A Renewed Interest in Murine Eye Movements
Orienting the eyes in space is a vital function for animals that rely on their visual system. Historically, eye movements in mice have predominantly been considered a way to stabilise or shift gaze following head movements, as observed in other afoveate visual animals (Land 2019; Kautzky and Busse 2020). However, technical difficulties in recording head-coupled eye movements of small, lateral-eyed animals, such as mice, hindered research in the field (Kautzky and Busse 2020). This and the prevailing assumption that mice preferentially rely on their whiskers explain the limited knowledge of murine eye movement dynamics and its higher-order control (Kautzky and Busse 2020). By contrast, perhaps paradoxically, a significant body of research focuses on the murine visual cortex, thanks to its amenability to observation and manipulation (Niell 2011). Recent technological advances have fostered a perspective shift on the function and control of murine eye movements and their integration within central visual processing pathways.
Eye movements in head- or body-restrained mice generated in an optokinetic drum were described as early as 1976 using direct observation through a microscope (Mitchiner, Pinto, and Vanable 1976). The advent of video-oculography allowed for increasingly precise monitoring of eye movements (John S. Stahl 2004b, 200; J. S. Stahl, van Alphen, and De Zeeuw 2000; Sakatani and Isa 2004), which confirmed that restrained mice do make horizontal eye movements akin to, and even faster than, human saccades (John S. Stahl 2004b; 2004a; Sakatani and Isa 2007; Wang et al. 2015; Itokazu et al. 2018; Samonds, Geisler, and Priebe 2018; Sato et al. 2019; Zahler et al. 2021). Nonetheless, the rich repertoire of eye movements displayed by freely-moving mice has only been recently characterised following the development of high-speed, head-mounted camera systems (Meyer et al. 2018; Meyer, O’Keefe, and Poort 2020). Leveraging these devices, diverse groups described the complex head/eye movement dynamics of mice performing naturalistic, visually-guided actions such as predation (Michaiel, Abe, and Niell 2020; Holmgren et al. 2021) and distance estimation (Boone et al. 2021; Parker et al. 2022a). In parallel, probing the neuronal circuits that generate and modulate eye movements in mice revealed that, similar to primates (Pouget 2015; Charles Pierrot-Deseilligny, Milea, and Müri 2004), the murine cortex is indeed implicated in oculomotor control (Liu, Huberman, and Scanziani 2016; Itokazu et al. 2018; Sato et al. 2019). High-density electrophysiological recordings and functional imaging techniques have also begun to elucidate the neural bases underlying visual flow information processing (Keller, Bonhoeffer, and Hübener 2012; Leinweber et al. 2017; Meyer et al. 2018; van Beest et al. 2021; Parker et al. 2022b; 2022c), and to identify the circuits governing the integration of visual flow with self-generated proprioceptive and vestibular signals (Vélez-Fort et al. 2018; Bouvier, Senzai, and Scanziani 2020; Guitchounts et al. 2020; Hennestad et al. 2021; Miura and Scanziani 2021; Keshavarzi et al. 2022; Parker et al. 2022b).
Here, we review recent developments in mouse eye movement research. First, we briefly describe the types of eye movements defined in primates and relate their kinematics and mechanisms to those observed in mice. In the second section, we focus on studies carried out in head-fixed mice, primarily aimed at dissecting the circuits underlying oculomotor control. In the third section, we highlight novel findings from freely-moving rodents, which helped advance hypotheses on the function of murine eye movements in naturalistic settings. Finally, we give a brief compendium of brain regions implicated in eye movements in mice and primates.
Types of Eye Movements and Their Categorisations
Five main types of eye movements are widely recognised in mammals: vestibulo-ocular reflexes, optokinetic reflexes, saccades, smooth pursuit, and vergence eye movements. A sixth type, fixational eye movements, is only sometimes included in these classifications; the definition of “fixation", together with that of “saccades'' and “gaze”, has indeed been the source of debate among researchers (Hessels et al. 2018). Eye movement types can also be categorised according to their kinematic properties (i.e. speed, acceleration, amplitude, direction), by their effect on the line of sight (gaze-stabilising or gaze-shifting), or based on their volitional control (reflexive or voluntary). Only a subset of the eye movement types described in primates has been reported in mice, and differences emerge when comparing those eye movement types shared by both groups, particularly regarding the direction of the eyes and their concomitance with head movements (Tehovnik et al. 2021). Additionally, mice, unlike primates, are lateral-eyed animals with larger monocular visual fields (approximately 180°) and a smaller binocular field, spanning approximately 50° and extending above the head of the animal (Sterratt et al. 2013; Holmgren et al. 2021). This section briefly describes the most common categorisations of eye movements and relates the findings in primates to rodents.
Gaze-Stabilising and Gaze-Shifting Eye Movements
The separation between gaze-stabilising and gaze-shifting eye movements is a particularly relevant functional classification. Gaze-stabilising eye movements compensate for head and visual world motion to stabilise the retina's image and enable clear vision (Schweigart et al. 1997). They typically manifest as a slow, centrifugal or centripetal pursuit-like phase (slow phase of nystagmus), at times followed by a fast, centripetal component (fast phase of the nystagmus, or resetting saccade) that recenters the eyes when their orbit reaches maximal eccentricity. While saccades are, by definition, gaze-shifting, they are also part of an eye movement sequence that stabilises gaze. In contrast to gaze-stabilising movements, gaze-shifting eye movements align the eyes on a target. In foveate animals, gaze-shifting movements centre the fovea on the target in either the fronto-parallel plane or depth. Despite the lack of fovea, gaze-shifting eye movements also occur in afoveate animals (Land 2019) such as mice (Meyer, O’Keefe, and Poort 2020; Parker et al. 2022c).
Figure 1.
Eye movement types are categorised by their origin (reflexive or voluntary) and function (gaze-shifting or stabilising). Asterisks mark eye movements observed in primates but not rodents. Note that only ocular drifts and tremors, but not microsaccades, have been described in rodents.
Figure 1.
Eye movement types are categorised by their origin (reflexive or voluntary) and function (gaze-shifting or stabilising). Asterisks mark eye movements observed in primates but not rodents. Note that only ocular drifts and tremors, but not microsaccades, have been described in rodents.
The Vestibulo-Ocular Reflex (VOR)
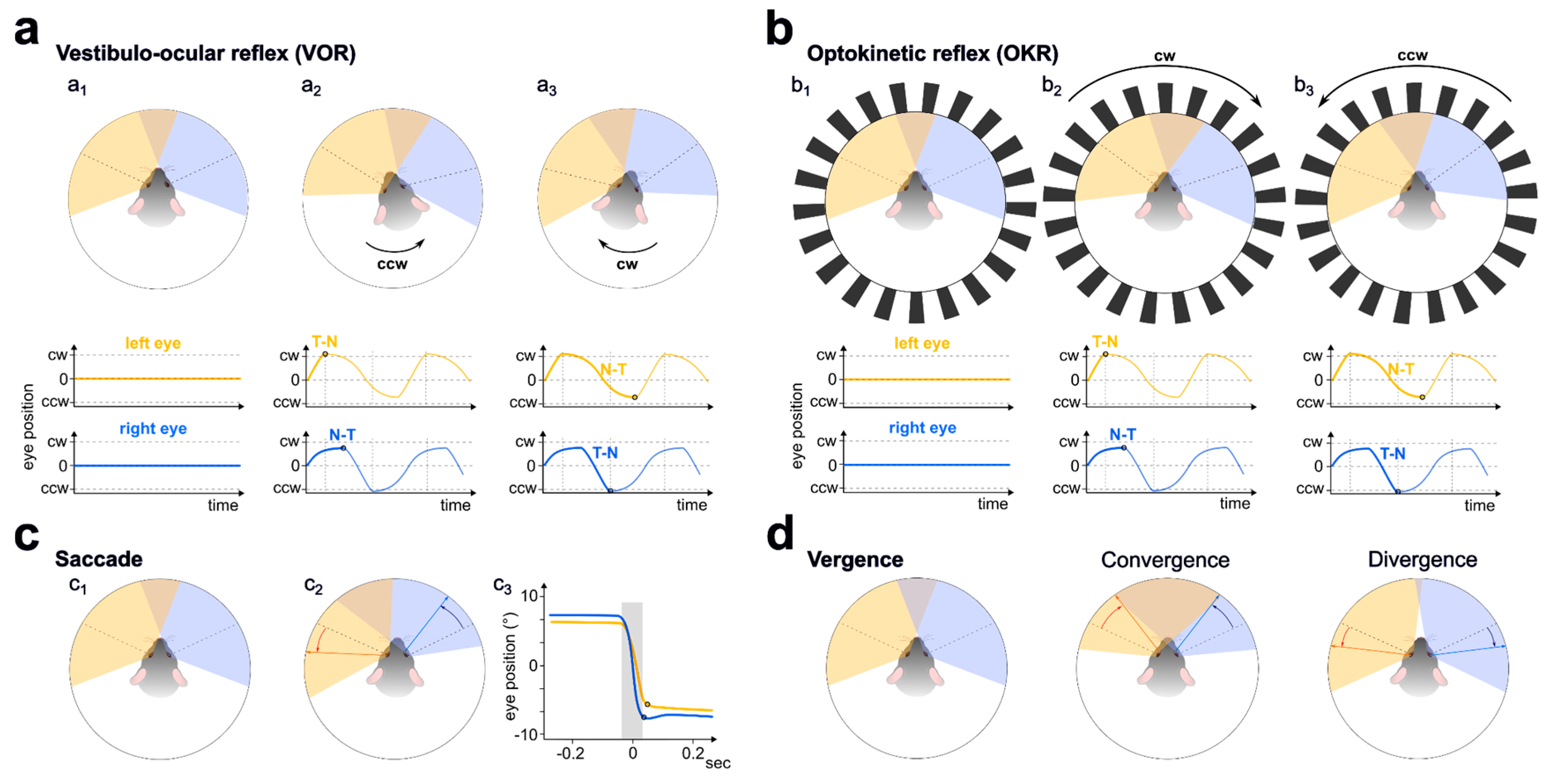
The vestibulo-ocular reflex (VOR) is a purely gaze-stabilising eye movement which generates conjugate eye movements with velocities equal and opposite to head movements along the rotational and translational axes (Figure 2a; Schweigart et al. 1997). The peripheral sensors of the VOR are the semicircular canals, responsible for sensing angular acceleration (generating the angular VOR, aVOR), and the otolith organs, which sense linear acceleration and the position of the head relative to gravity (generating translational VOR, tVOR; Straube and Büttner 2007). The VOR is a plastic and adaptive reflex: the gain of the VOR (i.e., the multiplicative factor between the driving head velocity and the compensatory eye velocity) can be modulated by the visual system. Increases and decreases in gain will result from the incoherent or coherent motion of the visual scene versus the head movement, respectively (Faulstich, Onori, and du Lac 2004). Additionally, VOR gain retention in humans could be increased by inserting consolidation intervals between training blocks (Mahfuz et al. 2018). While visual feedback is crucial for developing an appropriate VOR response, the VOR is similarly engaged in the dark (John S. Stahl 2004b). When visual stimuli are present, the VOR and the optokinetic reflex (OKR, see next section) operate in synergy (visuo-vestibular-optokinetic reflex, vVOR; John S. Stahl 2004b). The VOR in primates and mice is best adapted for mid-high frequency, transient movements of the head, as shown by its optimal gain under these stimulus conditions (Schweigart et al. 1997; John S. Stahl 2004b).
Figure 2.
Gaze-stabilising and gaze-shifting eye movements of mice. (a) Vestibulo-ocular reflex (VOR) in head-fixed or body-restrained mice. a1, upper panel: eye position at rest, gaze direction is indicated by dashed lines. Monocular and binocular fields of view (overlap) for the left and right eye are shaded in yellow and blue; the lower panel represents eye position over time for each eye. a2, upper panel: counterclockwise (CCW) movement of the head or platform causes conjugate contraversive eye movements; bottom panel: clockwise (CW) movement of the left and right eye in the temporo-nasal (T-N) and naso-temporal (N-T) direction, respectively. a3, upper panel: CW movement of the head or platform and respective N-T and T-N movement of the left and right eye in CCW direction (lower panel). (b) Optokinetic reflex (OKR) in head-fixed mice evoked by a rotating drum of black and white gratings. b1, eye position at rest, like in a1. b2, upper panel: movement of the gratings in CW direction; lower panel: eyes move in CW direction, following the visual stimulus. b3: like in b2, but gratings and eyes move in CCW direction. (c) Saccades in head-fixed mice. c1, eye position at rest. c2, a representative CCW saccade. c3, average amplitude and speed of saccades in head-fixed mice, modified from (Meyer, O’Keefe, and Poort 2020). The grey shaded area indicates the gaze-shifting saccade. (d) Vergence eye movements. Convergence: non-conjugate movement in the T-N direction. Divergence: non-conjugate eye movement in the N-T direction. Note that, in all cases, T-N movement is faster and larger in amplitude than the corresponding N-T movement, leading to the widening of the binocular field.
Figure 2.
Gaze-stabilising and gaze-shifting eye movements of mice. (a) Vestibulo-ocular reflex (VOR) in head-fixed or body-restrained mice. a1, upper panel: eye position at rest, gaze direction is indicated by dashed lines. Monocular and binocular fields of view (overlap) for the left and right eye are shaded in yellow and blue; the lower panel represents eye position over time for each eye. a2, upper panel: counterclockwise (CCW) movement of the head or platform causes conjugate contraversive eye movements; bottom panel: clockwise (CW) movement of the left and right eye in the temporo-nasal (T-N) and naso-temporal (N-T) direction, respectively. a3, upper panel: CW movement of the head or platform and respective N-T and T-N movement of the left and right eye in CCW direction (lower panel). (b) Optokinetic reflex (OKR) in head-fixed mice evoked by a rotating drum of black and white gratings. b1, eye position at rest, like in a1. b2, upper panel: movement of the gratings in CW direction; lower panel: eyes move in CW direction, following the visual stimulus. b3: like in b2, but gratings and eyes move in CCW direction. (c) Saccades in head-fixed mice. c1, eye position at rest. c2, a representative CCW saccade. c3, average amplitude and speed of saccades in head-fixed mice, modified from (Meyer, O’Keefe, and Poort 2020). The grey shaded area indicates the gaze-shifting saccade. (d) Vergence eye movements. Convergence: non-conjugate movement in the T-N direction. Divergence: non-conjugate eye movement in the N-T direction. Note that, in all cases, T-N movement is faster and larger in amplitude than the corresponding N-T movement, leading to the widening of the binocular field.
