Submitted:
04 June 2023
Posted:
05 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods and Materials
- Use experimental charge/discharge and heat transfer data for the A123 Systems 26650, 2.3 Ah lithium-ion battery to estimate the parameters of our SPM.
- Use the simulation results from the SPM and experimental data to train and test a DNN.
- Train and test the same DNN with a similar data set except it is derived from real time operation data for the extreme road test: Up Mount Sano in Huntsville, AL
2.1. COMSOL Simulation of the Cahn–Hilliard Single-Particle Model
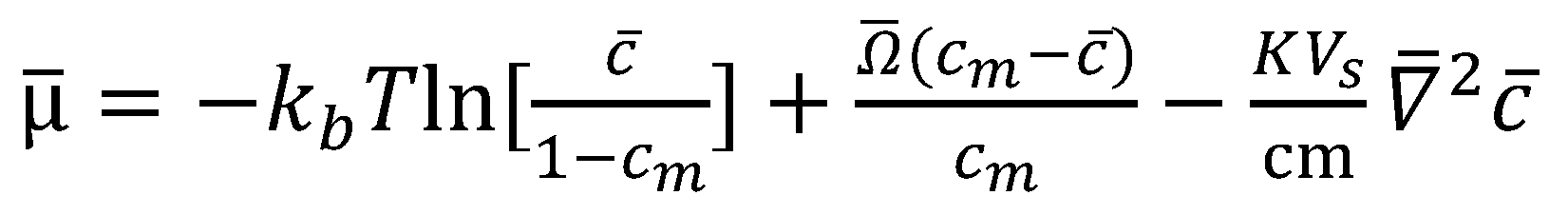
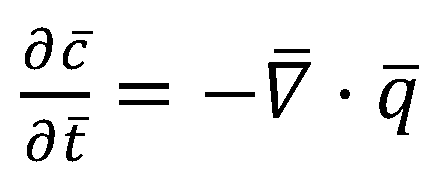
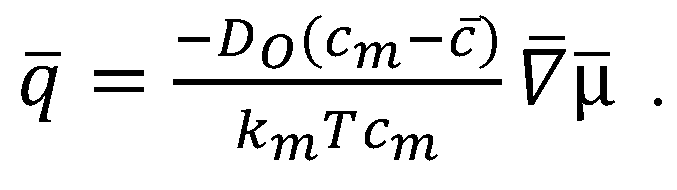
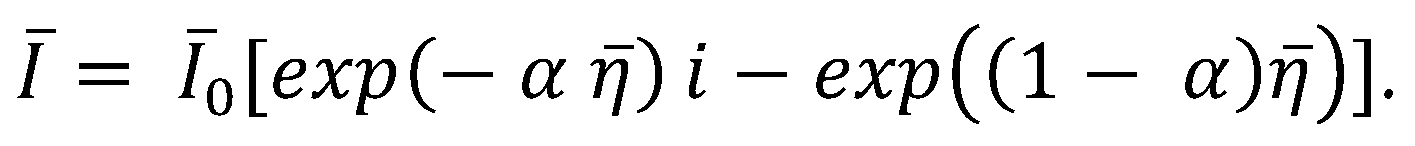
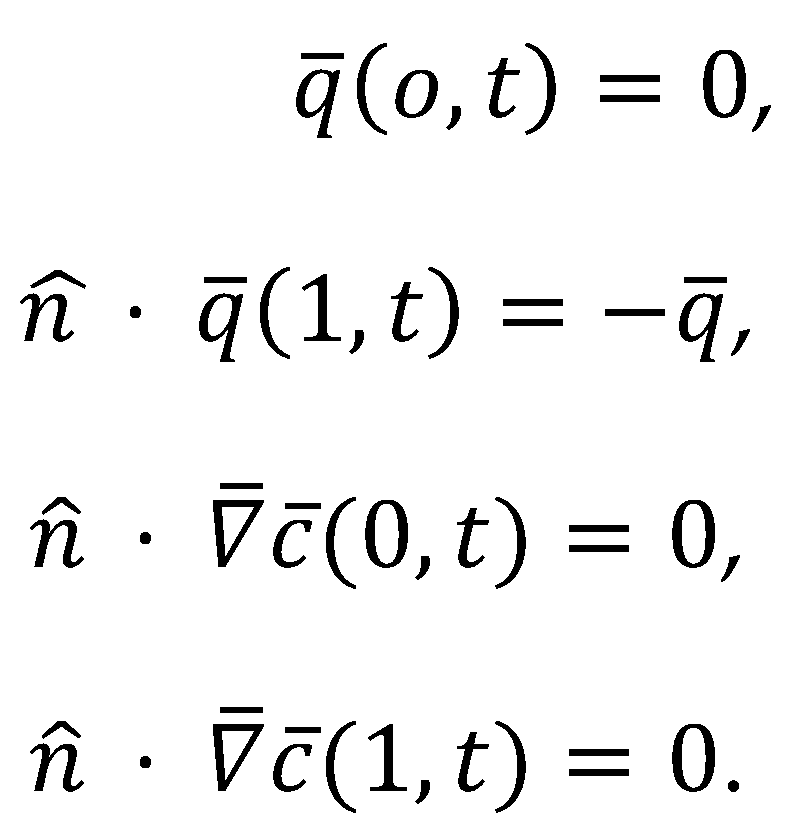
2.2. Coupling the Heat Transfer Model to the Cahn–Hilliard Equation
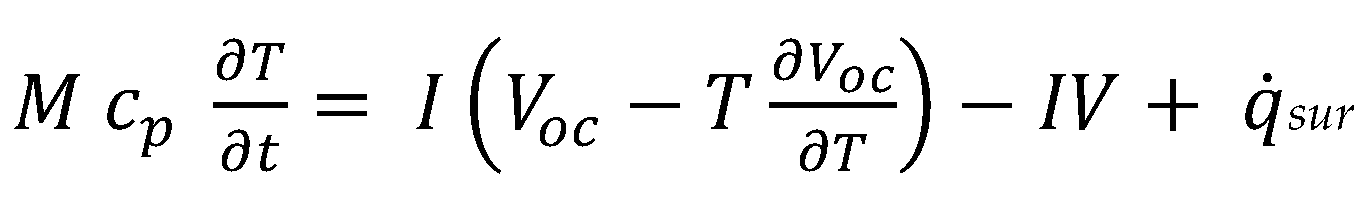
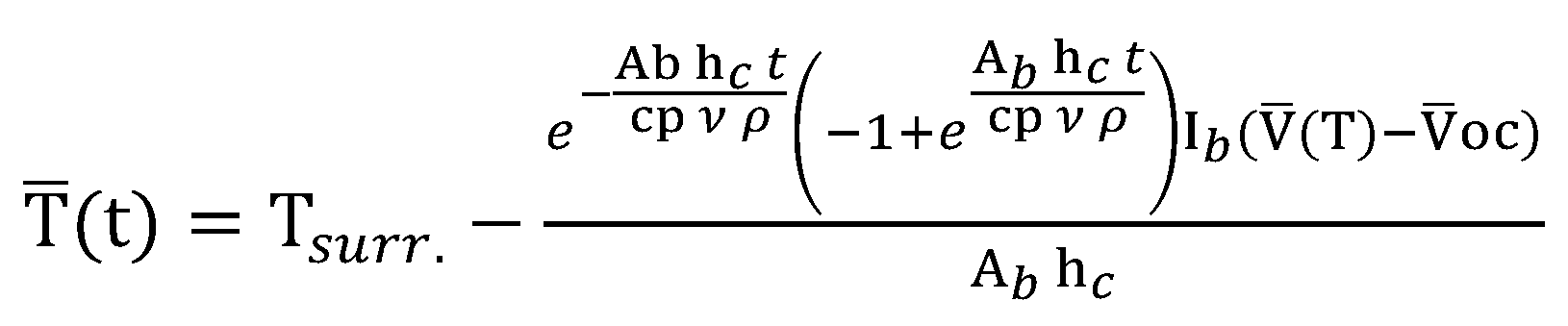
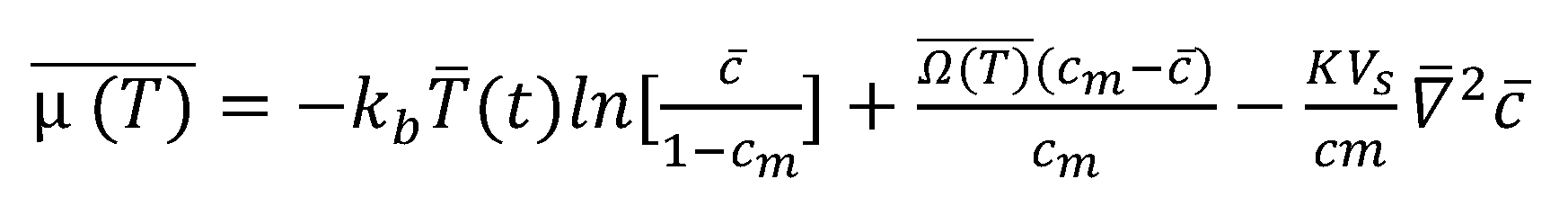
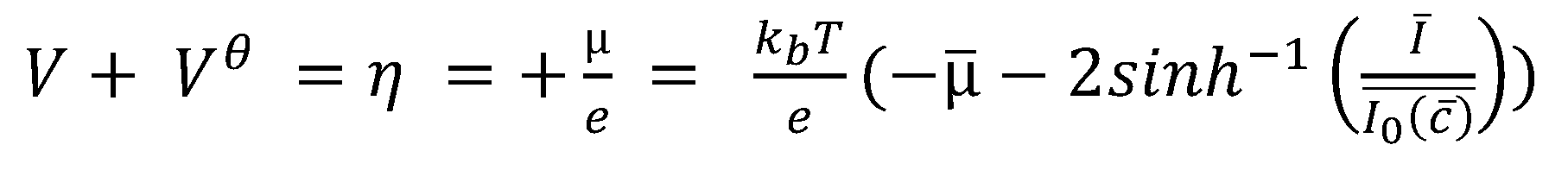
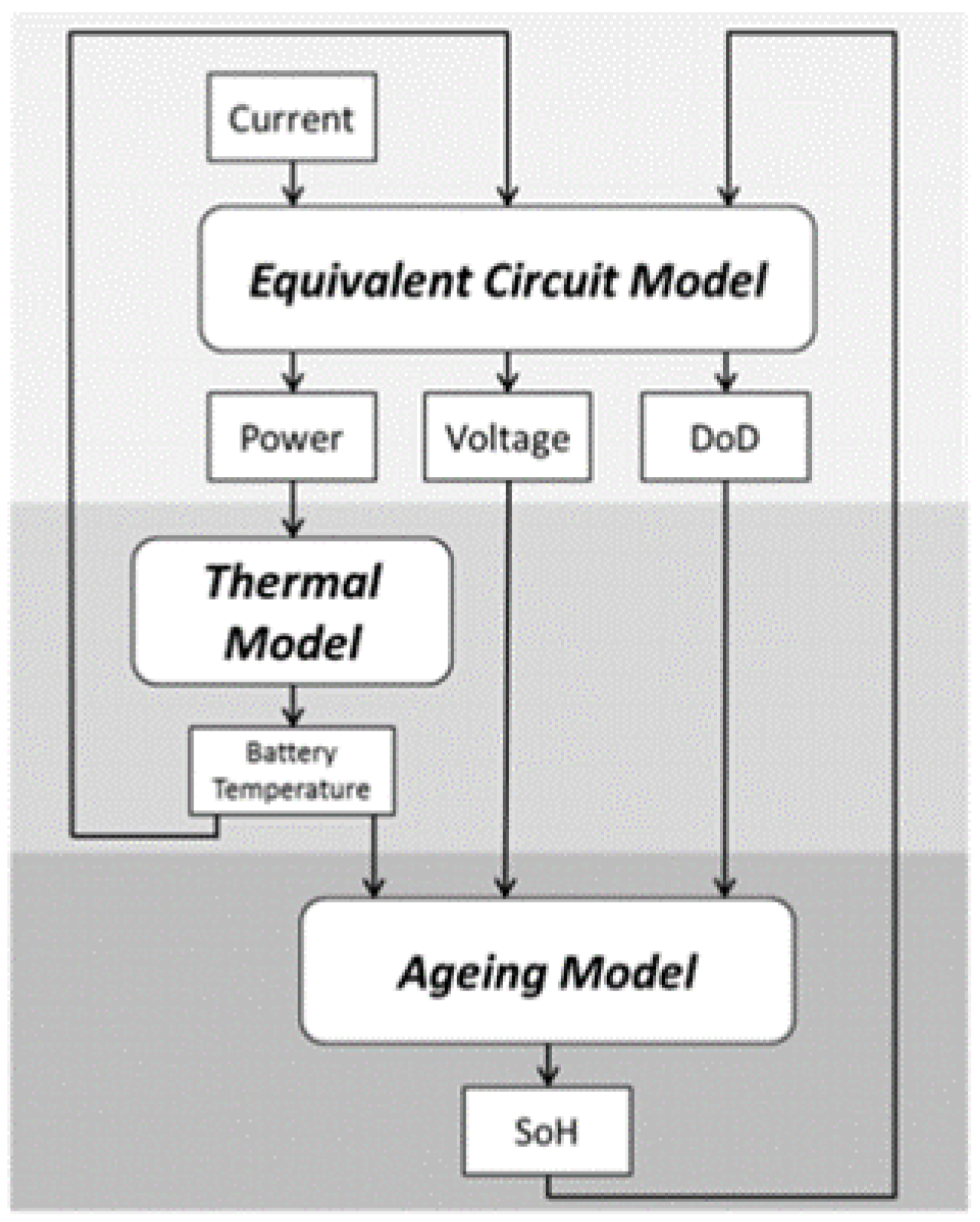
2.3. Digital Twin Configuration of BMS
2.4. Generating the Dataset from the COMSOL® Multiphysics Simulation
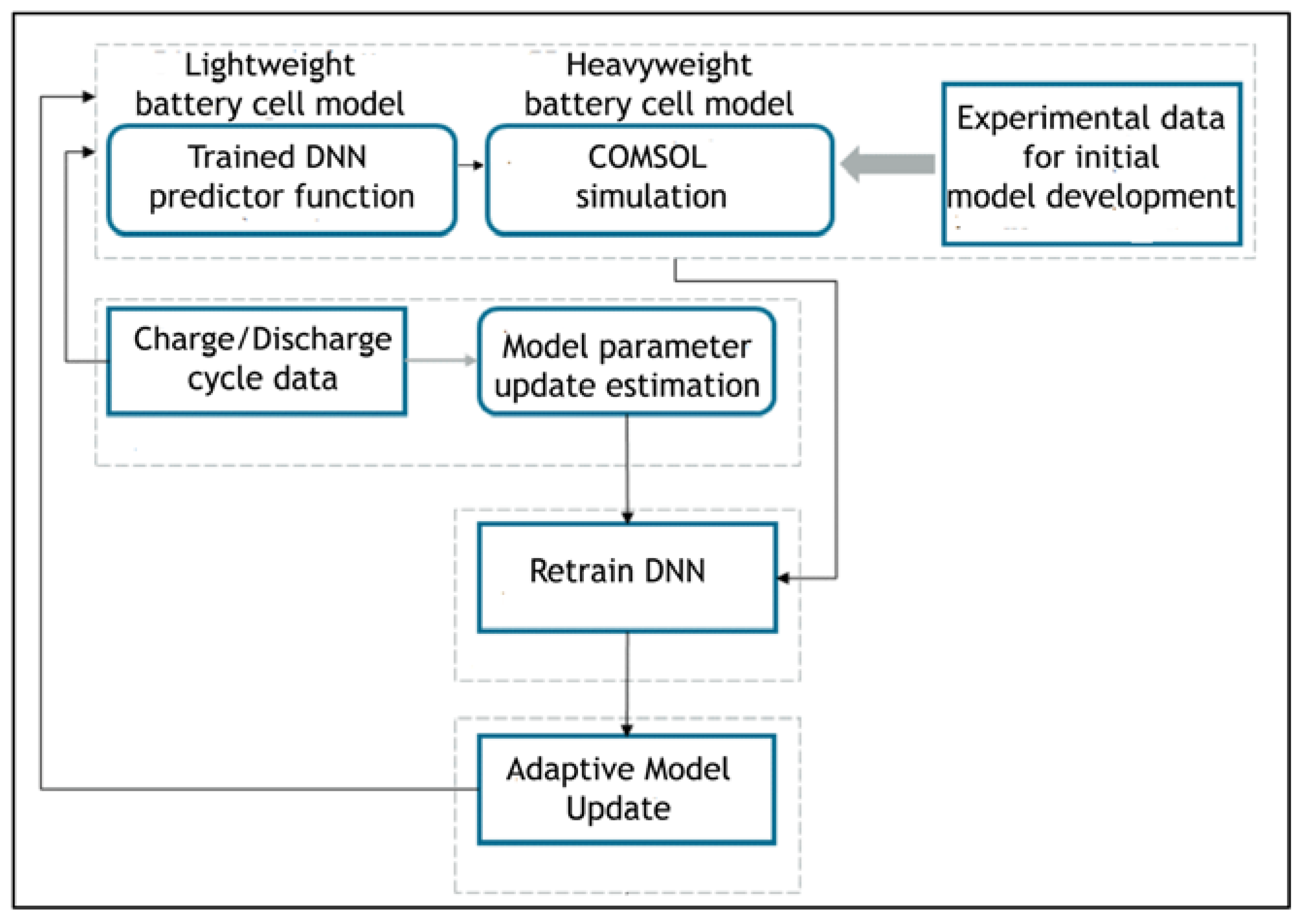
2.4. Developing the DNN
3. Results and Discussion
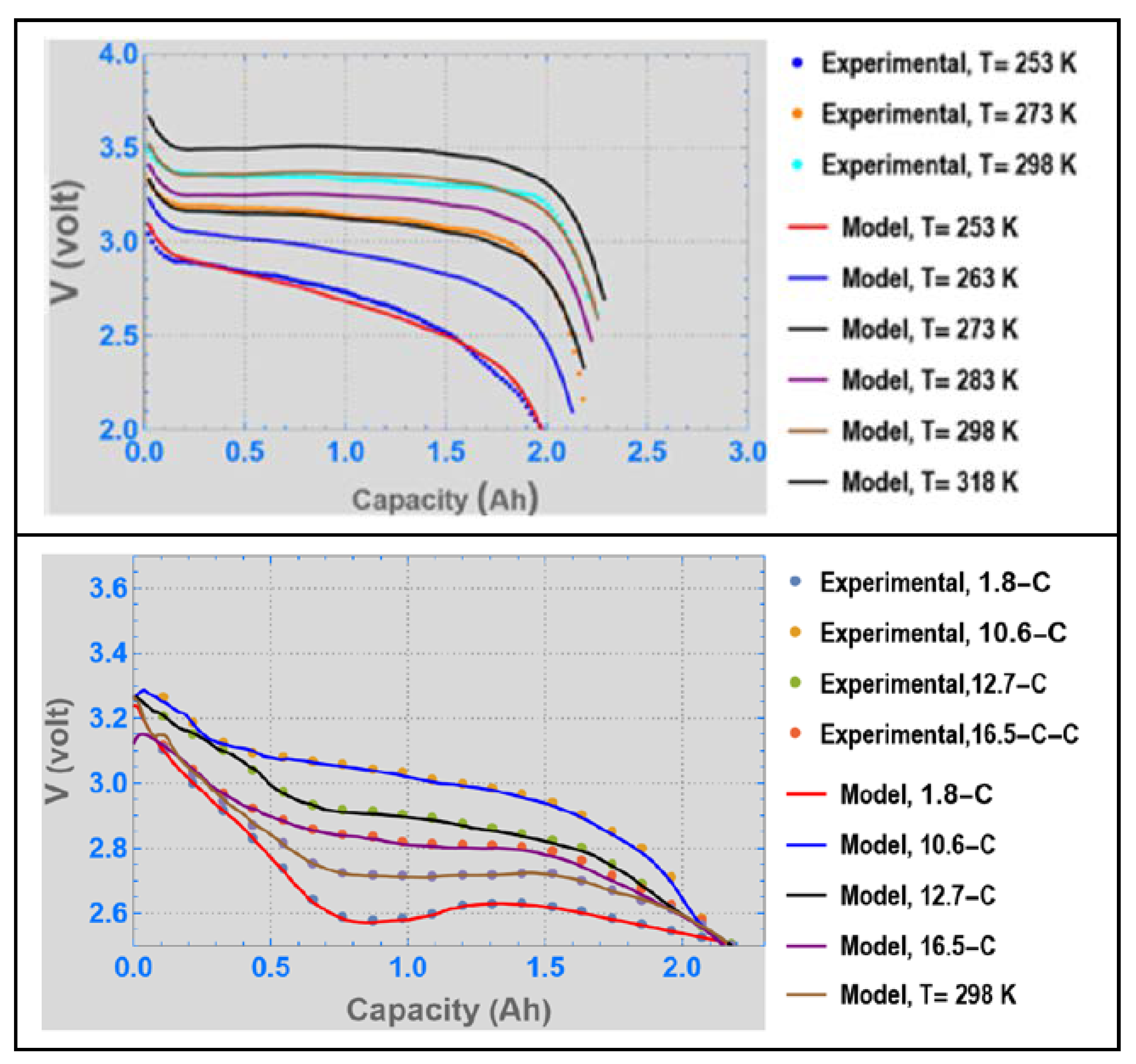

4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Ab | Battery surface area | Parameter | 4.12 × 10−2 [m2] |
| c | Concentration | Variable | [mol m−3] |
| Dimensionless concentration | Variable | ||
| cm | Maximum concentration | Parameter | 1.379 × 1028 [m−3] |
| cp | Specific heat coefficient | Parameter | 825 J [kg−1 K−1] |
| hc | Convection heat transfer coefficient | Parameter | 5.0 [W m−2 K−1] |
| I | Current | Variable | [A] |
| Dimensionless current | Variable | ||
| I0 | Current density | Parameter | 1.6 × 10−4 [A m−2] |
| Dimensionless current density | Parameter | ||
| Is | Current from online sensors | Variable | [A] |
| kb | Boltzmann constant | Constant | 3.13 × 109 [eV K−1] |
| q | Radial flux | Variable | [s−1 m−2] |
| Dimensionless radial flux | Variable | ||
| Heat loss to surroundings | Variable | [W ] | |
| Rp | Particle diameter | Parameter | 1 × 10−7 [m] |
| T | Temperature | Variable | [K[ |
| Tsurrr. | Surroundings temperature | Parameter | (253–298) [K] |
| Ts | Temperature from on-board sensor | Variable | [K] |
| V | Voltage | Variable | [V] |
