Submitted:
05 June 2023
Posted:
05 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study design
2.2. Ethical considerations
2.3. Patients and samples
2.4. Methods for detection of pathogen
2.4.1. Real-time PCR for qualitative and quantitative detection of P. jirovecii:
2.4.2. Staining methods for detection of P. jirovecii:
- Romanowski-Giemza staining (for trophozoites and cysts of P. jirovecii). Commercial Giemsa stain, modified solution (Sigma-Aldrich) was used. Dried thin smears were fixed with methyl alcohol for 5-10 minutes, dried, stained with a working solution of Giemsa stain for 20-22 minutes (the exposure was determined during the initial testing of the stain), washed with tap water and allowed to dry in a vertical position at room temperature.
- Toluidine blue staining (selective method for cysts of P. jirovecii). The thin smears from each clinical material were immersed for 5 minutes in sulfate reagent (prepared by mixing 25 ml diethyl ether and 25 ml concentrated sulfuric acid), rinsed with tap water, and stained with toluidine blue solution for 3 minutes. Differentiation was then performed in 2 shifts of isopropyl alcohol for 15-30 seconds, lightening with xylene and finally drying.
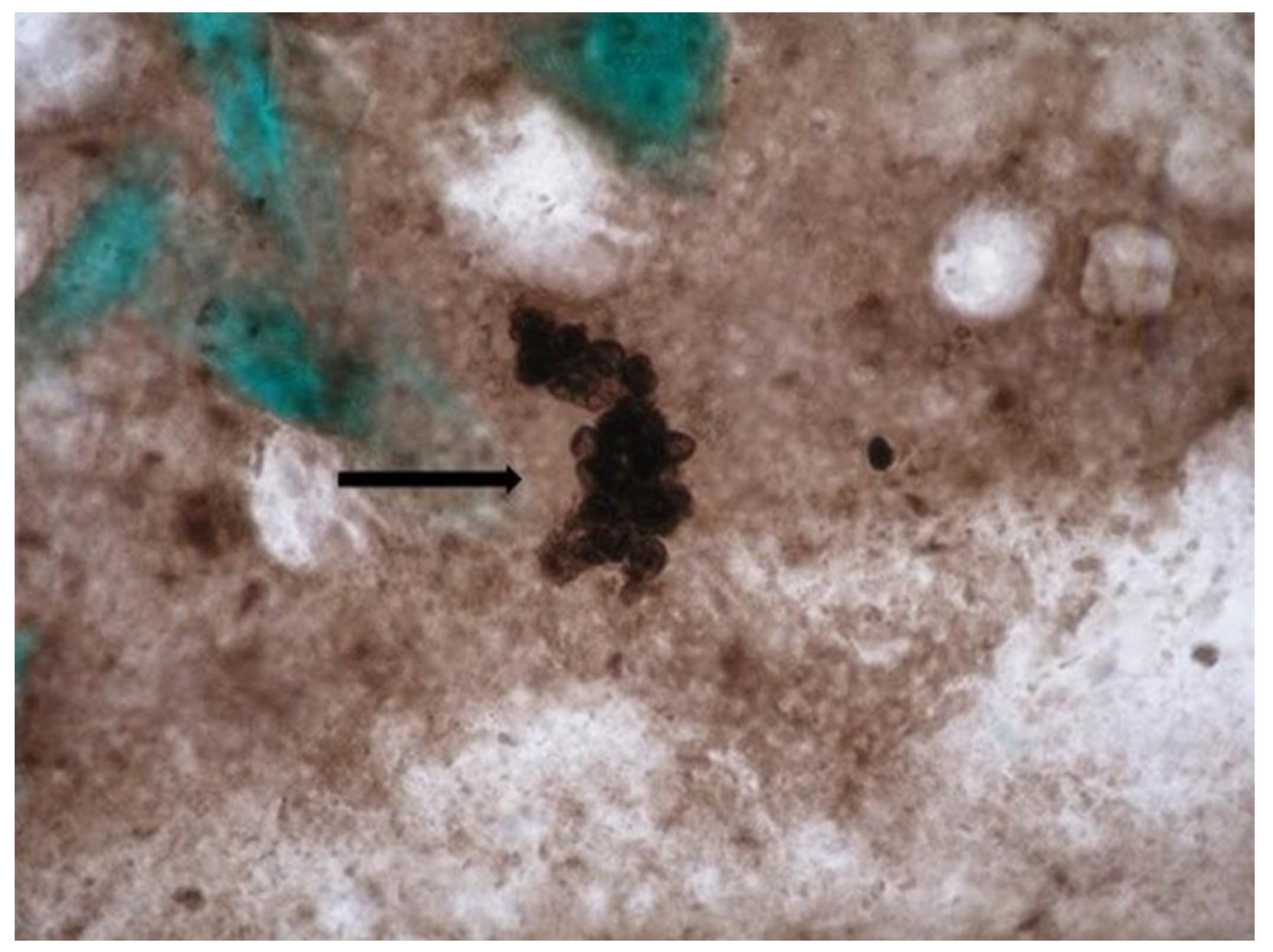
- Staining with methenamine-silver nitrate according to Gomori (for cysts of P. jirovecii). The method is considered the "gold standard" for microscopic visualization of P. jirovecii cysts. Microscopy Methenamine silver plating kit acc. to Gomori (Cat. No. 1.00820.0001; Merck KGaA, 64271 Darmstadt, Germany, Sigma-Aldrich Canada Co. or Millipore, Canada Ltd.) was used. The dried smears of the relevant clinical material were fixed for 30 minutes in 3.5% formalin and stained according to the manufacturer's protocol. The color of the cyst wall varies from gray to black (their surface membranes are visible).
- The samples were examined under a light microscope (Euromex IS.1153-Pli, The Netherlands) at 400x and 1000x magnification and visualized using color digital camera (Euromex DC.6000s, The Netherlands).
