Background
Patient Blood Management (PBM) is defined as the timely application of evidence based medical and surgical concepts designed to manage anemia, optimize hemostasis, and minimize blood loss in order to improve patient outcomes(1).
PBM encompasses all aspects of medical care including patient evaluation and clinical management surrounding disease management and the appropriate therapeutic decision-making process, including the application of appropriate agents with best indications, as well as identifying prevention to improve outcome and quality of life. As a result, one of many outcomes attributed to PBM is reduction of the need for allogeneic blood transfusions and reduce health-care costs, while ensuring that blood components are available for the patients who need them(2).
The three-pillar matrix of patient blood management [Shander et al 2012] is a model of evidence-based approaches to optimize erythropoiesis, minimising blood loss and harnessing the physiology of anemia while applying the most appropriate therapy(3). Notably, the approach is grounded on the following three pillars: (4) the optimization of the patient's endogenous red cell mass; (5) the control of all blood loss and (6)the harnessing and optimization of the patient-specific physiological tolerance of anemia, while administering the best therapy and adopting restrictive transfusion thresholds. PBM identifies patients who are planned for surgery and are at risk of transfusion and provides a management plan aimed at reducing or eliminating the need for allogeneic transfusion, thus reducing its inherent risks, inventory pressures and the escalating costs associated with transfusion. (7)
PBM pathways were first recommended in 2010 by the World Health Organisation, which called on member countries to apply the three-pillar policy; as recently as 2021, the WHO re-emphasised the urgent need for PBM to be applied in healthcare settings, based on epidemiological criteria and the worldwide prevalence of anaemic states. In fact, the worldwide prevalence of sideropenic anaemia is around 2.9 billions while for chronic inflammatory anaemia it is around 600 millions.(8)
Sideropenic anaemia is the most common disease in the world's population and accounts for about 45 % of patients who are candidates for elective surgery. (9)
This high prevalence is supported by multifactorial parameters: the underlying specialist pathology, the clinical characteristics of the patient, comorbidities, and phar The preoperative clinical assessment of the patient, the diagnosis of the anaemic state and its therapeutic resolution is of paramount importance for a care pathway with reduced clinical risks and pharmacological interference. (10)
Surgery of any kind is a major cause of blood mass loss directly proportional to the degree of invasiveness. The latter, coupled with cosmetic and healing impact, is a strong driver for surgeons to opt for the minimally invasive route. (11)
Reconstructive surgery provides the restoration of integrity of soft tissues damaged by trauma, surgery, congenital malformation, burns or infections. Microsurgery has a fundamental role in Reconstructive surgery (RS) and is based on the use of free tissue transfer (FTT). Microsurgical reconstruction techniques consist in harvesting of tissues that are separated from the native vascular sources of the donor site, transferred to a recipient site and revescularised by anastomosing to the vessels of the recipient site(12)
In these procedures, flaps perfusion and settling on the new site is crucial and there are some preoperative editable factors that have the potential of affecting the flap transfer's outcomes and their anastomoses patency. Some of these factors are ascribable to the time of surgery, such as the duration of surgery, the trophic conditions of the injured tissues, the anesthesiologic procedures which have a main role in the control of the hemodynamic stability and the local blood perfusion. Other factors are connected to the general clinical condition of the patient, especially comorbidities: hypercoagulability, diabetes, cardiovascular disease, collagen disease etc. These clinical conditions have a specific load on the free flap's outcomes.
Among these general factors, anemia is one well recognized leading aspect. (13-14)
For these patients it is essential to manage the anemia that generally occurs in the postoperative period and which plays a decisive role in the implantation of the flap and it is associated with a clinical and laboratory setting of chronic inflammation. (
Figure 1).
Methods
Anemia is a constant find in patients who underwent microsurgery. The preoperative anemia with value of haematocrit less than 30% and of Haemoglobin less than 10g/dl has been reported to compromise the perfusion of a free flap and to bring to flap necrosis (Hill et al. 2012) (15-16). In a previous study by Clark et al (2007), strong emphasis has been placed on maintaining the level of Hct >30% and of Hb>10g/dl before and after microsurgical procedures to reduce the risk of negative outcomes. (17)
The risk is enhanced due to the flap transfer itself, where the anastomosed vessels are perfused at different value of blood pressure, usually lower than the donor site; for this reason, an optimal blood oxygenation is mandatory to balance this transitory haemodynamic subversion.(18)
Nevertheless, there are clinical studies that do not confirm the results showed by Hill et al. (Mlodinow et al 2013), (19-20) so the correlation between anemia and microsurgical procedures’ outcomes is still controversial.
