with ions (Ca2+ in the environment during MICP, leading to subsequent mineral precipitation (Esme,et al.,2021).
Ronca and Zeller (1965) found that a certain functional relationship forms between the TL of sedimentary carbonate rocks and the average monthly maximum temperature in low-latitude regions and used this relationship to study the climate and temperature changes in the Earth by measuring TL of sedimentary carbonate rocks.
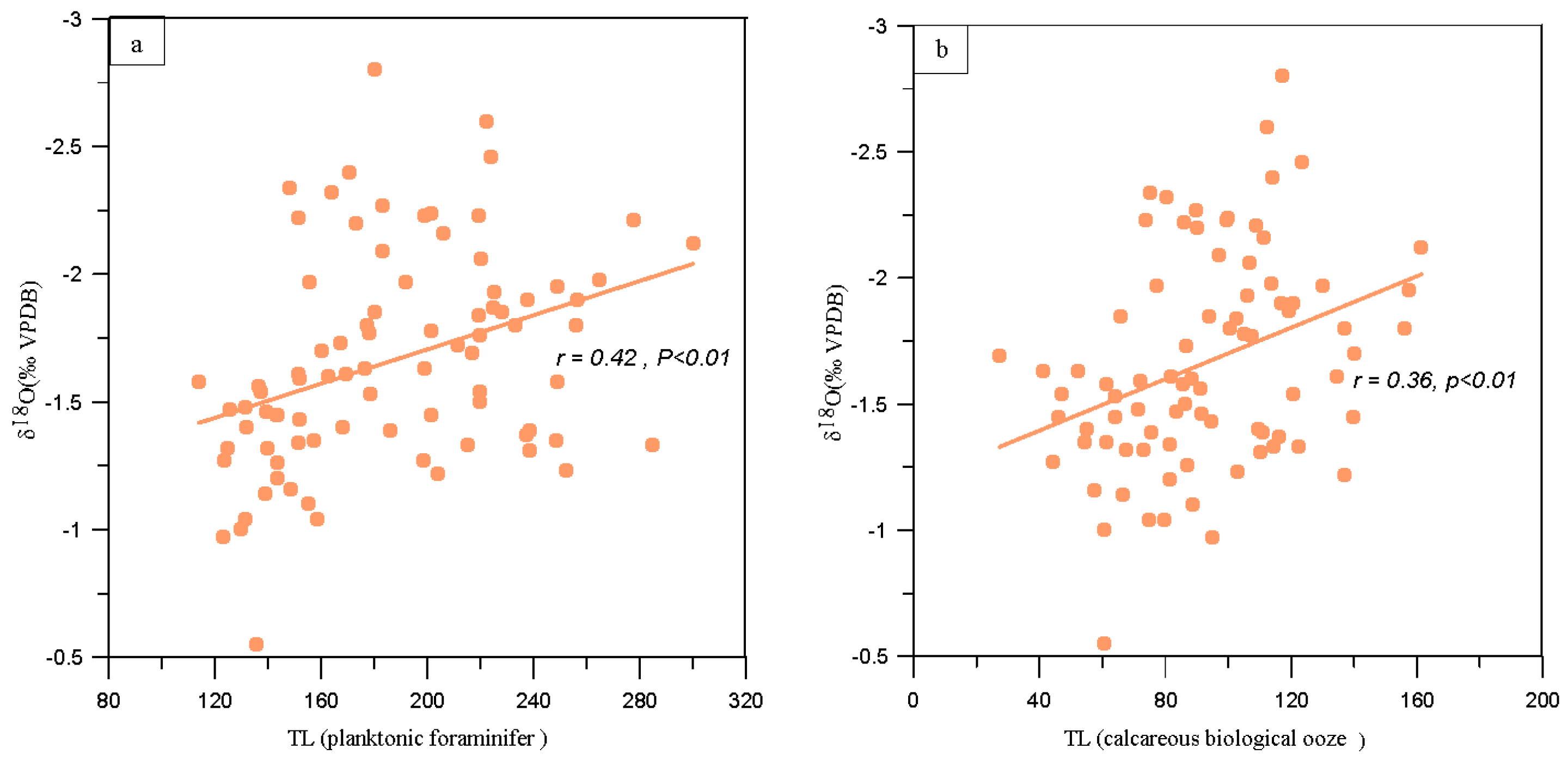
Statistical Product Service Solutions (SPSS) software correlation and significance tests are performed on TL of core MD81349 calcareous biological ooze and δ
18O, which is calculated in accordance with the statistical significance level of 99%. From the perspective of correlation coefficient, TL of foraminifer fossils extracted from the ooze is best correlated with δ
18O, and the correlation coefficient is
r=0.42 (
p<0.01). The two are significantly and positively correlated at the 99% significance level. As is common knowledge, planktonic foraminifer are strongly influenced by the environmental conditions of the near-surface sea in which they live, particularly due to their sensitivity to environmental temperature and salinity (Carla et.al.,2016). The correlation coefficient between TL of calcareous biological ooze and δ
18O is
r=0.36 (
p<0.01), as shown in
Figure 8.
