1. Introduction
Natural enzymes show several disadvantages as follows [
1]; (I) low stability (thermal and narrow pH range) (II) difficulty in recovery, and (III) no reusability of the enzyme. Commonly, for overcoming these drawbacks, the enzyme immobilization process has been developed [
2,
3,
4]. Another approach for overcome to these difficulties is utilizing highly stable nanoparticles with high enzyme-like activity in the enzyme-catalyzed reactions [
1,
5]. In fact, the recent progress in nanochemistry [
17] and material science open a new door for developing high-performance nano-supports such as MOFs [
6], catalytic materials [
7,
8], and nanoparticles with enzyme-like activity [
5,
9,
10,
11,
12,
13].
Nanozymes or nanoparticles with excellent enzyme-like activity are attracted much attention due to their stability and higher efficiency compared to natural enzymes [
1,
5]. Recently, nanozymes had been used for different applications for instance, analytical sensing of species, biocatalysis of reactions instead of natural enzymes, dye degradation [
14], water treatment [
15], food quality checking [
16] and etc.. Up to now a wide range of these nanomaterials, for example, MnO2 nanoparticles [
18], BiOI-NFs [
19], silver nanoparticles [
20], BSA-Au nanoclusters [
21,
22], SiO2-Fe3O4 NPs [
23], and some MOFs, e.g., NEQC-340 [
24], have been used as nanozymes with high pseudo-peroxidation activity. Since nanozymes can catalyze the oxidation of peroxidase substrates to their corresponding colored products, they have been used for analytical purposes [
15,
16]. For example, spectrophotometric measurement of several analytes such as cysteine [
25], hydrogen peroxide [
26], mercury ions [
27], triacetone triperoxide [
18], glucose [
28], and glutathione [
24] was performed by different nanozyme-based sensors. Recently catalytic property of gold nanozymes and their excellent peroxidase-like activity brought them up as safe, green, and high throughput alternatives of natural peroxidase enzymes to developed enzyme-mediated sensors [
21,
22,
27]. Usually 3,3',5,5'-tetramethylbenzidine (TMB), and 3,3'-diaminobenzidine (DAB) substrates have been used as the peroxidase substrates, and their corresponding oxidation products were utilized as the analytical probes for sensing aims [
9,
11,
13,
21,
22,
23,
24]. However, it is well-known that the affinity of these substrates for binding to enzyme/nanozyme is not the same, resulting in different kinetics indexes. As the BSA-gold nanozymes are considered artificial peroxidase enzymes, hence, the kinetics indexes of these nanozymes should also be varied by varying their substrate.
Hence, in this work, a comparative study was performed on the kinetics performances of BSA-gold nanozymes for enzyme-mediated oxidations of 3,3’,5,5’-thetramethylbenzidine, and 3,3’-diaminobenzidine. To do this, initially, BSA-gold nanozymes were synthesized via a protein-directed method utilizing bovine serum albumin (BSA) as both reductant and stabilizer. Thereafter, the as-prepared nanozymes were characterized by recording their TEM image. Afterward, the Michaelis–Menten model and the Lineweaver–Burk method were utilized for the estimation of kinetic parameters of BSA-gold nanozymes for both DAB and TMB.
2. Experimental Section
2.1. Materials
DAB was obtained from Sigma Aldrich Company. Deionized water was obtained from Zolal Teb chemical company (Iran). Other materials were obtained from Merck Company in their analytical or synthesis grades.
2.2. Instrumentations
A Metrohm 827 pH lab pH meter equipped with a combined glass electrode was applied for buffer preparation. An Ultrospec 4000 UV-Vis spectrophotometer manufactured by Pharmacia Biotech (Biochrom) Ltd. equipped with SWIFT Software was utilized for spectrophotometric assay of nanozyme activity toward oxidation of both DAB and TMB. The size and morphology of the as-prepared BSA-Au nanozymes were characterized by the TEM imaging method utilizing a transmission electron microscope (Zeiss, model EL10C) operated at an accelerating voltage of 80 kV.
