Submitted:
05 June 2023
Posted:
05 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
- HQM — Home Quarantined with Mild disease course
- HMO — Hospitalized with Moderate course
- HSV — Hospitalized oxygen-dependent patients with Severe symptoms
- HCR — Hospitalized Critical patients in ICU departments with artificial ventilation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; Lin, K.; Mansur, R.B.; Ho, R.C.; Rosenblat, J.D.; Miskowiak, K.W.; Vinberg, M.; Maletic, V.; McIntyre, R.S. Fatigue and Cognitive Impairment in Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing Long COVID in an International Cohort: 7 Months of Symptoms and Their Impact. eClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Nemati, H.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; Farjoud-Kouhanjani, M.; Jafari, A.; Sasannia, F.; Ashrafi, S.; Nazeri, M.; Nasiri, S.; Shahisavandi, M. Long COVID Syndrome-Associated Brain Fog. J. Med. Virol. 2022, 94, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.; Porcher, R.; Pane, I.; Ravaud, P. Course of Post COVID-19 Disease Symptoms over Time in the ComPaRe Long COVID Prospective e-Cohort. Nat. Commun. 2022, 13, 1812. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; Iotti, G.; Latronico, N.; Lorini, L.; Merler, S.; Natalini, G.; Piatti, A.; Ranieri, M.V.; Scandroglio, A.M.; Storti, E.; Cecconi, M.; Pesenti, A. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; Cookingham, J.; Coppa, K.; Diefenbach, M.A.; Dominello, A.J.; Duer-Hefele, J.; Falzon, L.; Gitlin, J.; Hajizadeh, N.; Harvin, T.G.; Hirschwerk, D.A.; Kim, E.J.; Kozel, Z.M.; Marrast, L.M.; Mogavero, J.N.; Osorio, G.A.; Qiu, M.; Zanos, T.P. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and Diabetes as High-Risk Factors for Severe Coronavirus Disease 2019 (Covid-19). Diabetes Metab. Res. Rev. 2021, 37, e3377. [Google Scholar] [CrossRef]
- Ahmed, S.; Zimba, O.; Gasparyan, A.Y. Thrombosis in Coronavirus Disease 2019 (COVID-19) through the Prism of Virchow’s Triad. Clin. Rheumatol. 2020, 39, 2529–2543. [Google Scholar] [CrossRef]
- Su, H.; Yang, M.; Wan, C.; Yi, L.X.; Tang, F.; Zhu, H.Y.; Yi, F.; Yang, H.C.; Fogo, A.B.; Nie, X.; Zhang, C. Renal Histopathological Analysis of 26 Postmortem Findings of Patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef]
- Li, X.; Ma, X. Acute Respiratory Failure in COVID-19: Is It “Typical” ARDS? Crit. Care 2020, 24, 198. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The Cytokine Storm in COVID-19: An Overview of the Involvement of the Chemokine/Chemokine-Receptor System. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines Including Interleukin-6 in COVID-19 Induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef]