| Dimensionless voltage | Variable | ||
| Single particle voltage for half-filled particle | Variable | [V] | |
| Cell voltage at 50 % SOC | Variable | [V] | |
| Vθ | Reference voltage | Constant | 3.42 [V] |
| VCH | Voltage simulated by the SPM | Variable | [V] |
| Dimensionless reference voltage | Constant | ||
| α | Electron transfer symmetry factor | Parameter | 0.5 |
| η | Activation potential | Variable | - |
| Dimensionless activation potential | Variable | ||
| Δϕeq | Near-equilibrium voltage | Variable | [V] |
| μ | Chemical potential | Variable | [eV] |
| Dimensionless chemical potential | Variable | ||
| Potential energy term from the SPM | Variable | - | |
| Potential energy estimated from Equation with 7 | Variable | - | |
| VOC | Open circuit voltage | ||
| ν | Battery volume | Parameter | 3.42 × 10−5 [m3] |
| ρ | Battery density | Parameter | 1824 [kg m−3] |
| Ω | Enthalpy of mixing | Parameter | 0.115 [eV] |
| Dimensionless enthalpy of mixing | Parameter |
References
- Tian, J.; Xiong, R.; Shen, W. Electrode aging estimation and open circuit voltage reconstruction for lithium-ion batteries. Energy Storage Mater. 2021, 37, 283–295. [Google Scholar] [CrossRef]
- Tian, J.; Xiong, R.; Shen, W.; Lu, J. State-of-charge estimation of LiFePO4 batteries in electric vehicles: A deep-learning enabled approach. Appl. Energy 2021, 291, 116812. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Dai, H.; Jiang, B.; Hu, X.; Lin, X.; Wei, X.; Pecht, M. Advanced battery management strategies for a sustainable energy future: Multilayer design concepts and research trends. Renew. Sustain. Energy Rev. 2021, 138, 110480. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, J.; Sun, Z.; Wang, L.; Xu, R.; Li, M.; Chen, Z. A comprehensive review of battery modeling and state estimation approaches for advanced battery management systems. Renew. Sustain. Energy Rev. 2020, 131, 110015. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium Batteries: Status, Prospects and Future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Fergus, J. Recent Developments in Cathode Materials for Lithium Ion Batteries. J. Power Sources 2010, 195, 939–954. [Google Scholar] [CrossRef]
- Shim, J. Electrochemical analysis for cycle performance and capacity fading of a lithium-ion battery cycled at elevated temperature. J. Power Sources 2002, 112, 222–230. [Google Scholar] [CrossRef]