Chronic Inflammatory Anemia (ACD) is a prevalent and persistent condition observed in patients undergoing Reconstructive Surgery, and its impact on tissue perfusion, particularly in relation to free flap viability, is significant. (21) In the context of Reconstructive Surgery, maintaining optimal tissue oxygenation is of paramount importance as it directly influences the healing process and overall surgical outcomes. By preventing blood hyperviscosity and minimizing the need for blood transfusions, clinicians can mitigate the immunosuppressive effects associated with transfusions and improve tissue oxygenation. (22)
ACD, characterized by impaired iron utilization and reduced red blood cell production, poses a challenge in achieving adequate tissue oxygenation during Reconstructive Surgery. (23-24)
Insufficient tissue perfusion due to chronic anemia compromises the viability of free flaps, which rely on robust blood supply for successful integration. Thus, adopting a comprehensive approach to manage ACD in this patient population becomes imperative. (25)
In order to optimize tissue oxygen delivery, it is crucial to implement strategies that minimize blood hyperviscosity and reduce the reliance on blood transfusions. (26)Addressing the underlying causes of ACD, such as inflammation or chronic disease processes, through targeted therapies can help improve erythropoiesis and alleviate anemia. Additionally, judicious use of iron supplementation, in combination with other erythropoietic agents, may be employed to enhance red blood cell production and restore hemoglobin levels. (27)
By prioritizing patient blood management principles, including preoperative optimization, meticulous intraoperative hemostasis, and innovative transfusion alternatives, the risk of complications associated with transfusions can be mitigated.(28) Techniques like intraoperative cell salvage, where shed blood is collected, processed, and reinfused, minimize the need for allogeneic blood transfusions and reduce immunosuppression. (29) Additionally, the use of advanced monitoring technologies, such as near-infrared spectroscopy, can provide real-time feedback on tissue oxygenation and guide interventions to maintain optimal perfusion.