5.2. Periodic variations of TL peak intensities associated with orbital forcing
The changes in global temperature and paleoclimate are mainly controlled by astronomy and tectonic factors, with global simultaneity. The strict regularity of the astronomical cycle enables the paleoclimatic cycle to have a strong temporal regularity because of the effect of the Earth’s orbital cycle, and the paleoclimate of the Earth is periodically changed. The most typical one is the Milankovitch cycle, which includes the period (100 ka) of the Earth’s orbit eccentricity (e), the inclination of the Earth’s axis (yellow intersection angle) (ε) period (54, 41 ka, etc.), and the annual difference in solar radiation (p) period (23, 19 ka, etc.). The analysis of the existence of the Milankovitch cycle in various indicators in marine sediments has become the basis for verifying whether it can reflect the changes in paleoclimate. δ18O, seawater surface temperature, CaCO3 percentage content, and C. davisiana content spectrum analysis results obtain 100, 42, 23, and 19 ka cycles (Hays et al.,1976). The frequency spectrum of δ18O, CO2 in the North Atlantic Ocean and Vostok ice core contains 107.5 ka, 41.5, 23.1, and 19.3 ka cycles, indicating that Milankovitch cycles (Ruddiman et al.,1986; Jouzel et al.,1987; Shackleton,2000) are found in high latitudes. The frequency spectrum analysis of TL found that it corresponded to the sunspot activity cycle and can be compared with 10Be, δ18O period comparison of polar ice core, such as 11-year Schwabe cycle, 22-year Hale cycle, 90-year Gleissberg cycle, and 200-year Suess cycle (Casstagnoli et al., 1984a,1984b; Casstagnoli and Bonino,1985;Casstagnoli et al., 1987,1988,1990,1997,1998).
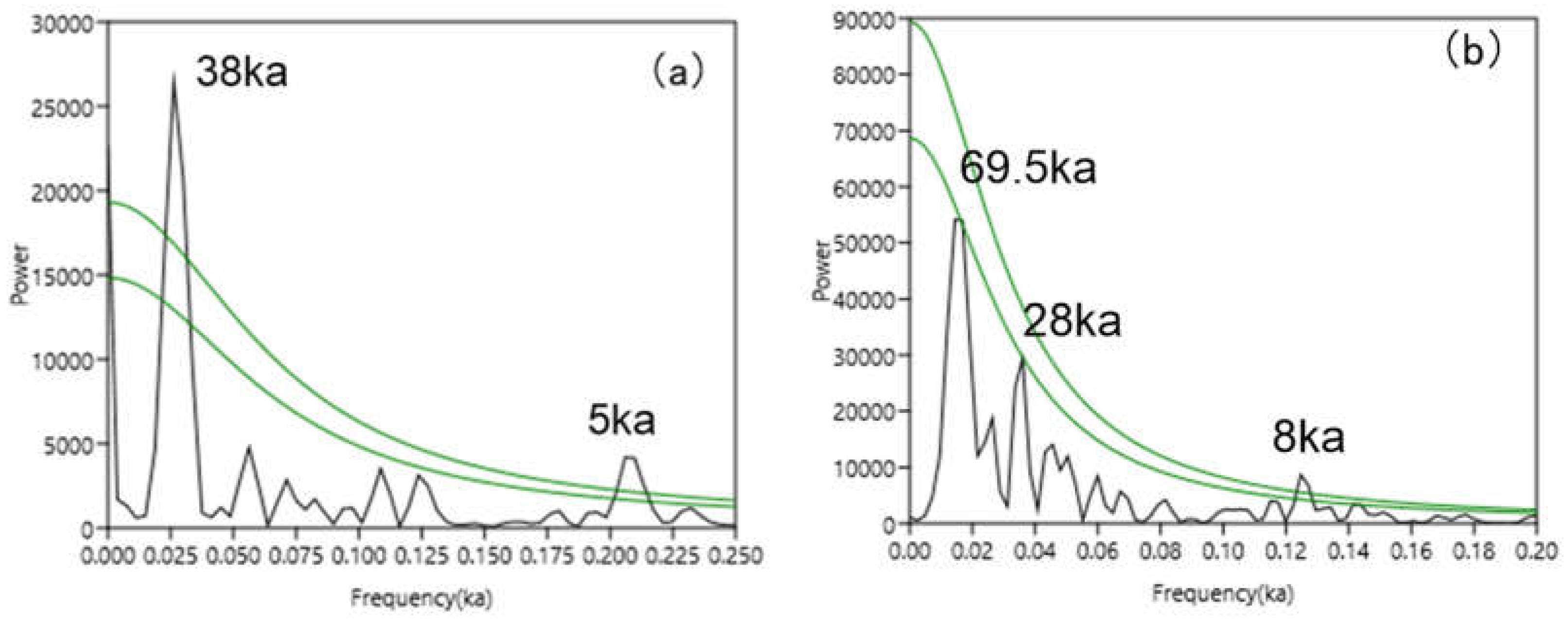
The TL- time series
of calcareous biological ooze
in MD81349 core deposited since 296ka B.P. was analyzed by spectrum analysis software (Hammer et al., 2001
). The result analysis show that: At the confidence levels above 90% , the change in TL is prominent in periods that are mainly close to 38 ka and 5 ka, as shown in
Figure 9(a). The TL cycle of 38 ka and 5 ka is stable and significant.
The age span of the core U1312B in 0–6.6 m is approximately 299 ka
. At the confidence levels above 90%,
TL spectrum analysis results show the period of 69.5 ka, 28 ka and 8 ka, as shown in
Figure 9(b). The 8 ka cycle is stable and significant.