- Ning, G.; Popov, B. Cycle Life Modeling of Lithium-Ion Batteries. J. Electrochem. Soc. 2004, 151, A1584. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Wang, Y.; Dan, D.; Zhang, Y.; Qian, Y.; Panchal, S.; Fowler, M.; Li, W.; Tran, M.; Xie, Y. A novel heat dissipation structure based on flat heat pipe for battery thermal management system. Int. J. Energy Res. 2022, 46, 15961–15980. [Google Scholar] [CrossRef]
- Yalçın, S.; Panchal, S.; Herdem, M.-S. A CNN-ABC model for estimation and optimization of heat generation rate and voltage distributions of lithium-ion batteries for electric vehicles. Int. J. Heat Mass Transf. 2022, 199, 123486. [Google Scholar] [CrossRef]
- Mevawalla, A.; Shabeer, Y.; Tran, M.K.; Panchal, S.; Fowler, M.; Fraser, R. Thermal Modelling Utilizing Multiple Experimentally Measurable Parameters. Batteries 2022, 8, 147. [Google Scholar] [CrossRef]
- Guo, M.; Sikha, G.; White, R. Single-Particle Model for A Lithium-Ion Cell: Thermal Behavior. Electrochem. Soc. 2011, 158, A122, Erratum in: J. Electrochem. Soc. 2011, 158, S11. [Google Scholar] [CrossRef]
- Cai, L.; White, R. Mathematical Modeling of a Lithium Ion Battery with Thermal Effects in COMSOL® Inc. Multiphysics (MP) Software. J. Power Sources 2011, 196, 5985–5989. [Google Scholar] [CrossRef]
- Krewer, U.; Roder, F.; Harinath, E.; Braatz, R.; Bedurftig, B.; Findeisen, R. Review—Dynamic Models of Li-Ion Batteries for Diagnosis and Operation: A Review and Perspective. J. Electrochem. Soc. 2018, 165, A3656–A3673. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Z.; Xiong, B.; Vilathgamuwa, D. Adaptive ensemble-based electrochemical–thermal degradation state estimation of lithium-ion batteries. IEEE Trans. Ind. Electron. 2021, 69, 6984–6996. [Google Scholar] [CrossRef]
- Li, Y.; Karunathilake, D.; Vilathgamuwa, D.M.; Mishra, Y.; Farrell, T.W.; Zou, C. Model Order Reduction Techniques for Physics-Based Lithium-Ion Battery Management. IEEE Ind. Electron. Mag. 2022, 16, 36–51. [Google Scholar] [CrossRef]
- Sakar, S.; Halim, Z.; El-Halwagi, M.; Khan, F. Electrochemical models: Methods and applications for safer lithium-ion battery operation. J. Electrochem. Soc. 2022, 169, 100501. [Google Scholar] [CrossRef]
- Yannliaw, B. Modeling of lithium ion cells? A simple equivalent-circuit model approach. Solid State Ion. 2004, 175, 835–839. [Google Scholar] [CrossRef]