Optokinetic Reflex (OKR)
In both primates and rodents, the optokinetic reflex (OKR), a purely gaze-stabilising eye movement, occurs in response to movements of large portions of the visual field on the retina (Figure 2b; Lappe and Hoffmann 2000). Eye movements during OKR responses are typically tuned to slow-moving visual stimuli, and in rodents, the initial eye acceleration is constrained compared to primates (Harvey, De’sperati, and Strata 1997). The OKR can be elicited by both binocular and monocular stimuli in both the fronto-parallel (horizontal) and anteroposterior plane (in-depth, also termed vergence OKR; Choi and Priebe 2020). During fronto-parallel OKR, eye movements are conjugate; during depth OKR, eyes move asymmetrically. Like the VOR, the OKR is a plastic reflex with adjustable gain. In mice, VOR and OKR are controlled by distinct gain-control circuitry (Faulstich, Onori, and du Lac 2004). In particular, mouse OKR gain can be potentiated by experience and constant exposure to visual stimuli (Faulstich, Onori, and du Lac 2004; Katoh et al. 2000; Kodama and du Lac 2016; Wakita et al. 2017), but also after vestibular damage (Liu, Huberman, and Scanziani 2016). In mice and other lateral-eyed animals, eye movements during the OKR show an asymmetry in velocity, with temporo-nasal (T-N) eye movements being faster than naso-temporal (N-T) ones (Kodama and du Lac 2016). While present in humans during monocular OKR, this asymmetry disappears upon the visual cortex-dependent development of binocular OKR (Brodsky 2019). In primates, slow eye movements of the OKR follow a biphasic response. In the initial phase, upon onset of visual field motion (open-loop phase of the OKR or ocular following response, OFR), eye movements are triggered with short latency, and eye acceleration is steep (Miles, Kawano, and Optican 1986). During the second phase of the OKR, eye velocity increases gradually to compensate for the movement of the visual stimulus (closed-loop phase; Miles, Kawano, and Optican 1986). The OFR reflects the engagement of the cortical smooth pursuit system (Takemura et al. 2007), thought to be absent in afoveate rodents. In fact, the initial phase of the OKR in mice shows only limited similarity to the primate OFR (Tabata et al. 2010). Furthermore, contrarily to primates, mouse eye velocity is steady or even gradually decreases during the closed-loop phase of the OKR when the stimuli are presented over long periods, a phenomenon related to an underdeveloped velocity storage system (van Alphen, Stahl, and De Zeeuw 2001; John S. Stahl 2004b; Kodama and du Lac 2016). Lastly, upon the termination of the visual stimulus, but once the OKR has been initiated, eyes will continue moving, an effect known as optokinetic after nystagmus (OKAN; Gygli et al. 2021) observed in both primates and mice (França de Barros et al. 2020).
Saccades
Saccades are gaze-shifting eye movements whose definition has been the source of debate and has followed numerous criteria (Figure 2c; Hessels et al. 2018). Functionally, saccades can be defined as eye movements performed to rapidly reposition the line of sight and the fovea between fixation periods (Hessels et al. 2018). A purely kinematic definition sees saccades as fast, conjugate, ballistic eye movements that occur in horizontal, vertical, or oblique directions. In mice, saccadic eye movements with high angular speed have been observed during both head-fixed and freely-moving conditions (John S. Stahl 2004b; Meyer, O’Keefe, and Poort 2020; Michaiel, Abe, and Niell 2020; Senzai and Scanziani 2022). The kinematics of saccades, termed “saccadic main sequence” (Bahill, Clark, and Stark 1975), are highly stereotyped, showing a regular relationship between amplitude, duration and velocity. In primates, the speed versus amplitude relationship is non-linear and shows saturation, causing the duration of saccades to scale linearly to their amplitude (Gibaldi and Sabatini 2021). In mice, on the other hand, the speed of saccades scales linearly with their amplitude, while the duration of saccades saturates relatively to their amplitude. Indeed, murine saccades are substantially faster than primates’ (Sakatani and Isa 2007). During a saccadic eye movement, a directional asymmetry like the one present in the OKR and VOR occurs, with saccades in the T-N direction being consistently faster and larger in amplitude compared to N-T ones (a phenomenon disputed in humans, see Takahashi et al. 2019). Lastly, saccade types can be further grouped according to their triggering stimulus and degree of volitional control (see BOX 1 for a detailed classification of saccade types).
Vergence
From a mechanical standpoint, vergence eye movements are defined as movements of the eyes in different directions: eyes can either converge (one or two eyes move towards each other) or diverge (one or two eyes move away from each other, Figure 3d). In primates, though, vergence is defined in relation to binocular vision, namely as a type of asymmetrical eye movement that serves to maintain binocular fusion in depth, by minimising horizontal, vertical and cyclodisparities (Schor et al. 2002; Wibble and Pansell 2020). In rodents, eye movements are largely non-conjugate (i.e. eyes moving in different directions), and vergence eye movements are observed as a consequence of the ‘ocular counter roll’, thus serving the tVOR(Meyer, O’Keefe, and Poort 2020; Michaiel, Abe, and Niell 2020; Holmgren et al. 2021; Wallace et al. 2013). Mice also show vergence eye movements in response to depth OKR (vergence OKR), albeit with a lower gain compared to primates, and are primarily driven by interocular velocity differences (IOVDs; Choi and Priebe 2020). In contrast to primates, changes in binocular disparity cues (CD) in the visual cortex did not elicit vergence eye movements in mice (Choi and Priebe 2020).
Smooth Pursuit
Smooth pursuit is defined as the ability to voluntarily follow a moving target while holding the gaze on it. For high-velocity targets, smooth pursuit movements are interrupted by “catch-up” saccades. Similarly to the OKR, the smooth pursuit is subdivided into an open-loop and a closed-loop phase. During open-loop, eye acceleration is exclusively dictated by the object’s velocity on the retina. During closed-loop, eye motion is also taken into account, and the pursuit system tries to nullify the retinal slip of the target (attempting to achieve a null difference between eye velocity and target velocity). In primates, smooth pursuit is best performed for horizontally versus vertically moving stimuli. Smooth pursuit movements, strictly defined as voluntary smooth gaze-shifting eye movements, have not been observed in rodents. Nonetheless, the neural circuits supporting smooth pursuit may be present in rodents (see OKR section).
Fixational Eye Movements
Fixational eye movements are a complex movement sequence composed of microsaccades, ocular drifts (low-frequency movement of the eye) and tremors (higher-frequency perturbations), attesting to the fact that eyes are never stationary (Collewijn and Kowler 2008; Rucci and Victor 2015). Functionally, fixational eye movements are gaze-stabilising, as they occur during intervals between gaze shifts and serve to maintain a stable image on the retina; from an oculomotor perspective, though, the gaze is rapidly and microscopically shifted during fixation intervals. Image stabilisation during ocular drifts and tremors is not perfect, and this residual motion on the retina was proven necessary for normal vision (Rucci and Victor 2015). Both residual motion and microsaccades have been proposed to prevent stimulus adaptation and to create synchronous population responses in retinal ganglion cells (RGCs; Rucci and Victor 2015). In mice, only ocular eye drifts, but no microsaccades were observed to date (Itokazu et al. 2018; Meyer, O’Keefe, and Poort 2020; Kodama and du Lac 2016).
Look-ahead fixations are orienting eye movements towards objects that will be manipulated in the subsequent steps of a task or naturalistic behaviour (Pelz and Canosa 2001). However, they are yet to be thoroughly described in primates, and only one report has described an equivalent behaviour in mice (Bergmann et al. 2022).
Eye Movements in Head-Fixed Mice
Until recently, head fixation has been the preferred experimental approach to study eye movements in mice. This approach allows precise control of the visual and vestibular stimuli presented to the animal and high temporal resolution when quantifying the resulting eye movements. This section will review recent developments related to the function and control of eye movements in head-fixed mice.
Subcortical and Cortical Mechanisms Underlying the Adaptability of Gaze-Stabilising Eye Movements
The slow phases of the VOR and OKR have been extensively investigated in rodents (Mitchiner, Pinto, and Vanable 1976), and detailed kinematic descriptions of these eye movements have been obtained with diverse methods (van Alphen, Stahl, and De Zeeuw 2001; John S. Stahl 2004b; Tabata et al. 2010; Migliaccio, Meierhofer, and Della Santina 2011; Kodama and du Lac 2016; Imai et al. 2016). On the other hand, only a few studies have described the neural circuits governing the adaptability and plasticity of gaze-stabilising eye movements. Briefly, gain control is done by pontine nuclei through cerebellar and mesencephalic mechanisms, which in turn can be modulated by cortical input.
In vertebrates, the gain of the OKR is typically highest for slow eye movements, complementing the operational range of the VOR (van Alphen, Stahl, and De Zeeuw 2001). The gain of OKR can be potentiated by vestibular lesions (Liu, Huberman, and Scanziani 2016) or by experiencing fast and high-frequency stimulation (Faulstich, Onori, and du Lac 2004; Wakita et al. 2017; Katoh et al. 2000). Likewise, the limited eye acceleration capacity during the OKR can be overcome, thus dismissing the view that OKR dynamics are limited by the properties of ON-DRGCs, the retinal cells primarily driving the reflex (Kodama and du Lac 2016). Notably, the gain of naso-temporal (N-T) and temporo-nasal (T-N) movements could be differentially and independently modulated (Kodama and du Lac 2016). Besides, unilateral flocculectomy caused diminished OKR gain in both ipsilateral and contralateral eyes due to faster eye deceleration and rebounding gaze drift (in both ipsilateral and contralateral eyes), or slower acceleration in the T-N OKR of the ipsilateral eye, exclusively (Kodama and du Lac 2016). These results revealed that distinct, functionally parallel circuits underlie the plasticity of T-N and N-T compensatory eye movements. A possible contribution of the nucleus prepositus hypoglossi (NPH) in support of N-T eye acceleration and rebound drift, similar to what is observed in monkeys (Kaneko 1999), remains to be experimentally confirmed. Similar asymmetries in the directionality of gain potentiation have been observed for the VOR (Voges et al. 2017). Potentiation of simple spiking activity from Purkinje cells in the flocculocerebellum correlated with VOR gain, but only for visual stimuli contraversive to the VOR stimulus (animal and visual field moving in opposite directions), in the N-T direction. Decreases in simple spikes during gain-decrease learning, however, only partially reflected the changes in VOR gain, suggesting that the locus for VOR gain-decrease learning is not located within the cerebellar cortex (Voges et al. 2017). Indeed, reduction in VOR and OKR gain using a “naturalistic” visual-vestibular mismatch protocol led to strong excitability changes at the level of the vestibular nuclei and simultaneous synaptic depression at the cerebellar-vestibular synapses (Carcaud et al. 2017; França de Barros et al. 2020).
Cortical contributions to the adaptability of the OKR have been evaluated by lesioning the cortex in various mammals: in cats (Tusa, Demer, and Herdman 1989), in newborn rats (Prusky et al. 2008) and primates (Zee et al. 1987). Lesions or inactivation of the visual cortex did not ablate the OKR in any of the species but instead reduced its gain and eye acceleration to high-frequency stimuli, especially in the N-T direction. Only recently, a similar role for the primary visual cortex (VISp) has been described in mice (Liu, Huberman, and Scanziani 2016). Here, corticofugal projections from the visual cortex to the nucleus of the optic tract and dorsoterminal nucleus (NOT/DT) support fronto-parallel OKR potentiation after lesions to the vestibular system or after persistent exposure to visual stimuli (Liu, Huberman, and Scanziani 2016). OKR stimulation did not significantly enhance the activity in the motor layers of the superior colliculus (SCm) or the ventral lateral geniculate nucleus of the thalamus (vLGN), and the activity did not correlate with OKR gain, confirming the cortex-specific contribution to OKR potentiation (Liu, Huberman, and Scanziani 2016). On the other hand, silencing VISp had no significant effect on vergence OKR movements in mice, although the gain was not measured in this study (Choi and Priebe 2020). Interestingly, in cats, VOR adaptation was shown to partially depend on the middle suprasylvian cortex (area 7, equivalent to the primate V4; Tusa, Demer, and Herdman 1989; Jocelyne Ventre 1985a). Despite widespread activation of the rodent cortex by vestibular stimulation (Ventre-Dominey 2014; Rancz et al. 2015), the contribution of the cortex to the gain of the VOR in mice is unknown.
These findings shed light on the neural circuits underlying the adaptability of gaze-stabilising eye movements in mice. Intriguingly, the strong asymmetry of gain and speed observed in the directionality of gaze-stabilising eye movements is supported by parallel subcortical circuits governing their plasticity. Finally, the adaptability of OKR in mice also depends on the visual cortex, a finding that highlights important similarities between the murine and the primate visual system.
Subcortical and Cortical Mechanisms Underlying the Generation of Stimulus-Oriented Saccades
In head-fixed conditions, mice perform horizontal eye movements kinematically akin to primate saccades. Recent studies have proposed functional roles for these eye movements and probed the neural substrates required to generate horizontal saccades in head-fixed mice.
In a recent report, horizontal saccades observed in head-fixed mice were triggered by natural scenes and were related to natural image statistics (Samonds, Geisler, and Priebe 2018). Saccades occurred in bursts interspersed by fixation intervals, and their size could be predicted by the minimum distance needed to increase population responses (i.e. decrease image correlation) of modelled simple and complex cells with distinct receptive field sizes, selectivity and tolerance (Samonds, Geisler, and Priebe 2018). Larger cortical receptive fields correlate inversely with visual acuity; thus, mice need to make larger saccades to promote decorrelation of visual input compared to other species (Samonds, Geisler, and Priebe 2018). Furthermore, monocularly deprived mice made larger saccades, potentially resulting from decreased visual acuity (Samonds, Geisler, and Priebe 2018). Whether retinal or cortical computations support this saccade initiation mechanism remains to be determined, although the cortical generation of saccades had previously been demonstrated in mice. Specifically, mice were trained to perform stimulus-oriented saccades towards a LED target (Itokazu et al. 2018). Mice showed directional asymmetries in saccadic velocities (T-N saccades were faster and larger than N-T saccades), and their saccades were faster than those typically performed by primates (reaching angular speeds of about 300 °/sec), a finding congruent with previously published results. Electrical stimulation of the supplementary motor areas (MOs), but not VISp or higher visual areas (HVAs) elicited saccades of variable amplitude, while acute silencing of the MOs impaired the acquired saccadic behaviour (Itokazu et al. 2018). Upon prolonged silencing of the ipsilateral MOs, contralateral cortical plasticity changes were sufficient to restore the acquired saccadic eye movement behaviour (Sato et al. 2019). MOs projections may exert control of saccade generation via their afferents to eye movement-related areas of the midbrain, including the SC or the contralateral striatum (Itokazu et al. 2018; Oh et al. 2014). Regardless of potential cortical control, or lack thereof, the SCm was shown to indeed trigger the generation of saccades in mice analogous to primates (Wang et al. 2015; Zahler et al. 2021). Specifically, activation (optogenetic and electrical) or inhibition of the SCm via optogenetics respectively triggered or altered the frequency of contralateral saccades (Wang et al. 2015; Zahler et al. 2021). SCm inhibition caused biases in saccade targets in one study (Zahler et al. 2021) following the known laterality of eye movement generation. In contrast, when SCm silencing was achieved via the activation of GABAergic neurons, saccade probability was slightly increased (Wang et al. 2020), possibly due to the engagement of long-range inhibition circuits (Essig, Hunt, and Felsen 2021). Furthermore, electrical stimulation of the SCm along the dorsoventral axis was shown to be sufficient to generate saccades with different amplitudes and directions, revealing that the topological organisation of saccade generator circuits in the murine SCm is similar to the primate’s (Wang et al. 2015).