5.3. TL of the core MD81349 to paleoclimate change response
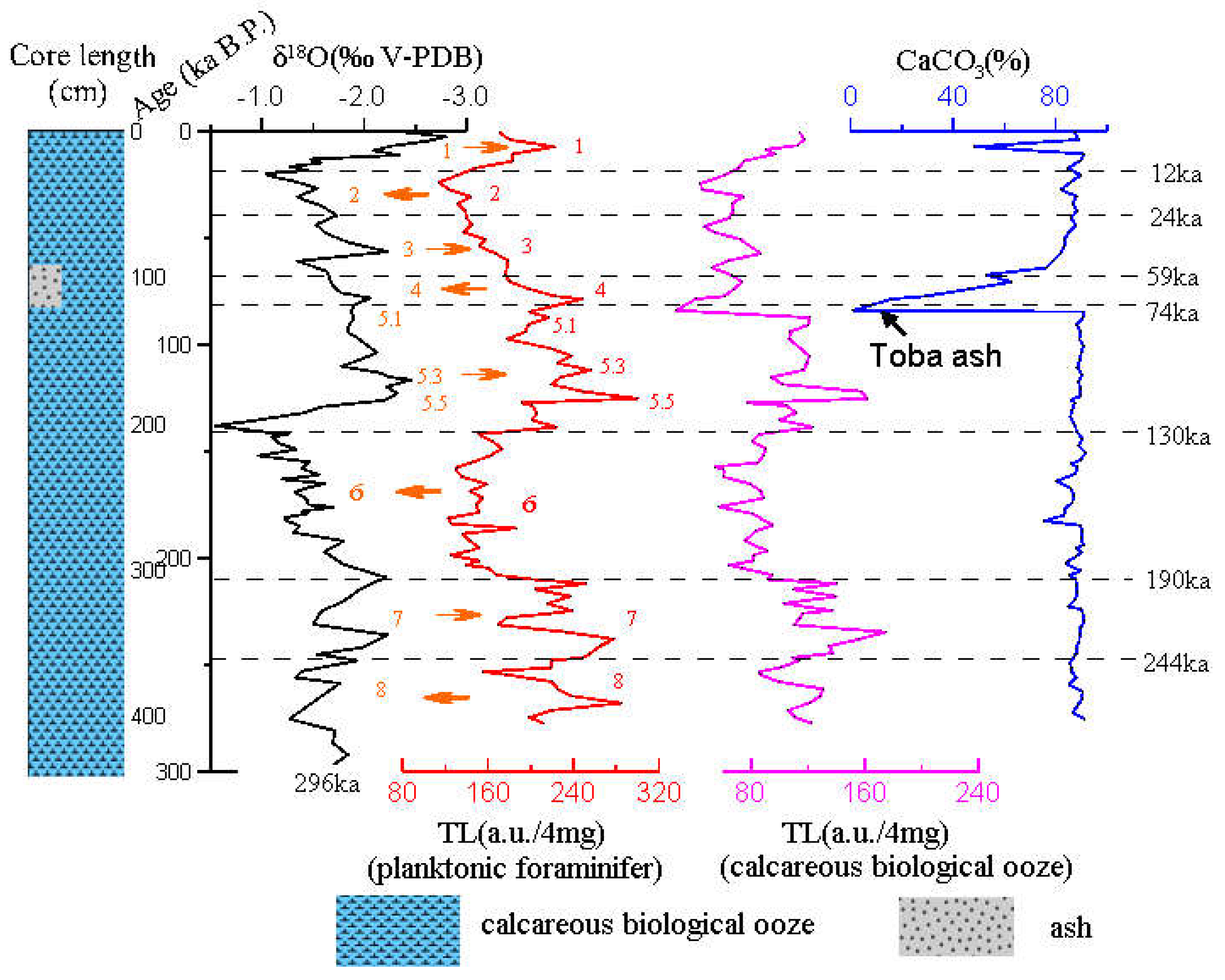
The δ
18O measured in
G.ruber is compared with the standard age (Lisiecki and Raymo, 2005), it corresponds to an age at approximately 296 ka B.P. .The core of 0–4.30 m records the evolution history of the ancient marine environment since the mid-Pleistocene and can be divided into MIS 1-8. The core was affected by abrupt tectonic–climatic events, where the most notable was the Toba volcanic ash formed around 75 ka (Rose and Chesner,1987) and evident volcanic ash deposits were formed. Thus, the core was mixed with many silicate clay components .The geological history represents the sedimentary environment from the mid-late Pleistocene to the Holocene. The measured TL data are plotted as a curve with depth and is compared with the δ
18O time curve, as shown in
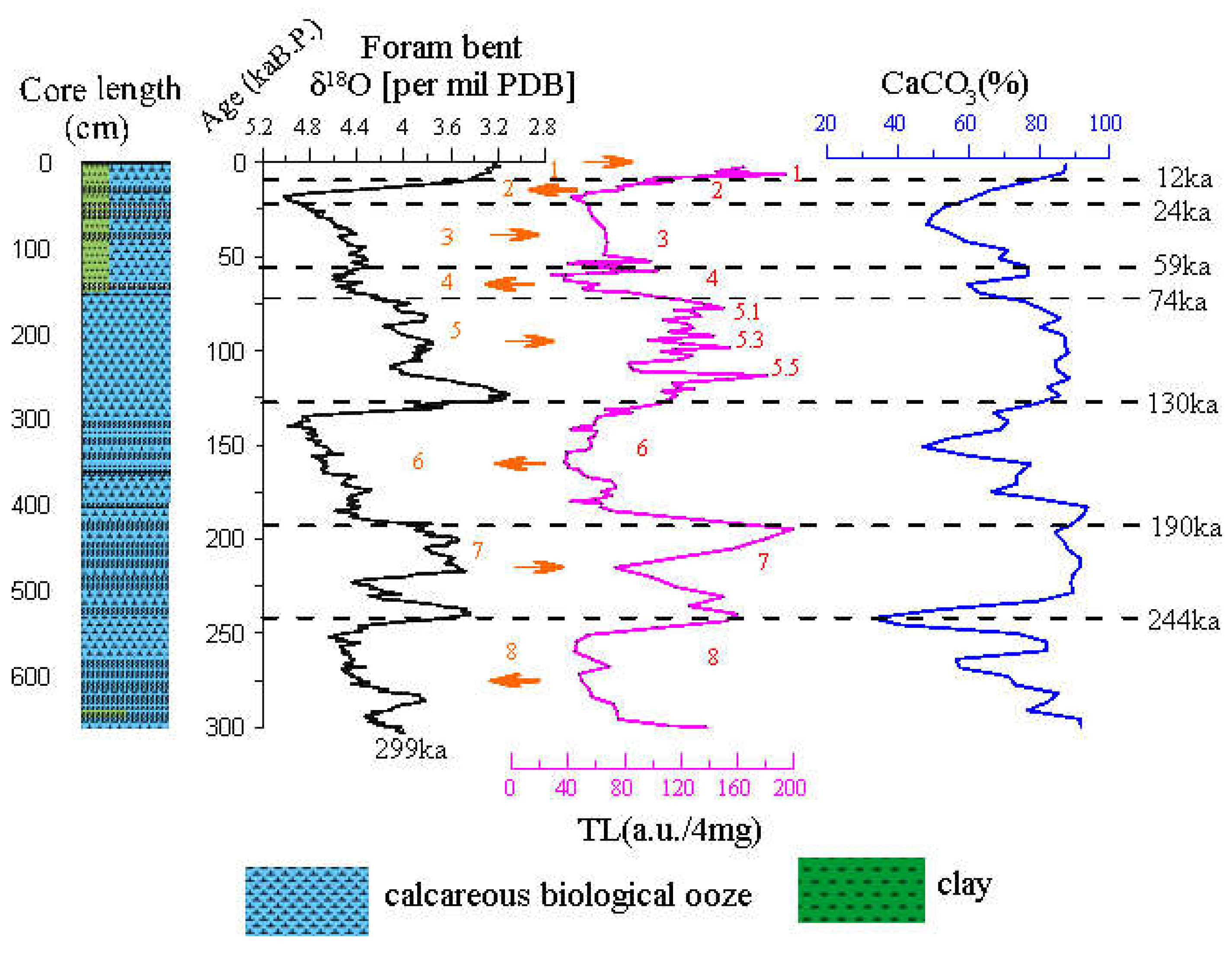
Figure 10. Comparative findings show that the ups and downs of TL profile show a significant corresponding relationship with the fluctuations of the oxygen isotope. The range of the increase in TL corresponds to the MIS 1, 5 and 7. The paleoclimate shows the characteristics of the interglacial period, as the interglacial period with the glacier cycle. The reduction range of TL corresponds to the MIS 2, 4, 6 and 8, showing the characteristics of the glacier. TL profile is particularly significant in the fifth period of climate change, which can be divided into 5.1, 5.3 and 5.5 subcycles. In addition, there is a temporal delay which needs further discussion between the beginning of the interglacial MIS 5e and the TL maximum labeled 5.5. Throughout the entire profile, TL profile can be divided into 1–8 and several sub stages from the newest to the oldest in the sequence of stratigraphic deposition. The only exception is that the early oxygen isotope in the fourth period is affected by the composition of volcanic ash (Toba ash), and TL value of calcareous biological ooze is suddenly decreased, indicating that volcanic ash experiences high temperature, and the residual TL is low.The TL of foraminifera shell was weakly affected by volcanic eruption. From the profile of CaCO
3, the volcanic ash deposited on the Toba volcanic eruption at 75 ka shows a clear manifestation that the global climate is rapidly cooled during this period.
5.4. TL of the core U1312B to paleoclimate change response