- Xie, Y.; Li, W.; Hu, X.; Tran, M.K.; Panchal, S.; Fowler, M.; Liu, K. Co-estimation of SOC and three dimensional SOT for lithium-ion batteries based on distributed spatial-temporal online correction. IEEE Trans. Ind. Electron. 2022, 70, 5937–5948. [Google Scholar] [CrossRef]
- Sekhar, R.; Shah, P.; Panchal, S.; Fowler, M.; Fraser, R. Distance to empty soft sensor for ford escape electric vehicle. Results Control. Optim. 2022, 9, 100168. [Google Scholar] [CrossRef]
- Verbrugge, M. Adaptive, multi-parameter battery state estimator with optimized time-weighting factors. J. Appl. Electrochem. 2007, 37, 605–616. [Google Scholar] [CrossRef]
- Hu, Y.; Yurkovich, S.; Guezennec, Y.; Yurkovich, B. Electro-thermal battery model identification for automotive applications. J. Power Sources 2011, 196, 449–457. [Google Scholar] [CrossRef]
- Perez, H.E.; Siegel, J.B.; Lin, X.; Stefanopoulou, A.G.; Ding, Y.; Castanier, M.P. Parameterization and Validation of an Integrated Electro-Thermal Cylindrical LFP Battery Model. In Renewable Energy Systems; Robotics; Robust Control; Single Track Vehicle Dynamics and Control; Stochastic Models, Control and Algorithms in Robotics; Structure Dynamics and Smart Structures; ASME 2012 5th Annual Dynamic Systems and Control Conference: Fort Lauderdale, Florida, USA, 2012; Volume 3. [Google Scholar]
- Torabi, F.; Ahmadi, P. Simulation Of Battery Systems; Academic Press: London, UK, 2019. [Google Scholar]
- Barreras, J.; Schaltz, E.; Andreasen, S. Datasheet-Based Modeling of Li-Ion Batteries. In Proceedings of the 2012 IEEE Vehicle Power and Propulsion Conference, Seoul, Republic of Korea, 9–12 October 2012. [Google Scholar]
- Zou, Z.; Xu, J.; Mi, C.; Cao, B.; Chen, Z. Evaluation of Model Based State of Charge Estimation Methods for Lithium-Ion Batteries. Energies 2014, 7, 5065–5082. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, J.; He, H.; Yu, Y.; Marco, J. Embedded distributed temperature sensing enabled multistate joint observation of smart lithium-ion battery. IEEE Trans. Ind. Electron. 2022, 70, 555–565. [Google Scholar] [CrossRef]
- Hu, X.; Sun, F.; Zou, Y. Estimation of state of charge of a lithium-ion battery pack for electric vehicles using an adaptive Luenberger observer. Energies 2010, 3, 1586–1603. [Google Scholar] [CrossRef]
- Kim, I. The novel state of charge estimation method for lithium battery using sliding mode observer. J. Power Sources 2006, 163, 584–590. [Google Scholar] [CrossRef]