In summary, in patients undergoing Reconstructive Surgery, Chronic Inflammatory Anemia (ACD) poses a continuous challenge to tissue oxygenation and flap viability.(30) By adopting a proactive approach that focuses on managing ACD, minimizing blood hyperviscosity, and reducing the immunosuppressive effects associated with transfusions, clinicians can optimize tissue oxyphoresis and ultimately enhance patient outcomes. (31) Embracing patient blood management strategies and integrating advancements in monitoring technologies are essential in Reconstructive Surgery to ensure successful outcomes and improve patient well-being. (32)
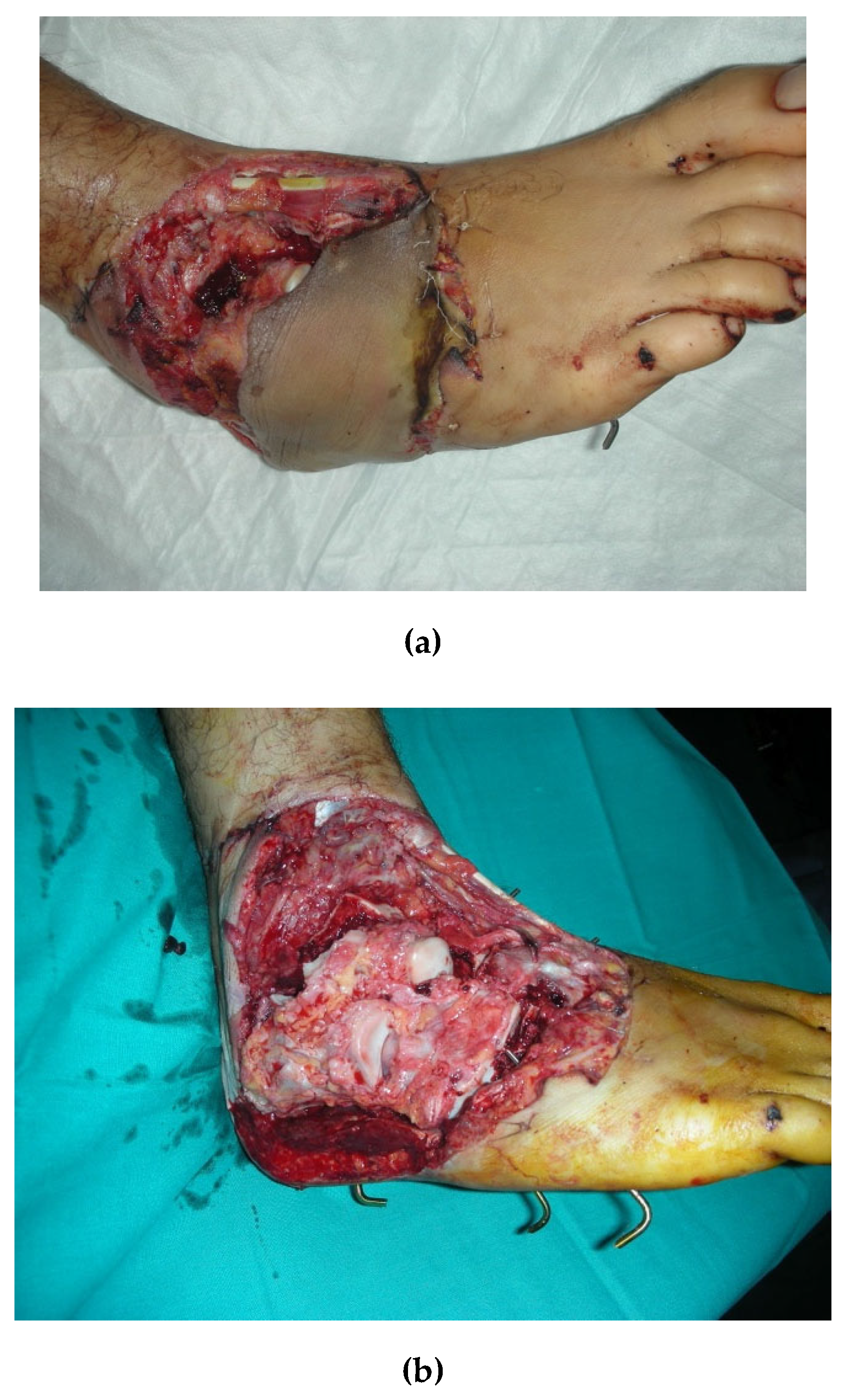
In order to contribute filling this gap in evidence we conducted a prospective observational study: from January 2017 to September 2019 we evaluated 16 patients (16 males, average age 33 years) who underwent microsurgical procedures for FTT. In all cases, the recipient site was the lower limb. All enrolled patients presented a loss of substance of soft tissues with bone exposure due to an open injury of the limb that was repaired with an Anterolateral Thigh Flap (
Figure 2). Haemoglobin (Hb) levels, Corpuscular Indexes, Transferrin saturation (TSAT), ferritin concentrations and creatinine clearance have been measured the first day after surgery (T0), after the first week (T1) and after five weeks (T2). At T0 all patients showed low hemoglobin levels (average 7.4 g/dl, STD 0,71 range 6,2-7,4 g dL-1), with an MCV of 72, MCH 28, MCHC 33, RDW 16, Serum iron 35, Ferritin 28, Ret% 1,36, TRF 277 and Creatinine Clearance 119. All patients were evaluated for the iron balance with the transferrin saturation percentage , sixteen patients had a percentage lower than 20% and 3 patients had TSAT% higher than 20%. Renal function and creatinine clearance level were normal for all patients considered.
(33)
We assessed all patients for their clinical condition, history, and comorbidities before starting therapy. Tabular views of baseline characteristics, and iron methabolism as well as red blood cells parameters are reported in
Table 1 and
Table 2.
Results
The collaboration between two departments (i.e. Department of Transfusion Medicine and of Reconstructive Surgery) resulted in the application of a therapeutic protocol with erythropoietic stimulating agents (ESAs) (Binocrit 6000 UI/week) and intravenous iron every other day, starting from the second day after surgery. (34) Thirteen patients received ESAs and ferric carboxymaltose (FCM) (500-1000 mg per session), three patients received ESAs and iron gluconate (1 vial every other day). Variation of the laboratory parameters from T0 to T1 and T2 is reported in Table 2.
No side effects have been observed and above all no rejection of the limb or flap.