The North Atlantic ice-rafted detritus belt ( “IRD belt”)(Dansgaard et al.,1993;Stein et al.,2006) is another hotspot for research.The age of the oxygen isotope in the 0–6.6 m section
is approximately 299ka B.P.. The core U1312B deposition sequence at station 1312 of the comprehensive ocean drilling IODP306 voyage represents the past 11 Ma depositions. The climate change on the millennium scale and the million-year scale and the interaction between air and sea under various boundary conditions can be studied. The core of
0–6.6 m is selected for TL analysis in this research and the corresponding ages in MIS1–8 stages are divided, representing the sedimentary environment from the mid-late Pleistocene to the Holocene.
A sample at 4 cm spacing is chosen for TL measurement, as shown in Figure 11. The characteristics of TL profile in core U1312B are similar to those in the core MD81349, showing that the high-value area corresponds to the oxygen isotope stages MIS1, 5 and 7 and reflecting the interglacial characteristics of the glacier cycle. The low-temperature TL region corresponds to the MIS 2, 4, 6 and 8 stages of oxygen isotopes, reflecting the ice age characteristics of the glacier cycle. TL profile is particularly significant in the fifth period of climate change, which can be divided into 5.1, 5.3, and 5.5 sub-cycles.Similar to the core MD81349 , there is a temporal delay between the start of inter ice MIS 5e and the TL maximum labeled 5.5 which could be related to IRD deposition during the deglaciation. This TL profile is significantly consistent with MD81349 near the northeast Indian Ocean equator, reflecting that TL is of similar changes in different latitudes, revealing the characteristics of universal regularity. It contains a small amount of clay at 0–2.5 m and exerts minimal effect on TL.
6. Conclusions
1. The TL -age profile of calcareous biological ooze reveals eight subglacial cycles and cyclical climate changes since 299 ka B.P. Interglacial period corresponds to enhanced TL. Ice age corresponds to a reduction in TL. The TL of glacial-interglacial cycle can be compared remotely.
2.The analysis results of TL spectrum show the relationship with the changes in the Earth’s orbital parameters and the corresponding astronomical cycle. Near the equator of the Northeast Indian Ocean,it is more significant that the short cycle of 38 ka and 5 ka while it is more significant that the cycle of 8 ka at the North Atlantic since the middle Pleistocene.