2.3. Synthesis of BSA-gold nanozymes
BSA-gold nanozymes were synthesized based on the previously reported method [
21], briefly, 5.0 mL of 10.0 mM HAuCl
4.4H
2O solution was introduced to 5.0 mL 50 mg mL
-1 bovine serum albumin (BSA), followed by stirring at 37 °C and adding 500 µL of 1.0 M NaOH. The solution was incubated at 37 °C for 12 h to synthesize the BSA-gold nanozymes.
2.4. Synthesis of BSA-gold nanozymes
For the DAB assay, 80.0 µL BSA- gold nanozymes were added into 1.3 mL of phosphate buffer solution (pH 7.0, 0.4 M) containing 200.0 µL of DAB (different concentrations) and 40.0 µL of HP (with a final concentration of 0.24 M) and thoroughly mixed at ambient temperature. The oxidation was followed for 25.0 min to complete the production of the corresponding indamine polymer (i.e., polyDAB). Thereafter, the nanozyme activity (nM sec-1) was measured by probing the absorbance of the resulting polyDAB at 460 nm considering a molecular extinction coefficient ɛ=5500 molar cm-1.
Regarding the TMB assay, 40 µL hydrogen peroxide solution (final concentrations of 0.24 M), 200 µL of TMB (different concentrations), and 80 µL of BSA-gold nanozymes were introduced to 1.3 mL of acetate buffer (0.3 M; pH, 0.4), followed by incubation for about 10 minutes at ambient temperature. After that, the absorbance of the blue-colored TMB-ox at 658 nm was used for nanozyme activity (nM sec-1) calculation considering a ɛ of 39000 M cm-1. Finally, the kinetic parameters of the as-prepared BSA-gold nanozymes for both DAB and TMB were investigated based on Michaelis–Menten equation and the Lineweaver–Burk method as the standard models for evaluation of the enzyme kinetics performances.
3. Results and discussion
3.1. Characterization of BSA-gold nanozymes
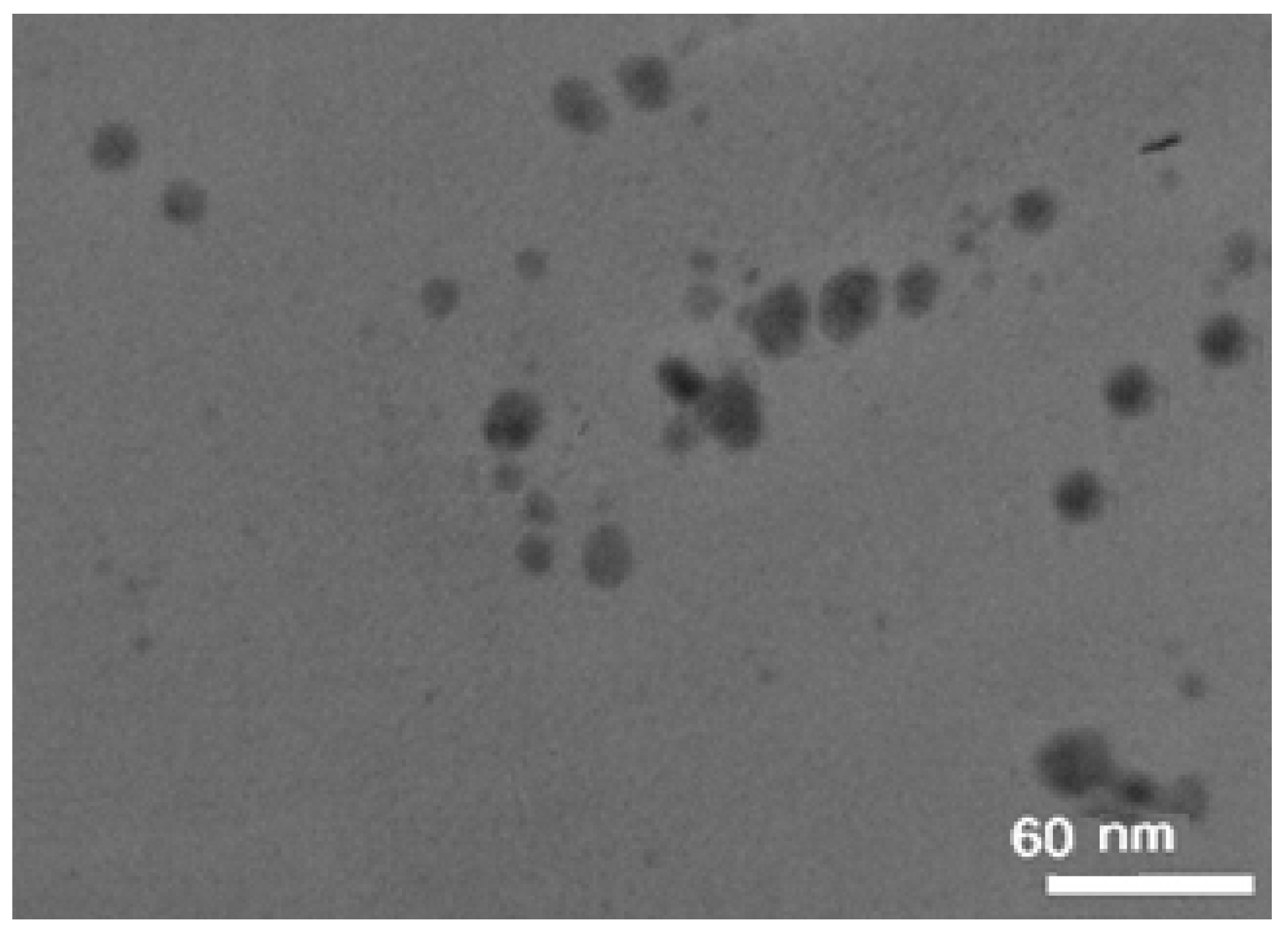
