Submitted:
05 June 2023
Posted:
06 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Description of Evogen Biogas Additive
2.2. Operation of the two full-scale biogas plants under investigation
2.3. Determination of Total Solids
2.4. Determination of Volatile Solids
2.6. Determination of FOS/TAC Ratio
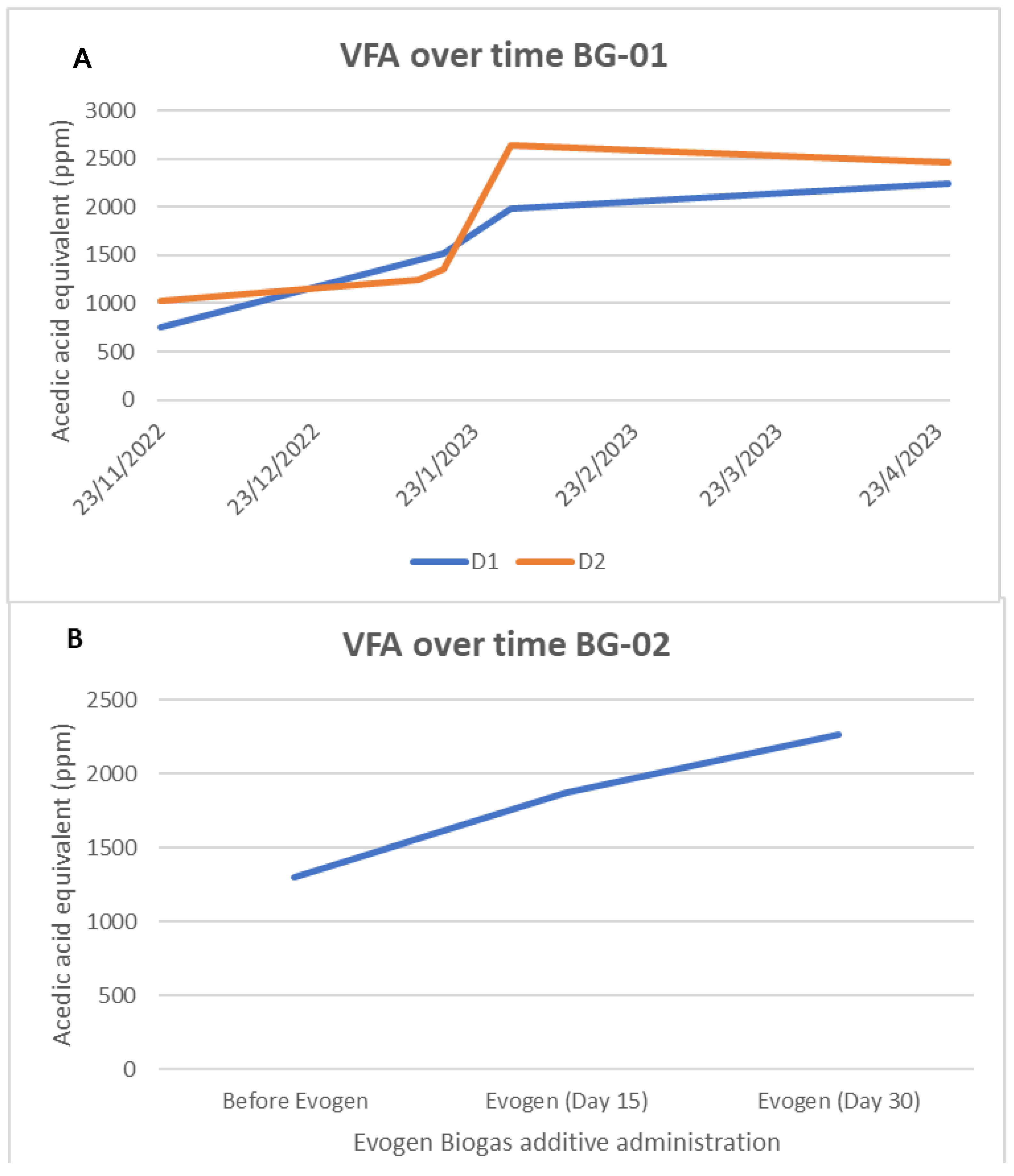
2.7. Determination of Volatile Fatty Acids Profile (VFAs)
2.8. Biochemical Methane Potential (BMP) assay
2.4. DNA extraction and 16S rRNA gene amplicon sequencing
2.5. Bioinformatics
3. Results and Discussion
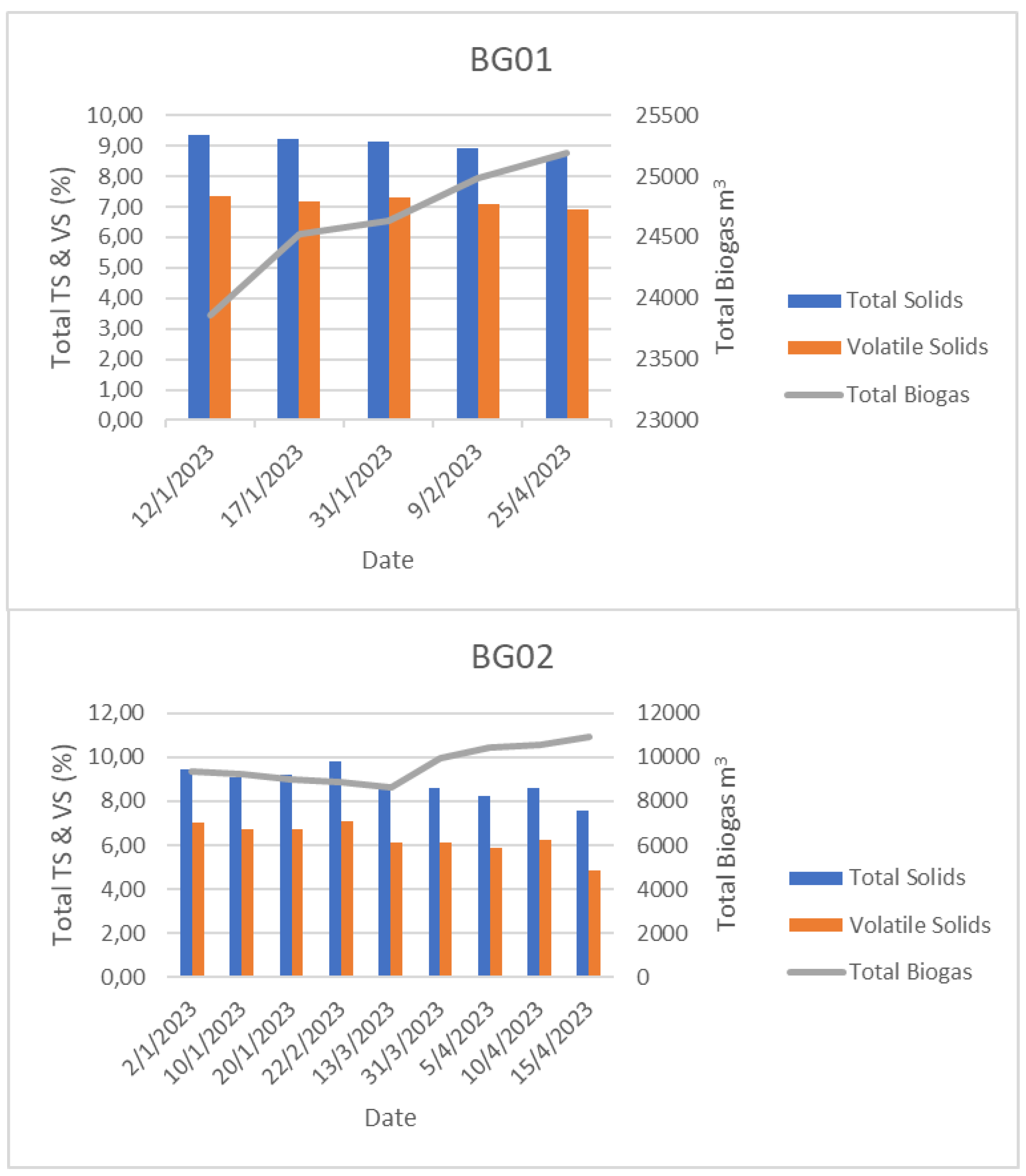
3.1. Physicochemical results during additive administration
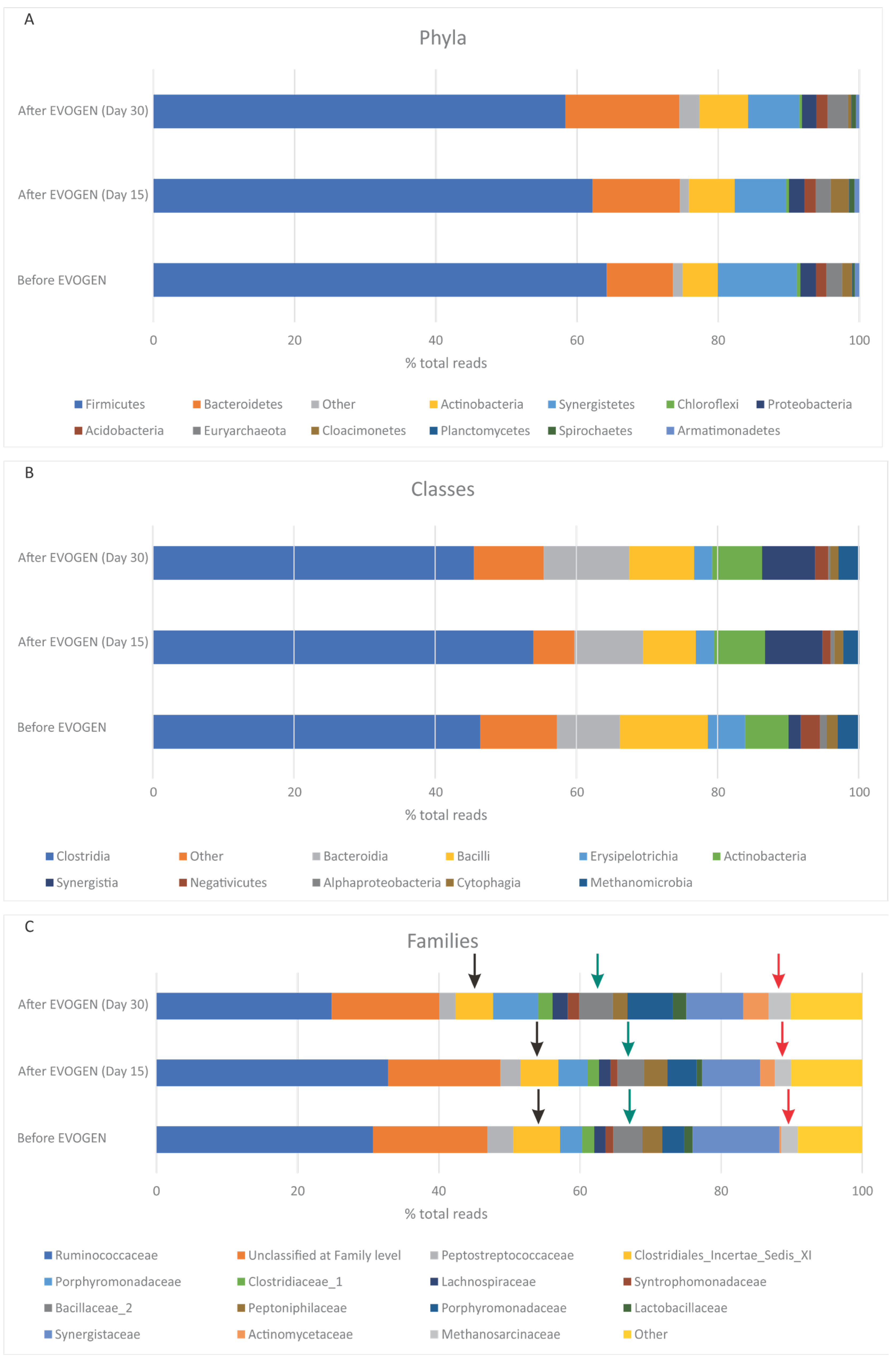
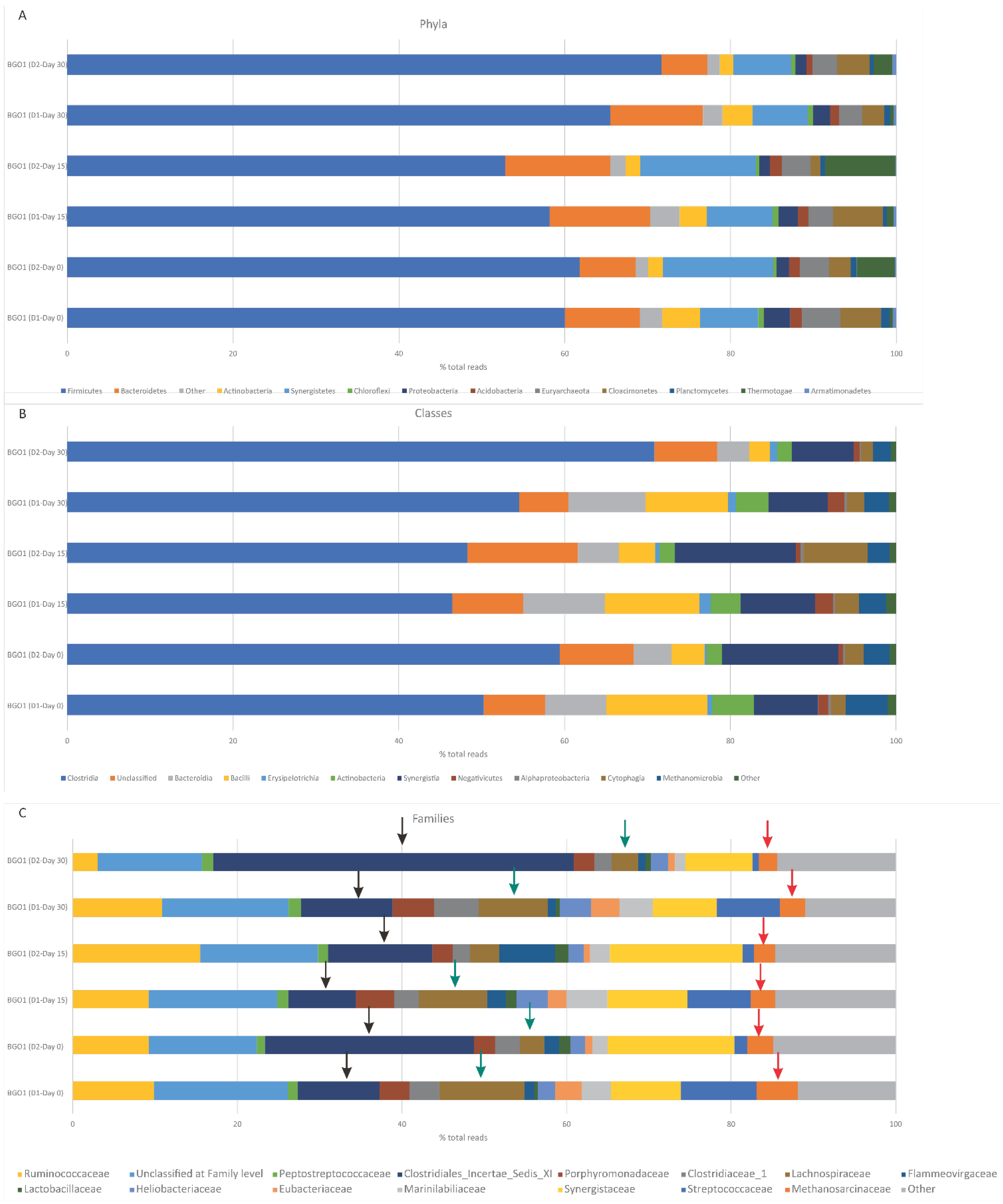
3.2. Microbiome Alternation during additive administration
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lisowyj, M.; Wright, M.M. A review of biogas and an assessment of its economic impact and future role as a renewable energy source. Rev. Chem. Eng. 2020, 36, 401–421. [Google Scholar] [CrossRef]