3.TL fluctuations have provided us a relationship between thermoluminescence and the temperature of formation of calcareous biological ooze.
4.Calcareous biological ooze is TL carrier minerals. Spurious TL intensity of 395 °C peak from the marine calcareous biological ooze is dose independent, regardless of the irradiation dose.
5.The impurity ions (e.g. Ba2+ and Mn2+ ) doped in carbonate are acting as activators of TL,while Ti2+ is acting as suppressant.
Funding
This study was supported by National Natural Science Fund (No. 10875067 and 40306017).
Acknowledgments
The samples were provided by the School of Ocean in China University of Geosciences (Beijing). TL analysis was immensely supported by the Radiation and Environmental Laboratory in China University of Geosciences (Beijing). Graduate student Li Bo completed the thermoluminescence measurement of u1312b core sample.
Declaration of competing interest
The authors declared that they have no conflicts of interest to this work.
References
- Aitken, M.J. Thermoluminescence Dating; Academic Press Inc.: London, UK, 1985. [Google Scholar]
- Berger, A.; Crucifix, M.; Hodell, D.A.; et al. Interglacials of the last 800,000 years. Rev. Geophys. 2016, 54, 162–219. [Google Scholar] [CrossRef]
- Biswas, R. H; Herman, F; King, G.E.; Braun, J. Thermoluminescence of feldspar as a multi-thermochronometer to constrain the temporal variation of rock exhumation in the recent past. Earth Planet. Sci. Lett. 2018, 495, 56–68. [Google Scholar] [CrossRef]
- Biswas, R.H.; Frédéric, H.; Georgina, E.K.; Benjamin, L.; Ashok, K.S. Surface paleothermometry using low-temperature thermoluminescence of feldspar. Clim. Past 2020, 16, 2075–2093. [Google Scholar] [CrossRef]
- Bothner, M.H.; Johnson, N.M. Natural TL dosimetry in Late Pleistocene Pelagic sediments. J. Geophys. Res. 1969, 74, 5331–5338. [Google Scholar] [CrossRef]
- Calderon, T.; Aguilar, M.; Jaquel, F.; Coy-yll, R. TL from natural calcites. J. Phys. C: Solid State Phys. 1984, 17, 2027–2038. [Google Scholar] [CrossRef]
- Carla, T.; Silvia, A.; Sara, R.; Gianna, V.; Salvatore, M. A foraminiferl δ18O record covering the last 2,200 years. Sci. Data 2016, 3, 160042. [Google Scholar] [CrossRef]
- Carmichael, L.A.; Sanderson, D.C.W.; Riain, S.N. TL measurement of calcite shells. Radiat. Meas. 1994, 23, 455–463. [Google Scholar] [CrossRef]
- Casstagnoli, G.C.; Bonino, G.; Attolini, M.R.; Galli, M. The 11y cycle in the TL profile of sea sediments. Nuovo C. C 1984, 7, 69–73. [Google Scholar] [CrossRef]
- Casstagnoli, G.C.; Bonino, G.; Attolini, M.R.; Beer, J. Solar cycles in the last centuries in 10Be and δ18O in polar ice and in TL signals of a sea sediment. Nuovo C. C 1984, 7, 235–244. [Google Scholar] [CrossRef]
- Casstagnoli, G.C.; Bonino, G. Comparison of TL profiles in recent sea cores. Nucl. Tracks Radiat. Meas. 1985, 10, 759–761. [Google Scholar] [CrossRef]
- Casstagnoli, G.C.; Bonino, G.; Attolini, M.R.; Nanni, T. The Schwabe cycle in the TL profiles of an Ionian sea core. Nuovo C. C 1987, 10, 315–322. [Google Scholar] [CrossRef]
- Castagnoli, G.C.; Bonono, G.; Provenzale, A. On the TL profile of an Ionian sea sediment:evidence of 137,118,12.1,and 10.8y cycles in the last two millennia. Nuovo C. C 1988, 11, 1–12. [Google Scholar] [CrossRef]
- Castagnoli, G.C.; Bonono, G.; Provenzale, A.; Serio, M. On The solar origin of the TL profile of the GT14 core. Sol. Phys. 1990, 127, 357–377. [Google Scholar] [CrossRef]
- Castagnoli, G.C.; Bonono, G.; Della, M.P.; Taricco, C. Record of TL in sea sediments in the last millennia. Nuovo C. C 1997, 20, 1–8. [Google Scholar]
- Castagnoli, G.C.; Bonono, G.; Della, M.P.; Taricco, C. On the solar origin of the 200y Suess wiggles: Evidence from TL in sea sediments. Nuovo C. C 1998, 21, 237–241. [Google Scholar]
- Channell, J.E.T.; Kanamatsu, T.; Sato, T.; Stein, R.; Alvarez Zarikian, C.A.; Malone, M.J. The Expedition 303/306 Scientists. Proceedings of the Integrated Ocean Drilling Program, Volume 303/306. [CrossRef]
- Chen, G.F.; Hu, C.Y.; Li, N.; Yi, Z.H. Thermoluminescence in response to the mass extinction event in Penglaitan Section in Laibin, Guangxi. Sci. China: Earth Sci. 2013, 56, 1350–1356. [Google Scholar] [CrossRef]
- Chen; MT; Farrell, J. Planktonic foraminifer faunal variations in the northeastern Indian Ocean:A high-resolution record of the past 800,000 years from site 758. Proc. Ocean Drill. Program Sci. Results 1990, 121, 125–126. [Google Scholar]
- Christodoulides, C.; Fremlin, J.H. TL of biological materials. Nature 1971, 232, 257–258. [Google Scholar] [CrossRef]