- (Kai, H.; Kai, M. Interactions of Coronaviruses with ACE2, Angiotensin II, and RAS Inhibitors—Lessons from Available Evidence and Insights into COVID-19. Hypertens. Res. 2020, 43, 648–654. [Google Scholar] [CrossRef]
- Pons, S.; Fodil, S.; Azoulay, E.; Zafrani, L. The Vascular Endothelium: The Cornerstone of Organ Dysfunction in Severe SARS-CoV-2 Infection. Crit. Care 2020, 24, 353. [Google Scholar] [CrossRef]
- Amraei, R.; Rahimi, N. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cells 2020, 9, 1652. [Google Scholar] [CrossRef]
- Long, B.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Cardiovascular Complications in COVID-19. Am. J. Emerg. Med. 2020, 38, 1504–1507. [Google Scholar] [CrossRef]
- Guzik, T.J.; Mohiddin, S.A.; Dimarco, A.; Patel, V.; Savvatis, K.; Marelli-Berg, F. M.; Madhur, M.S.; Tomaszewski, M.; Maffia, P.; D’Acquisto, F.; Nicklin, S.A.; Marian, A.J.; Nosalski, R.; Murray, E.C.; Guzik, B.; Berry, C.; Touyz, R.M.; Kreutz, R.; Dao, W.W.; Bhella, D.; Sagliocco, O.; Crea, F.; Thomson, E.C.; McInnes, I.B. COVID-19 and the Cardiovascular System: Implications for Risk Assessment, Diagnosis, and Treatment Options. Cardiovasc. Res. 2020, 116, 1666–1687. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of Comorbidities and Its Effects in Coronavirus Disease 2019 Patients: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- South, A.M.; Tomlinson, L.; Edmonston, D.; Hiremath, S.; Sparks, M.A. Controversies of Renin-Angiotensin System Inhibition during the COVID-19 Pandemic. Nat. Rev. Nephrol. 2020, 16, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Chary, M.A.; Barbuto, A.F.; Izadmehr, S.; Hayes, B.D.; Burns, M.M. COVID-19: Therapeutics and Their Toxicities. J. Med. Toxicol. 2020, 16, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Khashkhusha, T.R.; Chan, J.S.K.; Harky, A. ACE Inhibitors and COVID-19: We Don’t Know Yet. J. Card. Surg. 2020, 35, 1172–1173. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Theodoridou, K.; Poland, G. Influenza Immunization and COVID-19. Vaccine 2020, 38, 6078–6079. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; Zhang, Y.; Song, J.; Wang, S.; Chao, Y.; Yang, Z.; Xu, J.; Zhou, X.; Chen, D.; Xiong, W.; Xu, L.; Zhou, F.; Jiang, J.; Bai, C.; Zheng, J.; Song, Y. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Verma, R.; Lohana, P.; Lohana, A.; Ramphul, K. Acute Myocardial Infarction in COVID-19 Patients. A Review of Cases in the Literature. Arch. Med. Sci Atheroscler. Dis. 2021, 6, e169–e175. [Google Scholar] [CrossRef] [PubMed]