- Iglesias, R.; Muñoz, R.; Polanco, M.; Díaz, I.; Susmozas, A.; Moreno, A.D.; Guirado, M.; Carreras, N.; Ballesteros, M. Biogas from anaerobic digestion as an energy vector: Current upgrading development. Energies 2021, 14, 1–30. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, S.; Singh, D.; Shekher Giri, B.; Mishra, P.K. Barriers in biogas production from the organic fraction of municipal solid waste: A circular bioeconomy perspective. Bioresour. Technol. 2022, 362, 127671. [Google Scholar] [CrossRef] [PubMed]
- Werkneh, A.A. Biogas impurities: environmental and health implications, removal technologies and future perspectives. Heliyon 2022, 8, e10929. [Google Scholar] [CrossRef] [PubMed]
- Roubík, H.; Mazancová, J.; Banout, J.; Verner, V. Addressing problems at small-scale biogas plants: A case study from central Vietnam. J. Clean. Prod. 2016, 112, 2784–2792. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Sun, Y.; Yu, J.; Zheng, Z.; Li, S.; Cui, Z.; Hao, J.; Li, G. Effects of intermittent mixing mode on solid state anaerobic digestion of agricultural wastes. Chemosphere 2020, 248, 126055. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.P.; Nguyen, L.N.; Wang, Q.; Ngo, H.H.; Liu, Q.; Zhang, X.; Nghiem, L.D. Hydrogen sulphide management in anaerobic digestion: A critical review on input control, process regulation, and post-treatment. Bioresour. Technol. 2022, 346, 126634. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Kulig, A.; Lelicińska-Serafin, K. Odour Emissions of Municipal Waste Biogas Plants—Impact of Technological Factors, Air Temperature and Humidity. Appl. Sci. 2020, Vol. 10, Page 1093 2020, 10, 1093. [Google Scholar] [CrossRef]