- Dansgaard, W.; Johnsen, S.J.; Clausen, H.B.; Dahl-Jensen, D.; Gundestrup, N.S.; Hammer, C.U.; Hvidberg, C.S.; Steffensen, J.P.; SveinbjÖrnsdottir, A.E.; Jouzel, J.; Bond, G. Evidence for general instability of past climate from a 250-kyr ice-core record. Nature 1993, 364, 218–220. [Google Scholar] [CrossRef]
- Duller; GAT; Penkman, K. E.H.; Wintle, A.G. Assessing the potential for using biogenic calcites as dosemeters for luminescence dating. Radiat. Meas. 2009, 44, 429–433. [Google Scholar] [CrossRef]
- Engin, B.; Güven, O. Thermoluminescence dating of Denizli travertines from the southwestern part of Turkey. Appl. Radiat. Isot 1997, 48, 1257–1264. [Google Scholar] [CrossRef]
- Esme, I.; Dilek, T.; Mehmet, B.E.; Muhammed, H. Classification of TL features of CaCO3 with long short-term memory model. Luminescence 2021, 1–6. [Google Scholar] [CrossRef]
- Farrell, I.W.; Janecek, T.R. Late Neogene Paleoceangraphy and Paleoclimatology of the Northeast Indian Ocean (site758). Proc. Ocean Drill. Program Sci. Results 1990, 121, 297–350. [Google Scholar]
- Gerard, C.B.; Rusty, L. Iceberg dischardes into the North Atlantic on millennial time scales during the last glaciation. Science. 1995, 267, 1005–1010. [Google Scholar]
- Hammer, O.; Harper, D.; Ryan, P. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron 2001, 4, 1–9. [Google Scholar]
- Hays, J.D.; Imbrie, J.; Shackleton, N.J. Variations in the Earth orbit pacemaker of the ice age. Science 1976, 194, 1121–1132. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, J.J.; Wang, L.B.; Zhao, H.; Li, S.H. Equivalent dose estimation of calcite using isothermal thermoluminescence signals. Quat. Geochronol. 2022, 70, 1–10. [Google Scholar] [CrossRef]
- Huntley, D.J.; Johnson, H.P. TL as a potential means of dating siliceous ocean sediments. Can. J. Earth Sci. 1976, 13, 593–596. [Google Scholar] [CrossRef]
- Johnson, N.M. TL in biogenic calcium carbonate. J. Sediment. Res. 1960, 30, 305–313. [Google Scholar]
- Johnson, N.M. An empirical isothermal decay law for the TL of calcite. J. Geophys. Res. 1965, 70, 4653–4662. [Google Scholar] [CrossRef]
- Joseph, J.T.; Merlin, L.M.; Eduardo, G.Y.; Adam, C.C. Thermoluminescent microparticle thermal history sensors. Microsyst. Nanoeng. 2016, 2, 16037. [Google Scholar] [CrossRef]
- Jouzel, J.; Lorius, C.; Pettl, J.R.; Genthon, C.; Barkov; N. I.; Kotlyakov, V.M.; Petrov, V.M. Vostok ice core: a continuous isotope temperature record over the last climate cycle (160 000 years). Nature 1987, 329, 403–408. [Google Scholar] [CrossRef]
- Kononova, V.A.; Tarashchan, A.N. TL of carbonates from carbonatites. Int. Geol. Rev. 1970, 12, 272–280. [Google Scholar] [CrossRef]
- Lisiecki, L.E.; Raymo, M.E. A Pliocene-Pleistocene stack of 57 globally distributed benthic δ18O records. Paleoceanography 2005, 20, PA1003. [Google Scholar] [CrossRef]
- Liu, H.S.; Fang, N.Q.; Hou, S.L.; Chen, Y.X. Natural thermolum inescence of fossilforaminiferls as a potential proxy for deep sea temperature changes. Acta Oceanol. Sin. 2008, 27, 30–34. [Google Scholar]
- Liu, J.; Fang, N.; Wang, F.; Yang, F.; Ding, X. Features of ice-rafted debris (IRD) at IODP site U1312 and their palaeoenvironmental implications during the last 2.6 Myr. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 511, 364–378. [Google Scholar] [CrossRef]
- Martinson, D.G.; Pisias, N.G.; Hays, J.D.; John, I.; Moore, T.C.; Shackleton, N.J. Age dating and the orbital theory of the ice ages: development of a high resolution 0-300000 years chronostratigraphy. Quat. Res. 1987, 27, 1–29. [Google Scholar] [CrossRef]
- Medlin, W.L. TL of sedimentary rocks. World Pet. Cong Proc 6th Sect. 1963, 1, 63–77. [Google Scholar]
- Medlin, W.L. Color center growth curves in calcite. Journal Physical Chem. Solids 1967, 28, 1725–1733. [Google Scholar] [CrossRef]
- Nambi, K.S.V. TL investigations of natural calcite crystals of differing genesis. Thermochim. Acta 1978, 27, 61–67. [Google Scholar] [CrossRef]
- Ninagawa, K.; Takahashi, N.; Wada, T.; Yamamoto, I.; Yamashita, N.; Yamashita; Y. Thermo-luminescence measurements of a calcite shell for dating. Quat. Sci. Rev. 1988, 7, 367–371. [Google Scholar] [CrossRef]
- Noriyuki, T.; Atsushi, S.; Hiroshi, I.; Katsuyuki, H.; Takayuki, H. Thermoluminescence of coral skeletons: a high-sensitivity proxy of diagenetic alteration of aragonite. Sci. Rep. 2017, 7, 17969. [Google Scholar] [CrossRef]
- Pagonis, V.; Maniatis, Y.; Michael, C.; Bassiakos, Y. Spurious and Regererated Thermoluminescence in Calcite Powder Samples. Radiat. Meas. 1997, 27, 37–42. [Google Scholar] [CrossRef]