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovacs, J.A.; Masur, H. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA 2009, 301, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cattamanchi, A.; Davis, J.L.; Boon, S.D. , Kovacs, J.; Meshnick, S.; Miller, R.F.; Walzer, P.D.; Worodria, W.; Masur, H. HIV-Associated Pneumocystis Pneumonia. Proceedings of the American Thoracic Society 2011, 8, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Tasaka, S. Pneumocystis pneumonia in Human Immunodeficiency Virus-infected adults and adolescents: Current concepts and future directions. Clin. Med. Insights. Circ. Respir. Pulm. Med. 2015, 9, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Petrov, P.; Kurdova, R. Pneumocystosis. In Clinical parasitology and tropical medicine, 1st ed.; Petrov, P., Kurdova, R., Eds.; Publisher: East-West, Sofia, Bulgaria, 2016; pp. 198–205. (in Bulgarian) [Google Scholar]
- Morris, A.; Wei, K.; Afshar, K.; Huang, L. Epidemiology and clinical significance of Pneumocystis colonization. J. Infect. Dis. 2008, 197, 10–17. [Google Scholar] [CrossRef]
- Nevez, G.; Raccurt, C.; Vincent, P.; Jounieaux, V.; Dei-Cas, E. Pulmonary colonization with Pneumocystis carinii in human immunodeficiency virus-negative patients: assessing risk with blood CD4+ T cell counts. Clin. Infect. Dis. 1999, 29, 1331–1332. [Google Scholar] [CrossRef]
- Armbruster, C.; Pokieser, L.; Hassl, A. Diagnosis of Pneumocystis carinii pneumonia by bronchoalveolar lavage in AIDS patients. Acta Cytol. 1995, 39, 1089–1093. [Google Scholar]
- Sing, A.; Roggenkamp, A.; Autenrieth, I.B.; Heesemann, J. Pneumocystis carinii carriage in immunocompetent patients with primary pulmonary disorders as detected by single or nested PCR. J. Clin. Microbiol. 1999, 37, 3409–3410. [Google Scholar] [CrossRef]
- Koeva-Slavova, N.V. Peculiarities in the epidemiology and clinic of Pneumocystis pneumonia at NIIP in 1957-1960. Program and Abstracts of the National Paediatric Conference, Sofia, Bulgaria, 1960, Abstract pр.17-19. (in Bulgarian).