BSA-gold nanozymes were synthesized via a protein-directed method utilizing bovine serum albumin (BSA) as both reductant and stabilizer. Thereafter, the as-prepared nanozymes were characterized by recording their TEM image (
Figure 1). The results showed that the as-prepared BSA-gold nanozymes have a size distribution of 7.7-18.3 nm with a mean size of about 13.2 nm. As can be seen from
Figure 1, the as-prepared BSA-gold nanozymes reveal semi-spherical morphological properties with an approximately narrow size distribution which makes them suitable for enzyme-mimicking applications, considering this fact that the size of nanozymes can strongly affect their enzyme-like activity [
21].
3.2. Kinetics parameters of BSA-gold nanozyme for TMB oxidation
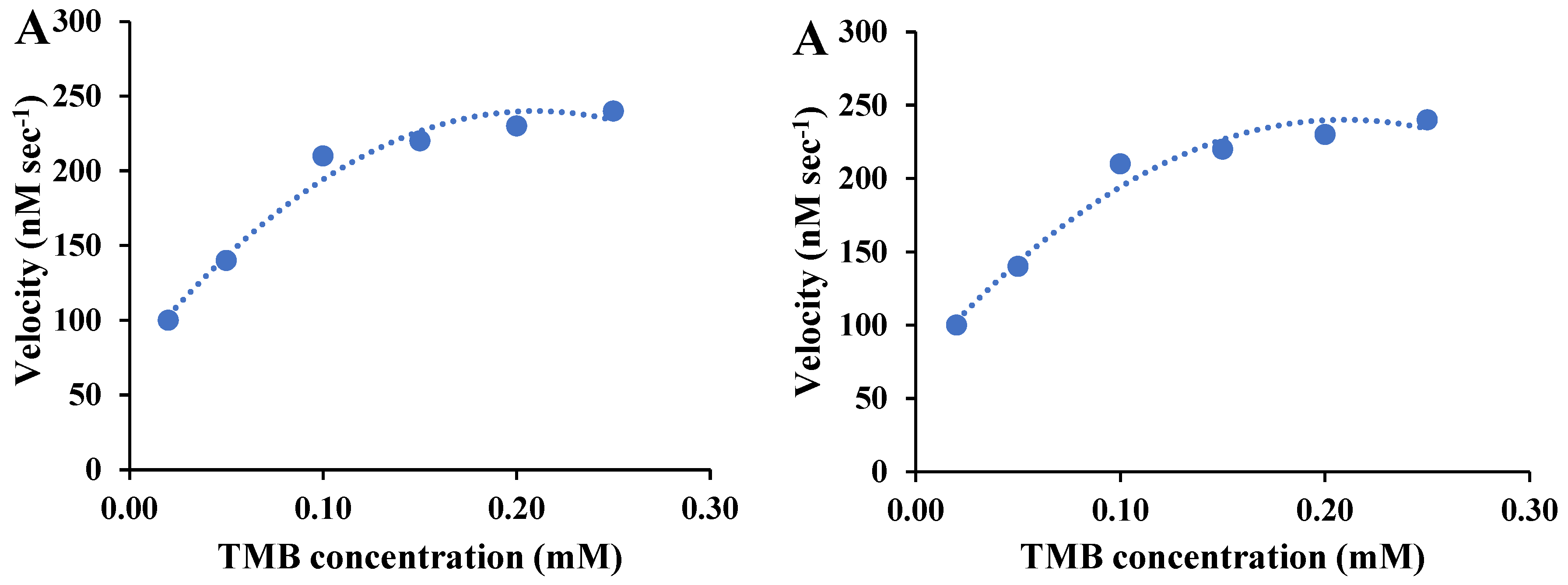
The kinetics studies of the as-prepared BSA-gold nanozymes were performed by measuring their activity as a function of DAB or TMB (i.e., enzyme-substrate) concentrations. The kinetic parameter, Vmax and Km was then calculated by using Michaelis–Menten and the linear plot of Lineweaver–Burk for both substrates. The Michaelis–Menten plot for oxidation of TMB catalyzed by BSA-gold nanozymes was shown in
Figure 2A, revealing that the rate of nanozyme-mediated oxidation of TMB was increased by increasing the substrate concertation and then leveled off. To estimate the kinetic parameters of BSA-gold nanozymes toward TMB oxidation, the Lineweaver–Burk plot was constructed (
Figure 2B). Based on
Figure 3B, the V
max and K
m of the as-prepared nanoymes toward TMB oxidation were calculated at about 263 nM sec
-1 and 0.03 mM, in order.
3.3. Kinetics parameters of BSA-gold nanozyme for DAB oxidation
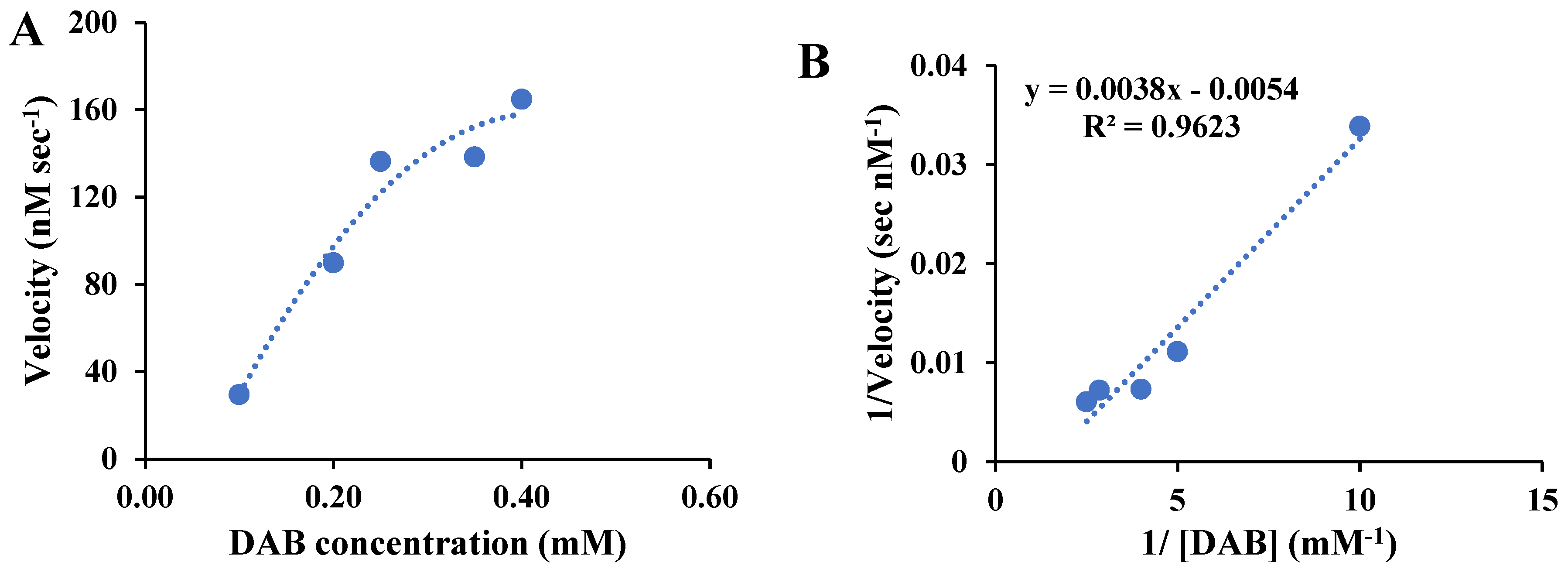