- Ponnusamy, V.P.; Ramasamy, V.; Anandalakshmi, K. Effect of preheating in biogenic shells-Thermostimulated luminescence and FTIR study. Indian J. Pure Appl. Phys. 2006, 44, 13–19. [Google Scholar]
- Qiu, Z.; Song, H.; Hu, C.; Wignall, P.B.; Song, H. Carbonate TL and its implication for marine productivity change during the Permian-Triassic transition. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 526, 72–79. [Google Scholar] [CrossRef]
- Ronca, L.B.; Zeller, E.J. TL as a function of climate and temperature. Am. J. Sci. 1965, 263, 416–428. [Google Scholar] [CrossRef]
- Roque, C.; Guibert, P.; Vartanian, E.; Bechtel, F.; Schvoerer, M. TL–dating of calcite: study of heated limestone fragments from Upper Paleolithic layers at Combe Saunière, Dordogne, France. Quat. Sci. Rev. 2001, 20, 935–938. [Google Scholar] [CrossRef]
- Rose, W.I.; Chesner, C.A. Dispersal of ash in the great Toba eruption,75ka. Geology 1987, 15, 913–917. [Google Scholar] [CrossRef]
- Rosenthal, Y.; Boyle, E.A.; Labeyrie, L.; Oppo, D. Glacial enrichments of authigenic Cd and U in Subantarctic sediments: A climatic control on the elements'oceanic budget? Paleoceanography 1995, 10, 395–413. [Google Scholar] [CrossRef]
- Ruddiman, W.F.; Raymo, M.; Mcintrye, A. Matayama 41 000 year cycles: North Atlantic ocean and Northern hemisphere ice sheets. Earth and Planet science letter 1986, 80, 117–129. [Google Scholar] [CrossRef]
- Shackleton, N.J. The 100,000-Year Ice-Age Cycle Identified and Found to Lag Temperature, Carbon Dioxide, and Orbital Eccentricity. Science 2000, 289, 1897–1902. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, A.; Charlis, C.D.; Fairbanks, R.G. Oxygen isotopes in biogenic silica: Global changes in ocean temperature and isotopic composition. Science 1992, 256, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.; Kanamatsu, T.; Alvarez Zarikian, C.A.; Higgins, S.; Zhai, Q. North Atlantic paleocenography: The last five million years. EOS. 87 PAGES 2006, 129, 133. [Google Scholar]
- Stirling, R.J.; Duller, G.A.T.; Roberts, H.M. Developing a single-aliquot protocol for measuring equivalent dose in biogenic carbonates. Radiat. Meas. 2012, 47, 725–731. [Google Scholar] [CrossRef]
- Sunta, C.M. A Review of TL of calcium fluoride, calcium sulphate and calcium carbonate. Radiat. Prot. Dosim. 1984, 8, 25–44. [Google Scholar] [CrossRef]
- Townsend, P.D.; Luff, B.J.; Wood, R.A. Mn2+ transitions in the TL emission spectra of calcite. Radiat. Meas 1994, 23, 433–440. [Google Scholar] [CrossRef]
- Vaz, J.E.; Zeller, E.J. TL of calcite from high gamma radiation doses. Amer. Miner. 1966, 51, 1156–1166. [Google Scholar]
- Wang, H.Y.; Li, C.; Hu, C.Y.; et al. Spurious Thermoluminescence Characteristics of the Ediacaran Doushantuo Formation (ca. 635–551 Ma) and Its Implications for Marine Dissolved Organic Carbon Reservoir. J. Earth Sci. 2015, 26, 883–892. [Google Scholar] [CrossRef]
- Wintle, A.G.; Huntley, D.J. TL dating of a deep-sea sediment core. Nature 1979, 279, 710–712. [Google Scholar] [CrossRef]
- Wintle, A.G.; Huntley, D.J. TL dating of ocean sediments. Can. J. Earth Sci. 1980, 17, 348–360. [Google Scholar] [CrossRef]
- Yassin, A.; Abdel, R. TL dosimetry using natural calcite. J. Taibah Univ. Sci. 2016, 10, 286–295. [Google Scholar]
- Zeller, E.J.; Wary, J.L.; Daniels, F. TL induced by pressure and by crystallization. J. Chem. Phys. 1955, 23, 2187. [Google Scholar] [CrossRef]
- Zeller, E.J.; Wary, J.L.; Daniels, F. Factors in age determination of carbonate sediments by, T.L. Bull. Am. Assoc. Pet. Geol. 1957, 41, 121–129. [Google Scholar]
- Zhang, J.J.; Wang, L.B. TL dating of calcite – Alpha effectiveness and measurement protocols. J. Lumin. 2020, 223, 117205–117213. [Google Scholar] [CrossRef]
Figure 1.
Location map of sampling and geography environment.
Figure 1.
Location map of sampling and geography environment.
Figure 2.
Calcareous biological ooze sample and sample splitter used in this study.
Figure 2.
Calcareous biological ooze sample and sample splitter used in this study.
Figure 3.
Core MD81349 FE-TEM imaging of foraminifer nannofossil ooze (microscopic images revealed by different imaging resolutions).
Figure 3.
Core MD81349 FE-TEM imaging of foraminifer nannofossil ooze (microscopic images revealed by different imaging resolutions).
Figure 4.
Core MD81349 foraminifer nannofossil ooze electron microscope analysis results.
Figure 4.
Core MD81349 foraminifer nannofossil ooze electron microscope analysis results.