All patients were studied for immunotransfusion aspects (blood group, direct and indirect Coombs test) on the first day of hospitalisation; no patients received blood units during the hospital stay.
After assessing normality with Shapiro Wilk Test, a null hypothesis was formulated stating no actual difference in between timepoints levels of hemoglobinm ferritin and TSAT values. A dependent samples two tailed omoschedastic t-test was then performed to reject the hypothesis, with results shown in
Table 2 and
Table 4.
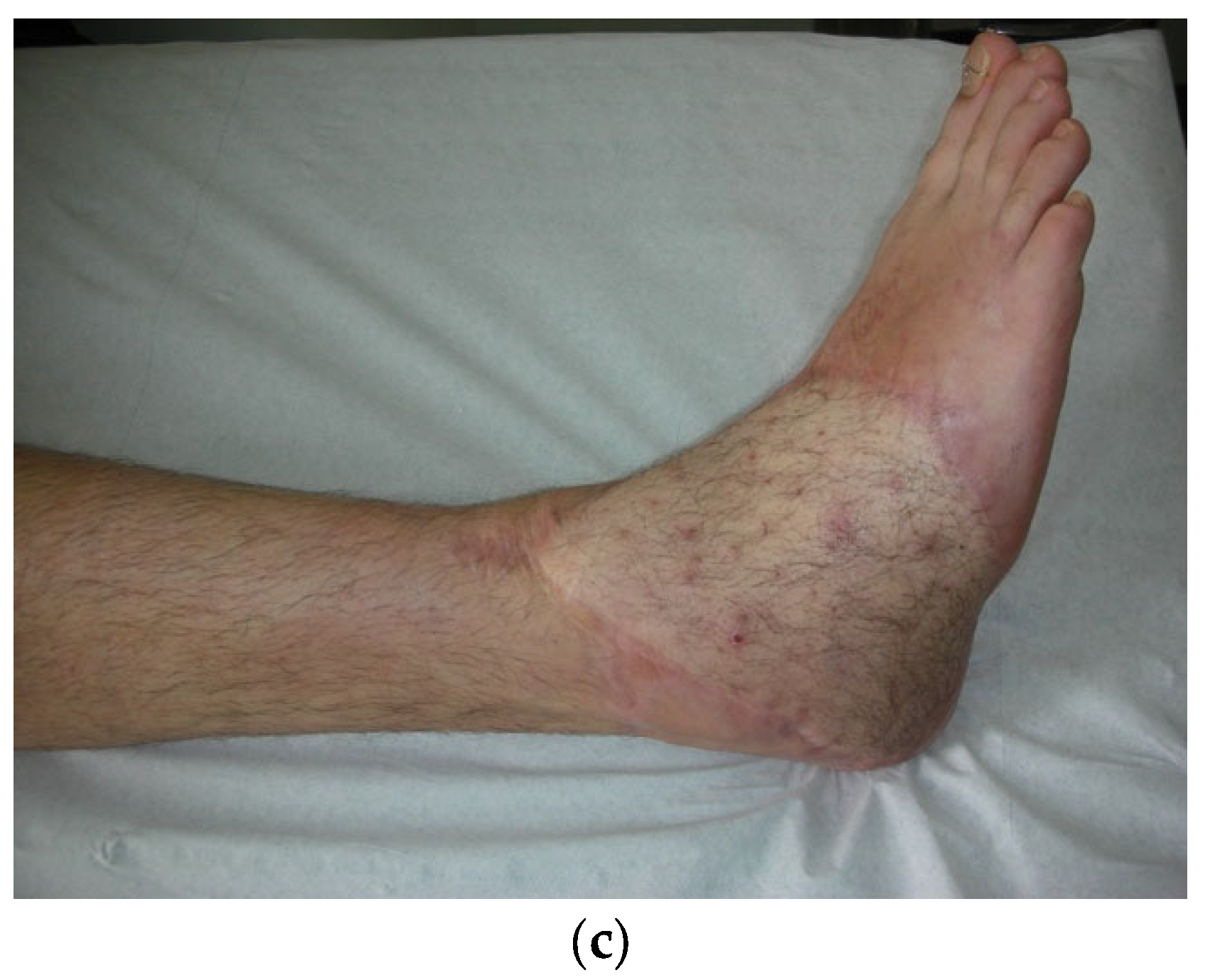
No immediate surgery-related complications occurred. In one case a superficial necrosis of the flap occurred for a venous congestion. Debridment of the necrotic tissues was performed and after 3 weeks of delivering negative pressure wound therapy, the superficial layer of the flap was successfully covered with a skin graft. (35)
Table 3.