To evaluate the kinetics performances of the as-prepared BSA-gold nanozymes for DAB oxidation, the Michaelis–Menten plot for DAB oxidation by hydrogen peroxide in the presence of the BSA-gold nanozymes as the peroxidase-mimicking agents were obtained, and the results are shown in
Figure 3A, revealed that the rate of nanozyme-mediated DAB oxidation was increased by increasing the substrate concertation and then leveled off which is same of the TMB oxidation but the TMB oxidation rate was found to be higher than that of the DAB oxidation by BSA-gold nanozymes. To explore more precise the kinetic performances of BSA-gold nanozymes toward DAB oxidation, the Lineweaver–Burk plot was constructed for estimation of Km and Vmax of BSA-gold enzymes-mediated oxidation of DAB (
Figure 3B), revealing a Vmax and Km of 185 nM sec
-1 and 0.72 mM, in order, for DAB oxidation.
3.4. Comparison of kinetic performances of BSA-gold nanozymes for different substrates
Table 1 shows the kinetic parameters of the as-prepared BSA-gold nanozymes for both DAB and TMB. As seen in this table, the V
max of DAB oxidation was found to be lower than that of the TMB oxidation which pointed to the fact that the catalytic efficiency of the as-prepared BSA-gold nanozymes toward TMB is significantly higher than their efficiency for the DAB. The ratio of V
max (TMB)/V
max (DAB) was calculated as 1.42, revealing that the catalytic efficiency of the as-prepared BSA-gold nanozymes for TMB oxidation is about 1.5-fold higher than their catalytic performances for DAB oxidation. The difference between the kinetic indexes of TMB and DAB may be related to their different oxidation pathways and their different reactivity. In fact, the DAB oxidizes via an n-electron irreversible oxidation pathway to produce an indamine polymer. While TMB nanozyme-mediated oxidation has occurred upon a 2-electron reversible mechanism for the production of a cation radical. These different pathways resulted in different kinetic performances. Besides, the K