Figure 5.
TL glow curve of planktonic foraminifer.
Figure 5.
TL glow curve of planktonic foraminifer.
Figure 6.
Comparison of TL intensity as a function of exposed dose. The red line is the sample received a 60Co gamma dose of 180 Gy curve,the green line is the natural signal curve of the same sample. The peak temperature of the calcareous biological ooze sample is approximately 395 ° C, which is similar to the "natural" signal, indicating that they may be caused by non radiation induced spurious TL, regardless of the irradiation dose (Pagonis et al.,1997).
Figure 6.
Comparison of TL intensity as a function of exposed dose. The red line is the sample received a 60Co gamma dose of 180 Gy curve,the green line is the natural signal curve of the same sample. The peak temperature of the calcareous biological ooze sample is approximately 395 ° C, which is similar to the "natural" signal, indicating that they may be caused by non radiation induced spurious TL, regardless of the irradiation dose (Pagonis et al.,1997).
Figure 7.
Cross plots between TL and 135Ba,47Ti (a) and 55Mn (b).135Ba concentrations show a significant positive correlation with TL(n = 92, r =0.32, p < 0.01) and 47Ti show a significant negative relationship (n = 92, r = −0.33, p < 0.01). 55Mn concentrations show a weakly positive relationship with TL (n = 92, r = 0.14).
Figure 7.
Cross plots between TL and 135Ba,47Ti (a) and 55Mn (b).135Ba concentrations show a significant positive correlation with TL(n = 92, r =0.32, p < 0.01) and 47Ti show a significant negative relationship (n = 92, r = −0.33, p < 0.01). 55Mn concentrations show a weakly positive relationship with TL (n = 92, r = 0.14).
Figure 8.
Cross plots between δ18O and TL. δ18O show a positive relationship with planktonic foraminifer TL(a) (n=73, r=0.42,p<0.01) and calcareous biological ooze TL(b) (n=73, r=0.36,p<0.01). Among them, 73 samples are consistent with the sampling depth of oxygen isotopes.
Figure 8.
Cross plots between δ18O and TL. δ18O show a positive relationship with planktonic foraminifer TL(a) (n=73, r=0.42,p<0.01) and calcareous biological ooze TL(b) (n=73, r=0.36,p<0.01). Among them, 73 samples are consistent with the sampling depth of oxygen isotopes.
Figure 9.
REDFIT spectrum analysis for MD81349 and U1312B TL over 300ka intervals. Numbers indicate the significant periodicities, and green lines indicate the 95% and 90% significant levels from up to down. REDFIT spectrum analysis was performed by Past 4.0 (Hammer et al., 2001). The parameters of this test are over sampling = 3, segments = 1, window = rectangle and Monte Carlo on. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.).
Figure 9.
REDFIT spectrum analysis for MD81349 and U1312B TL over 300ka intervals. Numbers indicate the significant periodicities, and green lines indicate the 95% and 90% significant levels from up to down. REDFIT spectrum analysis was performed by Past 4.0 (Hammer et al., 2001). The parameters of this test are over sampling = 3, segments = 1, window = rectangle and Monte Carlo on. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.).
Figure 10.
Core MD81349 composite records of δ18O, TL , CaCO3 vs. age based on the δ18O age model (Martinson et al.,1987). Depth of major ash layers (Toba ash) at 75 ka is labeled. The global climate is abruptly cooled during this period. Affected by volcanic ash, the TL of carbonate ooze decreased significantly, while the TL of foraminifera shell was weakly affected. Numbered intervals in the legend refer to interglacial and glacial stages. The dotted red curve shows the periodic variations in TL-peak of planktonic foraminifers samples and the magenta curve shows the periodic variations of calcareous biological ooze samples.
Figure 10.
Core MD81349 composite records of δ18O, TL , CaCO3 vs. age based on the δ18O age model (Martinson et al.,1987). Depth of major ash layers (Toba ash) at 75 ka is labeled. The global climate is abruptly cooled during this period. Affected by volcanic ash, the TL of carbonate ooze decreased significantly, while the TL of foraminifera shell was weakly affected. Numbered intervals in the legend refer to interglacial and glacial stages. The dotted red curve shows the periodic variations in TL-peak of planktonic foraminifers samples and the magenta curve shows the periodic variations of calcareous biological ooze samples.
Figure 11.
Profiles of TL ,CaCO3 of Core U1312B vs. δ18O and age based on the δ18O age model (LR04, Lisiecki and Raymo, 2005)). Numbered intervals in the legend refer to interglacial and glacial stages. CaCO3 is from Channell et al.(2006) and dotted with blue curve.
Figure 11.
Profiles of TL ,CaCO3 of Core U1312B vs. δ18O and age based on the δ18O age model (LR04, Lisiecki and Raymo, 2005)). Numbered intervals in the legend refer to interglacial and glacial stages. CaCO3 is from Channell et al.(2006) and dotted with blue curve.
Table 1.
Cores MD81349 (Liu et al., 2008) and IODP306-U1312B parameters[Channell et al.2006 ].
Table 1.
Cores MD81349 (Liu et al., 2008) and IODP306-U1312B parameters[Channell et al.2006 ].
| Regions |
Core |
Seawater depth(m) |
Core length(m) |
Latitude |
Longitude |
Age |
Core drillingTime |
| Northeast Indian Ocean |
MD81349 |
2505 |
4.30 |
1°01'0S |
89°22'0E |
296ka B.P. |
1981 |
| North Atlantic Ocean |
IODP306-U1312B(1H-2H)CC* |
3533.6 |
6.60 |
42°50.2150'N |
23°5.2652'W |
299ka B.P. |
2005 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).