- Sun, F.; Xiong, R.; He, H.; Li, W.; Aussems, J. Model-based dynamic multi-parameter method for peak power estimation of lithium-ion batteries. Appl. Energy 2012, 96, 378–386. [Google Scholar] [CrossRef]
- Xu, J.; Mi, C.; Cao, B.; Deng, J.; Chen, Z.; Li, S. The state of charge estimation of lithium-ion batteries based on a proportional integral observer. IEEE Trans. Veh. Technol. 2014, 63, 1614–1621. [Google Scholar]
- Wei, Z.; Dong, G.; Zhang, X.; Pou, J.; Quan, Z.; He, H. Noise-immune model identification and state-of-charge estimation for lithium-ion battery using bilinear parameterization. IEEE Trans. Ind. Electron. 2021, 68, 312–323. [Google Scholar] [CrossRef]
- Dong, G.; Wei, J.; Zhang, C.; Chen, Z. Online state of charge estimation and open circuit voltage hysteresis modeling of LiFePO4 battery using invariant imbedding method. Appl. Energy 2016, 162, 163–171. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, R.; He, H.; Shen, W. A lithium-ion battery pack state of charge and state of energy estimation algorithms using a hardware-in the-loop validation. IEEE Trans. Power Electron. 2017, 32, 4421–4431. [Google Scholar] [CrossRef]
- Padhi, A.K. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188. [Google Scholar] [CrossRef]
- Yamada, A.; Chung, S.C.; Hinokuma, K. ChemInform Abstract: Optimized LiFePO4 for Lithium Battery Cathodes. ChemInform 2010, 32. [Google Scholar]
- Huang, H.; Yin, S.; Nazar, L.F. Approaching Theoretical Capacity of LiFePO4 at Room Temperature at High Rates. Electrochem. Solid-State Lett. 2001, 4, A170. [Google Scholar] [CrossRef]
- Ravet, N.; Chouinard, Y.; Magnan, J.; Besner, S.; Gauthier, M.; Armand, M. Electroactivity of natural and synthetic triphylite. J. Power Sources 2001, 97–98, 503–507. [Google Scholar] [CrossRef]
- Chung, S.; Bloking, J.T.; Chiang, Y. Electronically conductive phospho-olivines as lithium storage electrodes. Nat. Mater. 2002, 1, 123–128. [Google Scholar] [CrossRef]
- Johns, P.A.; Roberts, M.R.; Wakizaka, Y.; Sanders, J.H.; Owen, J.R. How the electrolyte limits fast discharge in nanostructured batteries and supercapacitors. Electrochem. Commun. 2009, 11, 2089–2092. [Google Scholar] [CrossRef]
- Newman, J.; Tiedemann, W. Porous-Electrode Theory with Battery Applications. AIChE J. 1975, 21, 25–41. [Google Scholar] [CrossRef]