m value for DAB was found to be 24-fold higher than that for TMB. It is well-known that the K
m shows the affinity of an enzyme to its substrate which its lower value assigned to a higher affinity [
2,
3,
4]. Hence, the very higher K
m for DAB reveals the very lower affinity of DAB for binding to BSA-gold nanozyme active nodes compared to its alternative substrate, TMB. This difference can be related to the different reactivity of DAB and TMB.
4. Conclusions
Herein, a comparative study was performed on the kinetics performances of BSA-gold nanozymes for enzyme-mediated oxidations of 3,3’,5,5’-thetramethylbenzidine, and 3,3’-diaminobenzidine. The results showed that the Km value of BSA-gold nanozymes was 0.03 mM and 0.72 mM toward TMB and DAB, in order, to reveal the higher affinity (lower Km) of TMB for binding to nanozyme active nodes compared to its alternative substrate, DAB. In contrast, the Vmax was found to be 263 nM sec-1 and 185 nM sec-1 for nanozmye-mediated oxidation of TMB and DAB, respectively. The higher Vmax of the nanozyme-mediated oxidation of TMB revealed that the catalytic efficiency of BSA-Au nanozymes toward TMB oxidation is higher (about 1.5-fold) than that of the DAB oxidation. The difference between the kinetic indexes of TMB and DAB may be related to their different oxidation pathways and their different reactivity. In fact, the DAB oxidizes via an n-electron irreversible oxidation pathway to produce an indamine polymer. While TMB nanozyme-mediated oxidation has occurred upon a 2-electron reversible mechanism for the production of a cation radical. These different pathways resulted in different kinetic performances.