Saccades performed by mice in the studies mentioned above were elicited by training (Itokazu et al. 2018; Sato et al. 2019), using electrical or optogenetic stimulation (Wang et al. 2015; Itokazu et al. 2018; Sato et al. 2019), or “spontaneously” generated in the presence of a visual scene (Sakatani and Isa 2007; Samonds, Geisler, and Priebe 2018). Mice, however, were shown to innately (i.e. in the absence of training) perform stimulus-oriented, conjugate horizontal saccades towards tactile (air-puff and whisker stimulation) and, to a lesser extent, auditory stimuli (Zahler et al. 2021). Perhaps surprisingly, simple visual stimuli (blinking LED) did not evoke stimulus-targeted saccades in this study. Intriguingly, stimulus-evoked saccades were performed in close correspondence with attempted head movements in the same direction. Spontaneous gaze shifts in this study were instead preceded by slow orienting head movements, in line with what had been previously observed (Meyer, O’Keefe, and Poort 2020). Orienting eye movements were shown to occur in mice not only in response to a stimulus but also in a “look ahead” fashion. Bergmann et al. (2022) recently investigated saccades linked to navigation in a plus-maze in head-fixed mice and observed that saccades occurred together with asymmetrical whisking towards the direction of the future turn, regardless of body turns, as the animals moved backwards along the maze to restart the trial.
In sum, head-fixed mice show diverse saccadic behaviour occurring spontaneously or in response to various stimuli. Like in primates, mouse saccades can be generated in the superior colliculus and the supplementary motor cortex. Furthermore, saccade size in mice is related to the ensemble statistics of natural images, thought to decorrelate retinal input to prevent stimulus adaptation.
Section summary
In this section, we highlighted relevant aspects of eye movements performed by rodents under head-fixed, stimulus-controlled conditions. Neural correlates responsible for the adaptation and plasticity of gaze-stabilising reflexes were found both at the cortical and subcortical levels. In addition, but of no less importance, increasing data shows that head-fixed mice can perform conjugate, rapid eye movements comparable to primate saccades on the kinematic and - possibly - functional level.
Eye Movements in Freely-Moving Mice
Measuring eye movements in freely-moving, lightweight animals with lateral eyes, like rodents, is not trivial (Kautzky and Busse 2020). This challenge has been recently overcome using miniaturised magnetic (Payne and Raymond 2017) or camera-based eye-tracking devices in rats (Wallace et al. 2013) and mice (Meyer et al. 2018). Combined with recordings of head movements using a head-mounted inertial measurement unit, these studies were the first to describe head/eye coupling dynamics in mice, opening up the field for further research.
VOR-Linked Eye Movements: Head-Tilt Compensation and The “Saccade and Fixate” Behaviour
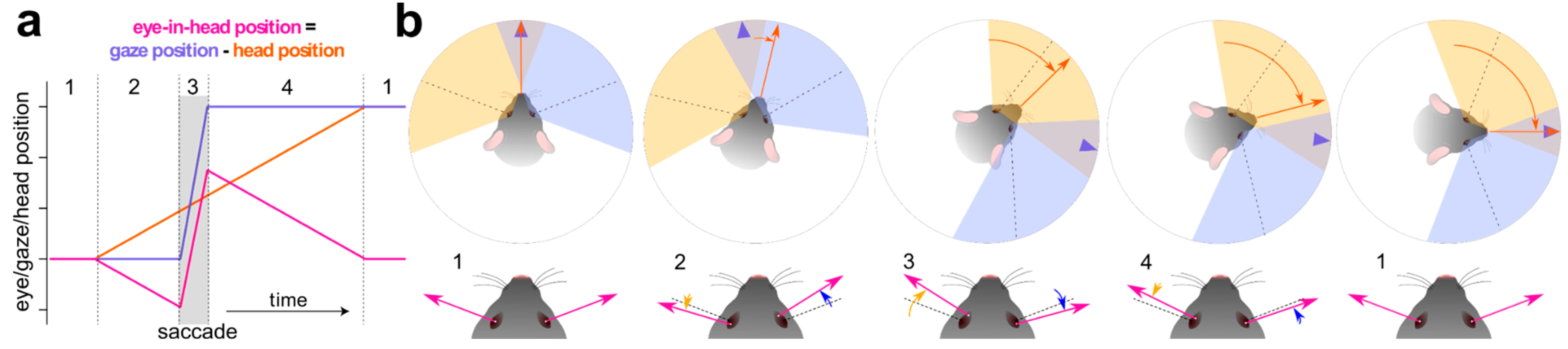
During open-field exploration, rat eye movements were shown to be predominantly non-conjugate (Wallace et al. 2013). As a function of the ocular counter roll, eyes move in opposite directions when the head rolls or in response to changes in head pitch (upward and outward for negative pitch and vice versa). This behaviour was also recently observed in freely-moving mice (Meyer, O’Keefe, and Poort 2020; Michaiel, Abe, and Niell 2020). Like rats, the mouse's average eye position is slightly more elevated than the horizontal plane (+30°) at the resting head position. Functionally, changes in eye position and inferred gaze direction during head tilt could serve to stabilise the visual field along the horizontal plane so that the celestial and the ground sampling portions of the retina are kept in place (Meyer, O’Keefe, and Poort 2020; Saleem 2020). Non-conjugate torsional movements linked to the VOR, similarly supporting gaze stabilisation, were also described by a recent study (Holmgren et al. 2021). Besides, compared to head-fixed mice, the degree and variability of divergence are increased in freely-moving mice, likely because of the vestibular compensatory reflexes initiated by head motion (Payne and Raymond 2017). However, not all eye movements of freely moving rodents proved to be gaze-stabilising. When trying to predict the eye movements in freely moving animals using pitch/roll head movements, Meyer et al. (2020) observed that a fraction of the eye movements could not be explained. This unexplained variability reflected conjugate eye movements generated during head yaw (Meyer, O’Keefe, and Poort 2020), similar to what had been previously observed (Payne and Raymond 2017). Conjugate eye movements were characterised by a triphasic sequence: an initial slow gaze-stabling slow movement (eyes and head counter-rotate), followed by a fast resetting saccade (eyes and head rotate in the same direction), and a final slow compensatory eye movement that recenters the gaze (Figure 3). In addition, saccades showed a faster speed in the T-N direction, similar to head-fixed animals, leading to changes in binocular field width during the head turn (Meyer, O’Keefe, and Poort 2020). This eye movement sequence, or behaviour, is the well-described “saccade and fixate” eye movement sequence, which is highly conserved across vertebrates (Land 2019) and occurs as a function of the angular VOR (Meyer, O’Keefe, and Poort 2020).
Figure 3.
Saccade and fixate eye movement sequence in freely-moving mice. (a) Representative trajectories of eyes in head, gaze, and head position over time during the saccade and fixate eye movement sequence. The shaded area in grey indicates the gaze-shifting saccade and the various phases of the movement are indicated with numbers. (b) Phases of the saccade and fixate behaviour. The orange arrow indicates the position and movement of the head; the purple arrow indicates gaze direction. 1, eye, gaze, and head position at rest. 2, the head turns in the CW direction and the eyes move in the opposite direction, as indicated by the zoom in the lower panel (slow, gaze-stabilising phase). 3, eyes saccade towards the direction of the head (fast, gaze-shifting phase). 4, eyes move in the opposite direction to the head, to recenter gaze after phase 3, as the head keeps turning (slow, gaze-stabilising phase).
Figure 3.
Saccade and fixate eye movement sequence in freely-moving mice. (a) Representative trajectories of eyes in head, gaze, and head position over time during the saccade and fixate eye movement sequence. The shaded area in grey indicates the gaze-shifting saccade and the various phases of the movement are indicated with numbers. (b) Phases of the saccade and fixate behaviour. The orange arrow indicates the position and movement of the head; the purple arrow indicates gaze direction. 1, eye, gaze, and head position at rest. 2, the head turns in the CW direction and the eyes move in the opposite direction, as indicated by the zoom in the lower panel (slow, gaze-stabilising phase). 3, eyes saccade towards the direction of the head (fast, gaze-shifting phase). 4, eyes move in the opposite direction to the head, to recenter gaze after phase 3, as the head keeps turning (slow, gaze-stabilising phase).
In sum, head/eye coupling dynamics in mice during free exploration resemble those previously described in rats. Non-conjugate and conjugate eye movements occur to compensate for changes in head tilt and yaw, respectively, according to vestibular input. Conjugate eye movements associated with head yaw follow a “saccade and fixate” movement pattern that serves to shift gaze, as observed in other afoveate animals.
Control and Reorganisation of Head/Eye Movements during Visually Guided Behaviours
Prey Capture and Object Exploration
Mice rely on vision to predate crickets, which they perform innately (Hoy et al. 2016). While they can successfully complete the capture in the dark, the latency is markedly increased (Hoy et al. 2016), suggesting that vision crucially supports this behaviour. Several labs have implemented variations of this prey capture task as a model to study the visual processing mechanisms underlying prey capture in mice.
Recent studies have demonstrated that the head/eye coupling dynamics observed during free exploration are preserved when mice perform visually guided tasks. First, the “saccade and fixate” pattern was also shown to be present during visually guided behaviours, as eyes did not change their position systematically when tracking a visual stimulus but instead moved to compensate for the orienting head movements (Meyer, O’Keefe, and Poort 2020). Second, torsional, gaze-stabilising eye movements during free exploration were also not significantly different from those performed during prey capture behaviour (Holmgren et al. 2021). However, while head/eye coupling dynamics were unaltered, head/eye movements became highly structured during prey capture tasks (Michaiel, Abe, and Niell 2020; Holmgren et al. 2021). Michaiel et al. (Michaiel, Abe, and Niell 2020) have shown that (i) mice maintain the head in a neutral pitch position, hence stabilising the eyes at a neutral vergence during approach; (ii) the prey is kept within the binocular visual field; (iii) mice employ a saccade and fixate strategy consisting of head turns and accompanying eye movements that progressively decrease in amplitude as a function of their distance to the target. Eye position at the end of the head turn did not increase target accuracy compared to head position, indicating that gaze shifts were purely compensatory for head movements (Michaiel, Abe, and Niell 2020). Separate experiments by Holmgren et al. (Holmgren et al. 2021) showed that mice perform orienting head/eye movements to maintain the prey in the part of the visual field with the least optic flow. This part corresponds to the point in space from which all motion appears to emanate during self-motion, termed the focus of expansion (FOE; Gibson 1950). It is within the FOE where the binocular overlap is maintained with the highest probability (Holmgren et al. 2021), strengthening the hypothesis that mice use binocular cues to track prey. In line with these findings and consistent with this hypothesis, it was shown that monocular enucleation affected all phases of the prey hunt (Johnson et al. 2021). In the same study, mice were shown to maintain a negative head pitch when approaching crickets (Johnson et al. 2021), a marked difference from Michaiel et al. (Michaiel, Abe, and Niell 2020). This discrepancy, however, is unlikely to reflect a biological difference, as the head pitch was measured in relation to different baseline values. It was further demonstrated that the last phase of the prey approach was characterised by a sharp elevation of head pitch, which stereotypically preceded a bite-and-grab event sequence (Johnson et al. 2021). How the eyes move during this sharp head movement and whether any additional mechanisms to prevent the ocular counter-roll reflex are in place remain unknown. Lastly, mice were shown to make compensatory head/eye movements that align the “focea” to the frontal-facing direction during open-field or object exploration (van Beest et al. 2021). Aligning the focea at this level positions it at the FOE, similar to what was observed by Holmgren et al. (Holmgren et al. 2021). The focea is a cortical region with enhanced spatial representation, mapping a portion of visual space lying slightly above the mouse (10-20° above ground (van Beest et al. 2021). This area contains an overrepresentation of binocular regions of space (i.e. more neurons sampling the binocular field of view) and neurons with smaller receptive fields in LM and AL (van Beest et al. 2021). It is tempting to compare the murine focea to the cortical region innervated by the fovea in other animals, as these show anatomical and functional resemblance. Nevertheless, further studies are needed to confirm the contribution of the focea to murine vision.
Thus, when mice move their head and eyes to track prey and navigate an environment, their retinas are repositioned to sample the behaviorally relevant portions of the visual field. During prey capture, as mice approach crickets, the FOE is sampled by the dorso-temporal portion of the retina (Holmgren et al. 2021). In mice, the dorso-temporal retina is enriched in Alpha-ON and Alpha-OFF sustained RGCs with centre-surround receptive fields (sONα- and sOFFα-RGCs) projecting to both to the thalamus and SC (Huberman, Feller, and Chapman 2008; Bleckert et al. 2014). Additionally, ipsilaterally projecting RGCs in the ventro-temporal portion of the retina (ipsi-RGCs, similarly targeting both thalamus and SC) were necessary for the completion of prey capture behaviour, as animals with ablated ipsi-RGCs were significantly less successful in converting approach phases to capture phases (Johnson et al. 2021). Ipsilaterally projecting RGCs are thought to support stereopsis in mammals (Wilks et al. 2013). In the mouse, ipsi-RGCs represent nine of the more than forty RGC types (Johnson et al. 2021). Four of the nine ipsi-RGC types (contrast encoding ipsi-RGCs, including sONα- and sOFFα-RGCs) were shown to respond to prey-mimetic stimuli in retinal preparations in vitro (Johnson et al., 2021). Notably, sONα- and sOFFα-RGCs in the ventro-temporal portion of the retina have smaller dendritic fields, indicating enhanced spatial resolution (Bleckert et al. 2014). Thus, dorsal and ventral specialisations of the temporal retina likely support prey-capture mice. The retinotopic organisation also suggests that ipsi-RGCs substantially project to the focea, a hypothesis that needs to be confirmed experimentally.
Altogether, these studies revealed that head/eye movements in mice are finely tuned and controlled to achieve optimal sampling of the visual world during exploration and prey capture. Furthermore, mice show retinal and cortical specialisations linked to its processing like other animals with functional binocular vision. Accordingly, both binocular and stereoscopic cues are required for efficient prey capture. Lastly, while resetting saccades linked to orienting head turns are readily documented in freely-moving mice, there is a paucity of data on saccades performed towards non-visual stimuli under freely-moving conditions.
Depth Perception
Depth perception in mammals is achieved using both monocular and binocular cues. Recent studies summarised below have shown that mice strongly rely on binocular cues to estimate distances during naturalistic, visually guided tasks but can also use monocular cues.
Two independent groups have recently employed naturalistic, non-head-restrained tasks to study the link between head/eye coupling, depth perception, and distance estimation in mice (Boone et al. 2021; Parker et al. 2022a). Mice were shown to rely on binocular cues to estimate distances in a gap-jumping task, but to resort to monocular strategies if binocular vision is hindered (Parker et al. 2022a). Consistent with the need for the animals to use motion parallax to estimate depth with only one eye, monocular deprivation or silencing of binocular VISp caused mice to perform more head/eye coupled movements. Interestingly, monocularly deprived mice kept their head in a sharper downward pitch position (Parker et al. 2022a), similar to when chasing crickets (Johnson et al. 2021). However, combined monocular deprivation and bilateral silencing of the monocular portions of VISp did not affect distance estimation (Parker et al. 2022a), suggesting that ipsilateral information from a single eye was sufficient for mice to estimate distances successfully. The increased head/eye movements, particularly the downward pitch, may then support the realignment of binocular VISp to the direction facing the animal and motion parallax. In either case, mice did not make systematic changes in eye vergence angles that could not be explained by head movements during the task (Parker et al. 2022a). Likewise, mice were shown to rely almost exclusively on binocular cues to perform a modified version of the cliff task (Boone et al. 2021). By ingeniously combining the cliff task with a pole descent task, mice were forced to align their head in the position optimal to process binocular cues, which they used to identify the nearest platform to land on (i.e. discriminate distances based on depth estimation; Boone et al. 2021). Interestingly, mouse behaviour was consistent with the disparity tuning measured in VISp (see BOX 3; Samonds, Choi, and Priebe 2019). Nonetheless, this study only provides indirect evidence for using absolute disparity selectivity in VISp to estimate depth. Depth perception can be confounded by non-stereoscopic binocular cues (Chopin et al. 2019), so further experiments are needed to clarify whether mice use stereoscopy or other cues to estimate depth. Why impaired binocular vision significantly altered the success of mice in the pole descent cliff task but not in the gap-jumping task needs further clarification.