- Doyle, M. Modeling of Galvanostatic Charge and Discharge of the Lithium/Polymer/Insertion Cell. J. Electrochem. Soc. 1993, 140, 1526. [Google Scholar] [CrossRef]
- Bazant, M. Theory of Chemical Kinetics and Charge Transfer Based on Nonequilibrium Thermodynamics. Acc. Chem. Res. 2013, 46, 1144–1160. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Ceder; Bazant, M. Intercalation dynamics in rechargeable battery materials: General theory and phase-transformation waves in LiFePO4. Electrochim. Acta 2008, 53, 7599–7613. [Google Scholar] [CrossRef]
- Painter, R.; Embry, I.; Sharpe, L.; Hargrove, S.K. A Reduced Order Thermal Model for Lithium Ion Batteries Derived from the Cahn-Hilliard Equation. In Proceedings of the 2020 COMSOL® Conference, Boston, MA, USA, 7–8 October 2020. [Google Scholar]
- Bai, P.; Cogswell, D.; Bazant, M.Z. Suppression of phase separation in LiFePO4 nanoparticles during battery discharge. Nano Lett. 2011, 11, 4890–4896. [Google Scholar] [CrossRef]
- Malik, R.; Abdellahi, A.; Ceder, G. A Critical Review of the Li Insertion Mechanisms in LiFePO4 Electrodes. J. Electrochem. Soc. 2013, 160, A3179–A3197. [Google Scholar] [CrossRef]
- Chen, L. Phase-Field Models for Microstructure Evolution, Annu. Rev. Mater. Res. 2002, 32, 113–140. [Google Scholar] [CrossRef]
- Yuan, C.; Gao, X.; Jia, Y.; Zhang, W.; Wu, Q.; Xu, J. Coupled crack propagation and dendrite growth in solid electrolyte of all-solid-state battery. Nano Energy 2021, 86, 106057. [Google Scholar] [CrossRef]
- Yuan, C.; Lu, W.; Xu, J. Unlocking the electrochemical–mechanical coupling behaviors of dendrite growth and crack propagation in all-solid-state batteries. Adv. Energy Mater. 2021, 11, 2101807. [Google Scholar] [CrossRef]
- Zeng, Y.; Bazant, M. Cahn-Hilliard Reaction Model for Isotropic Li-Ion Battery Particles. MRS Proc. 2013, 1542, 0201. [Google Scholar] [CrossRef]
- Painter, R.; Berryhill, B.; Sharpe, L.; Hargrove, S.K. A Single Particle Thermal Model for Lithium Ion Batteries. In Proceedings of the 2012 COMSOL® Conference, Boston, MA, USA, 3–5 October 2020. [Google Scholar]
- Painter, R.; Sharpe, L.; Hargrove, S.K. A Phase Field Model for Lithium Batteries. In Proceedings of the 2012 COMSOL® Conference, Boston, MA, USA, 3–5 October 2020. [Google Scholar]
- Sullivan, U.V. Published 2019. Available online: http://www.sullivanuv.com/wp-content/uploads/2014/06/A123-Cell-Datasheet-2008-08.pdf (accessed on 1 December 2019).