Average erythrocyte parameters at different study timepoints.
Table 3.
Average erythrocyte parameters at different study timepoints.
| Parameter |
T0 |
T1 |
T2 |
p T0 vs T1 |
p T1 vs T2 |
p T0 vs T2 |
| Haemoglobin |
7,4 |
8,43 |
10,49 |
<0.001 |
<0.001 |
<0.001 |
| Hematocrit |
24,2 |
28,31 |
36,69 |
<0.001 |
<0.001 |
<0.001 |
| MCV |
72,06 |
76,44 |
83,13 |
<0.001 |
<0.001 |
<0.001 |
| MCH |
27,94 |
31 |
33,63 |
<0.001 |
<0.001 |
<0.001 |
| MCH-C |
33,1 |
32,96 |
33,64 |
0.70 |
0.20 |
0.001 |
| RDW |
15,93 |
19,92 |
23,81 |
0.45 |
0.001 |
<0.001 |
Table 4.
Iron metabolism and kidney function at different study timepoints.
Table 4.
Iron metabolism and kidney function at different study timepoints.
| Parameter |
T0 |
T1 |
T2 |
p T0 vs T1 |
p T1 vs T2 |
p T0 vs T2 |
| Sideremia |
35 |
49,75 |
68,38 |
<0.001 |
<0.001 |
<0.001 |
| Ferritin |
28,56 |
120,63 |
150 |
<0.001 |
<0.001 |
0.003 |
| Reticolocites % |
1,36 |
1,66 |
2,24 |
0.10 |
<0.001 |
0.003 |
| TRF |
277,44 |
271,94 |
145,94 |
0.49 |
<0.001 |
<0.001 |
| Creatinin Clearence |
117,5 |
117,44 |
119 |
0.954 |
0.426 |
0.249 |
Discussion
The application of PBM strategies represents an evidence-based best practice in several surgical settings. The reason lies in the synergy between the healthcare risks reduction, the outcome improvement and health economic saving. (36)
Patient Blood Management (PBM) in surgical settings is an integral aspect of modern healthcare, emphasizing optimal utilization of blood products while prioritizing patient safety and outcomes. PBM represents a state-of-the-art approach that recognizes the potential risks associated with transfusions and aims to minimize them through a comprehensive and evidence-based strategy. (37)
The importance of PBM lies in its ability to enhance patient care across the perioperative continuum. By employing preoperative anemia management, judicious use of blood transfusions, and effective bleeding control, PBM strategies reduce the need for transfusions, thereby minimizing the associated risks and complications. This approach not only improves patient safety but also reduces healthcare costs and enhances overall surgical outcomes. (38)
The cornerstone of PBM is an individualized patient assessment, considering factors such as age, comorbidities, and surgical complexity. Preoperative optimization through the correction of anemia, nutritional support, and iron supplementation can significantly reduce the need for transfusions. Furthermore, employing minimally invasive surgical techniques and utilizing hemostatic agents during surgery aids in controlling bleeding and reducing blood loss. (39)
An essential component of PBM is the concept of a patient blood management team, comprising various healthcare professionals collaborating to implement tailored strategies. This interdisciplinary approach ensures effective communication, knowledge sharing, and adherence to evidence-based guidelines. Moreover, ongoing education and training programs for healthcare providers play a vital role in promoting awareness and implementing best practices related to PBM. (40)
The state of the art in PBM includes advanced laboratory testing, such as thromboelastography and point-of-care coagulation monitoring, which enable real-time assessment of coagulation status and guide transfusion decisions. Additionally, the use of cell salvage techniques, where shed blood is collected, processed, and reinfused back to the patient, reduces the reliance on allogeneic blood transfusions.