- Hewitt, J.; Holden, M.; Robinson, B.L.; Jewitt, S.; Clifford, M.J. Not quite cooking on gas: Understanding biogas plant failure and abandonment in Northern Tanzania. Renew. Sustain. Energy Rev. 2022, 165, 112600. [Google Scholar] [CrossRef]
- Liu, M.; Wei, Y.; Leng, X. Improving biogas production using additives in anaerobic digestion: A review. J. Clean. Prod. 2021, 297, 126666. [Google Scholar] [CrossRef]
- Paritosh, K.; Yadav, M.; Chawade, A.; Sahoo, D. Additives as a Support Structure for Specific Biochemical Activity Boosts in Anaerobic Digestion : A Review. 2020, 8, 1–17.
- Dompara, I.; Maragkaki, A.; Papastefanakis, N.; Floraki, C.; Vernardou, D.; Manios, T. Effects of Different Materials on Biogas Production during Anaerobic Digestion of Food Waste. 2023, 1–13.
- Fugol, M.; Prask, H.; Szlachta, J.; Dyjakon, A.; Pasławska, M.; Szufa, S. Improving the Energetic Efficiency of Biogas Plants Using Enzymatic Additives to Anaerobic Digestion. 2023, 1–12.
- Barua, S.; Dhar, B.R. Bioresource Technology Advances towards understanding and engineering direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 244, 698–707. [Google Scholar] [CrossRef]
- Eduok, S.; John, O.; Ita, B.; Inyang, E.; Coulon, F. Enhanced Biogas Production From Anaerobic Co-digestion of Lignocellulosic Biomass and Poultry Feces Using Source Separated Human Urine as Buffering Agent. 2018, 6, 1–9. 6.
- Tian, W.; Gu, L.; Li, S.; Deng, R.; Zhu, L.; Li, W.; Liu, F. Facilitating Digester Recovery from Acid Inhibition at High Organic Load Rates by Limited Calcium Peroxide Addition. ACS Sustain. Chem. Eng. 2022, 10, 8184–8195. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Sun, Y. Acclimation of Acid-Tolerant Methanogenic Culture for Bioaugmentation: Strategy Comparison and Microbiome Succession. ACS Omega 2020, 5, 6062–6068. [Google Scholar] [CrossRef]
- Leca, E.; Zennaro, B.; Sambusiti, C. Use of additives to improve collective biogas plant performances : A comprehensive review er o. 2023, 65. 65.