- Maleki, H. Thermal Properties of Lithium-Ion Battery and Components. J Electrochem Soc. 1999, 146, 947. [Google Scholar] [CrossRef]
- Chen, Y. Thermal Analysis of Lithium-Ion Batteries. J Electrochem Soc. 1996, 143, 2708. [Google Scholar] [CrossRef]
- Onda, K.; Ohshima, T.; Nakayama, M.; Fukuda, K.; Araki, T. Thermal behavior of small lithium-ion battery during rapid charge and discharge cycles. J Power Sources. 2006, 158, 535–542. [Google Scholar] [CrossRef]
- Thomas, K.; Newman, J. Thermal Modeling of Porous Insertion Electrodes. J. Electrochem. Soc. 2003, 150, A176. [Google Scholar] [CrossRef]
- Forgez, C.; Vinh Do, D.; Friedrich, G.; Morcrette, M.; Delacourt, C. Thermal modeling of a cylindrical LiFePO4/graphite lithium-ion battery. J. Power Sources 2010, 195, 2961–2968. [Google Scholar] [CrossRef]
- Youngki, K. Power Capability Estimation Accounting for Thermal and Electrical Constraints for Lithium-Ion Batteries. Ph.D. Thesis, University of Michigan, Midsize city, MI, USA, 2014. [Google Scholar]
- Singh, S.; Weeber, M.; Birke, K. Implementation of digital twin: Approach, Functionaliyies and Benefits, Batteries; MDPI, 2021. [Google Scholar]