Acknowledgments
The authors gratefully thank the Hormozi Laboratory of Chemistry and Biochemistry for the support of this work.
References
- Zhou, Y., Liu, B., Yang, R., & Liu, J. (2017). Filling in the gaps between nanozymes and enzymes: challenges and opportunities. Bioconjugate chemistry, 28(12), 2903-2909. [CrossRef]
- Hormozi Jangi, S. R., & Akhond, M. (2021). High throughput urease immobilization onto a new metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) for water treatment and safe blood cleaning. Process Biochemistry, 105, 79-90. [CrossRef]
- Hormozi Jangi, S. R., & Akhond, M. (2022). Introducing a covalent thiol-based protected immobilized acetylcholinesterase with enhanced enzymatic performances for biosynthesis of esters. Process Biochemistry, 120, 138-155. [CrossRef]
- Hormozi Jangi, S. R., Akhond, M., & Dehghani, Z. (2020). High throughput covalent immobilization process for improvement of shelf-life, operational cycles, relative activity in organic media and enzymatic kinetics of urease and its application for urea removal from water samples. Process Biochemistry, 90, 102-112. [CrossRef]
- Hormozi Jangi, A. R., Hormozi Jangi, M. R., & Hormozi Jangi, S. R. (2020). Detection mechanism and classification of design principles of peroxidase mimic based colorimetric sensors: A brief overview. Chinese Journal of Chemical Engineering, 28(6), 1492-1503. [CrossRef]
- Hormozi Jangi, S. R. (2023). Synthesis and characterization of magnesium-based metal-organic frameworks and investigating the effect of coordination solvent on their biocompatibility. Chemical Research and Nanomaterials, 1(4), 1-9.
- Hormozi Jangi S. R.; Akhond M. (2020). High throughput green reduction of tris (p-nitrophenyl) amine at ambient temperature over homogenous AgNPs as H-transfer catalyst. Journal of Chemical Sciences, 132, 1-8. [CrossRef]
- Hormozi Jangi, S. R. (2023). Low-temperature destructive hydrodechlorination of long-chain chlorinated paraffins to diesel and gasoline range hydrocarbons over a novel low-cost reusable ZSM-5@ Al-MCM nanocatalyst: a new approach toward reuse instead of common mineralization. Chemical Papers, 1-15. [CrossRef]
- Hormozi Jangi, S. R., & Dehghani, Z. (2023). Spectrophotometric quantification of hydrogen peroxide utilizing silver nanozyme. Chemical Research and Nanomaterials. https://crn.shiraz.iau.ir/article_701960.html?lang=en.
- Zeng, X., Ruan, Y., Chen, Q., Yan, S., & Huang, W. (2023). Biocatalytic cascade in tumor microenvironment with a Fe2O3/Au hybrid nanozyme for synergistic treatment of triple negative breast cancer. Chemical Engineering Journal, 452, 138422. [CrossRef]
- Hormozi Jangi, S.R. (2023) Evaluating the Effect of Shelf-Storage, Daylight, and Air Oxygen on the Peroxidase-like Activity of Unmodified Silver Nanoparticles. Preprints.org, 2023052106. [CrossRef]
- Yang, X., Xiang, J., Su, W., Guo, J., Deng, J., Tang, L., ... & Zhao, J. (2023). Modulating Pt nanozyme by using isolated cobalt atoms to enhance catalytic activity for alleviating osteoarthritis. Nano Today, 49, 101809. [CrossRef]
- Hormozi Jangi, S. R. (2023). Effect of daylight and air oxygen on nanozymatic activity of unmodified silver nanoparticles: Shelf-stability. Qeios. [CrossRef]
- Chen, X., Wang, Y., Feng, M., Deng, D., Xie, X., Deng, C., ... & Yang, X. (2023). Dual-active-site Fe/Cu single-atom nanozymes with multifunctional specific peroxidase-like properties for S2− detection and dye degradation. Chinese Chemical Letters, 34(6), 107969. [CrossRef]
- Bittencourt, G. A., de Souza Vandenberghe, L. P., Martínez-Burgos, W. J., Valladares-Diestra, K. K., de Mello, A. F. M., Maske, B. L., ... & Soccol, C. R. (2023). Emerging contaminants bioremediation by enzyme and nanozyme-based processes–a review. Iscience. [CrossRef]