In summary, while binocular vision appears to be the preferred way to estimate depth, mice do not systematically change their vergence eye angle to align focus in-depth as primates do. Instead, mice move their head and make compensatory eye movements to estimate distance accurately. Impaired performance upon binocular silencing of the VISp suggests that binocular fusion is required for depth estimation and that mice plausibly rely on changes in disparity tuning to judge distances.
Section summary
This section summarised recent findings on head/eye movement dynamics in freely-moving mice. In unrestrained conditions, mice use their head to optimally sample visual information both during open-field exploration and visually guided tasks. Eye movements are tightly coupled to the VOR: non-conjugate eye movements stabilise the gaze along the horizontal plane following head tilt. Conjugate eye movements are part of a saccade and fixate strategy to shift gaze following head yaw. While conjugate across both eyes, horizontal saccades show asymmetries in the naso-temporal versus temporo-nasal velocities, hindering the maintenance of the binocular field’s width during the head turn. Therefore both non-conjugate and conjugate eye movements undermine stable stereopsis in mice. Regardless, mice were shown to rely on binocular vision during naturalistic tasks and marked binocular disparity tuning is present throughout the visual cortex. Loss of binocularity was also linked to an increased frequency of head/eye coupled movements when estimating distances. Whether loss of binocularity similarly disrupts head/eye movements during prey capture and how lack of stereoscopy shifts the relative contributions of change in disparity tuning versus interocular eye velocity differences is not yet known.
Concluding Remarks and Future Perspectives
Over the last decades, the appeal of the murine visual system has dramatically increased among system neuroscientists (Huberman and Niell 2011). Large-scale recordings and manipulation of neural activity have recently been combined with high-resolution video-oculography in mice (Parker et al. 2022c; 2022b; Meyer et al. 2018; Abdolrahmani et al. 2021; Liu, Huberman, and Scanziani 2016; Samonds, Choi, and Priebe 2019). This, together with the strive to probe neural circuits in naturalistic tasks (Miller et al. 2022), has ushered in a series of studies endowed with unprecedented translational and ethological relevance. Indeed, while relating all primate eye movement behaviours and neuronal substrates to murine ones is wishful thinking (Huberman and Niell 2011), emerging discrepancies should be used to refine or refute hypotheses on neural homology across species (Glickfeld and Olsen 2017). Brain structures evolve to process ethologically relevant stimuli; evolutionarily conserved structures, such as the superior colliculus, show a remarkable degree of functional and anatomical similarity across vertebrates, as highlighted in this review (for an in-depth review on SC, see Isa et al. 2021, for a review on the topic). To what extent this applies to evolutionarily younger structures and processes, like the top-down influence of the visual cortex, is an open and timely question that we are beginning to tackle. Recent transformative studies have demonstrated that eye movements in mice are primarily coupled to the VOR during freely-moving exploration (Meyer et al. 2018; Meyer, O’Keefe, and Poort 2020; Michaiel, Abe, and Niell 2020; Holmgren et al. 2021). In this context, three compelling observations are to be made. First, while head/eye coupling dynamics are strictly maintained during visually guided behaviours, head/eye movements are finely controlled and optimised to use binocular cues. Second, while head-fixed mice perform attempted head rotations when they perform saccadic eye movements, the dynamics of the head movements change according to the saccade-initiating trigger (spontaneous vs stimulus-oriented, (Zahler et al. 2021). Third, several groups have provided evidence of retinal (Johnson et al. 2021), subcortical and cortical (van Beest et al. 2021) specialisations supporting distinct phases of visual behaviours. It is thus plausible that the mouse cortex exerts control over eye movement behaviours observed in both head-fixed and freely-moving mice; causal relationships are now to be established by perturbing the system in ethologically relevant settings. In conclusion, we believe that mouse eye movement dynamics is a powerful yet poorly exploited diagnostic tool for investigating the input-output transformation exerted by mammalian subcortical and cortical circuits in ethologically relevant experimental settings.
Glossary
Binocular vision is the ability to process monocular images simultaneously.
Binocular fusion is the second grade of binocular vision; both monocular images, which are different due to the position of the eyes in the head, are merged into a single one. Binocular fusion requires both eyes to focus on the same object.
Stereopsis: is the highest grade of binocular vision, supporting our ability to see in three dimensions. Stereopsis relies on binocular disparity tuning, which occurs after binocular fusion. Stereopsis facilitates depth perception and distance estimation thanks to changes in disparity tuning with self-motion.
Disparity-tuning refers to the response of neurons in VISp and HVAs to differences in monocular images (binocular disparity). Disparity tuning drives cortex-dependent vergence eye movements in primates but not in mice. Crossed disparities occur when the object is beyond the focus plane; uncrossed disparities occur when the object is before the focus plane. Disparity-tuned neurons (also known as stereo-sensitive neurons) are described in terms of their sensitivity to left and right eye stimuli (receptive fields) or to the sum or subtraction of the images presented to both eyes. Disparity tuning does not imply stereopsis.
Random dot stereograms are stimuli used to test stereopsis. They are correlated or anti-correlated patterns presented to both eyes; when binocular fusion occurs, a 3D depth perception is created from the 2D images.
Interocular velocity differences are binocular cues not requiring binocular fusion to elicit eye movements. IOVDs are the differences in object motion in both eyes (i.e. monocular cues deriving from both eyes). IOVDs were shown to drive cortex-independent vergence eye movements in head-fixed mice.
Parallax is the apparent displacement of an object from two different lines of sight. Motion parallax refers to the phenomenon that objects closer to the observer move faster than those further away and can function as a monocular depth perception cue.
Conjugate and non-conjugate eye movements are movements of both eyes occurring in the same direction (i.e. N-T for the right eye and T-N for the left, or vice versa) or movements of both eyes in opposite directions, respectively.
Convergent and divergent eye movements are non-conjugate eye movements occurring in the T-N or N-T direction for both eyes, respectively.
Saccadic suppression refers to the reduction of sensitivity in the visual system immediately before and after a saccade. Serves to minimise perturbations of visual flow caused by the saccade. Saccadic suppression acts both peripherally and centrally.
Corollary discharge refers to a copy of the motor command sent to sensory cortices upon movement planning or initiation. The corollary discharge signal suppresses the sensory activity driven by the reafference (i.e. the sensory stimuli derived from the planned motor movement).
List of abbreviations
ACA, anterior cingulate area
AOS, accessory optic system
AOT, accessory optic tract
CN, caudate nucleus
DS-RGC, direction-sensitive retinal ganglion cells
DT, dorsal terminal nucleus
FEF, frontal eye fields
FOE, field of expansion
FOR, fastigial nucleus of the cerebellum
HVAs, higher visual areas (including RSPagl)
INC, interstitial nucleus of Cajal
IO, inferior olive
IOVD, interocular velocity differences
LT, lateral terminal nucleus
MDJ, medial diencephalic junction
MOs, supplementary motor area
MST, medial superior temporal cortex
MTC, medial temporal cortex
MT, medial terminal nucleus
NOT, nucleus of the optic tract
N-T, naso-temporal
NPH, nucleus prepositus hypoglossi
OFR, ocular following response
OKAN, optokinetic after nystagmus
OKR, optokinetic reflex
OMV, oculomotor vermis
PFC, prefrontal cortex
PMRF, pontomedullary reticular formation
PPC, posterior parietal cortex
PPRF, paramedian pontine reticular formation
RGC, retinal ganglion cell
RSPd, dorsal retrosplenial cortex
RSPv, ventral retrosplenial cortex
SC, superior colliculus
SCm, intermediate-deep superior colliculus
SCs, superficial superior colliculus
SEF, supplementary eye fields
SNr, substantia nigra
Str, striatum
T-N, temporo-nasal
V1, primary visual cortex (primate)
VISp, primary visual cortex (rodent)
VISam, anteromedial visual cortex
VISal, anterolateral visual cortex
VISpm, posteromedial visual cortex
VISrl, rostrolateral visual cortex
VOR, vestibulo-ocular reflex
aVOR, angular vestibulo-ocular reflex
vVOR, visual vestibulo-ocular reflex
dLGN, dorsal lateral geniculate nucleus
dlPFC, dorsolateral prefrontal cortex
ipsi-RGC, ipsilaterally projecting RGCs
tVOR, translational vestibulo-ocular reflex
BOX 1-Diversity of Saccades
Reflexive, Exogenously-Driven Saccades
Stimulus-oriented saccades (also known as visually-guided or reflexive saccades) are triggered by an external stimulus (exogenously-driven sensorimotor saccades). They reposition the gaze to target the stimulus optimally and do not require strong volitional control (McDowell et al. 2008). A quantitative description of spontaneous saccades performed by head-fixed mice was provided by Sakatani and Isa (Sakatani and Isa 2007). In mice, the saccadic main sequence is conserved for both spontaneous and electrically evoked saccades (Wang et al. 2015). Stimulus-oriented saccades also occur while viewing a static visual scene, as it has been demonstrated that natural image statistics correlate with saccade size in primates and mice (Samonds, Geisler, and Priebe 2018).
Express saccades are stimulus-oriented saccades with extremely short latency, as low as 110 ms (McDowell et al. 2008). Stimulus-oriented saccades with such short latencies have also been reported in mice (Zahler et al. 2021).
Resetting saccades occur during gaze-stabilising eye movements (OKR and VOR). They represent the quick phase of the jerk nystagmus (John S. Stahl 2004b), occurring in the same direction as the head movement during VOR while in the opposite direction of the visual stimulus during OKR. Resetting saccades are not under volitional control in either mice or primates. Strictly speaking, resetting saccades do compensate for head movements; recent reports in mice (Meyer, O’Keefe, and Poort 2020; Michaiel, Abe, and Niell 2020) describe resetting saccades as “compensatory saccades”, although this definition may be misleading due to the term used in a human clinical context (see below). Finally, resetting saccades in mice and humans are slower than stimulus-oriented saccades, although the directional bias in velocity persists (Sakatani and Isa 2007; Garbutt, Harwood, and Harris 2001).
Microsaccades, like stimulus-oriented saccades, are fast ballistic eye movements showing a “main sequence” and can only be operationally distinguished from other saccade types (Martinez-Trujillo 2022). Microsaccades are typically defined as involuntary eye movements that occur during fixation, although evidence shows that microsaccades can be under a certain degree of voluntary control (Willeke et al. 2019). Microsaccades could prevent stimulus adaptation on the retina, counteract ocular drift, and shift spatial attention when the eyes are free to move (Rolfs 2009). Stimulus-oriented saccades and microsaccades likely share control mechanisms and functions (Rucci and Victor 2015). Even though their amplitude is microscopic, they shift the visual image over several retinal receptors. Microsaccades have not yet been reported in mice.
Compensatory saccades occur in humans as compensation for a defective VOR, prevalent in older, healthy adults (Anson et al. 2016). Compensatory saccades have not yet been described in mice (but see resetting saccades above).
Voluntary, Endogenously-Driven Saccades
Endogenously driven eye movements are of great experimental value to study top-down processes like attention and memory (Zhao et al. 2012). Even though higher cognitive processes like selective visual attention have been identified in mice (Wang and Krauzlis 2018), the following types of saccades have not yet been reported.
Predictive saccades occur when the gaze is fixated on the future location of a target that is moving predictably (McDowell et al. 2008).
Antisaccades are intentional gaze shifts away from a visual stimulus (Rommelse, Van der Stigchel, and Sergeant 2008). Misdirections of antisaccades are linked to dysfunctions of the frontal cortices (Rommelse, Van der Stigchel, and Sergeant 2008).
Memory-guided saccades occur without a visual stimulus and are guided by the memory of a previously presented stimulus (C. Pierrot-Deseilligny et al. 1991). Deficits of memory-guided saccades are related to dysfunctions of the frontal cortices (C. Pierrot-Deseilligny et al. 1991).
BOX 2-Brain Circuits Underlying the Generation and Control of Eye Movements
Mechanistically, the movement of the eyes within the eye socket is generated by the contraction of six extraocular muscles, innervated by the oculomotor nuclei (Edinger-Westphal, nucleus of Darkschewitsch, and main oculomotor nuclei) in the midbrain and brainstem (see Sparks 2002 for an extensive review on subcortical control of eye movements). Generating the motor command requires cortical and subcortical integration of externally and internally generated neural activity. Historically, it was thought that each eye movement type was governed by discrete neural circuits operating in parallel, a view no longer prevailing (John S. Stahl 2004b; Büttner-Ennever and Horn 1997). To help orient the reader, we list brain areas involved in eye movements, primarily in primates and, where information is available, in mice (see Table 1 and Figure 4 for a summary).
Table 1.
list of brain regions known to contribute to the generation of eye movements in primates and mice. The colour code indicates the species: primate, mouse, or both.
Table 1.
list of brain regions known to contribute to the generation of eye movements in primates and mice. The colour code indicates the species: primate, mouse, or both.
| |
Cortical |
Subcortical
(other than oculomotor nuclei) |
| OKR (slow phases) |
MT, MST, PPC, V1, VISp
|
AOS, vestibular nuclei, vestibulocerebellum |
| VOR (slow phases) |
PPC |
Vestibular nuclei, vestibulocerebellum, AOS (vVOR) |
| Saccades |
PFC (dlPFC, FEF, SEF, ACA); PPC
MOs
|
Basal ganglia (CN, Str, SNr); SCm, NRTP, PPRF, INC, MDJ; cerebellar OMV and FN
|
| Vergence |
FEF (area 8), V1 |
SCm, NRTP, vestibulocerebellum |
| Smooth pursuit |
FEF, MT, MST |
SCm, NRTP, vestibulocerebellum, cerebellar OMV and FN |
| Fixational eye movements |
FEF, PPC |
rostral SCm, PPRF |
Figure 4.