- Available online: https://docplayer.net/20772679-Managing-a123-cells-with-fma-cell-balancing-technologies.html (accessed on 1 March 2022).
- Pivelait, B.; Rentel, C.; Plett, G.; Marcel, M.; Carmen, D. An advanced battery management system for lithium-ion batteries, 2011 NDIA Ground Vehicle Systems Engineering and Technology Symposium; Power and Mobility (P&M) Mini-Symposium August 9-11 Dearborn Michigan.
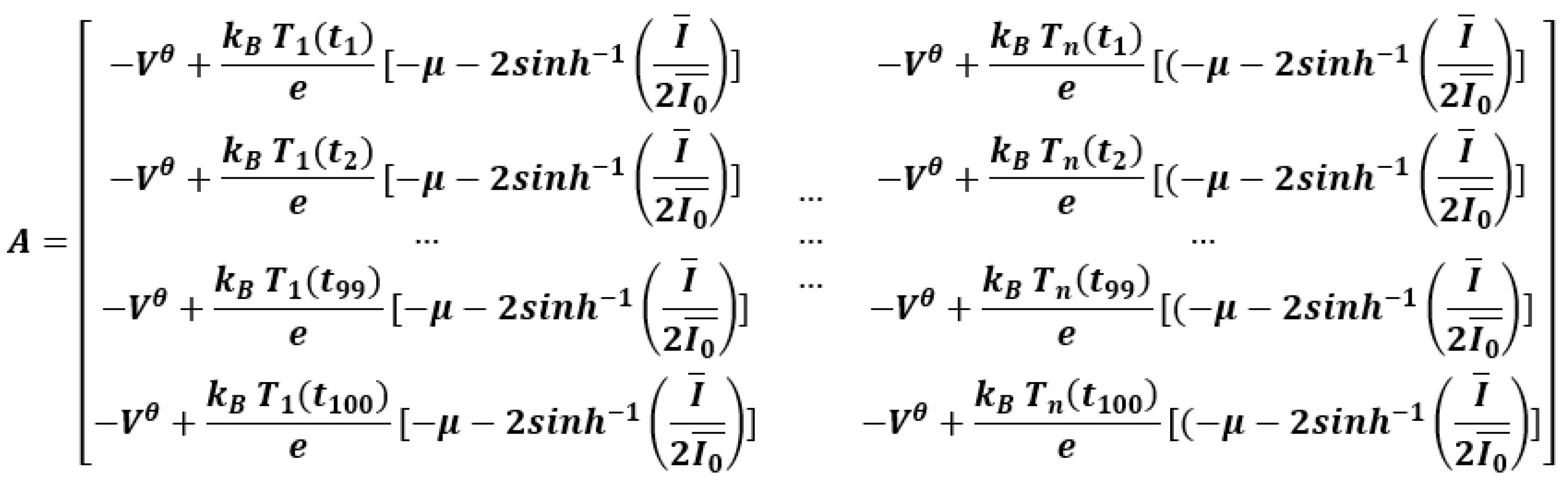
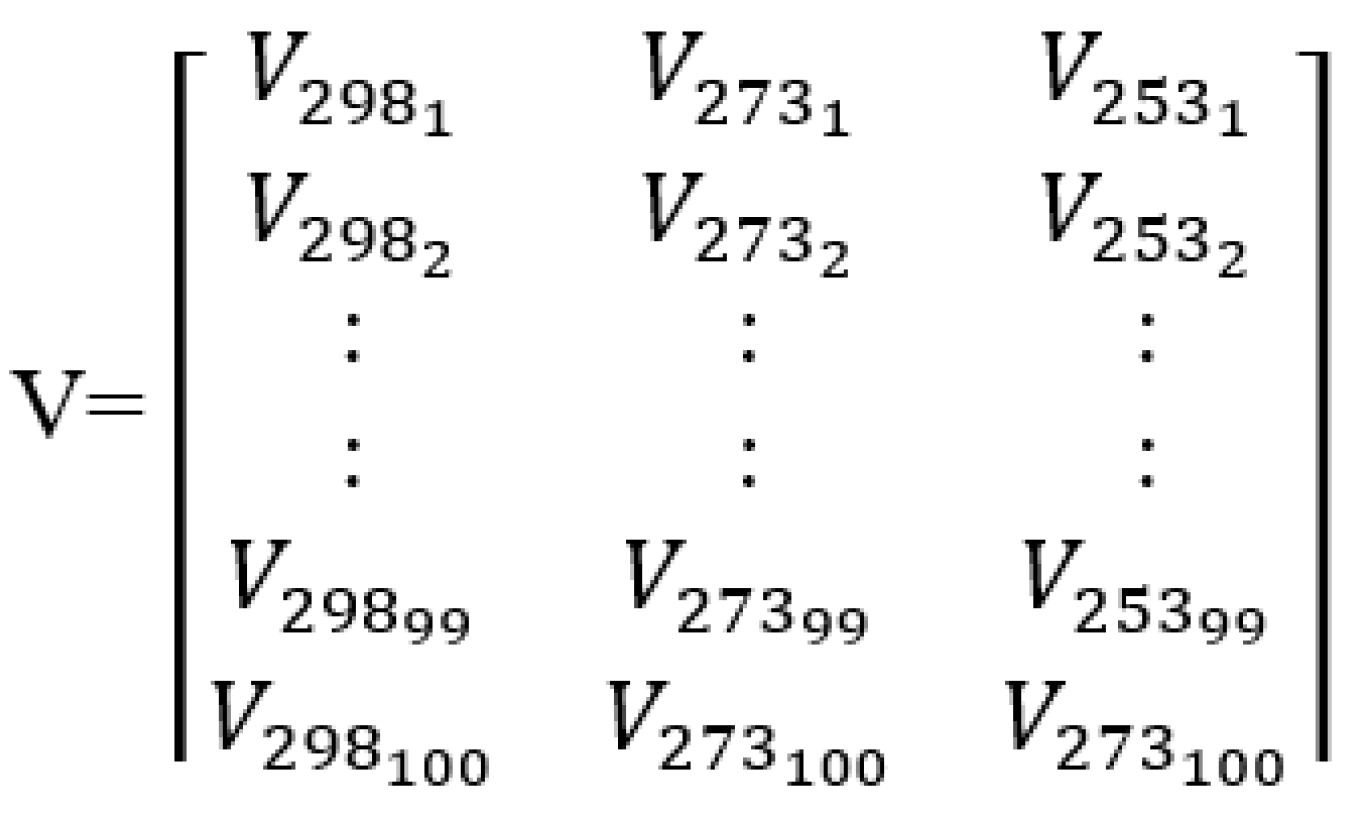


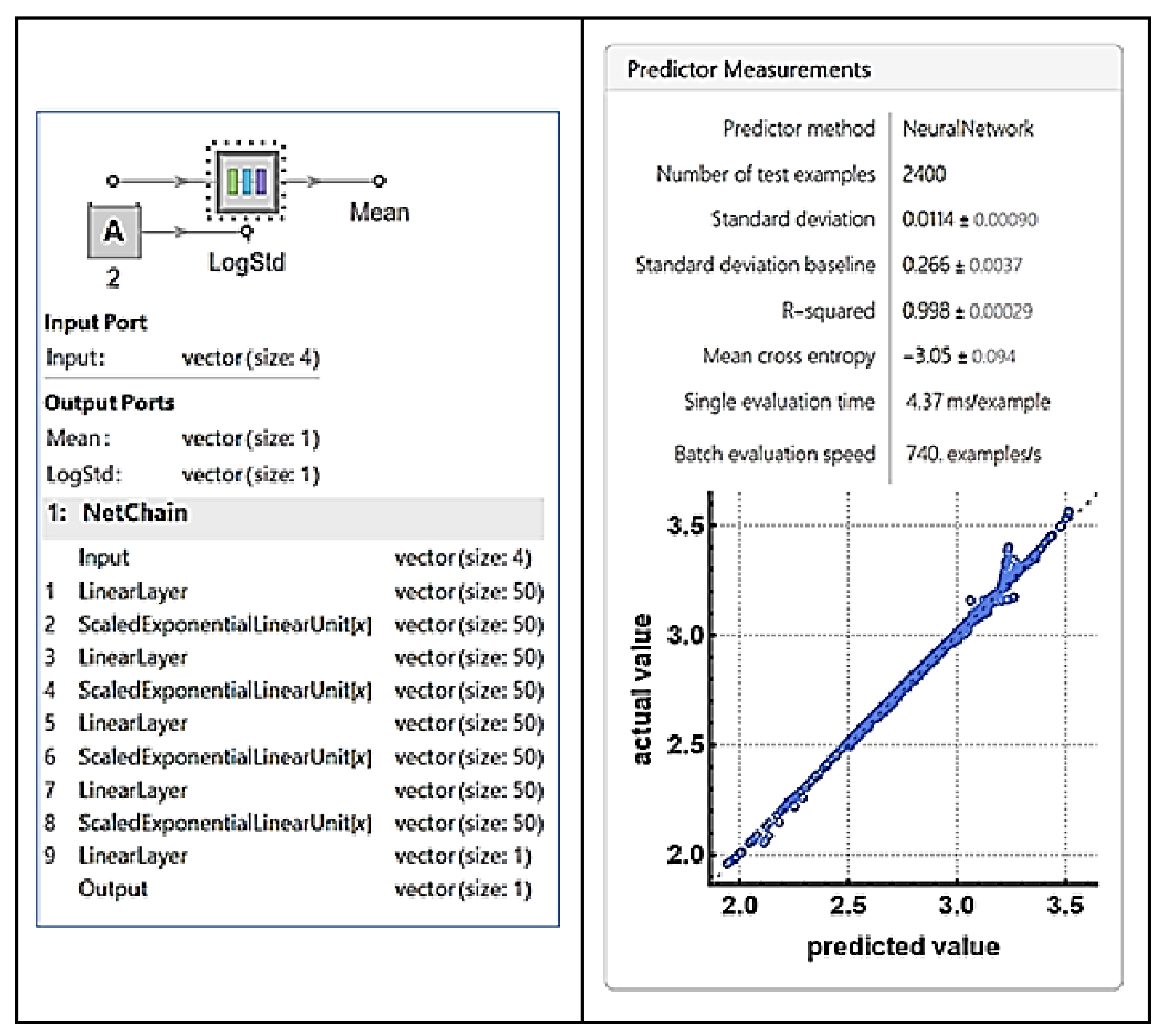
| Nominal capacity and voltage | 2.3 Ah, 3.3 V |
|---|---|
| Internal impedance (1 kHz AC) | 8 mΩ typical |
| Internal resistance 10 A, 1 s DC | 10 mΩ typical |
| Recommended charge method | 3 A to 3.6 V CCCV, 45 min |
| Recommended fast charge current | 10 A to 3.6 CCCV |
| Maximum continuous discharge | 70 A |
| Pulse discharge at 10 s | 120 A |
| Cycle life at 10 C discharge | Over 1000 cycles |
| Recommended pulse charge/discharge cutoff | 3.8 V to 1.6 V |
| Operating temperature range | 243 K to 333 K |
| Core cell weight | 70 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