- Gamcheva, C.S. Study on pneumocystosis in infancy. PhD Thesis, National Center of Infectious and Parasitic Diseases, Sofia, Bulgaria, 1980. (in Bulgarian). [Google Scholar]
- Kurdova, R. Contemporary problems of opportunistic parasitoses and their diagnosis, In Proceedings of the IV national seminar on the topic: Modern methods for immunodiagnostics in the field of infectology, Sofia, Bulgaria,1999; Abstract, pр. 4-5. (in Bulgarian). (in Bulgarian).
- Dikov, I.; Plochev, K.; Topov, J.; et al. First case of AIDS in Bulgaria in a Bulgarian citizen. Epidemic Microbiol. Infect. Dis. 1999, 1, 35–38 (in Bulgarian). (in Bulgarian). [Google Scholar]
- Dikov, I.; Plochev, K.; Duhovnikova, T.; et al. Clinical and laboratory studies on the HIV-infectious process in Bulgaria. Modern Medicine 1989, 10, 15–18 (in Bulgarian). (in Bulgarian). [Google Scholar]
- Kurdova-Mincheva, R.; Tsvetanov, J.; Dikov, I. Pneumocystosis in a patient who died of AIDS - electron microscopy. Epidemic Microbiol. Inf. Dis. 1990, 27, 31–38 (in Bulgarian). (in Bulgarian). [Google Scholar]
- Kurdova, R.; Jordanova, D. Opportunistic parasitoses and HIV infection in Bulgaria. Infectology 2000, 37, 16–19 (in Bulgarian). (in Bulgarian). [Google Scholar]
- Kurdova, R.; Marinova, T.; Jordanova, D.; Ivanova, M.; Tzvetkova, N.; Rainova, I. Opportunistic parasitic diseases associated with HIV infection in Bulgaria. In Proceedings of the IX European Multicolloquium of Parasitology, Valencia, Spain, 2004; 1009(P); pp. 435–440. [Google Scholar]
- Tsvetkova, N.D.; Harizanov, R.N.; Ivanova, A.I.; Strashimirov, D.T.; Grozdeva, R.S.; Yancheva-Petrova, N.S. Recurrent Pneumocystis jirovecii pneumonia in an HIV-infected patient: A case report. GSC Advanced Research and Reviews 2021, 7, 083–086. [Google Scholar] [CrossRef]
- Sokulska, M.; Kicia, M.; Wesołowska, M.; Hendrich, A.B. Pneumocystis jirovecii - from a commensal to pathogen: clinical and diagnostic review. Parasitol. Res. 2015, 114, 3577–3585. [Google Scholar] [CrossRef] [PubMed]
- Fauchier, T.; Hasseine, L.; Gari-Toussaint, M.; Casanova, V.; Marty, P. M.; Pomares, C. Detection of Pneumocystis jirovecii by Quantitative PCR To Differentiate Colonization and Pneumonia in Immunocompromised HIV-Positive and HIV-Negative Patients. J. Clin. Microbiol. 2016, 54, 1487–1495. [Google Scholar] [CrossRef]
- Gal, S. Le.; Pougnet, L.; Damiani, C.; Fréalle, E.; Guéguen, P.; Virmaux, M.; Ansart, S.; Jaffuel, S.; Couturaud, F.; Delluc, A.; Tonnelier, J.-M.; Castellant, P.; Le Meur, Y.; Le Floch, G.; Totet, A.; Menotti, J.; Nevez, G. Pneumocystis jirovecii in the air surrounding patients with Pneumocystis pulmonary colonization. Diagn. Microbiol. Infect. Dis. 2015, 82, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Mühlethaler, K.; Bögli-Stuber, K.; Wasmer, S.; von Garnier, C. , Dumont, P.; Rauch, A.; Mühlemann, K.; Garzoni, C. Quantitative PCR to diagnose Pneumocystis pneumonia in immunocompromised non-HIV patients. Eur. Respir. J. 2012, 39, 971–978. [Google Scholar] [CrossRef]