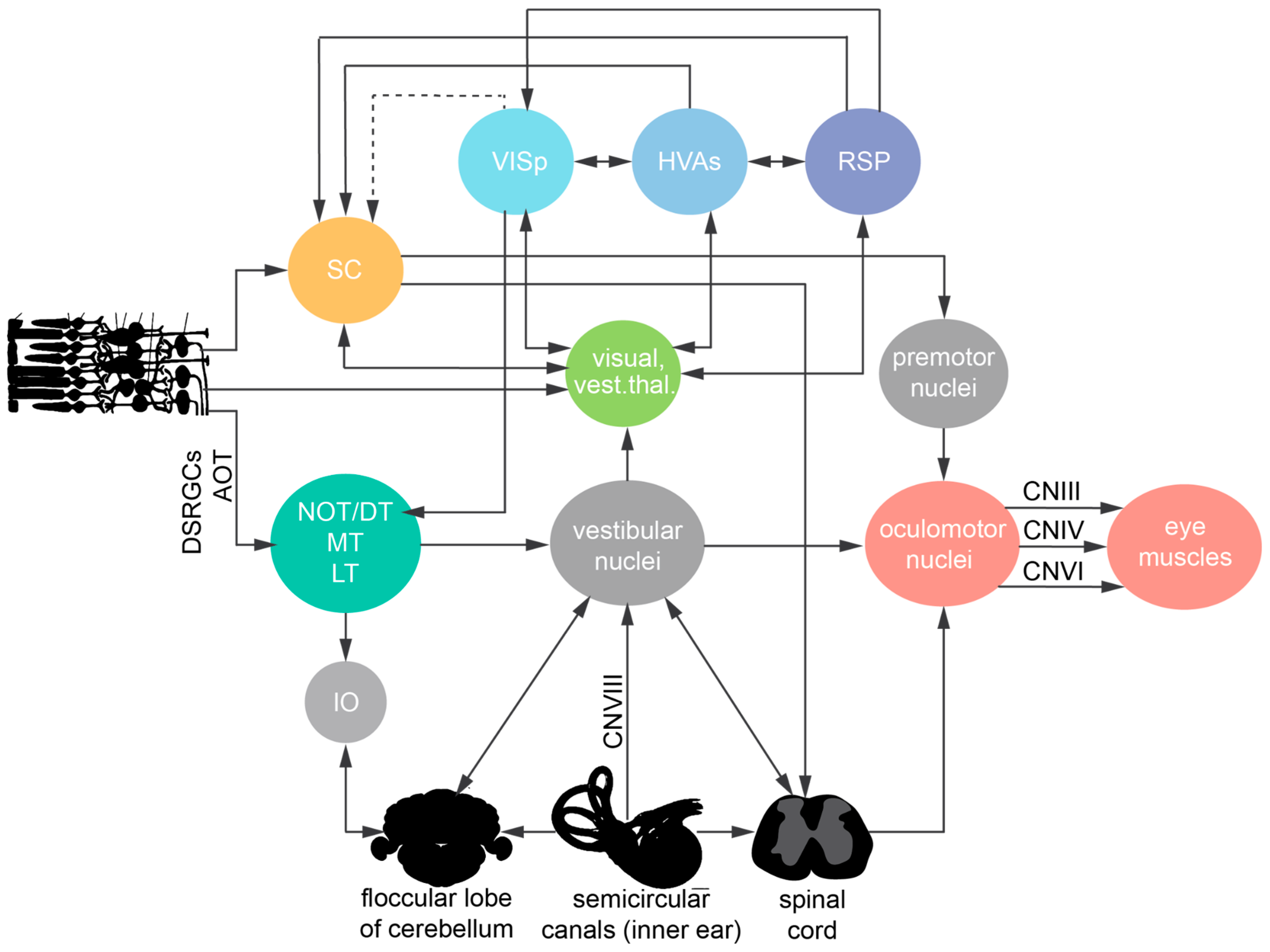
Peripheral afferents and brain regions involved in visuo-vestibular signal processing and transformation into an eye movement command in mice. Visual, vestibular, and proprioceptive input signals from the sensory periphery reach the vestibular nuclei. Stimulus integration occurs both at the level of vestibular nuclei, the thalamus, and the cortex. Subcortical regions (superior colliculus and NOT/DT) that generate eye movements receive strong cortical innervation from layer 5 pyramidal neurons. Eye muscle contraction is initiated upon commands generated via the oculomotor nuclei, which in turn receive innervation from the vestibular nuclei and premotor nuclei in the brainstem.
Figure 4.
Peripheral afferents and brain regions involved in visuo-vestibular signal processing and transformation into an eye movement command in mice. Visual, vestibular, and proprioceptive input signals from the sensory periphery reach the vestibular nuclei. Stimulus integration occurs both at the level of vestibular nuclei, the thalamus, and the cortex. Subcortical regions (superior colliculus and NOT/DT) that generate eye movements receive strong cortical innervation from layer 5 pyramidal neurons. Eye muscle contraction is initiated upon commands generated via the oculomotor nuclei, which in turn receive innervation from the vestibular nuclei and premotor nuclei in the brainstem.
Subcortical Areas
The superior colliculus (SC), the homologue of the optic tectum found in non-mammalian vertebrates (Isa et al. 2021), occupies a central role in the generation of horizontal and vertical eye movements in both primates and mice. A direct pathway from the retina (retinotectal pathway) and parallel pathways from the visual cortices (corticotectal) converge in the SC (Gandhi and Katnani 2011; Isa et al. 2021). Upon multimodal stimulus integration in its superficial layers (SCs), a motor command is generated from the intermediate and deep layers of the SC (SCm) to instruct the midbrain and brainstem oculomotor nuclei (see (Isa et al. 2021) for a review). In primates and mice, bursting neurons in the SCm drive ipsiversive saccades (saccades in the direction of the recorded SCm, but see Zhang et al. 2022). Furthermore, the burst firing of neural populations along the anteroposterior axis of the SCm is optimally tuned for saccades with distinct amplitudes and directions (see Gandhi and Katnani 2011 for a review). In the brainstem of primates, reticular nuclei in the pons (paramedian pontine reticular formation PPRF; tegmental pontine reticular nucleus, NRTP), the mesodiencephalic junction (MDJ) and the interstitial nucleus of Cajal (INC) are known as saccade generators receiving innervation from the SCm. These nuclei operate in conjunction with the pontomedullary reticular formation (PMRF), which controls orienting head movements. Neurons in the PPRF generate conjugate horizontal saccades by innervating medial and lateral rectus muscles; neurons in the MDJ and the INC innervate inferior rectus and oblique muscles, whose contraction determines the vertical and torsional components of the saccade (see Isa et al. 2021; Sparks 2002). The generation of horizontal smooth pursuit movements is also controlled by the SCm, the NRTP, and the dorsolateral pontine nucleus (DLPN; Ilg and Thier 2008). The rostral portion of the SCm is implicated in fixational eye movements, particularly microsaccades (Basso, Krauzlis, and Wurtz 2000). Bilateral lesions of the SC have also been shown to alter vergence eye movements in humans (Ohtsuka, Maeda, and Oguri 2002). In mice, the firing patterns in the homologous brainstem nuclei during saccades, or their innervation by the SCm, remain largely unexplored. Despite the conserved nature of brainstem circuits, further research in this direction is needed.
Within the basal ganglia of primates, the caudate nucleus (CN), the substantia nigra pars reticulata (SNr), and the striatum (Str) form an indirect saccade generator pathway that operates via cortical disinhibition of SCm neurons (Okihide Hikosaka, Takikawa, and Kawagoe 2000). While inhibitory nigrotectal projections have been functionally characterised in mice (Kaneda et al. 2008), their contribution to saccade generation remains to be demonstrated.
In both primates and mice, the nuclei of the accessory optic system (AOS) and the vestibular nuclei mediate gaze stabilisation, as they trigger eye movements in response to retinal slip (Giolli, Blanks, and Lui 2006; Dhande et al. 2013) or vestibular signals generated by head-motion, respectively. The AOS comprises the terminal nuclei of the accessory optic tract (DT, MT, LT) located in the midbrain; the nucleus of the optic tract (NOT) in the pretectal area is often considered part of the AOS as well, as it operates in close conjunction with the DT (Giolli, Blanks, and Lui 2006). The NOT/DT supports image stabilisation in the horizontal axis, while the MT and the LT do so in the vertical axis. The nuclei of the AOS are subserved by contralaterally projecting direction-sensitive RGCs (DS-RGCs; Masseck and Hoffmann 2009), which have large receptive fields, optimally respond to slow stimuli in a preferred direction, and target the AOS exclusively (Giolli, Blanks, and Lui 2006). In mammals, efferents of the AOS primarily target the ipsilateral vestibular nuclei, inferior olive (IO), and pontine nuclei, sparing the oculomotor nuclei and cerebellar cortex (Masseck and Hoffmann 2009).
In the cerebellum, the oculomotor vermis (OMV) and the oculomotor region of the fastigial nucleus (FN) are part of the cortico-ponto-cerebellar circuit governing conjugate horizontal eye movements, best described in primates (see Kheradmand and Zee 2011 for a review). The OMV and FN exert a modulatory function on the accuracy of saccade amplitude and direction, and on eye acceleration during smooth pursuit. Neurons within the vestibulocerebellum, particularly the flocculus/paraflocculus and the nodulus/uvula, are involved in the control and initiation of smooth pursuit, vergence eye movements, gaze holding and VOR reflexes (Kheradmand and Zee 2011). Lastly, the vestibulocerebellum is strongly involved in the adaptive plasticity of gaze-stabilising reflexes (Kodama and du Lac 2016; Carcaud et al. 2017), as well as in the coordination of subtypes of eye movements (Kheradmand and Zee 2011).
Cortical Areas
Electrical stimulation, lesion and functional imaging studies revealed a constellation of cortical areas within the frontal, temporal and parietal lobes supporting the generation of horizontal and vertical eye movements in primates. Even though these areas operate in synergy, and in some cases sequentially, some degree of task specialisation exists. The prefrontal and parietal cortices are implicated in triggering (frontal eye fields, FEF), motor planning and learning (supplementary eye field, SEF; posterior parietal cortex, PPC), modulation (anterior cingulate area, ACA; PPC) or inhibition (dorsolateral prefrontal cortex, dlPFC) of saccades (see (Charles Pierrot-Deseilligny, Milea, and Müri 2004; Pouget 2015) for a review). Lesions of the FEF also disrupted smooth pursuit eye movements (Lynch 1987), and it was shown that an area anterior to the FEF (area 8; (Gamlin 2002) is implicated in generating vergence and fixational eye movements (see (Krauzlis, Goffart, and Hafed 2017) for a review on the topic). Moreover, the parietal cortex is involved in the control and gain modulation of both OKR and VOR (J. Ventre and Faugier-Grimaud 1986; Jocelyne Ventre 1985a; 1985b). Within the temporal lobe, the medial temporal (MT) and medial superior temporal (MST) areas are engaged during smooth pursuit eye movements (including the OFR; Takemura et al. 2007; Kawano et al. 1994). Short-latency vergence eye movements (occurring in response to anticorrelated binocular disparity cues) depend on the early processing of binocular cues in the visual cortex and can occur without stereopsis (Masson, Busettini, and Miles 1997).
The contribution of the cortex to the generation of eye movements in rodents is less extensively described but gaining interest. For instance, the supplementary motor cortex (MOs) was shown to be necessary for the generation of contralateral saccadic eye movements in mice (Itokazu et al. 2018). Regarding gaze stabilisation, the primary visual cortex (VISp) was shown to promote adaptive plasticity of OKR via cortico-fugal projections to the midbrain (NOT/DT) upon vestibular lesion (Liu, Huberman, and Scanziani 2016). Finally, in rodents, vestibular signals are processed by a widespread network of cortical areas, including primary and higher visual areas (Rancz et al. 2015; Vélez-Fort et al. 2018; Bouvier, Senzai, and Scanziani 2020; Guitchounts et al. 2020; Hennestad et al. 2021; Keshavarzi et al. 2022; Parker et al. 2022b). Visuo-vestibular integration already occurs at the level of the vestibular nuclei in the brainstem to drive gaze-stabilising reflexes. However, gain control and other modulatory influence are implemented predominantly at subsequent stages at the level of the thalamus, cortex and cerebellum (see K. Cullen 2019; K. E. Cullen and Zobeiri 2021).
Neuromodulation of Eye Movements
Dopaminergic, noradrenergic and cholinergic neurotransmission have been strongly implicated in visual attention orienting in primates and mice (Noudoost and Moore 2011b; Chudasama and Robbins 2004; Li et al. 2021). While selective visual attention has been demonstrated in mice, its effect, if any, on eye movements is unclear (Wang and Krauzlis 2018). Within the basal ganglia of non-human primates, reward signals in the caudate nucleus (CN) are used to disinhibit collicular neurons that burst before saccade generation (O. Hikosaka and Wurtz 1985). In primates, saccades are indeed faster when a reward is expected, a process linked to dopaminergic signalling (Chen et al. 2014, 201). The application of dopamine agonists in the FEF of monkeys was also linked to increased ipsiversive saccadic target selection (Noudoost and Moore 2011a). Conversely, depleting dopamine in the CN disrupted the saccadic main sequence and voluntary saccades in primates (Kato et al. 1995). Accordingly, fatigue-related reduction of saccade velocity has been linked to noradrenaline and dopamine depletion (Connell et al. 2017). While monoamines modulated saccade velocity, inhibition of monoamine reuptake did not affect smooth pursuit movements (Connell et al. 2017). Lastly, acetylcholine was shown to play a role in the fixation of the eyes after a saccade in cats (Navarro-López et al. 2004), presumably by modulating the presynaptic release of PPRF neurons and local excitability of tonic firing neurons in the NPH nucleus as shown in rats (Navarro-López et al. 2004).
BOX 3-Motion-Induced Signals in the Visual Cortex
The pervasiveness of task-irrelevant and task-relevant movement signals in cortical sensory areas is a well-described phenomenon in the mouse (see (Parker et al. 2020) for a review). Proprioceptive (i.e. head, eye, body position or movement) and locomotor signals in primary sensory areas have been proposed to represent a corollary discharge signal, used to i) inhibit the sensory reafference generated by the self-motion (Schneider, Sundararajan, and Mooney 2018; Miura and Scanziani 2022), ii) generate a prediction of the sensory input generated by self-movements (Leinweber et al. 2017), or iii) modulate the gain of visual responses via neuromodulatory signals (hypothesised by Leinweber et al. 2017). In the context of visual processing, head/eye movements and locomotion signals constantly undermine the perceptual stability of the world and, in agreement with the corollary discharge hypothesis, they are differentially encoded by neurons in the murine visual cortex (Vélez-Fort et al. 2018; Bouvier, Senzai, and Scanziani 2020; Guitchounts et al. 2020; Hennestad et al. 2021; Abdolrahmani et al. 2021; Keshavarzi et al. 2022; Parker et al. 2022b). Specifically, visual and vestibular inputs contribute to angular head-velocity tuning in the cortex, with HVAs being more strongly tuned to a combination of both stimuli than to each stimulus alone (Hennestad et al. 2021). Neurons in VISp and the HVAs VISpm, VISam, Visa (Abdolrahmani et al. 2021) and VISrl, VISa, VISal (Itokazu et al. 2018) were also shown to increase firing prior to stimulus-elicited saccades, with premotor activity being as much as 4-fold larger than responses to visual stimuli (Abdolrahmani et al. 2021). On the other hand, new lines of evidence on visual sampling in mice, marmosets and macaque monkeys suggest that head/eye movements trigger a stereotyped sequence of neuronal responses across VISp only when the movements are linked to a shift in gaze (Parker et al. 2022c; Talluri et al. 2022). Thus, the motor/vestibular component of gaze-stabilising eye movements was not represented in the primary visual cortex, but whether it is in HVAs remains unknown. Locomotor signals were also shown to modulate neurons in the visual cortex. Species-specific differences emerge when comparing locomotion-induced activity in the visual cortex. Specifically, while neuronal activity in VISp positively correlated with running in mice (Niell and Stryker 2010), firing in the primary visual cortex was suppressed during locomotion in marmosets, albeit with a lower modulation gain (Liska et al. 2022). These diverging results may be partially explained by anatomical differences between monkeys and mice, whose visual cortex is more strongly innervated by premotor areas (Hovde et al. 2022; Leinweber et al. 2017; Markov et al. 2014) and whose neuromodulatory systems impinge on both inhibitory and disinhibitory mechanisms (Disney and Robert 2019). Lastly, it was recently shown in mice that a non-visual directional signal relayed by the lateral posterior nucleus of the thalamus (the homologue of the primate pulvinar) is required to achieve saccadic suppression in VISp (Miura and Scanziani 2022), hence to instruct the neurons in VISp that the motion perceived on the retina is self-generated. Mechanisms of saccadic suppression may involve the relay of a corollary discharge signal generated in SCm upon the initiation of movement via the pulvinar, as shown for monkeys, or rely on proprioceptive signals produced from the extraocular eye muscles (see Wurtz et al. 2011 for a review).