In conclusion, Patient Blood Management in surgical settings represents an important paradigm shift in healthcare, focusing on individualized patient care, optimizing blood utilization, and minimizing transfusion-related risks. By implementing evidence-based strategies, fostering interdisciplinary collaboration, and embracing technological advancements, PBM improves patient outcomes, enhances safety, and ensures efficient resource allocation. Embracing PBM as a standard of care in surgical settings can lead to significant advancements in healthcare and ultimately benefit patients worldwide. (41)
Reconstructive surgery is a relatively innovative application of PBM strategies: it is fundamental to preserve the surgical reconstruction and, at the same time, the current oxyphoresis of the FTT. Wound contamination in severe open limb trauma often leads to deep infection. The strategy employed in such cases involves early debridment of the wound, combined with intensive and repeated washing and repair of the loss of substance by FTT.
In patients with open and contaminated trauma, it is quintessential to perform infection control through irrigation and debridment within the first hours after trauma, and to cover residual loss of substance with vascularised flaps. Flap survival depends on optimal tissue perfusion and oxygenation; to this regard, anaemia plays a fundamental role. Haematocrit levels should be maintained above 30 per cent, also to minimize the number of postoperative transfusions; there is evidence that blood transfusions are associated with both infectious and noninfectious complications. (42)
In this setting, the preoperative anemia has a dual pathogenesis, both due to post-surgical inflammation and perioperative bleeding and infection. Notably, the inflammation impedes iron turnover through a blockade of the regulatory hormone hepcidin, resulting in a condition of chronic inflammatory anemia. Thus, this type of patients has multifactorial anemia with hyperferritinemic status.
Allogeneic blood transfusion often represents a life-saving therapy, particularly in patients with acute bleeding. However, literature and hemovigilance systems expose several associated risks with this therapy: primarily, immunosuppressive and blood hyperviscosity constitute factors that could lead to rejection of the reconstructed flap, and thus a therapeutic failure.
Scientific evidence has allowed the use of the described therapeutic protocol involving the use of biosimilars, to bypass the hepcidin-induced iron blockade and to stimulate erythropoiesis. The therapeutic use of intravenous iron assists the action of erythrocyte reproductive stimulation.
The results achieved through the implementation of this therapy have consistently demonstrated a notable and progressive improvement in various erythrocyte parameters. These positive outcomes include a steady increase in red blood cell count, hematocrit, and hemoglobin levels, collectively indicating enhanced oxygen-carrying capacity within the bloodstream. By maintaining optimal levels of hemoglobin, a key component of erythrocytes responsible for binding and transporting oxygen, this therapy ensures the preservation of optimal tissue oxyphoresis.
The therapy's effectiveness is further underscored by its ability to sustain the desired hemoglobin levels over time. This maintenance of adequate hemoglobin concentrations plays a critical role in facilitating the efficient delivery of oxygen to tissues throughout the body. As oxygen is indispensable for cellular metabolism and tissue health, ensuring optimal tissue oxygenation is of paramount importance in supporting physiological processes, promoting wound healing, and minimizing the risk of complications.
The observed progressive increase in erythrocyte parameters reflects the therapy's impact on improving red blood cell production, optimizing iron utilization, or enhancing erythropoiesis, depending on the underlying cause of the anemia. By addressing the root cause of anemia, this therapy effectively restores erythrocyte function, leading to improved oxygen-carrying capacity and overall tissue oxygenation.
The consistent elevation in erythrocyte parameters also suggests that this therapy promotes the long-term sustainability of improved blood parameters, thereby reducing the likelihood of relapses or fluctuations in hemoglobin levels. This stability in erythrocyte parameters is crucial in maintaining sustained tissue oxyphoresis and ensuring the ongoing delivery of oxygen to organs and tissues, supporting their normal physiological functions.
Overall, the results obtained with this therapy showcase its efficacy in progressively enhancing erythrocyte parameters, sustaining optimal hemoglobin levels, and thereby optimizing tissue oxyphoresis. By improving oxygen delivery to tissues, this therapy holds significant promise in promoting overall patient well-being, facilitating efficient wound healing, and enhancing physiological processes that rely on adequate tissue oxygenation. (43)
All patients were evaluated for transfusion tests, but no patients received blood or other blood component transfusions, decreasing the risks of flap rejection and infection.
Preliminary data of our protocol show an optimal therapeutic response, in a surgical setting with few scientific literature data. The enrollment of other patients will allow us to validate this therapeutic protocol with statistically significant data.
Conclusions
Patient Blood Management shifts the focus from the product to the patient and considers the patient's blood as a resource that should be stored and managed appropriately as a standard of care.