- Chabé, M.; Dei-Cas, E.; Creusy, C.; Fleurisse, L; Respaldiza, N.; Camus, D.; Durand-Joly, I. Immunocompetent hosts as a reservoir of Pneumocystis organisms: Histological and RT-PCR data demonstrate active replication. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 89–97. [CrossRef]
- Cano, S.; Capote, F.; Pereira, A.; Calderon, E.; Castillo, J. Pneumocystis carinii pneumonia in patients without predisposing illnesses: acute episode and follow-up of five cases. CHEST 1993, 104, 376–381. [Google Scholar] [CrossRef]
- Ide, H.; Yamaji, Y.; Tobino, K.; Okahisa, M.; Murakami, K.; Goto, Y.; Sueyasu, T.; Nishizawa, S.; Yoshimine, K.; Munechika, M.; Oya, M.; Hiraki, Y. Pneumocystis jirovecii Pneumonia in an Immunocompetent Japanese Man: A Case Report and Literature Review. Case Rep. Pulmonol. 2019, 2019, 1–5. [Google Scholar] [CrossRef]
- Dunbar, A.; Schauwvlieghe, A.; Algoe, S.; van Hellemond, J. J.; Reynders, M.; Vandecasteele, S.; Boelens, J.; Depuydt, P.; Rijnders, B. Epidemiology of Pneumocystis jirovecii Pneumonia and (Non-)use of Prophylaxis. Front. Cell. Infect. Microbiol. 2020, 15, 10:224. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.J.; Lueck, C.; Ziesing, S.; Stoll, M.; Haller, H.; Gottlieb, J.; Eder, M.; Welte, T.; Hoeper, M.M.; Scherag, A.; David, S. Clinical course, treatment and outcome of Pneumocystis pneumonia in immunocompromised adults: a retrospective analysis over 17 years. Crit. Care 2018, 22, 307. [Google Scholar] [CrossRef]
- Shoji, K.; Michihata, N.; Miyairi, I.; Matsui, H.; Fushimi, K.; Yasunaga, H. Recent epidemiology of Pneumocystis pneumonia in Japan. J. Infect. Chemother. 2020, 26, 1260–1264. [Google Scholar] [CrossRef]
| Demographic data | Age groups (range) | Gender | Total N (%) |
||||
|---|---|---|---|---|---|---|---|
| 0-12 months |
1-9 years |
10-18 years |
> 18 years | Male sex | Female sex | ||
| No of cases | 25 | 36 | 31 | 128 | 137 | 83 | 220 |
|
Real-time PCR positive |
3 | 2 | 2 | 31 | 32 | 6 | 38 (17.3%) |
|
Real-time PCR negative |
22 | 34 | 29 | 97 | 105 | 77 | 182 (82.7%) |
|
Light microscopy(RG1/ TB2/ GMS3), positive |
0 | 0 | 0 | 5 | 5 | 0 | 5 (2.3%) |
|
Light microscopy, negative |
25 | 36 | 31 | 123 | 132 | 83 | 215 (97.7%) |
| Groups distributed by immunological status and clinical presentation (primary diagnosis) | |||||||
| Group 1 - patients without data of immunosuppression | 23 | 34 | 26 | 70 | 86 | 67 | 153 |
| pneumonia | 7 | 1 | 1 | 14 | 16 | 7 | 23 (15%) |
| respiratory distress syndrome | 0 | 0 | 1 | 0 | 1 | 0 | 1 (0.7%) |