- Li, Z., Hu, J., Zhan, Y., Shao, Z., Gao, M., Yao, Q., ... & Wang, L. (2023). Coupling Bifunctional Nanozyme-Mediated Catalytic Signal Amplification and Label-Free SERS with Immunoassays for Ultrasensitive Detection of Pathogens in Milk Samples. Analytical Chemistry, 95(15), 6417-6424. [CrossRef]
- Hormozi Jangi, S. R., & Akhond, M. (2021). Ultrasensitive label-free enantioselective quantification of d-/l-leucine enantiomers with a novel detection mechanism using an ultra-small high-quantum yield N-doped CDs prepared by a novel highly fast solvent-free method. Sensors and Actuators B: Chemical, 339, 129901. [CrossRef]
- Hormozi Jangi, S. R., Akhond, M., & Absalan, G. (2020). A field-applicable colorimetric assay for notorious explosive triacetone triperoxide through nanozyme-catalyzed irreversible oxidation of 3, 3′-diaminobenzidine. Microchimica Acta, 187, 431. [CrossRef]
- Ahmadi-Leilakouhi, B., Hormozi Jangi, S. R., & Khorshidi, A. (2023). Introducing a novel photo-induced nanozymatic method for high throughput reusable biodegradation of organic dyes. Chemical Papers, 77(2), 1033-1046. [CrossRef]
- Geng, X., Xue, R., Liang, F., Liu, Y., Wang, Y., Li, J., & Huang, Z. (2023). Synergistic effect of silver nanoclusters and graphene oxide on visible light-driven oxidase-like activity: Construction of a sustainable nanozyme for total antioxidant capacity detection. Talanta, 259, 124565. [CrossRef]
- Hormozi Jangi, S. R., Akhond, M., & Absalan, G. (2020). A novel selective and sensitive multinanozyme colorimetric method for glutathione detection by using an indamine polymer. Analytica Chimica Acta, 1127, 1-8. [CrossRef]
- Akhond, M., Hormozi Jangi, S. R., Barzegar, S., & Absalan, G. (2020). Introducing a nanozyme-based sensor for selective and sensitive detection of mercury (II) using its inhibiting effect on production of an indamine polymer through a stable n-electron irreversible system. Chemical Papers, 74, 1321-1330. [CrossRef]
- Hormozi Jangi, S. R., Davoudli, H. K., Delshad, Y., Hormozi Jangi, M. R., & Hormozi Jangi, A. R. H. (2020). A novel and reusable multinanozyme system for sensitive and selective quantification of hydrogen peroxide and highly efficient degradation of organic dye. Surfaces and Interfaces, 21, 100771. [CrossRef]
- Hormozi Jangi, S. R., & Akhond, M. (2020). Synthesis and characterization of a novel metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) and its application for constructing a reusable nanozyme-based sensor for selective and sensitive glutathione quantification. Microchemical Journal, 158, 105328. [CrossRef]
- Singh, A. K., Bijalwan, K., Kaushal, N., Kumari, A., Saha, A., & Indra, A. (2023). Oxidase-like Nanozyme Activity of Manganese Metal–Organic Framework Nanosheets for Colorimetric and Fluorescence Sensing of l-Cysteine. ACS Applied Nano Materials. [CrossRef]
- Li, T., Wang, Y., Liu, W., Fei, H., Guo, C., & Wei, H. (2023). Nanoconfinement-Guided Construction of Nanozymes for Determining H2O2 Produced by Sonication. Angewandte Chemie International Edition, 62(12), e202212438. [CrossRef]
- Zhang, K., Luo, M., Rao, H., Liu, H., Li, J., Chen, J., ... & Xue, Z. (2023). Integrating plasmonic and nanozyme responses of gold nanoparticles for enhancing photothermometric sensing. Sensors and Actuators B: Chemical, 134067. [CrossRef]
- Chen, J., Liu, X., Zheng, G., Feng, W., Wang, P., Gao, J., ... & Wang, Q. (2023). Detection of glucose based on noble metal nanozymes: Mechanism, activity regulation, and enantioselective recognition. Small, 19(8), 2205924. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).