- Rojas-García, M.; Vázquez, B.; Torres-Poveda, K.; Madrid-Marina, V. Lethality Risk Markers by Sex and Age-Group for COVID-19 in Mexico: A Cross-Sectional Study Based on Machine Learning Approach. BMC Infect. Dis. 2023, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- (Hamer, M.; Gale, C.R.; Kivimäki, M.; Batty, G.D. Overweight, Obesity, and Risk of Hospitalization for COVID-19: A Community-Based Cohort Study of Adults in the United Kingdom. Proc. Natl. Acad. Sci. USA 2020, 117, 21011–21013. [Google Scholar] [CrossRef] [PubMed]
- Bastolla, U.; Chambers, P.; Abia, D.; Garcia-Bermejo, M.-L.; Fresno, M. Is Covid-19 Severity Associated With ACE2 Degradation? Front. Drug Discov. 2022, 1, 5. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Y.; Ke, Q.; Wang, Y.; Gong, Z.; Chen, X.; Cai, Y.; Li, S.; Sun, Y.; Peng, X.; Ji, Y.; Zhang, T.; Wu, W.; Cui, L.; Wang, Y. ApoE4 Associated with Severe COVID-19 Outcomes via Downregulation of ACE2 and Imbalanced RAS Pathway. J. Transl. Med. 2023, 21, 103. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, Y.H.; Jung, S.W.; Kim, D.J.; Park, S.H.; Song, S.J.; Jeong, K.H.; Moon, J.Y.; Ihm, C.-G.; Lee, T.W.; Kim, J.S.; Sohn, I.S.; Lee, S-Y.; Kim, D-O.; Kim, Y.G. Sex-Related Differences in the Intratubular Renin-Angiotensin System in Two-Kidney, One-Clip Hypertensive Rats. Am. J. Physiol. Renal Physiol. 2019, 317, F670–F682. [Google Scholar] [CrossRef]
- Pouremamali, A.; Babaei, A.; Malekshahi, S.S.; Abbasi, A.; Rafiee, N. Understanding the Pivotal Roles of ACE2 in SARS-CoV-2 Infection: From Structure/Function to Therapeutic Implication. Egypt J. Med. Hum. Genet. 2022, 23, 103. [Google Scholar] [CrossRef]
- Mortaz, E.; Jamaati, H.; Roofchayee, N. D.; Sheikhzade, H.; Mirenayat, M.; Sadeghi, M.; Lookzadeh, S.; Dezfuli, N.K.; Folkerts, G.; Mumby, S.; Garssen, J.; Adcock, I.M. Decreased Serum Levels of Angiotensin Converting Enzyme (ACE)2 and Enhanced Cytokine Levels with Severity of COVID-19: Normalisation upon Disease Recovery. Heliyon 2022, 8, e08957. [Google Scholar] [CrossRef]
- Maza, M.; Úbeda, M.; Delgado, P.; Horndler, L.; Llamas, M.A.; van Santen, H.M.; Alarcón, B.; Abia, D.; García-Bermejo, L.; Serrano-Villar, S.; Bastolla, U.; Fresno, M. ACE2 Serum Levels as Predictor of Infectability and Outcome in COVID-19. Front. Immunol. 2022, 13, 836516. [Google Scholar] [CrossRef]
- Florescu, S.; Stanciu, D.; Zaharia, M.; Kosa, A.; Codreanu, D.; Fareed, K.; Kidwai, A.; Kaye, C.; Coutts, A.; MacKay, L.; et al. Effect of Angiotensin-Converting Enzyme Inhibitor and Angiotensin Receptor Blocker Initiation on Organ Support–Free Days in Patients Hospitalized With COVID-19: A Randomized Clinical Trial. JAMA 2023, 329, 1183–1196. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; An, Y. ACE2 Shedding and the Role in COVID-19. Front. Cell. Infect. Microbiol. 2022, 11, 789180. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; Guan, L.; Wei, Y.; Li, H.; Wu, X.; Xu, J.; Tu, S.; Zhang, Y.; Chen, H.; Cao, B. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Salah, H.M.; Mehta, J.L. Hypothesis: Sex-Related Differences in ACE2 Activity May Contribute to Higher Mortality in Men Versus Women With COVID-19. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 114–118. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Cannarella, R.; Condorelli, R.A.; Torre, F.; Aversa, A.; Calogero, A.E. Sex-Specific SARS-CoV-2 Mortality: Among Hormone-Modulated Ace2 Expression, Risk of Venous Thromboembolism and Hypovitaminosis D. Int. J. Mol. Sci. 2020, 21, 2948. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, L.A.; Noonan, J.; Wang, X.; Peter, K. Higher Mortality of COVID-19 in Males: Sex Differences in Immune Response and Cardiovascular Comorbidities. Cardiovasc. Res. 2020, 116, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, H.C.; Sukumaran, V.; Al-Ruweidi, M.K.A.A.; Shurbaji, S. Do Changes in ACE-2 Expression Affect SARS-CoV-2 Virulence and Related Complications: A Closer Look into Membrane-Bound and Soluble Forms. Int. J. Mol. Sci. 2021, 22, 6703. [Google Scholar] [CrossRef] [PubMed]
- Emilsson, V.; Gudmundsson, E.F.; Aspelund, T.; Jonsson, B.G.; Gudjonsson, A.; Launer, L.J.; Lamb, J.R.; Gudmundsdottir, V.; Jennings, L.L.; Gudnason, V. Serum Levels of ACE2 Are Higher in Patients with Obesity and Diabetes. Obes. Sci. Pract. 2021, 7, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Hrebenyk, M.; Maslii, S.; Shevchuk, O.; Korda, M.; Vari, S.G. Impact of High Blood Pressure and Antihypertensive Treatment on COVID-19 Severity (Retrospective Observational Study in Ternopil Region, Ukraine). In Materials of 17th RECOOP Bridges in Life Sciences, Video Conference; Prague, Check Republic, 2022, pp 72–73.

| 0–45 days after the last negative PCR (59.6%) | 46–90 days after the last negative PCR (40.4%) | ||||||
|---|---|---|---|---|---|---|---|
| 344 | 233 | ||||||
| Total number | Females (66.9%) | Males (33.1%) | Average age | Seronegative patients (control) | |||
| 577 | 386 | 191 | 50.63 ± 13.08 years | 30 | |||
| Patients based on COVID-19 severity were divided into groups | |||||||
| HQM (45.6%) | HMO (37.3%) | HSV (14.2%) | HCR (2.9%) | ||||
| 263 | 215 | 82 | 17 | ||||
| Groups | ACE2, ng/mL n = 440 |
|
|---|---|---|
| n | Me [Lq; Uq] | |
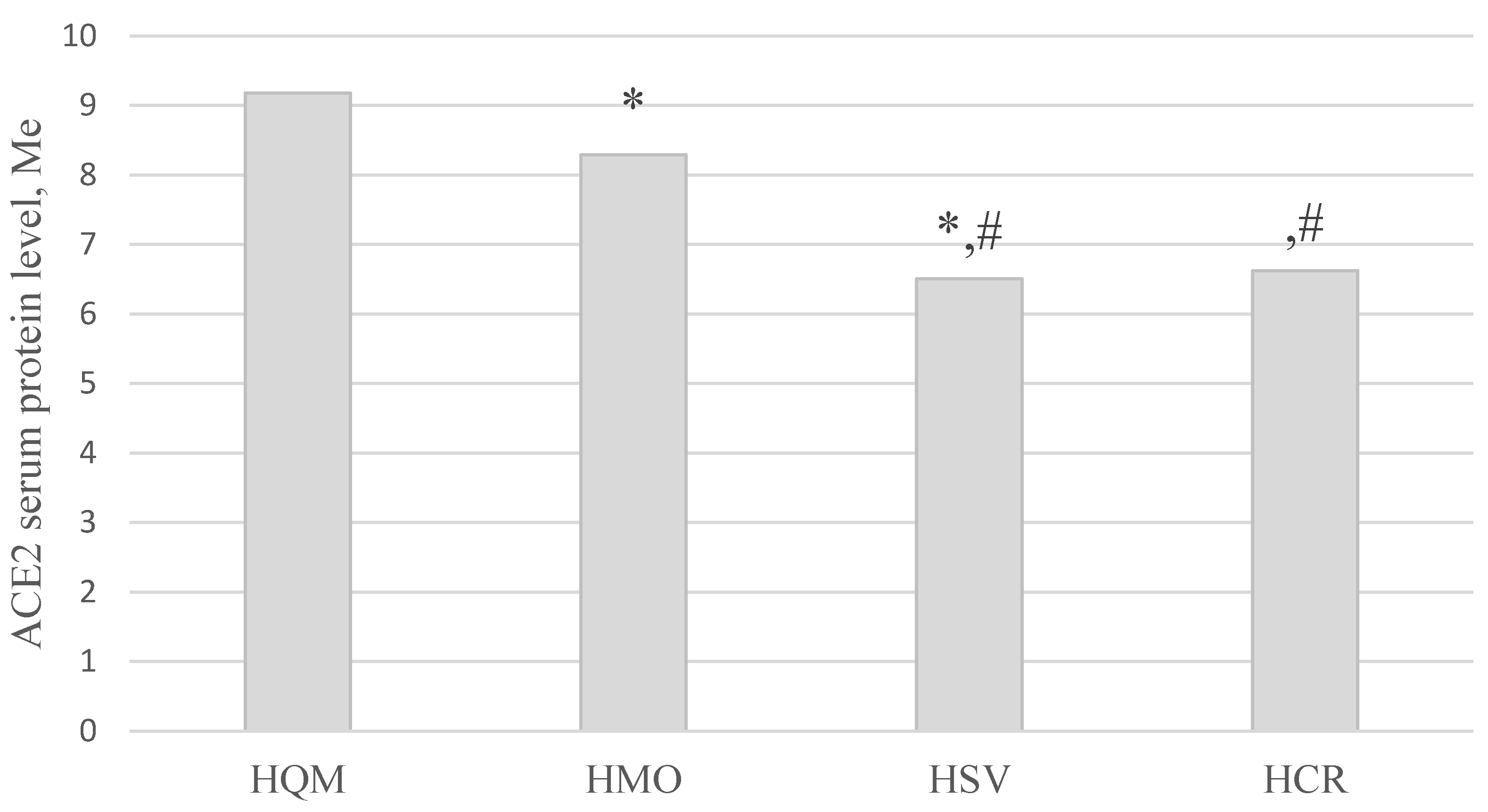
| HQM (1) | 155 | 9.19 [8.36; 10.05] |
| HMO (2) | 156 | 7.37 [5.75; 9.32] |
| HSV (3) | 82 | 6.49 [5.28; 7.25] |
| HCR (4) | 17 | 6.65 [5.35; 7.35] |
| Control group (5) | 30 | 7.69 [6.82; 9.09] |
| Kruskal-Wallis test | H = 104.53; p < 0.001* | |
| Multiple groups comparisons | p1-2, 1-3, 1-4, 1-5 < 0.001*, p2-3 < 0.05*, p 3-5 < 0.05* | |
| Group | ACE2, ng/mL; n = 410 | ||
| M | F | ||
| HQM (A) | n | 54 | 101 |
| Me [Lq; Uq] |