| pharyngitis | 0 | 0 | 0 | 1 | 1 | 0 | 1 (0.7%) |
| respiratory failure | 1 | 0 | 0 | 2 | 1 | 2 | 3 (1.9%) |
| dyspnea | 0 | 0 | 1 | 3 | 1 | 3 | 4 (2.6%) |
| pulmonary abscess | 0 | 0 | 0 | 1 | 1 | 0 | 1 (0.7%) |
| bronchitis | 0 | 1 | 0 | 1 | 0 | 0 | 2 (1.3%) |
| fatigue | 0 | 0 | 0 | 2 | 2 | 0 | 2 (1.3%) |
| hemoptysis | 0 | 0 | 0 | 3 | 1 | 2 | 3 (1.9%) |
| cough | 15 | 32 | 23 | 36 | 56 | 50 | 106 (69.3%) |
| COVID-19 | 0 | 0 | 0 | 7 | 4 | 3 | 7 (4.6%) |
|
Real-time PCR positive |
3 | 0 | 1 | 2 | 6 | 0 | 6 (3.9%) |
|
Real-time PCR negative |
20 | 34 | 25 | 68 | 80 | 67 | 147 (96.1%) |
|
Light microscopy, positive |
0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Group 2 - patients with compromised immune system |
2 | 2 | 5 | 58 | 51 | 16 | 67 |
| HIV infection | 0 | 1 | 0 | 46 | 42 | 5 | 47 (70%) |
| hematological malignancy | 1 | 0 | 1 | 5 | 4 | 3 | 7 (10.5%) |
| interstitial pulmonary fibrosis | 0 | 0 | 0 | 3 | 2 | 1 | 3 (4.5%) |
| nephrotic syndrome | 0 | 1 | 2 | 0 | 1 | 2 | 3 (4.5%) |
| solid organ transplantation | 1 | 0 | 1 | 0 | 2 | 0 | 2 (3%) |
| long-term use of inhaled corticosteroids due to bronchiectasis and asthma | 0 | 0 | 0 | 4 | 0 | 4 | 4 (6%) |
| disseminated lupus | 0 | 0 | 1 | 0 | 0 | 1 | 1 (1.5%) |
|
Real-time PCR positive |
0 | 2 | 1 | 29 | 26 | 6 | 32 (47.8%) |
|
Real-time PCR negative |
2 | 0 | 4 | 29 | 25 | 10 | 35 (52.2%) |
|
Light microscopy, positive |
0 | 0 | 0 | 5 (GMS3) | 5 | 0 | 5 (7.5%) |
|
Light microscopy, negative |
2 | 2 | 5 | 53 | 46 | 16 | 62 (92.5%) |
| Patients | Staining method Specimen type - induced sputum |
Real-time quantitative PCR | Ct | |
|---|---|---|---|---|
| GMS |
P. jirovecii DNA concentration (copies/µӏ) |
|||
| In 1µl of the reaction solution | In 200 µӏ of the initial sample | |||
| P1 HIV+ | Clusters of cysts | 5,035 х 105 | 1,007 х 108 | 18.074 |
| P2 HIV+ | Clusters of cysts | 4,669 x 105 | 9,338 x 107 | 18.176 |
| P3 HIV+ | Single cysts | 2,179 х 101 | 4,358 х 103 | 31.566 |
| P4 HIV+ | Single cysts | 5,790 х 101 | 1,158 х 104 | 30.254 |
| P5 HIV+ | Single cysts | 4,703 х 102 | 9,406 х 104 | 27.441 |
| Type of clinical specimen | Patients/age group | Real-time quantitative PCR |
Ct |
|
|---|---|---|---|---|
| Concentration of P. jirovecii DNA (copies/µl) | ||||
| In 1µl of the reaction solution | In 200 µӏ of the initial sample | |||
| Tracheal aspirate | A 4-month-old baby with pneumonia | 0,8123 х 103 | 0,162480 х 106 | 35.37 |
| A 6-month-old baby with severe interstitial pneumonia | 359,6 х 103 | 71,92 х 106 | 26.67 | |
| Bronchoalveolar lavage | A 60-year-old man with interstitial pulmonary fibrosis | 1,265 х 103 | 253 х 106 | 24.87 |
| A 45-year-old man with bilateral interstitial pneumonia | 87,52 х 103 | 17,504 х 106 | 29.69 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