- Tale, V.P.; Maki, J.S.; Struble, C.A.; Zitomer, D.H. Methanogen community structure-activity relationship and bioaugmentation of overloaded anaerobic digesters. Water Res. 2011, 45, 5249–5256. [Google Scholar] [CrossRef] [PubMed]
- Lovato, G.; Kovalovszki, A.; Alvarado-morales, M.; Domingues, A.; Angelidaki, I. Modelling bioaugmentation : Engineering intervention in anaerobic digestion. 2021, 175. 175.
- Shanmugam, S.; Sun, C.; Chen, Z.; Wu, Y.R. Enhanced bioconversion of hemicellulosic biomass by microbial consortium for biobutanol production with bioaugmentation strategy. Bioresour. Technol. 2019, 279, 149–155. [Google Scholar] [CrossRef]
- Mistry, A.N.; Kachenchart, B.; Pinyakong, O.; Assavalapsakul, W.; Jitpraphai, S.M.; Somwangthanaroj, A.; Luepromchai, E. Bioaugmentation with a defined bacterial consortium: A key to degrade high molecular weight polylactic acid during traditional composting. Bioresour. Technol. 2023, 367, 128237. [Google Scholar] [CrossRef] [PubMed]
- Additive, E.B. Optimising the AD circular economy. Filtr. + Sep. 2021, 58, 8–10. [Google Scholar]
- Król, M.; Syguła-Cholewińska, J.; Sawoszczuk, T. Zeolite-Supported Aggregate as Potential Antimicrobial Agents in Gypsum Composites. Materials (Basel). 2022, 15. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Cho, W. Il; Chung, M.S. Bacillus spores: a review of their properties and inactivation processing technologies. Food Sci. Biotechnol. 2020, 29, 1447. [Google Scholar] [CrossRef]
- Danilova, I.; Sharipova, M. The Practical Potential of Bacilli and Their Enzymes for Industrial Production. Front. Microbiol. 2020, 11, 1782. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Chen, Y.; Ndegwa, P. Association between methane yield and microbiota abundance in the anaerobic digestion process: A meta-regression. Renew. Sustain. Energy Rev. 2021, 135, 110212. [Google Scholar] [CrossRef]
- Yi, J.; Dong, B.; Jin, J.; Dai, X. Effect of increasing total solids contents on anaerobic digestion of food waste under mesophilic conditions: Performance and microbial characteristics analysis. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Chen, Y.; Ndegwa, P. Association between methane yield and microbiota abundance in the anaerobic digestion process: A meta-regression. Renew. Sustain. Energy Rev. 2021, 135, 110212. [Google Scholar] [CrossRef]
- Tzun-Wen Shaw, G.; Liu, A.C.; Weng, C.Y.; Chou, C.Y.; Wang, D. Inferring microbial interactions in thermophilic and mesophilic anaerobic digestion of HOG waste. PLoS One 2017, 12, 1–22. [Google Scholar]
- Gao, M.; Guo, B.; Zhang, L.; Zhang, Y.; Liu, Y. Microbial community dynamics in anaerobic digesters treating conventional and vacuum toilet flushed blackwater. Water Res. 2019, 160, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Mutungwazi, A.; Ijoma, G.N.; Ogola, H.J.O.; Matambo, T.S. Physico-Chemical and Metagenomic Profile Analyses of Animal Manures Routinely Used as Inocula in Anaerobic Digestion for Biogas Production. Microorganisms 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yang, Y.; Xu, K.; Long, X.; Zhang, Y.; Liu, H.; Chen, T.; Li, J. Distinguishing anaerobic digestion from electrochemical anaerobic digestion: Metabolic pathways and the role of the microbial community. Chemosphere 2023, 326, 138492. [Google Scholar] [CrossRef]