The fundamental objectives of PBM are: improvement of clinical patient safety , prevention of avoidable transfusion through timely management of all modifiable risk factors and consequent reduction of management costs.
There is much scientific evidence, clinical trials and guidelines that emphasise and encourage the application of PBM pathways, but to date the level of international and national application is uneven and sometimes disorganised and therefore not effective.
This course of treatment represents an innovative modality within this surgical setting. Its implementation will ensure the definition of new endpoints but above all the overcoming of decisive clinical phases for these patients
Conflicts of Interest
The authors declare that they have not conflict of interest .
References
- Shander A. et al “Patient blood management in Europe” Br J Anaesth. 2012 Jul;109(1):55-68.
- Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR: “ Activity-based costs of blood transfusions in surgical patients at four hospitals. “ Transfusion 2010; 50:753– 655–701.
- Suzanne ET, Michael HC. Clinical strategies to avoid blood transfusion. Anaesth Intensive Care Med 2013;14:48 50.
- Susan M. Goobie : Patient Blood Management is a new standard of Care to optimize Blood Health , September 2022 , International Anesthesia Research.
- Mejer J, Filipescu D, Kozek-Langenecker S et al. Intraoperative transfusion practices in Europe. Br J Aneaesth 2016:Feb;116(2):255-61.
- Van Bommel J, Siegemund M, Henny CP, Ince C. Heart, kidney, and intestine have different tolerances for anemia. Transl Res. 2008 Feb;151(2):110-7.
- Mahecic TT, Dunserb M, Meierb J. RBC Transfusion Triggers: is there anything new? Transfus Med Hemother 2020;47:361-368. [CrossRef]
- Bennett S, Ayoub A, Tran A, et al. Current practices in perioperative blood management for patients undergoing liver resection: a survey of surgeons and anesthesiologists. Transfusion. 2018;58:781– 787.
- Leahy MF, Hofmann A, Towler S, Trentino KM, Burrows SA, Swain SG, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017 Jun;57(6):1347-58.
- Leahy MF, Hofmann A, Towler S, Trentino KM, Burrows SA, Swain SG, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017 Jun;57(6):1347-58.
- Meybohm P, Herrmann E, Steinbicker AU, Wittmann M, Gruenewald M, Fischer D, et al; PBM-study Collaborators. Patient Blood Management is Associated With a Substantial Reduction of Red Blood Cell Utilization and Safe for Patient’s Outcome: A Prospective, Multicenter Cohort Study With a Noninferiority Design. Ann Surg. 2016 Aug;264(2):203-11.
- Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. British Medical Journal 2015; 350: h1354.
- Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C, et al.Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2016 Oct;10(5):CD002042.
- Retter A, Wyncoll D, Pearse R et al. Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. British Journal of Haematology 2013;160:445-464.
- Napolitano LM, Kurek S, Luchette F et al. Clinical Practice Guideline: red blood cell transfusion in adult trauma and critical care. The Journal of Trauma: Injury, Infection, and Critical Care, 67(6), 1439–1442. [CrossRef]
- Boening A, Boedeker RH, Scheibelhut C, Rietzschel J, Roth P, Schònburg M. Anemia before coronary artery bypass surgery as additional risk factor increases the perioperative risk. Ann Thorac Surg. 2011 Sep;92(3):805-10.
- Karkouti K, Grocott HP, Hall R, Jessen ME, Kruger C, Lerner AB, et al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth. 2015 Apr; 62(4):377-8.
- Culleton BF, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Hemmelgarn BR. Impact of anemia on hospitalization and mortality in older adults. Blood. 2006 May;]07(10):3841-6.
- Clevenger B, Richards T. Pre-operative anaemia. Anaesthesia.2015;70 Suppl 1:20-8, e6-8.
- Holst LB, Haase N, Wetterslev J, et al; TRISS Trial Group; Scandinavian Critical Care Trials Group: Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med 2014;371:1381–1391.
- Hovaguimian F, Myles PS. A context specific systematic review and meta-analysis of randomized control trials.Anesthesiology 2016; 125:46-61.
- Baker L, Park L et al. Intraoperative red blood cell transfusion decision-making. Ann Surg 2021;274:86-96.
- Bennett S, Baker LK, Martel G, et al. The impact of perioperative red blood cell transfusions in patients undergoing liver resection: a systematic review. HPB (Oxford). 2017;19:321–330.