BOX 4-Binocular Disparity-Tuning in the Visual Cortex
Binocular disparity-tuned neurons are found throughout the visual system in mice (Chioma, Bonhoeffer, and Hübener 2020). Neurons in the binocular regions of both VISp and HVAs, containing the largest representation of the binocular field (Garrett et al. 2014), were shown to respond to retinal disparity depending on the elevation of the visual field (Chioma, Bonhoeffer, and Hübener 2020; 2019). Disparity-tuned neurons in area VISrl were preferentially activated by close visual stimuli in contrast to areas VISp and VISlm (Chioma, Bonhoeffer, and Hübener 2019). Moreover, area VISlm responded more strongly to random dot stereograms than areas VISp and VISrl (Chioma, Bonhoeffer, and Hübener 2020). These findings are similar to the disparity tuning discrepancies observed between V1 and V2 in primates (Chioma, Bonhoeffer, and Hübener 2020). Furthermore, mice can be trained to discriminate crossed (far) and uncrossed (close) disparities while head-fixed (Samonds, Choi, and Priebe 2019). While the degree of disparity tuning observed in VISp is sufficient to explain the behavioural success of mice performing the pole descent cliff task (Boone et al. 2021), causal manipulations need to be performed to establish the function of VISp disparity tuned neurons during this the pole-descent task.
BOX 5-Methods for Eye Tracking in Freely-Moving Mice
Eye tracking using magnetic search coils was the first method developed to record high-resolution eye movements in freely-moving mice (Payne and Raymond 2017). Different to the search coil used in primates, this technique relies on placing a miniaturised magnet onto the surface of the eyes. The magnet’s rotation is then measured with an external sensor. Video-oculography, instead, consists of recording pupil movement with head-mounted, high-speed cameras, first employed in rats (Wallace et al. 2013) and subsequently in mice. Meyer, Poort et al. (Meyer et al. 2018) were the first to combine an inertial measurement unit (IMU) with head-mounted CMOS image sensors with a frame rate of around 60 Hz, arguably suboptimal to measure saccade kinetics. The cameras record signals reflected by infrared “hot” mirrors (reflecting infrared while passing visible light) positioned in front of each eye. The 2D position of the pupil is transformed into 3D angular coordinates based on the anatomical structure of the eyeball by fitting a circular function (Sakatani and Isa 2004). Variations of this setup, employing cameras or LEDs instead of IMUs to track head position, have been employed by independent labs (Michaiel, Abe, and Niell 2020; Holmgren et al. 2021). The pupil and head position measurement was combined with neuronal activity recordings in VISp using tetrodes (Meyer et al. 2018) or linear silicon probes (Parker et al. 2022b). In parallel to video-oculography, hardware and software solutions were developed to see the world through the eye of the mouse. One strategy has been to direct an additional camera (“world camera”) towards the outside world (Parker et al. 2022b) and transform head-centric coordinates into retino-centric coordinates using a shifter network (Yates et al. 2021; Parker et al. 2022b). To approximate cyclotorsion, a TiO2 spot can be applied on the cornea and tracked in each image frame (Holmgren et al. 2021). Alternatively, cyclotorsion can be approximated with models based on variations in the head pitch (Parker et al. 2022b; 2022c). Without a world camera, a digitised version of the behavioural arena and room can be rendered onto each eye during offline analyses (Holmgren et al. 2021).
Outstanding Questions
Active vision in mice and the perception of a stable world
The framework known as active sensing, which postulates that perception is achieved through active exploration of the environment by our sensory organs (Gibson 1979), has been widely adopted by independent groups in recent years. Active sensing is easily reconciled with the predictive processing hypothesis, according to which brains actively infer the sensory consequences of the organism’s actions to create an expected view of the world (Bar 2009; Friston 2005; Keller and Mrsic-Flogel 2018). In the context of mouse vision, little is known about how cortical and subcortical areas support perception by relating visual processing to orienting movements. Is the integration of bottom-up information with top-down modulatory signals in HVAs (Galloni, Ye, and Rancz 2022; Jia et al. 2022) needed to produce a seamless sequence of eye movements during free exploration? Does the visual cortex direct compensatory and gaze-shifting eye movements in freely-exploring mice in response to the integrated angular head velocity signals?
Peripheral, cortical, and subcortical behavioural specialisations: how are eye movements integrated?
Specialisations in the murine retina (Johnson et al. 2021; Kim et al. 2020) and central nervous system (Hoy, Bishop, and Niell 2019) support different aspects of visually guided behaviours. Head/eye movements are accordingly organised to optimise visual perception (Holmgren et al. 2021; van Beest et al. 2021). These studies also identified the dorsal (Holmgren et al. 2021) and ventral (Johnson et al. 2021) temporal retina, together with correspondingly innervated portions of the binocular visual cortex (Chioma, Bonhoeffer, and Hübener 2019; 2020), as critical hotspots involved in mouse visual behaviour and navigation. It is known that, in humans, orienting towards salient cues can elude cortical control (express saccades). Orienting (Masullo et al. 2019) and fight-or-flight behaviours (Hoy, Bishop, and Niell 2019; Yilmaz and Meister 2013) have been studied in the mouse and related to the activity of the SC. In parallel, retinotectal projections, of which a population exclusively targets the SC, are the most abundant in mice (Ellis et al. 2016). Surprisingly, although the responses of retinotectal neurons were recently described in vivo (Sibille et al. 2022), a causal link between retinotectal pathways and escape/orienting behaviours needs to be made. In addition, whether the same head/eye coupling observed during exploration is maintained to deploy colliculus-mediated fight-or-flight responses or whether VOR-suppressing mechanisms exist remains unknown.
What is the function of binocular vision in mice?
Due to the prevalence of non-conjugate eye movements during exploration and velocity asymmetries of conjugate saccades, there is no stable basis for stereopsis freely-moving mice. In fact, in the absence of binocular fusion, stereopsis based on changes in disparity tuning cannot occur. Notwithstanding a small and unstable binocular field, loss of binocularity by ablating ipsilaterally projecting RGCs, monocular deprivation, or silencing the binocular portion of the visual cortex profoundly affects mouse behaviour. Furthermore, monocular deprivation causes the reorganisation of head/eye coupling during distance estimation, presumably to increase motion parallax-derived cues or to shift gaze to optimally promote sampling of the visual field by specialised portions of the cortex. Are IOVDs or changes in disparity-tuning used to estimate depth in naturalistic conditions? (see Choi and Priebe, 2020; Parker et al., 2022; also proposed by Johnson et al., 2021). Alternatively, and as suggested by other authors, does binocular vision support contrast sensitivity in dim light conditions (Speed et al., 2019, Johnson et al., 2021)?
Leading eyes, leading heads
Eye movements can be equally driven by exogenous and endogenous signals (McDowell et al. 2008). Mice are skilled at navigating through environments equipped with distal visual cues (Morris water maze), and they chase crickets using head turns coupled with a saccade and fixate strategy (Michaiel, Abe, and Niell 2020). Thus, while mice cannot track crickets with their eyes alone, they do so with their head and eyes combined (the mouse saccade is a head/eye gaze shift, as shown by Michaiel et al., 2020; P. R. L. Parker et al., 2022b). What are the circuits implicated in tracking different salient stimuli, and which eye movements occur under these conditions? (see Solié et al., 2022 for recent findings on the subcortical circuits governing orientation to salient stimuli).
Saccades and eye movements in head-fixed versus freely-moving mice
Eye movements of freely-moving mice are fundamentally different from those observed during head fixation. This is likely due to the lack of head movements and the ensuing vestibular input in head-fixed conditions. Nevertheless, even during head-fixed conditions, “spontaneous” saccades are preceded by ipsilateral attempted head movements similar in kinetics to saccade and fixate movements (Zahler et al. 2021; Meyer, O’Keefe, and Poort 2020). Saccades evoked by strong sensory stimuli also occur in close correspondence to attempted orienting head movements but lack the initial slow head-movement attempt, typical of the saccade and fixate eye movement sequence (Zahler et al. 2021). What is the interaction between saccades and head movements in these different scenarios? Are these parallel neuronal pathways, and what degree of cross-talk exists between them? Are the motor commands to initiate saccades and head movements generated in distinct brain areas? What are the relative contributions of the cortex and subcortical structures during gaze-shifting versus gaze-stabilising head/eye movements? Does the superior colliculus occupy a central role in either case? Interestingly, stimulation of deep layers of the primate SC induces a head turn, a concomitant gaze-shifting eye movement, and a gaze-stabilising eye movement (Sparks 2002); this pattern significantly resembles the “saccade and fixate” strategy employed by freely-moving mice, possibly indicating that the same circuit is involved in both cases.
Acknowledgements
We thank Antonin Blot, Anna Montagnini and Jasper Poort for their comments on the manuscript. The project leading to this publication has received funding from the Excellence Initiative of Aix-Marseille Université - A*Midex, a French "Investissements d'Avenir" programme" AMX-20-CE-06.
References
- Abdolrahmani, Mohammad, Dmitry R. Lyamzin, Ryo Aoki, and Andrea Benucci. 2021. “Attention Separates Sensory and Motor Signals in the Mouse Visual Cortex.” Cell Reports 36 (2). [CrossRef]
- Alphen, A. M. van, J. S. Stahl, and C. I. De Zeeuw. 2001. “The Dynamic Characteristics of the Mouse Horizontal Vestibulo-Ocular and Optokinetic Response.” Brain Research 890 (2): 296–305. [CrossRef]
- Anson, Eric R., Robin T. Bigelow, John P. Carey, Quan-Li Xue, Stephanie Studenski, Michael C. Schubert, Konrad P. Weber, and Yuri Agrawal. 2016. “Aging Increases Compensatory Saccade Amplitude in the Video Head Impulse Test.” Frontiers in Neurology 7. https://www.frontiersin.org/articles/10.3389/fneur.2016.00113.
- Bahill, A. Terry, Michael R. Clark, and Lawrence Stark. 1975. “The Main Sequence, a Tool for Studying Human Eye Movements.” Mathematical Biosciences 24 (3): 191–204. [CrossRef]
- Basso, Michele A., Richard J. Krauzlis, and Robert H. Wurtz. 2000. “Activation and Inactivation of Rostral Superior Colliculus Neurons During Smooth-Pursuit Eye Movements in Monkeys.” Journal of Neurophysiology 84 (2): 892–908. [CrossRef]
- Beest, Enny H. van, Sreedeep Mukherjee, Lisa Kirchberger, Ulf H. Schnabel, Chris van der Togt, Rob R. M. Teeuwen, Areg Barsegyan, et al. 2021. “Mouse Visual Cortex Contains a Region of Enhanced Spatial Resolution.” Nature Communications 12 (1): 4029. [CrossRef]
- Bergmann, Ronny, Keisuke Sehara, Sina E. Dominiak, Jens Kremkow, Matthew E. Larkum, and Robert N. S. Sachdev. 2022. “Coordination between Eye Movement and Whisking in Head-Fixed Mice Navigating a Plus Maze.” ENeuro 9 (4): ENEURO.0089-22.2022. [CrossRef]
- Bleckert, Adam, Gregory W. Schwartz, Maxwell H. Turner, Fred Rieke, and Rachel O. L. Wong. 2014. “Visual Space Is Represented by Nonmatching Topographies of Distinct Mouse Retinal Ganglion Cell Types.” Current Biology: CB 24 (3): 310–15. [CrossRef]
- Boone, Howard C., Jason M. Samonds, Emily C. Crouse, Carrie Barr, Nicholas J. Priebe, and Aaron W. McGee. 2021. “Natural Binocular Depth Discrimination Behavior in Mice Explained by Visual Cortical Activity.” Current Biology 31 (10): 2191-2198.e3. [CrossRef]
- Bouvier, Guy, Yuta Senzai, and Massimo Scanziani. 2020. “Head Movements Control the Activity of Primary Visual Cortex in a Luminance-Dependent Manner.” Neuron 108 (3): 500-511.e5. [CrossRef]
- Brodsky, Michael C. 2019. “Monocular Nasotemporal Optokinetic Asymmetry—Unraveling the Mystery.” Journal of American Association for Pediatric Ophthalmology and Strabismus 23 (5): 249–51. [CrossRef]
- Büttner-Ennever, J. A., and A. K. Horn. 1997. “Anatomical Substrates of Oculomotor Control.” Current Opinion in Neurobiology 7 (6): 872–79. [CrossRef]
- Carcaud, Julie, Filipa França de Barros, Erwin Idoux, Daniel Eugène, Lionel Reveret, Lee E. Moore, Pierre-Paul Vidal, and Mathieu Beraneck. 2017. “Long-Lasting Visuo-Vestibular Mismatch in Freely-Behaving Mice Reduces the Vestibulo-Ocular Reflex and Leads to Neural Changes in the Direct Vestibular Pathway.” ENeuro 4 (1). [CrossRef]
- Chen, Lewis L., Y. Mark Chen, Wu Zhou, and William D. Mustain. 2014. “Monetary Reward Speeds up Voluntary Saccades.” Frontiers in Integrative Neuroscience 8 (June). [CrossRef]
- Choi, Veronica, and Nicholas J. Priebe. 2020. “Interocular Velocity Cues Elicit Vergence Eye Movements in Mice.” Journal of Neurophysiology 124 (2): 623–33. [CrossRef]
- Chopin, Adrien, Samantha Wenyan Chan, Bahia Guellai, Daphné Bavelier, and Dennis Michael Levi. 2019. “Binocular Non-Stereoscopic Cues Can Deceive Clinical Tests of Stereopsis.” Scientific Reports 9 (1): 5789. [CrossRef]
- Chudasama, Yogita, and Trevor W. Robbins. 2004. “Dopaminergic Modulation of Visual Attention and Working Memory in the Rodent Prefrontal Cortex.” Neuropsychopharmacology 29 (9): 1628–36. [CrossRef]
- Collewijn, Han, and Eileen Kowler. 2008. “The Significance of Microsaccades for Vision and Oculomotor Control.” Journal of Vision 8 (14): 20.1-21. [CrossRef]
- Connell, Charlotte J. W., Benjamin Thompson, Jason Turuwhenua, Alexa Srzich, and Nicholas Gant. 2017. “Fatigue-Related Impairments in Oculomotor Control Are Prevented by Norepinephrine-Dopamine Reuptake Inhibition.” Scientific Reports 7 (1): 42726. [CrossRef]
- Cullen, Kathleen. 2019. “Vestibular Processing during Natural Self-Motion: Implications for Perception and Action.” Nature Reviews. Neuroscience 20 (6): 346–63. [CrossRef]
- Cullen, Kathleen E, and Omid A Zobeiri. 2021. “Proprioception and the Predictive Sensing of Active Self-Motion.” Current Opinion in Physiology 20 (April): 29–38. [CrossRef]
- Dhande, Onkar S., Maureen E. Estevez, Lauren E. Quattrochi, Rana N. El-Danaf, Phong L. Nguyen, David M. Berson, and Andrew D. Huberman. 2013. “Genetic Dissection of Retinal Inputs to Brainstem Nuclei Controlling Image Stabilization.” Journal of Neuroscience 33 (45): 17797–813. [CrossRef]
- Essig, Jaclyn, Joshua B. Hunt, and Gidon Felsen. 2021. “Inhibitory Neurons in the Superior Colliculus Mediate Selection of Spatially-Directed Movements.” Communications Biology 4 (1): 719. [CrossRef]
- Faulstich, Bernd Michael, Kimberly Ann Onori, and Sascha du Lac. 2004. “Comparison of Plasticity and Development of Mouse Optokinetic and Vestibulo-Ocular Reflexes Suggests Differential Gain Control Mechanisms.” Vision Research, The Mouse Visual System: From Photoreceptors to Cortex, 44 (28): 3419–27. [CrossRef]
- França de Barros, Filipa, Louise Schenberg, Michele Tagliabue, and Mathieu Beraneck. 2020. “Long Term Visuo-Vestibular Mismatch in Freely Behaving Mice Differentially Affects Gaze Stabilizing Reflexes.” Scientific Reports 10 (1): 20018. [CrossRef]
- Gamlin, Paul D. R. 2002. “Neural Mechanisms for the Control of Vergence Eye Movements.” Annals of the New York Academy of Sciences 956 (April): 264–72. [CrossRef]
- Gandhi, Neeraj J., and Husam A. Katnani. 2011. “Motor Functions of the Superior Colliculus.” Annual Review of Neuroscience 34 (1): 205–31. [CrossRef]
- Garbutt, S., M. Harwood, and C. Harris. 2001. “Comparison of the Main Sequence of Reflexive Saccades and the Quick Phases of Optokinetic Nystagmus.” The British Journal of Ophthalmology 85 (12): 1477–83. [CrossRef]
- Gibaldi, Agostino, and Silvio P. Sabatini. 2021. “The Saccade Main Sequence Revised: A Fast and Repeatable Tool for Oculomotor Analysis.” Behavior Research Methods 53 (1): 167–87. [CrossRef]
- Gibson, James J. 1950. The Perception of the Visual World. Boston: Houghton Mifflin.