9.05 [8.19; 10.70] |
9.22 [8.53; 9.94] |
|
| HMO (B) | n | 60 | 96 |
| Me [Lq; Uq] | 7.65 [6.32; 9.53] |
6.93 [5.61; 9.04] |
|
| HSV (C) | n | 41 | 41 |
| Me [Lq; Uq] |
6.67 [4.95; 7.25] |
6.05 [5.33; 7.17] |
|
| HCR (D) | n | 9 | 8 |
| Me [Lq; Uq] |
6.60 [5.35; 6.88] |
7.05 [5.47; 7.41] |
|
| Kruskal-Wallis test | H = 41.35, p < 0.001* |
H = 60.93, p < 0.001* |
|
| Multiple groups comparisons | p A-B, A-C, A-D, B-C < 0.05* | p A-B, A-C, A-D < 0.05* | |
| Group | 0–45 days | 46–90 days |
|---|---|---|
| HQM | 9.41 [8.85; 10.88] | 8.94 [7.43; 9.54]* |
| HCR | 5.16 [4.75; 6.34] | 6.92 [6.60; 7.41]* |
| Parameters | HQM (1) | HMO (2) | HSV (3) | HCR (4) | Statistical significance |
| Weight, kg | 76.02 ± 17.31 |
83.24# ± 14.88 |
88.70# ± 18.04 |
93.41# ± 15.40 |
pANOVA < 0.001* p1-2; p1-3; p1-4 < 0.001* |
| Height, cm | 166.43 ± 13.35 |
168.25 ± 9.26 |
168.99 ± 9.67 |
168.76 ± 9.58 |
pANOVA = 0.186 |
| BMI, kg/m2 | 27.17 ± 5.68 |
29.45# ± 4.76 |
31.01# ± 5.29 |
32.80# ± 4.91 |
pANOVA < 0.001* p1-2; p1-3; p1-4 < 0.001* |
| WC, cm | 90.91 ± 14.92 |
99.66# ± 13.49 |
106.63# ± 12.38 |
109.29# ± 9.58 |
pANOVA < 0.001* p1-2; p1-3; p1-4 < 0.001* p2-3 < 0.001* p2-4 = 0.031* |
| HC, cm | 105.24 ± 11.46 |
109.12# ± 9.48 |
111.35# ± 10.33 |
114.06# ± 11.71 |
pANOVA < 0.001* p1-2; p1-3; p1-4 < 0.01* |
| WHtR | 0.54 ± 0.09 |
0.59# ± 0.08 |
0.63# ± 0.07 |
0.65# ± 0.06 |
pANOVA < 0.001* p1-2; p1-3; p1-4 < 0.001* p2-3 = 0.002* p2-4 = 0.038* |
| WHR | 0.86 ± 0.10 |
0.91 ± 0.09 |
0.96 ± 0.08 |
0.96 ± 0.09 |
pANOVA < 0.001* p1-2; p1-3; p1-4 < 0.001* p2-3 = 0.001* |
| AO (WC ≥ 94 cm [M], ≥ 80 cm [W])* | 179 (69.65%) |
186 (88.57%) |
76 (96.20%) |
17 (100.00%) |
χ2 = 45.79; p < 0.001* |
| AO (WHtR ≥ 0.5) | 175 (67.83%) |
185 (88.94%) |
77 (97.47%) |
17 (100.00%) |
χ2 = 55.02; p < 0.001* |
| AO (WHR ≥0.90 [M]; ≥0.85 [W]) | 137 (53.31%) |
158 (75.24%) |
72 (91.14%) |
17 (100.00%) |
χ2 = 58.18; p < 0.001* |
| Group | Diabetes Mellitus | ||
|---|---|---|---|
| absent | present | ||
| HQM (A) | n | 150 | 5 |
| Me [Lq; Uq] |
9.18 [8.35; 9.9]) |
11.16 # [9.53; 12.77] |
|
| HMO (B) | n | 138 | 18 |
| Me [Lq; Uq] |
7.11 [5.57; 9.26]) |
8.21 # [7.19; 9.57] |
|
| HSV (C) | n | 62 | 20 |
| Me [Lq; Uq] |
6.20 [4.90; 7.06] |
7.14 # [5.67; 8.96] |
|
| HCR (D) | n | 13 | 4 |
| Me [Lq; Uq] |
6.34 [4.97; 6.88] |
7.37 # [7.26; 7.41] |
|
| Kruskal-Wallis test | H = 98.02 p < 0.001* |
H = 11.92 p = 0.008* |
|
| Multiple groups comparisons | p A-B, A-C, A-D, B-C < 0.05* |
p A-C < 0.05* | |
| COVID-19 | 1 — HP, n = 338 |
2 — HP + DM, n = 88 |
3 — DM, N = 17 |
4 — No HP, No DM, n = 500 | p < 0,05 |
| Diagnosis | 5.58 ± 0.42 | 4.80 ± 0.38 | 8.33 ± 1.60 | 5.01 ± 0,34 | р1-3 = 0.037, р2-3 = 0.0003, р3-4 = 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