- Carr, S.; Buan, N.R. Insights into the biotechnology potential of Methanosarcina. Front. Microbiol. 2022, 13, 4577. [Google Scholar] [CrossRef]
- Pyzik, A.; Ciezkowska, M.; Krawczyk, P.S.; Sobczak, A.; Drewniak, L.; Dziembowski, A.; Lipinski, L. Comparative analysis of deep sequenced methanogenic communities: Identification of microorganisms responsible for methane production. Microb. Cell Fact. 2018, 17, 1–16. [Google Scholar] [CrossRef]
- Rodger, B.B.; Andrew, E.D.; Eugene, R.W. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; APHA: Washington, D.C. : American Public Health Association, 2017. [Google Scholar]
| Biogas Plant | BG01 | BG02 |
|---|---|---|
|
ElectricalPower Capacity |
2 MW | 1 MW |
| Pre-tank | 1 pre-tank | 1 pre-tank |
| Daily Supply | 45 tn/d: corn silage 8 tn/d: LD recirculation from storage tank 8 tn/d: glycerol 21 tn/d: waste residues 20 tn/d: pomace (olive, fruits) |
8 tn/d: mix corn silage, potatoes, sunflower 7 tn/d: Rye silage 3tn/d: Beetroot 30 tn/day: chicken manure (solid) 2tn/day: liquid digested residue (after separator) 20tn/d: Organic waste (food waste, etc.) 30tn/d: Whey 60tn/d: Cattle manure (liquid) |
| Feeding Rate | 2,1 tn/ 30min | 160 tn/day |
| 1st Digester (D1) | 4000 m3 | 4250 m3 |
| Temperature | 44 oC | 41 oC |
| HRT | 50 days | 40 days |
| Stirring | Constantly | Constantly |
| 2nd Digester (D2) | 2.800 m3 | - |
| Temperature | 39,5 oC | - |
| HRT | 20 days | - |
| Recirculation of Digested Residue | Yes | Yes |
| Biogas Plant | Sampling Day | pH (μοph) | FOS (mgc/l) | TAC (mgCaCO3) | FOS/TAC ratio | TS (%) | VS (%) | NFE (%DM) | Fats (%) | Proteins (%) | Fibers (%OM) | Theoritical gas yield (L/kg) | Methane (%) | N-NH4 (mgΝ/L) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BG01 (D1) | Day 0 | 8.10 | 2321 | 10592 | 0.22 | 9.36 | 7.37 | - | - | - | - | - | - | 2608 | |
| Day 15 | 7.70 | 2562 | 10595 | 0.24 | 8.91 | 7.10 | - | - | - | - | - | - | 1547 | ||
| Day 40 | 7.40 | 2498 | 10408 | 0.24 | 8.66 | 6.93 | - | - | - | - | - | - | 3156 | ||
| BG01 (D2) | Day 0 | 7.70 | 4432 | 9533 | 0.47 | 7.60 | 5.49 | - | - | - | - | - | - | 944 | |
| Day 15 | 7.70 | 4342 | 6348 | 0.68 | 7.50 | 5.31 | - | - | - | - | - | - | 1079 | ||
| Day 40 | 8.50 | 3724 | 11748 | 0.32 | 7.47 | 5.09 | - | - | - | - | - | - | 2045 | ||
| BG02 (D1) | Day 0 | Before Evogen | 7.90 | 4451 | 14377 | 0.31 | 9.44 | 7.01 | 14.80 | 0.56 | 3.89 | 1.16 | 37.00 | 62.30 | 2609 |
| Day 15 | 8.10 | 2368 | 12203 | 0.19 | 8.56 | 6.10 | 4.95 | 0.51 | 3.75 | 1.41 | 30.20 | 63.90 | 1559 | ||
| Day 30 | During Evogen | 8.10 | 2689 | 14344 | 0.19 | 8.57 | 6.09 | - | - | - | - | - | - | 3044 | |
| Day 40 | 8.20 | 2985 | 14270 | 0.21 | 8.58 | 6.24 | 8.10 | 0.32 | 3.62 | 1.61 | 30.70 | 62.50 | 2861 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