- Wu HL, Tai YH, Lin SP, et al. The impact of blood transfusion on recurrence and mortality following colorectal cancer resection: a propensity score analysis of 4,030 patients. Sci Rep. 2018;8:13345.
- Hallet J, Tsang M, Cheng ES, et al. The impact of perioperative red blood cell transfusions on long- term outcomes after hepatectomy for colorectal liver metastases. Ann Surg Oncol. 2015;22:4038– 4045.
- Hart S, Cserti-Gazdewich CM, McCluskey SA. Red cell transfusion and the immune system. Anaesthesia. 2015;70(Suppl 1):38–45.e13-6.
- Weiskopf RB, Viele MK, Feiner J et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA 1998 21;279(3):217-21.
- Rossmiller SR, Cannady SB, Ghanem TA, Wax MK. Transfusion criteria in free flap surgery. Otolaryngol Head Neck Surg. 2010 Mar;142(3):359-64. https://doi.org/10.1016/j.otohns.2009.11.024. Epub 2010 Jan 22. PMID: 20172381.
- Danan D, Smolkin ME, Varhegyi NE, Bakos SR, Jameson MJ, Shonka DC Jr. Impact of blood transfusions on patients with head and neck cancer undergoing free tissue transfer. Laryngoscope. 2015 Jan;125(1):86-91. Epub 2014 Aug 14. PMID: 25124183. [CrossRef]
- Hill JB, Patel A, Del Corral GA, Sexton KW, Ehrenfeld JM, Guillamondegui OD, Shack RB – Preoperative anemia predicts thrombosis and free flap in microvascular reconstruction. Ann Plast Surg, 2012 Oct; 69(4):346-7.
- Clark JRJ, McCluskey SAS, Hall F, Lipa J, Neligan P, Brown D, Gilbert R – Predictors of morbidity following free flap reconstruction for cancer of the head and neck. Head Neck, 2007 Dec; 29:1090-1101.
- Mlodinow AS, Ver Halen JP, Rambachan A, Gaido J, Kim JY – Anemia is not a predictor of free flap failure: a review of NSQIP data. Microsurgery, 2013 Sep; 33(6):432-8.
- Longo, B., Giacalone, M., D’Orsi, G., Gagliano, E., Vannucchi, L., Ferracci, A., Iundusi, R., Tarantino, U. and Cervelli, V. 2023. Microsurgical reconstruction of lower extremity in homozygosity of C677T MTHFR gene mutation: case report and review of the literature. Plastic Reconstructive and Regenerative Surgery. 3, (Feb. 2023), 98–105. [CrossRef]
- Silverberg DS et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol 2000; 35(7):1731744.
- Sjӧberg D, Holmstrom T, Larsson M, et al. Anemia in a population based IBD cohort (I CURE): still high prevalence after 1 year, especially among pediatric patients. Inflamm Bowel Dis 2014; 20: 2266-70.
- Timothy G.J. et al. Anemia in Emergency Department: Evaluation and Treatment. Em Med Practice 2013; 15: 1-16.
- Liumbruno G, Bennardello F, Lattanzio A et al. Raccomandazioni SIMTI sul corretto uso degli emocomponenti e dei plasmaderivati. Ed. SIMT, 1° edizione settembre 2008.
- Shah A, Stanworth SJ, McKechnie S. Evidence and triggers for the transfusion of blood and blood products. Anaesthesia 2015, 70, suppl. 1, 10-19).
- Robertson CS, Hannay J et al. Effect of Erythropoietin and Transfusion Threshold on Neurological Recovery After Traumatic Brain Injury A Randomized Clinical Trial JAMA. 2014;312(1):36-47. [CrossRef]
- B.J. Hunt, S. Allard, D. Keeling, D. Norfolk, S.J. Stanworth, K. Pendry, H. British Committee for Standards in. A practical guideline for the haematological management of major haemorrhage, Br J Haematol 2015; 170:788-803.
- S. Padhi, S. Kemmis-Betty, S. Rajesh, J. Hill, M.F. Murphy, G. Guideline Development. Blood transfusion: Summary of NICE guidance, BMJ 2015; 351:h5832.
- Carson LJ et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet 1996; 348(9034): 1055-60.
- Walsh TS, McArdle F et al. Does the storagetime of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients? Critical Care Medicine, 2004; 32, 364-371.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).