- Giolli, Roland A., Robert H.I. Blanks, and Fausta Lui. 2006. “The Accessory Optic System: Basic Organization with an Update on Connectivity, Neurochemistry, and Function.” In Progress in Brain Research, 151:407–40. Elsevier. [CrossRef]
- Glickfeld, Lindsey L., and Shawn R. Olsen. 2017. “Higher-Order Areas of the Mouse Visual Cortex.” Annual Review of Vision Science 3 (1): 251–73. [CrossRef]
- Guitchounts, Grigori, Javier Masís, Steffen B.E. Wolff, and David Cox. 2020. “Encoding of 3D Head Orienting Movements in the Primary Visual Cortex.” Neuron 108 (3): 512-525.e4. [CrossRef]
- Gygli, Jan, Fausto Romano, Christopher J. Bockisch, Nina Feddermann-Demont, Dominik Straumann, and Giovanni Bertolini. 2021. “Effect of the Stimulus Duration on the Adaptation of the Optokinetic Afternystagmus.” Frontiers in Neurology 12. https://www.frontiersin.org/articles/10.3389/fneur.2021.518133.
- Harvey, R.J, C De’sperati, and P Strata. 1997. “The Early Phase of Horizontal Optokinetic Responses in the Pigmented Rat and the Effects of Lesions of the Visual Cortex.” Vision Research 37 (12): 1615–25. [CrossRef]
- Hennestad, Eivind, Aree Witoelar, Anna R. Chambers, and Koen Vervaeke. 2021. “Mapping Vestibular and Visual Contributions to Angular Head Velocity Tuning in the Cortex.” Cell Reports 37 (12). [CrossRef]
- Hessels, Roy S., Diederick C. Niehorster, Marcus Nyström, Richard Andersson, and Ignace T. C. Hooge. 2018. “Is the Eye-Movement Field Confused about Fixations and Saccades? A Survey among 124 Researchers.” Royal Society Open Science 5 (8): 180502. [CrossRef]
- Hikosaka, O., and R. H. Wurtz. 1985. “Modification of Saccadic Eye Movements by GABA-Related Substances. II. Effects of Muscimol in Monkey Substantia Nigra Pars Reticulata.” Journal of Neurophysiology 53 (1): 292–308. [CrossRef]
- Hikosaka, Okihide, Yoriko Takikawa, and Reiko Kawagoe. 2000. “Role of the Basal Ganglia in the Control of Purposive Saccadic Eye Movements.” Physiological Reviews 80 (3): 953–78. [CrossRef]
- Holmgren, Carl D, Paul Stahr, Damian J Wallace, Kay-Michael Voit, Emily J Matheson, Juergen Sawinski, Giacomo Bassetto, and Jason ND Kerr. 2021. “Visual Pursuit Behavior in Mice Maintains the Pursued Prey on the Retinal Region with Least Optic Flow.” ELife 10 (October): e70838. [CrossRef]
- Hoy, Jennifer L., Iryna Yavorska, Michael Wehr, and Cristopher M. Niell. 2016. “Vision Drives Accurate Approach Behavior during Prey Capture in Laboratory Mice.” Current Biology 26 (22): 3046–52. [CrossRef]
- Huberman, Andrew D., Marla B. Feller, and Barbara Chapman. 2008. “Mechanisms Underlying Development of Visual Maps and Receptive Fields.” Annual Review of Neuroscience 31: 479–509. [CrossRef]
- Huberman, Andrew D., and Cristopher M. Niell. 2011. “What Can Mice Tell Us about How Vision Works?” Trends in Neurosciences 34 (9): 464–73. [CrossRef]
- Ilg, Uwe J., and Peter Thier. 2008. “The Neural Basis of Smooth Pursuit Eye Movements in the Rhesus Monkey Brain.” Brain and Cognition 68 (3): 229–40. [CrossRef]
- Imai, Takao, Yasumitsu Takimoto, Noriaki Takeda, Atsuhiko Uno, Hidenori Inohara, and Shoichi Shimada. 2016. “High-Speed Video-Oculography for Measuring Three-Dimensional Rotation Vectors of Eye Movements in Mice.” PLOS ONE 11 (3): e0152307. [CrossRef]
- Isa, Tadashi, Emmanuel Marquez-Legorreta, Sten Grillner, and Ethan K. Scott. 2021. “The Tectum/Superior Colliculus as the Vertebrate Solution for Spatial Sensory Integration and Action.” Current Biology 31 (11): R741–62. [CrossRef]
- Itokazu, Takahide, Masashi Hasegawa, Rui Kimura, Hironobu Osaki, Urban-Raphael Albrecht, Kazuhiro Sohya, Shubhodeep Chakrabarti, et al. 2018. “Streamlined Sensory Motor Communication through Cortical Reciprocal Connectivity in a Visually Guided Eye Movement Task.” Nature Communications 9 (1): 338. [CrossRef]
- Johnson, Keith P., Michael J. Fitzpatrick, Lei Zhao, Bing Wang, Sean McCracken, Philip R. Williams, and Daniel Kerschensteiner. 2021. “Cell-Type-Specific Binocular Vision Guides Predation in Mice.” Neuron 109 (9): 1527-1539.e4. [CrossRef]
- Kaneda, Katsuyuki, Kaoru Isa, Yuchio Yanagawa, and Tadashi Isa. 2008. “Nigral Inhibition of GABAergic Neurons in Mouse Superior Colliculus.” Journal of Neuroscience 28 (43): 11071–78. [CrossRef]
- Kaneko, C. R. 1999. “Eye Movement Deficits Following Ibotenic Acid Lesions of the Nucleus Prepositus Hypoglossi in Monkeys II. Pursuit, Vestibular, and Optokinetic Responses.” Journal of Neurophysiology 81 (2): 668–81. [CrossRef]
- Kato, M., N. Miyashita, O. Hikosaka, M. Matsumura, S. Usui, and A. Kori. 1995. “Eye Movements in Monkeys with Local Dopamine Depletion in the Caudate Nucleus. I. Deficits in Spontaneous Saccades.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 15 (1 Pt 2): 912–27. [CrossRef]
- Katoh, Akira, Hiromasa Kitazawa, Shigeyoshi Itohara, and Soichi Nagao. 2000. “Inhibition of Nitric Oxide Synthesis and Gene Knockout of Neuronal Nitric Oxide Synthase Impaired Adaptation of Mouse Optokinetic Response Eye Movements.” Learning & Memory 7 (4): 220–26. [CrossRef]
- Kautzky, Magdalena, and Laura Busse. 2020. “Vision: How Mice Control Their View.” Current Biology 30 (11): R635–37. [CrossRef]
- Kawano, K., M. Shidara, Y. Watanabe, and S. Yamane. 1994. “Neural Activity in Cortical Area MST of Alert Monkey during Ocular Following Responses.” Journal of Neurophysiology 71 (6): 2305–24. [CrossRef]
- Keller, Georg B., Tobias Bonhoeffer, and Mark Hübener. 2012. “Sensorimotor Mismatch Signals in Primary Visual Cortex of the Behaving Mouse.” Neuron 74 (5): 809–15. [CrossRef]
- Keshavarzi, Sepiedeh, Edward F. Bracey, Richard A. Faville, Dario Campagner, Adam L. Tyson, Stephen C. Lenzi, Tiago Branco, and Troy W. Margrie. 2022. “Multisensory Coding of Angular Head Velocity in the Retrosplenial Cortex.” Neuron 110 (3): 532-543.e9. [CrossRef]
- Kheradmand, Amir, and David S. Zee. 2011. “Cerebellum and Ocular Motor Control.” Frontiers in Neurology 2: 53. [CrossRef]
- Kodama, Takashi, and Sascha du Lac. 2016. “Adaptive Acceleration of Visually Evoked Smooth Eye Movements in Mice.” The Journal of Neuroscience 36 (25): 6836–49. [CrossRef]
- Krauzlis, Richard J., Laurent Goffart, and Ziad M. Hafed. 2017. “Neuronal Control of Fixation and Fixational Eye Movements.” Philosophical Transactions of the Royal Society B: Biological Sciences 372 (1718): 20160205. [CrossRef]
- Land, Michael. 2019. “Eye Movements in Man and Other Animals.” Vision Research 162 (September): 1–7. [CrossRef]
- Lappe, Markus, and Klaus-Peter Hoffmann. 2000. “Optic Flow and Eye Movements.” In International Review of Neurobiology, edited by Markus Lappe, 44:29–47. Neuronal Processing of Optic Flow. Academic Press. [CrossRef]
- Leinweber, Marcus, Daniel R. Ward, Jan M. Sobczak, Alexander Attinger, and Georg B. Keller. 2017. “A Sensorimotor Circuit in Mouse Cortex for Visual Flow Predictions.” Neuron 95 (6): 1420-1432.e5. [CrossRef]
- Li, S., C. May, A. J. Hannan, K. A. Johnson, and E. L. Burrows. 2021. “Assessing Attention Orienting in Mice: A Novel Touchscreen Adaptation of the Posner-Style Cueing Task.” Neuropsychopharmacology 46 (2): 432–41. [CrossRef]
- Liu, Bao-hua, Andrew D. Huberman, and Massimo Scanziani. 2016. “Cortico-Fugal Output from Visual Cortex Promotes Plasticity of Innate Motor Behaviour.” Nature 538 (7625): 383–87. [CrossRef]
- Lynch, J. C. 1987. “Frontal Eye Field Lesions in Monkeys Disrupt Visual Pursuit.” Experimental Brain Research 68 (2): 437–41. [CrossRef]
- Mahfuz, M. Muntaseer, Michael C. Schubert, William V. C. Figtree, Christopher J. Todd, and Americo A. Migliaccio. 2018. “Human Vestibulo-Ocular Reflex Adaptation: Consolidation Time Between Repeated Training Blocks Improves Retention.” JARO: Journal of the Association for Research in Otolaryngology 19 (5): 601–10. [CrossRef]
- Martinez-Trujillo, Julio. 2022. “Corollary Discharge: Linking Saccades and Memory Circuits in the Human Brain.” Current Biology 32 (14): R774–76. [CrossRef]
- Masseck, Olivia Andrea, and Klaus-Peter Hoffmann. 2009. “Comparative Neurobiology of the Optokinetic Reflex.” Annals of the New York Academy of Sciences 1164 (1): 430–39. [CrossRef]
- Masson, G. S., C. Busettini, and F. A. Miles. 1997. “Vergence Eye Movements in Response to Binocular Disparity without Depth Perception.” Nature 389 (6648): 283–86. [CrossRef]
- McDowell, Jennifer E., Kara A. Dyckman, Benjamin P. Austin, and Brett A. Clementz. 2008. “Neurophysiology and Neuroanatomy of Reflexive and Volitional Saccades: Evidence from Studies of Humans.” Brain and Cognition 68 (3): 255–70. [CrossRef]
- Meyer, Arne F., John O’Keefe, and Jasper Poort. 2020. “Two Distinct Types of Eye-Head Coupling in Freely Moving Mice.” Current Biology 30 (11): 2116-2130.e6. [CrossRef]
- Meyer, Arne F., Jasper Poort, John O’Keefe, Maneesh Sahani, and Jennifer F. Linden. 2018. “A Head-Mounted Camera System Integrates Detailed Behavioral Monitoring with Multichannel Electrophysiology in Freely Moving Mice.” Neuron 100 (1): 46-60.e7. [CrossRef]
- Michaiel, Angie M, Elliott TT Abe, and Cristopher M Niell. 2020. “Dynamics of Gaze Control during Prey Capture in Freely Moving Mice.” ELife 9 (July): e57458. [CrossRef]
- Migliaccio, Americo A., Robert Meierhofer, and Charles C. Della Santina. 2011. “Characterization of the 3D Angular Vestibulo-Ocular Reflex in C57BL6 Mice.” Experimental Brain Research. Experimentelle Hirnforschung. Experimentation Cerebrale 210 (3–4): 489–501. [CrossRef]
- Miles, F. A., K. Kawano, and L. M. Optican. 1986. “Short-Latency Ocular Following Responses of Monkey. I. Dependence on Temporospatial Properties of Visual Input.” Journal of Neurophysiology 56 (5): 1321–54. [CrossRef]
- Miller, Cory T., David Gire, Kim Hoke, Alexander C. Huk, Darcy Kelley, David A. Leopold, Matthew C. Smear, Frederic Theunissen, Michael Yartsev, and Cristopher M. Niell. 2022. “Natural Behavior Is the Language of the Brain.” Current Biology 32 (10): R482–93. [CrossRef]
- Mitchiner, J.C., L.H. Pinto, and J.W. Vanable. 1976. “Visually Evoked Eye Movements in the Mouse (Mus Musculus).” Vision Research 16 (10): 1169-IN7. [CrossRef]
- Miura, Satoru K., and Massimo Scanziani. 2021. “Erasing Motion: Scrambling Direction Selectivity in Visual Cortex during Saccades.” bioRxiv. https://www.biorxiv.org/content/10.1101/2021.03.30.437338v2.
- Niell, Cristopher M. 2011. “Exploring the Next Frontier of Mouse Vision.” Neuron 72 (6): 889–92. [CrossRef]
- Noudoost, Behrad, and Tirin Moore. 2011a. “CONTROL OF VISUAL CORTICAL SIGNALS BY PREFRONTAL DOPAMINE.” Nature 474 (7351): 372–75. [CrossRef]
- Noudoost, Behrad, and Tirin Moore. 2011b. “The Role of Neuromodulators in Selective Attention.” Trends in Cognitive Sciences 15 (12): 585–91. [CrossRef]
- Oh, Seung Wook, Julie A. Harris, Lydia Ng, Brent Winslow, Nicholas Cain, Stefan Mihalas, Quanxin Wang, et al. 2014. “A Mesoscale Connectome of the Mouse Brain.” Nature 508 (7495): 207–14. [CrossRef]
- Ohtsuka, Kenji, Sachie Maeda, and Naomi Oguri. 2002. “Accommodation and Convergence Palsy Caused by Lesions in the Bilateral Rostral Superior Colliculus.” American Journal of Ophthalmology 133 (3): 425–27. [CrossRef]
- Parker et al. 2022a. “Distance Estimation from Monocular Cues in an Ethological Visuomotor Task ELife.” 2022. https://elifesciences.org/articles/74708.
- Parker, Philip R. L., Elliott T. T. Abe, Emmalyn S. P. Leonard, Dylan M. Martins, and Cristopher M. Niell. 2022b. “Joint Coding of Visual Input and Eye/Head Position in V1 of Freely Moving Mice.” Neuron, September. [CrossRef]
- Parker, Philip R. L., Dylan M. Martins, Emmalyn S. P. Leonard, Nathan M. Casey, Shelby L. Sharp, Elliott T. T. Abe, Matthew C. Smear, Jacob L. Yates, Jude F. Mitchell, and Cristopher M. Niell. 2022c. “A Dynamic Sequence of Visual Processing Initiated by Gaze Shifts.” bioRxiv. https://www.biorxiv.org/content/10.1101/2022.08.23.504847v1.
- Payne, Hannah L, and Jennifer L Raymond. 2017. “Magnetic Eye Tracking in Mice.” Edited by Geoffrey Schoenbaum. ELife 6 (September): e29222. [CrossRef]
- Pelz, Jeff B, and Roxanne Canosa. 2001. “Oculomotor Behavior and Perceptual Strategies in Complex Tasks.” Vision Research 41 (25): 3587–96. [CrossRef]
- Pierrot-Deseilligny, C., S. Rivaud, B. Gaymard, and Y. Agid. 1991. “Cortical Control of Memory-Guided Saccades in Man.” Experimental Brain Research 83 (3): 607–17. [CrossRef]
- Pierrot-Deseilligny, Charles, Dan Milea, and René M. Müri. 2004. “Eye Movement Control by the Cerebral Cortex.” Current Opinion in Neurology 17 (1): 17–25.
- Pouget, P. 2015. “The Cortex Is in Overall Control of ‘Voluntary’ Eye Movement.” Eye 29 (2): 241–45. [CrossRef]
- Prusky, Glen T., Byron D. Silver, Wayne W. Tschetter, Nazia M. Alam, and Robert M. Douglas. 2008. “Experience-Dependent Plasticity from Eye Opening Enables Lasting, Visual Cortex-Dependent Enhancement of Motion Vision.” The Journal of Neuroscience 28 (39): 9817–27. [CrossRef]
- Rancz, Ede A., Javier Moya, Florian Drawitsch, Alan M. Brichta, Santiago Canals, and Troy W. Margrie. 2015. “Widespread Vestibular Activation of the Rodent Cortex.” The Journal of Neuroscience 35 (15): 5926–34. [CrossRef]
- Rolfs, Martin. 2009. “Microsaccades: Small Steps on a Long Way.” Vision Research 49 (20): 2415–41. [CrossRef]
- Rommelse, Nanda N. J., Stefan Van der Stigchel, and Joseph A. Sergeant. 2008. “A Review on Eye Movement Studies in Childhood and Adolescent Psychiatry.” Brain and Cognition 68 (3): 391–414. [CrossRef]
- Rucci, Michele, and Jonathan D. Victor. 2015. “The Unsteady Eye: An Information-Processing Stage, Not a Bug.” Trends in Neurosciences 38 (4): 195–206. [CrossRef]
- Sakatani, Tomoya, and Tadashi Isa. 2004. “PC-Based High-Speed Video-Oculography for Measuring Rapid Eye Movements in Mice.” Neuroscience Research 49 (1): 123–31. [CrossRef]
- Sakatani, Tomoya, and Tadashi Isa. 2007. “Quantitative Analysis of Spontaneous Saccade-like Rapid Eye Movements in C57BL/6 Mice.” Neuroscience Research 58 (3): 324–31. [CrossRef]
- Saleem, Aman B. 2020. “Two Stream Hypothesis of Visual Processing for Navigation in Mouse.” Current Opinion in Neurobiology 64 (October): 70–78. [CrossRef]
- Samonds, Jason M., Veronica Choi, and Nicholas J. Priebe. 2019. “Mice Discriminate Stereoscopic Surfaces Without Fixating in Depth.” Journal of Neuroscience 39 (41): 8024–37. [CrossRef]
- Samonds, Jason M., Wilson S. Geisler, and Nicholas J. Priebe. 2018. “Natural Image and Receptive Field Statistics Predict Saccade Sizes.” Nature Neuroscience 21 (11): 1591–99. [CrossRef]
- Sato, Takashi R, Takahide Itokazu, Hironobu Osaki, Makoto Ohtake, Tetsuya Yamamoto, Kazuhiro Sohya, Takakuni Maki, and Tatsuo K Sato. 2019. “Interhemispherically Dynamic Representation of an Eye Movement-Related Activity in Mouse Frontal Cortex.” Edited by Naoshige Uchida and Richard B Ivry. ELife 8 (November): e50855. [CrossRef]
- Schor, Clifton M., James S. Maxwell, Jefrey McCANDLESS, and Erich Graf. 2002. “Adaptive Control of Vergence in Humans.” Annals of the New York Academy of Sciences 956 (1): 297–305. [CrossRef]
- Schweigart, G, T Mergner, I Evdokimidis, S Morand, and W Becker. 1997. “Gaze Stabilization by Optokinetic Reflex (OKR) and Vestibulo-Ocular Reflex (VOR) During Active Head Rotation in Man.” Vision Research 37 (12): 1643–52. [CrossRef]
- Senzai, Yuta, and Massimo Scanziani. 2022. “A Cognitive Process Occurring during Sleep Is Revealed by Rapid Eye Movements.” Science 377 (6609): 999–1004. [CrossRef]
- Sparks, David L. 2002. “The Brainstem Control of Saccadic Eye Movements.” Nature Reviews Neuroscience 3 (12): 952–64. [CrossRef]
- Stahl, J. S., A. M. van Alphen, and C. I. De Zeeuw. 2000. “A Comparison of Video and Magnetic Search Coil Recordings of Mouse Eye Movements.” Journal of Neuroscience Methods 99 (1–2): 101–10. [CrossRef]
- Stahl, John S. 2004a. “Eye Movements of the Murine P/Q Calcium Channel Mutant Rocker, and the Impact of Aging.” Journal of Neurophysiology 91 (5): 2066–78. [CrossRef]
- Stahl, John S. 2004b. “Using Eye Movements to Assess Brain Function in Mice.” Vision Research 44 (28): 3401–10. [CrossRef]
- Sterratt, David C., Daniel Lyngholm, David J. Willshaw, and Ian D. Thompson. 2013. “Standard Anatomical and Visual Space for the Mouse Retina: Computational Reconstruction and Transformation of Flattened Retinae with the Retistruct Package.” PLOS Computational Biology 9 (2): e1002921. [CrossRef]
- Straube, Andreas, and Ulrich Büttner, eds. 2007. Neuro-Ophthalmology: Neuronal Control of Eye Movements. Developments in Ophthalmology, Vol. 40. Basel Freiburg [Breisgau]: Karger.
- Tabata, Hiromitsu, Naoki Shimizu, Yoshiro Wada, Kenichiro Miura, and Kenji Kawano. 2010. “Initiation of the Optokinetic Response (OKR) in Mice.” Journal of Vision 10 (1): 13. [CrossRef]
- Takahashi, Koji, Osamu Tanaka, Yosuke Kudo, Eriko Sugawara, and Ken Johkura. 2019. “Adduction-Abduction Asymmetry in Saccades During Video-Oculographic Monocular Recording: A Word of Caution.” Neuro-Ophthalmology 43 (5): 284–88. [CrossRef]
- Takemura, Aya, Yumi Murata, Kenji Kawano, and F. A. Miles. 2007. “Deficits in Short-Latency Tracking Eye Movements after Chemical Lesions in Monkey Cortical Areas MT and MST.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 27 (3): 529–41. [CrossRef]
- Tehovnik, E.J., E. Froudarakis, F. Scala, S.M. Smirnakis, S.S. Patel, and A.S. Tolias. 2021. “Visuomotor Control in Mice and Primates.” Neuroscience & Biobehavioral Reviews 130 (November): 185–200. [CrossRef]
- Tusa, R. J., J. L. Demer, and S. J. Herdman. 1989. “Cortical Areas Involved in OKN and VOR in Cats: Cortical Lesions.” Journal of Neuroscience 9 (4): 1163–78. [CrossRef]
- Vélez-Fort, Mateo, Edward F. Bracey, Sepiedeh Keshavarzi, Charly V. Rousseau, Lee Cossell, Stephen C. Lenzi, Molly Strom, and Troy W. Margrie. 2018. “A Circuit for Integration of Head- and Visual-Motion Signals in Layer 6 of Mouse Primary Visual Cortex.” Neuron 98 (1): 179-191.e6. [CrossRef]
- Ventre, J., and S. Faugier-Grimaud. 1986. “Effects of Posterior Parietal Lesions (Area 7) on VOR in Monkeys.” Experimental Brain Research 62 (3): 654–58. [CrossRef]
- Ventre, Jocelyne. 1985a. “Cortical Control of Oculomotor Functions. I. Optokinetic Nystagmus.” Behavioural Brain Research 15 (3): 211–26. [CrossRef]
- Ventre, Jocelyne. 1985b. “Cortical Control of Oculomotor Functions. II. Vestibulo-Ocular Reflex and Visual-Vestibular Interaction.” Behavioural Brain Research 17 (3): 221–34. [CrossRef]
- Ventre-Dominey, Jocelyne. 2014. “Vestibular Function in the Temporal and Parietal Cortex: Distinct Velocity and Inertial Processing Pathways.” Frontiers in Integrative Neuroscience 8: 53. [CrossRef]
- Voges, Kai, Bin Wu, Laura Post, Martijn Schonewille, and Chris I. De Zeeuw. 2017. “Mechanisms Underlying Vestibulo-Cerebellar Motor Learning in Mice Depend on Movement Direction.” The Journal of Physiology 595 (15): 5301–26. [CrossRef]
- Wakita, Ryo, Soshi Tanabe, Kazunari Tabei, Asako Funaki, Takuma Inoshita, and Tomoo Hirano. 2017. “Differential Regulations of Vestibulo-Ocular Reflex and Optokinetic Response by β- and A2-Adrenergic Receptors in the Cerebellar Flocculus.” Scientific Reports 7 (June): 3944. [CrossRef]
- Wallace, Damian J., David S. Greenberg, Juergen Sawinski, Stefanie Rulla, Giuseppe Notaro, and Jason N. D. Kerr. 2013. “Rats Maintain an Overhead Binocular Field at the Expense of Constant Fusion.” Nature 498 (7452): 65–69. [CrossRef]
- Wang, Lupeng, and Richard J. Krauzlis. 2018. “Visual Selective Attention in Mice.” Current Biology 28 (5): 676-685.e4. [CrossRef]
- Wang, Lupeng, Mingna Liu, Mark A. Segraves, and Jianhua Cang. 2015. “Visual Experience Is Required for the Development of Eye Movement Maps in the Mouse Superior Colliculus.” Journal of Neuroscience 35 (35): 12281–86. [CrossRef]
- Wang, Lupeng, Kerry McAlonan, Sheridan Goldstein, Charles R. Gerfen, and Richard J. Krauzlis. 2020. “A Causal Role for Mouse Superior Colliculus in Visual Perceptual Decision-Making.” Journal of Neuroscience 40 (19): 3768–82. [CrossRef]
- Wibble, Tobias, and Tony Pansell. 2020. “Optokinetic Stimulation Induces Vertical Vergence, Possibly through a Non-Visual Pathway.” Scientific Reports 10 (1): 15544. [CrossRef]
- Wilks, Tenelle A., Alan R. Harvey, Jennifer Rodger, Tenelle A. Wilks, Alan R. Harvey, and Jennifer Rodger. 2013. Seeing with Two Eyes: Integration of Binocular Retinal Projections in the Brain. Functional Brain Mapping and the Endeavor to Understand the Working Brain. IntechOpen. [CrossRef]
- Willeke, Konstantin F., Xiaoguang Tian, Antimo Buonocore, Joachim Bellet, Araceli Ramirez-Cardenas, and Ziad M. Hafed. 2019. “Memory-Guided Microsaccades.” Nature Communications 10 (1): 3710. [CrossRef]
- Zahler, Sebastian H, David E Taylor, Joey Y Wong, Julia M Adams, and Evan H Feinberg. 2021. “Superior Colliculus Drives Stimulus-Evoked Directionally Biased Saccades and Attempted Head Movements in Head-Fixed Mice.” ELife 10 (December): e73081. [CrossRef]
- Zee, D. S., R. J. Tusa, S. J. Herdman, P. H. Butler, and G. Gücer. 1987. “Effects of Occipital Lobectomy upon Eye Movements in Primate.” Journal of Neurophysiology 58 (4): 883–907. [CrossRef]
- Zhang, Tong, Tatiana Malevich, Matthias P. Baumann, and Ziad M. Hafed. 2022. “Superior Colliculus Saccade Motor Bursts Do Not Dictate Movement Kinematics.” Communications Biology 5 (1): 1–14. [CrossRef]
- Zhao, Min, Timothy M. Gersch, Brian S. Schnitzer, Barbara A. Dosher, and Eileen Kowler. 2012. “Eye Movements and Attention: The Role of Pre-Saccadic Shifts of Attention in Perception, Memory and the Control of Saccades.” Vision Research 74 (December): 40–60. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).